Abstract
The influence of visual experience deprivation on changes in synaptic plasticity during postnatal development was studied in the ventral part of the rat medial vestibular nuclei (vMVN). We analysed the differences in the occurrence, expressed as a percentage, of long-term depression (LTD) and long-term potentiation (LTP) induced by high frequency stimulation (HFS) of the primary vestibular afferents in rats reared in the light (LR) and those in the dark (DR). In LR rats, HFS only induced LTD in the early stages of development, but the occurrence of LTD progressively decreased to zero before their eyes opened, while that of LTP enhanced from zero to about 50%. Once the rats' eyes had opened, LTD was no longer inducible while LTP occurrence gradually reached the normal adult value (70%). In DR rats, a similar shift from LTD to LTP was observed before their eyes opened, showing only a slightly slower LTD decay and LTP growth, and the LTD annulment was delayed by 1 day. By contrast, the time courses of LTD and LTP development in DR and LR rats showed remarkable differences following eye opening. In fact, LTD occurrence increased to about 50% in a short period of time and remained high until the adult stage. In addition, the occurrence of LTP slowly decreased to less than 20%. The effect of light-deprivation was reversible, since the exposure of DR rats to light, 5 days after eye opening, caused a sudden disappearance of LTD and a partial recover of LTP occurrence. In addition, we observed that a week of light deprivation in LR adult rats did not affect the normal adult LTP occurrence. These results provide evidence that in a critical period of development visual input plays a crucial role in shaping synaptic plasticity of the vMVN, and suggest that the visual guided shift from LTD to LTP during development may be necessary to refine and consolidate vestibular circuitry.
Long-term potentiation (LTP) and long-term depression (LTD) are opposite forms of activity-dependent changes in synaptic efficacy which may underlie learning and memory (Bliss & Collingridge, 1993; Malenka, 1994; Malenka et al. 2000; Martin et al. 2000). It has been suggested that LTP and LTD could also contribute to the stabilization and elimination of synapses that occurs during the process of pathway refinement in the developing brain (Bear et al. 1987; Constantine-Paton et al. 1990; Goodman & Shatz, 1993; Jessel & Kandel, 1993; Bear & Malenka, 1994; Katz & Shatz, 1996; Buonomano & Merzenich, 1998). There is also increasing evidence to support the hypothesis that LTP contributes to the formation of new synapses (Engert & Bonhoeffer, 1999; Shi et al. 1999; Toni et al. 1999) as well as the selective strengthening and stabilization of appropriately targeted connections, while LTD may facilitate the loss of inappropriate synaptic contacts by causing a synapse weakening (Artola & Singer, 1993; Katz & Shatz, 1996; Buonomano & Merzenich, 1998; Lo & Mize, 2000; Allen et al. 2003; Heynen et al. 2003).
In many regions of the rat's brain, the expression of LTD and LTP markedly changes during development (Dudek & Bear, 1993; Battistin & Cherubini, 1994; Kirkwood et al. 1995; Lo & Mize, 2002) and the prevalence of LTP over LTD, or vice versa, coincides with critical periods for the establishment of functional synaptic connections. For example, LTP is induced during the critical periods of barrel field and ocular dominance plasticity (Tsumoto, 1992; Bear & Kirkwood, 1993; Crair & Malenka, 1995; Kirkwood et al. 1995; Buonomano & Merzenich, 1998) and its progressive decline runs parallel with the maturation of the functional properties of the visual cortex (Kirkwood & Bear, 1994; Kirkwood et al. 1995; Dudek & Friedlander, 1996; Sermasi et al. 1999; Rittenhouse et al. 1999), while in other brain areas, like the hippocampus and superior colliculus, the induction of LTD precedes the developmental onset of LTP (Dudek & Bear, 1993; Lo & Mize, 2002; Wasling et al. 2002).
Our recent study in rat brainstem slices shows that changes in the expression of long-term synaptic phenomena also occur in the medial vestibular nuclei (MVN) during a critical period of development (Puyal et al. 2003). In fact, while high frequency stimulation (HFS) of the primary vestibular afferents induces LTD in the MVN during the first stages of postnatal development, it only evokes LTP in adulthood (Capocchi et al. 1992; Grassi et al. 1996; Grassi & Pettorossi, 2001; Puyal et al. 2003). Therefore, the probability of inducing LTD progressively decreases and that of LTP increases throughout development. This change coincides with the period of maturation of rat vestibular nuclei, corresponding to the first month of postnatal life, during which the secondary vestibular neurones develop morphological and functional characteristics typical of the adult stage (Lannou et al. 1979; Curthoys, 1982; Dutia et al. 1995; Johnston & Dutia, 1996; Dutia & Johnston, 1998; Murphy & du Lac, 2001).
The mechanisms guiding the developmental changes in the synaptic plasticity of the MVN remain unclear. Glutamate and GABA receptors are assumed to be primarily involved in the developmental synaptic plasticity in many brain areas (Monyer et al. 1994; Fox et al. 1996; Hensch et al. 1998; Fagiolini & Hensch, 2000; Knott et al. 2002). Therefore, one of the factors responsible for the shift from LTD to LTP in developing vestibular nuclei, might be the different stages of maturation of N-methyl-d-aspartate (NMDA) and group I metabotropic glutamate receptors (mGluR1 and mGluR5), which play opposite roles in vestibular synaptic plasticity. In fact, the expression of NMDA and mGluR1, which are facilitatory to LTP (Capocchi et al. 1992; Grassi et al. 1996; Grassi & Pettorossi, 2001; Grassi et al. 2002) shows a progressive increase which runs parallel to that of LTP (Sans et al. 2000; Puyal et al. 2003), while that of mGluR5, which plays an inhibitory role on LTP (Grassi et al. 2002), shows a decrease which runs parallel to that of LTD (Puyal et al. 2003).
An interesting finding is that the major change in the developmental shift from LTD to LTP occurs in the MVN soon after the rats open their eyes (about P14), when LTD is no longer observed and the frequency of LTP quickly reaches the adult value (Puyal et al. 2003). This suggests that the neural activity that is normally supplied by visual experience plays a crucial role in shaping the synaptic plasticity of the MVN.
