Abstract
Photoinhibition of photosystem II was studied in vivo with bean (Phaseolus vulgaris) plants grown in the presence of 0.3 (control), 4, or 15 μm Cu2+. Although photoinhibition, measured in the presence of lincomycin to block concurrent recovery, is faster in leaves of Cu2+-treated plants than in control leaves, thylakoids isolated from Cu-treated plants did not show high sensitivity to photoinhibition. Direct effects of excess Cu2+ on chloroplast metabolism are actually unlikely, because the Cu concentration of chloroplasts of Cu-treated plants was lower than that of their leaves. Excess Cu in the growth medium did not cause severe oxidative stress, collapse of antioxidative defenses, or loss of photoprotection. Thus, these hypothetical effects can be eliminated as causes for Cu-enhanced photoinhibition in intact leaves. However, Cu treatment lowered the leaf chlorophyll (Chl) concentration and reduced the thylakoid membrane network. The loss of Chl and sensitivity to photoinhibition could be overcome by adding excess Fe together with excess Cu to the growth medium. The addition of Fe lowered the Cu2+ concentration of the leaves, suggesting that Cu outcompetes Fe in Fe uptake. We suggest that the reduction of leaf Chl concentration, caused by the Cu-induced iron deficiency, causes the high photosensitivity of photosystem II in Cu2+-treated plants. A causal relationship between the susceptibility to photoinhibition and the leaf optical density was established in several plant species. Plant species adapted to high-light habitats apparently benefit from thick leaves because the rate of photoinhibition is directly proportional to light intensity, but photosynthesis becomes saturated by moderate light.
Cu is an essential trace element for all higher plants, and has several roles in metabolic processes in plants (Maksymiec, 1997). In chloroplasts, Cu is needed as a cofactor of plastocyanin (Lolkema and Vooijs, 1986; Raven et al., 1999). Micromolar concentrations of Cu in growth medium, corresponding to 20 to 30 μg of Cu 1 mg−1 dry weight of leaf tissue, are toxic to most plants (Ouzounidou et al., 1992). The mechanism of Cu toxicity to photosynthetic electron transport has been widely studied in vitro, and inhibition of the donor and acceptor side of photosystem II (PSII) have been suggested (Mohanty et al., 1989; Schröder et al., 1994; Jegerschöld et al., 1995; Yruela et al., 1996a).
Cu2+ has been shown to increase susceptibility to photoinhibition in vitro using isolated thylakoids (Cedeno-Maldonado and Swader, 1972; Pätsikkä et al., 2001) or PSII particles (Jegerschöld et al., 1995; Yruela et al., 1996b). Excess Cu-induced susceptibility to photoinhibition is particularly severe in intact leaves (Pätsikkä et al., 1998), but the underlying mechanism has remained unclear. Reduction of chlorophyll (Chl) concentration (Baszyñski et al., 1988; Lidon and Henriques, 1991; Pätsikkä et al., 1998; Quartacci et al., 2000) has been observed to accompany Cu excess concomitant with ultrastructural changes in chloroplasts, such as reduction of thylakoid membranes (Elefteriou and Karataglis, 1989). Excess Cu may interfere with the biosynthesis of the photosynthetic machinery and may modify the pigment and protein components of photosynthetic membranes (Lidon and Henriques, 1991; Maksymiec et al., 1994). Cu-induced lipid peroxidation has also been suggested to be the reason for the membrane degeneration (Luna et al., 1994; Gallego et al., 1996).
In the present study, we addressed the question of why does an excess of Cu2+, supplied in plant growth medium, so efficiently enhance photoinhibition determined in the presence of lincomycin in vivo. We conclude that the primary effect of Cu2+ is simply a decrease in the Chl concentration of leaves, and that the reduced screening by Chl makes the leaves more susceptible to photoinhibition. The results show that changes in the Chl concentration of leaves must always be taken into account when measuring the effects of various stress conditions or transgenes on the susceptibility of plants to photoinhibition.
RESULTS
Photoinhibition of Thylakoids Isolated from Control and Cu-Treated Plants
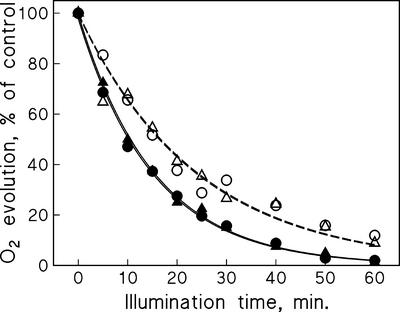
We have previously shown that the presence of 15 μm Cu2+ in the growth medium of bean (Phaseolus vulgaris) plants increases the reaction rate constant of photoinhibition (kPI) from the control value of 0.25 to 0.92 h−1 when measured from intact leaves illuminated in the presence of lincomycin (Pätsikkä et al., 1998). To localize the reason for this higher photosensitivity, we illuminated thylakoids isolated from control and Cu-treated plants with strong light, at the photosynthetic photon flux density (PPFD) of 1,000 or 2,000 μmol m−2 s−1, as indicated. Figure 1 shows that photoinhibition proceeded at exactly the same rate in thylakoids isolated from Cu-treated and control plants. The lack of sensitivity to photoinhibition in the thylakoids isolated from the Cu-treated plants prompted us to measure the Cu2+ concentration of intact chloroplasts isolated from Cu2+-treated and control leaves to see whether our thylakoid isolation procedure washes off Cu2+ that is free in stroma or bound to thylakoids in vivo. The results (Table I) show that when plants were grown in the presence of excess Cu2+, the Cu2+:PSII ratio of chloroplasts increased less than the overall Cu2+:PSII ratio calculated from the Cu2+ concentration of the whole leaves, indicating that Cu2+ did not specifically accumulate in the chloroplasts. Because ion transport through the chloroplast envelope is an active process, we find it unlikely that leakage of Cu from chloroplasts to the medium during the isolation could explain the low Cu content of the chloroplasts. The measured Cu2+:PSII ratio of the chloroplasts was so low that it would have virtually no effect on photoinhibition in isolated thylakoids (Pätsikkä et al., 2001). The fact that the in vivo effect is a 3.7-fold increase in kPI (Pätsikkä et al., 1998) indicates that the high photosensitivity of PSII in Cu2+-treated plants is caused by indirect effects of Cu2+.
Figure 1.
Photoinhibition of oxygen evolution in thylakoids isolated from control bean leaves (○) and from leaves of bean plants grown in the presence of 15 μm Cu2+ (▵). Isolated thylakoids were illuminated at 1,000 (white symbols, dashed line) or 2,000 μmol photons m−2 s−1 (black symbols, solid line).
Table I.
