Abstract
Etomidate, an intravenous imidazole general anaesthetic, is thought to produce anaesthesia by modulating or activating ionotropic Cl−-permeable GABAA receptors. Chromaffin cells are known to express functional GABAA receptors with properties similar to their neuronal counterparts. We have shown that activation of the GABAA receptors, with specific GABAA agonists, leads to cellular excitation. Our goal was to determine whether etomidate mimicked this response and to explore the functional consequences of this activation. Imaging experiments with the Ca2+-indicator dye fura-2 were used to assay [Ca2+]i. Bovine adrenal chromaffin cells were superfused with a variety of GABAA-selective drugs to determine their effects on [Ca2+]i. Amperometric measurements were used to assay catecholamine release in real-time. We show that bovine adrenal chromaffin cells were excited by etomidate at clinically relevant concentrations. Etomidate directly activated GABAA receptors found in chromaffin cells thereby elevating [Ca2+]i. The effects of etomidate were mimicked by the specific GABAA agonist muscimol and blocked by the specific antagonist bicuculline. Our data show that low concentrations of etomidate modulated GABAA receptor activation by muscimol. Blockade of voltage-dependent Ca2+ channels prevented the elevation of [Ca2+]i by GABA. Application of etomidate directly to the chromaffin cells elicited robust catecholamine secretion from these cells. The data indicate that clinically relevant concentrations of etomidate can directly activate GABAA receptors, which, due to the positive anion equilibrium potential, depolarizes chromaffin cells. This depolarization activates voltage-dependent Ca2+ channels thereby stimulating catecholamine release. Our data suggest that circulating catecholamine levels may be elevated after etomidate application.
Historically, anaesthetics were thought to non-specifically modulate membrane lipid fluidity, thereby exerting their anaesthetic actions. More recently this hypothesis has fallen out of favour. Anaesthetics are thought to act by directly interacting with membrane protein targets, generally thought to be neurotransmitter receptors and perhaps ion channels (Franks & Lieb, 1994; Krasowski & Harrison, 1999; Campagna et al. 2003). Anaesthetics are known to modulate a variety of targets including gamma-aminobutyric acid type-A (GABAA), glycine, neuronal nicotinic acetylcholine, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-d-aspartate (NMDA) and serotonin type-3 (5-HT3) receptors (Krasowski & Harrison, 1999; Campagna et al. 2003). Voltage-dependent channels may be sensitive to anaesthetics but at concentrations higher than used clinically (Franks & Lieb, 1994; Krasowski & Harrison, 1999; Campagna et al. 2003). Etomidate is a widely-used intravenous general anaesthetic that is particularly useful in patients who have myocardial dysfunction because it produces minimal haemodynamic change in these patients (Angelini et al. 2001; Rothermel, 2003). Etomidate is thought to act on GABAA receptors at clinically relevant concentrations (Belelli et al. 2003; Reynolds et al. 2003). At these same concentrations etomidate has no effect on glycine, AMPA, NMDA, 5-HT3 or nicotinic receptors (Krasowski & Harrison, 1999; Belelli et al. 2003; Reynolds et al. 2003) but there are effects at higher concentrations (Belelli et al. 2003). It is interesting that etomidate is thought to potentiate GABA-mediated transmission at clinically relevant concentrations (in the range from 1 to 8 μm; Giese & Stanley, 1983; Yang & Uchida, 1996; Belelli et al. 1999) but at higher concentrations is thought to directly activate GABAA receptors (Belelli et al. 1997; Moody et al. 1997). Other drugs that also potentiate GABAergic neurotransmission, such as diazepam or phenobarbital, are not efficacious anaesthetics (Schulz & Macdonald, 1981; Sigel et al. 1990; Moody et al. 1997). Thus, it seems likely that either etomidate acts on other targets, or that it targets different kinds of GABAA receptors than do diazepam or phenobarbital. Another possibility is that direct activation of GABAA receptors may mediate part of the anaesthetic response to etomidate (Schulz & Macdonald, 1981; Sigel et al. 1990; Belelli et al. 1997; Moody et al. 1997).
GABA is the primary inhibitory neurotransmitter in the adult brain, with ionotropic Cl−-permeable GABAA receptors mediating the majority of fast inhibitory synaptic transmission. It has been estimated that approximately one-third of all synapses in the brain are GABAergic (Krasowski & Harrison, 1999). In neonatal animals activation of Cl−-permeable GABA receptors is excitatory and appears to depend on the expression of a Na+–K+ – 2Cl− cotransporter (NKCC) which driven by Na+ and K+ gradients, acts to accumulate intracellular Cl− (Ben-Ari, 2002), leading to a depolarized Cl− equilibrium potential (ECl) (Alvarez-Leefmans et al. 1988; Rohrbough & Spitzer, 1996; Sun & Murali, 1999). The change from excitation to inhibition appears to involve the expression of the K+–Cl− cotransporter, KCC2 (Payne et al. 1996), which lowers intracellular Cl− levels resulting in a hyperpolarized ECl (Rivera et al. 1999; Ben-Ari, 2002; Owens & Kriegstein, 2002). We previously showed that bovine chromaffin cells from juvenile animals (4–5 months old) are excited by GABA. Activation of GABAA receptors depolarizes the cells, opens voltage-dependent Ca2+ channels, elevates [Ca2+]i and promotes the release of catecholamines (Xie et al. 2003). The extrapolated anion reversal potential in these cells was ∼−28 mV indicating a resting intracellular anion concentration of ∼50 mm. Expression of KCC2 protein was not detected in the juvenile chromaffin cells. In contrast, clear expression of NKCC1 was observed, which explains the presence of the depolarized anion potential. Depolarizing GABAA actions have also been observed in some adult neurones (Alvarez-Leefmans et al. 1988; Staley et al. 1996).
In this manuscript we explore the chromaffin cell response to etomidate. Exposing these cells to etomidate caused a large and rapid elevation in [Ca2+]i, which required Ca2+ influx from the extracellular space. The elevation of [Ca2+]i required the activation of GABAA receptors; the action was mimicked by the selective GABAA agonist muscimol and blocked by the selective GABAA antagonist bicuculline. Exposing chromaffin cells to etomidate elicited significant catecholamine secretion. Although we show that etomidate can potentiate GABA responses, the responses outlined in this manuscript resulted from etomidate directly activating the GABAA receptors and occurred at clinically relevant concentrations. Responses to etomidate were observed at concentrations as low as 1 μm and the EC50 was 5.8 μm. The results suggest that some GABAA receptors can be directly activated at low concentrations of etomidate and that these effects may mediate some of the anaesthetic responses to the drug. Etomidate is used in surgeries where patients have cardiovascular problems, as it has beneficial haemodynamic properties relative to other anaesthetics. Our data suggest that circulating catecholamine levels may be elevated after etomidate application which may help explain the beneficial haemodynamic properties of this anaesthetic.
Methods
Cell culture
Juvenile bovine adrenal glands, from animals 4–5 months old, were obtained from a local slaughterhouse. Glands were harvested immediately following death. Chromaffin cells were prepared by digestion with collagenase followed by density gradient centrifugation as previously described (Xie et al. 2003). The cells were plated onto collagen-coated coverslips (at a density of 0.3–0.4 × 106 cells ml−1 for [Ca2+]i measurements) and maintained in an incubator at 37°C in an atmosphere of 93% air and 7% CO2 with a relative humidity of 90%. Fibroblasts were effectively suppressed with cytosine-arabinoside (10 μm), leaving relatively pure chromaffin cell cultures. Half of the culture medium was exchanged every day. This medium consisted of Dulbecco's modified Eagle's medium (DMEM)\F12 (1 : 1) (Gibco) supplemented with fetal bovine serum (10%), glutamine (2 mm), penicillin/streptomycin (100 units ml−1/100 μg ml−1), cytosine arabinoside (10 μm) and 5-fluorodeoxyuridine (10 μm).
[Ca2+]i measurements
Experiments were conducted 1–5 days after chromaffin cells were prepared. Cells were incubated in Hanks' balanced salt solution (HBSS) with 2 μm acetoxymethyl ester form of fura-2 (fura-2 AM) and 1 mg ml−1 albumin for 45 min and then washed in a fura-2-free solution for 1 h. Fura-2 loading and wash was carried out at 37°C. The coverslip was transferred to an experimental chamber for recording. Backgrounds at 340 nm and 380 nm were obtained using an area of the coverslip devoid of cells. Data were continuously collected throughout the experiment using a Coho series 4920 cooled CCD camera. On each coverslip, 10–40 chromaffin cells were selected and individually imaged. Image pairs (one at 340 nm and 380 nm) were obtained every 2 s by averaging 16 frames at each wavelength. Backgrounds were subtracted from the individual wavelengths, and the 340 nm image was divided by the 380 nm image to provide a ratiometric image. Ratios were converted to free [Ca2+]i by comparing data to fura-2 calibration curves made in vitro by adding fura-2 (50 μm free acid) to solutions that contained known concentrations of calcium (0 to 2000 nm). The recording chamber was continuously perfused with fresh solution from gravity-fed reservoirs. All drugs were applied via this perfusion system.