The neurones in the MVN receive vestibular sensory signals and respond to moving visual stimuli, which drive eye movements during vestibulo-ocular (VOR) and optokinetic reflexes (OKR). There is considerable evidence to show a modification of the VOR gain in response to altered visual environments demonstrating that visual slip helps maintain the VOR (Gonshor & Melvill Jones, 1976; Melvill Jones & Davies, 1979; Miles & Eighmy, 1980). The importance of retinal slip during the VOR development has also been confirmed by the reduction of the VOR gain observed in animals reared in the dark (Collewijn, 1977; Harris & Cynader, 1981; Kennedy et al. 1982; Horn et al. 1996; Goode et al. 2001). The fact that the coincidence of vestibular and visual information during development seems to be crucial for the full maturation of the functional characteristics of MVN neurones, suggests that visual experience also drives the progress of mechanisms, like LTD and LTP, that might be necessary for the light-guided refining and consolidation of vestibular circuitry.
The aim of this study was to verify a potential dependence of vestibular synaptic plasticity development on visual experience, by analysing whether the occurrence of LTD and LTP was modified in brainstem slices of light-deprived rats during postnatal development.
Methods
Animals and slice preparation
The experiments were performed on 260 brainstem slices obtained from 68 Wistar rats (Harlan-Nossan, Italy) at various stages of postnatal (P) development from P6 to adult (P32). The animals were used in accordance with the ethical guidelines of the Bioethical Committee of the University of Perugia. Throughout the study a group of animals (49) was housed in total darkness until they were killed, and a group (14) was maintained in the dark until 5 days after opening their eyes and then transferred to normal light–dark conditions. During dark rearing, once a week, when the animals were transferred to a clean box, they were briefly (less than 5 min) exposed to low-intensity red light illumination. In addition, normal light-reared adult animals (5) were transferred to dark conditions for a week and then killed. Under ether anaesthesia, the animals were decapitated, and the cranium opened to expose the entire brain. The methods for preparing and maintaining the slices have been reported previously (Capocchi et al. 1992; Grassi et al. 1995). In brief, transverse 350 μm thick slices, containing the MVN, were incubated in warmed (30–31°C) oxygenated ACSF, transferred after 1 h to an interface-type recording chamber and perfused at a rate of 2 ml min−1. The ACSF contained (mm): NaCl 124, KCl 3, KH2PO4 1.25, NaHCO3 26, CaCl2 3.4, MgSO4 2.5, d-glucose 10 and l-ascorbate 2.
Electrophysiological recording and stimulating technique
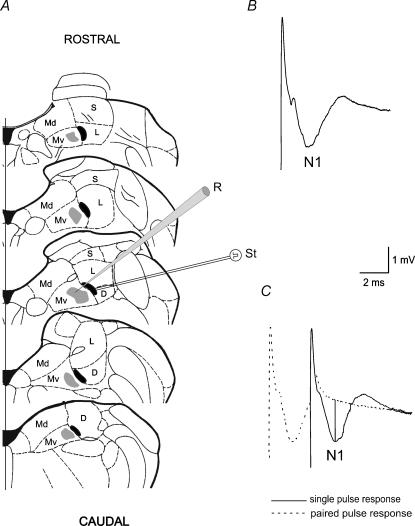
The field potentials elicited by vestibular afferent stimulation, were recorded in the ventral part of the MVN (vMVN) with 2 m sodium chloride-filled micropipettes (resistance 3–10 MΩ) (Fig. 1). The recorded field potentials consisted of a large negative wave (N1), which follows the artefact and represents the monosynaptic activation of the secondary vestibular neurones (Fig. 1B and C). Usually, the field potentials were similar to those recorded in the adult animals, but they were evoked by lower stimulus intensity. As reported previously (Grassi et al. 1996) the postsynaptic nature of the N1 wave was verified by a 3 ms interval paired-pulse test, which caused the disappearance of the N1 wave (Fig. 1C), and by using a Ca2+-free solution (not shown). The recorded potentials were amplified, filtered with a wideband filter (0–10 kHz) and stored in a computer equipped with a data acquisition card (AT-MIO-16E-2, National Instruments, Austin, TX, USA).
Figure 1. Recordings in the ventral part of the MVN.
A, recording sites in the ventral portion of the MVN (grey areas) and stimulating zones (black areas) are plotted on the diagrams of 0.5-mm-spaced brainstem slices. B, typical field potential recorded in the vMVN. C, single pulse response is superimposed onto a 3 ms paired pulse stimulation to show how the N1 peak negative voltage was calculated compared with the baseline (vertical line). Abbreviations: D, descending vestibular nucleus; Md, dorsal part of the medial vestibular nucleus; Mv, ventral part of the medial vestibular nucleus; L, lateral vestibular nucleus; S, superior vestibular nucleus; R, recording electrode; St, stimulating electrode.
One bipolar NiCr-stimulating electrode was placed at the point where the vestibular afferents enter the MVN, which is in a narrow zone at the medial border of the lateral or descending vestibular nucleus (Fig. 1A). The distance between stimulating and recording electrodes was about 1 mm. We did not use more lateral positions, because, as previously reported (Grassi et al. 1999; Grassi & Pettorossi, 2000), the probability of eliciting field potentials was very low when the stimulating distance increased. However, our previous studies show no difference in the results between medial and lateral stimulation. In addition, in all our previous studies we were never able to evoke any measurable potential when the stimulating electrode was placed outside the loci where the vestibular afferents were probably localized and, in some cases, clearly visible. This was also confirmed by histological examination. This also rules out the possibility that the elicited responses are due to activation of fibres mediating internuclear interaction.
Stimulus test parameters were: intensity, 20–100 μA; duration, 0.07 ms and frequency, 0.06 Hz. We adjusted stimulus intensity so that the amplitude of the N1 wave was 40–60% of the maximum as determined by an input/output curve. HFS consisted of four bursts at 100 Hz applied with alternated polarity for 2 s with a 5 s interval. In our previous study (Puyal et al. 2003) we showed that HFS induces LTD in the vMVN from P6 to P13 with a probability which progressively decreases to 0 while the probability of inducing LTP increases and reaches the adult value (70–75% of the cases) soon after eye opening (Puyal et al. 2003).
The recording and stimulating sites were marked, in each slice, by passing DC current of 50–100 μA for 10–20 s and subsequently verified by histological analysis.
