Abstract
Self-referencing H+-selective microelectrodes were used to measure extracellular H+ fluxes from horizontal cells isolated from the skate retina. A standing H+ flux was detected from quiescent cells, indicating a higher concentration of free hydrogen ions near the extracellular surface of the cell as compared to the surrounding solution. The standing H+ flux was reduced by removal of extracellular sodium or application of 5-(N-ethyl-N-isopropyl) amiloride (EIPA), suggesting activity of a Na+–H+ exchanger. Glutamate decreased H+ flux, lowering the concentration of free hydrogen ions around the cell. AMPA/kainate receptor agonists mimicked the response, and the AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) eliminated the effects of glutamate and kainate. Metabotropic glutamate agonists were without effect. Glutamate-induced alterations in H+ flux required extracellular calcium, and were abolished when cells were bathed in an alkaline Ringer solution. Increasing intracellular calcium by photolysis of the caged calcium compound NP-EGTA also altered extracellular H+ flux. Immunocytochemical localization of the plasmalemma Ca2+–H+-ATPase (PMCA pump) revealed intense labelling within the outer plexiform layer and on isolated horizontal cells. Our results suggest that glutamate modulation of H+ flux arises from calcium entry into cells with subsequent activation of the plasmalemma Ca2+–H+-ATPase. These neurotransmitter-induced changes in extracellular pH have the potential to play a modulatory role in synaptic processing in the outer retina. However, our findings argue against the hypothesis that hydrogen ions released by horizontal cells normally act as the inhibitory feedback neurotransmitter onto photoreceptor synaptic terminals to create the surround portion of the centre-surround receptive fields of retinal neurones.
Neuronal activity is often accompanied by changes in extracellular pH (for review see Chesler, 2003; Deitmer & Rose, 1996). Light-induced shifts in extracellular hydrogen ion concentrations have been observed in the retinas of both vertebrates (Oakley & Wen, 1989) and invertebrates (Coles et al. 1996). The light-induced responses of second order retinal neurones are highly sensitive to changes in extracellular pH. Kleinschmidt (1991) found that light-induced responses of horizontal cells were almost entirely abolished by changing extracellular pH in the tiger salamander retina from pH 7.8 to 7.2. A similar sensitivity of second order retinal neurones to extracellular H+ was described by Harsanyi & Mangel (1993) and Barnes et al. (1993), the latter reporting that light-sensitive responses of horizontal and bipolar cells were exponentially dependent upon extracellular pH. Barnes et al. (1993) provided strong evidence that this modulation of synaptic transmission was due to the marked sensitivity of photoreceptor calcium channels to extracellular protons: elevated concentrations of H+ acted to shift the calcium-activation curve of photoreceptors to depolarized levels and also reduced the calcium conductance. Circadian changes in retinal extracellular pH have also been described, with a significant increase in the extracellular H+ concentration occurring during subjective night (Dmitriev & Mangel, 2000, 2001). It has been proposed that this increased level of acidity in the subjective night might serve to initiate many of the adaptive changes that occur as the retina adjusts to changes in lighting conditions.
Horizontal cells receive direct input from photoreceptors and play a key role in establishing the surround portion of the centre–surround receptive fields of retinal neurones. The molecular mechanism(s) by which horizontal cells exert their inhibitory influences remains under debate. The high sensitivity of retinal neurones to changes in extracellular pH has lent support to the hypothesis that hydrogen ions released by horizontal cells might act as the agent mediating lateral inhibition (Verweij et al. 1996; Kamermans & Spekreijse, 1999). Little is known about the extrusion of H+ or hydrogen ion equivalents by horizontal cells. One reason for this lack of information is the complex cellular composition of the intact retina, where the extracellular pH is the result of the activity of many different cell types. An additional difficulty stems from the invaginating structure of the photoreceptor synapse, where the processes of horizontal and bipolar cells are tucked within and surrounded by the synaptic pedicle of photoreceptors. This structure makes accurate measurements of extracellular pH using H+-selective microelectrodes extremely difficult to obtain. To overcome these limitations, we have sought to measure extracellular H+ fluxes directly from single, isolated retinal horizontal cells maintained in primary culture. A major technical challenge limiting such experiments has been electrical noise and drift inherent in H+-selective microelectrodes. The magnitude of this electrical drift and noise is likely to be large enough to obscure the small responses expected from single cells. In the present study we have used H+-sensitive microelectrodes in a self-referencing format, which greatly enhances the stability and useful sensitivity of these electrodes and eliminates much of the drift and noise normally present (Smith et al. 1999; Smith & Trimarchi, 2001). Here we demonstrate that this technique permits the detection of H+ fluxes from retinal isolated horizontal cells of the skate. We further demonstrate that glutamate, the likely neurotransmitter released by photoreceptors, induces marked alterations in the measured H+ fluxes, and that this modulation requires the presence of extracellular calcium. Our data suggest that activation of horizontal cells by glutamate may indeed lead to alterations of extracellular levels of pH, but argue against the hypothesis that protons released by horizontal cells feed back onto photoreceptor synaptic terminals to promote lateral inhibition within the retina.