Amperometric measurement of catecholamine release from populations of cells
The recording bath was designed so that it was small (< 1 cm in diameter) and the volume of the bath was kept at around 300–350 μl. Carbon fibre electrodes (Dagan Instruments) were backfilled with 3 m KCl and held at a potential of +700 mV using a modified Warner PC-501 patch clamp amplifier. Electrodes were positioned within the bathing medium. In this configuration the electrode detected increases in the local catecholamine concentration due to release from neighbouring cells. Amperometric data from the patch clamp was recorded continuously using AxoTape (an 8-pole Bessel filter was set at 300–500 Hz and acquired at 1 kHz). The cells were washed with HBSS for several minutes prior to application of etomidate or high K+-containing solutions. Under control conditions (HBSS), stopping the flow of the solution in the recording bath for 90–120 s did not produce any change in amperometric current, indicating that the cells were not releasing appreciable amounts of catecholamine under basal conditions. Cells were stimulated by stopping the flow of the bath solution for 15–30 s and then directly applying a 50 μl bolus, using a pipette, of one of the following: HBSS (negative control), high K+ (50 mm) (positive control) or etomidate (100 μm; final bath concentration of ∼20 μm). After 30–60 s the flow of HBSS through the bath was resumed to wash away the drug and any released catecholamine.
Amperometric measurement of catecholamine release from individual cells
Carbon fibre electrodes were fabricated with 7-μm diameter carbon fibres (Fortafil Fibres Inc., Knoxville, TN, USA). The carbon fibre was threaded through a glass capillary pipette (Drummond Scientific, Broomall, PA, USA), and pulled with a vertical glass pipette puller (Narashige, Tokyo, Japan). An epoxy seal between the carbon fibre and glass was created by dipping the glass–fibre tip into a 14% hardener/resin mixture (w/w), pre-heated to ∼100°C (hardener, meta-phenylene diamene; resin, 828 Epon Resin; Miller-Stephenson Chemical Co., Morton Grove, IL, USA). Electrodes were baked overnight at 66°C. The glass portion of the tip was painted with Sylgard (Dow Corning, Midland, MI, USA) and then baked at 66°C for at least 1 h. On the day of the experiment the carbon fibre was cut with a scalpel blade. The electrode was backfilled with 3 m KCl. The electrode was held at +700 mV using an EPC-8 amplifier (HEKA Electronics, Lambrecht, Germany) to oxidize catecholamine transmitter. The amperometric signal was low-pass filtered at 1 kHz (8-pole Bessel; Warner Instruments, Hamden, CT, USA). A 16-bit analog-to-digital converter (National Instruments Corp., Austin, TX, USA) was interfaced with in-house written data acquisition software. The amperometric signal was acquired at 5 kHz and stored on a personal computer. Data were analysed with a program kindly supplied by Eugene Mosharov (Columbia University). This program identified amperometric spikes and then integrated the area under each event to provide quantal size. The detection threshold for each event was set at five times the baseline root mean square (RMS) of the noise; spikes were automatically detected. For these experiments, the recording chamber was continuously perfused with fresh solution from gravity-fed reservoirs. All drugs were applied via this perfusion system.
Solutions
Experiments were carried out in Hanks' balanced salt solution (HBSS; Gibco) which contained (mm): NaCl 138, KCl 5, CaCl2 1.3, KH2PO4 0.3, MgSO4 0.8, Na2HPO4 0.3, d-glucose 5.6 and Hepes 20. Nominally Ca2+-free HBSS was prepared by treating Ca2+-free, Mg2+-free, HCO3-free HBSS with Chelex-100, then adding MgCl2 to a final concentration of 1 mm. The etomidate solution contained polyethylene glycol (PG; 35% v/v). PG was tested to determine whether it could elicit a response. Muscimol hydrobromide (Calbiochem) was prepared as a 25 mm stock in H2O. Muscimol was diluted to a final concentration with HBSS. (–)-Bicuculline methiodide (Sigma) was prepared as a 100 mm stock in H2O and then diluted to a final concentration of 100 μm with HBSS. Lanthanum chloride (Sigma) was prepared as a 100 mm stock in H2O and then diluted to a final concentration of 1 mm with HBSS. The solution containing sodium thiocyanate (Sigma) contained (mm): NaSCN 140, CaCl2 1.3, KCl 5, MgCl2 1, glucose 5.6 and Hepes 20; pH 7.35. For the amperometry experiments the bath contained HBSS. All experiments were carried out at room temperature (∼23°C).
Results
Intracellular calcium measurements: etomidate elevated intracellular calcium
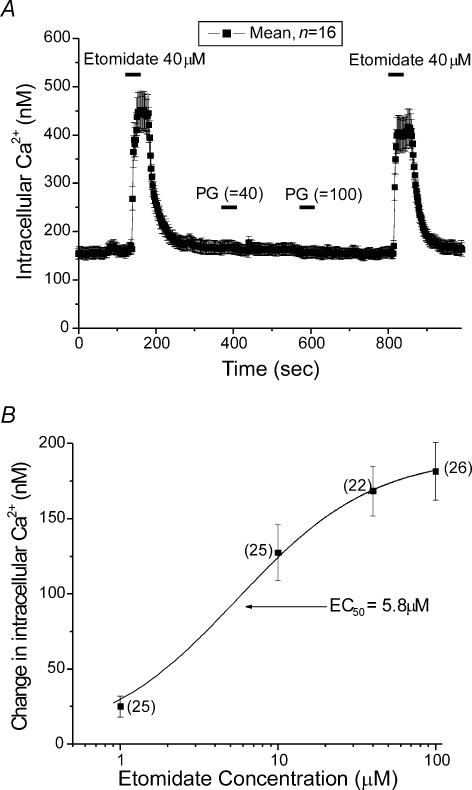
Chromaffin cells were tested after 1–5 days in primary culture. A coverslip that contained cells was transferred to an experimental chamber for each experiment. The microscope was adjusted until a field of view was obtained that had dozens of chromaffin cells visible. [Ca2+]i measurements from 10 to 40 cells per coverslip were collected every 2 s. Figure 1 shows representative data from such an experiment where [Ca2+]i, assayed with fura-2, was averaged from 16 cells and then plotted. At the bar labelled Etomidate, 40 μm etomidate was perfused into the bath, producing an elevation in [Ca2+]i of ∼300 nm. The etomidate was in a solution that was 35% PG (labelled PG in the figure). Following application of the drug, PEG was applied twice. At the first bar labelled PG, 24 mm PG, a concentration identical to the 40 μm etomidate solution, was applied without effect. At the second bar PG was applied at 60 mm, a concentration similar to that in a 100 μm etomidate solution, again without effect. Etomidate (40 μm) was re-applied at the end of the experiments and produced virtually the same response observed during the first application. The average change in [Ca2+]i elicited by application of 40 μm etomidate was 220.9 ± 19.3 nm (s.e.m., n = 40), while in the same group of cells, application of 60 mm PG was without effect. These data suggest that etomidate elevates [Ca2+]i and that the carrier, propylene glycol has no effect on resting [Ca2+]i.
Figure 1. Etomidate elevates [Ca2+]i in chromaffin cells.
A, mean [Ca2+]i in 16 individual chromaffin cells was determined every 2 s throughout the experiment (see Methods for details). Note that each time point is mean ± s.e.m. Etomidate (40 μm) was applied in a Ca2+-containing HBSS solution during the time indicated by the bars. The data are from a single representative experiment. The etomidate solution contained PG (35% v/v). We tested PG by itself twice; first at the concentration found in the 40 μm etomidate solution (24 mm PG) and then at the concentration found in 100 μm etomidate solution (60 mm PG). Both applications of PG were without effect. B, etomidate dose–response relation for [Ca2+]i elevation. The mean elevation of [Ca2+]i is plotted as a function of log10 of the concentration of etomidate that elicited the elevation. Data are from cells in Ca2+-based HBSS solution. The number of cells is indicated next to each data point along with standard error bars. The data was fitted by a curve defined by the function Y = Ymax × 1/1 + (EC50/X) in which Y is the [Ca2+]i elevation and X is the etomidate concentration.
Figure 1B provides a more detailed analysis of the response of chromaffin cell to etomidate. The average change in [Ca2+]i observed in response to different etomidate concentrations is plotted. Responses were observed at concentrations as low as 1 μm etomidate, which produced an [Ca2+]i elevation of 25 ± 7 nm (n = 25). Responses at 100 μm were not significantly different from those observed at 40 μm. The data were well fitted with a standard dose–response equation. The EC50 provided by the fitting function (see legend) was 5.8 μm.
