Abstract
Recent evidence suggests a role for vasoactive intestinal polypeptide (VIP) in active vasodilatation and it has been shown that VIP-mediated vasodilatation includes a nitric oxide (NO) and histamine component. Thus, the purpose of this study was to determine the role of H1 and H2 histamine receptors and to examine a potential interaction between NO and histamine receptors in cutaneous active vasodilatation. Eleven subjects were instrumented with four microdialysis fibres. Site 1 served as a control and site 2 was perfused with l-NAME to inhibit nitric oxide synthase. Site 3 was perfused with either the H1 antagonist pyrilamine maleate or the H2 antagonist cimetidine. Site 4 was perfused with l-NAME plus pyrilamine maleate or l-NAME plus cimetidine. Laser-Doppler flowmetry (LDF) was used as an index of skin blood flow and cutaneous vascular conductance (CVC) was calculated as LDF/mean arterial pressure and normalized to maximal vasodilatation achieved via 28 mm sodium nitroprusside infusion. During whole body heating, subjects' sublingual temperature increased a minimum of 0.8°C. In the H1 antagonist studies, CVC in l-NAME, pyrilamine, and combined l-NAME plus pyrilamine sites was significantly reduced compared with control (P < 0.001). The l-NAME and combined l-NAME plus pyrilamine sites were significantly reduced compared with pyrilamine only sites (P < 0.05) but no significant differences were observed between sites. In the H2 receptor antagonist studies, CVC in control sites was not significantly different from cimetidine sites. There was no difference between the l-NAME and combined l-NAME plus cimetidine sites but these sites were significantly attenuated compared with control and cimetidine only sites (P < 0.05). These data suggest the rise in skin blood flow during whole body heating contains an H1 histamine receptor component but do not support an H2 histamine receptor component. Furthermore, part of the NO-dependent component of active vasodilatation can be explained by H1 receptor activation.
Increases in skin blood flow and sweating are the primary autonomic means by which humans regulate core temperature in response to heat stress. Under normothermic conditions, sympathetic adrenergic nerves impart a level of tonic vasoconstriction on the cutaneous vasculature (Grant & Holling, 1938). The initial increase in skin blood flow upon exposure to heat stress is by passive withdrawal of this tonic vasoconstriction (Grant & Holling, 1938; Roddie et al. 1957b). At a given core temperature threshold, further increases in skin blood flow are mediated by an active vasodilator mechanism, which also mediates the sweat response (Grant & Holling, 1938; Roddie et al. 1957a). Kellogg et al. (1995) demonstrated that the active vasodilator system is under the control of cholinergic nerves, as both the sweat and skin blood flow response to heat stress were abolished with botulinum toxin treatment, which inhibits pre-synaptic release of cholinergic vesicles.
The predominant theory regarding the mechanism of cutaneous active vasodilatation is the cotransmission theory. The cotransmission theory suggests acetylcholine and an unknown neurotransmitter(s) are released simultaneously from sympathetic cholinergic nerves in human skin (Grant & Holling, 1938; Kellogg et al. 1995). Fox & Hilton (1958), and later Kellogg et al. (1995), observed that the sweat response, but not the increase in skin blood flow, was abolished in the presence of atropine, suggesting acetylcholine mediates the sweat response and an unknown neurotransmitter(s) co-released with acetylcholine mediates the increase in skin blood flow. These observations and the proposed theory of sympathetic cholinergic cotransmission mechanism of active vasodilatation are limited to the cutaneous vasculature.
It has been well established that nitric oxide (NO) contributes to the rise in skin blood flow during whole body heating (Kellogg et al. 1998, 2003; Shastry et al. 1998) and our laboratory has shown NO acts in a synergistic manner with the unknown neurotransmitter(s) (Wilkins et al. 2003). Bennett et al. (2003) have recently demonstrated an attenuated skin blood flow response to whole body heating when vasoactive intestinal polypeptide (VIP) 10–28, an analogue of VIP, was administered to the skin via microdialysis. In this context, our laboratory has recently shown that exogenous VIP-mediated vasodilatation in human skin includes a substantial NO component, and that part of this NO component can be explained by H1 histamine receptor activation (Wilkins et al. 2004). That VIP-mediated vasodilatation includes a NO and H1 histamine receptor component suggests that a fraction of the vasodilatation from VIP may be due to an interplay between the NO and H1 histamine pathways. Taken together, these data suggest a potential role for VIP in active vasodilatation.
