Abstract
Large calyceal synapses are often regarded as simple relay points, built for high-fidelity and high-frequency synaptic transmission and a minimal requirement for synaptic plasticity, but this view is oversimplified. Calyceal synapses can exhibit surprising activity-dependent developmental plasticity. Here we compare basal synaptic transmission and activity-dependent plasticity at two stereotypical calyceal synapses in the auditory pathway, the endbulb and the calyx of Held. Basal synaptic transmission was more powerful at the calyx than the endbulb synapse: the amplitude of evoked AMPA receptor-mediated excitatory postsynaptic currents (eEPSCs) was significantly greater at the calyx, as were the release probability, and the number of release sites. The quantal amplitude was smaller at the calyx, consistent with the smaller amplitude of spontaneous miniature EPSCs at this synapse. High-frequency trains of stimuli revealed that the calyx had a larger readily releasable pool of vesicles (RRP), less tetanic depression and less asynchronous transmitter release. Activity-dependent synaptic plasticity was assessed in congenitally deaf mutant mice (dn/dn). Previously we showed that a lack of synaptic activity in deaf mice increases synaptic strength at the endbulb of Held via presynaptic mechanisms. In contrast, we have now found that deafness does not affect synaptic transmission at the calyx synapse, as eEPSC and mEPSC amplitude, release probability, number of release sites, size of RRP, tetanic depression and asynchronous release were unchanged compared to normal mice. Synaptic transmission at the calyx synapse is more powerful and has less capacity for developmental plasticity compared to the endbulb synapse.
Sound localization requires highly specialized synapses in the auditory pathway for precise processing of binaural cues. Minute differences in the arrival time and intensity level of sound at each ear are detected by neurones in the superior olivary complex. Interaural time differences (ITD) are detected by neurones in the medial superior olive (MSO), while interaural level differences (ILD) are noted by neurones in the lateral superior olive (LSO). The neuronal circuits for detecting ITDs and ILDs are distinct, yet both contain synapses that are specialized for rapid, high-fidelity signalling. This specialization is necessary for superior olivary neurones to function as coincident detectors of binaural information, particularly for LSO neurones which receive monosynaptic input from the ipsilateral ear and disynaptic input from the contralateral ear. Rapid high-fidelity transmission is made possible by giant calyceal terminals in direct contact with the soma of the postsynaptic neurone. Auditory nerve fibres from the ear give rise to calyceal-type endbulb of Held terminals as they contact spherical and globular bushy cells in the brainstem anteroventral cochlear nucleus (AVCN). Both spherical and globular bushy cells receive endbulb terminals but those on the spherical bushy cells are fewer and larger. The calyx of Held terminals arise from the axons of globular bushy cells which target principal cells in the contralateral medial nucleus of the trapezoid body (MNTB).
The two giant calyceal terminals share morphological and physiological features. Both terminals show developmental changes in morphology from a spoon-shaped swelling to a finger-like structure that covers 40–60% of the target soma (Ryugo & Fekete, 1982; Kuwabara et al. 1991; Kandler & Friauf, 1993; Smith et al. 1998; Satzler et al. 2002; Taschenberger et al. 2002). The area of the calyx terminal is greater than the endbulb terminal yet both terminals contain hundreds of active zones with clusters of spherical vesicles apposed to slightly curved postsynaptic densities (Neises et al. 1982; Ryugo et al. 1996, 1997; Smith et al. 1998; Rowland et al. 2000; Nicol & Walmsley, 2002; Satzler et al. 2002; Taschenberger et al. 2002; Lee et al. 2003). Both synapses also share developmental changes in physiology as postsynaptic glutamatergic NMDA receptors are replaced by AMPA receptors at maturity (Bellingham et al. 1998; Chuhma & Ohmori, 1998; Taschenberger & von Gersdorff, 2000; Futai et al. 2001; Joshi & Wang, 2002). The AMPA receptor-mediated responses show robust short-term depression to high-frequency stimulation yet are among the fastest and largest in the central nervous system, allowing presynaptic action potentials to generate postsynaptic spikes with very few failures and at very high frequencies, up to 800 Hz (Wu & Kelly, 1993; Taschenberger & von Gersdorff, 2000; Oleskevich & Walmsley, 2002; Schneggenburger et al. 2002).
Despite numerous similarities, the two calyceal synapses occur at different positions in the neuronal circuits for sound localization. Endbulb synapses occur earlier in the ITD and ILD circuits than calyx synapses. Is this positional difference reflected in a functional difference? Previous studies show that the endbulb but not the calyx synapse can improve the degree of phase-locking (the ability to generate spikes at a preferred phase of the stimulus period) of its excitatory input from the auditory nerve (Smith et al. 1998; Paolini et al. 2001). Also, different types and numbers of afferents supplement the calyceal input in the AVCN and MNTB regions, possibly resulting in different degrees of signal modulation. The bushy cells at the endbulb synapse receive diverse inhibitory inputs from the cochlear nucleus, the trapezoid nucleus and the superior paraolivary nucleus (Wu & Oertel, 1986; Roberts & Ribak, 1987; Schofield, 1991; Kolston et al. 1992; Schofield, 1994). Pre- and postsynaptic modulation may occur at the calyx synapse via excitatory and inhibitory afferents to the presynaptic terminal and postsynaptic MNTB principal cells (Trussell, 2002; Von Gersdorff & Borst, 2002).
Here we investigate synaptic transmission at two calyceal synapses to determine whether functional differences are present in these superficially similar synapses. We investigate the parameters of evoked and spontaneous synaptic transmission in the first detailed comparison of the endbulb and calyx synapse. In addition to basic transmission, we probe how the synapses react to perturbations in synaptic input using deaf mutant mice. Previously we reported that a lack of auditory input to the endbulb of Held synapse resulted in numerous changes including evoked EPSC amplitude, release probability, tetanic depression and the frequency of asynchronous release (Oleskevich & Walmsley, 2002). Here we investigate whether these changes in synaptic transmission propagate along the auditory pathway to the calyx of Held synapse. We show that the two giant terminals have different basal properties of synaptic transmission and respond differently to the absence of synaptic input.
