Abstract
The vanilloid receptor TRPV1, previously known as VR1, has been implicated in pain sensation under both physiological and pathological conditions. The channel is highly expressed in sensory ganglion neurones and is activated by a range of noxious stimuli including irritant chemicals, acids and heat. In order to understand the structural basis underlying this polymodal activation and the regulation by intracellular signalling pathways, we have investigated the functional roles of the cytoplasmic C-terminal of rat TRPV1. A mutant with the maximal truncation of the distal C-terminal encompassing the last 88 residues was constructed. Of interest, this mutant exhibited a Ca2+-dependent functional loss; it was irresponsive to capsaicin in the presence of extracellular Ca2+, but fully functional otherwise. Further studies of this construct revealed that extracellular Ca2+ alone could activate the channel, and that the activation required protein kinase C (PKC) phosphorylation at S502, an event that was up-regulated by external Ca2+ entry. We compared the truncation mutant with wild-type TRPV1 and demonstrated that it had a significantly increased sensitivity to PKC phosphorylation. These results suggest the distal C-terminal of TRPV1 can inhibit phosphorylation-induced potentiation of the wild-type channel. They also call into question some established functions of the distal C-terminal of TRPV1, including its roles in agonist binding and functional desensitization. We suggest that the functional loss of the truncation mutant, in the presence of extracellular Ca2+, was not due to disruption of agonist binding or gating, but rather to desensitization promoted by unstimulated extracellular Ca2+ entry.
The vanilloid receptor TRPV1 has been implicated in perception of peripheral pain (Caterina et al. 1997). The receptor has many of the functional traits of polymodal nociceptors including the C and type II Aδ fibres. Heat, acid and irritant chemicals such as capsaicin, the pungent ingredient of hot chilli peppers, all activate the channel at conditions that are similar to those found in normal physiological states. Moreover, these stimuli in combination cause cross-sensitization of the receptor, leading to an apparent increase in its sensitivity compared to any individual stimulus when applied alone. This unique polymodal responsiveness has led to suggestion that the receptor may function as an ‘integrating’ detector for noxious stimuli, enabling the cell to assimilate and respond to complex changes in the physiological environment.
TRPV1 also plays a role in pathological pain (Caterina et al. 2000; Davis et al. 2000). The receptor responds to many of the products released from tissue damage and neurogenic inflammation, such as extracellular protons, bradykinin, arachidonic acid and other lipid metabolites, nerve growth factor (NGF) and others (Levine & Taiwo, 1990; Julius & Basbaum, 2001). Some of the components (e.g. pH and lipid metabolites) have a direct effect on the channel itself, while others exert their effects through surface receptor-mediated intracellular pathways. The primary sequence of TRPV1 predicts many putative phosphorylation sites for a variety of protein kinases, most notably protein kinase C (PKC), protein kinase A (PKA), and Ca2+−calmodulin-dependent kinase II (CaMK II). Of these, PKC has been shown to enhance TRPV1-mediated responses (Cesare et al. 1999; Premkumar & Ahern, 2000; Vellani et al. 2001; Numazaki et al. 2002; Crandall et al. 2002; Bhave et al. 2003), PKA has been reported to both sensitize the channel activity and regulate the desensitization of the receptor (Lopshire & Nicol, 1998; De Petrocellis et al. 2001; Hu et al. 2002; Bhave et al. 2002; Vulcu et al. 2003; Mohapatra & Nau, 2003), and CaMK II has been implicated in NGF-induced sensitization (Bonnington & McNaughton, 2003).
While it is remarkable that a single receptor can mediate such a large and diverse range of stimuli, the underlying mechanisms are not well understood. Here we are concerned with the functional roles of the cytosolic C-terminal of rat TRPV1. Several functional sites on the C-terminal of the channel have been described. For example, a Walker motif for nucleotide binding (L726-W740) and two PKC phosphorylation sites (T704 and S800) have been shown to up-regulate the channel activity (Kwak et al. 2000; Numazaki et al. 2002). Two PKA phosphorylation sites, S774 and S820, were found to regulate channel desensitization (Mohapatra & Nau, 2003). A region around E761 in the cytoplasmic tail was suggested to form the ligand-recognition site for capsaicin binding (Jung et al. 2002). The last 72 residues were reported to be essential to a steep temperature-dependence of TRPV1 and important for regulation of capsaicin, low pH and voltage-induced responses (Vlachova et al. 2003). More recently, a region in the distal C-terminal between L777 and S820 was identified as a site for phosphatidylinositol-4,5-bisphosphate (PIP2) binding, where the hydrolysis of PIP2 mediates the NGF effect (Prescott & Julius, 2003). A similar region was reported for Calmodulin (CaM) binding and determines the Ca2+-dependent desensitization (Numazaki et al. 2003). Figure 1 summarizes the regions and sites on the C-terminal of rat TRPV1 that have been studied. It emerges that the cytosolic tail of the receptor has a diverse range of functions in both activation and regulation of the channel.
Figure 1. Functional domains of distal C-terminal of TRPV1.
The distal C-terminal of TRPV1 has been implicated in a variety of functions ranging from ligand binding to functional regulations. The molecular regions identified for these functions are indicated. Of them, 726–740 forms a Walker motif for ATP binding, S800 and T704 for PKC phosphorylation, S774 and S820 for PKA phosphorylation, E761 for ligand recognition, 777–820 for PIP2 binding and 767–801 for CaM association. The distal half of the terminal also confers specific thermal sensitivity. The truncation mutants that were studied here include TRPV1-Δ88 and TRPV1-Δ40, involving deletions of the last 88 and 40 residues, respectively. The TRPV1-Δ88 mutant represents a functional channel with the maximal truncation.
