Abstract
We found persistent abnormalities in the recovery of membrane excitability in long-term regenerated motor nerve fibres in the cat as indicated in the companion paper. These abnormalities could partly be explained by membrane hyperpolarization. To further investigate this possibility, we compared the changes in excitability in control nerves and long-term regenerated cat nerves (3–5 years after tibial nerve crush) during manoeuvres known to alter axonal membrane Na+–K+ pump function: polarization, cooling to 20°C, reperfusion after 10 min ischaemia, and up to 60 s of repetitive stimulation at 200 Hz. The abnormalities in excitability of regenerated nerves were reduced by depolarization and cooling and increased by hyperpolarization and during postischaemia. Moreover, the time course of recovery of excitability from repetitive stimulation and ischaemia was prolonged in regenerated nerves. Our data are consistent with an increased demand for electrogenic Na+–K+ pumping in regenerated nerves leading to membrane hyperpolarization. Such persistent hyperpolarization may influence the ability of the axon to compensate for changes in membrane potential following normal repetitive activity.
At the completion of maturation after Wallerian degeneration, regenerated axons show abnormalities in both passive (Vizoso & Young, 1948) and active (Kocsis & Waxman, 1983) membrane properties. The functional consequence of these persistent abnormalities is unknown.
Peripheral fibres with a reduced safety factor for conduction due to demyelination are easily blocked by trains of impulses due to excessive membrane hyperpolarization induced by electrogenic sodium pumping (Bostock & Grafe, 1985). A large repertoire of internodal channels (Waxman & Ritchie, 1993) is required in peripheral axons to maintain conduction at various membrane potentials (Chiu & Ritchie, 1984). Information about the accommodative properties of internodal ion channels in vitro was obtained from undissected axons by injecting current pulses and measuring changes in membrane potential – ‘electrotonus’ (Baker et al. 1987). In normal axons, excess depolarization is compensated by outward rectification through K+ channels (Bostock & Baker, 1988) and excessive hyperpolarization is compensated through ‘inward rectification’ mediated by both Na+ and K+ ions (Baker et al. 1987; Eng et al. 1990; Birch et al. 1991). It is not known whether regenerating peripheral axons show persistent alterations in the resting membrane potential or the ability to compensate for physiological variations in membrane potential.
Information about membrane potential in vivo can be obtained by threshold tracking (Bostock et al. 1998) when there are coherent changes in a number of excitability measures (Kiernan et al. 2000). Direct membrane hyperpolarization causes a decrease in axonal excitability, a decrease in the slope of the current–threshold relationship consistent with increased input resistance, a ‘fanning-out’ of responses during threshold electrotonus, an increase in super-excitability, and a decrease in both chronaxie and refractory period (Kiernan & Bostock, 2000). Similar hyperpolarizing excitability changes were seen during acute hypokalaemia (Kuwabara et al. 2002), reperfusion after ischaemia (Kiernan & Bostock, 2000) and recovery after activity (Vagg et al. 1998). Direct membrane depolarization causes excitability changes in the opposite direction (Kiernan & Bostock, 2000). Depolarizing excitability changes were also seen in hyperkalaemia (Kiernan et al. 2002b) and during ischaemia (Kiernan & Bostock, 2000). Changes in excitability consistent with depolarization were also seen with cooling, though the temperature sensitivity differed among measures (Kiernan et al. 2001).
We have recently explored, by serial electrophysiological observations, the recovery of membrane excitability in long-term regenerated motor nerve fibres of cat (Moldovan & Krarup, 2004). We found persistent abnormalities that could partly be explained by resting membrane hyperpolarization. To further investigate this possibility, we compared the changes in excitability in control nerves and long-term regenerated cat nerves (3–5 years after tibial nerve crush) during various conditions associated with a change in membrane potential. We tested the direct effect of depolarization and hyperpolarization by persistent DC pulses, depolarization occurring during cooling and hyperpolarization during reperfusion after ischaemia. To investigate the physiological significance of changes in membrane potential we tested the recovery in excitability after trains of impulses.
Brief preliminary reports have been published on some of these results (Moldovan & Krarup, 2003; Moldovan et al. 2001a,b).
Methods
Animals and electrophysiological investigations
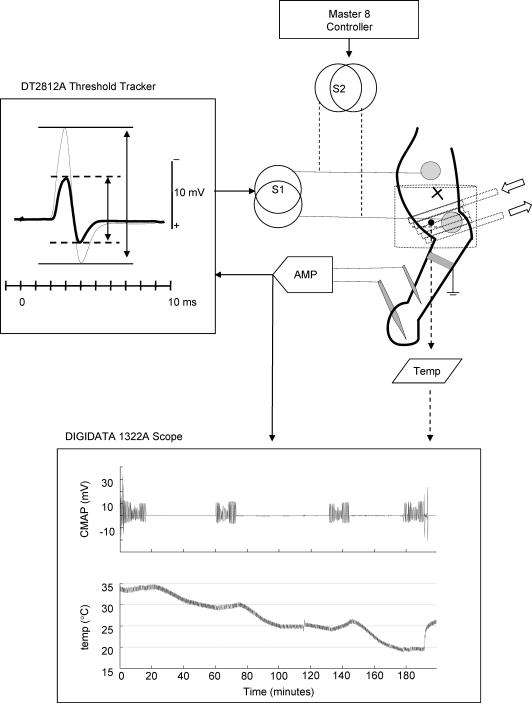
The studies were carried out on the same nine young adult cats described in the companion paper (Moldovan & Krarup, 2004). The experiments were approved by the Danish National Animal Experiment Committee. Both tibial nerves of three cats were crushed just distal to the branches to the deep toe flexor muscles during deep anaesthesia induced with pentobarbital (40 mg kg−1), and maintained by repeated intravenous injections of a 10% dilution. The cats were housed in a communal cage where they were allowed to move freely, and resumed normal locomotion 3–4 days after surgery. No gait alterations, self-mutilation or trophic disturbances were observed as a result of the lesion. All cats stayed healthy during the observation period. Electrophysiological investigations were carried out in control nerves and regenerated nerves at 39 and 60 months of regeneration under anaesthesia obtained by a mixture of 0.7 ml ketamine (10 mg kg−1) and 0.4 ml xylazine (2 mg kg−1) given by intramuscular injection, maintained by subcutaneous injection as needed, and monitored by the suppression of the corneal reflex elicited by air puff. The tibial nerve was stimulated at ankle and compound muscle action potentials (CMAPs) were recorded from plantar muscles. These procedures have been previously described in detail (Moldovan & Krarup, 2004). A diagram of the experimental set-up is presented in Fig. 1.