Methods
Whole-cell recordings were made from 120 neurones, comprising bushy cells (n = 61) in the AVCN and principal cells (n = 59) in the MNTB. Thin parasagittal slices (150 µm) of AVCN and transverse slices of MNTB were prepared from 11- to 16-day-old deafness mutant mice (dn/dn) and normal CBA mice, following decapitation in accordance with local guidelines (Oleskevich & Walmsley, 2002). Neurones were visualized using infra-red differential interference contrast (DIC) optics and recordings were performed at room temperature (22–25°C). Slices were superfused with an artificial cerebrospinal fluid (ACSF) containing (mm): 130 NaCl, 3 KCl, 1.3 Mg2SO4, 2 CaCl2, 1.25 NaH2PO4, 26.2 NaHCO3, 10 glucose, equilibrated with 95% O2, 5% CO2. Patch electrodes (3–6 MΩ resistance) contained (mm): 120 CsCl, 4 NaCl, 4 MgCl2, 0.001 CaCl2, 10 Hepes, 2 Mg-ATP, 0.2 GTP-tris, and 10 EGTA (pH 7.3; 300 mOsm). Series resistance, which was < 10 MΩ, was compensated by >80%.
Excitatory postsynaptic currents (EPSCs), recorded under voltage clamp (holding potential −60 mV), were evoked by focal stimulation of afferent fibres using an extracellular electrode filled with ACSF in the AVCN or a bipolar tungsten microelectrode positioned at the brainstem midline close to the MNTB (0.1 ms; 20–90 V; 0.2 Hz). Trains of stimuli consisted of 15 pulses at 100 Hz at 30 s intervals. Stimulation intensity was set at 1.5 times threshold for all experiments. The synaptic currents were recorded and filtered at 10 kHz with an Axopatch 200B amplifier (Axon Instruments) before being digitized at 20 kHz. Mean peak amplitudes were measured as the mean of 30–150 single evoked responses. Excess variance in the amplitude of the synaptic currents was minimized by using a caesium chloride-based internal solution to block potassium conductances, and by adding QX-314 intracellularly to block sodium channels. Spontaneous miniature excitatory postsynaptic currents (mEPSCs) were detected using a sliding template procedure which detected all spontaneous events with amplitudes greater than 2.5–4 standard deviations of the background noise (Clements & Bekkers, 1997). Approximately 200–1000 miniature events were collected for each cell for frequency measurements. Data acquisition and analysis was performed using AxoGraph 4.9 (Axon Instruments).
Variance–mean analysis was used to estimate three parameters of synaptic function: the average amplitude of the postsynaptic response to a vesicle of transmitter (Qav); the average probability of vesicle release from a release site (Pr); and the number of independent release sites (N). These three parameters can be estimated from the relationship between the variance and mean amplitude of evoked EPSCS recorded under different release probability conditions (Clements & Silver, 2000). Release probability conditions were modulated by increasing or decreasing the extracellular calcium concentration (0.2–3.0 mm). To compare Qav with mean mEPSC amplitude, a correction factor was calculated from the ratio of peak amplitude : charge for the evoked and miniature EPSCs, and applied to the Qav value from the variance–mean analysis. This corrects for an underestimate of mean Qav as the contribution of individual quanta to evoked EPSC peak amplitude will be less than expected due to the asynchrony of release (Isaacson & Walmsley, 1995; Bellingham et al. 1998).
Bicuculline methochloride (10 µm; Tocris) (+)-2-amino-5-phosphonopentanoic acid (D-AP5; 30 µm; Tocris), tetrodotoxin (TTX; 1 µm; Alomone), tetra-acetoxymethyl ester (30 µm; EGTA-AM; Molecular Probes) and strychnine hydrochloride (1 µm; Sigma) were added, as indicated, to the Ringer's solution and applied by bath perfusion. Lidocaine N-ethyl bromide (QX-314; 2 mm; RBI) was added to the patch electrode solution. Results are expressed as means ± standard error of the mean (s.e.m.) and statistical tests of significance were determined with parametric tests (t test; factorial analysis of variance (ANOVA) with Fischer PLSD post hoc test; restricted maximum likelihood (REML) test). The mEPSC frequency values showed a skewed distribution and therefore were converted to log scale for statistical comparison between the two calyces.
Data from the endbulb synapse of a previously published report (Oleskevich & Walmsley, 2002) were re-analysed and used as comparison data against the calyx synapse. In most cases, new data from additional experiments at the endbulb synapse have been included. All data at the calyx synapse are new.
Results
Synaptic transmission at the calyx versus endbulb synapse
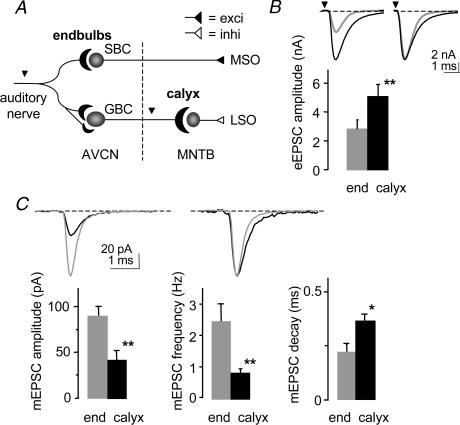
Synaptic transmission was compared at two calyceal terminals in the central auditory pathway of normal mice. Focal stimulation of the auditory nerve evoked synaptic responses at the endbulb of Held–bushy cell connection in the AVCN (endbulb synapse; n = 26) while bipolar stimulation at the brainstem midline evoked responses at the calyx of Held–principal cell connection in the MNTB (calyx synapse; n = 31; Fig. 1A). Evoked responses were AMPA receptor-mediated, isolated by the addition of strychnine, bicuculline and D- AP5. The mean amplitude of evoked AMPA excitatory postsynaptic currents (eEPSCs) was almost two-fold greater at the calyx (5.1 ± 0.8 nA; n = 11) versus endbulb synapse (2.9 ± 0.5 nA; n = 21; P < 0.01; Fig. 1B; Table 1). The eEPSC responses were also slower at the calyx synapse with the mean time constant of decay greater at the calyx (0.75 ± 0.07 ms; n = 11) versus endbulb synapse (0.48 ± 0.02 ms; n = 13; P < 0.001).
Figure 1. Synaptic transmission is different at the calyx synapse.
A. schematic of the brainstem auditory pathway showing the auditory nerve which branches to form an endbulb of Held-spherical bushy cell (SBC) synapse and smaller multiple connections with globular bushy cells (GBC) in the anteroventral cochlear nucleus (AVCN). Projections of the GBC give rise to the calyx of Held-principal cell synapse in the contralateral medial nucleus of the trapezoid body (MNTB). MSO and LSO = medial and lateral superior olivary complexes. Arrowheads = stimulation sites. Broken line = midline. B. summary data showing that the mean amplitude of AMPA-evoked excitatory postsynaptic currents (eEPSC) is significantly greater (80%) at the calyx (black bar) versus the endbulb (grey bar) synapse. Inset: averaged representative traces from individual cells (endbulb P13; calyx P12; normalized on right). **Significant difference (P < 0.01) from normal animals for all figures. C, the spontaneous miniature AMPA EPSCs (mEPSCs) were significantly smaller, less frequent and had slower decay kinetics at the calyx versus the endbulb synapse. Inset: averaged representative traces from individual cells (endbulb P15; calyx P12; normalized on right). *Significant difference (P < 0.05) from normal animals. Error bars are s.e.m.