The vasoactive properties of histamine in human skin can be demonstrated by its role in the wheal and flare response (Fjellner & Hagermark, 1981; Clough, 1999; Clough & Church, 2002), which has been shown to be attenuated in the presence of H1 histamine receptor antagonists (Greaves et al. 1977; Fjellner & Hagermark, 1981; Saxena et al. 1983; Patwardhan et al. 1984). The H2 histamine receptor has been shown to be involved in cutaneous vasodilatation induced by histamine delivered via iontophoresis (Grossmann et al. 1999) and via intradermal injection (Greaves et al. 1977; Greaves & Davies, 1982; Johnson et al. 1984). Additionally, a relationship between NO and histamine has been shown to exist in human skin. Clough (1999) and colleagues (Clough et al. 1998) have shown that intradermal injections of histamine result in significant increases in NO production. From these data the authors concluded that the production of NO contributes to a portion of histamine-mediated vasodilatation in human skin.
Based on the characteristics of exogenous VIP-mediated vasodilatation and the interplay between NO and histamine, if VIP is indeed involved in active vasodilatation then it is plausible that a portion of the skin blood flow response during whole body heat stress could be explained by H1 and/or H2 histamine receptors and NO. However, there have been no studies examining this potentially important vasodilatory pathway in active vasodilatation. Therefore the purpose of this study was to determine the role of H1 and H2 histamine receptors in active vasodilatation and to determine if there is an interaction between NO and histamine receptors in active vasodilatation. We hypothesized that the increase in skin blood flow during whole body heating would be attenuated in the presence of an H1 receptor antagonist but not in the presence of an H2 receptor antagonist.
Methods
Subjects
Five males (mean age 21 ± 1 years) and six females (mean age 22 ± 1 years) participated in two separate protocols. Written informed consent was obtained from each subject prior to participation and this study was approved by the Institutional Review Board of the University of Oregon. The protocols in this study conformed to the guidelines as set forth by the Declaration of Helsinki. All subjects were healthy, normotensive, non-smokers, non-diabetics, and were not on any medications with the exception of oral contraceptives.
Instrumentation
All protocols were performed in a thermoneutral laboratory with the subjects in the supine position and the experimental arm at heart level. Subjects' electrocardiogram was continuously monitored throughout each protocol (CardioCap, Datex-Ohmeda, Tewksbury, MA, USA). Blood pressure was monitored via brachial auscultation every 5 min for the duration of each protocol.
In each protocol, subjects were instrumented with four microdialysis fibres (MD 2000, Bioanalytical Systems, West Lafayette, IN, USA) on the ventral surface of the forearm. Each microdialysis fibre had a 10-mm long, 20 kDa molecular weight cut-off membrane. Microdialysis fibres were placed with a 25-gauge needle inserted in the dermal layer of the skin. The microdialysis fibre was then threaded through the lumen of the needle and the needle was withdrawn leaving the membrane in place. During the trauma resolution period, each microdialysis fibre was perfused with Ringer solution at a rate of 2 µl min−1 with a microinfusion pump (CMA/102, CMA Microdialysis, Stockholm, Sweden) for 90–120 min.
Red blood cell (RBC) flux was used as an index of skin blood flow via laser-Doppler flowmetry (MoorLAB, Moor Instruments, Devon, UK). Integrated laser-Doppler probes were placed on the skin directly above each microdialysis membrane.
To control whole body temperature, subjects wore a water-perfused suit that covered the entire body except head, hands, feet and experimental arm. Subjects' oral temperature (Tor) was monitored with a sublingual thermistor for 10 min prior to heating and for the duration of the heating period. During trauma resolution and baseline collection period, thermoneutral water (33°C) was perfused through the suit to clamp whole body temperature. Subjects were covered with a water-impermeable rain suit for the duration of the heating to minimize evaporative heat loss and 50°C water was pumped through the water-perfused suit to raise subjects' oral temperature at least 0.8°C above baseline.
Specific protocols
Protocol 1
The aim of this protocol was to determine the contribution of H1 histamine receptors to the rise in skin blood flow during whole body heating. Six subjects were instrumented with four microdialysis fibres as described above. Site one served as a control site and was continuously perfused with sterile Ringer solution. The second site received 10 mm of the NO synthase inhibitor NG.-nitro-l-arginine-methyl ester (l-NAME; CalBiochem, San Diego, CA, USA) dissolved in sterile Ringer solution. This concentration of l-NAME has been shown previously to adequately inhibit NO synthase in human skin (Kellogg et al. 1999; Minson et al. 2001, 2002). A third site received 500 µm of the H1 receptor antagonist pyrilamine maleate (Sigma, St Louis, MO, USA) dissolved in sterile Ringer solution. Previous studies in our laboratory were performed to determine that this dose of pyrilamine maleate significantly attenuated the vasodilatation to exogenously administered histamine without independently causing vasodilatation (Wilkins et al. 2004). The fourth site was perfused with l-NAME combined with pyrilamine maleate (10 mm and 500 µm final concentrations, respectively). This site was used to examine a potential interaction between NO and H1 histamine receptor activation during whole body heating.