Figure 1. Experimental set-up.
Schematic representation of the stimulation and recording arrangements. The tibial nerve lesion was performed proximal in the leg (see text). The active electrode was placed at the ankle over the tibial nerve. At this level, a rubber tube was coiled for temperature experiments (arrows) and a blood pressure cuff (shown in outline) was inflated for ischaemia–reperfusion experiments. The temperature at the ankle was monitored on an external digital scope (Digidata 1322A). The cooling was done slowly and the excitability was tested only after the temperature was stable (lower panel). A DT2812A A/D board was used in conjunction with the QTRAC software to investigate the changes in threshold. The stimulus (S1) was automatically adjusted to maintain peak-to-peak amplitude of the threshold response at 40% of the supramaximal response (left panel). To investigate the effects of polarization and repetitive stimulation, an additional stimulator (S2) driven by a Master 8 controller was used.
Excitability testing
Investigations were performed using a recently described protocol designed to measure a number of excitability parameters in the human nerves (Kiernan et al. 2000). Stimulation and recording were controlled by a standard threshold tracking software (QTRAC, copyright Institute of Neurology, London). The full sequence of tests included: (i) stimulus–response curves for 0.2 and 1.0 ms test currents; (ii) threshold electrotonus induced by 100 ms polarizing currents set to +40% (depolarizing) and −40% (hyperpolarizing) of the control threshold; (iii) current–threshold relationship tested with 1 ms pulses at the end of 200 ms polarizing currents altered in a ramp fashion from 50% (depolarizing) to −100% (hyperpolarizing) of the control threshold; (iv) recovery of excitability from 2 to 200 ms after a supramaximal conditioning stimulus.
Reference values
The full sequence of excitability tests were obtained in regenerated and control nerves at 37°C. The animal was placed on a thermostatically heated rubber pad and covering it with hydrophobic cotton. An additional feed-back operated heating lamp was used to maintain the skin temperature over the investigated tibial nerve at 35°C.
Cooling
The temperature dependency of excitability was investigated in 39-month-regenerated nerves and control nerves (n = 6). Cooling was induced by means of a circulating water thermostat (Holm & Halby, Heto HMT 200/CBN 8-30) through a thin (5 mm diameter) rubber tube loosely coiled (5–8 times) around the stimulating electrode at the ankle. The instantaneous temperature was monitored using a temperature sensor and a digital acquisition system (Axon Instruments Digidata 1322A) (Fig. 1). It was previously suggested that excitability is more sensitive to changes in temperature than to steady-state temperature (Kiernan et al. 2001). Hence in this study cooling was done slowly (∼1°C in 5 min). The full sequence of excitability tests was investigated at 35°, 30°, 25° and 20°C only after the temperature had been stable for at least 15 min (Fig. 1).
Ischaemia
Ischaemia of the foot was induced with a blood pressure cuff inflated over the systolic pressure (Fig. 1). In this study a short (10 min) ischaemic episode was tested to minimize the effect of ischaemic conduction block (Bostock et al. 1991a). Due to spatial constraints, the cuff had to be placed over the cathode at the ankle. During the inflation of the cuff it was not possible to ascertain that the relationship between the cathode and the tibial nerve remained unchanged. Therefore changes in threshold during ischaemia were not investigated. The threshold changes during reperfusion were slow (data not shown) so the full sequence of excitability measures could be carried out at 5 and 30 min postischaemia.
Persistent polarization
At 60 months after crushing the effects of polarization on excitability measures were compared with findings in control nerves (n = 6). Previous studies testing the effect of polarization on excitability (Kiernan & Bostock, 2000) or the ability of polarization to compensate for pathology (Kiernan et al. 2002a) expressed the size of polarizing currents in absolute values. In this study, the multiple excitability protocol was altered so the threshold current could be visually adjusted to a target value prior to the actual excitability testing. By means of an additional constant current stimulator, external polarizing currents were delivered to produce a threshold alteration that was a fixed fraction of the ‘resting’ threshold (Fig. 1).
Repetitive stimulation
Recovery of excitability after repetitive stimulation was investigated in 60-month-regenerated nerves and control nerves (n = 6). Supramaximal stimuli (0.1 ms) were delivered at 200 Hz for 30 s and for 1 min by means of an external constant current stimulator driven by a Master-8 controller (AMPI, Inc.) (Fig. 1). The observed changes in excitability following repetitive stimulation were too rapid to be monitored with the multiple excitability protocol. We designed a simple protocol to monitor only the changes in threshold, similar to other studies (Kiernan et al. 1996). The threshold was estimated every 0.8 s using proportional tracking. All valid threshold estimates (within 15% of target) were averaged over 30 s intervals.
Data analysis
Off-line threshold estimates were computed using the sigmoidal stimulus–response relationship as previously described (Moldovan & Krarup, 2004). All data analysis and presentation was performed using a custom-made program implemented in MATLAB 6.5 (MathWorks, Inc.). Values of several parameters where derived from each electrophysiological test for numerical analysis. The naming and calculations are the same as those used in the companion paper (Moldovan & Krarup, 2004) except for latency (ms) that was measured from the 1.0 ms stimulus onset to the peak of CMAP averaged over the whole recording period.
Statistics
The parameters were stored in an SQL database to facilitate non-parametric statistical comparisons using SPSS 11.5 (SPSS, Inc.). Results in numbers are given as means ± s.e.m. unless otherwise specified. Comparisons were carried out using Mann-Whitney non-parametric tests except when otherwise specified.
Results
No electrophysiological differences in excitability were noted between 39 and 60 months after crush so maturation was considered complete at these times. When compared with control nerves, regenerated nerves showed slightly slower conduction and several persistent abnormalities in excitability: reduced chronaxie, increased rheobase, reduced relative refractory period (RRP), reduced resting and minimal I–V slopes and markedly increased threshold reduction during hyperpolarizing electrotonus (Moldovan & Krarup, 2004).