Table 1.
Properties of synaptic transmission at two calyceal synapses
| Endbulb | Calyx | |||
|---|---|---|---|---|
| Normal | Deafness | Normal | Deafness | |
| Evoked EPSC amplitude (nA) | 2.9 ± 0.5 | 4.8 ± 0.6* | 5.1 ± 0.8 | 6.0 ± 1.2 |
| Miniature EPSC amplitude (pA) | 87 ± 8 | 99 ± 8 | 42 ± 7 | 50 ± 7 |
| Release probability (Pr) | 0.49 ± 0.10 | 0.77 ± 0.08* | 0.78 ± 0.07 | 0.77 ± 0.05 |
| Quantal amplitude from variance—mean (pA) | 105 ± 13 | 103 ± 13 | 33 ± 4 | 57 ± 10 |
| Number of release sites | 90 ± 20 | 97 ± 13 | 490 ± 50 | 540 ± 110 |
| Tetanic depression (%) | 88 ± 1 | 93 ± 1** | 72 ± 4 | 73 ± 5 |
| Asynchronous EPSC frequency (Hz) | 7 ± 1 | 98 ± 21** | 2 ± 1 | 3 ± 1 |
Significant difference (P < 0.05) with normal animals
significant difference (P < 0.01) with normal animals.
The spontaneous miniature EPSCs (mEPSCs) were significantly smaller, less frequent and had slower kinetics at the calyx versus endbulb synapse. The mean peak amplitude of mEPSCs was approximately two times smaller at the calyx synapse (n = 5) compared to the endbulb synapse (n = 21; P < 0.01; Fig. 1C; Table 1) and the frequency of mEPSCs was less at the calyx (0.8 ± 0.3 Hz: n = 14) versus the endbulb synapse (2.7 ± 0.6 Hz; n = 21; P < 0.005; Fig. 1C). The decay kinetics were significantly slower at the calyx (0.37 ± 0.03 ms: n = 4) than endbulb synapse (0.22 ± 0.04 ms; n = 8; P < 0.05; Fig. 1C), a trend similar to the kinetics of the eEPSCs. At the calyx synapse, the mEPSC decay kinetics were faster than the eEPSC decay (P < 0.01).
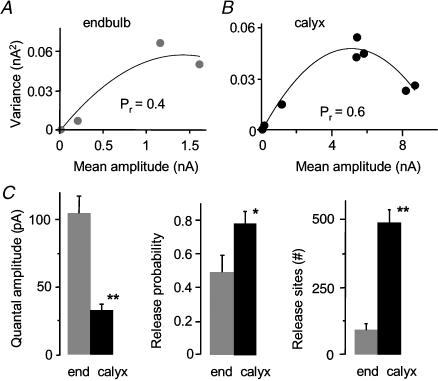
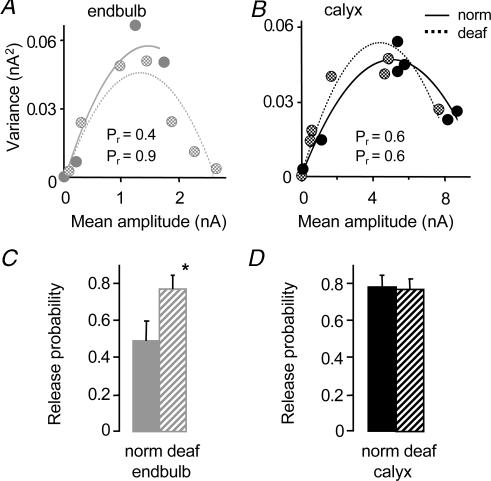
To investigate whether differences in eEPSCs and mEPSCs between the endbulb and calyx synapse could be explained by differences in quantal parameters, we used variance–mean analysis to estimate quantal amplitude (Qav), the average probability for releasing at least one vesicle from an active zone following a single pre-synaptic action potential (Pr), and number of release sites (N; Fig. 2). A visual inspection of the parabolic fit to the variance–mean relationship suggests a difference in Pr and N between the endbulb and calyx synapse (Fig. 2A and B). We observed a 60% larger mean release probability at the calyx (0.78 ± 0.07; n = 7) versus endbulb synapse (0.49 ± 0.10; n = 5; P < 0.05) at the standard external calcium concentration of 2 mm (Fig. 2C). The mean Qav (corrected for asynchrony, see Methods) was significantly smaller at the calyx versus endbulb synapse (P < 0.0001), consistent with the difference in peak amplitude measures of mEPSCs (Fig. 1C; Table 1). The number of release sites was 400% greater at the calyx versus the endbulb synapse (P < 0.0001; Table 1). The combination of larger Pr and larger N presumably underlies the observed larger eEPSC amplitude at the calyx.
Figure 2. Release probability and number of release sites are different at the calyx synapse.
A,B, variance–mean analysis of individual cells shows difference in quantal parameters between the endbulb and calyx synapse (initial slope of parabola proportional to quantal amplitude; curvature of parabola related to release probability and number of release sites). C, summary data showing that quantal amplitude is significantly smaller at the calyx synapse. Release probability (probability of releasing at least one vesicle from a release site), and number of release sites (N) are significantly greater at the calyx versus the endbulb synapse. (Endbulb data in C from Oleskevich & Walmsley, 2002.) *Significant difference (P < 0.05) from normal animals. Error bars are s.e.m.