Methods
Cell dissociation
The skate used for these studies (Raja erinacea and R. ocellata) were obtained from the Marine Biological Laboratory in Woods Hole, MA, USA. They were maintained for up to 3 weeks in a tank of circulating artificial seawater kept at 14°C. The protocol for obtaining isolated retinal cells has been described by Malchow et al. (1990). Skates were anaesthetized with 1 g gal−1 (∼0.26 g l−1)MS 222 (tricaine, Sigma), cervically transected and double-pithed. Eyes were enucleated, cut in half, and eyecups cut into quarters and immersed in a solution of skate-modified Leibowitz culture medium (L-15, Sigma) containing 2 mg ml−1 papain and 1 mg ml−1 cysteine for 5 min. Retinas were isolated and placed in papain/cysteine-containing L-15 for 45 min with continuous gentle agitation, then washed eight times in skate-modified culture medium lacking papain/cysteine, and mechanically agitated through a 5 ml graduated pipette. The cellular suspension (1–2 drops) was placed in 35 mm culture dishes (Falcon 3001) pre-loaded with 3 ml modified culture medium. Dishes were maintained at 14°C; in later experiments, cells were kept at 4°C, as cells tended to survive longer at this temperature. In experiments using Fura-2, cells were placed on coverslips coated with protamine and convanavalian-A as described by Kreitzer et al. (2003).
Preparation of electrodes
H+-selective microelectrodes were prepared as described by Smith et al. (1999). In brief, thin-walled glass capillary tubes (outer diameter, 1.5 mm) were pulled to produce tip diameters of 2–4 μm, baked at 200°C for at least 4–6 h, then coated with silane by exposing the pipettes to N,N-dimethyltrimethylsilyalamine (Fluka Chemica) vapour in a hood for 30 min at 200°C. Electrodes were baked for an additional 4–6 h at 200°C. Silanized pipettes were back-filled with 100 mm KCl; fluid was forced to the tip by air pressure supplied to the back of the pipette. The pipette tip was placed in contact with a highly selective H+-selective resin (hydrogen ionophore 1-cocktail B, Fluka Chemica) and approximately 50 μm of resin drawn up. The voltage generated across this cocktail is directly proportional to the log of the free H+ ion activity. The time response of these electrodes was checked using a Warner fast superfusion system (model SF-77B). The time constant for H+-selective electrodes to respond to a change in pH from 7.0 to 8.0 was 32 ± 6 ms (n = 4), and 33 ± 7 ms when changing from pH 8.0 to 7.0. These response times are very close to the limit of solution exchange of the superfusion system itself (∼25 ms as measured examining changes in liquid junction potentials).
Self-referencing recordings
Microelectrodes were used in a self-referencing format, which greatly enhances the sensitivity and stability of recordings. Microelectrodes were moved alternately between a point close (within 1–2 μm) to the cell membrane and a known distance away (typically 30 μm). The voltage difference between the readings reflects the difference in free ion concentrations at these positions. The frequency of movement was 0.3 Hz. Electrodes were connected to an IonAmp (version 3.1) high impedance voltage-follower via a Ag/AgCl wire inserted into the back of the micropipette; the return electrode was a Ag/AgCl wire in the bath. The position of the electrode was controlled using a Microstep control box (version 6.0) permitting submicrometre control of electrode placement. Electrode movement and data collection were controlled by IonView 32 software run on a 200 MHz computer. Electrode movement was observed on a television monitor throughout experiments; results in which mechanical drift of the electrode was detected were discarded. All amplifiers and software were the creation of the BioCurrents Research Center, Woods Hole, MA, USA. The electrical signal of the H+ electrode was sampled at 1 kHz; the first third of collected data points from each cycle of electrode movement was discarded to eliminate movement artifacts. The mean voltages at the two extremes of translation were calculated and entered into a running average encompassing 10 data points at a time.