Baseline data were collected for 10 min and thermoneutral water was pumped through the water-perfused suit. Following the baseline period, the microdialysis fibres were perfused with their respective drugs via a microinfusion pump for 30 min at a rate of 2 µl min−1 prior to whole body heating and were continuous throughout the heating period. Subjects were then heated for 45–50 min, raising Tor at least 0.8°C. After a rise in Tor of 0.8°C, subjects' temperature was maintained at this level until a stable 10 min plateau in skin blood flow was achieved. At the end of the heating protocol, each microdialysis fibre was perfused with 28 mm sodium nitroprusside (SNP; Nitropress, Abbot Laboratories, Chicago, IL, USA) at a rate of 4 µl min−1 to achieve maximal skin blood flow. This dose of SNP has been shown previously to cause maximal vasodilatation in human skin (Kellogg et al. 1999; Minson et al. 2001, 2002).
Protocol 2
The aim of this protocol was to determine the contribution of H2 histamine receptors to the rise in skin blood flow during whole body heating. For this protocol, five subjects were instrumented with four microdialysis fibres as described above. Sites one and two received the same treatments as in protocol 1. The third site received 2 mm of the H2 receptor antagonist cimetidine (Sigma; St Louis, MO, USA). Previous studies in our laboratory were performed to determine that this concentration of cimetidine sufficiently antagonized the vasodilatory effects of exogenous histamine without independently causing vasodilatation (Wilkins et al. 2004). The fourth microdialysis site was perfused with l-NAME combined with cimetidine (10 mm and 2 mm final concentrations, respectively). This site was used to examine a potential interaction between NO and H2 receptor activation during whole body heating.
As in protocol 1, a 10 min baseline collection period was followed by a 30 min drug infusion (rate 2 µl min−1) prior to whole body heating. All drug infusions were continuous throughout the heating period. Subjects were then heated for 45–50 min, raising Tor at least 0.8°C. After a rise in Tor of 0.8°C, subjects' temperature was maintained at this level until a stable 10 min plateau in skin blood flow was achieved. At the end of the heating period, all microdialysis sites were perfused with 28 mm SNP (rate 4 µl min−1) to achieve maximal skin blood flow.
Follow-up protocol
The purpose of the follow-up protocol was to determine the effects of an oral second-generation H1 histamine receptor antagonist on the skin blood flow response to exogenous histamine. Four subjects were asked to ingest 360 mg of fexofenadine and were instrumented with three microdialysis fibres and laser-Dopplers as described above. Two subjects ingested fexofenadine 1 h prior to histamine infusion and two subjects ingested fexofenadine 4 h prior to arrival at the laboratory. Ingestion of fexofenadine at these two time periods corresponded to peak plasma and skin concentrations of fexofenadine (Russel et al. 1998; Grant et al. 1999a,b), respectively, prior to infusion of exogenous histamine. The first microdialysis site was continuously perfused with sterile Ringer solution and served as a control to examine the efficacy of oral fexofenadine to exogenous histamine. The second site was perfused with 500 µm pyrilamine to determine any additive effects of first and second generation H1 histamine receptor antagonists. The third site was perfused with 2 mm cimetidine to determine any additive effects of a second generation H1 receptor antagonist and an H2 receptor antagonist. The two treatment sites were perfused with their respective antagonists for 30 min after which a 5 pmol dose of histamine (2 µl volume over 30 s) was perfused through each microdialysis fibre. We have shown previously that this dose of histamine delivered via microdialysis elicits a demonstrable but submaximal increase in skin blood flow (∼70% CVCmax; Wilkins et al. 2004). Following histamine infusion, skin blood flow was allowed to return to baseline and each site was perfused with 28 mm SNP to elicit maximal dilatation.
Data collection and analysis
Data were digitized and stored at 20 Hz on a personal computer and were analysed offline using signal processing software (Windaq, Dataq Instruments, Akron, OH, USA). A stable 5 min period of skin blood flow was used for analysis of baseline, whole body heating plateau, and maximal skin blood flow. Cutaneous vascular conductance (CVC) was calculated as RBC flux/mean arterial pressure and normalized to maximal values during 28 mm SNP infusion.