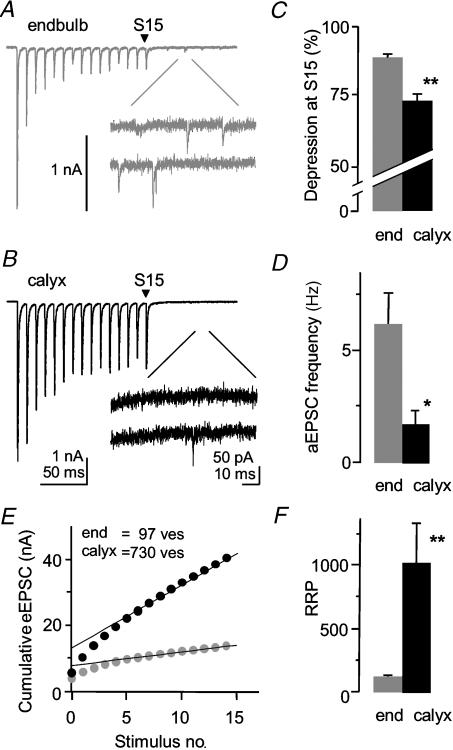
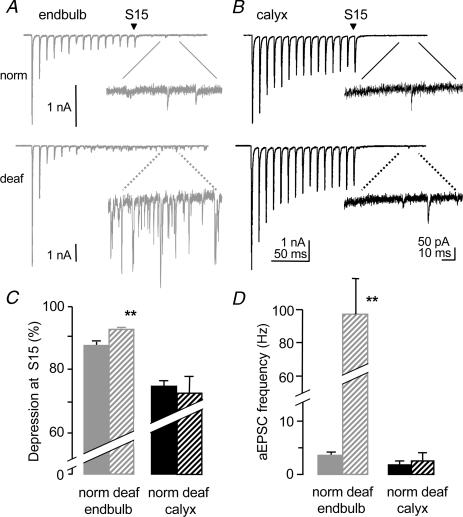
Physiological responses to sound stimuli involve bursts of high-frequency activity at the endbulb and calyx synapse. Synaptic transmission is known to depress with high-frequency firing primarily due to presynaptic depletion of vesicles and postsynaptic AMPA receptor desensitization (for review see von Gersdorff & Borst, 2002). Short-term depression or tetanic depression was induced by 15 pulses at high-frequency stimulation (100 Hz; Fig. 3A and B). The calyx synapse showed less tetanic depression than the endbulb synapse. The final AMPA eEPSC in the train (S15) was depressed by 72 ± 4% of the initial eEPSC (n = 5) at the calyx synapse while the final eEPSC was depressed by 88 ± 1% at the endbulb synapse (n = 6; P < 0.003, Fig. 3C).
Figure 3. Tetanic depression and asynchronous release are reduced at the calyx synapse.
A,B, fifteen pulses of high-frequency stimulation (100 Hz) caused less tetanic depression at the calyx versus the endbulb synapse. Spontaneous asynchronous release was reduced at the calyx versus the endbulb synapse in a 100 ms time period after the high-frequency train. Individual traces of individual cells. C, the amount of depression of the last stimulus (S15) relative to the first stimulus was significantly less at the calyx synapse (endbulb P14; calyx P14). D, the frequency of spontaneous asynchronous miniature EPSCs (aEPSCs) is significantly less at the calyx versus the endbulb synapse in normal mice. (Endbulb data in B and D from Oleskevich & Walmsley, 2002.) E, readily releasable pool size is significantly greater at the calyx synapse. Cumulative eEPSC amplitudes were measured from 15 stimuli at high-frequency (100 Hz). Extrapolation of linear fit yields y-intercept (NQav). Data from two individual cells. F, the number of vesicles in the readily releasable pool (RRP), calculated from NQav/Qav, is significantly greater at the calyx synapse. *Significant difference (P < 0.05) from normal animals. Error bars are s.e.m.
A high-frequency train of stimuli can induce a flurry of delayed asynchronous miniature EPSCs (aEPSCs). Calcium accumulates in the presynaptic terminal during repeated stimulation and causes the random release of vesicles. The frequency of the aEPSCs has been related to the calcium buffering capacities of the presynaptic terminal and if sufficiently high, may deplete the readily releasable pool of vesicles (RRP) and exacerbate tetanic depression (Hagler & Goda, 2001; Oleskevich & Walmsley, 2002; Otsu et al. 2004). The frequency of the aEPSCs was measured in a 100 ms time period after the final stimulus (Fig. 3A and B). The frequency of aEPSCs was significantly less at the calyx synapse (2 ± 0.6; n = 5) versus the endbulb synapse (7 ± 1 Hz; n = 6; P < 0.01; Fig. 3D). The lower frequency of aEPSCs at the calyx synapse is compatible with less depletion of the RRP and less tetanic depression observed at this synapse.
The larger release probability at the calyx synapse could be influenced by the number of docked and primed vesicles at the release site (Murthy & Stevens, 1998; Walmsley et al. 1998; Schikorski & Stevens, 1999; Taschenberger et al. 2002). We therefore measured the size of the RRP using a technique first described at a nerve–muscle synapse (Elmqvist & Quastel, 1965; Schneggenburger et al. 1999). Trains of stimuli (100 Hz) were applied to the endbulb and calyx synapse and the cumulative EPSC amplitude (NQav) was plotted versus stimulus number (Fig. 3E). A linear fit was applied from stimulus number 10–15 and the y-intercept of the linear fit provided an estimate of NQav. The number of vesicles (ves) in the RRP, calculated from NQav/Qav, was almost 10-fold greater in the calyx (1025 ves, n = 5) versus endbulb synapse (112 ves, n = 7; P < 0.005; Fig. 3F; Table 1). Using the mean value of N from the variance–mean analysis, the RRP size per release site was not statistically different between the two synapses (endbulb, 1.2 ± 0.2 ves (release site)−1; calyx, 2.1 ± 0.6 ves (release site)−1). The probability of release for a single vesicle from the readily releasable pool (Pves), calculated from the ratio of the first EPSC amplitude and the RRP, was not significantly different between the endbulb (0.53 ± 0.03) and calyx synapses (0.49 ± 0.03).
Effect of deafness is different at the calyx versus endbulb synapse
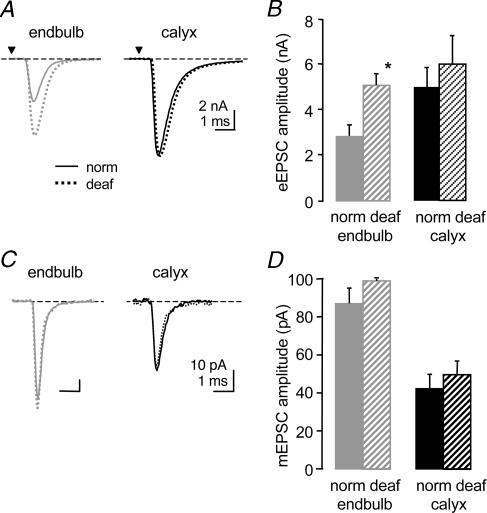
In addition to the observed differences in basal synaptic transmission between the two calyceal synapses, we investigated the modulation of synaptic transmission following alterations in synaptic input. We have previously shown that the endbulb synapse undergoes significant modulation by a lack of synaptic input (Oleskevich & Walmsley, 2002). Here we investigate whether synaptic transmission at the calyx synapse is also affected in congenitally deaf mice. We used deafness mutant mice (dn/dn) which are profoundly deaf from birth due to abnormal hair cells and thus little or no evoked synaptic activity reaches the endbulb or calyx synapses. Whole-cell recordings were made from bushy cells in the AVCN of deaf mice (n = 35) and from principal cells in the MNTB of deaf mice (n = 28; Fig. 4). There was no difference in age groups between deaf and normal animals at the endbulb or calyx synapse for measurements of eEPSCs, mEPSCs, release probability, tetanic depression and aEPSC frequency. At the endbulb synapse, the mean amplitude of eEPSCs was significantly greater in the deaf mice (normal, 2.9 ± 0.5 nA; deaf, 4.8 ± 0.6 nA, n = 34; P < 0.02; Fig. 4B). In contrast, eEPSC amplitudes at the calyx synapse were similar between normal and deaf mice (normal, 5.1 ± 0.8 nA, n = 11; deaf, 6.0 ± 1.2 nA, n = 7).