Electrodes were calibrated using commercially purchased pH standards: pH 6.00, pH 7.00 and pH 8.00 (SB104-1, SB108-1, and SB112-1, respectively, Fisher Scientific). Only electrodes possessing Nernst slopes between 45 and 60 mV (pH unit)−1 were used. Control experiments demonstrated that electrodes retained their Nernstian response characteristics in normal skate extracellular solutions ranging in pH from 6.0 to 10.0. Electrodes also responded in a Nernstian fashion to pH changes ranging from 5.5 to 8.0 in solutions in which sodium had been replaced by N-methyl-d-glucamine (NMDG).
Solutions and drug application
Chemicals were purchased from Sigma Chemical Co (St Louis, MO, USA) unless otherwise indicated. The extracellular solution used in most experiments contained (mm); NaCl 270, KCl 6, CaCl2 4, MgCl2 1, urea 360, Hepes 2, glucose 10; pH was adjusted to 7.60 with 1 m or 5 m NaOH. The urea is common to all elasmobranch Ringer solutions, and such concentrations are typically detected in the plasma of these species. In experiments examining the sodium dependence of the responses, sodium was replaced by equal molar amounts of NMDG; pH was adjusted to 7.60 with 1 m HCl. Solutions containing nominally zero calcium were prepared by replacing the normal 4 mm CaCl2 by 4 mm MgCl2. In experiments examining the effect of alkaline extracellular solution, the 2 mm Hepes was replaced by 2 mm of the pH buffer AMPSO (which has a pKa of 9.0), and the solution adjusted to pH 9.50 using NaOH.
Measurement of H+ fluxes from isolated cells relies on the establishment of a H+ gradient generated at the outer membrane which declines by diffusion away from the cell. The small pH gradients expected to be generated by isolated cells would probably be significantly disturbed or eliminated by rapid superfusion of solutions around the cell. Consequently, we applied solutions by adding 1 ml of solution quickly to the bath by a hand-held pipette. A typical experiment began by replacing the culture medium completely with solution containing 2 mm Hepes; the final volume of fluid in the dish was set to 3 ml. A cell was then located, and the pH-selective electrode placed 1 μm from the cell membrane. Differential extracellular recordings were made for several minutes to ensure a steady baseline reading. Normal extracellular solution (1 ml) was then applied to ensure that application of the fluid itself did not alter the measured flux. Some time (usually several minutes) later, 1 ml of the same solution containing the test compound was added. The pH of the solution containing the test compound was adjusted to the same as the normal extracellular solution to within 0.01 pH units. The concentrations listed throughout reflect the final concentration of the drug after complete mixing. Unless otherwise specified, drugs remained in the dish during the remainder of the recording.
We conducted separate control experiments to ensure that drugs did not alter the ability of H+-selective electrodes to sense changes in pH. Two types of control experiments were performed for all compounds used. First, H+ gradients were measured from a H+ source pipette in the presence and absence of a drug. Source pipettes were prepared by pulling glass capillary tubes to produce a final tip diameter between 40 and 50 μm. Skate extracellular solution containing 10 mm Hepes was adjusted to pH 7.40, and agar (0.5%) then added to the solution and heated. Hot solution was injected into the back of the pipettes and cooled at room temperature for an hour. H+ source electrodes were mounted on 35 mm culture dishes with wax and immersed in normal extracellular solution containing 2 mm Hepes, pH 7.60, for 30 min to allow H+ flux to stabilize. H+-selective microelectrodes were positioned so that the near position was no more than 10 μm from the source pipette. As in experiments with cells, dishes were initially filled with 3 ml solution. The second type of control experiment involved calibration of electrodes at pH 6.0, 7.0 and 8.0 in the absence and then presence of the drug. Such controls revealed that 100 μm of the calcium channel blocker nifedipine altered the characteristics of the H+-selective electrodes, making it unsuitable for use in the present experiments. All other compounds used were found to be without effect on H+ sensors at the concentrations indicated.