To determine the relative contribution of NO and histamine and the interaction between histamine and NO, the plateau in skin blood flow achieved during whole body heating was analysed in each protocol using a one-way repeated measures ANOVA. The point at which whole body heating began and the point at which baseline skin blood flow significantly deviated from baseline was used to determine the onset of vasodilatation. The time to plateau was measured from the initiation of whole body heating to the point at which skin blood flow values stabilized at a Tor of 0.8°C. A one-way repeated measures ANOVA was used to analyse the time to onset of vasodilatation and the time to plateau during whole body heating. Data from the follow-up protocol were analysed using one-way repeated measures ANOVA. The Holm-Sidak post hoc test was used to determine where significances occurred. A P value < 0.05 was considered statistically significant. All data are presented as means ± s.e.m.
Results
The infusion of l-NAME or the respective histamine receptor antagonists did not affect baseline skin blood flow values in either protocol.
Protocol 1
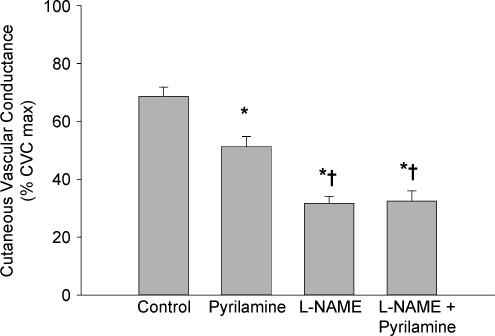
In the control sites, CVC increased during whole body heating to 69 ± 3% CVCmax. CVC was significantly reduced in l-NAME sites (32 ± 2% CVCmax; P < 0.001) and in pyrilamine sites (51 ± 3% CVCmax; P < 0.001) compared with the control sites. The combined l-NAME plus pyrilamine sites (33 ± 3% CVCmax) were significantly reduced compared with control sites (P < 0.001). Additionally, the l-NAME only sites and the combined l-NAME plus pyrilamine sites were significantly reduced compared with the pyrilamine only sites (P < 0.001). However, there was no difference between the l-NAME only sites and the combined l- NAME plus pyrilamine sites (P = 0.841). The group data are summarized in Fig. 1.
Figure 1. Group mean (± s.e.m.) CVC data from protocol 1.
Values are from the plateau in skin blood flow during whole body heating. Compared with control, pyrilamine significantly reduced CVC. Similarly, l-NAME significantly reduced CVC compared with control. l-NAME plus pyrilamine attenuated CVC compared with control and the pyrilamine only sites but was not significantly different from l-NAME only sites. *P < 0.001 versus control site; †P < 0.05 versus pyrilamine only site.
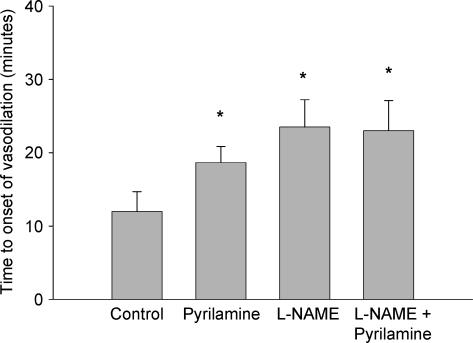
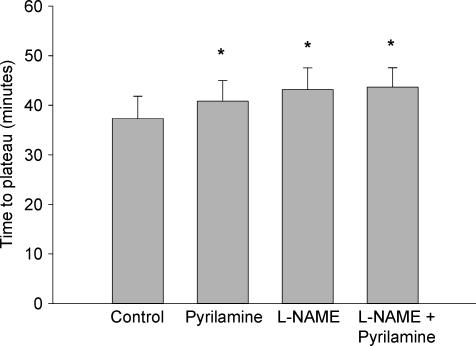
Figure 2 presents the mean group data for the time to the onset of vasodilatation after the initiation of whole body heating. The time to the onset of vasodilatation was significantly delayed in the l-NAME (24 ± 4 min), pyrilamine (19 ± 2 min), and combined l-NAME plus pyrilamine (23 ± 4 min) sites (P < 0.05 for all conditions) when compared with the control site (12 ± 3 min) but there was no difference between the three treatment sites. The mean group data for the time required to reach a plateau in skin blood flow during whole body heating is presented in Fig. 3. Similar to the time to the onset of vasodilatation, the time to plateau in skin blood flow during whole body heating was significantly delayed in the l-NAME (43 ± 4 min), pyrilamine (41 ± 4 min), and combined l-NAME plus pyrilamine (44 ± 4 min) sites when compared with the control sites (37 ± 4 min; P < 0.05) but there was no difference detected between the three treatment sites.
Figure 2. Time (min ± s.e.m.) to onset of vasodilatation following initiation of whole body heating.
The onset of vasodilatation was significantly delayed in all three treatment sites compared with the control site. *P < 0.05 versus control site.
Figure 3. Time (min ± s.e.m.) to plateau in skin blood flow during whole body heating.