In our previous studies (Grassi et al. 1996; Grassi & Pettorossi, 2000, 2001) we established the induction of HFS long-term effects by combining the analysis of the evoked field potential amplitude with that of the single unit latency. Since discrepancy in the results from these two analyses has never been seen, we considered the addition of the single unit examination unnecessary, and it would have exceedingly increased the number of experiments.
Data collection
We measured the amplitude of the N1 wave as the difference between the wave peak negative voltage and a baseline influenced by the electrical stimulus decay (Fig. 1C). To quantify this voltage decay, responses to the 3 ms interval paired-pulse test were recorded before and after HFS application, as the second response of the paired-pulse stimulation represented only the electrical stimulus.
To evaluate the effect of HFS the wave amplitudes were measured every 15 s and expressed as a percentage of the baseline (the mean of the responses recorded within the first 5 min of each experiment). To compare the effects within a single experiment, we considered the average value and s.d. within a 5 min interval before HFS and at the HFS steady state. In each experiment the differences between the values were evaluated using analyses of variance (ANOVA) and Tukey's post hoc test. Statistical significance was established at P < 0.05.
To show the different effects of HFS on N1 amplitude during postnatal development (P6–P32) we evaluated the occurrence of LTP and LTD by calculating, in each animal, the number of LTP and LTD following HFS and expressing it as a percentage of all the responses. For each animal, two to six slices were used and in each slice HFS was delivered only once. For each age we calculated the mean and s.d. of percentage values from three to six animals. The age of the animals was considered as the day before (indicated by E−) or after (E+) eye opening and not as postnatal days. For the ages before eye opening, the probable eye opening day was considered P14. The percentages of LTP and LTD obtained at each age were compared with those previously obtained at the same ages in light-reared (LR) rats (Puyal et al. 2003). To do this we reconsidered our previous data by grouping them according to the eye opening time of each animal. To determine the time course of the developmental changes in LTP and LTD occurrence a linear regression line, a first-order exponential decay (y = y0 + Ae−(x−x○)/t) and 4th order polynomial curves were fitted to the data points representing the percentage values of the long-term effect occurrence in a single animal. The goodness fit was established by χ2 for the exponential functions, and R for regression and polynomial curves.
Because LTP induced by HFS at different ages showed different time courses, we also calculated the rise time of LTP as the time (in minutes) from HFS necessary to reach the potentiation steady state. The values before eye opening were obtained from consecutive age groups, while those after eye opening from a single age group. The values were compared with those of LR rats (Puyal et al. 2003) reconsidered according to the animal eye opening time.
To establish the significance of the differences in the amplitude, rise time and occurrence of long-term effects among the experiments we used analyses of variance (ANOVA) and Tukey's post hoc test. Statistical significance was established at P < 0.05. In order to statistically analyse the LTP and LTD changes induced by visual deprivation during development, we grouped data of consecutive ages. The time interval for grouping data was chosen to maintain a sufficient temporal resolution (2 or 3 days). This allowed us to increase the number of observations and reach a valid statistical evaluation.
Results
Effect of HFS on the field potentials elicited in the vMVN during postnatal development in light-reared rats
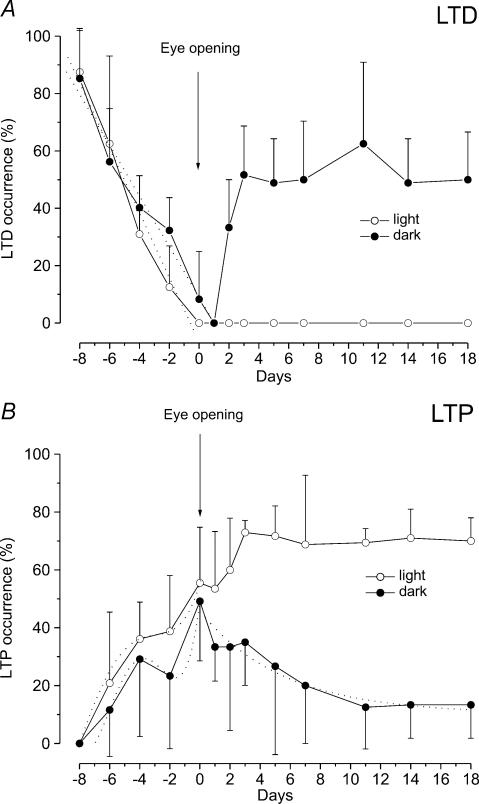
In our previous paper (Puyal et al. 2003) we examined the effect of HFS on the field potential N1 wave in slices from developing rats (P6–P21). To compare the effects of HFS on light-reared (LR) and dark-reared (DR) rats, in this study we have reconsidered the previous data regarding the occurrence of LTP and LTD by grouping them according to the time of eye opening of each animal. In addition, the occurrence of LTP and LTD is reported as the mean and s.d. of the percentage of the events evaluated in each animal. Our previous results show that before eye opening, the occurrence of LTD progressively decreases while that of LTP increases (Fig. 2). At E–8, HFS induced LTD in 87.5 ± 14.4% of the examined cases (n = 13) while LTP was not inducible (Fig. 2). In the following days the occurrence of LTD decreased and it was null at the time of eye opening, while that of LTP increased and remained stable at about 36–38% of the examined cases before eye opening (Fig. 2). In all the other cases HFS was ineffective. At the time of eye opening, LTP occurrence suddenly increased to about 55.5 ± 19.2% (n = 14) and then more gradually reached the adult value (about 70%) in the following days (Fig. 2B). By contrast, after eye opening LTD was no longer revocable (Fig. 2A).
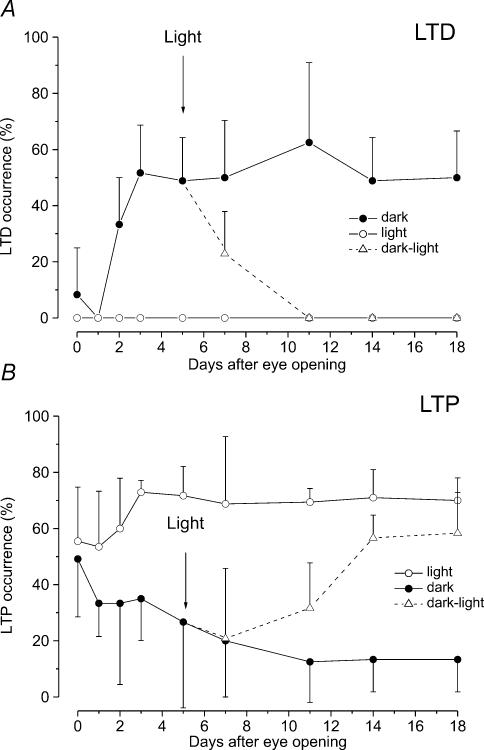
Figure 2. Effect of light deprivation on the occurrence of LTD and LTP during postnatal development.