Intracellular calcium and pH imaging
Intracellular levels of calcium were monitored using one of two techniques. The first employed the ratiometric indicator dye, Fura-2 (Molecular Probes) used as described by Haugh et al. (1995). In experiments examining the effects of caged calcium on H+ flux, the acetoxymethyl ester (AM) forms of the single wavelength calcium indicator dye Oregon Green (Molecular Probes) and the caged calcium compound NP-EGTA (Molecular Probes) were used. Isolated cells were bathed in 5 μm Oregon Green-AM and 8 μm NP-EGTA-AM with 0.2% DMSO and 0.02% Pluronic F-127 for 35–45 min at 14°C, rinsed and left to stand for 1 h. Cells were then exposed to 20 μm kainate for 20 s to permit loading of the NP-EGTA with calcium and then washed in normal extracellular solution; experiments were conducted 10–30 min after calcium loading. We employed the ratiometric pH indicator dye BCECF (Molecular Probes), used as decribed by Haugh-Scheidt & Ripps (1998), to examine changes in intracellular pH induced by glutamate and kainate. Note, however, that BCECF and related compounds derived from fluorescien have been reported to interfere with Ca2+-H+-ATPase activity (Gatto & Milanick, 1993).
Measurements of intracellular calcium and pH were obtained using a Zeiss Atto-fluor imaging system equipped with an infrared light source and infrared light-sensitive camera to permit observation of the cell and self-referencing electrode during experiments. A Zeiss Axiovert S100TV microscope equipped with a 40× LD phase acroplan objective (NA, 0.60) was used. For Fura-2 experiments, excitation was achieved using a single light source equipped with alternating filters that let through 340 and 380 nm UV light. Emission was monitored at 510 nm. In experiments using Oregon Green, excitation was achieved using 488 nm light and emission monitored at 530 nm. For intracellular pH measurements, light was passed through alternating filters for 488 nm and 460 nm light. Fluorescence emission was monitored at 520 nm. Calibration of the pH signal was obtained by equilibrating pH across the cell membrane to known values by bathing the cells in extracellular solution containing 10 μm nigericin and 100 mm KCl set to pH 5, 7 and 9 (Haugh-Scheidt & Ripps, 1998).
Statistical treatment
Student's paired t tests were used throughout to determine statistical significance, with a criterion of P < 0.01 selected as indicating significantly different distributions. Data are presented throughout as the mean ± s.e.m.
Immunocytochemistry
Isolated cells were fixed 2 h after plating onto glass coverslips treated with concanavalin-A. Cells were fixed by adding 3 drops of 2.5% paraformaldehyde to dishes containing 3 ml skate modified L-15 solution. After 5 min the medium was washed and replaced with 2.5% paraformaldehyde, allowed to stand for 2 h, then washed three times over 30 min using a blocking solution composed of 10% goat serum and 0.1% Triton X-100 in PBS. Cells were incubated overnight at 4°C in primary antibody (5F10, Affinity BioReagents, diluted 1/200 with blocking solution) known to recognize the plasmalemma Ca2+–H+-ATPase present in cells of many species. Cells were again washed three times in 30 min with blocking solution, incubated in darkness for 3 h with secondary antibody (goat anti-mouse IgG fluorescein conjugate, Calbiochem, diluted 1/500), then washed three times over 30 min with PBS. Coverslips were mounted on slides with Pro-Long anti-fade kit (Molecular Probes) and covered with a larger glass coverslip. Slides were dried overnight and sealed the following day with clear nail polish. Cells were viewed using a Zeiss confocal microscope.