The time to plateau in skin blood flow was significantly delayed in all three treatment sites compared with the control site. *P < 0.05 versus control site.
Protocol 2
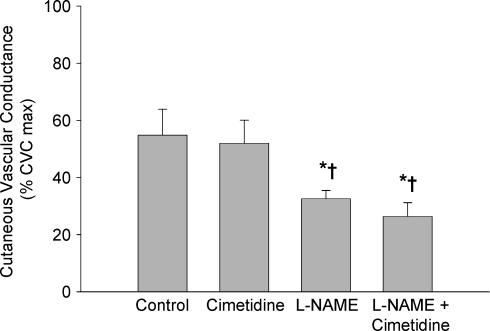
Figure 4 presents the group data from protocol 2. CVC in the control sites were not significantly different from the cimetidine sites (P = 0.680). The l-NAME-treated sites were significantly reduced compared with both the control sites and the cimetidine sites (P < 0.05 for both conditions). Similarly, the combined l-NAME plus cimetidine sites were significantly reduced compared with the control and cimetidine only sites (P < 0.05 for both conditions). There were no differences detected between the l-NAME only sites and the combined l-NAME plus cimetidine sites (P = 0.368).
Figure 4. Group mean (±s.e.m.) CVC data from protocol 2.
Values are from the plateau in skin blood flow during whole body heating. Treatment with an H2 antagonist did not significantly reduce CVC compared with control. l-NAME sites and sites treated with l-NAME plus cimetidine were significantly reduced compared with control and cimetidine only sites but were not different from each other. *P < 0.05 versus control sites; †P < 0.05 versus cimetidine only sites.
The time to onset of vasodilatation (data not shown) in the control sites averaged 20 ± 5 min, which was not significantly different from the cimetidine sites (18 ± 4 min; P = 0.453). However, the onset of vasodilatation was significantly delayed in the l-NAME (24 ± 5 min) and the combined l-NAME plus cimetidine (27 ± 6 min) when compared with both the control and cimetidine only sites (both P < 0.01). Additionally, the time to plateau (data not shown) was not significantly different between control (40 ± 5 min) and cimetidine only (40 ± 4 min). Similar to the onset of vasodilatation, the l-NAME only (47 ± 4 min) and combined l-NAME plus cimetidine (48 ± 4 min) sites were significantly delayed when compared with both the control and cimetidine only sites (both P < 0.05).
Follow-up protocol
As there were no differences in the data between the two separate ingestion periods, the data for this protocol have been grouped. The plateau in CVC following histamine infusion averaged 72 ± 5% CVCmax in control sites, which is similar to values we have previously observed in control sites (∼70% CVCmax; Wilkins et al. 2004). The average CVC in pyrilamine sites was 25 ± 4% CVCmax and 15 ± 3% CVCmax in cimetidine sites (data not shown). In this protocol, the skin blood flow response to 5 pmol histamine in control sites was similar to values we have previously reported (Wilkins et al. 2004) and the two treatment sites were significantly attenuated compared with the control sites (P < 0.001 for both treatment sites) suggesting a lack of inhibitory effect on the skin blood flow response to exogenous histamine with oral H1 histamine receptor antagonists.
Discussion
This is the first study designed to examine a potential role for H1 and H2 histamine receptors in cutaneous active vasodilatation in humans. A secondary goal of our study was to examine a potential interaction between histamine receptors and NO. The main finding of this study was that H1 histamine receptor activation contributes to a portion of the rise in skin blood flow during whole body heat stress. This was shown by a diminished skin blood flow response during whole body heat stress in the presence of a selective H1 receptor antagonist (Fig. 1). These data also demonstrate that a portion of the NO component of active vasodilatation was due to H1 receptor activation as the sites receiving only NO synthase inhibition and sites receiving NO synthase inhibitor plus H1 receptor antagonist were not significantly different from each other, but were significantly attenuated compared with sites receiving the H1 receptor antagonist alone (Fig. 1). However, a substantial portion of the NO component cannot be explained by H1 receptor activation. Conversely, the H2 isoform of the histamine receptor does not appear to be directly involved in active vasodilatation during heat stress (see Fig. 2).
It has been well documented that NO is required for the full expression of active vasodilatation in humans (Kellogg et al. 1998, 2003; Shastry et al. 1998; Wilkins et al. 2003). Shibasaki et al. (2002) have shown that in the early stages of whole body heating a portion of the NO production may be due to acetylcholine. These authors demonstrated a lower core temperature threshold for active vasodilatation in the presence of the acetylcholinesterase inhibitor neostigmine and a rightward shift in the core temperature threshold when neostigmine was combined with l-NAME (Shibasaki et al. 2002). The data from the present study suggest that there are at least two additional pathways by which NO can be produced during active vasodilatation. A portion of the NO component may be explained by the activation of H1 histamine receptors, where histamine or another substance bind to the H1 histamine receptors and induce NO production. However, a large portion of the NO component appears to be independent of H1 histamine receptors. This H1 histamine receptor-independent portion may be due to direct stimulation of NO production, possibly by the active vasodilator substance co-released with acetylcholine or by histamine stimulating NO directly from the endothelium.