Each point on the graphs represents the mean ± s.d. of percentage occurrence of LTD (A) and LTP (B) after HFS in different age groups of LR (open circle) and DR (filled circle) rats. For LR animals we reconsidered our previous data (Puyal et al. 2003) by grouping them according to the eye opening time. A, the dotted lines show the linear regression lines fitting the values of LTD occurrence before eye opening. Note the slight delay of the LTD decrease in DR rats compared with LR rats, demonstrated by the different slope of the regression lines (LR rats: slope 12.49, R = −0.870; DR rat: slope 9.18, R = −0.901). Two days after eye opening LTD reappears in DR rats and its occurrence increases in the following days. B, before eye opening the dotted lines represent the 4th order polynomial curves fitting the LTP data points in LR (R = 0.678) and DR rats (R = 0.589). Note the abrupt enhancement of LTP frequency just at the time of eye opening. After eye opening the dotted line represents the first-order exponential decay (t = 6.04, χ2: 54.7) fitting the LTP occurrence data in DR rats. Note that LTP frequency decreases in DR rats, while it increases in LR rats.
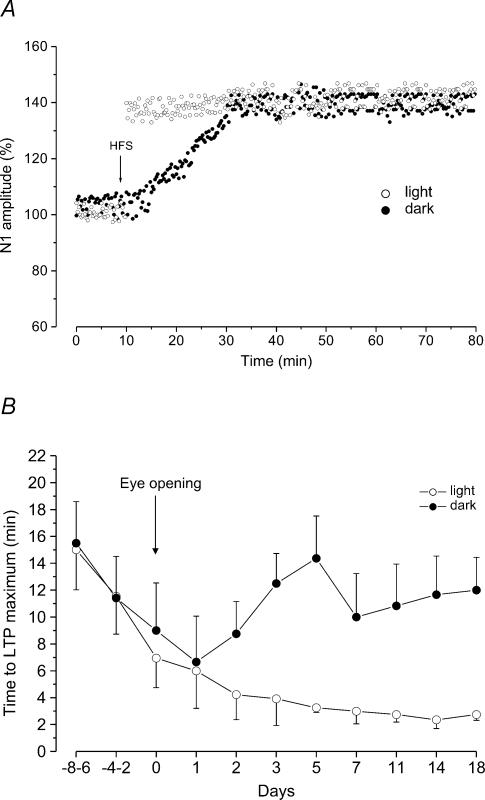
As far as the amplitudes of LTD and LTP are concerned, they were quite constant throughout development. The HFS-dependent depression was similar at all ages and was characterized by a reduction of N1 amplitude to 80 ± 5% of the control (n = 30). In addition, when LTP was induced the N1 wave increased to 131.2 ± 8.5% of the control (n = 131) and it was not statistically different compared with that observed in the adult animals. By contrast, the rise time of LTP was significantly longer in the early stages of development and progressively decreased to reach the adult values soon after eye opening (Fig. 4B).
Figure 4. Effect of light deprivation on rise time of LTP induced by HFS during postnatal development.
A, time courses of LTP induced by HFS at 5 days after eye opening in LR (filled dots) and DR (open dots) rats. The arrow indicates the time of HFS. Note the consistent increase of LTP rise time in DR animals. B, plot showing the rise time of LTP induced by HFS at different ages in LR (open circles) and DR (filled circles) rats. Each point represents the mean ± s.d. of LTP rise time (min). The values before the eye opening are obtained from three consecutive age groups, while those after the eye opening from a single age group. For LR animals the values are those obtained in our previous study (Puyal et al. 2003) adjusted to the eye opening time. Note that light deprivation induces a significant increase of LTP rise time beginning 2 days after eye opening.
Effect of HFS on the field potentials elicited in the vMVN during postnatal development in dark-reared rats
As in LR rats, the occurrence of LTD showed a progressive decrease before eye opening, even though at each age it was slightly higher compared with that of LR rats (Fig. 2A). In fact, while at E–8, LTD occurrence was 85.2 ± 17.4% (n = 13), a similar value to that obtained in LR rats at this age, at E–2 it was 32.3 ± 11.4% (n = 13), while in LR rats LTD only occurred in 12 ± 14.4% of the cases (n = 17). This delay in LTD decrease is evidenced by the different slope of the regression lines in DR and LR rats. In addition, the annulment of LTD occurred 1 day later compared with LR rats (Fig. 2A). Statistical analysis was performed by grouping data from E–6 to E–4 and from E–2 to E0. This resulted in a significant difference between DR and LR rats in the E–2–E0 group (Tukey's test: P < 0.05). As far as the occurrence of LTP is concerned, as in LR rats, it increased progressively before eye opening, but showed lower values than those of LR rats (Fig. 2B). In fact, at E–2 LTP occurrence was 23.3 ± 25.1% (n = 13) in DR rats and 38.7 ± 19.3% (n = 17) in LR rats (Fig. 2B). However, E–6, E–4, E–2 and E0 grouped data did not show significant differences. It is interesting to note that in both DR and LR rats the increase of LTP occurrence showed a pause at time E–2 and a peak at time E0. The 4th order polynomial fittings show this biphasic behaviour (Fig. 2B).
By contrast, marked differences in the occurrence of LTD and LTP between DR and LR rats were observed 1 or 2 days after eye opening (Fig. 2). In fact, LTD could be re-evoked at E+2 (Fig. 2A) in 33.3 ± 16.7% (n = 14) of the cases and later on, its occurrence suddenly increased. At E+3, the LTD occurrence was 51.6 ± 17% (n = 19), a value which can only be observed in the first stages of development in LR rats, and it remained stable at about this value throughout the period we examined. The LTD reappearance was associated with an exponential decay of the LTP occurrence, which was 12.5 ± 14.4% (n = 13) at E+11 (Fig. 2B), and remained steady low in the adult stage (E+18) (Fig. 2B). Statistical analysis of LTP and LTD occurrence in LR and DR rats showed that all values obtained after eye opening were significantly different (Tukey's test: P < 0.05).