Cu:PSII ratio measured from leaves of control (0.3 μm Cu2+) and Cu-treated (4 μm Cu2+) bean plants and from chloroplasts isolated from these plants
| Treatment | Chl of Leaves | Chl a:b | Cu2+:PSII |
|---|---|---|---|
| μg cm−2 | |||
| Control leaves | 37.2 ± 4.2 | 3.9 | 11 |
| Control chloroplasts | * | * | 3.0 |
| Leaves grown with 4 μm Cu2+ | 12.0 ± 3.2 | 5.1 | 53 |
| Chloroplasts from plants grown with 4 μM Cu2+ | * | * | 5.5 |
The ChI concentration and ChI a:b ratio were measured from three trifoliate leaves collected from each treatment. The Cu concentration of isolated intact chloroplasts was determined from dried samples containing 1 mg of Chl. The Cu2+:PSII ratio was estimated by assuming a Chl:PSII ratio of 440 in control and 392 in Cu-treated leaves. An asterisk indicates that the parameter was not measured.
Cu2+ Induces Moderate Oxidative Stress
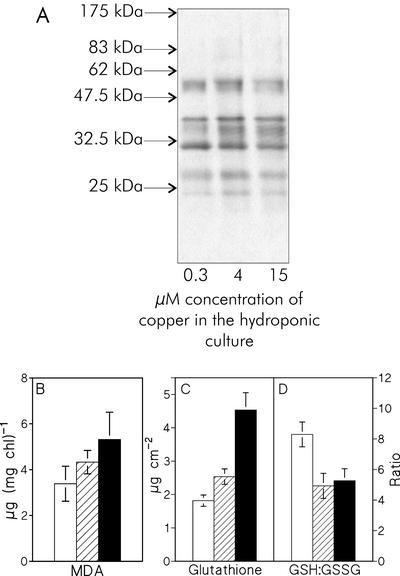
As Cu is known to cause alteration of the lipid composition and is suggested to mediate oxidative stress in plants, we checked if the in vivo photoinhibition was due to increased oxidation of cellular components. We looked for signs of Cu-induced oxidation in proteins and lipids, and for changes in the reduction state of glutathione, a major reductant of plant leaves. All three assays pointed to distinct but only moderate oxidative stress due to growth at excess Cu2+. Little if any increase in the number of carbonyl groups in thylakoid proteins was seen in plants grown in the presence of excess Cu2+ in the growth medium compared with control plants (Fig. 2A). MDA, a product of lipid peroxidation, showed a slight increase when measured from thylakoids isolated from Cu2+-treated plants (Fig. 2B). The MDA measurement assumes that the Chl:lipid ratio does not change due to the Cu treatments, and therefore, the MDA values represent an upper limit for the Cu-treated plants. Moreover, when the MDA concentration was calculated against thylakoid protein concentration, the increasing trend disappeared (data not shown), suggesting that the increase in lipid peroxidation is small in the Cu-treated plants. The total glutathione concentration of the leaf tissue increased (Fig. 2C), but the ratio of reduced to oxidized glutathione (GSH:GSSG) decreased considerably (Fig. 2D).
Figure 2.
A, Oxyblot visualizing carbonyl groups in thylakoid proteins. The thylakoids were isolated from control bean plants (0.3 μm Cu2+) and from bean plants grown at 4 and 15 μm Cu2+. Each sample contained 7.5 μg of soluble protein. The arrows mark the positions of Mr standards. B, The amount of malondealdehyde (MDA) of the thylakoids isolated from the bean leaves, measured with the thiobarbituric acid method. C, The amount of total glutathione determined from the bean leaves; D, the ratio of GSH to GSSG. In B through D, white, hatched, and black bars correspond to plants grown in the presence of 0.3 (control), 4, and 15 μm Cu2+ in growth medium, respectively. Each bar represents the mean of three independent experiments, and the error bars show se.
Excess Cu2+ Does Not Collapse Antioxidative Defense or Affect Photoprotection
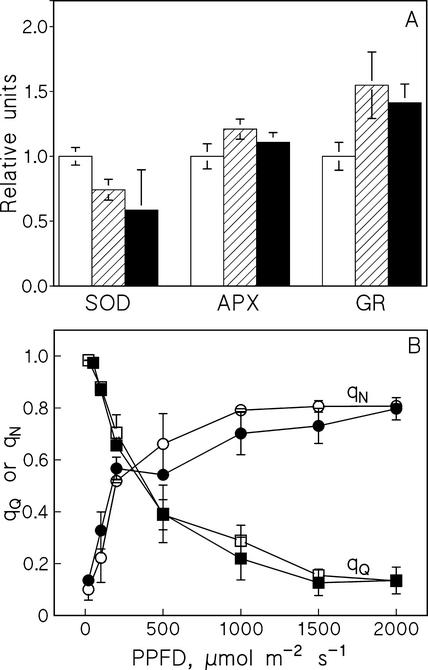
A possible reason for symptoms of oxidative stress might be that Cu2+ greatly reduces the efficiency of antioxidative defense. A collapse of antioxidative defense might also sensitize PSII to photoinhibition. We first explored this possibility by measuring the activities of three major antioxidative enzymes, superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR). Growth in the presence of excess Cu2+ caused a slight decrease in SOD activity, whereas GR activity increased and APX activity remained at the same level as in control leaves (Fig. 3A). These results reveal that the presence of excess Cu2+ in growth medium did not caused a collapse of the antioxidative defense system and Cu2+ did not induce a strong activation of antioxidative enzymes.
Figure 3.
A, Activities of SOD, APX, and GR measured from the first trifoliate leaves of bean plants after 2 weeks of growth in the presence of 0.3 μm Cu2+ (control plants; white bars), 4 μm Cu2+ (hatched bars), or 15 μm Cu2+ (black bars). B, The coefficient of nonphotochemical (qN, circles) and photochemical (qQ, squares) quenching of Chl fluorescence in leaves of control beans (white symbols) and leaves of bean plants grown in the presence of 4 μm Cu2+ (black symbols). Fluorescence was measured with a PAM fluorometer after 5 min of illumination at each PPFD, and far-red illumination was used to measure F0 ' after each white light illumination period. Each data point shows the mean of four independent experiments, and the error bars show se.
Because changes in the biochemical defense mechanisms apparently did not explain the effect of Cu2+ on susceptibility to photoinhibition in vivo, we next turned to a biophysical mechanism attributed to protection of PSII against high light. The coefficient of nonphotochemical quenching of Chl fluorescence (qN) is an indicator of the efficiency by which excitation energy is converted to heat. The results (Fig. 3B) show that the Cu2+ treatment did not affect qN measured between PPFD values of 20 and 2,000 μmol m−2 s−1. The NPQ parameter that can be measured without measuring the light-induced value of initial fluorescence (F0') showed an identical light-intensity dependence as qN in control and Cu-treated plants, but with the higher amplitude of 0.04 to 2.5 (data not shown). Furthermore, the qQ parameter, indicating the efficiency by which light absorbed by PSII is dissipated by photosynthesis, was also insensitive to growth under excess Cu2+ (Fig. 3B).