An alternative explanation regarding the H1 histamine receptor and NO data could be due to cross-activation between the downstream second-messenger systems of the H1 receptors and NO. In this model, the H1 histamine receptor pathway and the NO pathway are independent but redundant pathways. Data from isolated coronary arteries (Jiang et al. 1992) suggest that increased levels of cAMP can activate protein kinase G. In this context, it is possible that the activation of H1 histamine receptors increases the levels of cAMP while NO increases cGMP levels. The H1 receptor-induced increases in cAMP could then result in activation of protein kinase G and, ultimately cGMP, to levels above those achieved from NO alone.
In human skin it has been shown that neuropeptides, including VIP, can induce NO from the endothelium (Bull et al. 1996; Klede et al. 2003). Along these lines, Wilkins et al. (2004) have demonstrated a clear NO component as well as an H1, but not an H2, histamine receptor component to exogenous VIP-mediated vasodilatation. The data of Wilkins et al. (2004) also suggest that only a fraction of VIP-mediated vasodilatation is directly due to VIP, as the majority of the vasodilatation can be explained by H1 histamine receptor activation and NO. Additionally, receptors for VIP have been located in human skin (Kummer et al. 1990; Fischer et al. 2002) and on mast cells in human skin (Groneberg et al. 2003) and the vasodilatation due to intradermal VIP injection has been shown to be attenuated in the presence of an H1 histamine receptor antagonist (Fjellner & Hagermark, 1981). In the context of active vasodilatation, Bennett et al. (2003) have observed an attenuated skin blood flow response during whole body heating in the presence of VIP10-28, an analogue of VIP. Taken together, these data suggest a possible interaction between VIP, H1 histamine receptor activation and NO in cutaneous active vasodilatation.
Using the data from the present study and building on the available evidence, we have constructed a working model for the mechanism of cutaneous active vasodilatation. During the early stages of heat stress, VIP is co-released with acetylcholine, where acetylcholine binds to muscarinic receptors on the endothelium to induce NO and VIP may bind to VIP receptors on cutaneous arterioles to directly mediate a portion of the vasodilatation. Additionally, VIP could also bind to receptors on mast cells, thereby resulting in histamine release via degranulation of the mast cells while simultaneously stimulating NO production from the endothelium. It is then possible that histamine released from mast cells binds to the H1 isoform of the histamine receptors and directly causes vasodilatation while also stimulating further increases in NO production. Although it cannot be ruled out during whole body heating, it seems unlikely that NO is produced in response to an increase in endothelial shear stress, as NO does not appear to contribute to the rise in skin blood flow during reactive hyperaemia, a condition that significantly increases the shear stress on the endothelium of the cutaneous arterioles (Wong et al. 2003; Zhao et al. 2004).
A limitation to our proposed model of active vasodilatation is that although we have demonstrated that a portion of active vasodilatation was mediated by H1 histamine receptor activation, the data from this study must be viewed as indirect evidence regarding a role for histamine in active vasodilatation. In this study, we specifically antagonized the histamine receptors and, as such, we cannot discount the possibility that other vasoactive substances are binding to the H1 histamine receptors resulting in vasodilatation. Thus, we also cannot conclude that histamine is released or is directly involved in cutaneous active vasodilatation.
The reason for the lack of an apparent H2 histamine receptor-mediated mechanism in active vasodilatation is unclear. The H2 isoform of the histamine receptor is known to exist on human skin blood vessels (Greaves et al. 1977; Greaves & Davies, 1982) and data from several laboratories have shown an H2 histamine receptor-mediated vasodilatation in human skin following intradermal histamine injections (Greaves et al. 1977; Greaves & Davies, 1982; Johnson et al. 1984) as well as when histamine was delivered to the skin via iontophoresis (Grossmann et al. 1995, 1999). However, in agreement with the data in this study is the fact that Wilkins et al. (2004) failed to observe an attenuation of the vasodilatation induced by exogenous VIP in the presence of an H2 histamine receptor antagonist.