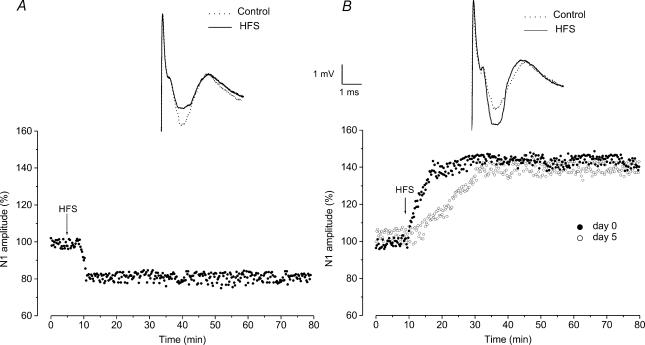
We also analysed the features of LTD and LTP in DR rats. As far as the amplitudes of LTD and LTP are concerned, they were not statistically different compared with those previously observed in the LR rats. In fact, LTD was characterized by a reduction of N1 amplitude to 79.6 ± 5.4% (n = 77) and LTP by an increase of N1 to 132.3 ± 7.4% (n = 52) (Fig. 3B and C). By contrast, the rise time of LTP, which similarly to LR rats progressively decreased before eye opening, showing values (15.5 ± 3 min, n = 2, at E–8–E–6 and 6.6 ± 3.4 min, n = 4, at E+1) which were not statistically different from those in LR rats, significantly increased 2 days after eye opening (P < 0.05) (Fig. 4B). In fact, at E+3, the LTP rise time was 12.5 ± 2.2 min (n = 6) and remained longer than that of LR rats until the adult stage (11.6 ± 2.8 min, n = 2, at E+18) (Fig. 4A and B).
Figure 3. Effects induced by HFS in the vMVN during postnatal development of dark-reared rats.
A and B, time courses of HFS effects on the amplitude of field potential N1 wave in DR rats. The N1 wave amplitude was measured every 15 s, expressed as a percentage of the baseline and plotted as a function of time in a single experiment. The arrows show the time of HFS. A, typical time course of LTD induced by HFS during the early stages of development. At the top: averaged field potentials (10 sweeps) before (dotted line, control) and after HFS (continuous line, HFS). B, time courses of LTP induced by HFS on the eye opening day (filled dots, day 0) and 5 days after eye opening (open dots, day 5). Note that the increase of N1 amplitude after HFS is delayed at day 5 compared with that at day 0. At the top: averaged field potentials (10 sweeps) before (dotted line, control) and after HFS (continuous line, HFS).
Reversibility of light deprivation effect
We verified whether the effect of light deprivation on the occurrence of LTD and LTP could be reversed by putting DR rats in the light, 5 days after eye opening. The occurrence of LTD and LTP was assayed 2, 6, 9 and 13 days after light exposure. Two days of light exposure caused a significant decrease of LTD occurrence from 48.86 ± 15.35% (n = 13) to 22.9 ± 15% (n = 13) (P < 0.05) and LTD was no longer inducible 6 days after light exposure (Fig. 5A). The probability of inducing LTP, which was 26.66 ± 30.55% (n = 13) at the eye opening time, was reduced to 20.82 ± 24.99% (n = 13) 2 days after light exposure, a value which was not significantly different compared with that obtained in DR rats at this age (20%, n = 14). Later on, the occurrence of LTP increased and reached 56.65 ± 8.13% (n = 16) only 9 days after light exposure (Fig. 5B). At 13 days of light exposure there was only a slight further increase to 58.33 ± 14.43% (n = 12) (Fig. 5B). The last two values remained significantly different from those of the LR rats (P < 0.05). In addition, the rise time of LTP did not recover fully; at this age it was significantly longer (5.2 ± 1.4 min, n = 7, P < 0.05) compared with that of LR rats. These findings reflect an incomplete recovery of LTP.
Figure 5. Effect of light exposure on LTD and LTP occurrence in dark-reared rats.
Each point on the graphs represents the mean ± s.d. of percentage occurrence of LTD (A) and LTP (B) after eye opening in different age groups of DR (filled circles), LR (open circles) and DR rats which were exposed to light 5 days after eye opening (triangles and dotted line). The arrows indicate the time when the animals were exposed to light. Note that the LTD occurrence decreased to zero in a short period of time after light exposure, while the increase of LTP occurrence was delayed and LTP did not reach the levels observed in LR animals.
Effect of light deprivation in adulthood
In order to verify whether light deprivation was also able to influence the effect of HFS in adulthood, we analysed the occurrence of LTP in slices from adult LR rats (P35, n = 11), which were maintained in the dark for a week. This light deprivation period did not interfere with the normal capability of vestibular synapses to be potentiated by HFS. In fact, HFS induced LTP in 73 ± 2.4% of the examined cases and had no effect in the other ones (not shown), a value which was not statistically different compared with that of normal adult rats. In addition, no statistical difference was observed in both the amplitude and rise time of LTP.
Discussion
Our previous study in LR rats demonstrates that the long-term effects induced by HFS of the primary vestibular afferents in the rat vMVN shift from LTD to LTP during a critical period of postnatal development (P6–P21) (Puyal et al. 2003). The occurrence of LTD progressively decreases and that of LTP increases before the time of eye opening (about P14). Thereafter, LTD can no longer be induced, while LTP occurs more frequently and reaches its full development a week after eye opening. Moreover, the rise time of LTP is progressively shortened suggesting a progressive maturation of the mechanisms underlying the full expression of LTP (Puyal et al. 2003).