Chl Concentration and Chloroplast Ultrastructure
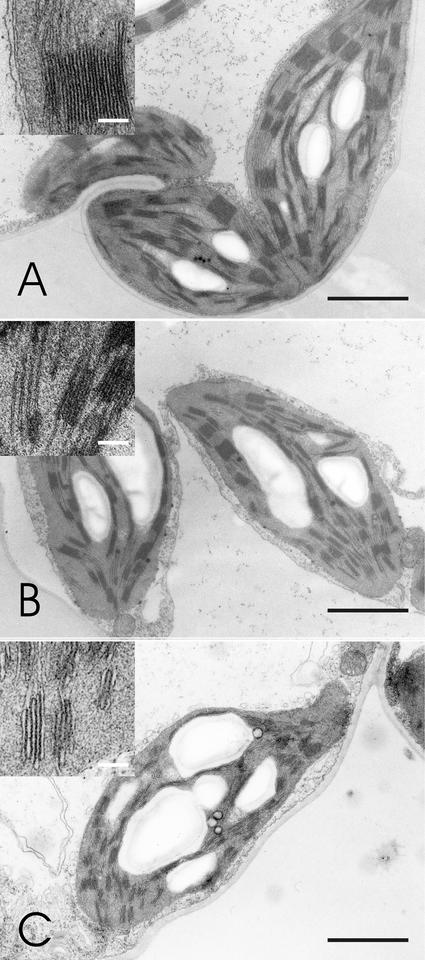
Because oxidative stress apparently did not provide an explanation for the increased photosensitivity of PSII in Cu2+-treated plants, we next investigated structural changes in chloroplasts. The Chl concentration of the leaves was lowered by the Cu2+ treatment, together with an increase in the Chl a:b ratio (Table I). Electron micrographs from leaves of plants grown at 0.3 (control), 4, and 15 μm Cu2+ (Fig. 4) show that the lowered Chl concentration was accompanied by a reduction of the thylakoid membrane structure in plants grown in the presence of excess Cu (Fig. 4).
Figure 4.
Electron micrographs of bean chloroplasts. A, Chloroplasts of a control plant. B, Chloroplasts of a plant grown in the presence of 4 μm Cu2+. C, Chloroplasts of a plant grown in the presence of 15 μm Cu2+. Black bar = 2 μm. The upper left corner of each image shows a magnification of a grana stack; white bar = 0.2 μm.
Cu2+ Predisposes Leaves to Photoinhibition through Reduction of Chl Concentration
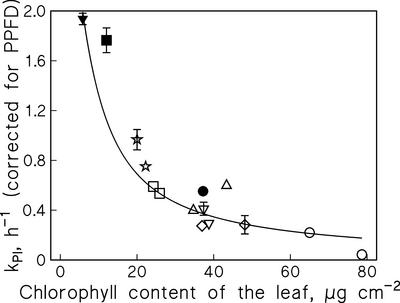
The direct proportionality between kPI and light intensity (Tyystjärvi and Aro, 1996) implies that photoinhibition is slow in optically thick samples, and in vitro experiments with isolated thylakoids show that the attenuation of light by sample absorption lowers kPI, according to the Lambert-Beer law (Pätsikkä et al., 2001). A similar dependence of kPI on Chl concentration was found in vivo by measuring kPI in six different plant species, all collected from an open habitat, exhibiting 4-fold variation of Chl concentration per leaf area (Fig. 5, white symbols). Furthermore, the kPI values measured from Cu-treated bean leaves (Fig. 5, black symbols) fall on the same curve, indicating that the reduced Chl concentration of the Cu-treated plants fully explains the Cu-induced increase in kPI.
Figure 5.
Dependence of photoinhibition on Chl concentration. Lincomycin-treated leaves of Sinapis alba (⋆), Alliaria petiolata (□), Plantago major (▵), Tilia platyphyllos (⋄), Alchemilla vulgaris (▿), and Aesculus hippocastanum (○) were illuminated at the PPFD of 1,500 μmol m−2 s−1. The kPI values are based on measurements of oxygen evolution. The kPI values of control bean leaves (●) and from leaves of beans grown in the presence of 4 μm Cu2+ (▪) or 15 μm Cu2+ (▴) were multiplied by 1.5 to compensate for the lower PPFD (1,000 μmol m−2 s−1) used to obtain these values. The bean data is from Pätsikkä et al. (1998). The line is the best fit to Equation 1, each data point corresponds to an independent experiment, and the error bars, drawn if larger than the symbol, indicate se of the curve fit.
Excess Cu in the Growth Medium Causes Fe Deficiency in Bean Leaves
One mechanism by which excess Cu2+ decreases the leaf Chl concentration is competition between Fe and Cu in the roots. Analysis of basic elements of bean plants grown for 2 weeks in the presence of 4 μm Cu2 revealed that the 5-fold increase in the leaf Cu concentration was accompanied by 4-fold decrease in their Fe concentration (Table II). In a separate experiment, we added excess Cu (4 μm) and 4.5-fold excess of Fe (113 μm) to the hydroponic medium. After the 2-week growth in this medium, the concentrations of Cu and Fe were measured from the leaves, and kPI was determined by illuminating the leaves at 1,000 μmol m−2 s−1 in the presence of lincomycin. The Fe addition did return the kPI value close to the control level, the leaves appeared green (Table II), and the leaf Fe concentration increased considerably. The Cu concentration of these plants was still higher than in control plants, but lower than in plants treated with excess Cu alone.
Table II.
The amount of Cu and Fe in the control and Cu-treated bean plants and in the plants grown with excess Cu and Fe
| Treatment | Color | kPI | Fe in Leaves | Cu in Leaves |
|---|---|---|---|---|
| h−1 | mg/kg dry wt | |||
| Control leaves | Green | 0.28 ± 0.03 | 242 | 12 |
| 4 μm Cu2+ | Yellowish green | 0.67 ± 0.09 | 62 | 65 |
| 4 μm Cu2− plus 113 μm Fe2+ | Green | 0.32 ± 0.02 | 142 | 22 |
Three trifoliate leaves collected from separate plants were pooled for the measurement of basic elements. The rate constant of photoinhibition, kPI, was determined on the basis of photoinhibition experiments at PPFD of 1,000 μmol photons m−2 s−1. The kPI values show mean and se of six independent experiments.