Effects of persistent polarization
Compensating for persistent abnormalities
To test whether the persistent abnormalities in regenerated nerves could be explained by changes in membrane potential, we tried to empirically determine a polarization value that compensated for the excitability abnormalities in regenerated nerves. The investigated range was limited to weak currents (−50% to +50% of threshold) to avoid possible tissue damage under the electrodes during the 10–15 min of continuous polarization. No significant changes in latency or amplitude of the CMAP were observed during the investigated range of polarization.
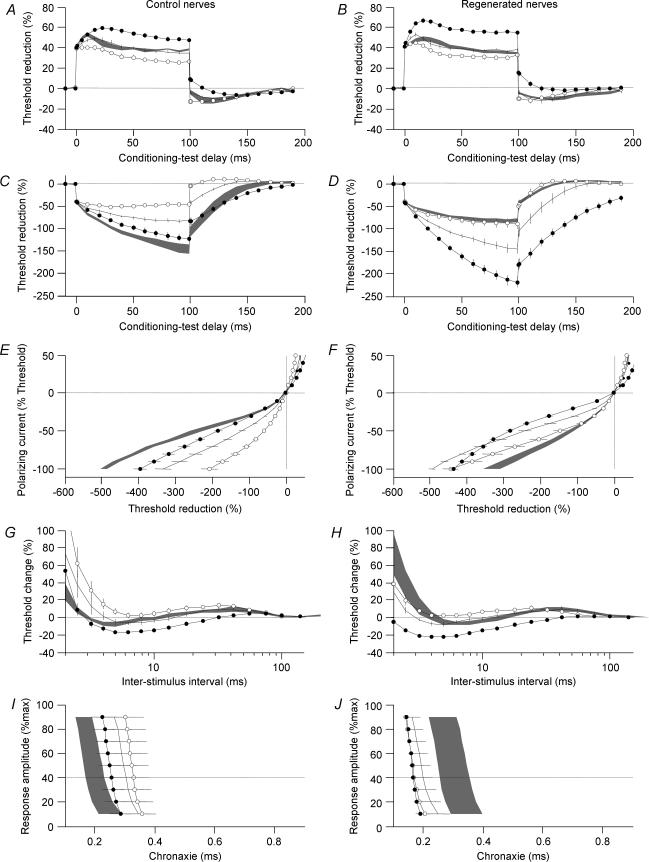
Depolarization of 25% of threshold normalized TEh(Peak) in regenerated nerves (Fig. 2D), and also normalized the RRP (Fig. 2H) and resting I–V slope (Fig. 2F). Moreover, a 25% hyperpolarization caused responses in control nerve to resemble regenerated nerve (Fig. 2C).
Figure 2. Influence of weak polarizing currents on excitability measures.
Excitability was investigated in control nerves (left) and 60-month-regenerated nerves (right) in normal conditions (lines) during persistent depolarization (open symbols) and during persistent hyperpolarization (filled symbols). The persistent polarization currents were set to 25% of threshold. To facilitate visual comparisons grey areas represent measurements in the absence of polarization from regenerated nerves (left) and normal nerves (right). Error bars and grey areas indicate ± s.e.m. Graphs illustrate 40% depolarizing threshold electrotonus (A and B), 40% hyperpolarizing threshold electrotonus (C and D), current–threshold relationship (E and F), recovery cycle for 1 ms pulses (G and H) and chronaxie (I and J).
Not all excitability measures could be compensated by depolarization. Most notably we found no difference in the responses to early or late depolarization (TEd(peak), TEd(plateau)) when comparing regenerated and control nerves: in both depolarization and hyperpolarization caused ‘fanning’-in and ‘fanning’-out (P < 0.05), respectively, compared to the changes that occurred in the absence of polarization (Fig. 2A and B).
Sensitivity to polarization
We found that chronaxie and minimum I–V slope seemed less affected by polarization (Fig. 2I and J) than other excitability parameters. It was therefore ascertained whether sensitivity to polarization differed between parameters. A previous study (Kiernan & Bostock, 2000) suggested that for weak polarizing currents the change in excitability is approximately linear. Thus we calculated the influence of polarization on each parameter from the linear fit: y = a + bx, where ‘x’ is the polarizing current, ‘a’ the resting value of the parameter, and ‘b’ the estimated slope of the parameter per unit polarization. We expressed the polarizing current as a fraction of the resting threshold, and hence the normalized slope (b/a) × 100 describes the per cent of parameter change for 1% of threshold polarization. A steeper normalized slope per percentage polarization indicates greater sensitivity to polarization. The largest fractional change for 1% polarization was seen in RRP in both normal (3.35%) and regenerated (3.12%) nerves.
The minimum I–V slope and chronaxie that were not normalized during depolarization of regenerated nerves were found to be almost independent of polarization in regenerated nerves. Nevertheless, in normal nerves these indices showed a small change to polarization (Fig. 2E and I).
Effects of cooling
The temperature sensitivity (percentage change per degree Centigrade) of each parameter was calculated from the regression line a + b(t − 30) as b/a × 100, where t is the temperature monitored at the site of stimulation (Kiernan et al. 2001).
Nerve conduction and CMAP amplitude
As temperature decreases, sodium channel gating is slowed, leading to slowing of the inward current responsible for depolarization (Schwarz & Eikhof, 1987) at each node of Ranvier along the cooled nerve segment. The increase in latency was similar in normal (3.3% per °C) and regenerated (3.9% per °C) nerves. This may suggest that Na+ channel function is normal in regenerated nerves.
A discrepancy (P < 0.05) was observed in changes in amplitude. During cooling amplitude increased in normal nerves (0.8% per °C) and decreased in regenerated nerves (1.4% per °C). As the temperature decreases, inactivation of Na+ channels is also slowed and there is an increase in the duration of individual action potentials that contribute to the CMAP (Ludin & Beyeler, 1977; Bolton et al. 1981). This may suggest an abnormality in repolarization or a difference in phase summation and cancellation of regenerated motor units.