We report here yet another function of the distal half of the C-terminal of rat TRPV1. By seeking a minimal channel with the longest truncation of the distal C-terminal, we constructed a mutant that has the last 88 residues removed. The truncation, as expected from previous reports (Jung et al. 2002; Vlachova et al. 2003), caused a nearly complete loss of response to capsaicin. But surprisingly, this functional loss was Ca2+-dependent. In the absence of extracellular Ca2+, the mutant channel retained near normal activity to various stimuli including heat, low pH and capsaicin. While trying to understand the mechanism underlying this condition-specific loss of function, we found that extracellular Ca2+ alone activates the construct. The activation appears to be indirect, involving PKC phosphorylation at S502. PKC phosphorylation occurred in the wild-type too, but was insufficient to activate the channel, apparently because of the presence of the C-terminal tail. Taken together, our data suggest that the distal C-terminal of TRPV1 has an inhibitory effect on the sensitivity of the channel to PKC phosphorylation, and that the functional loss of the truncation mutant in the presence of extracellular Ca2+ was caused by the desensitization resulting from unstimulated Ca2+ entry, rather than by an impairment of the activation and gating machinery.
Methods
Mutagenesis
Rat TRPV1 from dorsal root ganglia cloned in pFROG vector was generously provided by Dr David Julius (Caterina et al. 1997; Jordt et al. 2000). Site-directed mutagenesis was performed using the overlap-extension polymerase chain reaction (PCR) method. Truncations of the C-terminal were done by either inserting a stop codon at the respective position, or through PCR amplification of a primer with appropriate restriction sites (5′, SacII; 3′, XbaI). All recombinant constructs were confirmed by restriction enzyme digestion and by DNA sequence analysis.
Channel expression
HEK293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetal bovine serum (Hyclone Laboratories Inc.) with 1% penicillin-streptomycin, incubated at 37°C in 5% CO2, and transfected at a confluence of approximately 80% using the standard calcium phosphate precipitation method. Either green fluorescent protein (GFP) or human CD8 lymphocyte antigen (0.5 μg (0.2 ml)−1) was cotransfected as a surface marker. Electrophysiological recordings took place 10–28 h after transfection. For cells cotransfected with CD8, antibody-coated beads were used to visually identify the transfected cells (Dynabeads M450 CD8, Dynal, Lake Success, NY, USA). More than 90% of the bead-decorated cells appeared to express the channel (Jurman et al. 1994).
The oocyte expression system was used for single-channel recordings, as previously described (Hui et al. 2003). Female Xenopus laevis frogs were anaesthetized by immersion in 0.2% tricaine methanesulphonate, and segments of ovarian lobes were removed through a small abdominal incision. The incision was sutured closed and the frogs were allowed to recover for approximately 4 weeks before a second batch of oocytes was harvested. When individual frogs no longer yielded acceptable oocytes, they were killed by an overdose of anaesthetic. All experiments and animal handling were in compliance with National Institutes of Health approved guidelines. Stage V and VI oocytes were separated by enzymatic digestion with collagenase, and then hand-selected 1 or 2 days after harvesting for microinjection of the channel cRNA. Typically, each oocyte received 10–30 ng cRNA. The injected oocytes were incubated in ND96 solution supplemented with 2.5 mm sodium pyruvate, 100 units ml−1 penicillin and 100 μg ml−1 streptomycin at 18°C for 2–7 days before use. For RNA preparation, rat TRPV1 in pFROG vector was linearlized by MluI digest. Capped cRNA was synthesized using the mMessage mMachine kit (Ambion). The final cRNA was resuspended in RNase-free water to approximately 1 ng nl−1, and kept at −80°C.
Electrophysiology
Conventional whole-cell and excised patch-clamp recording methods were used. Patch pipettes were fabricated from borosilicate glass (Sutter Instrument, Novato, CA, USA), coated with Sylgard (Dow-Corning, Midland, MI, USA), and fire-polished to a resistance of 0.5–2.5 MΩ for whole-cell recordings and 6–10 MΩ for single-channel recordings. Currents were amplified using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA) and recorded through a BNC-2090/MIO acquisition system (National Instruments, Austin, TX, USA) using a custom-designed software based on Labview 5.1 (National Instruments). Whole-cell recordings were typically filtered at 1 kHz and sampled at 5 kHz, and single-channel recordings were filtered at 10 kHz and sampled at 25 kHz. Currents were evoked from a holding potential of either −60 mV (inward) or +60 mV (outward). All experiments except those on heat activation were conducted at room temperature (21–25°C).
The control bath solution for HEK 293 cells contained (mm): NaCl 140, KCl 5, Hepes 10 and glucose 30; pH adjusted to 7.4 with NaOH. The solution was supplemented with 1.8 mm Ca2+ for the Ca2+-containing bath solution or 5 mm EGTA for the Ca2+-free bath solution. The internal pipette solution consisted of (mm): CsCl 140 and Hepes 10; pH adjusted to 7.4 with CsOH. In a subset of experiments, the pipette solution was added with calcium buffers, including 5 mm EGTA, 10 mm 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N-tetra-acetic acid (BAPTA) or 10 mm EGTA with 8 mm Ca2+ (140 nm free Ca2+; MaxChelator, Stanford University). Although the pipette solution contained no ATP, the relatively high concentration of extracellular glucose was used to maintain intracellular ATP (Simmons & Schneider, 1998). The bath and pipette solutions for single-channel recordings in oocytes were symmetrical and contained 100 mm sodium gluconate and 10 mm NaCl instead of 140 mm NaCl, and other components were the same as the Ca2+-free bath solution for HEK 293 cells.
Unless otherwise stated, all drugs and enzymes were purchased from Sigma-Aldrich (Sigma, St Louis, MO, USA). Capsaicin and capsazepine were obtained from Precision Biochemicals (Vancouver, BC, Canada). They were dissolved in 100% ethanol to make a 1 mm stock solution, stored at 4°C and diluted into the recording solution at appropriate concentrations before experiments (0.001–0.1% final ethanol). Low pH (5.5) solutions were buffered using 2-(N-morpholino)ethanesulphonic acid (MES). Solutions were titrated to their nominal pH at room temperature (21–25°C). Protein kinase reagents PKI 14–22 (Calbiochem), bisindolylmaleimide (BIM; Sigma) and phorbol myristate acetate (PMA; LC Laboratories) were all prepared in a 1 mm stock solution, where BIM was dissolved in dimethyl sulphoxide (DMSO), PMA in ethanol and PKI in distilled water. Working solutions were made fresh daily from stocks kept at −20°C. Drug delivery was controlled by a gravity-driven local perfusion system (ALA Scientific Instruments). The perfusion solutions were the same as the bath solutions except for the addition of appropriate agonists or antagonists.