DISCUSSION
Excess Cu2+ causes an elevated susceptibility to photoinhibition of PSII in vitro and in vivo (Jegerschöld et al., 1995; Pätsikkä et al., 1998, 2001), but the molecular basis of the increased photosensitivity has remained unclear. It has long been known that high concentrations of Cu2+, when added to the incubation medium of isolated thylakoids, inhibit PSII electron transfer activity on the acceptor side (Yruela et al., 1996) and finally cause the release of the external polypeptides of the oxygen-evolving complex on the donor side of PSII (Arellano et al., 1995; Jegerschöld et al., 1995; Yruela et al., 2000; Pätsikkä et al., 2001).
The Cu2+:PSII ratio measured from chloroplasts of plants grown hydroponically at the toxic concentration of 4 μm Cu2+ was only twice as high as that of control leaves (Table I), corroborating the earlier finding that excess Cu does not specifically accumulate in chloroplasts (Lolkema and Vooijs, 1986; Baszyñski et al., 1988; Quartacci et al., 2000). Strict regulation of metal transport is a crucial factor of heavy metal tolerance in plants (Hall, 2002), and the small increase in the Cu2+ concentration of the chloroplast compartment suggests that Cu transport is a highly regulated process even in plants suffering from excess Cu2+. Although Cu-treated plants are more susceptible to photoinhibition than control plants (Pätsikkä et al., 1998), the Cu concentrations measured from chloroplasts of Cu-treated plants are below the concentration range required to predispose isolated thylakoids to photoinhibition in vitro. Thylakoids isolated from leaves of Cu2+-treated plants, whose PSII is highly sensitive to photoinhibition in vivo (Pätsikkä et al., 1998), were found to be equally resistant to high light as control thylakoids (Fig. 1). These data indicate that the mechanisms by which high Cu concentrations induce photosensitivity of PSII in vitro are of little importance in vivo.
The present study aimed at solving the mechanism of the Cu2+-induced enhancement of photoinhibition in vivo. Cu2+ is often reported to cause oxidative stress in plants (Weckx and Clijsters, 1996; Navari-Izzo et al., 1998; Gupta et al., 1999), and PSII is a possible target for inhibition by reactive oxygen species. In particular, PSII is sensitive to exogenously generated singlet oxygen (1O2; Knox and Dodge, 1985; Kim et al., 1993). Thus, it is conceivable that a production of 1O2 by a hypothetical Cu2+-dependent mechanism outside of PSII might simultaneously cause specific inhibition of PSII and severe general symptoms of oxidative stress in other parts of the plant leaf. We looked for such symptoms in thylakoid proteins and lipids, and we found signs of enhanced activity of reactive oxygen (Fig. 2). However, these signs were far too mild to account for the 4-fold increase of the susceptibility to photoinhibition in these Cu-treated plants. Furthermore, analysis of biochemical and biophysical antioxidative defense (Figs. 2 and 3) shows that these defense systems were in good shape in plants after 2 weeks of growth with excess Cu2+. The increased glutathione concentration (Fig. 2C) is apparently a response to oxidative stress or directly to heavy metal excess (Alscher, 1989; Xiang and Oliver, 1998; Cuypers et al., 2000). Cu may oxidize sulfhydryl groups of proteins, and the conversion of GSH to GSSG drives the re-reduction of these groups (Uribe and Stark, 1982; Demidchik et al., 1997). The Cu-induced increase in total glutathione (Fig. 2C) may be caused by a feedback mechanism triggered by the thereby lowered GSH:GSSG ratio.
The failure to explain the Cu2+ effect on photoinhibition solely with oxidative stress or antioxidative defense mechanisms prompted us to explore the possibility that the Cu2+-induced enhancement of photoinhibition in vivo is mediated through Cu-induced interference in chloroplast development. The reduction in leaf Chl concentration (Table I) was most probably caused by the Cu-mediated Fe deficiency as the Fe concentration of the leaves decreased with increasing Cu2+ concentrations (Table II). Moreover, the amelioration of the effects of excess Cu2+ by excess Fe2+ in the growth medium (Table II) suggests that Cu2+ and Fe2+ compete in ion uptake and in metabolic processes of the leaf (Schmidt et al., 1997). This conclusion gets further support from the results of Ouzounidou et al. (1998), who showed that the toxic effects of Cu to photosynthesis are reduced considerably with simultaneous high concentration of Fe inside the leaf, due to antagonist interaction between Cu and Fe.
Mechanisms by which excess concentrations of other heavy metals inhibit Fe uptake have been studied earlier (Wallace et al., 1992; Sárvári et al., 1999). To be specific, Fe(III) and Cu(II) reductase activities in root cell plasma membranes are induced by Fe and Cu deficiency (Welch et al., 1993), suggesting that the uptake of these two metals may use partially common pathways. The reductase catalyzes a key step in Fe uptake in dicots (Schmidt, 1999). Fe deficiency also leads to an increase in Cu and Mn content of pea (Pisum sativum) leaves (Iturbe-Ormaetxe et al., 1995).
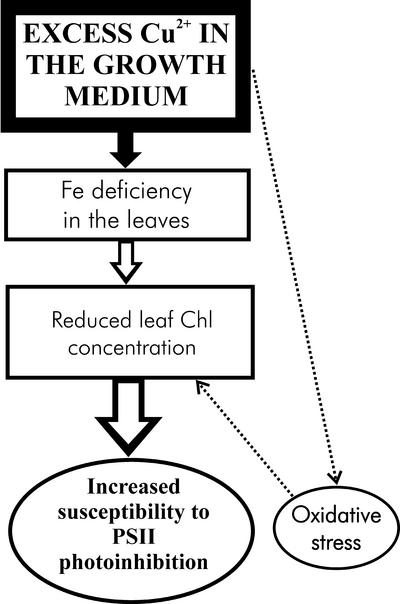
Excess heavy metals cause similar symptoms in chloroplast ultrastructure (decrease in grana and stroma thylakoids per chloroplast) as Fe deficiency (Spiller and Terry, 1980; Taylor and Foy, 1985; Ouzounidou et al., 1992). Fe is needed in biosynthesis of Chl, and symptoms of Fe deficiency include diminished Chl concentration of leaves, increased Chl a:b ratio, and decreased photosynthetic activity (Abadía et al., 1989; Ouzounidou et al., 1992; Fodor et al., 1995). These features are also apparent in bean plants grown in the presence of excess Cu2+ (Table I; Figs. 1 and 4), suggesting that these symptoms may actually have been caused by Fe deficiency, although our data cannot exclude additional influence of excess Cu. Fe deficiency does not seem to affect the efficiency of the photosystems, but instead lowers photosynthetic performance by decreasing the number of photosynthetic units per leaf area (Spiller and Terry, 1980; Abadía et al., 2000; Morales et al., 2000). The reduction of the grana structure (Fig. 4) is consistent with the increased Chl a:b ratio and may indicate that synthesis of the photosystem cores takes metabolic preference over the synthesis of the light-harvesting complex II. Figure 6 summarizes the effect of excess Cu on photoinhibition in vivo.