Intact retinas were fixed for 2 h in cold 4% paraformaldehyde in 0.1 m PBS, pH 7.4, rinsed twice (30 min each) in cold buffer and stored at 4°C in buffer containing 0.1% sodium azide. The tissue was cryoprotected by stepping it through cold 20% and 30% sucrose overnight, then mounted with OCT embedding medium (Miles) and sectioned at 8–20 μm. Sections were picked up on Superfrost Plus Slides (Fisher), washed in PBS at room temperature, and placed in block buffer (4% goat serum, 2% bovine gamma globulin and 0.3% Triton X-100 in PBS) for 30 min. The block buffer was removed and primary antibodies applied to the sections, allowed to incubate overnight, and then washed three times (10 min each) in PBS before incubation with Cye-3-labelled goat anti-mouse secondary antibody (Jackson Laboratories) for 2 h. This was followed by three additional 10-min washes. The sections were coverslipped with Vectashield (Vector Laboratories) and viewed using a Zeiss confocal microscope.
Calculation of total H+ flux
As described by Smith et al. (1999), the value for the flux of an ion in an unbuffered solution is dependent upon the diffusion coefficient for that ion, the ‘background’ concentration of the ion, and the distance over which the self-referencing electrode travels. The movement of ions through a medium is governed by Fick's law:
where JH+ is flux (μmol cm−2 s−1) of hydrogen ions; D the diffusion constant (for hydrogen ions in H2O, 9.22 × 10−5 cm2 s−1), and dc/dx the gradient as specified by a change in the ion activity (mol cm−3) over a given distance (cm) (Smith et al. 1994). Strictly defined, all measurements made with ion-selective sensors are of the free ion concentration or activity, not total concentration (cf. Ammann et al. 1987; Voipio et al. 1994; Smith et al. 1999). Therefore, in this paper when ion concentrations are referred to it represents the free ion concentration or is the concentration gradient as specified by a change in concentration (μmol cm−3) over a given distance (cm) (Smith et al. 1994). In order to calculate flux, we must obtain the value for dc/dx. In the present experimental framework, eqn (1) can be rewritten as eqn (2):
where ΔC is the difference in ionic activity (μmol cm−3) between two positions, one close to the cell membrane and the other a fixed distance away, and Δr is the excursion value (cm). ΔC can be obtained using eqn (3):
where ΔV is the differential reading (mV) measured by the self referencing system; CB is the background concentration of free H+; and S is the Nernstian slope of the electrode (mV), typically 56 mV for H+. As described by Smith et al. (1999), the signal measured using self-referencing electrodes underestimates actual flux due to the response times of the electrode and the electronics. The constant nature of this underestimate allows the use of a correction factor, necessitating that the calculated value for ΔC be multiplied by 1.5.
These equations allow us to obtain the flux of H+ ions through an unbuffered solution. However, a large proportion of the H+ flux produced by the cell is masked from the H+ sensor because it is bound to Hepes buffer. It is possible to calculate a value ‘R’ to represent the ratio of H+ flux bound to buffer and unbound H+. As derived from Arif et al. (1995), the ratio can be obtained using eqn (4):
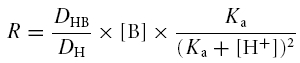 |
where R is the ratio of H+ flux (bound to buffer) to free (unbound) H+; DHB is the diffusion coefficient for protonated buffer (0.62 × 10−5 cm2 s−1); DH is the diffusion coefficient for H+ ions (9.22 × 10−5 m2 s−1); [B] is the buffer concentration in mol cm−3, which in the present set of experiments was usually 2.0 × 10−6 mol cm−3 (that is, equivalent to 2.0 × 10−3m); and Ka (the dissociation constant for the buffer) is 3.16 × 10−11 mol cm−3. This same equation can be derived as well from the work of Demarest & Morgan (1995). The total flux of H+ can be calculated using eqn (5):
That is, total H+ flux from a cell is equal to the sum of the hydrogen ions detected by the sensor plus the hydrogen ions bound to Hepes which is not detectable by the sensor.