Cooling-induced depolarization
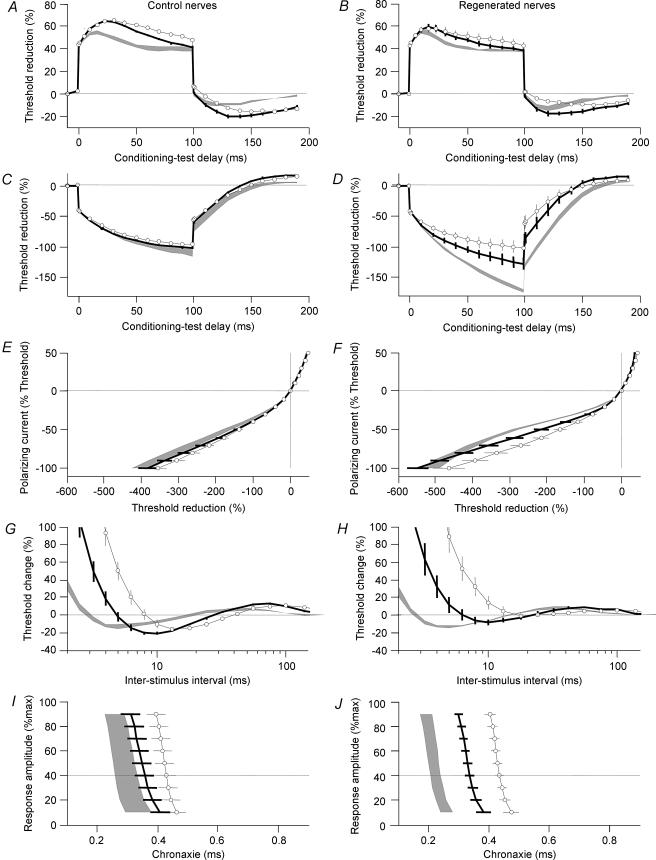
Excitability indices showed marked changes with cooling (Fig. 3). There was a decrease in response to hyperpolarizing electrotonus (Fig. 3C, D, E and F), an increase in RRP (Fig. 3G and H), a decrease in superexcitability and an increase in chronaxie (Fig. 3I and J) all consistent with resting membrane depolarization due to a shift in equilibrium potential. The largest change was seen in RRP both for normal (9.7% per °C) and regenerated (15.8% per °C) nerves. This was attributed to the fact that both increased depolarization and slowing of Na+ gating contribute to the increase in RRP. The changes in chronaxie, superexcitability and hyperpolarizing electrotonus were more pronounced in regenerated nerves than in normal nerves (P < 0.05).
Figure 3. Cooling from 35 to 25°C.
Normal nerves (left) and 39-month-regenerated nerves (right) were investigated at 35°C (grey areas), 30°C (lines) and to 25°C (open symbols). Error bars and grey areas indicate ± s.e.m. Graphs illustrate 40% depolarizing threshold electrotonus (A and B), 40% hyperpolarizing threshold electrotonus (C and D), current–threshold relationship (E and F), recovery cycle for 1 ms pulses (G and H) and chronaxie (I and J).
At 25°C, the peak threshold increase to the hyperpolarizing current (TEh(peak)) and the chronaxie of regenerated nerves were similar to normal nerves indicating that the effect of cooling was larger in regenerated than control nerve (P < 0.05). By contrast, during cooling superexcitability in regenerated nerves (−4 ± 2%) became smaller than in control (−16 ± 2%, P < 0.05).
Slowing of accommodation
The peak percentage threshold reduction to the depolarizing current (TEd(peak)) was increased by cooling to 25°C suggesting that the effect of reduced accommodation by slowing the gating of K+ channels was greater than the depolarizing effect. This effect appeared larger in control nerves but did not reach statistical significance.
With further cooling, at 20°C a striking discrepancy was observed between control and regenerated nerves (Fig. 4). The response to depolarizing electrotonus flattened in regenerated nerves (Fig. 4A) indicating that the effect of depolarization became stronger than the reduced accommodation. This suggests that a further source of depolarization is triggered in regenerated nerves when cooling from 25 to 20°C, and this was supported by the tendency of regenerated nerves to show even greater changes than control during RRP (Fig. 4D) and chronaxie (Fig. 4E), whereas the effects during hyperpolarizing electrotonus were indistinguishable in regenerated and control nerve (Fig. 4B).
Figure 4. Excitability measures in normal (open symbols) and regenerated (line) nerves at 20°C.
Error bars and grey areas indicate ± s.e.m. Grey areas indicate regenerated nerves at 35°C. Graphs illustrate +40% depolarizing threshold electrotonus (A), 40% hyperpolarizing threshold electrotonus (B), current–threshold relationship (C), recovery cycle for 1 ms pulses (D) and chronaxie (E).
Reperfusion after ischaemia
The effects of ischaemia on peripheral nerves are well documented (Lundberg & Oscarsson, 1954). Ischaemia distal to compression was shown to produce depolarization due to inhibition of the Na+–K+ pump and subsequent extracellular accumulation of K+ (Bostock et al. 1991a,b). With release from ischaemia, the Na+–K+ pump is driven to restore the ionic balance across the membrane and this leads to membrane hyperpolarization (Bostock et al. 1991a).
Reperfusion induced hyperpolarization
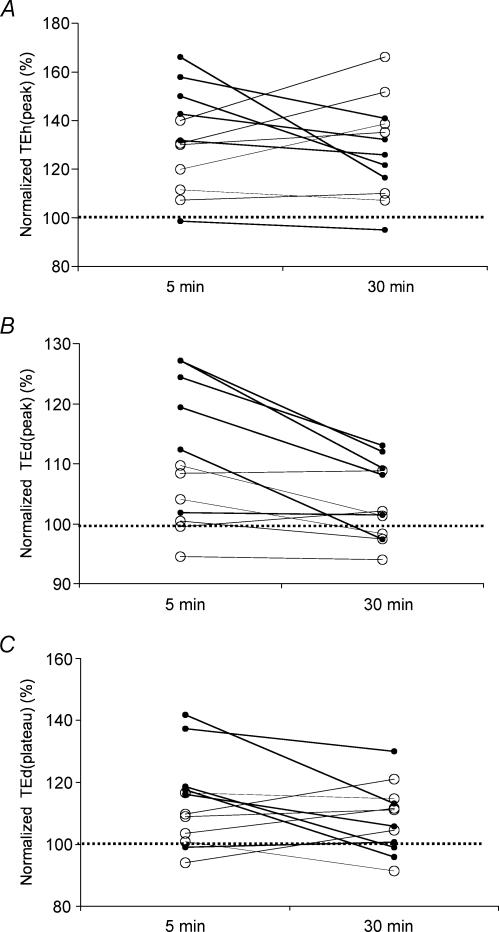
During reperfusion after ischaemia no persistent changes in latency and amplitude were apparent and both control and regenerated nerves showed excitability changes consistent with hyperpolarization. The changes in threshold electrotonus were the most pronounced but there was also a noticeable reduction in RRP and chronaxie (data not shown). While normal nerves showed an increase in TEd(peak) consistent with postischaemic hyperpolarization, no such change was observed in regenerated nerves (Fig. 5B). The increase in TEd(plateau) (the mean threshold reduction between 90 and 100 ms of depolarization) was less apparent (Fig. 5C). This indicates that with slight hyperpolarization the ‘fanning-out’ of depolarizing electrotonus may not be apparent in regenerated nerves.