Temperature control
Cells were placed in a narrow, rectangular chamber. Heat stimulus was applied by exchanging the entire content of the recording bath through an in-line SH-27B heater powered by a TC-324B temperature controller (Warner Instruments). The actual temperature of the recording was monitored during an experiment using a miniature thermocouple (Warner Instruments) placed about 1 mm from the pipette tip. The temperature drop between the tip and the thermocouple was less than 0.5°C throughout the entire tested temperature range (40–50°C) (Liu et al. 2003). The reported temperature corresponds to the readout of the thermocouple without corrections.
Results
Mutants lacking the distal cytosolic tail retain wild-type TRPV1 responses to capsaicin, low pH and heat in Ca2+-free solution
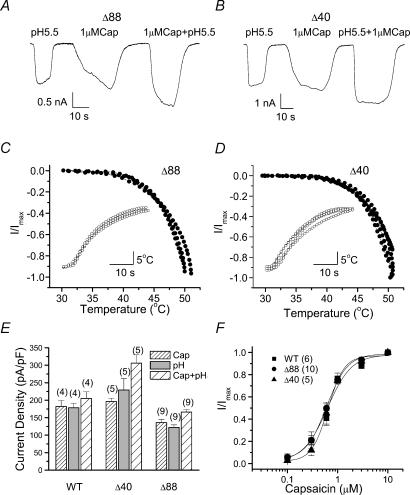
In an effort to identify functional roles of the cytoplasmic C-terminal of TRPV1, we studied the responses of mutant channels with different extent of deletion of the distal cytosolic tail (Fig. 1). Our experiments were first performed in Ca2+-free bath solutions. The mutant channels were tested with capsaicin, low pH and heat, three prototype stimuli for wild-type TRPV1. Figure 2A–D shows current responses from two representative constructs, one with the last 40 residues truncated (TRPV1-Δ40), and the other with the last 88 residues removed (TRPV1-Δ88). Both TRPV1-Δ40 and TRPV1-Δ88 were functional, producing large currents in response to all three stimuli. When capsaicin and low pH were applied simultaneously, they produced a larger current than either of the stimuli alone (the increase of open probability (Po) was even larger because of reduced conductance at low pH), suggesting that the cross-sensitization between stimuli was also retained in the truncation mutants.
Figure 2. The truncated mutants retain normal activity at the whole-cell level in Ca2+-free solutions.
A and B, whole-cell currents evoked by capsaicin, low pH and the combination of both for TRPV1-Δ88 and TRPV1-Δ40. Large and robust ionic currents were observed for both truncation mutants. C and D, heat responses of the mutant channels as a function of temperature. Each curve represents a recording from an individual cell. The insets show the time course of the elevation of the solution temperature. E, maximal currents of wild-type and mutant channels tested in the three conditions, 1 μm capsaicin, pH 5.5 and the combination of the two. Currents were normalized by membrane capacitance to account for variations in cell sizes. The number of cells in each trial is indicated over each bar. F, dose–response curves of capsaicin for the wild-type and the mutant channels. Each point describes mean ±s.e.m. from WT (n = 6), TRPV1-Δ40 (n = 5) and TRPV1-Δ88 (n = 10) experiments. The currents were normalized by the maximal responses obtained at 10 μm capsaicin in each experiment. The smooth curves are the best fits by the Hill equation, with wild-type: EC50, 0.6 ± 0.1; Hill coefficient (nH), 1.8 ± 0.5; TRPV1-Δ88: EC50, 0.6 ± 0.04; nH, 2.1 ± 0.3; TRPV1-Δ40: EC50, 0.7 ± 0.1; nH, 2.7 ± 0.4. All recordings were made from transiently transfected HEK293 cells in Ca2+-free solutions at a holding potential of −60 mV. Cap, capsaicin; WT, wild-type; Δ40, TRPV1-Δ40; Δ88, TRPV1-Δ88.
Figure 2E shows the current density (normalized by cell capacitance) of the mutant channels in comparison with that of the wild-type. Although the maximal current of TRPV1-Δ88 was somewhat less than that of the wild-type, the absolute currents were substantial and generally in the nanoamp range on a typical HEK293 cell. In addition, the capsaicin dose–response curves were similar in the truncated and the wild-type channels (Fig. 2F). These results suggest that the truncation of the distal cytosolic tail did not cause significant changes in ligand binding and gating of the channel.
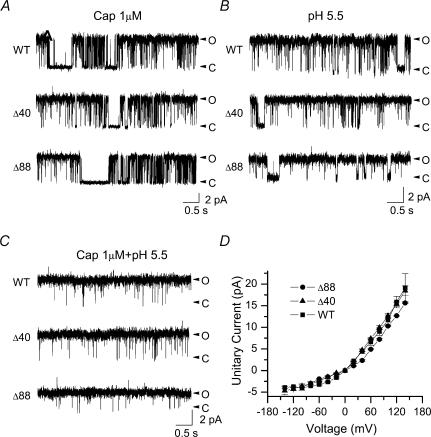
The function of the truncation mutants was further investigated at the single-channel level. Figure 3A–C illustrates representative single-channel activity of the wild-type, TRPV1-Δ88 and TRPV1-Δ40 in response to capsaicin, low pH and the combination of both. The kinetics of the gating, although not quantified, appeared similar for all three constructs. With capsaicin and low pH alone (Fig. 3A and B), the activity consisted of clusters of openings separated by relatively long gap durations (Hui et al. 2003). When the two stimuli were combined, the channel became fully open (Fig. 3C). Our previous analysis of acid-induced potentiation of the capsaicin response in the wild-type TRPV1 indicated that full activation was accompanied by an elongation of openings and a shortening of closures (Ryu et al. 2003). The same pattern occurred in the mutant channels (Fig. 3C). The similarity in gating behaviour between wild-type and the mutant channels, together with their ability to be fully open at low pH and high capsaicin, suggests that the gating machinery of the channel was largely untouched by the truncation of the distal C-terminal regions of the channel.