To determine whether the effects of an oral dose of an H1 histamine receptor antagonist could attenuate the vasodilatation to exogenous histamine in a fashion similar to studies in which we delivered an H1 antagonist via microdialysis, we performed studies on four subjects who were administered 360 mg orally of the second-generation H1 antagonist fexofenadine. Based on previous data, we infused histamine via microdialysis and observed the skin blood flow response 1 h after fexofenadine ingestion, which corresponds to peak plasma concentrations of fexofenadine, as well as at time points corresponding to peak concentrations in human skin (4 and 6 h post-ingestion) (Russel et al. 1998; Grant et al. 1999a,b). In no case did we observe an attenuation of the cutaneous vasodilatation due to exogenous histamine infusion with oral fexofenadine. Based on these observations, we used pyrilamine delivered via microdialysis as our H1 histamine receptor antagonist to ensure adequate antagonism of the H1 histamine receptors. The discrepancy between our observations and previous data using oral administration of fexofenadine may be due to the different methods employed. While we used microdialysis to deliver histamine to the skin and attempted to attenuate only the histamine-induced vasodilatation, the studies cited above used intradermal injections of histamine and attempted to attenuate the subsequent wheal and flare response. Thus, it is possible that the trauma following an intradermal injection is involved in the wheal and flare response, as we have not observed a wheal and flare response to histamine delivered via microdialysis.
In conclusion, this is the first study to demonstrate that H1 histamine receptor activation contributes to active vasodilatation. The skin blood flow response during whole body heating was significantly attenuated in the presence of an antagonist specific for the H1 histamine receptor isoform. However, no effect was observed when an H2 receptor-specific antagonist was delivered to the skin. Moreover, our data suggest that a portion of the NO component observed during active vasodilatation is due to H1 receptor activation, while a larger portion of the NO component is independent of H1 receptor activation and may be due to VIP-induced NO production.
Acknowledgments
The authors would like to thank all of the subjects who participated in this study. This study was supported by a grant from the National Institutes of Health (Minson, R01HL70928).
References
- Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL. Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature in humans. J Physiol. 2003;552:223–232. doi: 10.1113/jphysiol.2003.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull HA, Hothersall J, Chowdhury N, Cohen J, Dowd PM. Neuropeptides induce release of nitric oxide from human dermal microvascular endothelial cells. J Invest Dermatol. 1996;106:655–660. doi: 10.1111/1523-1747.ep12345471. [DOI] [PubMed] [Google Scholar]
- Clough GF. Role of nitric oxide in the regulation of microvascular perfusion in human skin in vivo. J Physiol. 1999;516:549–557. doi: 10.1111/j.1469-7793.1999.0549v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough GF, Bennett AR, Church MK. Measurement of nitric oxide concentration in human skin in vivo using dermal microdialysis. Exp Physiol. 1998;83:431–434. doi: 10.1113/expphysiol.1998.sp004126. [DOI] [PubMed] [Google Scholar]
- Clough GF, Church MK. Vascular responses in the skin: an accessible model of inflammation. News Physiol Sci. 2002;17:170–174. doi: 10.1152/nips.01378.2001. [DOI] [PubMed] [Google Scholar]
- Fischer TC, Dinh QT, Peiser C, Loser C, Fischer A, Groneberg DA. Simultaneous detection of receptor mRNA and ligand protein in human skin tissues. Cutan Pathol. 2002;29:65–71. doi: 10.1034/j.1600-0560.2002.290201.x. [DOI] [PubMed] [Google Scholar]
- Fjellner B, Hagermark O. Studies on pruritogenic and histamine-releasing effects of some putative peptide neurotransmitters. Acta Derm Venereol. 1981;61:245–250. [PubMed] [Google Scholar]
- Fox RH, Hilton SM. Bradykinin formation in human skin as a factor in heat vasodilatation. J Physiol. 1958;142:219–232. doi: 10.1113/jphysiol.1958.sp006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JA, Danielson L, Rihoux JP, DeVos C. A double-blind, single-dose, crossover comparison of cetirizine, ebastine, epinastine, fexofenadine, terfenadine, and loratadine versus placebo: suppression of histamine-induced wheal and flare response for 24 h in healthy male subjects. Allergy. 1999a;54:700–707. doi: 10.1034/j.1398-9995.1999.00032.x. [DOI] [PubMed] [Google Scholar]
- Grant JA, Danielson L, Rihoux JP, DeVos C. A comparison of cetirizine, ebastine, epinastine, fexofenadine, terfenadine and loratadine, versus placebo in suppressing the cutaneous response to histamine. Int Arch Allergy Immunol. 1999b;118:339–340. doi: 10.1159/000024126. [DOI] [PubMed] [Google Scholar]