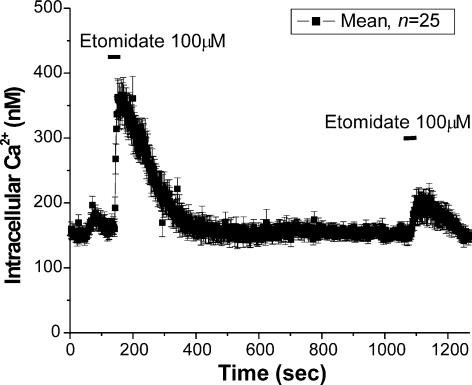
Multiple responses to higher concentrations of etomidate (> 40 μ m) were less reproducible than to lower concentrations (< 40 μm), suggesting desensitization to higher concentrations of the anaesthetic. Figure 2 shows responses from 25 cells to two applications of etomidate (100 μm). The response to the second application was significantly smaller than the first. Application of high concentrations of etomidate always elicited smaller subsequent responses, while application of lower concentrations typically allowed for multiple reproducible responses (see Fig. 1A).
Figure 2. High doses of etomidate produce desensitizing responses.
Mean [Ca2+]i in 25 individual chromaffin cells. The cells were exposed to etomidate (100 μm), during the times indicated. Note that the second response was significantly smaller than the first. Run-down in the response to multiple exposures of etomidate was observed at concentrations greater than 40 μm.
Extracellular calcium was required to observe the response to etomidate
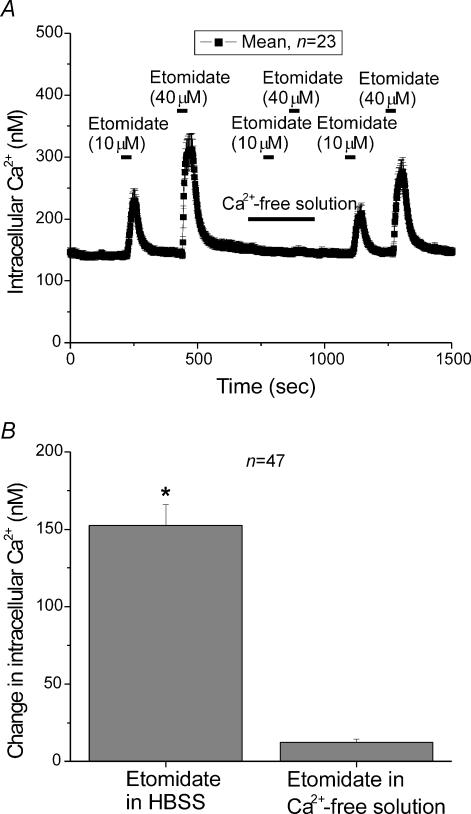
Figure 3A shows the response of 23 cells to either 10 μm or 40 μm etomidate, in the presence or absence of extracellular Ca2+. Etomidate (10 μm) was perfused into the bath (as indicated), producing a large elevation in [Ca2+]i. After allowing the cell to recover, 40 μm etomidate was perfused into the bath, producing an even larger change in [Ca2+]i. After recovery, cells were perfused with a Ca2+-free solution and then etomidate was re-applied at 10 μm and 40 μm, without any changes in [Ca2+]i. Subsequently, Ca2+ was re-introduced into the bath and then etomidate was re-applied again at 10 μm and 40 μm, this time producing responses not significantly different from the original responses.
Figure 3. The elevation of [Ca2+]i produced by etomidate required Ca2+ influx into the cells.
A, mean [Ca2+]i in 23 individual chromaffin cells. Etomidate was applied at both 10 μm and 40 μm in Ca2+-containing HBSS or in a Ca2+-free HBSS solution during the time indicated by the bars. B, the mean increase in [Ca2+]i in 47 cells treated with 40 μm etomidate was 152.4 ± 13.5 nm in the Ca2+-containing HBSS, while in the Ca2+-free HBSS the increase was only 12.4 ± 2 nm.
Figure 3B shows the average response of chromaffin cells to etomidate. In this experiment etomidate (40 μm) produced a [Ca2+]i elevation of 152 ± 13.5 nm when applied in the presence of extracellular Ca2+ but produced virtually no change of [Ca2+]i in extracellular Ca2+-free conditions. These data suggest that entry of Ca2+ into the chromaffin cells from the extracellular space is required for the response to etomidate. While it is possible that release of Ca2+ from intracellular stores plays a role in the responses observed here, perhaps via Ca2+-dependent Ca2+ release, such release does not appear to initiate the measured response.
Etomidate elevates [Ca2+]i via activation of GABAA receptors
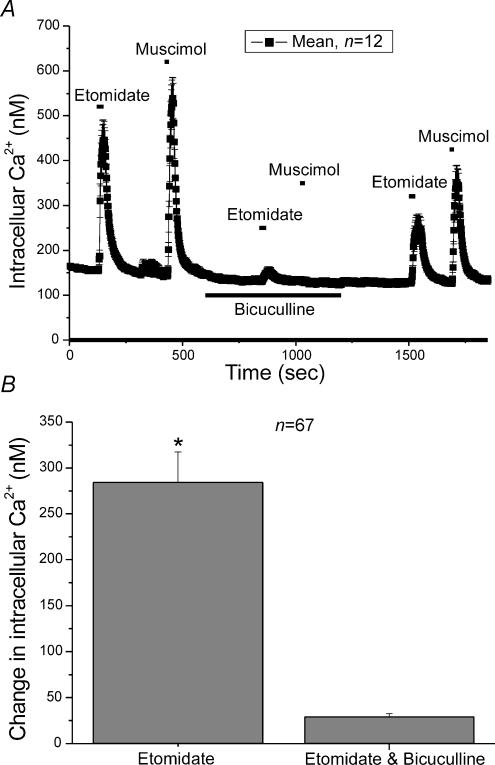
Etomidate is thought to selectively activate GABAA receptors. In an earlier study we showed that activation of GABAA receptors produced an increase in [Ca2+]i (Xie et al. 2003). To verify the involvement of GABAA receptors in the etomidate response, the specific GABAA receptor antagonist bicuculline was applied to chromaffin cells. Both etomidate (40 μm) and muscimol (5 μm) produced large elevations in [Ca2+]i when applied to a group of chromaffin cells (Fig. 4A). In the presence of bicuculline, the elevation of [Ca2+]i observed with either etomidate or muscimol was almost completely blocked (Fig. 4A). Partial recovery of the response to both etomidate and muscimol was observed upon washout of the bicuculline. Etomidate (40 μm) produced an average elevation in [Ca2+]i of 284 ± 33.1 nm (n = 67) when applied in the absence of bicuculline but this increase was almost completely blocked, to 29.2 ± 3.5 nm, by bicuculline (Fig. 4B). The data demonstrate that etomidate and muscimol operate via the same mechanism, activation of GABAA receptors.
Figure 4. The GABAA antagonist bicuculline blocked the effects of etomidate on [Ca2+]i.
A, mean [Ca2+]i in 12 individual chromaffin cells was determined every 2 s. The cells were exposed to either etomidate (40 μm) or muscimol (5 μm) during the times indicated. Both drugs were first added to the bath in the absence of bicuculline and then re-applied in the presence of 100 μm bicuculline. The bicuculline was washed out of the bath and etomidate and muscimol were re-applied. B, the mean increase in [Ca2+]i in response to application of 40 μm etomidate in either the presence or absence of bicuculline. Etomidate elevated [Ca2+]i by 284 ± 33.2 nm (n = 67), when applied in the absence of bicuculline. This increase was only 29.2 ± 3.5 nm in the presence of bicuculline.
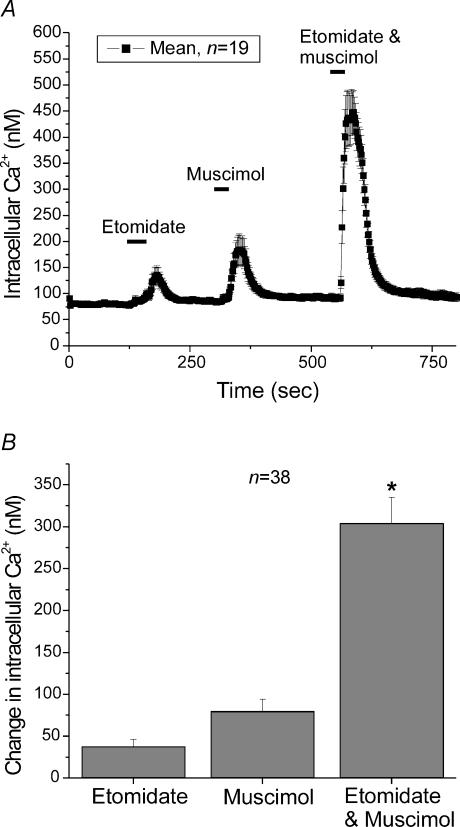
Figure 5 shows the supra-additive effects of applying muscimol, a selective GABAA agonist, and etomidate simultaneously (both drugs were used at non-saturating concentrations). At the first bar in Fig. 5, 4 μm etomidate was introduced into the bath. This concentration of etomidate produced a small elevation in [Ca2+]i. Then 1 μm muscimol was introduced into the bath. This concentration of muscimol produced a modest elevation in [Ca2+]i. When both drugs were applied simultaneously, a large increase in [Ca2+]i was observed suggesting that the drugs were supra-additive, under these conditions. Thus, low concentrations of etomidate can potentiate responses to GABA (or in this case muscimol), as has been described in neurones (Yang & Uchida, 1996; Reynolds et al. 2003), in addition to the direct effects on the GABAA receptors shown above.