Figure 5. Changes in threshold electrotonus during reperfusion.
The effects of reperfusion were investigated in normal nerves (filled symbols) and 39-month-regenerated nerves (open symbols) at 5 min postischaemia and 30 min postischaemia. The parameters derived from threshold electrotonus are normalized to the pre-ischaemic levels (100% dotted line). The variations for each nerve are shown for TEh(peak) (A), TEd(peak) (B) and TEd(plateau) (C).
Time course of recovery of excitability after ischaemia
In 5 out of 6 regenerated nerves (Fig. 5A) there was a delayed increase in TEh(peak) at 30 min postischaemia while control nerves showed a clear trend of recovery. This discrepancy suggests that recovery from ischaemia may be delayed in regenerated nerves.
Recovery of threshold after repetitive stimulation
Long trains of impulses induce hyperpolarization of peripheral axons due to activation of the Na+–K+ pump by Na+ entry (Ritchie & Straub, 1957; Bergmans & Michaux, 1970; Schoepfle & Katholi, 1973; Bostock & Grafe, 1985).
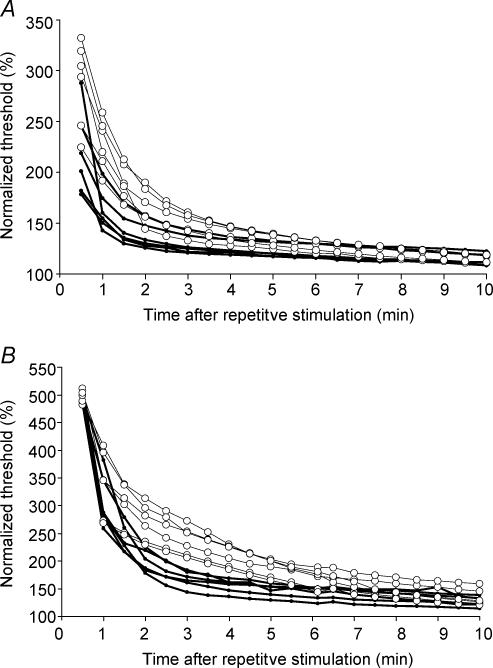
We investigated the effect of repetitive stimulation on nerve excitability in 60-month-regenerated nerves following 30 s and 1 min of supramaximal 200 Hz repetitive stimulation (Fig. 6). The first 30 s following activity were not included to allow for the recovery of blocked CMAP, and no persistent changes in latency or amplitude were detected. Both control and regenerated nerves showed a marked increase in threshold following activity that was more pronounced after 1 min (Fig. 6B) than after 30 s (Fig. 6A) of repetitive stimulation. The maximal increase in threshold after 1 min tetanic stimulation was similar in control and regenerated nerves, whereas the recovery in excitability was considerably slower in regenerated nerves and this effect appeared more pronounced after 1 min of repetitive stimulation (Fig. 6). The time course of these findings suggests that the Na+–K+ pumping activity may differ in regenerated and control nerves.
Figure 6. Repetitive stimulation.
The effects of repetitive stimulation on threshold were investigated in normal nerves (filled symbols) and 60-month-regenerated nerves (open symbols). Trains of 200 Hz were delivered for 30 s (A) and 60 s (B). Thresholds were normalized to the pre-test values (100%).
Discussion
In the companion paper we suggested that regenerated motor axons of cat could be hyperpolarized at rest (Moldovan & Krarup, 2004). In this study, we compared the changes in excitability in control nerves and long-term regenerated cat nerves at various membrane potentials. We found further evidence of hyperpolarization in regenerated nerves. Moreover, our findings suggest that the persistent hyperpolarization could be attributed to an increased activity of the Na+–K+ pump.
Evidence supporting membrane hyperpolarization
We found (Moldovan & Krarup, 2004) that long-term regenerated nerves show an abnormally high threshold increase during hyperpolarizing electrotonus, a decrease in the resting I–V slope, a decrease in RRP and a decrease in chronaxie, all consistent with membrane hyperpolarization (Kiernan & Bostock, 2000). The most convincing evidence obtained in the present study that the persistent abnormalities in excitability observed in regenerated nerves could be attributed to a change in resting membrane potential came from polarization experiments. Most abnormalities could be compensated by depolarization of regenerated nerves and could be reproduced by hyperpolarizing normal nerves. Depolarization induced by cooling also normalized the abnormalities in excitability in regenerated nerves (except for RRP).
One limitation of the threshold tracking studies is that the assessment of membrane properties at the site of stimulation depends on the muscle response. Conduction in experimentally demyelinated nerve fibres is easily blocked by hyperpolarization (Bostock & Grafe, 1985). Thus if hyperpolarization leads to conduction block in regenerated nerve fibres the maximal amplitude of CMAP will decrease and there will be an ‘erroneous’ increase in threshold. This was not the case in our study because no changes in control threshold were observed for the duration of excitability testing.
While most measures of axonal excitability of cat tibial nerves were very similar to those described in human median nerves (Kiernan et al. 2000), inward rectification seemed less apparent over the range of hyperpolarization tested. With persistent hyperpolarization, an inward rectification became noticeable in the current–threshold relationship of regenerated but not control nerves (Fig. 2F). This discrepancy could also be explained by the resting hyperpolarization of regenerated nerves.