Figure 6.
Model of the in vivo mechanism by which excess Cu makes PSII more susceptible to photoinhibition. The main primary effect of excess Cu is Fe deficiency, which causes the metabolic disturbances leading to reduction of the Chl concentration in leaves. Leaves with low Chl concentration are sensitive to photoinhibition. Cu2+-Induced oxidative stress may enhance the symptoms of Fe deficiency.
Fe deficiency may not cause oxidative stress, although the activities of antioxidative enzymes are low in Fe-depleted plants (Iturbe-Ormaetxe et al., 1995). We suggest that the symptoms of oxidative stress observed in the Cu-treated plants are mainly caused by the presence of toxic amounts of Cu in the leaves.
The finding that leaves with less Chl are more susceptible to photoinhibition may seem surprising at first sight, but the theory of the relationship between Chl concentration and photoinhibition is straightforward. The rate constant kPI is directly proportional to light intensity (Jones and Kok, 1966; Tyystjärvi and Aro, 1996), implying that the probability of a given PSII unit to lose its activity in unit time depends on the rate of photon absorption by the photoreceptor(s) of photoinhibition belonging to that particular PSII. In general, the fraction of incident light (I0) caught by one absorbing molecule is curvilinearly related to the optical thickness of the sample. For example, if an optically thick sample already absorbs 99% of incident light, an increase in the concentration of the sample cannot cause more than 1% increase in the number of quanta absorbed. According to the Lambert-Beer law, the total intensity absorbed is I0(1 − e−c), where the constant c is a function of the composition and thickness of the sample. The numerical value of c is directly proportional to concentration (approximately the number of absorbing molecules). Because the average intensity absorbed by one absorber is proportional to total absorption divided by the number of absorbers, kPI follows the equation:
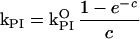 |
Eq. 1 |
where kPI0 is the limiting value of kPI when the optical density of the sample approaches zero. The result is valid irrespective of the identity of the photoreceptor, provided that the dilution of the sample does not involve major changes in the pigment ratios. We have earlier demonstrated that photoinhibition of isolated thylakoids shows a perfect fit to Equation 1 (Pätsikkä et al., 2001), and the present study shows that the prediction holds for intact leaves, too (Fig. 5). The direct proportionality between kPI and light intensity ensures that Equation 1 is valid in spite of the steep light intensity gradient in a leaf. The chlorotic leaves of Cu-treated plants are more susceptible to photoinhibition than control leaves, but such a difference disappears when thylakoids isolated from the Cu2+-treated and control leaves are illuminated at the same Chl concentration. Thus, although the chlorosis caused by Fe deficiency drastically enhances susceptibility to photoinhibition in plants grown with excess Cu2+, Cu does not play any direct role in the light tolerance of PSII in vivo.
It should be noted that the susceptibility to photoinhibition, measured in the presence of lincomycin, is only one parameter defining the photosensitivity of the photosynthetic machinery in natural conditions. In particular, the efficiency by which the photoinhibitory damage is repaired is of great importance (Tyystjärvi et al., 1992). Exposure to intense light during Fe deficiency may also trigger photoprotective responses (Morales et al., 2000).
Leaf Chl concentration has a crucial role for the susceptibility of the leaves to photoinhibition, implying that Chl concentration should always be taken into account when effects of mutation, chemical treatment, environmental condition or plant developmental stage on photoinhibition of intact leaves are under study. Consequences of a simple photochemical law may often explain experimental results more adequately than complicated physiological arguments. Furthermore, it is well known that the thickness of plant leaves tends to increase with increasing irradiance of the habitat (Lambers et al., 1998). We suggest that protection against photoinhibition is an important ecophysiological factor affecting the thickness of plant leaves. A plant growing in a sunny habitat benefits from thick leaves not only because they can store more water, but also because thick leaves offer protection against photoinhibition.
MATERIALS AND METHODS
Bean (Phaseolus vulgaris) Plants
Bean plants (cv Dufrix) were grown in the absence or presence of added CuSO4 · 5H2O at final concentrations of 4 and 15 μm in hydroponic culture as described by Pätsikkä et al. (1998). The full-strength Hoagland medium was buffered by the addition of 2 mm MES-KOH, pH 5.5. The micromolar concentrations of the trace elements were 18 (Cl), 9 (Mn), 0.3 (Cu), 0.8 (Zn), 46 (B), and 0.1 (Mo). Fe was added from freshly made solution to reach the final concentration of 25 μm (or 113 μm, as indicated) FeSO4 · 7H2O in the growth medium. Three to five leaf discs (10 cm2) collected from the first trifoliate leaves of 4-week-old bean plants were pooled for each enzyme or metabolite determination. Immediately after termination of the light treatment, the thylakoids were isolated as described by Pätsikkä et al. (1998).
Photoinhibition Treatments
In vitro photoinhibition of isolated thylakoids was measured as described by Pätsikkä et al. (1998). Before all in vivo photoinhibition treatments, the harvested bean leaves were kept for 3 h under dim light with the petioles in lincomycin solution (1 g L−1) to inhibit chloroplast protein synthesis. Before illumination, in the middle and at the end of appropriate illumination period, thylakoids were isolated from part of the leaf material, and oxygen evolution was immediately measured from these thylakoids. The kPI was calculated by fitting of the loss of oxygen evolution to the first-order reaction equation.
The plant material used for the determination of the relationship between kPI and the Chl concentration of the leaves was collected from an open habitat, where light conditions were relatively uniform, and thus, the differences in leaf Chl concentration reflect morphological differences between the species, not adaptations to sun or shade. Before the experiments, the plants or attached leaves were kept in the dark for 24 h to reduce the starch content. Whole individuals of white mustard (S. alba), garlic mustard (A. petiolata), greater plantain (P. major), and lady's mantle (A. vulgaris) were taken to the laboratory and darkened there, whereas leaves of large-leaved lime (T. platyphyllos) and horse chestnut (A. hippocastaneum) were darkened in situ by covering lower branches of these trees with a black veil. After the dark treatment, the leaves were detached, lincomycin treated, and then illuminated in white light using a 1,200-W daylight lamp at the PPFD of 1,500 μmol m−2 s−1 at 20°C, and samples for thylakoid isolation were cut after 0, 1.5, and 3 h of illumination.