- Grant RT, Holling HE. Further observations on the vascular responses of the human limb to body warming: evidence for sympathetic vasodilator nerves in the normal subject. Clin Sci. 1938;3:273–285. [Google Scholar]
- Greaves MW, Davies MG. Histamine receptors in human skin: indirect evidence. Br J Dermatol. 1982;107(Suppl. 23):101–105. doi: 10.1111/j.1365-2133.1982.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Greaves M, Marks R, Robertson I. Receptors for histamine in human skin blood vessels: a review. Br J Dermatol. 1977;97:225–228. doi: 10.1111/j.1365-2133.1977.tb15071.x. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Welker P, Fischer TC, Dinh QT, Grutzkau A, Peiser C, Wahn U, Henz BM, Fischer A. Down regulation of vasoactive intestinal polypeptide receptor expression in atopic dermatitis. J Allergy Clin Immunol. 2003;111:1099–1105. doi: 10.1067/mai.2003.1477. [DOI] [PubMed] [Google Scholar]
- Grossmann M, Jamieson MJ, Kellogg DL, Jr, Kosiba WA, Pergola PE, Crandall CG, Shepherd AM. The effect of iontophoresis on the cutaneous vasculature: evidence for current-induced hyperemia. Microvasc Res. 1995;50:444–452. doi: 10.1006/mvre.1995.1070. [DOI] [PubMed] [Google Scholar]
- Grossmann M, Jamieson MJ, Kirch W. Histamine response and local cooling in the human skin: involvement of H1- and H2-receptors. Br J Clin Pharmacol. 1999;48:216–222. doi: 10.1046/j.1365-2125.1999.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Colbran JL, Francis SH, Corbin JD. Direct evidence for cross-activation of c-GMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem. 1992;267:1015–1019. [PubMed] [Google Scholar]
- Johnson CE, Weiner JS, Wagner DS, McLean JA. Effect of H1- and H2-receptor blockade on the inhibition of immediate cutaneous reactions. Clin Pharm. 1984;3:60–64. [PubMed] [Google Scholar]
- Kellogg DL, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilatation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilatation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Zhao JL, Friel C, Roman LJ. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J Appl Physiol. 1999;48:216–222. doi: 10.1152/japplphysiol.00826.2002. [DOI] [PubMed] [Google Scholar]
- Klede M, Clough G, Lischetzki G, Schmelz M. The effect of the nitric oxide synthase inhibitor N-nitro-L-arginine-methyl ester on neuropeptide-induced vasodilatation and protein extravasation in human skin. J Vasc Res. 2003;40:105–114. doi: 10.1159/000070707. [DOI] [PubMed] [Google Scholar]
- Kummer W, Herbst WM, Heym C. Vasoactive intestinal polypeptide receptor-like immunoreactivity in human sweat glands. Neurosci Lett. 1990;110:239–243. doi: 10.1016/0304-3940(90)90853-2. [DOI] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilatation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Patwardhan MD, Muley MP, Manaker MS. Involvement of H1- and H2-receptors in triple response to histamine in human volunteers. Indian J Physiol Pharmacol. 1984;28:335–337. [PubMed] [Google Scholar]
- Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. J Physiol. 1957a;136:489–497. doi: 10.1113/jphysiol.1957.sp005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddie IC, Shepherd JT, Whelan RF. The vasomotor nerve supply to the skin and muscle of human forearm. Clin Sci. 1957b;16:67–74. [PubMed] [Google Scholar]
- Russel T, Stoltz M, Weir S. Pharmacokinetics, pharmacodynamics, and tolerance of single- and multiple-dose fexofenadine hydrochloride in healthy male volunteers. Clin Pharmacol Ther. 1998;64:612–621. doi: 10.1016/S0009-9236(98)90052-2. [DOI] [PubMed] [Google Scholar]
- Saxena RC, Das M, Dixit KS, Bhargava KP. Involvement of H1 & H2 receptors in histamine-induced skin response in humans. Indian J Med Res. 1983;78:277–280. [PubMed] [Google Scholar]
- Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilatation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine release from cholinergic nerves contributes to cutaneous vasodilatation during heat stress. J Appl Physiol. 2002;93:1947–1952. doi: 10.1152/japplphysiol.00036.2002. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide (VIP) -mediated vasodilatation in human skin. J Appl Physiol. 2004;97:1291–1298. doi: 10.1152/japplphysiol.00366.2004. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilatation in humans. J Physiol. 2003;548:963–969. doi: 10.1113/jphysiol.2002.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol. 2003;95:504–510. doi: 10.1152/japplphysiol.00254.2003. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Pergola PE, Roman LJ, Kellogg DL. Bioactive nitric oxide concentration does not increase during reactive hyperemia in human skin. J Appl Physiol. 2004;96:628–632. doi: 10.1152/japplphysiol.00639.2003. [DOI] [PubMed] [Google Scholar]