Figure 3. The truncated mutants retain near normal single-channel activity.
A–C, single-channel currents from wild-type and truncation mutants evoked by different stimuli. Downward deflection represents channel opening. D, unitary current–voltage relationship activated by 1 μm capsaicin at normal pH. The TRPV1-Δ88 mutant exhibited a reduced unitary current at positive membrane potentials, while TRPV1-Δ40 was approximately the same as the wild-type. Data were summarized from three to four patches. All single-channel currents were recorded from Xenopus oocytes at +60 mV in the outside-out configuration. Cap, capsaicin; WT, wild-type; Δ40, TRPV1-Δ40; Δ88, TRPV1-Δ88.
The truncation mutants appeared to differ slightly from the wild-type in conductance. Figure 3D shows the I–V relationship of the unitary currents for the three constructs, with 1 μm capsaicin as the stimulus. While TRPV1-Δ40 had a conductance similar to the wild-type, TRPV1-Δ88 showed a consistently smaller unitary current especially at positive voltages. The reduction of TRPV1-Δ88 was ∼26% at +60 mV (wild-type, 6.65 pA; TRPV1-Δ88, 4.93 pA). Similar decreases were also observed with low pH or both capsaicin and low pH (Fig. 3B and C), suggesting that the changes were not stimulus-specific and probably reflect an involvement of some distal C-terminal residues in the permeation pathway of the channel.
While the truncation of the last 88 residues preserved the function of the channel, the further deletion of two more residues (TRPV1-Δ90) resulted in a nearly complete loss of responsiveness to either capsaicin or low pH (data not shown). Increasing capsaicin concentration to as high as 40 μm remained ineffective, indicating that the irresponsiveness was not due to a weakened capsaicin binding. The last 88 residues therefore represent the largest region on the distal cytosolic tail that could be truncated without disrupting the function of the channel.
TRPV1-Δ88 becomes irresponsive in Ca2+-containing solution
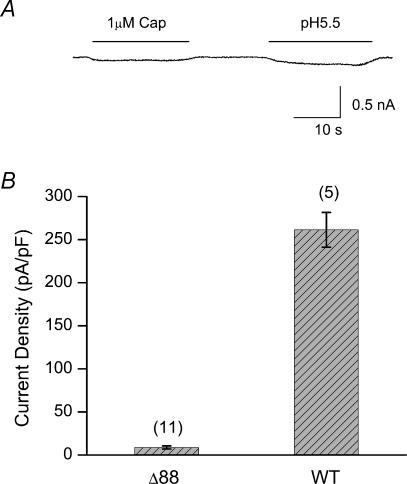
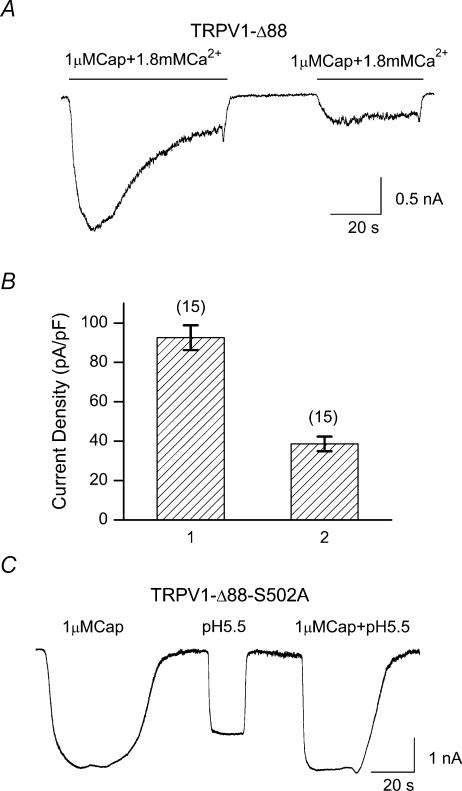
Previous studies suggest that only the last ∼70 residues of rat TRPV1 could be truncated before a significant loss of function (Jung et al. 2002; Vlachova et al. 2003). Our results on TRPV1-Δ88 mutant were clearly different. One difference we noticed between our studies and the reported ones is that our experiments were conducted in Ca2+-free conditions, while the previous studies used Ca2+-containing solutions. We therefore tested whether extracellular Ca2+ was the source for the apparent discrepancy. Figure 4A shows a response from TRPV1-Δ88, where the bath solution contained 1.8 mm Ca2+, but was otherwise identical to the solution used in Fig. 2. In the presence of extracellular Ca2+, no significant current was activated by either capsaicin or low pH. Figure 4B quantifies the current density for the wild-type and the mutant channel. The mutant channel produced only ∼10% of the wild-type current. This result was similar to those reported in the literature, suggesting that extracellular Ca2+ was the origin of the functional loss of the truncation mutant.
Figure 4. Functional loss of TRPV1-Δ88 in the presence of extracellular Ca2+.
A, current response recorded from a HEK293 cell expressing TRPV1-Δ88 in a bath solution containing 1.8 mm Ca2+. The construct was nearly irresponsive to either capsaicin or low pH. B, current density of the wild-type and TRPV1-Δ88 measured in the presence of extracellular Ca2+. Capsaicin at 1 μm was used as the stimulus. The mutant channel retained only a portion (< 3%) of the wild-type current. Holding potential (Vh), −60 mV. Cap, capsaicin; WT, wild-type; Δ88, TRPV1-Δ88.