Figure 4. Deafness affects synaptic transmission differently at the calyx synapse.
A. the mean amplitude of eEPSC at the endbulb synapse is greater in deaf (broken line) versus normal mice (solid line) while the eEPSC at the calyx synapse is similar in deaf and normal mice. Averaged representative traces from individual cells (endbulb norm P13; endbulb deaf P15; calyx norm P12; calyx deaf P13). B. summary data showing significant increase in mean amplitude (110%) due to deafness at the endbulb synapse but no difference at the calyx synapse. C, the peak amplitude of mEPSCs is unchanged at the endbulb and calyx synapse in deaf versus normal mice. Averaged representative traces from individual cells (endbulb norm P12; endbulb deaf P15; calyx norm P15; calyx deaf P13). D, summary data show no significant difference in the peak amplitude of mEPSCs in deaf versus normal mice at the endbulb and calyx synapse. (Some endbulb data from Oleskevich & Walmsley, 2002.) *Significant difference (P < 0.05) from normal animals. Error bars are s.e.m.
The lack of synaptic input did not affect spontaneous mEPSCs at the endbulb synapse in terms of mean peak amplitude (normal, 87 ± 8 pA; deaf, 99 ± 8 pA, n = 23; Fig. 5C and D), frequency (normal, 2.7 ± 0.6 Hz; n = 21; deaf, 2.7 ± 0.5 Hz, n = 22) or the time constant of decay (normal, 0.22 ± 0.04 ms, n = 8; deaf, 0.19 ± 0.02 ms, n = 7). At the calyx synapse, the mean peak amplitude of mEPSCs was not different in deaf mice (normal, 42.3 ± 7 pA, n = 5; deaf, 49 ± 7 pA, n = 5; Fig. 4C and D). The decay time-constant was not different (normal, 0.37 ± 0.03 ms, n = 4; deaf, 0.33 ± 0.03 ms, n = 5) and the frequency was significantly greater at the calyx synapse in deaf mice (normal, 0.83 ± 0.32 Hz, n = 14; deaf, 2.0 ± 0.7 Hz, n = 22; P < 0.05).
Figure 5. Deafness affects quantal parameters differently at the calyx versus the endbulb synapse.
A,B, variance–mean analysis of individual cells shows difference in release probability (curvature of parabola) between deaf (broken line) and normal mice (solid line) at the endbulb but not the calyx synapse. Quantal amplitude (initial slope of parabola) was similar in deaf versus normal mice at both synapses. C, summary data showing release probability at the endbulb synapse is 60% greater in deaf (hatched grey bar) versus normal mice (solid grey bar). (Endbulb data in C from Oleskevich & Walmsley, 2002.) D, release probability at the calyx synapse is similar in deaf (hatched black bar) and normal mice (solid black bar). *Significant difference (P < 0.05) from normal animals. Error bars are s.e.m.
The lack of synaptic input in deafness mutant mice has previously been shown to increase release probability at the endbulb synapse (Oleskevich & Walmsley, 2002). Here we compare the effect of deafness on quantal parameters at the calyx synapse using variance–mean analysis in deaf and normal mice. Figure 5A and B illustrates the variance–mean plot for an individual endbulb and calyx synapse in deaf and normal mice. Release probability at physiological calcium concentrations was significantly greater in deaf versus normal mice at the endbulb synapse (normal, 0.49 ± 0.10; n = 5; deaf, 0.77 ± 0.08, n = 6; P < 0.05; Fig. 5C) but similar at the calyx synapse (normal, 0.78 ± 0.07, n = 7; deaf, 0.77 ± 0.05, n = 9; Fig. 5D). The quantal amplitude and number of release sites was not affected by deafness at either the endbulb or calyx synapse (Table 1). These results suggest the endbulb and calyx synapse respond differently to perturbations in presynaptic activity. Deafness increases release probability at the endbulb synapse but at the calyx synapse, where release probability is larger, deafness causes no further increase in release probability.
The two calyceal synapses respond differently to trains of stimuli in deafness mutant mice. Tetanic depression at the endbulb synapse was significantly greater in deaf (93 ± 1% of initial EPSC; n = 18) versus normal mice (88 ± 1% of initial EPSC; n = 6; P < 0.001; Fig. 6A, C). At the calyx synapse, tetanic depression was similar between deaf (73 ± 5% of the initial eEPSC; n = 5) and normal mice (72 ± 4% of the initial eEPSC; n = 5; Fig. 6B, C). The effect of deafness on the frequency of aEPSCs was pronounced at the endbulb synapse where a 10-fold greater rate of aEPSC release was observed in deaf mice (normal, 7 ± 1 Hz; n = 6; deaf, 98 ± 21 Hz, n = 18, P < 0.01, Fig. 6A inset and Fig. 6D). The higher rate of aEPSC release could contribute to the larger tetanic depression observed at the endbulb synapse in deaf mice. No change in aEPSC frequency was observed at the calyx synapse of normal (n = 5) and deaf mice (n = 5; Fig. 6B inset and Fig. 6D; Table 1). There was no effect of deafness on the RRP, the RRP per release site or Pves at the endbulb or calyx synapse (data not shown).
Figure 6. Deafness affects tetanic depression and asynchronous release differently at the calyx synapse.
A. high-frequency stimulation induces greater tetanic depression in deaf (bottom trace) versus normal mice (top trace) at the endbulb synapse. Asynchronous release is dramatically greater in deaf mice (broken lines for bottom inset). Individual traces of individual cells (endbulb norm P14; endbulb deaf P16; calyx norm P14; calyx deaf P13). B, tetanic epression and asynchronous release are unchanged in deaf (bottom) versus normal (top) mice at the calyx synapse. C, in summary, tetanic depression is significantly greater at the endbulb synapse in deaf mice (hatched grey bar) yet unchanged at the calyx synapse in deaf mice (hatched back bar). D, the frequency of aEPSCs at the endbulb synapse is significantly greater in deaf mice while the frequency at the calyx synapse is similar in deaf and normal mice. (Endbulb data in C and D from Oleskevich & Walmsley, 2002.) *Significant difference (P < 0.05) from normal animals. Error bars are s.e.m.