Results
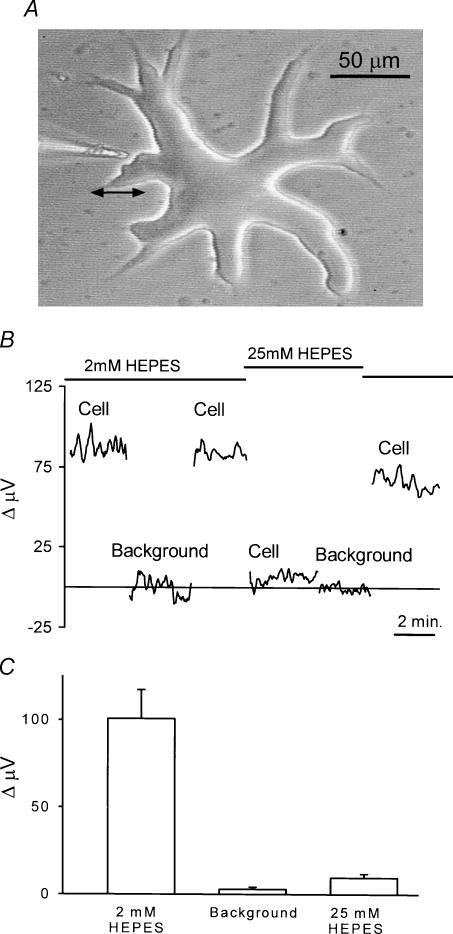
An external horizontal cell typical of the ones used for this study is shown in Fig. 1A. These cells are very large and easy to distinguish from other neuronal cell types. The photomicrograph also shows the placement of a H+-sensitive microelectrode near the edge of the cell. In all experiments, electrodes were placed within 1–2 μm of the cell membrane. To make differential H+ recordings, measurements were first made with the electrode in this position, and then at a position (usually 30–50 μm) distant from the cell. In the case of the cell in Fig. 1A, the electrode was moved back to the left, as indicated by the double arrow. This process was repeated at a rate of 0.3 Hz; as mentioned in the Methods, the first third of the collected data points from each cycle of electrode movement was discarded to eliminate movement artifacts.
Figure 1. Self-referencing recordings from isolated skate horizontal cells using a H+-selective microelectrode.
A, an isolated cell obtained via enzymatic dissociation from the retina of the skate. The large size and thick cell soma permit ready identification of this cell as an external horizontal cell (Malchow et al. 1990). To the left is shown a H+-selective microelectrode positioned in the ‘near pole’ recording position. Differential recordings were made by laterally translating the electrode to a position 50 μm distant from the cell and computing the difference in signal between the near and far poles of the recording. The double arrow indicates the movement from near to far positions. B, a self-referencing differential recording from a single horizontal cell. The differential signal with the cell bathed in a skate Ringer solution containing 2 mm of the pH buffer Hepes was approximately 85 μV. Moving the electrode 250 μm (Background) resulted in a differential response close to 0 μV. Moving the electrode back to its original position restored the 85 μV signal. The solution was then replaced by one containing 25 mm Hepes; recording at the same location resulted now in a much smaller signal. This signal was still larger than that obtained at a background location in the same 25 mm Hepes solution. Replacing the solution with one containing 2 mm Hepes brought the signal near its original amplitude. C, average results from eight cells; recordings were first made with the cell bathed in 2 mm Hepes and the electrode 1 μm from the cell membrane. Differential recordings were then obtained at a position at least 250 μm from the cell (Background). The solution around the cell was then changed to one containing 25 mm Hepes and the response recorded at the original position of the electrode near the cell.
Figure 1B shows the differential signals obtained from one horizontal cell recorded in this fashion. The lefthand portion of the trace (labelled ‘Cell’) was obtained with the cell bathed in a solution containing 2 mm pH buffer Hepes adjusted to pH 7.60. A differential signal of approximately 85 μV was detected by the electrode. Ion fluxes recorded by this method represent net changes, with the signal reflecting the balance of ion movement both in and out of the cell. The positive polarity indicates a higher concentration of H+ ions next to the cell membrane as compared to the point 50 μm distant. After several minutes, the electrode was moved in the vertical plane to a position far removed from the cell (‘Background’). At this position, the signal was close to 0 μV; that is, the free H+ concentration was essentially identical at the two positions. Returning the electrode to its initial position close to the cell restored the 85 μV differential signal. The solution surrounding the cell was then changed to one containing 25 mm Hepes also adjusted to pH 7.60. The higher concentration of this pH buffer was expected to reduce the extracellular concentration of free H+ near the cell and thus reduce the signal detected by the electrode. As can be seen, the size of the signal decreased to about 10 μV. This value was still greater than the differential signal obtained when the electrode was again moved to a background location. Bathing the cell once again in 2 mm Hepes largely restored the differential signal. Figure 1C shows averaged responses obtained from eight cells. A consistent differential signal of the order of 100 μV in size was detected, whereas practically no differential signal was observed at locations far removed from cells. A signal was also detectable in 25 mm Hepes that was significantly greater than that at background locations, but significantly less than that obtained in 2 mm Hepes.