Figure 5. Submaximal concentrations of etomidate and muscimol produced elevations of [Ca2+]i that were supra-additive.
A, mean [Ca2+]i in 19 individual chromaffin cells was determined every 2 s. The cells were exposed to either etomidate (4 μm) or muscimol (1 μm) during the times indicated. After allowing the cells to recover, etomidate and muscimol were applied simultaneously. B, the mean increase in [Ca2+]i in response to application of 4 μm etomidate, 1 μm muscimol or the combination of 4 μm etomidate and 1 μm muscimol. Etomidate by itself elevated [Ca2+]i by 37.6 ± 8.4 nm (n = 38), muscimol by itself elevated [Ca2+]i by 79.6 ± 14.6 nm (n = 38) while etomidate and muscimol applied together elevated [Ca2+]i by 303.7 ± 31.5 (n = 38).
Voltage-dependent Ca2+ channels probably mediate Ca2+ influx into chromaffin cells
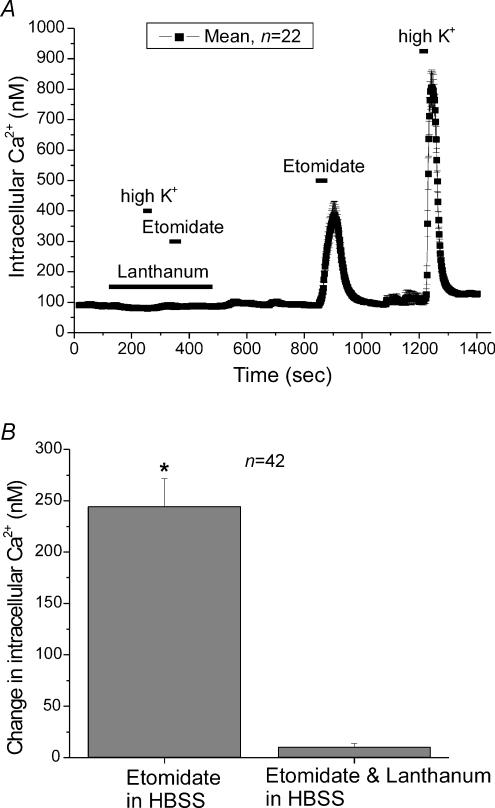
We wanted to verify that etomidate operated through the activation of voltage-dependent Ca2+ channels. Another way to activate voltage-dependent Ca2+ channels is to depolarize cells; high K+-based solutions depolarize cells and activate voltage-dependent Ca2+ channels. In turn, this elevates [Ca2+]i, and elicits robust catecholamine secretion. Ca2+ channel blockers such as La3+, block the Ca2+ influx produced by high-K+ stimulation. Would La3+ also block the response to etomidate? La3+ (1 mm) blocked the Ca2+ influx elicited by either high-K+ (50 mm K+) or etomidate (40 μm). In Fig. 6, the bar at the bottom shows when La3+ was introduced into the bath. In the presence of La3+ neither etomidate nor high K+-containing solutions elicited significant changes in [Ca2+]i. Washing La3+ from the bath and then re-applying both etomidate and high K+-containing solution, subsequently produced large changes in [Ca2+]i. In a previous study we showed that Cd2+ blocked the Ca2+ influx elicited by the activation of GABAA receptors (Xie et al. 2003).
Figure 6. The Ca2+ influx observed after activation of GABAA receptors by etomidate involves voltage-dependent Ca2+ channels.
A, mean [Ca2+]i in 22 individual chromaffin cells was determined every 2 s. La3+ (1 mm), a non-selective Ca2+ channel blocker, was superfused onto the cells during the time indicated by the bar. In the presence of La3+ neither depolarization of chromaffin cells with a high K+-containing solution (see Methods) nor the application of 40 μm etomidate elevated [Ca2+]i. After washing La3+ out of the bath, increases in [Ca2+]i were observed in response to both etomidate and the high K+-containing solution. Note the high K+-containing solution contained 2 mm Ca2+, not the 1.3 mm Ca2+ found in the usual HBSS. The data are from a single representative experiment. B, the mean increase in [Ca2+]i in 42 cells treated with etomidate in the absence of La3+ was 244.2 ± 27 nm, while in the presence of La3+ the increase was only 10.3 ± 3.4 nm. Note that the response to etomidate was obtained after exposing cells to La3+ and may have been somewhat diminished.
Figure 6B summarizes average data from 42 cells showing that La3+ completely blocks the [Ca2+]i elevation observed with etomidate. These results suggest that etomidate activates voltage-dependent Ca2+ channels, thereby producing the Ca2+ influx which elevates [Ca2+]i.
Nicotinic receptors are not involved in the elevated [Ca2+]i response to etomidate
In chromaffin cells, ACh release from pre-synaptic splanchnic neurones activates neuronal nicotinic receptors (nAChRs) which elicit action potentials in chromaffin cells. Ca2+ influx into chromaffin cells is mediated by both voltage-dependent Ca2+ channels as well as nicotinic receptors. Recent experiments suggest that transient receptor potential (TRP) channels may also allow Ca2+ into cells.
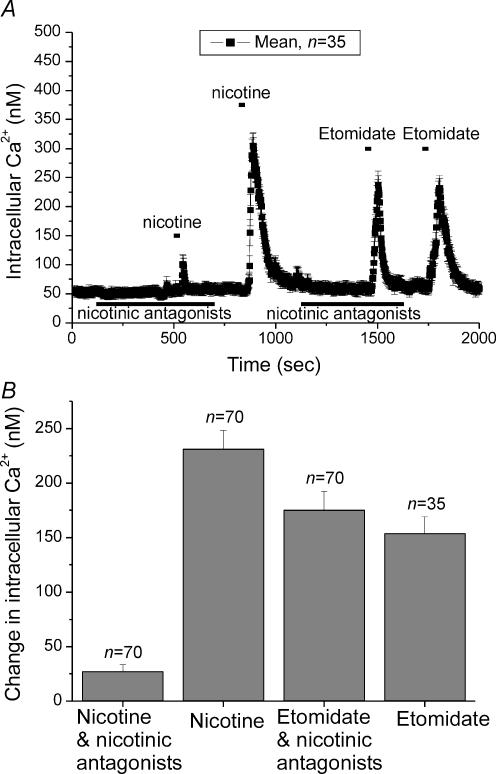
Although etomidate is not thought to act on nAChRs at physiologically relevant concentrations, only a few subunit combinations have been tested (Belelli et al. 2003; Reynolds et al. 2003). Thus we decided to test the involvement of these receptors in the elevated [Ca2+]i response to etomidate. Figure 7A shows [Ca2+]i averaged from 35 cells. In the presence of a cocktail of nicotinic antagonists (methyllycaconitine, 1 μm; mecamylamine, 20 μm; dihydro-β-erythroidine, 20 μm; all from Research Biochemicals International, Natick, MA, USA), the response to nicotine (100 μm) was effectively blocked, but etomidate (40 μm) still produced a large elevation in [Ca2+]i not different from that observed in control conditions. Figure 7B summarizes data averaged from 70 cells showing that the nicotinic antagonists effectively blocked the response to nicotine (231.3 ± 17 nm, in absence of antagonists; 26.9 ± 6.5 nm, in the presence of antagonists; n = 70 for both), but had little effect on responses to etomidate (153.8 ± 15.5, n = 35 in absence of antagonists; 175.1 ± 17.2, n = 70, in the presence of antagonists). Thus, our data suggest that the elevation of [Ca2+]i observed upon application of etomidate does not involve the activation of nicotinic receptors.
Figure 7. Nicotinic ACh receptor antagonists did not block the response to etomidate.