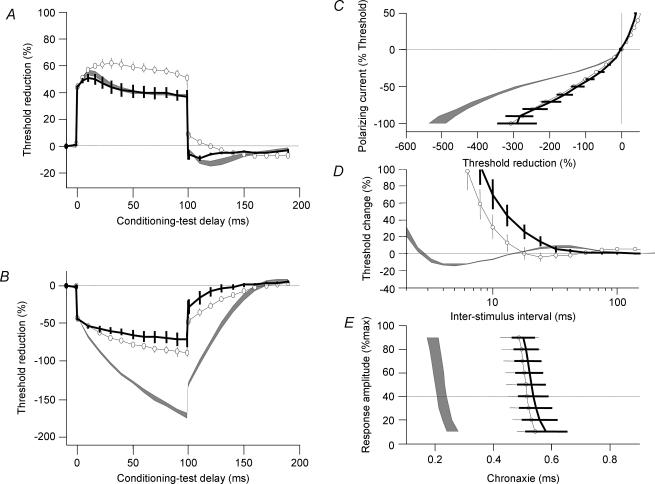
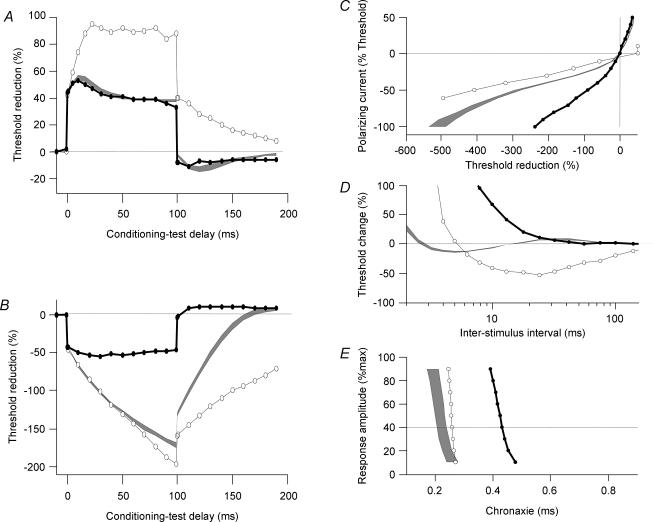
At 35°C chronaxie was virtually independent of small polarizing currents in regenerated nerves but not in control nerves. Chronaxie is a measure of excitability of the nodal membrane and depends on the passive membrane time constant and a ‘local’ response due to a non-inactivating voltage-dependent ‘threshold conductance’ (Bostock & Rothwell, 1997). The small but noticeable (P < 0.05) difference in sensitivity to polarization of chronaxie between normal and regenerated nerves could be explained by the fact that the ‘threshold conductance’ of regenerated nerves is blocked by hyperpolarization. With cooling chronaxie showed even greater prolongation than normal nerves (Fig. 3I and J) suggesting that the voltage dependency was increased as the ‘threshold current’ was activated. In order to test this hypothesis we subjected a regenerated nerve at 20°C to strong hyperpolarization (Fig. 7). We found that hyperpolarization brought the chronaxie back to the 35°C values by switching off the effect of ‘threshold current’. The same tendency was seen in RRP (Fig. 7D) but the recovery was limited, probably by the temperature slowing of Na+ channel inactivation.
Figure 7. Effect of cooling and hyperpolarization.
Measurements in one regenerated nerve at 20°C (black line) and the same nerve at 20°C during 100% hyperpolarization (open symbols). Grey area indicates regenerated nerves at 35°C. Graphs illustrate +40% depolarizing threshold electrotonus (A), 40% hyperpolarizing threshold electrotonus (B), current–threshold relationship (C), recovery cycle for 1 ms pulses (D) and chronaxie (E).
Minimum I–V slope showed only minimal voltage or temperature dependency and remained reduced in regenerated nerves (Fig. 3E and F). Thus it is a much better indicator of passive membrane properties than chronaxie.
Abnormalities not supported by membrane hyperpolarization
In regenerated nerves the recovery in responses to depolarizing electrotonus appeared complete while the threshold increase during hyperpolarizing electrotonus remained highly abnormal (Moldovan & Krarup, 2004). In the context of resting membrane hyperpolarization alone, the threshold response to depolarizing electrotonus should have also been abnormal. The response to hyperpolarizing electrotonus was more sensitive to persistent polarization in regenerated than in normal nerves (Fig. 2A and B). Nevertheless, the effects of polarization on depolarizing electrotonus were similar in regenerated and control nerves (Fig. 2A and B). We previously suggested (Moldovan & Krarup, 2004) that this discrepancy was due to increased accommodation during depolarization rather than indicating absent inward rectification during hyperpolarization. This was supported by a more pronounced inward rectification in regenerated than in normal nerves during persistent hyperpolarization (Fig. 2F). In addition, the relative insensitivity to polarization of depolarizing as opposed to hyperpolarizing threshold electrotonus in regenerated nerves was also observed during cooling (Fig. 3B) and reperfusion after ischaemia (Fig. 5). Furthermore, with cooling, superexcitability became smaller in regenerated than in control nerves. The degree of superexcitability depends on the spread of depolarization from the internode (Barrett & Barrett, 1982) and is limited by K+ channels, especially fast K+ channels (Bostock et al. 1981). The combination of these findings supports the suggestion that a persistent abnormality in rectifying K+ channels may exist in regenerated nerves. It was previously shown in rat that early regenerated axons show a marked sensitivity to K+ channel blocking agents (Kocsis et al. 1982). Some controversy exists as to whether this abnormality is persistent (Kocsis & Waxman, 1983) or disappears with time (Ritchie, 1982; Brismar et al. 1987). A possible explanation for this discrepancy was provided by the presence of some even more abnormally short internodes in regenerated nerves (Hildebrand et al. 1985; Brismar et al. 1987) responsible for the initial sensitivity to K+ blocking agents, which are later removed by Schwann cell remodelling (Kocsis & Waxman, 1987). It appears that in cat nerves the functional abnormality in K+ channels is persistent up to 5 years following nerve regeneration in the absence of important Schwann cell remodelling.