Extracellular Ca2+ activates TRPV1-Δ88 in Ca2+-free solution
It seems paradoxical that the truncation of an intracellular domain can lead to a functional loss in a way that depends on extracellular Ca2+. To explore the underlying mechanisms, we applied Ca2+ to TRPV1-Δ88 transfected cells in a Ca2+-free bath (Fig. 5A). It was surprising to find that application of extracellular Ca2+ alone produced a current. The current slowly developed over a time course of many seconds, but it was robust (Fig. 5B). The same stimulus evoked no detectable current in normal HEK293 cells (data not shown), indicating that the current was conducted through TRPV1-Δ88.
Figure 5. Extracellular Ca2+ activates TRPV1-Δ88.
A and B, when cells were bathed in the Ca2+-free solution, application of extracellular Ca2+ alone induced a current in the absence of agonist. The Ca2+-evoked current was substantial as compared to that of 1 μm capsaicin. C and D, inhibition of Ca2+-evoked currents by intracellular Ca2+ chelation. Dialysis of cells with 10 mm BAPTA was sufficient to abolish the Ca2+ response. E, leak current detected in the whole-cell mode. Cells were bathed in the recording medium for > 5 min before recordings. All data were recorded from transiently transfected HEK293 cells at a Vh of −60 mV. Cap, capsaicin; Czp, capsazepine.
This Ca2+ activation was not seen in wild-type TRPV1, raising the question of whether Ca2+ acts on the channel directly from the extracellular side or through intracellular signalling pathways. We explored the issue by chelation of intracellular Ca2+. With 10 mm BAPTA in the patch pipette, extracellular Ca2+ was no longer effective (Fig. 5C). On average, the current with BAPTA was only ∼2% of that induced by 1 μm capsaicin, considerably less than that obtained in the absence of BAPTA. This significant reduction therefore points to a Ca2+-dependent, intracellular event underlying the activity in the presence of external Ca2+.
The fact that Ca2+ exerted its effect from the intracellular side requires initial Ca2+ entry from the extracellular bath before the channel is further activated. As one possible mechanism, we examined whether the truncation mutant had spontaneous activity in the absence of stimuli. Figure 5E shows a recording of the leak current in a cell transfected with the mutant channel. In the presence of extracellular Ca2+, a significant leak current was detected. The leak was substantially larger than that from non-transfected cells or cells transfected with the wild-type. Furthermore, the leak current was antagonized by the specific TRPV1 inhibitor capsazepine (Fig. 5E), confirming that it was carried by the expressed construct. These data suggest that the truncation mutant indeed allows extracellular Ca2+ entry in the absence of stimulus.
PKC phosphorylation underlies Ca2+ activation of TRPV1-Δ88
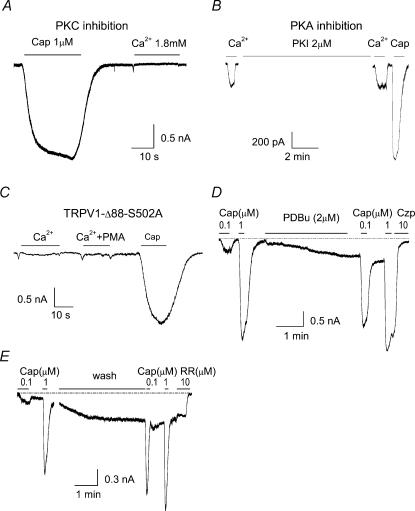
To probe the intracellular mechanism responsible for the Ca2+ activation of TRPV1-Δ88, we investigated the roles of protein kinases, particularly those that are known to regulate wild-type TRPV1. Figure 6A and B shows the effects of PKA and PKC inhibitors on the Ca2+-evoked currents from TRPV1-Δ88. Preincubation with 2 μm BIM, a specific inhibitor for PKC, was able to inhibit the current induced by 1.8 mm Ca2+ (Fig. 6A). However PKI 14–22, a membrane-permeable inhibitor for PKA, failed even at a relatively high concentration of 2 μm (Fig. 6B). H89, another inhibitor of PKA, was also ineffective at 2 μm under similar conditions (data not shown). The results therefore alluded to PKC phosphorylation as one possible event leading to Ca2+ activation of TRPV1-Δ88.
Figure 6. PKC phosphorylation at S502 underlies Ca2+-activation of TRPV1-Δ88.
A and B, effects of protein kinase inhibitors on Ca2+-evoked currents. For PKC inhibition, cells were pre-incubated with BIM (2 μm) for 1 h. For PKA inhibition, cells were perfused for 10 min with 2 μm PKI 14–22, which is a membrane-permeable PKA inhibitor. C, mutation of S502 to alanine in TRPV1-Δ88 abolished the Ca2+-evoked current. D and E, leak current and potentiation of capsaicin responses (0.1 μm) induced by application of 2 μm PDBu (D) and intracellular dialysis of 140 nm free Ca2+ through the pipette solution containing 10 mm EGTA and 8 mm Ca2+ (E). All recordings were made at Vh of −60 mV. Cap, capsaicin; RR, ruthenium red.
The involvement of PKC phosphorylation was corroborated by mutagenesis experiments. Among the PKC phosphorylation sites that have been identified on the wide-type (Numazaki et al. 2002), S502 is the only one remaining in TRPV1-Δ88. We therefore constructed the mutant with S502 replaced by alanine (TRPV1-Δ88-S502A) and examined its responsiveness to extracellular Ca2+. Figure 6C shows the currents recorded from a HEK293 cell expressing this construct. Although capsaicin (10 μm) evoked a large response, extracellular calcium was no longer effective, suggesting that phosphorylation at S502 is crucial for the occurrence of the Ca2+ current.