A, mean [Ca2+]i in 35 individual chromaffin cells was determined every 2 s. A cocktail of nicotinic antagonists (methyllycaconitine,1 μm; mecamylamine, 20 μm; and dihydro-β-erythroidine, 20 μm) was added, as indicated. This cocktail suppressed the response to nicotine but not etomidate. B, the mean elevation of [Ca2+]i in chromaffin cells exposed to nicotine (100 μm) in HBSS solution was 231.3 ± 17 nm (n = 70) in absence of nicotinic antagonists and 26.9 ± 6.5 nm (n = 70) in the presence of antagonists. The mean elevation of [Ca2+]i exposed to etomidate (40 μm) in HBSS solution was 153.8 ± 15.5 nm (n = 35) in absence of nicotinic antagonists 175.1 ± 17.2 nm (n = 70) in the presence of antagonists.
Evidence of a depolarized anion equilibrium potential in chromaffin cells
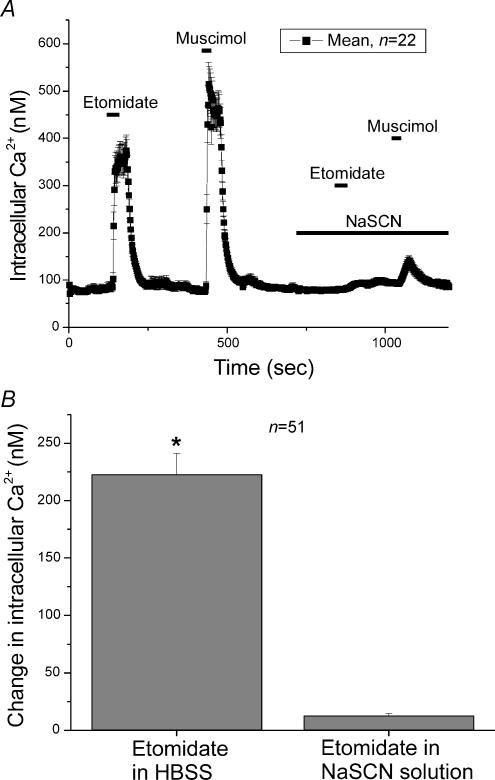
In order for GABA to depolarize a cell sufficiently to activate voltage-dependent Ca2+ channels, the anion equilibrium potential must be quite depolarized. To test this hypothesis we altered the Cl− reversal potential by replacing most of the extracellular Cl− by SCN−, an anion that is more permeant than Cl− itself in GABAA receptors and other chloride channels. Replacing Cl− by SCN−typically results in a negative shift in the anion equilibrium potential. If the shift in the anion reversal potential is large enough it would preclude activation of voltage-dependent Ca2+ channels in response to etomidate or muscimol. Indeed when cells were exposed to either 40 μm etomidate or 5 μm muscimol in the presence of SCN− (140 mm) only a small elevation of [Ca2+]i was observed (Fig. 8A). The mean response of chromaffin cells to etomidate in a solution containing SCN− was an elevation in [Ca2+]i of 12.4 ± 2 nm, but an elevation in [Ca2+]i of 222.7 ± 18.4 nm (n = 51) was observed when applied in HBSS (Fig. 8B). The data support the idea that the anion equilibrium potential of the cells is such that application of muscimol produces a depolarization that is strong enough to activate voltage-dependent Ca2+ channels.
Figure 8. Evidence for a depolarized ECl in chromaffin cells.
A, mean [Ca2+]i in 22 individual chromaffin cells was determined every 2 s. After the first applications of 40 μm etomidate or 5 μm muscimol, the anion equilibrium potential of the chromaffin cells was altered by exposing them to 140 mm SCN−, an anion which is more permeant in GABAA receptors than Cl−. Replacing most of the Cl− by SCN− should result in a negative shift in the Cl− equilibrium potential. When cells were re-exposed to either 40 μm etomidate or 5 μm muscimol in the presence of SCN− only a small elevation of [Ca2+]i was observed. B, the mean elevation of [Ca2+]i in chromaffin cells exposed to 40 μm etomidate in HBSS solution was 222.7 ± 18.4 nm (n = 51). When etomidate was applied during superfusion with 140 mm SCN− the increase was only 12.4 ± 2 nm.
Etomidate-induced catecholamine release
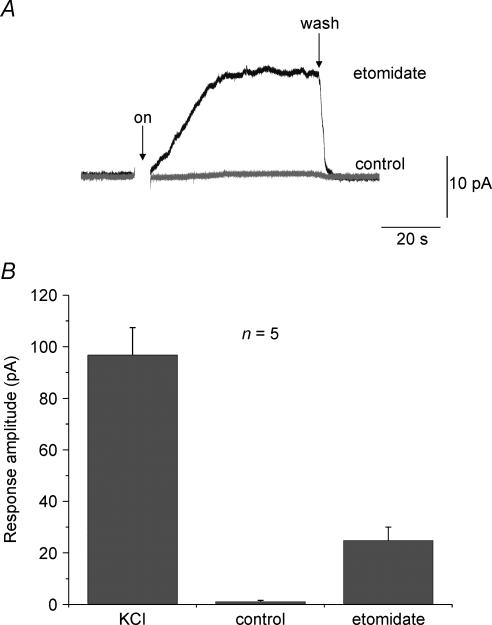
The robust elevations of [Ca2+]i observed upon exposing cells to etomidate suggest that the anaesthetic may be able to elicit catecholamine secretion in chromaffin cells. Figure 9 shows that in fact, etomidate does promote secretion. An amperometric electrode was positioned inside a small bath which contained chromaffin cells. The electrode sampled the bath concentration of catecholamine. At the time indicated by the arrow, a solution containing control HBSS solution (Fig. 9, grey trace) or etomidate (40 μm; Fig. 9, black trace) was introduced into the bath. Prior to introduction of each of the solutions the bath flow was stopped. This allowed any catecholamine released by the cells to accumulate in the bath. The etomidate-containing solution elicited secretion. However, application of control solution (HBSS) caused no increase in bath catecholamine concentration.
Figure 9. Activation of GABAA receptors by etomidate elicits catecholamine secretion in bovine chromaffin cells.
An amperometric electrode, used to sample the catecholamine concentration, was positioned inside a small bath that contained chromaffin cells. A, amperometric traces recorded from cells sequentially exposed to two different solutions. At the arrow a solution containing control HBSS solution (grey trace) or etomidate (40 μm, black trace) was introduced into the bath. Prior to introduction of each of the solutions the bath flow was stopped. This allowed any catecholamine released by the cells to accumulate in the bath. Stopping the bath flow, without etomidate, caused no increase in bath catecholamine concentration. The etomidate-containing solution elicited secretion. B, mean catecholamine release as measured by the peak amperometric response was averaged from five experiments. Although not as effective as KCl, etomidate application clearly resulted in significant release.
Figure 9B shows data averaged from five experiments. This figure shows that etomidate elicited slightly more than one-quarter the secretion of that produced by a solution containing 50 mm K+ (labelled KCI).
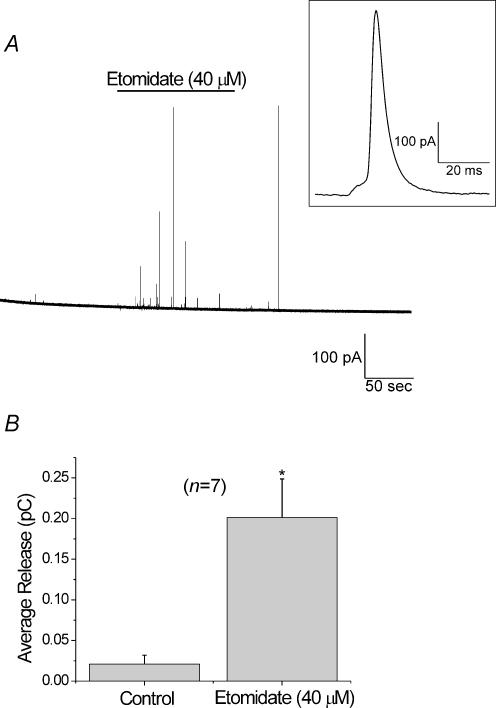
In order to rule out catecholamine release via plasma membrane transporters working in reverse, amperometric measurements were made from individual cells. In these experiments a carbon fibre electrode was gently pushed against a cell. Data were sampled continuously. Figure 10A shows the amperometric current observed from a cell perfused for 2 min with HBSS prior to exposing it to etomidate (40 μm) for 2 min. This was followed by ∼3 min where the cell was allowed to recover in HBSS. The current spikes correspond to amperometric events. The inset shows a single event on an expanded time scale. Integrating the area under each amperometric event provides a measure of the catecholamine content of individual vesicles. The sum all of the current integrals is proportional to the total catecholamine released. Figure 10B compares the sum of the amperometric current integrals in 2-min periods in the absence and presence of etomidate (40 μm), in seven cells. Both the total number of events (39 events in control conditions, versus 219 events in the presence of etomidate), as well as the sum of the current integrals, were significantly larger in the presence of etomidate. Taken together our data indicate that in addition to elevating [Ca2+]i, etomidate also promotes the vesicular release of catecholamines.
Figure 10. Activation of GABAA receptors by etomidate elicits catecholamine secretion from individual chromaffin cells.