Increased demand for Na+–K+ pumping
We previously suggested that the most likely mechanism that could describe long-term hyperpolarization of regenerated axons is the over-activity of the Na+–K+ pump (Moldovan & Krarup, 2004). In normal nerves, hyperpolarization induced by repetitive activity (Ritchie & Straub, 1957; Bergmans & Michaux, 1970; Schoepfle & Katholi, 1973; Bostock & Grafe, 1985) and reperfusion after ischaemia (Bostock et al. 1991a,b) is attributed to an increase in activity of the Na+–K+ pump driven by increased Na+ entry. In regenerated nerves we found that the recovery in excitability after both ischaemia (Fig. 5) and repetitive stimulation (Fig. 6) were prolonged in regenerated nerves. This suggested that the demand for Na+–K+ pumping may have been increased in regenerated nerves. Further evidence of a persistent increase in Na+–K+ pumping activity in regenerated nerves was obtained from cooling experiments. With decreasing temperature, the depolarizing shift in excitability seemed larger in regenerated nerves consistent with a reduction of the Na+–K+ pumping activity with temperature. Furthermore, the marked depolarization observed when cooling from 25 to 20°C could indicate a severe reduction or even block in the Na+–K+ pumping activity (Fig. 4). Such additional depolarization in regenerated nerve was supported by the strong fanning-out induced by persistent hyperpolarization during threshold electrotonus (Fig. 7A and B).
Increased Na+–K+ pump activity might be associated with an increased number of nodes (Vizoso & Young, 1948) and a normal Na+ channel density per node (Querfurth et al. 1987), and thus an increased Na+ influx that would activate the Na+–K+ pump to a greater extent in regenerated than in control nerves. Nevertheless, the effect should normalize with time after activity and this does not seem to be the case. It is unlikely that the slow rate of stimulation used in excitability testing is sufficient to maintain the hyperpolarization of generated nerves. A possible explanation for the persistent hyperactivity of the Na+–K+ pump could be a continuous intra-axonal flow of Na+ from dispersed regions with internodal or blood–nerve barrier abnormalities (Kiernan et al. 2002a). Such aberrant internodes (Hildebrand et al. 1985; Brismar et al. 1987) were shown to occur in regenerated nerves of rat but their functional consequences were unclear.
Similar abnormalities in excitability to those found in early regenerated cat nerves were recently described in multifocal motor neuropathy in humans and were also attributed to hyperpolarization (Kiernan et al. 2002a). Human axons were shown to hyperpolarize with natural activity associated with voluntary contraction (Vagg et al. 1998) and this may precipitate conduction block in patients with multifocal motor neuropathy (Kaji et al. 2000). It is possible that hyperpolarization may also have a negative consequence on repetitive conduction and energy demand in regenerated nerves. It is of possible relevance that similar changes to those observed in regenerated nerve were found in patients with chronic demyelinating neuropathy (Sung et al. 2004) since remyelinated internodes are shorter and the number of nodes of Ranvier per unit length are increased also in this condition. It is, however, unknown whether the hyperpolarization following remyelination has more severe consequences for the safety of conduction than that following regeneration and maturation.
Acknowledgments
We are grateful to Jesper Sørensen for his help with the surgical procedures. The project was supported by Lundbeck Foundation, the Novo Nordisk Foundation, the Danish Medical Research Council, the Ludvig and Sarah Elsass Foundation and the Foundation for Research in Neurology.
References
- Baker M, Bostock H, Grafe P, Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol. 1987;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans J, Michaux J. Hyperpolarization evoked in single human nerve fibres by rhythmically repeated tetanizations. Arch Int Physiol Biochim. 1970;78:569–570. [PubMed] [Google Scholar]
- Birch BD, Kocsis JD, Di Gregorio F, Bhisitkul RB, Waxman SG. A voltage- and time-dependent rectification in rat dorsal spinal root axons. J Neurophysiol. 1991;66:719–728. doi: 10.1152/jn.1991.66.3.719. [DOI] [PubMed] [Google Scholar]
- Bolton CF, Sawa GM, Carter K. The effects of temperature on human compound action potentials. J Neurol Neurosurg Psychiatry. 1981;44:407–413. doi: 10.1136/jnnp.44.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Baker M. Evidence for two types of potassium channel in human motor axons in vivo. Brain Res. 1988;462:354–358. doi: 10.1016/0006-8993(88)90564-1. [DOI] [PubMed] [Google Scholar]
- Bostock H, Baker M, Grafe P, Reid G. Changes in excitability and accommodation of human motor axons following brief periods of ischaemia. J Physiol. 1991a;441:513–535. doi: 10.1113/jphysiol.1991.sp018765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Baker M, Reid G. Changes in excitability of human motor axons underlying post-ischaemic fasciculations: evidence for two stable states. J Physiol. 1991b;441:537–557. doi: 10.1113/jphysiol.1991.sp018766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 1998;21:137–158. doi: 10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bostock H, Grafe P. Activity-dependent excitability changes in normal and demyelinated rat spinal root axons. J Physiol. 1985;365:239–257. doi: 10.1113/jphysiol.1985.sp015769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Rothwell JC. Latent addition in motor and sensory fibres of human peripheral nerve. J Physiol. 1997;498:277–294. doi: 10.1113/jphysiol.1997.sp021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Sears TA, Sherratt RM. The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J Physiol. 1981;313:301–315. doi: 10.1113/jphysiol.1981.sp013666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T, Hildebrand C, Berglund S. Voltage-clamp analysis of nodes of Ranvier in regenerated rat sciatic nerve. Brain Res. 1987;409:227–235. doi: 10.1016/0006-8993(87)90706-2. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Ritchie JM. On the physiological role of internodal potassium channels and the security of conduction in myelinated nerve fibres. Proc R Soc Lond B Biol Sci. 1984;220:415–422. doi: 10.1098/rspb.1984.0010. [DOI] [PubMed] [Google Scholar]