Discussion
Synaptic transmission at both the endbulb and calyx synapse is fast and powerful. The two synapses share morphological and physiological similarities including extensive synaptic contact with their target soma and high-frequency action potential firing. However, our studies demonstrate significant differences in synaptic transmission at these two giant calyceal synapses. The calyx synapse has a larger evoked response, release probability, number of release sites and RRP size than the endbulb synapse. Our estimates of release probability for a vesicle from an active zone following an action potential (Pr, 0.8) are consistent with previous estimates at the calyx terminal using coefficient of variation analysis (release probability 0.87 at postnatal age 9–11 days (Chuhma & Ohmori, 1998) but greater than estimates using variance–mean analysis of eEPSCs trains (0.25–0.4 at postnatal age 8–10 days (Meyer et al. 2001). Differences in reported estimates of release probability must consider developmental changes in release probability (Taschenberger & von Gersdorff, 2000; Iwasaki & Takahashi, 2001) and estimates of release probability for a single vesicle from the readily releasable pool (Pves) (Schneggenburger et al. 1999; Wu & Borst, 1999; Sakaba & Neher, 2001). Our estimates of release sites (500) are consistent with other estimates of release sites: 637 (Meyer et al. 2001); 700 (Taschenberger et al. 2002); 554 (Satzler et al. 2002). Release probability depends on the number of docked and primed vesicles per release site and, in keeping with this, the calyx synapse showed a greater release probability and a larger RRP per release site (2.1 vesicles per release site) although variability in the latter estimate precluded statistical significance (Murthy & Stevens, 1998; Walmsley et al. 1998; Schikorski & Stevens, 1999; Taschenberger et al. 2002). Our estimate of the size of the RRP at the calyx synapse (1000 ves) is similar to other studies: 940 (Taschenberger & von Gersdorff, 2000); 1100 (Satzler et al. 2002); 700 (Schneggenburger et al. 1999); 600 (Wu & Borst, 1999); but less than 3000–5000 (Sun & Wu, 2001, using capacitance measures). At the endbulb synapse, our estimate of the number of release sites (91) was consistent with a previous estimate of the number of active zones (155), while the RRP size (100 ves) was dissimilar to an estimate of the total number of docked vesicles (1200) which may have included vesicles that were docked but not primed for release (O'Dell et al. 1991; Schikorski & Stevens, 1999; Nicol & Walmsley, 2002).
Short-term depression during high-frequency simulation
In response to high-frequency stimulation, the postsynaptic AMPA response quickly depresses to a steady-state level at both the calyx and endbulb synapse. This short-term or tetanic depression is primarily caused by depletion of the readily releasable pool of vesicles and desensitization of postsynaptic AMPA receptors but may also include presynaptic mechanisms (for review see von Gersdorff & Borst, 2002). The response is reduced substantially (by 70%) at the calyx synapse during a train of stimuli, consistent with previous findings (72% and 75% depression at the calyx synapse and 93% depression at the endbulb synapse in the avian nucleus magnocellularis) (Taschenberger & von Gersdorff, 2000; Brenowitz & Trussell, 2001; Joshi & Wang, 2002). Some studies suggest that the steady-state level of depression is determined by the rate of vesicle depletion versus vesicle replenishment and is independent of the initial release probability (O'Donovan & Rinzel, 1997). However, a correlation between release probability and tetanic depression has been demonstrated at endbulb synapses in the mouse and chick, and here at the calyx synapse where a higher release probability was observed with less tetanic depression (Brenowitz et al. 1998; Brenowitz & Trussell, 2001; Oleskevich & Walmsley, 2002). Less tetanic depression was also associated with a larger RRP size, in agreement with previous developmental studies at the calyx synapse (Taschenberger & von Gersdorff, 2000; Iwasaki & Takahashi, 2001).
Delayed asynchronous transmitter release
The gradual accumulation of residual calcium in the presynaptic terminal during a train of stimuli is thought to cause a delayed asynchronous release of transmitter. Delayed asynchronous release may be especially significant in neuronal pathways that commonly respond with spike trains, such as the auditory pathway. Asynchronous release has been related to the calcium buffering capacity in the presynaptic terminal. The application of calcium buffers can reduce the frequency of aEPSCs at the calyx synapse of young animals, and in hippocampal cultures (Chuhma et al. 2001; Hagler & Goda, 2001; Otsu et al. 2004). At the endbulb synapse in deafness mutant mice, the application of the membrane-permeable calcium buffer, EGTA-AM, reduced asynchronous release and relieved tetanic depression (Oleskevich & Walmsley, 2002). This suggests that high levels of asynchronous release are sufficient to deplete the RRP and contribute to tetanic depression (Hagler & Goda, 2001; Otsu et al. 2004). However, low levels of asynchronous release may result in less tetanic depression as observed at the calyx synapse (less asynchronous release and less tetanic depression compared to the endbulb synapse). The lower level of aEPSC release may relate to enhanced calcium buffering capacity in the calyx compared to the endbulb terminal. Differences in calcium-binding proteins, in particular calbindin, may underlie differences in calcium buffering capacities at these two calyceal terminals (Caicedo et al. 1996; Caicedo et al. 1997).
Spontaneous miniature transmitter release
Spontaneous miniature EPSCs were significantly smaller, less frequent and slower at the calyx synapse, compared with the endbulb synapse. The observed mEPSC amplitude (42 pA) is consistent with previous values reported at the calyx synapse which range from 30 to 70 pA (Chuhma & Ohmori, 1998; Taschenberger & von Gersdorff, 2000; Iwasaki & Takahashi, 2001; Sahara & Takahashi, 2001). The frequency of mEPSCs at the calyx (0.8 Hz) was slightly lower than in previous reports 1.0–2.5 Hz (Barnes-Davies & Forsythe, 1995; Sahara & Takahashi, 2001). The inverse relationship between mEPSC frequency and release probability at the calyx synapse is in agreement with the hypothesis that the protein complex necessary for evoked transmitter release is distinct from the complex involved in spontaneous release (Deitcher et al. 1998; Washbourne et al. 2001). Such a separation of release mechanisms could also explain the observation of a lower mEPSC frequency and a greater number of release sites and RRP size at the calyx synapse. The decay time of mEPSCs at the calyx (1 ms) was in agreement with other reported decay times (0.3–2 ms) (Chuhma & Ohmori, 1998; Iwasaki & Takahashi, 2001; Sahara & Takahashi, 2001), and significantly slower at the calyx than the endbulb synapse (0.2 ms). The difference between synapses could be related to different AMPA receptor subunits and/or desensitization.