In the present study we show that visual experience has a key role in determining the time course of synaptic plasticity maturation up to the final adult stage, since visual deprivation prevents the normal development of vestibular plasticity and reverts it toward the early stages. In fact, in DR rats, LTP occurrence was remarkably reduced and LTD was still inducible after eye opening. In addition, the rise time of LTP was considerably longer. The powerful influence of visual input in changing the plastic properties of vestibular neurones is also supported by the reversal of the visual deprivation effects caused by light exposure. However, the reversibility does not seem to be complete as shown by the persistence of a lower LTP occurrence and longer LTP rise time, when the rats were exposed to light 5 days after eye opening. Conversely, the preclusion of vision beyond the developing period had no influence on LTP occurrence. This suggests that light is an important stimulus for the normal development of vestibular plasticity only during a critical period, beginning from the eye opening time and ending at the completeness of LTP development.
As to the synaptic plasticity development before the eye opening time, we expected this process to be genetically guided, because visual signals are impeded by eyelid closure. However, a slight delay in the time course of LTD disappearance and LTP increase was observed, suggesting a modest experiential influence even during the very early stages of development. This is conceivable since luminous signals, which are able to reach the retina even in the presence of eyelid closure, might in some way activate the optokinetic pathway. In any case, regardless of this minor influence, we can state that most of the plastic changes that take place before eye opening appear to be genetically guided, while those ensuing after eye opening seem to be powerfully influenced by visual experience. In addition, before eye opening the probability of inducing LTP shows a biphasic increase with a peak just at the eye opening time both in DR and LR rats. This suggests a genetic role also in this biphasic behaviour and emphasizes the necessity of a precise temporal coincidence between the time of LTP development and visual input arrival.
We therefore suggest that light signals, or more properly, optokinetic signals which reach vestibular neurones, may modulate the complex series of events underlying synaptic plasticity phenomena and, during a critical period, may be responsible for the shift from LTD to LTP.
It is not yet possible to understand how this influence occurs; we can only hypothesize a possible mechanism. In our previous studies we showed that the mechanism sustaining vestibular LTP is complex. In fact, it involves different steps including pre- and postsynaptic events, as well as activation of different glutamate receptors and retrograde messengers which may play key roles in the induction or in full expression of LTP. Therefore, a block at any of these steps could provoke remarkable changes in LTP–LTD occurrence as well as in LTP rise time.
Glutamate receptors within vestibular nuclei show notable modifications of their expression during postnatal development (Sans et al. 2000; Puyal et al. 2003) which might be important for the full maturation of the vestibular system. In particular, the expression of group I mGluRs in the MVN shows changes which parallel those of LTD and LTP occurrence. In fact, the expression of mGluR5, which plays an inhibitory role on the induction of LTP and mediates LTD (Grassi et al. 2002; Puyal et al. 2003), decreases from birth to adulthood, while that of mGluR1, which is facilitatory on LTP, shows a progressive increase (Puyal et al. 2003). Therefore, visual dependent activity reaching MVN might interfere with maturation of these receptors and cause the shift of receptor expression dominance from mGluR5 to mGluR1. To verify the crucial role of mGluRs in the visual deprivation effects further electrophysiological and anatomical studies should be performed to clarify whether the pharmacology of LTD and LTP of DR rats is similar to that of LR ones, and whether visual deprivation causes a change in mGluR1 and mGluR5 expression.
Interestingly, the timing of long-term effect modification observed after eye opening both in dark and light re-exposure conditions suggests a different susceptibility of LTP and LTD to visual input manipulations. In fact, while LTD re-appearance in DR animals and LTD disappearance after light exposure occur in a very short period of time, LTP changes are significantly delayed. It therefore seems that the change of LTD precedes that of LTP, and LTD may represent an inhibitory event counteracting the full development of LTP. If the different expression of mGluRs is the mechanism at the basis of the long-term effect shift, a sequence of influences might be inferred in which the mGluR5 expression is powerfully inhibited by the visual signal and that of mGluR1 is subsequently enhanced by the reduction of mGluR5.
As to the significance of LTD/LTP shift during vestibular development, we think that it may occur mainly to shape the vestibular circuitry and modulate the synaptic efficacy depending on functional needs, a role that is also suggested in other systems. In fact, it has been demonstrated that LTP plays a key role in cortical map construction since it is induced during critical periods of barrel field and ocular dominance plasticity (Tsumoto, 1992; Bear & Kirkwood, 1993; Crair & Malenka, 1995; Kirkwood et al. 1995; Buonomano & Merzenich, 1998; Sermasi et al. 1999). In addition, it has been widely proposed that LTD drives the rapid, activity-dependent reduction of responses to irrelevant sensory inputs, that is a prominent mechanism for map plasticity in visual and somatosensory cortex (Glazewski & Fox, 1996; Rittenhouse et al. 1999; Wallace et al. 2001). Even though direct evidence for LTD during map plasticity is lacking, it has recently been shown that monocular deprivation during the sensitive period induces LTD in visual cortex (Heynen et al. 2003) and LTD-like depression also participates in the loss of responses in somatosensory cortex following whisker deprivation (Allen et al. 2003).
This evidence in other parts of the central nervous system seems to support our hypothesis that in vestibular nuclei the change from LTD to LTP during the critical period is necessary to shape vestibular circuitry. This is also supported by the effect of visual deprivation on vestibular functional development, consisting in a remarkable reduction in DR animals of the gains of vestibulo-ocular and optokinetic reflexes, which still remains when the animals are re-exposed to light (Collewijn, 1977; Harris & Cynader, 1981; Kennedy et al. 1982; Horn et al. 1996; Goode et al. 2001). The importance of visual input for VOR development is evident, since the optokinetic signal represents the most important error signal for consolidating correct central vestibular connections and eliminating erroneous ones. Therefore, before the optokinetic information is available, we expect the system to express LTD to avoid consolidation of synapses which cannot be correctly validated by experience. Of course, since eye opening occurs at the end of the second postnatal week, during the period that precedes eye opening, there is a gradual decay of LTD and an increase of LTP to prepare vestibular synaptic plasticity for the visual experience. Therefore, it would seem conceivable that if the visual input is prevented, the system reactivates the LTD mechanism to impede erroneous synapse consolidation. Consequently, it is possible that the induction of LTD in developing vestibular nuclei is important to avoid strengthening of synaptic connections without visual experience and the induction of LTP may be necessary to establish correct spatial connections during the experiential period.