To gain further insight into how PKC phosphorylation is involved in Ca2+ activation of TRPV1-Δ88, we performed two other experiments, one involving PKC activation with phorbol dibutyrate (PDBu; 2 μm), and the other intracellular dialysis of the cytoplasm with buffered calcium. In the latter case, 10 mm EGTA and 8 mm Ca2+ were included in the pipette solution to give rise to ∼140 nm free Ca2+ (MaxChelator, Stanford University). In both experiments, we monitored the change of the leak current and the potentiation of submaximal capsaicin responses. As shown in Fig. 6D and E, either PDBu or intracellularly buffered calcium produced a leak current that was blocked by the TRPV1 inhibitors, capsazepine (10 μm) and ruthenium red (5 μm). In addition, both treatments caused profound potentiation of responses to 0.1 μm capsaicin, suggesting that the channel was probably phosphorylated. Taken together, these data further suggest that external Ca2+ entry could lead to activation of the truncation mutant and that the activation process involves PKC phosphorylation of the channel.
TRPV1-Δ88 has a higher sensitivity to PKC phosphorylation than wild-type TRPV1
An intriguing question is why wild-type TRPV1 is not activated by extracellular Ca2+ in the absence of a stimulus, given that it is also potentiated by phosphorylation at S502. There are two possibilities to account for the difference. One is that the cytosolic tail of rat TRPV1 protects the wild-type channel from being phosphorylated in the resting state. Alternatively, the channel can be phosphorylated, but the phosphorylation alone is inadequate to sufficiently open the channel. Both possibilities point to an inhibitory role for the cytosolic tail. To distinguish between them, we tested whether the wild-type can be phosporylated under resting conditions. Figure 7A shows a current recorded from a HEK293 cell expressing wild-type TRPV1. The cell was initially perfused with 1 μm PMA, with or without Ca2+, for up to 5 min, and then tested with both a low (0.1 μm) and a high (1 μm) concentration of capsaicin. PMA alone, or in combination with Ca2+, did not produce a detectable current in the wild-type. However following the treatment with PMA, 0.1 μm capsaicin evoked a current as large as activation by 1 μm capsaicin. Normally, in the absence of PMA treatment, the current induced by 0.1 μm capsaicin amounts to only a fraction of the current given by 1 μm capsaicin. The result therefore suggests that the channel could be phosphorylated in the resting state.
Figure 7. Distal C-terminal modulates the potentiation effect induced by PKC phosphorylation.
A, application of 1 μm PMA, with or without Ca2+, produced no detectable currents on HEK293 cells transiently transfected with the wild-type TRPV1. But subsequent application of low capsaicin (0.1 μm) elicited a large response comparable to that of 10 μm capsaicin, suggesting that the channel was phosphorylated prior to capsaicin application. B and C, current responses to application of capsaicin alone and together with 0.5 μm PMA for wild-type (B) and TRPV1-Δ88 (C). D, ratios of the increase of the current after PMA treatment. TRPV1-Δ88 showed a higher level of potentiation by PMA, at both 0.1 and 0.5 μm concentrations, than the wild-type. The capsaicin concentration was 0.1 μm. Cap, capsaicin; WT, wild-type.
The high sensitivity of TRPV1-Δ88 to PKC phosphorylation was also assessed by measuring the phosphorylation-induced potentiation of capsaicin responses. Figure 7B and C shows recordings of capsaicin currents evoked with and without PMA in the wild-type and truncation mutants. For both constructs, application of PMA potentiated the channel activity. The relative sensitivity to PMA is quantified in Fig. 7D. Data from two PMA concentrations, 0.1 μm and 0.5 μm, are presented. In both cases, the truncation mutant exhibited a higher level of potentiation than the wild-type. The difference was 2- to 3-fold depending on the PMA concentration. This result provides further support that the effect of PKC phosphorylation of TRPV1 is down-regulated by the distal C-terminal in the wild-type channel.
Desensitization as a cause for functional loss of TRPV1-Δ88 in Ca2+-containing solution
What caused the functional loss of TRPV1-Δ88 in the presence of extracellular Ca2+ (Fig. 4)? Given the large Ca2+ activation observed in the Ca2+-free bath, one candidate is the desensitization promoted by external Ca2+ entry. Essential to the mechanism is whether the truncation mutant retained wild-type desensitization following the removal of the distal C-terminal. Figure 8A illustrates a recording from a HEK293 cell expressing TRPV1-Δ88 in response to consecutive stimuli of capsaicin. The cell, incubated in the Ca2+-free solution, was given a first perfusion with 1 μm capsaicin along with 1.8 mm Ca2+ for approximately 1 min, followed by a wash with the Ca2+-free solution, and then a second perfusion with capsaicin in combination with Ca2+. As evident from the trace, the construct desensitized, and the desensitization process resembled that of the wild-type, involving both an acute inactivation within a prolonged application of agonist and a reduction of current between two adjacent stimuli (tachyphalaxis) to the subsequent exposure. The extent of desensitization was strong, with the second peak of the response being less than half of the first on average (Fig. 8B). However, the acute desensitization appeared to be slower than that of the wild-type under a similar condition, though the difference remains to be quantified.
Figure 8. Desensitization of TRPV1-Δ88.
A, current responses of TRPV1-Δ88 to two repeated applications of 1 μm capsaicin in the presence of 1.8 mm Ca2+. Cells were bathed in Ca2+-free solutions. B, plot of the normalized peak current density during the two consecutive capsasaicin applications. C, current responses to capsaicin and pH recorded from cells expressing the construct TRPV1-Δ88-S502 in Ca2+-containing solution. The mutation S502A rescued the channel activity of the truncation mutant in Ca2+-containing bath solutions. The perfusion solutions contained no Ca2+. Currents were recorded at Vh of −60 mV.
As a further support for the desensitization proposition, we tested the mutant TRPV1-Δ88-S502A in the Ca2+-containing solution. Figure 8C shows responses of the construct to application of capsaicin and/or low pH. Both capsaicin and low pH produced large currents. Previous experiments showed that this construct was irresponsive to extracellular Ca2+ due to disruption of PKC phosphorylation. The result therefore suggests that blockade of external Ca2+ entry, which prevents desensitization from occurring, can salvage the function of the truncation mutant. It also further argues that phosphorylation of S502 was essential to Ca2+ activation, and eventually desensitization of the construct.