Determination of GSH and GSSG
Five leaf discs were thoroughly homogenized in liquid nitrogen, and 5 mL of 0.15% (w/v) sodium ascorbate solution was added (Grill et al., 1979). The homogenate was filtered through Miracloth and the filtrate was centrifuged at 30,000g for 15 min (0°C). Supernatants were incubated at 100°C for 4 min to denature proteins, and centrifuged as described above. One milliliter of reaction mixture contained 200 μL thylakoid suspension, 0.2 mm NADPH, 0.6 mm 5,5′-dithio-bis(2-nitrobenzoic acid; DTNB), and 50 units of glutathione reductase. The reagents were used as stock solutions in a buffer containing 125 mm Na-phosphate and 6.3 mm Na-EDTA, pH 7.5 (Griffith, 1980). The reduction of DTNB was followed at 412 nm for 2 min at 30°C. For measuring the amount of GSSG in the supernatant, the GSH of the sample was derivatized by adding 2 μm 2-vinylpyridine 100 μL−1 supernatant and mixing them vigorously for 1 min (Griffith, 1980). The reduction rate of DTNB was measured after 20 and 40 min as in the total glutathione assay. Total glutathione and GSSG were quantified by comparing with the standard curves done with the purified reduced and oxidized forms of glutathione (Sigma, St. Louis). The amount of GSH was calculated by subtracting the amount of GSSG from the total glutathione.
APX Activity
Three leaf discs were homogenized in liquid nitrogen. The leaf powder was further homogenized in 3 mL of buffer consisting of 0.1 m Tricine-KOH (pH 8.0), 1 mm dithiothreitol, 10 mm MgCl2, 50 mm KCl, 1 mm EDTA, and 0.1% (w/v) Triton X-100 (Foyer et al., 1989), and was filtered through Miracloth. Activity of APX was measured at 265 nm by following the decrease in absorbance in reaction buffer containing the leaf extract, 0.1 m HEPES-KOH (pH 7.8), 125 μm ascorbic acid, 0.1 mm H2O2, and 1 mm EDTA.
SOD Activity
Six leaf discs were homogenized in liquid nitrogen. Six milliliters of buffer (0.1 m HEPES-KOH, pH 7.8, and 1 mm EDTA) was added, and the sample was thawed and filtered through Miracloth. The increase of A560 was monitored in 1 mL of reaction mixture containing 50 mm HEPES-KOH, pH 7.8, 0.5 mm EDTA, 0.5 mm nitroblue tetrazolium, 4 mm xanthine, 0.05 units xanthine oxidase, and 0 to 200 μL of diluted leaf extract (Beauchamp and Fridovich, 1971; Arisi et al., 1998).
GR Activity
Three leaf discs were homogenized in liquid nitrogen and in 3 mL of extraction buffer containing 150 mm HEPES-KOH (pH 8.0), 1.0 mm EDTA, and 0.1% (w/v) Triton X-100. The homogenized extract was filtered trough Miracloth and was centrifuged at 12,000g for 5 min. Activity of GR was determined as in Foyer et al. (1995) in a reaction buffer containing 50 mm HEPES-KOH, pH 8.0, 1.0 mm EDTA, 0.1 mm NADPH, and 1.0 mm GSSG by following the oxidation of NADPH at 340 nm.
Thiobarbituric Acid Reactive Substances
The thiobarbituric acid reactive substances were determined by a procedure based on the method of Heath and Packer (1968). Three hundred microliters of thylakoid suspension containing 600 μg of protein was homogenized by vortexing in 0.5 mL of 10% (w/v) trichloric acid, 0.5 mL of 0.6% (w/v) thiobarbituric acid, and 1.5 mL of 1% (v/v) H3PO4. The thylakoid extracts were incubated for 30 min at 95°C, chilled on ice, and centrifuged at 4,000g for 10 min (at 4°C). The MDA standards (0.25–7.5 μg of 1, 1, 3, 3-tetraethoxypropane adjusted to final volume of 200 μL with distilled water) were treated as the thylakoid samples, and MDA was quantified from the supernatants of the thylakoid samples by 535-nm absorbance. The A535 was measured from the thylakoid supernatants and MDA standards. Unspecific absorption at 600 nm was subtracted from the 535 nm values.
Electron Microscopy
Leaf sections (1 × 1 mm) were cut, fixed in glutaraldehyde (5% [w/v] in 100 mm sodium phosphate buffer, pH 7.5), postfixed in 3% (w/v) glutaraldehyde (dissolved in 100 mm sodium phosphate buffer, pH 7.0), and 1% (w/v) osmium tetroxide (dissolved in 100 mm sodium phosphate buffer, pH 7.0) for 3 h. The samples were dehydrated in a graded ethanol series, and washed with propyleneoxide. The samples were then embedded in propylene oxide:epon (1:1; v/v) overnight and were finally embedded in epon for 4 to 6 h. Ultrathin sections were cut with a microtome (Reichert Jung, Ultracut E.; Reichert Optische Werke, Wien, Austria) and placed on Cu grids. The sections were stained with uranyl acetate (40 min at 40°C) and lead citrate (5 min at 20°C) corresponding to the ready-made commercial products Ultrostain 1 and 2, respectively (Leica, Wetzlar, Germany), and were examined with electron microscopy (10-SX; JEOL, Tokyo).
Oxyblot of Thylakoid Proteins
The samples for detection of protein oxidation were prepared according to the protocol of the Oxyblot protein oxidation detection kit (Intergen, Purchase, NY). The separation of proteins, immunoblotting, and visualizing of the oxidized proteins were done as described in Pätsikkä et al. (2001).
Determination of Cu and Fe Concentration in Leaves and Isolated Chloroplasts
The analysis of Cu and Fe concentration of the leaves was done with a plasma emission spectrophotometer (ICP-AES; Applied Research Laboratories, Lausanne, Switzerland). For the measurement of the Cu concentration of the chloroplast compartment, intact chloroplasts were prepared as in Zhang et al. (1999). Isolated chloroplasts were dried to pellets containing 1 mg of Chl as described by Šeršen et al. (1997), and their Cu concentration was measured by x-ray radionuclide fluorescence analysis according to Havránek et al. (1989).
Protein Assay, Chl Determination, and Estimation of Chl:PSII Ratio
Protein contents were determined as described by Bradford (1976) using immunoglobulin G as a protein standard. Chl concentration was measured from thylakoid samples according to Porra et al. (1989) or from leaf discs according to Inskeep and Bloom (1985). The Chl:PSII ratio of control leaves was assumed to be 440, and the PSII:PSI ratio does not change due to the Cu treatments (Pätsikkä et al., 1998). The Chl:PSII ratio of Cu-treated plants was then calculated to be 392 by assuming that the Cu-induced increase in the Chl a:b ratio is caused by loss of light-harvesting complex II with Chl a:b ratio of 1.