Activity-dependent plasticity
Most properties of synaptic transmission were not altered at the calyx synapse in deaf mice, in sharp contrast to the endbulb synapse. Presynaptic plasticity was observed at the endbulb synapse when a lack of synaptic input caused a greater eEPSC amplitude, release probability, tetanic depression, and frequency of delayed aEPSCs (Oleskevich & Walmsley, 2002). Surprisingly, the calyx synapse in deaf mice showed no change in any of the measured properties of evoked synaptic transmission and only small changes in spontaneous transmission. This suggests that the endbulb synapse is capable of greater developmental plasticity than the calyx synapse when presented with changes in synaptic input. It is possible that once alterations in synaptic input are registered at the modified endbulb/globular bushy cell synapse in the AVCN, further modification is not necessary at subsequent calyx/principal cells synapses in the MNTB. Alternatively, the MNTB may be more of a ‘hard-wired’ relay region than the AVCN. Plasticity in the AVCN has been established previously as the output from globular bushy cells shows improved synchronization and phase-locking compared to auditory nerve input (Smith et al. 1998; Paolini et al. 2001). This signal conditioning role of the endbulb synapse contrasts with the calyx synapse in the MNTB. The calyx synapse is regarded as a simple sign-inverting relay synapse, converting glutamatergic excitatory input into glycinergic inhibitory output, and faithfully transforming presynaptic action potentials into postsynaptic spikes (Smith et al. 1998; Futai et al. 2001; Paolini et al. 2001). However, evidence is accumulating for modulation of synaptic transmission at the calyx synapse by glycinergic and GABAergic inhibitory afferents to the MNTB, which cover 20% of the somatic area of MNTB principal cells (Kuwabara et al. 1991; Ostapoff et al. 1997; Smith et al. 1998). Sound-driven inhibition of principal cells in the MNTB can affect postsynaptic spike activity (Kopp-Scheinpflug et al. 2003) and recent data show that evoked glycinergic inhibition can influence excitatory transmission and action potential generation in these cells (Awatramani et al. 2004).
Although the endbulb and calyx share many features typical of calyceal synapses, they also exhibit significant differences in their evoked and spontaneous synaptic transmission properties. Further, their contrasting response to an absence of synaptic input indicates that different mechanisms of plasticity are operating at these not-so-simple relay synapses.
Acknowledgments
The authors are grateful to Drs John Bekkers, John Clements and Tony Paolini for helpful discussions and comments on the manuscript, and for financial support from the National Health and Medical Research Council, Australia.
References
- Awatramani GB, Turecek R, Trussell LO. Inhibitory control at a synaptic relay. J Neurosci. 2004;24:2643–2647. doi: 10.1523/JNEUROSCI.5144-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. J Physiol. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Lim R, Walmsley B. Developmental changes in EPSC quantal size and quantal content at a central glutamatergic synapse in rat. J Physiol. 1998;511:861–869. doi: 10.1111/j.1469-7793.1998.861bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz S, David J, Trussell L. Enhancement of synaptic efficacy by presynaptic GABA (B) receptors. Neuron. 1998;20:135–141. doi: 10.1016/s0896-6273(00)80441-9. [DOI] [PubMed] [Google Scholar]
- Brenowitz S, Trussell L. Minimizing synaptic depression by control of release probability. J Neurosci. 2001;21:1857–1867. doi: 10.1523/JNEUROSCI.21-06-01857.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, d'Aldin C, Eybalin M, Puel JL. Temporary sensory deprivation changes calcium-binding proteins levels in the auditory brainstem. J Comp Neurol. 1997;378:1–15. doi: 10.1002/(sici)1096-9861(19970203)378:1<1::aid-cne1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Caicedo A, d'Aldin C, Puel JL, Eybalin M. Distribution of calcium-binding protein immunoreactivities in the guinea pig auditory brainstem. Anat Embryol. 1996;194:465–487. doi: 10.1007/BF00185994. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Koyano K, Ohmori H. Synchronisation of neurotransmitter release during postnatal development in a calyceal presynaptic terminal of rat. J Physiol. 2001;530:93–104. doi: 10.1111/j.1469-7793.2001.0093m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Ohmori H. Postnatal development of phase-locked high-fidelity synaptic transmission in the medial nucleus of the trapezoid body of the rat. J Neurosci. 1998;18:512–520. doi: 10.1523/JNEUROSCI.18-01-00512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophys J. 1997;73:220–229. doi: 10.1016/S0006-3495(97)78062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Silver RA. Unveiling synaptic plasticity: a new graphical and analytical approach. Trends Neurosci. 2000;23:105. doi: 10.1016/s0166-2236(99)01520-9. [DOI] [PubMed] [Google Scholar]
- Deitcher DL, Ueda A, Stewart BA, Burgess RW, Kidokoro Y, Schwarz TL. Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J Neurosci. 1998;18:2028–2039. doi: 10.1523/JNEUROSCI.18-06-02028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D, Quastel DM. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965;178:505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai K, Okada M, Matsuyama K, Kobayashi K, Takahashi T. High-fidelity transmission acquired by developmental loss of NMDA receptors in the medial nucleus of the trapezoid body in mice. J Neurosci. 2001;21:2234–2239. doi: 10.1523/JNEUROSCI.21-10-03342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Goda Y. Properties of synchronous and asynchronous release during pulse train depression in cultured hippocampal neurons. J Neurophysiol. 2001;85:2324–2334. doi: 10.1152/jn.2001.85.6.2324. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Counting quanta: direct measurements of transmitter release at a central synapse. Neuron. 1995;15:875–884. doi: 10.1016/0896-6273(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental regulation of transmitter release at the calyx of Held in rat auditory brainstem. J Physiol. 2001;534:861–871. doi: 10.1111/j.1469-7793.2001.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi I, Wang LY. Developmental profiles of glutamate receptors and synaptic transmission at a single synapse in the mouse auditory brainstem. J Physiol. 2002;540:861–873. doi: 10.1113/jphysiol.2001.013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. J Comp Neurol. 1993;328:161–184. doi: 10.1002/cne.903280202. [DOI] [PubMed] [Google Scholar]