- Eng DL, Gordon TR, Kocsis JD, Waxman SG. Current-clamp analysis of a time-dependent rectification in rat optic nerve. J Physiol. 1990;421:185–202. doi: 10.1113/jphysiol.1990.sp017940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C, Kocsis JD, Berglund S, Waxman SG. Myelin sheath remodelling in regenerated rat sciatic nerve. Brain Res. 1985;358:163–170. doi: 10.1016/0006-8993(85)90960-6. 10.1016/0006-8993(85)90960-6. [DOI] [PubMed] [Google Scholar]
- Kaji R, Bostock H, Kohara N, Murase N, Kimura J, Shibasaki H. Activity-dependent conduction block in multifocal motor neuropathy. Brain. 2000;123:1602–1611. doi: 10.1093/brain/123.8.1602. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Bostock H. Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain. 2000;123:2542–2551. doi: 10.1093/brain/123.12.2542. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Burke D, Andersen KV, Bostock H. Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve. 2000;23:399–409. doi: 10.1002/(sici)1097-4598(200003)23:3<399::aid-mus12>3.0.co;2-g. 10.1002/(SICI)1097-4598(200003)23:3<399::AID-MUS12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Cikurel K, Bostock H. Effects of temperature on the excitability properties of human motor axons. Brain. 2001;124:816–825. doi: 10.1093/brain/124.4.816. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Guglielmi JM, Kaji R, Murray NM, Bostock H. Evidence for axonal membrane hyperpolarization in multifocal motor neuropathy with conduction block. Brain. 2002a;125:664–675. doi: 10.1093/brain/awf041. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Mogyoros I, Burke D. Changes in excitability and impulse transmission following prolonged repetitive activity in normal subjects and patients with a focal nerve lesion. Brain. 1996;119:2029–2037. doi: 10.1093/brain/119.6.2029. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Walters RJ, Andersen KV, Taube D, Murray NM, Bostock H. Nerve excitability changes in chronic renal failure indicate membrane depolarization due to hyperkalaemia. Brain. 2002b;125:1366–1378. doi: 10.1093/brain/awf123. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG. Long-term regenerated nerve fibres retain sensitivity to potassium channel blocking agents. Nature. 1983;304:640–642. doi: 10.1038/304640a0. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG. Ionic channel organization of normal and regenerating mammalian axons. Prog Brain Res. 1987;71:89–101. doi: 10.1016/s0079-6123(08)61816-6. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG, Hildebrand C, Ruiz JA. Regenerating mammalian nerve fibres: changes in action potential waveform and firing characteristics following blockage of potassium conductance. Proc R Soc Lond B Biol Sci. 1982;217:77–87. doi: 10.1098/rspb.1982.0095. [DOI] [PubMed] [Google Scholar]
- Kuwabara S, Kanai K, Sung JY, Ogawara K, Hattori T, Burke D, Bostock H. Axonal hyperpolarization associated with acute hypokalemia: multiple excitability measurements as indicators of the membrane potential of human axons. Muscle Nerve. 2002;26:283–287. doi: 10.1002/mus.10169. 10.1002/mus.10169. [DOI] [PubMed] [Google Scholar]
- Ludin HP, Beyeler F. Temperature dependence of normal sensory nerve action potentials. J Neurol. 1977;216:173–180. doi: 10.1007/BF00313618. 10.1007/BF00313618. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Oscarsson O. Anoxic depolarization of mammalian nerve fibers. Acta Physiol Scand. 1954;30:99–110. [PubMed] [Google Scholar]
- Moldovan M, Krarup C. Abnormalities in excitability of cat motor axons years after crush. Muscle Nerve Suppl. 2003;12:S123. [Google Scholar]
- Moldovan M, Krarup C. Persistent abnormalities of membrane excitability in regenerated mature motor axons in cat. J Physiol. 2004;560:795–806. doi: 10.1113/jphysiol.2004.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan M, Sørensen J, Krarup C. Temperature dependence of excitability indices in long term regenerating motor axons after a crush lesion in cat. J Periph Nerv Sys. 2001a;6:162. [Google Scholar]
- Moldovan M, Sørensen J, Krarup C. Excitability indices in long term regenerating crushed motor axons of cat after ischaemia and cooling. Soc Neurosci Abstract. 2001b;27:366.6.. [Google Scholar]
- Querfurth HW, Armstrong R, Herndon RM. Sodium channels in normal and regenerated feline ventral spinal roots. J Neurosci. 1987;7:1705–1716. doi: 10.1523/JNEUROSCI.07-06-01705.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM. Sodium and potassium channels in regenerating and developing mammalian myelinated nerves. Proc R Soc Lond B Biol Sci. 1982;215:273–287. doi: 10.1098/rspb.1982.0042. [DOI] [PubMed] [Google Scholar]
- Ritchie JM, Straub RW. The hyperploarization which follows activity in mammalian non-medullated fibers. J Physiol. 1957;136:80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfle GM, Katholi CR. Posttetanic changes in membrane potential of single medullated nerve fibers. Am J Physiol. 1973;225:1501–1507. doi: 10.1152/ajplegacy.1973.225.6.1501. [DOI] [PubMed] [Google Scholar]
- Schwarz JR, Eikhof G. Na currents and action potentials in rat myelinated nerve fibres at 20 and 37 degrees C. Pflugers Arch. 1987;409:569–577. doi: 10.1007/BF00584655. [DOI] [PubMed] [Google Scholar]
- Sung JY, Kuwabara S, Kaji R, Ogawara K, Mori M, Kanai K, Nodera H, Hattori T, Bostock H. Threshold electrotonus in chronic inflammatory demyelinating polyneuropathy: correlation with clinical profiles. Muscle Nerve. 2004;29:28–37. doi: 10.1002/mus.10516. 10.1002/mus.10516. [DOI] [PubMed] [Google Scholar]
- Vagg R, Mogyoros I, Kiernan MC, Burke D. Activity-dependent hyperpolarization of human motor axons produced by natural activity. J Physiol. 1998;507:919–925. doi: 10.1111/j.1469-7793.1998.919bs.x. 10.1111/j.1469-7793.1998.919bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizoso AD, Young JZ. Internode length and fibre diameter in developing and regenerating nerves. J Anat. 1948;82:110–134. [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Ritchie JM. Molecular dissection of the myelinated axon. Ann Neurol. 1993;33:121–136. doi: 10.1002/ana.410330202. [DOI] [PubMed] [Google Scholar]