Fluorescence Quenching Analysis
Fluorescence quenching of the dark-adapted bean leaves was measured with a fluorometer (PAM 101; Heinz Walz, Effeltrich, Germany) using the saturating pulse method. After a 30-min dark adaptation, F0 was induced by illuminating the leaf for 3 s with weak modulated measuring beam, after which a saturating light pulse (5,000 μmol m−2 s−1 white light, applied for 2 s) was given to induce the Fmax. The leaf was then allowed to adapt to seven successive irradiance levels (5-min illumination at 20–2,000 μmol m−2 s−1 at 20°C). At the end of each adaptation period, a saturating pulse was first fired to measure F′max, and then the leaf was illuminated for 50 s with far-red light to measure F′0. The qN and qQ parameters were calculated with the FIP fluorescence software (QA Data, Turku, Finland) according to van Kooten and Snel (1990) and NPQ as defined by Bilger and Björkman (1991).
ACKNOWLEDGMENTS
We thank Hannu Raitio from the Finnish Forest Research Institute at Parkano station for the analysis of basic elements, Dr. Bumbalova from the Comenius University for the radionuclide fluorescence analysis, and Päivi Sarvikas and Saija Sirkiä for assistance in the laboratory.
Footnotes
This study was financially supported by the Academy of Finland.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.004788.
LITERATURE CITED
- Abadía A, Lemoine Y, Trémolières A, Ambard-Bretteville F, Rémy R. Iron deficiency in pea: effects on pigment, lipid and pigment-protein complex composition of thylakoids. Plant Physiol Biochem. 1989;27:679–687. [Google Scholar]
- Abadía J, Morales F, Abadía A. Photosystem II efficiency in low chlorophyll, iron-deficient leaves. Plant Soil. 2000;215:183–192. [Google Scholar]
- Alscher RG. Biosynthesis and antioxidant function of glutathione in plants. Physiol Plant. 1989;77:457–464. [Google Scholar]
- Arellano JB, Lázaro JJ, López GJ, Báron M. The donor side of PSII as copper-inhibitory site: fluorescence and polarographic studies. Photosynth Res. 1995;45:127–134. doi: 10.1007/BF00032584. [DOI] [PubMed] [Google Scholar]
- Arisi AC, Cornic G, Jouanin L, Foyer CH. Overexpression of FeSOD in transformed poplar modifies the regulation of photosynthesis at low CO2 partial pressures or following exposure to prooxidant herbicide methyl violagen. Plant Physiol. 1998;117:565–574. doi: 10.1104/pp.117.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baszyñski T, Tukendorf A, Ruszkowska M, Skórzyñska E, Maksymiec W. Characteristics of the photosynthetic apparatus of copper non-tolerant spinach exposed to excess copper. J Plant Physiol. 1988;132:708–713. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bilger W, Björkman O. Temperature dependence of violaxanthin deepoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva pariflora L. Planta. 1991;184:226–234. doi: 10.1007/BF00197951. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cedeno-Maldonado A, Swader JA. The cupric ion as an inhibitor of photosynthetic electron transport in isolated chloroplasts. Plant Physiol. 1972;50:698–701. doi: 10.1104/pp.50.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers A, Vangronsveld J, Clijsters H. Biphasic effect of copper on the ascorbate-glutathione pathway in primary leaves of Phaseolus vulgaris seedlings during the early stages of metal assimilation. Physiol Plant. 2000;110:512–517. [Google Scholar]
- Demidchik V, Sokolik A, Yurin V. The effect of Cu2+ on ion transport systems of the plant plasmalemma. Plant Physiol. 1997;114:1313–1325. doi: 10.1104/pp.114.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefteriou EP, Karataglis S. Ultrastructural and morphological characteristics of cultivated wheat growing on copper-polluted fields. Bot Acta. 1989;102:134–140. [Google Scholar]
- Fodor F, Böddi B, Sárvári É, Záray G, Cseh E, Láng F. Correlation of iron content, spectral forms of chlorophyll and chlorophyll-proteins in iron deficient cucumber (Cucumis sativus) Physiol Plant. 1995;93:750–756. [Google Scholar]
- Foyer CH, Dujardyn M, Lemoine Y. Responses of photosynthesis and the xanthophyll and ascorbate-glutathione cycles to changes in irradiance, photoinhibition and recovery. Plant Physiol Biochem. 1989;27:751–760. [Google Scholar]
- Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995;109:1047–1057. doi: 10.1104/pp.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego SM, Benavides MP, Tomaro ML. Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci. 1996;121:151–159. [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpuridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Grill D, Esterbauer H, Klösch U. Effect of sulphur dioxide on glutathione in leaves of plants. Environ Pollution. 1979;19:187–197. [Google Scholar]
- Gupta M, Cuypers A, Vangronsveld J, Clijsters H. Copper affects the enzymes of the ascorbate-glutathione cycle and its related metabolites in the roots of Phaseolus vulgaris. Physiol Plant. 1999;106:262–267. [Google Scholar]
- Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot. 2002;53:1–11. [PubMed] [Google Scholar]
- Havránek E, Bumbálová A, Harangozó M. A contribution to the sample preparation in radionuclide X-ray fluorescence analysis. J Radioanal Nucl Chem Lett. 1989;135:321–331. [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Inskeep W, Bloom PR. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Moran JF, Arrese-Igor C, Gogorcena Y, Klucas RV, Becana M. Activated oxygen and antioxidant defences in iron-deficient pea plants. Plant Cell Environ. 1995;18:421–429. [Google Scholar]
- Jegerschöld C, Arellano JB, Schröder WP, van Kan PJM, Barón M, Styring S. Copper (II) inhibition of electron transfer through photosystem II studied by EPR spectroscopy. Biochemistry. 1995;34:12747–12754. doi: 10.1021/bi00039a034. [DOI] [PubMed] [Google Scholar]
- Jones LW, Kok B. Photoinhibition of chloroplast reactions: kinetics and action spectra. Plant Physiol. 1966;41:1037–1043. doi: 10.1104/pp.41.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Han GH, Kim JM, Jung J. In situ susceptibilities of photosystems I and II to photosensitized inactivation via singlet oxygen mechanism. Photochem Photobiol. 1993;57:1069–1074. [Google Scholar]
- Knox JP, Dodge AD. The photodynamic action of eosin, a singlet-oxygen generator: the inhibition of photosynthetic electron transport. Planta. 1985;164:30–34. doi: 10.1007/BF00391022. [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapin IIIFS, Pons TL. Response of photosynthesis to light. In: Lambers H, Chapin FS III, Pons TL, editors. Plant Physiological Ecology. New York: Springer Verlag; 1998. pp. 25–46. [Google Scholar]