An amperometric electrode was positioned so that it gently touched a cell. Each event corresponds to the release of an individual vesicle. A, a representative 7-min amperometric trace with multiple amperometric events is shown. This cell was exposed to etomidate (40 μm) for 2 min, as indicated. The inset shows an individual amperometric event on an expanded time scale (corresponding to the largest event observed in the presence of etomidate). B, the area under each of the amperometric events, (as shown in the inset), was integrated in order to obtain the quantal size. Control data were obtained in the 2-min period prior to etomidate application, while the data obtained for etomidate correspond to the 2-min exposure to etomidate. Summing the integrals of individual spikes from seven cells provided a measurement of the total catecholamine released during the periods that cells were in either control conditions or in etomidate.
Co-application of ACh and etomidate
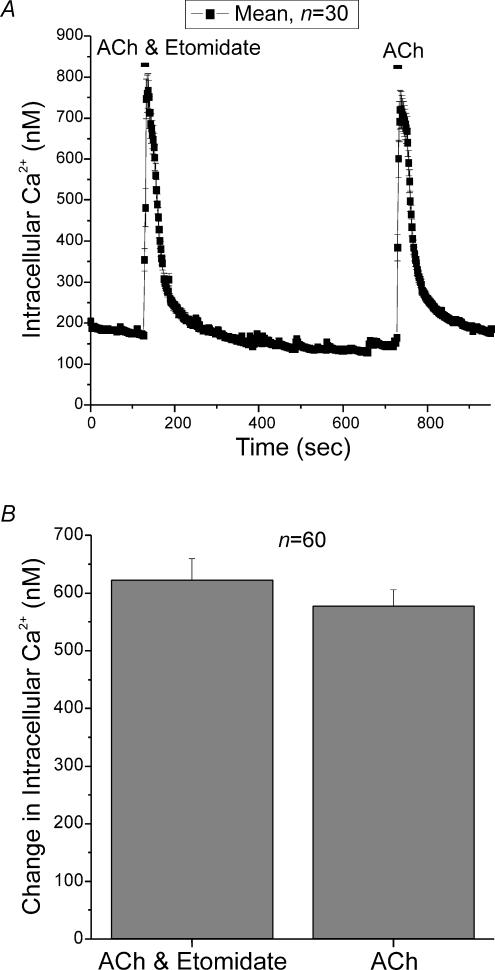
ACh released by pre-synaptic splanchnic nerve terminals is thought to be the physiological activator of adrenal chromaffin cells. In some cells activation of GABAA receptors is depolarizing but is still inhibitory (Zhang & Jackson, 1993). In chromaffin cells, there is considerable controversy regarding whether GABA can inhibit responses to high-K+ or ACh, even though GABA itself is excitatory (Kataoka et al. 1986; Fujimoto et al. 1987; Kitayama et al. 1990, 1991). We had previously shown that muscimol did not diminish ACh responses. Figure 11 shows that etomidate did not suppress responses to ACh either. Figure 11A shows that responses to ACh (100 μm) were virtually identical in the presence or absence of etomidate. The order of presentation of ACh or ACh plus etomidate did not affect the responses (not shown). Figure 11B shows mean data from 60 cells; responses to ACh were unaffected by etomidate. In this set of experiments ACh elevated [Ca2+]i by 622 ± 36.8 nm in the presence of etomidate and by 578 ± 28.2 nm in the absence of etomidate.
Figure 11. Co-application of ACh and etomidate demonstrates that etomidate did not interfere with the activation of nicotinic ACh receptors.
A, elevations in [Ca2+]i in response to ACh (100 μm), in the presence and absence of etomidate (40 μm). The order of presentation of ACh or ACh plus etomidate did not affect the responses (not shown). B, the mean increase in [Ca2+]i in 60 cells treated with ACh or ACh plus etomidate. ACh elevated [Ca2+]i by 622 ± 36.8 nm in the presence of etomidate and by 578 ± 28.2 nm in the absence of etomidate.
Discussion
In this manuscript we describe the response of chromaffin cells to the intravenous anaesthetic etomidate. We show that clinically relevant concentrations of etomidate directly activate GABAA receptors which then depolarize the cells sufficiently to activate voltage-gated Ca2+ channels. The resulting Ca2+ influx elicits catecholamine release. While it is possible that release of Ca2+ from intracellular stores plays a role in the responses observed, perhaps via Ca2+-dependent Ca2+ release, such release does not appear to initiate the measured response. Our results suggest that application of etomidate under clinical conditions will result in elevated levels of circulating catecholamines. Whether the release of catecholamines in vitro can explain the haemodynamical stability of etomidate in vivo remains to be determined.
Etomidate is a popular general anaesthetic. It is rapidly distributed and rapidly cleared which explains its rapid onset and short duration of action. Etomidate is thought to directly activate GABAA receptors, but only at high concentrations (Yang & Uchida, 1996; Belelli et al. 1997; Moody et al. 1997). At clinically relevant concentrations it is thought to potentiate GABA-mediated synaptic transmission (Ashton & Wauquier, 1985; Proctor et al. 1986; Yang & Uchida, 1996; Belelli et al. 1997). In our study we found that the EC50 for the elevation of [Ca2+]i in chromaffin cells was ∼5.8 μm. Clear elevations in [Ca2+]i were observed in response to application of etomidate as low as 1 μm. Chromaffin cells can release GABA upon stimulation (Kataoka et al. 1984; Oset Gasque et al. 1985, 1990) which raises the possibility that etomidate potentiated the response to endogenously released GABA. We believe this possibility to be unlikely, but not impossible, as the cells were continuously perfused and there were small numbers of cells in a relatively large bath. Any released GABA should be rapidly washed away. More likely, it appears that clinically relevant concentrations of etomidate, defined as concentrations in the range of 1–8 μm (Giese & Stanley, 1983; Yang & Uchida, 1996; Belelli et al. 1999), directly activated the GABAA receptors found in chromaffin cells. If similar channels are found in the CNS, then they too will respond to low concentrations of etomidate. Other drugs that potentiate GABAergic neurotransmission, such as diazepam or phenobarbital, are not effective anaesthetics (Schulz & Macdonald, 1981; Sigel et al. 1990; Moody et al. 1997). One possible explanation for the difference between etomidate and diazepam/phenobarbital is that there is a subpopulation of GABAA receptors, like those found in chromaffin cells, that can be directly activated by clinical concentrations of etomidate. For these types of GABAA receptors, etomidate would both potentiate GABAergic transmission as well as activate the receptors. Part of the anaesthetic response to etomidate would be due to direct receptor activation (Belelli et al. 1997; Moody et al. 1997). Of course there are other possible explanations for the differences between etomidate and diazepam/phenobarbital. The drugs may target different types of GABAA receptors preferentially, or they may act on different targets altogether.
Etomidate is used in surgeries where patients have cardiovascular problems, as it has beneficial haemodynamic properties relative to other anaesthetics. For instance, propofol can cause hypotension during surgery (Minami et al. 1996), even though, like etomidate, it acts at GABAA receptors. It is interesting that propofol inhibits catecholamine release from the adrenal medulla by interfering with Na+ influx through voltage-dependent Na+ channels as well as nAChRs (Minami et al. 1996). Our data suggest a different mechanism of action for etomidate. Figures 9 and 10 show that, unlike propofol, etomidate directly elicits catecholamine release from chromaffin cells. Also unlike propofol, etomidate does not interfere with the actions of ACh on nicotinic receptors (Fig. 11). These mechanistic differences may give rise to the clinical differences observed.
Although we show that etomidate stimulates catecholamine release from chromaffin cells, elevation of circulating catecholamine levels in vivo has not been observed (Mazerolles et al. 1996). If the increase in catecholamine levels is small, the level of change may not have been detected. Interestingly, these authors found that pentobarbital decreased catecholamine level while etomidate caused no change. Our data show that etomidate stimulates catecholamine release in chromaffin cells even though the elevations in [Ca2+]i described in this study are relatively modest. Submicromolar levels of intracellular Ca2+ can stimulate exocytosis from chromaffin cells (TerBush & Holz, 1992). Alternatively, it is possible that the depolarization induced by etomidate causes chromaffin cells to fire action potentials, which are not resolved by our imaging system. It is also worth noting that we measured the average response of the entire cell and that Ca2+ is substantially higher in microdomains near the release sites.