- Kolston J, Osen KK, Hackney CM, Ottersen OP, Storm-Mathisen J. An atlas of glycine- and GABA-like immunoreactivity and colocalization in the cochlear nuclear complex of the guinea pig. Anat Embryol (Berl) 1992;186:443–465. doi: 10.1007/BF00185459. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Lippe WR, Dorrscheidt GJ, Rubsamen R. The medial nucleus of the trapezoid body in the gerbil is more than a relay: comparison of pre- and postsynaptic activity. J Assoc Res Otolaryngol. 2003;4:1–23. doi: 10.1007/s10162-002-2010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara N, DiCaprio RA, Zook JM. Afferents to the medial nucleus of the trapezoid body and their collateral projections. J Comp Neurol. 1991;314:684–706. doi: 10.1002/cne.903140405. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Cahill HB, Ryugo DK. Effects of congenital deafness in the cochlear nuclei of Shaker-2 mice: an ultrastructural analysis of synapse morphology in the endbulbs of Held. J Neurocytol. 2003;32:229–243. doi: 10.1023/B:NEUR.0000010082.99874.14. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Neher E, Schneggenburger R. Estimation of quantal size and number of functional active zones at the calyx of held synapse by nonstationary EPSC variance analysis. J Neurosci. 2001;21:7889–7900. doi: 10.1523/JNEUROSCI.21-20-07889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF. Synaptic vesicles retain their identity through the endocytic cycle. Nature. 1998;392:497. doi: 10.1038/33152. [DOI] [PubMed] [Google Scholar]
- Neises GR, Mattox DE, Gulley RL. The maturation of the end bulb of Held in the rat anteroventral cochlear nucleus. Anat Rec. 1982;204:271–279. doi: 10.1002/ar.1092040312. [DOI] [PubMed] [Google Scholar]
- Nicol MJ, Walmsley B. Ultrastructural basis of synaptic transmission between endbulbs of Held and bushy cells in the rat cochlear nucleus. J Physiol. 2002;539:713–723. doi: 10.1113/jphysiol.2001.012972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell TJ, Kandel ER, Grant SG. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991;353:558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ, Rinzel J. Synaptic depression: a dynamic regulator of synaptic communication with varied functional roles. Trends Neurosci. 1997;20:431–433. doi: 10.1016/s0166-2236(97)01124-7. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Walmsley B. Synaptic transmission in the auditory brainstem of normal and congenitally deaf mice. J Physiol. 2002;540:447–455. doi: 10.1113/jphysiol.2001.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapoff EM, Benson CG, Saint Marie RL. GABA- and glycine-immunoreactive projections from the superior olivary complex to the cochlear nucleus in guinea pig. J Comp Neurol. 1997;381:500–512. doi: 10.1002/(sici)1096-9861(19970519)381:4<500::aid-cne9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Otsu Y, Shahrezaei V, Li B, Raymond LA, Delaney KR, Murphy TH. Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J Neurosci. 2004;24:420–433. doi: 10.1523/JNEUROSCI.4452-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini AG, FitzGerald JV, Burkitt AN, Clark GM. Temporal processing from the auditory nerve to the medial nucleus of the trapezoid body in the rat. Hear Res. 2001;159:101–116. doi: 10.1016/s0378-5955(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Ribak CE. GABAergic neurons and axon terminals in the brainstem auditory nuclei of the gerbil. J Comp Neurol. 1987;258:267–280. doi: 10.1002/cne.902580207. [DOI] [PubMed] [Google Scholar]
- Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. J Neurosci. 2000;20:9135–9144. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Fekete DM. Morphology of primary axosomatic endings in the anteroventral cochlear nucleus of the cat: a study of the endbulbs of Held. J Comp Neurol. 1982;210:239–257. doi: 10.1002/cne.902100304. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Pongstaporn T, Huchton DM, Niparko JK. Ultrastructural analysis of primary endings in deaf white cats: morphologic alterations in endbulbs of Held. J Comp Neurol. 1997;385:230–244. doi: 10.1002/(sici)1096-9861(19970825)385:2<230::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Wu MM, Pongstaporn T. Activity-related features of synapse morphology: a study of endbulbs of held. J Comp Neurol. 1996;365:141–158. doi: 10.1002/(SICI)1096-9861(19960129)365:1<141::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Sahara Y, Takahashi T. Quantal components of the excitatory postsynaptic currents at a rat central auditory synapse. J Physiol. 2001;536:189–197. doi: 10.1111/j.1469-7793.2001.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Quantitative relationship between transmitter release and calcium current at the calyx of Held synapse. J Neurosci. 2001;21:462–476. doi: 10.1523/JNEUROSCI.21-02-00462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satzler K, Sohl LF, Bollmann JH, Borst JG, Frotscher M, Sakmann B, Lubke JH. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci. 2002;22:10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative fine-structural analysis of olfactory cortical synapses. Proc Natl Acad Sci U S A. 1999;96:4107–4112. doi: 10.1073/pnas.96.7.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Meyer AC, Neher E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron. 1999;23:399–409. doi: 10.1016/s0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 2002;25:206–212. doi: 10.1016/s0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Superior paraolivary nucleus in the pigmented guinea pig: separate classes of neurons project to the inferior colliculus and the cochlear nucleus. J Comp Neurol. 1991;312:68–76. doi: 10.1002/cne.903120106. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Projections to the cochlear nuclei from principal cells in the medial nucleus of the trapezoid body in guinea pigs. J Comp Neurol. 1994;344:83–100. doi: 10.1002/cne.903440107. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J Neurophysiol. 1998;79:3127–3142. doi: 10.1152/jn.1998.79.6.3127. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu LG. Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynaptic currents at a central synapse. Neuron. 2001;30:171–182. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Leao RM, Rowland KC, Spirou GA, Von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Modulation of transmitter release at giant synapses of the auditory system. Curr Opin Neurobiol. 2002;12:400–404. doi: 10.1016/s0959-4388(02)00335-5. [DOI] [PubMed] [Google Scholar]
- Von Gersdorff H, Borst JG. Short-term plasticity at the calyx of held. Nat Rev Neurosci. 2002;3:53–64. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Alvarez FJ, Fyffe RE. Diversity of structure and function at mammalian central synapses. Trends Neurosci. 1998;21:81–88. doi: 10.1016/s0166-2236(97)01170-3. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Cansino V, Mathews JR, Graham M, Burgoyne RD, Wilson MC. Cysteine residues of SNAP-25 are required for SNARE disassembly and exocytosis, but not for membrane targeting. Biochem J. 2001;357:625–634. doi: 10.1042/0264-6021:3570625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Borst JG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–832. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- Wu SH, Kelly JB. Response of neurons in the lateral superior olive and medial nucleus of the trapezoid body to repetitive stimulation: intracellular and extracellular recordings from mouse brain slice. Hear Res. 1993;68:189–201. doi: 10.1016/0378-5955(93)90123-i. [DOI] [PubMed] [Google Scholar]
- Wu SH, Oertel D. Inhibitory circuitry in the ventral cochlear nucleus is probably mediated by glycine. J Neurosci. 1986;6:2691–2706. doi: 10.1523/JNEUROSCI.06-09-02691.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]