However, LTP cannot only be useful for building up vestibular circuitry, since its occurrence remains almost constant throughout adulthood. LTP maintenance in vestibular nuclei has to be related to another characteristic of the vestibular system, which regards the ability to modulate its activity in response to different functional needs. For example, MVN neurone activity should plastically change, together with that of cerebellar circuitry, in the presence of visual modifications as occurs during visuo-vestibular calibration (Lisberger & Sejnowski, 1992; du Lac et al. 1995; Raymond et al. 1996). Therefore, as in the hippocampus, where LTP is thought to form the basis for learning and memory (Bliss & Collingridge, 1993; Malenka & Nicoll, 2000; Martin et al. 2000), vestibular LTP should be maintained to contribute to the adaptive readjustments of vestibular neurone responses.
Our results have been obtained in Wistar albino rats since we used this strain of animals in all our previous studies of the vestibular plasticity and its development. The fact that we found such a big visual deprivation effect in animals with poor optokinetic reflexes, might lead us to conclude that in normal viewing animals, the influence of optic flow on vestibular maturation and plasticity should be even more evident.
In conclusion, our results show that visual information plays a crucial role in shaping the synaptic plasticity of MVN neurones. This suggests that the neural activity normally supplied by visual experience may drive the progress of mechanisms like LTD and LTP that seem to be necessary for the light-guided refining and consolidation of vestibular circuitry. In addition, this study shows that also for the vestibular system, there is a critical period early in the development during which visual experience is fundamental for the full maturation of LTP. During development the LTP mechanism allows the reinforcement of functionally significant synaptic connections within vestibular neuronal circuitry, and in adulthood can be useful for adapting and consolidating the efficacy of vestibular neurone responsiveness to environmental requirements.
Acknowledgments
This research was supported by a grant from the Italian Ministry of University and Scientific Research. We wish to thank H. A. Giles for English language advice, and D. Bambagioni for technical assistance.
References
- Allen CB, Celiken T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. 10.1016/0166-2236(93)90081-V. [DOI] [PubMed] [Google Scholar]
- Battistin T, Cherubini E. Developmental shift from long-term depression to long-term potentiation at the mossy fiber synapse in the rat hippocampus. Eur J Neurosci. 1994;6:1750–1755. doi: 10.1111/j.1460-9568.1994.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Bear MF, Cooper LN, Ebner FF. A physiological basis for a theory of synapse modification. Science. 1987;237:42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- Bear MF, Kirkwood A. Neocortical long-term potentiation. Curr Opin Neurobiol. 1993;3:197–202. doi: 10.1016/0959-4388(93)90210-p. [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;22:567–631. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Capocchi G, Della Torre G, Grassi S, Pettorossi VE, Zampolini M. NMDA-mediated long term modulation of electrically evoked field potentials in the rat medial vestibular nuclei. Exp Brain Res. 1992;90:546–550. doi: 10.1007/BF00230937. [DOI] [PubMed] [Google Scholar]
- Collewijn H. Optokinetic and vestibulo-ocular reflexes in dark-reared rabbits. Exp Brain Res. 1977;27:287–300. doi: 10.1007/BF00235504. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debsk E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. Postnatal developmental changes in the response of rat primary horizontal semicircular canal neurons to sinusoidal angular accelerations. Exp Brain Res. 1982;47:295–300. doi: 10.1007/BF00239389. [DOI] [PubMed] [Google Scholar]
- du Lac S, Raymond JL, Sejnowski TJ, Lisberger SG. Learning and memory in the vestibulo-ocular reflex. Ann Rev Neurosci. 1995;18:409–441. doi: 10.1146/annurev.ne.18.030195.002205. 10.1146/annurev.neuro.18.1.409. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Friedlander MJ. Developmental down-regulation of LTD in cortical layer IV and its independence of modulation by inhibition. Neuron. 1996;16:1097–1106. doi: 10.1016/s0896-6273(00)80136-1. 10.1016/S0896-6273(00)80136-1. [DOI] [PubMed] [Google Scholar]
- Dutia MB, Johnston AR. Development of action potentials and apamin-sensitive after-potentials in mouse vestibular nucleus neurones. Exp Brain Res. 1998;118:148–154. doi: 10.1007/s002210050266. 10.1007/s002210050266. [DOI] [PubMed] [Google Scholar]
- Dutia MB, Lotto RB, Johnston AR. Post-natal development of tonic activity and membrane excitability in mouse medial vestibular nucleus neurones. Acta Otolaryngol (Suppl) 1995;502:101–104. doi: 10.3109/00016489509125201. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fox K, Glazewski S, Chen CM, Silva A, Li X. Mechanisms underlying experience-dependent potentiation and depression of vibrissae responses in barrel context. J Physiol (Paris) 1996;90:263–269. doi: 10.1016/s0928-4257(97)81436-2. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Fox K. The time-course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J Neurophysiol. 1996;75:1714–1729. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- Gonshor A, Melvill Jones G. Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. J Physiol. 1976;256:381–414. doi: 10.1113/jphysiol.1976.sp011330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode CT, Maney DL, Rubel EW, Fuchs AF. Visual influences on the development and recovery of the vestibuloocular reflex in the chicken. J Neurophysiol. 2001;85:1119–1128. doi: 10.1152/jn.2001.85.3.1119. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Grassi S, Della Torre G, Capocchi G, Zampolini M, Pettorossi VE. The role of GABA in NMDA-dependent long term depression (LTD) of rat medial vestibular nuclei. Brain Res. 1995;699:183–191. doi: 10.1016/0006-8993(95)00895-w. 10.1016/0006-8993(95)00895-W. [DOI] [PubMed] [Google Scholar]
- Grassi S, Francescangeli E, Goracci F, Pettorossi VE. Platelet activating factor and group I metabotropic glutamate receptors interact for full development and maintenance of long term potentiation in the rat medial vestibular nuclei. Neuroscience. 1999;94:549–559. doi: 10.1016/s0306-4522(99)00284-5. 10.1016/S0306-4522(99)00284-5. [DOI] [PubMed] [Google Scholar]