GABA plays an important role in the physiology of the adrenal medulla: (1) chromaffin cells possess the enzymes that synthesize and degrade GABA (Fernandez-Ramil et al. 1983; Kataoka et al. 1984); (2) GABA is taken up by chromaffin cells and released in response to depolarizing stimuli (Kataoka et al. 1984; Oset Gasque et al. 1985, 1990); (3) GABA-immunoreactive nerve fibres are found throughout the adrenal medulla (Kataoka et al. 1986; Oomori et al. 1993) and bundles of GABA immunoreactive nerve fibres run into the medulla with varicosities often in close contact with chromaffin cells (Kataoka et al. 1986; Oomori et al. 1993); and (4) chromaffin cells express GABAA receptors (Bormann & Clapham, 1985; Amenta et al. 1988; Peters et al. 1989). Functionally it has been shown that GABA can elicit catecholamine release from isolated perfused adrenal glands (Sangiah et al. 1974; Kataoka et al. 1984; Kitayama et al. 1984; Fujimoto et al. 1987; Gonzalez et al. 1992). Thus agents, like anaesthetics, that either potentiate or directly activate GABAA receptors are expected to play an important role in normal physiological functioning of chromaffin cells. The responses that we report are similar to those we observed using muscimol to activate GABAA receptors (Xie et al. 2003).
Ionotropic GABAA receptors are usually associated with inhibition, but during development, GABAA-mediated depolarization has been observed in many regions of the CNS (Mueller et al. 1984; Luhmann & Prince, 1991; Yuste & Katz, 1991; Chen et al. 1996; Owens et al. 1996; Eilers et al. 2001; Ben-Ari, 2002). The action of GABAA receptors in neurones is determined by the transmembrane Cl− gradient and the resultant ECl (Staley et al. 1996). The depolarized ECl observed is due, at least in some cases, to a member of the NKCC family (Alvarez-Leefmans et al. 1988; Rohrbough & Spitzer, 1996; Sun & Murali, 1999), which driven by Na+ and K+ gradients, acts to accumulate intracellular Cl− (Ben-Ari, 2002). Expression of another cotransporter that normally lowers intracellular Cl−, KCC2 (Payne et al. 1996), appears to mediate the developmental shift towards inhibition (Rivera et al. 1999; Ben-Ari, 2002; Owens & Kriegstein, 2002). We had previously shown that chromaffin cells from animals 4–5 months old did not express KCC2, as expected from animals of this age (Xie et al. 2003). Rather these cells keep expressing the NKCC1 cotransporter typically found in embryonic cells. This result provides a molecular explanation for the depolarization produced by activation of GABAA receptors by etomidate. Data from our current study are consistent with a depolarized anion equilibrium potential. In cells where etomidate has inhibitory effects on release, as in SH-SY5Y neuroblastoma cells (Sikand et al. 1997), it is likely that either cells have a hyperpolarized anion equilibrium potential or that etomidate acts on targets other than GABAA receptors.
GABA responses are more complex than simply altering membrane potential. Activation of GABAA receptors induced c-Fos immunoreactivity and increased brain-derived neurotrophic factor (BDNF) mRNA expression in embryonic hippocampal neurones, but failed to have any effect in older neurones (Berninger et al. 1995). GABA stimulates DNA synthesis (LoTurco et al. 1995; Haydar et al. 2000) and appears to regulate KCC2 expression (Ganguly et al. 2001). It will be of interest to determine whether the GABA-mimetic actions of anaesthetics, such as etomidate, can also mediate some of these other responses.
The subunit composition of the GABAA receptors in chromaffin cells that responded to etomidate remains unknown. Immunoblots indicate the presence of α1, α4, β1–3 and γ2 subunits in chromaffin cells (Parramon et al. 1995). Several studies have examined GABAA receptor subunit composition and responsiveness to etomidate. Uchida et al. (1995) showed that responses to etomidate were prolonged in γ2-containing receptors, but that equivalent potentiation of GABA responses was observed in receptors with or without γ2 subunits. The ability of etomidate to modulate and activate receptors is dependent on the β subunit (Belelli et al. 1997; Sanna et al. 1997). β subunits can directly bind etomidate (Belelli et al. 1999). Receptors with β2 or β3 but not β1 are highly sensitive to etomidate (Belelli et al. 1997; Sanna et al. 1997). Single point mutations in β subunits could strongly suppress GABA-modulatory and GABA-mimetic actions of etomidate (Belelli et al. 1997). Furthermore, α1γ2-containing receptors were not activated by etomidate but homomeric β1 receptors or β1γ2 receptors produced large responses to etomidate (Sanna et al. 1997). There may be two separate loci where etomidate acts: one for potentiation and another for direct activation of receptors. Single point mutations could reduce the ability of etomidate to activate the GABAA receptors but still retain ability to potentiate GABA responses (Moody et al. 1997) (but see (Belelli et al. 1997; Cestari et al. 2000) who argue that a single site on GABAA receptors mediates the actions of etomidate). Sedation and anaesthesia may be mediated by distinct GABAA receptors with different subunit composition (Reynolds et al. 2003). A genetically modified mouse with an etomidate-insensitive β2 subunit showed that loss of consciousness was mediated through β3-containing receptors while β2 subunits mediated the sedative properties (Reynolds et al. 2003). Of interest, it was recently shown that the tuberomammillary nucleus plays a key role in the sedative properties of anaesthetics (Nelson et al. 2002). Jurd et al. (2003) showed that a single point mutation in the β3 subunit of a genetically modified mouse did not affect responses to volatile anaesthetics but did eliminate responses to etomidate. It would be of interest to see whether the etomidate-induced catecholamine secretion (or elevation in [Ca2+]i) are modified in chromaffin cells from the β2 or β3 mutant animals described above.
Acknowledgments
We would like to thank the Brain Foundation for awards to Z. X. and A. P. F.
References
- Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta F, Collier WL, Erdo SL, Giuliani S, Maggi CA, Meli A. GABAA receptor sites modulating catecholamine secretion in the rat adrenal gland: evidence from 3H-muscimol autoradiography and in vivo functional studies. Pharmacology. 1988;37:394–402. doi: 10.1159/000138494. [DOI] [PubMed] [Google Scholar]
- Angelini G, Ketzler JT, Coursin DB. Use of propofol and other nonbenzodiazepine sedatives in the intensive care unit. Crit Care Clin. 2001;17:863–880. doi: 10.1016/s0749-0704(05)70184-6. [DOI] [PubMed] [Google Scholar]
- Ashton D, Wauquier A. Modulation of a GABA-ergic inhibitory circuit in the in vitro hippocampus by etomidate isomers. Anesth Analg. 1985;64:975–980. [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci U S A. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Muntoni AL, Merrywest SD, Gentet LJ, Casula A, Callachan H, Madau P, Gemmell DK, Hamilton NM, Lambert JJ, Sillar KT, Peters JA. The in vitro and in vivo enantioselectivity of etomidate implicates the GABAA receptor in general anaesthesia. Neuropharmacology. 2003;45:57–71. doi: 10.1016/s0028-3908(03)00144-8. [DOI] [PubMed] [Google Scholar]
- Belelli I, Pistis I, Peters JA, Lambert JJ. General anaesthetic action at transmitter-gated inhibitory amino acid receptors. Trends Pharmacol Sci. 1999;20:496–502. doi: 10.1016/s0165-6147(99)01405-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Berninger B, Marty S, Zafra F, Da Penha Berzaghi M, Thoenen H, Lindholm D. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development. 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- Bormann J, Clapham DE. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985;82:2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- Cestari IN, Min KT, Kulli JC, Yang J. Identification of an amino acid defining the distinct properties of murine beta1 and beta3 subunit-containing GABA(A) receptors. J Neurochem. 2000;74:827–838. doi: 10.1046/j.1471-4159.2000.740827.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, Van Den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol. 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers J, Plant TD, Marandi N, Konnerth A. GABA-mediated Ca2+ signalling in developing rat cerebellar Purkinje neurones. J Physiol. 2001;536:429–437. doi: 10.1111/j.1469-7793.2001.0429c.xd. 10.1111/j.1469-7793.2001.0429c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ramil JM, Sanchez-Prieto J, Canadas S, Gonzalez MP. GABA-T in bovine medulla cells: kinetic properties and comparison with GABA-T from other tissues. Rev Esp Fisiol. 1983;39:299–303. [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Kataoka Y, Guidotti A, Hanbauer I. Effect of gamma-aminobutyric acid A receptor agonists and antagonists on the release of enkephalin-containing peptides from dog adrenal gland. J Pharmacol Exp Ther. 1987;243:195–199. [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Giese JL, Stanley TH. Etomidate: a new intravenous anesthetic induction agent. Pharmacotherapy. 1983;3:251–258. doi: 10.1002/j.1875-9114.1983.tb03266.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez MP, Oset-Gasque MJ, Castro E, Bugeda J, Arce C, Parramon M. Mechanism through which GABAA receptor modulates catecholamine secretion from bovine chromaffin cells. Neuroscience. 1992;47:487–494. doi: 10.1016/0306-4522(92)90263-2. 10.1016/0306-4522(92)90263-2. [DOI] [PubMed] [Google Scholar]
- Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Fujimoto M, Alho H, Guidotti A, Geffard M, Kelly GD, Hanbauer I. Intrinsic gamma aminobutyric acid receptors modulate the release of catecholamine from canine adrenal gland in situ. J Pharmacol Exp Ther. 1986;239:584–590. [PubMed] [Google Scholar]
- Kataoka Y, Gutman Y, Guidotti A, Panula P, Wroblewski J, Cosenza-Murphy D, Wu JY, Costa E. Intrinsic GABAergic system of adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984;81:3218–3222. doi: 10.1073/pnas.81.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S, Morita K, Dohi T, Tsujimoto A. The nature of the stimulatory action of gamma-aminobutyric acid in the isolated perfused dog adrenals. Naunyn Schmiedebergs Arch Pharmacol. 1984;326:106–110. doi: 10.1007/BF00517305. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Morita K, Dohi T, Tsujimoto A. GABAergic modulation of catecholamine release from cultured bovine adrenal chromaffin cells. Evidence for the involvement of Cl−-dependent Ca2+ entry. Naunyn Schmiedebergs Arch Pharmacol. 1990;341:419–424. doi: 10.1007/BF00176334. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Nakatsukasa Y, Morita K, Dohi T, Tsujimoto A. Pharmacological evidence for the possible involvement of repetitive action potentials in facilitation by GABA of catecholamine secretion in bovine adrenal chromaffin cells. Br J Pharmacol. 1991;102:706–710. doi: 10.1111/j.1476-5381.1991.tb12237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55:1278–1303. doi: 10.1007/s000180050371. 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. 10.1016/0896-6273(95)90008-X. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Mazerolles M, Senard JM, Verwaerde P, Tran MA, Montastruc JL, Virenque C, Montastruc P. Effects of pentobarbital and etomidate on plasma catecholamine levels and spectral analysis of blood pressure and heart rate in dogs. Fundam Clin Pharmacol. 1996;10:298–303. doi: 10.1111/j.1472-8206.1996.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Minami K, Yanagihara N, Segawa K, Tsutsui M, Shigematsu A, Izumi F. Inhibitory effects of propofol on catecholamine secretion and uptake in cultured bovine adrenal medullary cells. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:572–578. doi: 10.1007/BF00169178. [DOI] [PubMed] [Google Scholar]
- Moody EJ, Knauer C, Granja R, Strakhova M, Skolnick P. Distinct loci mediate the direct and indirect actions of the anesthetic etomidate at GABA(A) receptors. J Neurochem. 1997;69:1310–1313. doi: 10.1046/j.1471-4159.1997.69031310.x. [DOI] [PubMed] [Google Scholar]
- Mueller AL, Taube JS, Schwartzkroin PA. Development of hyperpolarizing inhibitory postsynaptic potentials and hyperpolarizing response to gamma-aminobutyric acid in rabbit hippocampus studied in vitro. J Neurosci. 1984;4:860–867. doi: 10.1523/JNEUROSCI.04-03-00860.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–984. doi: 10.1038/nn913. 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- Oomori Y, Iuchi H, Nakaya K, Tanaka H, Ishikawa K, Satoh Y, Ono K. Gamma-aminobutyric acid (GABA) immunoreactivity in the mouse adrenal gland. Histochemistry. 1993;100:203–213. doi: 10.1007/BF00269093. 10.1007/BF00269093. [DOI] [PubMed] [Google Scholar]
- Oset Gasque MJ, Canadas Correas S, Masso Cordoba JM, Gonzalez Gonzalez MP. Uptake of GABA by bovine adrenal medulla slices. Naunyn Schmiedebergs Arch Pharmacol. 1985;329:372–375. doi: 10.1007/BF00496370. [DOI] [PubMed] [Google Scholar]
- Oset-Gasque MJ, Castro E, Gonzalez MP. Mechanisms of [3H] gamma-aminobutyric acid release by chromaffin cells in primary culture. J Neurosci Res. 1990;26:181–187. doi: 10.1002/jnr.490260207. [DOI] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Parramon M, Gonzalez MP, Oset-Gasque MJ. Pharmacological modulation of adrenal medullary GABAA receptor: consistent with its subunit composition. Br J Pharmacol. 1995;116:1875–1881. doi: 10.1111/j.1476-5381.1995.tb16676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J Biol Chem. 1996;271:16245–16252. doi: 10.1074/jbc.271.27.16245. 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- Peters JA, Lambert JJ, Cottrell GA. An electrophysiological investigation of the characteristics and function of GABAA receptors on bovine adrenomedullary chromaffin cells. Pflugers Arch. 1989;415:95–103. doi: 10.1007/BF00373146. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Mynlieff M, Dunwiddie TV. Facilitatory action of etomidate and pentobarbital on recurrent inhibition in rat hippocampal pyramidal neurons. J Neurosci. 1986;6:3161–3168. doi: 10.1523/JNEUROSCI.06-11-03161.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DS, Rosahl TW, Cirone J, O'Meara GF, Haythornthwaite A, Newman RJ, et al. Sedation and anesthesia mediated by distinct GABA (A) receptor isoforms. J Neurosci. 2003;23:8608–8617. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Spitzer NC. Regulation of intracellular Cl− levels by Na+-dependent Cl− cotransport distinguishes depolarizing from hyperpolarizing GABAA receptor-mediated responses in spinal neurons. J Neurosci. 1996;16:82–91. doi: 10.1523/JNEUROSCI.16-01-00082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel LK. Newer pharmacologic agents for procedural sedation of children in the emergency department-etomidate and propofol. Curr Opin Pediatr. 2003;15:200–203. doi: 10.1097/00008480-200304000-00011. 10.1097/00008480-200304000-00011. [DOI] [PubMed] [Google Scholar]
- Sangiah S, Borowitz JL, Yim GK. Actions of GABA, picrotoxin and bicuculline on adrenal medulla. Eur J Pharmacol. 1974;27:130–135. doi: 10.1016/0014-2999(74)90209-x. 10.1016/0014-2999(74)90209-X. [DOI] [PubMed] [Google Scholar]
- Sanna E, Murgia A, Casula A, Biggio G. Differential subunit dependence of the actions of the general anesthetics alphaxalone and etomidate at gamma-aminobutyric acid type A receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1997;51:484–490. [PubMed] [Google Scholar]
- Schulz DW, Macdonald RL. Barbiturate enhancement of GABA-mediated inhibition and activation of chloride ion conductance: correlation with anticonvulsant and anesthetic actions. Brain Res. 1981;209:177–188. doi: 10.1016/0006-8993(81)91179-3. 10.1016/0006-8993(81)91179-3. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Trube G, Mohler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Sikand KS, Hirota K, Smith G, Lambert DG. Etomidate inhibits [3H]noradrenaline release from SH-SY5Y human neuroblastoma cells. Neurosci Lett. 1997;236:87–90. doi: 10.1016/s0304-3940(97)00766-0. 10.1016/S0304-3940(97)00766-0. [DOI] [PubMed] [Google Scholar]
- Staley K, Smith R, Schaack J, Wilcox C, Jentsch TJ. Alteration of GABAA receptor function following gene transfer of the CLC-2 chloride channel. Neuron. 1996;17:543–551. doi: 10.1016/s0896-6273(00)80186-5. 10.1016/S0896-6273(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Sun D, Murali SG. Na+-K+-2Cl− cotransporter in immature cortical neurons: a role in intracellular Cl− regulation. J Neurophysiol. 1999;81:1939–1948. doi: 10.1152/jn.1999.81.4.1939. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Holz RW. Barium and calcium stimulate secretion from digitonin-permeabilized bovine adrenal chromaffin cells by similar pathways. J Neurochem. 1992;58:680–687. doi: 10.1111/j.1471-4159.1992.tb09771.x. [DOI] [PubMed] [Google Scholar]
- Uchida I, Kamatchi G, Burt D, Yang J. Etomidate potentiation of GABAA receptor gated current depends on the subunit composition. Neurosci Lett. 1995;185:203–206. doi: 10.1016/0304-3940(95)11263-v. 10.1016/0304-3940(95)11263-V. [DOI] [PubMed] [Google Scholar]
- Xie Z, Currie KP, Cahill AL, Fox AP. Role of Cl− co-transporters in the excitation produced by GABAA receptors in juvenile bovine adrenal chromaffin cells. J Neurophysiol. 2003;90:3828–3837. doi: 10.1152/jn.00617.2003. [DOI] [PubMed] [Google Scholar]
- Yang J, Uchida I. Mechanisms of etomidate potentiation of GABAA receptor-gated currents in cultured postnatal hippocampal neurons. Neuroscience. 1996;73:69–78. doi: 10.1016/0306-4522(96)00018-8. 10.1016/0306-4522(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. 10.1016/0896-6273(91)90243-S. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Jackson MB. GABA-activated chloride channels in secretory nerve endings. Science. 1993;259:531–534. doi: 10.1126/science.8380942. [DOI] [PubMed] [Google Scholar]