Discussion
We have described a novel function of the distal C-terminal of rat TRPV1 on the regulation of its sensitivity to PKC phosphorylation. The finding is based on the study of a truncation mutant with the last 88 residues removed. When transiently expressed in HEK293 cells, the mutant channel exhibited a loss of function in a Ca2+-dependent manner. In the absence of extracellular Ca2+, the channel showed activity similar to that of the wild-type channel at both whole-cell and single-channel levels. To understand how extracellular Ca2+ caused the functional loss, we showed that extracellular Ca2+ alone could activate the channel in the absence of agonist, and that the activation resulted from PKC phosphorylation of S502. We also showed that wild-type TRPV1 could be phosphorylated, but the phosphorylation was insufficient to open the channel. The results indicate that the distal C-terminal of TRPV1 has an inhibitory role counteracting the stimulatory effect of PKC phosphorylation. We propose that the functional loss by the truncation mutant in the presence of extracellular Ca2+ occurred as a result of desensitization. Mutants that lacked the distal C-terminal became hypersensitive to phosphorylation, leading to unstimulated channel opening and thereby an elevation of intracellular Ca2+, which eventually amounted to a level sufficient to desensitize the channel.
The inhibitory role exerted by the C-terminal tail of the channel could be physiologically important. Phosphorylation is known to have a stimulatory effect on TRPV1, but little is known about how such a stimulatory effect is balanced within the channel. Without appropriate balancing, the channel can become over-reactive even to a basal level of phosphorylation. Although basal PKC activity is probably low under normal physiological conditions, our experiments on the Ca2+-activated current from the truncated mutant suggest that it can reach a level adequate to activate nearly half of the maximal current induced by 1 μm capsaicin. Therefore, the channel can be ‘auto-phosphorylated’ to a significant extent even at rest. Excitable cells, such as sensory neurones where TRPV1 is natively expressed, maintain a large repertoire of receptors, many of which are prone to modulation by phosphorylation. It is conceivable that the activation of kinases that target one type of receptor may inevitably lead to phosphorylation of other receptors. Without proper balancing, these phosphorylated receptors may form an oscillatory loop, leading to unstimulated but substantial channel activity as observed in the TRPV1-Δ88 truncation mutant. The C-terminal of TRPV1 could provide a negative feedback to balance the stimulatory effect of phosphorylation, and fine-tune the responsiveness of the channel.
While our data suggest that the Ca2+ activation of the truncation mutant required PKC phosphorylation, the precise molecular events that underlie this process may be complicated. The initial Ca2+ entry could arise from the basal activity of the construct, as suggested by the leak current in the presence of extracellular Ca2+. It could also arise from other mechanisms such as the Ca2+ release-activated channel (CRAC) (Parekh & Penner, 1997; Prakriya & Lewis, 2003). In our experiments where cells were incubated in Ca2+-free solution and then perfused with Ca2+-containing solutions, a large current spike was always observed preceding the activation of the expressed channels. The external Ca2+ entry can also up-regulate PKC activity in a variety of ways. For example, with its effect on membrane translocation of PKC (Lenz et al. 2002), the Ca2+ entry can lead to an increase of the basal activity of PKC. Cells also appear to express a repertoire of phospholipase C (PLCs) that become activated when the intracellular Ca2+ concentration is high. Such a pathway will lead to hydrolysis of PIP2, which has downstream effects to further elevate intracellular Ca2+ levels, and activate PKC. Common to all these mechanisms is that Ca2+ entry and PKC phosphorylation play into a positive feedback loop, where the Ca2+ entry amplifies the PKC activity, which in turn further activates the channel, thereby allowing a further increase of Ca2+ entry. The slow time course of the current evoked by Ca2+ is probably a result of this complex cascade within the cell.
We have used phorbol-esters as surrogate substrates for PKC in our phosphorylation experiments, and the results were interpreted as an effect of phosphorylation. There have been reports suggesting that PMA interferes with agonist binding of TRPV1 (Chuang et al. 2001). Given the structural similarity of PMA to resiniferotoxin (RTX), a strong agonist for TRPV1, it is conceivable that PMA may exert its effect either entirely or partially through a direct mediation of agonist binding instead of phosphorylation. In our experiments on wild-type channels, we found that PMA alone produced little activity, though an increase in baseline noise was occasionally observed. When applied together with capsaicin on excised macropatches it did not potentiate the capsaicin response either (data not shown). On the other hand, PDBu, a less hydrophobic analogue, exerted a similar effect on potentiation of capsaicin responses in the truncation mutant channels. The large potentiation effect of these compounds on capsaicin-activated current in the whole-cell configuration therefore must arise from an intracellular pathway such as PKC phosphorylation. However, it is possible that they may have an additional effect to potentiate the phosphorylation-induced effect (Bhave et al. 2003). In that case, the apparent potentiation observed in our data would represent the combined effect of both phosphorylation and its downstream interaction with the compounds.
Our results on TRPV1-Δ88 raise questions about some of the established functions of the distal C-terminal of TRPV1. It has been suggested that the N- and C-cytosolic tails, together with the region between S3 and S4 (Jordt & Julius, 2002; Gavva et al. 2004), form the ligand recognition site for capsaicin binding, and two particular residues, Arg114 and Glu761, located in the two respective regions, are critical for capsaicin binding (Jung et al. 2002). The distal half of the C-terminal has also been suggested to confer specific heat sensitivity and to be required for functional channel activation (Vlachova et al. 2003). These results are incompatible with our findings on TRPV1-Δ88, which lacks these regions. The disparity suggests that some of the unexpected functional consequences caused by truncation of the C-terminal regions may have confounded the interpretation of previous studies. Both studies used the loss of channel activity to infer specific functions of the structural perturbations. As seen in TRPV1-Δ88, such a loss of channel activity may be a secondary, rather than a direct, effect of the truncation. Therefore the loss of function does not necessarily correspond to alternation or disruption of ligand binding or channel gating. For example, the loss of [3H]RTX binding in some N- and C-terminal mutant channels (Jung et al. 2002) could be explained if the mutations altered the ligand binding affinity of the desensitization state(s). It is also possible that RTX binding may involve different sites than capsaicin binding and require the cytosolic termini. The truncation of the distal C-terminal also alters the sensitivity to PKC phosphorylation. Such an effect may have also contributed to some of the reported functional changes, but was apparently not taken into consideration.