- Lidon FC, Henriques FS. Limiting step of photosynthesis of rice plants treated with varying copper levels. J Plant Physiol. 1991;138:115–118. [Google Scholar]
- Lolkema PC, Vooijs R. Copper tolerance in Silene cucubalus. Planta. 1986;167:30–36. doi: 10.1007/BF00446365. [DOI] [PubMed] [Google Scholar]
- Luna CM, González CA, Trippi VS. Oxidative damage caused by excess of copper in oat leaves. Plant Cell Physiol. 1994;35:11–15. [Google Scholar]
- Maksymiec W. Effect of copper on cellular processes in higher plants. Photosynthetica. 1997;34:321–342. [Google Scholar]
- Maksymiec W, Russa R, Urbanic-Sypniewska, Baszyñski T. Effect of excess Cu on the photosynthetic apparatus of runner bean leaves treated at two different growth stages. Physiol Plant. 1994;91:715–721. [Google Scholar]
- Mohanty N, Vass I, Demeter S. Copper toxicity affects photosystem II electron transport at the secondary quinone acceptor, QB. Plant Physiol. 1989;90:175–179. doi: 10.1104/pp.90.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales F, Belkhodja R, Abadía A, Abadía J. Photosystem II efficiency and mechanisms of energy dissipation in iron-deficient, field-grown pear trees (Pyrus communis L.) Photosynth Res. 2000;63:9–21. doi: 10.1023/A:1006389915424. [DOI] [PubMed] [Google Scholar]
- Navari-Izzo F, Quartacci MF, Pinzino C, Dalla Vecchia F, Sgherri CLM. Thylakoid-bound and stromal enzymes in wheat treated with excess copper. Physiol Plant. 1998;104:630–638. [Google Scholar]
- Ouzounidou G, Elefteriou EP, Karataglis S. Ecophysiological and ultrastructural effects of copper in Thlapsi ochroleucum (Cruciferae) Can J Bot. 1992;70:947–957. [Google Scholar]
- Ouzounidou G, Ilias I, Tranopoulou H, Karataglis S. Amelioration of copper toxicity by iron on spinach physiology. J Plant Nutr. 1998;21:2089–2101. [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b with four different solvents: verification of the concentration of chlorophyll by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- Pätsikkä E, Aro E-M, Tyystjärvi E. Increase in the quantum yield of photoinhibition contributes to copper toxicity in vivo. Plant Physiol. 1998;117:619–627. doi: 10.1104/pp.117.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pätsikkä E, Aro E-M, Tyystjärvi E. Mechanism of copper-enhanced photoinhibition in thylakoid membranes. Physiol Plant. 2001;113:142–150. [Google Scholar]
- Quartacci MF, Pinzino C, Sgherri CLM, Dalla Vecchia F, Navari-Izzo F. Growth in excess copper induces changes in the lipid composition and fluidity of PSII-enriched membranes in wheat. Physiol Plant. 2000;108:87–93. [Google Scholar]
- Raven JA, Evans MCW, Korb R. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth Res. 1999;60:111–149. [Google Scholar]
- Sárvári É, Fodor F, Cheh E, Varga A, Záray G, Zolla L. Relationship between changes in ion content of leaves and chlorophyll-protein composition in cucumber under Cd and Pb stress. Z Naturforsch. 1999;54c:746–753. [Google Scholar]
- Schmidt W. Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol. 1999;141:1–26. [Google Scholar]
- Schmidt W, Bartels M, Tittel J, Fühner C. Physiological effects on iron acquisition processes in Plantago. New Phytol. 1997;135:659–666. [Google Scholar]
- Schröder WP, Arellano JB, Bittner T, Barón M. Flash-induced absorption spectroscopy studies of copper interaction with photosystem II in higher plants. J Biol Chem. 1994;52:32865–32870. [PubMed] [Google Scholar]
- Seršen F, Král'ová K, Bumbálová A, Svajlenová O. The effect of Cu(II) ions with tridentate Schiff base ligand upon photosynthetic apparatus. J Plant Physiol. 1997;151:299–305. [Google Scholar]
- Spiller S, Terry N. Limiting factors in photosynthesis: Iron stress diminishes photochemical capacity by reducing the number of photosynthetic units. Plant Physiol. 1980;65:121–125. doi: 10.1104/pp.65.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ, Foy CD. Differential uptake and toxicity of ionic and chelated copper in Triticum aestivum. Can J Bot. 1985;63:1271–1275. [Google Scholar]
- Tyystjärvi E, Ali-Yrkkö K, Kettunen R, Aro E-M. Slow degradation of the D1 protein is related to the susceptibility of low-light-grown pumpkin plants to photoinhibition. Plant Physiol. 1992;100:1310–1317. doi: 10.1104/pp.100.3.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyystjärvi E, Aro E-M. The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA. 1996;93:2213–2218. doi: 10.1073/pnas.93.5.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe EG, Stark B. Inhibition of photosynthetic energy conversion by cupric ion. Plant Physiol. 1982;69:1040–1045. doi: 10.1104/pp.69.5.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten OF, Snel JHF. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- Wallace A, Wallace GA, Cha JW. Some modifications in trace metal toxicities and deficiencies in plants resulting from interactions with other elements and chelating agents: the special case of iron. J Plant Nutr. 1992;15:1589–1598. [Google Scholar]
- Weckx JEJ, Clijsters HMM. Oxidative damage and defense mechanisms in primary leaves of Phaseolus vulgaris as a result of root assimilation of toxic amounts of copper. Physiol Plant. 1996;96:506–512. [Google Scholar]
- Welch RM, Norvel WA, Schaefer SC, Shaff JE, Kochian LV. Induction of iron(III) and copper(II) reduction in pea (Pisum sativum L.) roots by Fe and Cu status: Does the root-cell plasmalemma Fe(III)-chelate reductase perform a general role in regulating cation uptake? Planta. 1993;190:555–561. [Google Scholar]
- Yruela I, Alfonso M, Barón M, Picorel R. Copper effect on the protein composition of photosystem II. Physiol Plant. 2000;110:551–557. [Google Scholar]
- Yruela I, Gatzen G, Picorel R, Holzwarth AR. Cu(II)-inhibitory effect on photosystem II from higher plants: a picosecond time-resolved fluorescence study. Biochemistry. 1996a;35:9469–9474. doi: 10.1021/bi951667e. [DOI] [PubMed] [Google Scholar]
- Yruela I, Pueyo JJ, Alonso PJ, Picorel R. Photoinhibition of photosystem II from higher plants: effect of copper inhibition. J Biol Chem. 1996b;271:27408–27415. doi: 10.1074/jbc.271.44.27408. [DOI] [PubMed] [Google Scholar]
- Xiang C, Oliver DJ. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell. 1998;10:1539–1550. doi: 10.1105/tpc.10.9.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Paakkarinen V, van Wijk KJ, Aro E-M. Cotranslational assembly of the D1 protein into photosystem II. J Biol Chem. 1999;274:16062–16067. doi: 10.1074/jbc.274.23.16062. [DOI] [PubMed] [Google Scholar]