- Grassi S, Frondaroli A, Pettorossi VE. Different metabotropic glutamate receptors play opposite roles in synaptic plasticity of the rat medial vestibular nuclei. J Physiol. 2002;543:795–806. doi: 10.1113/jphysiol.2002.023424. 10.1113/jphysiol.2002.023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi S, Pettorossi VE. Role of nitric oxide in long-term potentiation of the rat medial vestibular nuclei. Neuroscience. 2000;101:157–164. doi: 10.1016/s0306-4522(00)00334-1. 10.1016/S0306-4522(00)00334-1. [DOI] [PubMed] [Google Scholar]
- Grassi S, Pettorossi VE. Synaptic plasticity in the medial vestibular nuclei: role of glutamate receptors and retrograde messengers in rat brainstem slices. Prog Neurobiol. 2001;64:527–553. doi: 10.1016/s0301-0082(00)00070-8. 10.1016/S0301-0082(00)00070-8. [DOI] [PubMed] [Google Scholar]
- Grassi S, Pettorossi VE, Zampolini M. Low frequency stimulation cancels the high frequency-induced long lasting effects in the rat medial vestibular nuclei. J Neurosci. 1996;16:3373–3380. doi: 10.1523/JNEUROSCI.16-10-03373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LR, Cynader M. The eye movements of the dark-reared cat. Exp Brain Res. 1981;44:41–56. doi: 10.1007/BF00238748. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, Bear MF. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6:854–862. doi: 10.1038/nn1100. 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- Horn ER, Sebastian CE, Ebeling K. Altered gravitational conditions affect the early development of the static vestibulo-ocular reflex in lower vertebrates. Ann N Y Acad Sci. 1996;781:635–638. doi: 10.1111/j.1749-6632.1996.tb15744.x. [DOI] [PubMed] [Google Scholar]
- Jessel TM, Kandel ER. Synaptic transmission: a bidirectional and self-modifiable form of cell-cell communication. Cell. 1993;72:1–30. doi: 10.1016/s0092-8674(05)80025-x. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Dutia MB. Postnatal development of spontaneous tonic activity in mouse medial vestibular nucleus neurones. Neurosci Lett. 1996;219:17–20. doi: 10.1016/s0304-3940(96)13152-9. 10.1016/S0304-3940(96)13152-9. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Courjon JH, Flandrin JM. Vestibulo-ocular reflex and optokinetic nystagmus in adult cats reared in stroboscopic illumination. Exp Brain Res. 1982;48:279–287. doi: 10.1007/BF00237224. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. J Neurosci. 1994;14:3404–3412. doi: 10.1523/JNEUROSCI.14-05-03404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. 10.1016/S0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Lannou J, Precht W, Cazin L. The postnatal development of functional properties of central vestibular neurons in the rat. Brain Res. 1979;175:219–232. doi: 10.1016/0006-8993(79)91002-3. 10.1016/0006-8993(79)91002-3. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Sejnowski TJ. Motor learning in a recurrent network model based on the vestibulo-ocular reflex. Nature. 1992;360:159–161. doi: 10.1038/360159a0. 10.1038/360159a0. [DOI] [PubMed] [Google Scholar]
- Lo FS, Mize RR. Synaptic regulation of L-type Ca2+ channel activity and long-term depression during refinement of the retinocollicular pathway in developing rodent superior colliculus. J Neurosci. 2000;20:RC58. doi: 10.1523/JNEUROSCI.20-03-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Mize RR. Properties of LTD and LTP of retinocollicular synaptic transmission in the developing rat superior collicus. Eur J Neurosci. 2002;15:1421–1432. doi: 10.1046/j.1460-9568.2002.01979.x. 10.1046/j.1460-9568.2002.01979.x. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78:535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 2000;285:1870–1874. doi: 10.1126/science.285.5435.1870. 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Martin SP, Grimwood PD, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649. doi: 10.1146/annurev.neuro.23.1.649. 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Melvill Jones G, Davies PRT. Adaptation of cat vestibulo-ocular reflex to 200 days of optically reversed vision. Brain Res. 1979;103:551–554. doi: 10.1016/0006-8993(76)90454-6. 10.1016/0006-8993(76)90454-6. [DOI] [PubMed] [Google Scholar]
- Miles FA, Eighmy BB. Long-term adaptive changes in primate vesibuloocular reflex. I. Behavioral observations. J Neurophysiol. 1980;43:1406–1425. doi: 10.1152/jn.1980.43.5.1406. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Du Lac S. Postnatal development of spike generation in rat medial vestibular nucleus neurons. J Neurophysiol. 2001;85:1899–1906. doi: 10.1152/jn.2001.85.5.1899. [DOI] [PubMed] [Google Scholar]
- Puyal J, Grassi S, Dieni C, Frondaroli A, Demémes D, Raymond J, Pettorossi VE. Developmental shift from long-term depression to long-term potentiation in the rat medial vestibular nuclei: role of group I metabotropic glutamate receptors. J Physiol. 2003;553:427–443. doi: 10.1113/jphysiol.2003.051995. 10.1113/jphysiol.2003.051995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neural learning machine? Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- Rittenhouse CD, Shouval HZ, Paradiso MA, Bear M. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397:347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- Sans NA, Montcouquiol ME, Raymond J. Postnatal development changes in AMPA and NMDA receptors in the rat vestibular nuclei. Brain Res Dev Brain Res. 2000;123:41–52. doi: 10.1016/s0165-3806(00)00082-1. [DOI] [PubMed] [Google Scholar]
- Sermasi E, Tropea D, Domenici L. A new form of synaptic plasticity is transiently expressed in the developing rat visual cortex: a modulatory role for visual experience and brain-derived neurotrophic factor. Neurosci. 1999;91:163–173. doi: 10.1016/s0306-4522(98)00598-3. 10.1016/S0306-4522(98)00598-3. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Tsumoto T. Long-term potentiation and long-term depression in the neocortex. Prog Neurobiol. 1992;39:209–228. doi: 10.1016/0301-0082(92)90011-3. 10.1016/0301-0082(92)90011-3. [DOI] [PubMed] [Google Scholar]
- Wallace H, Glazewski S, Liming K, Fox K. The role of cortical activity in experience-dependent potentiation and depression of sensory responses in rat barrel cortex. J Neurosci. 2001;21:3881–3894. doi: 10.1523/JNEUROSCI.21-11-03881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasling P, Hanse E, Gustafsson B. Long-term depression in the developing hippocampus: low induction threshold and synapse non specificity. J Neurosci. 2002;22:1823–1830. doi: 10.1523/JNEUROSCI.22-05-01823.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]