The distal C-terminal of TRPV1 has also been implicated as a structural determinant for desensitization of the channel (Numazaki et al. 2003). A region of ∼35 residues on the C-terminal tail (38–72 from the C-terminal end) was identified as a site for CaM binding and the deletion of the region reportedly caused a nearly complete loss of tachyphalaxis. This is different from our observation on TRPV1-Δ88, which does not have the suggested CaM binding region, but retains both acute desensitization and tachyphalaxis. Some of the differences may have resulted from the use of different experimental protocols. We used prolonged, instead of brief, application of agonist. A brief pulse of stimulus may limit the amount of Ca2+ entry and therefore the extent of desensitization. This may not be a problem with the wild-type because its desensitization is relatively fast; but truncation mutants, as suggested by TRPV1-Δ88, have a reduced desensitization rate. As a result, the reduction of current between two adjacent stimuli (tachyphalaxis) decreases and may go unnoticed if the stimulus does not last sufficiently long and is not often repeated. Our results on TRPV1-Δ88 would suggest that if a prolonged stimulus had been used, the tachyphalaxis would have been discernable, even after the deletion of the CaM binding region, though the extent of tachyphalaxis could be quantitatively different. It is appealing to argue that the essential machinery for the desensitization of the channel is located outside the distal C-terminus.
The truncation mutant TRPV1-Δ88 appears to delineate the largest region on the cytosolic C-terminal that could be removed from a functional TRPV1. The truncation of two more residues generated a non-functional channel. It is unknown how the additional truncation caused a complete loss of function, whether because the protein was improperly folded, or the gating machinery was disrupted. The surface expression, or targeting, does not seem to be the problem. Channels lacking the entire C-terminal retain the same distribution pattern of immunoreactivity as the wild-type when expressed in HEK293 cells (Vlachova et al. 2003).
Acknowledgments
We thank Drs Fred Sacks and Anthony Auerbach for reading the manuscript and Dr Chunguang Zhang for experimental assistance. This work was supported by grants R01-RR11114 and R01-GM65994 from National Institutes of Health.
References
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci U S A. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5),P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Crandall M, Kwash J, Yu W, White G. Activation of protein kinase C sensitizes human VR1 to capsaicin and to moderate decreases in pH at physiological temperatures in Xenopus oocytes. Pain. 2002;98:109–117. doi: 10.1016/s0304-3959(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Harrison S, Bisogno T, Tognetto M, Brandi I, Smith GD, Creminon C, Davis JB, Di Geppetti P. The vanilloid receptor (VR1)-mediated effects of anandamide are potently enhanced by the cAMP-dependent protein kinase. J Neurochem. 2001;77:1660–1663. doi: 10.1046/j.1471-4159.2001.00406.x. 10.1046/j.1471-4159.2001.00406.x& V. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, et al. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Bhave G, Gereau RW. Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KY, Liu BY, Qin F. Capsaicin activation of the pain receptor, VR1: multiple open states from both partial and full binding. Biophys J. 2003;84:2957–2968. doi: 10.1016/S0006-3495(03)70022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to ‘hot’ chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, Kim S, Oh U. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem. 2002;277:44448–44454. doi: 10.1074/jbc.M207103200. 10.1074/jbc.M207103200. [DOI] [PubMed] [Google Scholar]
- Jurman ME, Boland LM, Liu Y, Yellen G. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 1994;17:876–881. [PubMed] [Google Scholar]
- Kwak J, Wang MH, Hwang SW, Kim TY, Lee SY, Oh U. Intracellular ATP increases capsaicin-activated channel activity by interacting with nucleotide-binding domains. J Neurosci. 2000;20:8298–8304. doi: 10.1523/JNEUROSCI.20-22-08298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz JC, Reusch HP, Albrecht N, Schultz G, Schaefer M. Ca2+-controlled competitive diacylglycerol binding of protein kinase C isoenzymes in living cells. J Cell Biol. 2002;159:291–302. doi: 10.1083/jcb.200203048. 10.1083/jcb.200203048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Taiwo YO. Hyperalgesic pain: a review. Anesth Prog. 1990;37:133–135. [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hui K, Qin F. Thermodynamics of heat activation of single capsaicin ion channels VR1. Biophys J. 2003;85:2988–3006. doi: 10.1016/S0006-3495(03)74719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci U S A. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by PKCe and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. CRAC channels: activation, permeation, and the search for a molecular identity. Cell Calcium. 2003;33:311–321. doi: 10.1016/s0143-4160(03)00045-9. 10.1016/S0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Ryu SJ, Liu BY, Qin F. Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J Gen Physiol. 2003;122:45–61. doi: 10.1085/jgp.200308847. 10.1085/jgp.200308847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons MA, Schneider CR. Regulation of M-type potassium current by intracellular nucleotide phosphates. J Neurosci. 1998;18:6254–6260. doi: 10.1523/JNEUROSCI.18-16-06254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachova V, Teisinger J, Suankova K, Lyfenko A, Ettrich R, Vyklicky L. Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J Neurosci. 2003;23:1340–1350. doi: 10.1523/JNEUROSCI.23-04-01340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulcu SD, Rupp J, Wiwie C, Gillen C, Jostock R, Nawrath H. The cAMP pathway sensitizes VR1 expressed in oocytes from Xenopus laevis and in CHO cells. Pharmacology. 2003;69:38–43. doi: 10.1159/000071265. 10.1159/000071265. [DOI] [PubMed] [Google Scholar]