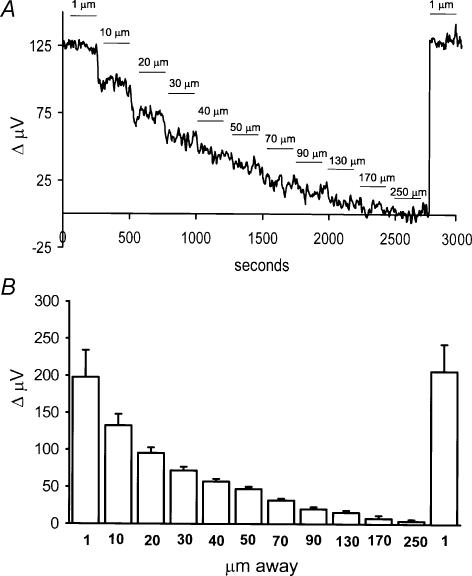
The H+ signal was strongly dependent upon the distance of the electrode from the cell. Figure 2A shows a recording from a single cell examined as a function of distance away from the cell. The numbers above the trace indicate the position of the electrode tip from the cell at its nearest point; the far position of the electrode was an additional 30 μm from the near position. When the electrode was more than about 170 μm away from the cell, the differential signal fell nearly to 0 μV. Moving the electrode back to the original position restored the size of the original signal. The average signal obtained from six cells is plotted in Fig. 2B. The righthand column reflects the return of the signal with the electrode positioned in its original location, about 1 μm from the cell.
Figure 2. Signal size as a function of distance of the electrode away from the cell membrane.
A, recording obtained from a single cell. The numbers above the trace indicate the distance of the electrode tip from the cell membrane with the electrode in the near-pole recording position. The solution contained 2 mm Hepes. B, average responses as a function of distance obtained from six cells.
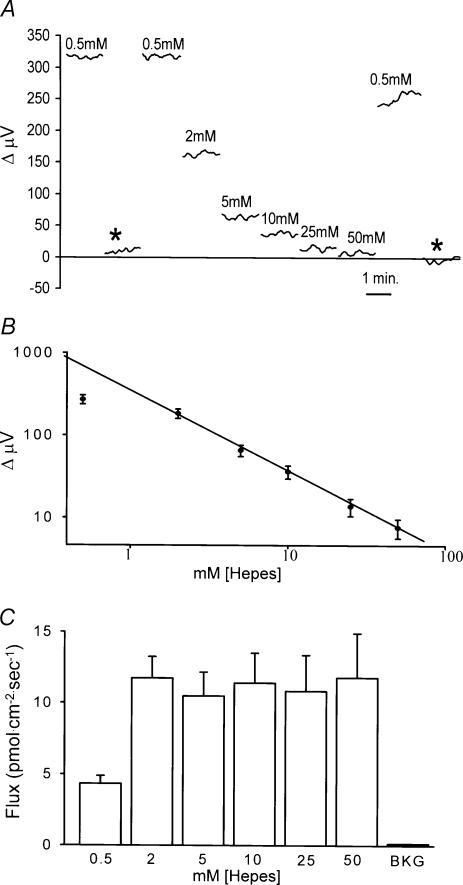
If the signal reflects H+ ions, then it should vary as a function of the concentration of pH buffer in the bath. Figure 3 shows that this was indeed the behaviour observed. Figure 3A shows recordings from a single cell bathed in a solution containing 0.5 mm Hepes adjusted to pH 7.60. The electrode was then moved to a background location far removed from the cell (asterisk), and very little signal was detected. Moving the electrode back to the initial location restored the signal. The solution was then carefully replaced by one containing 2 mm Hepes; the response detected by the electrode at the same position next to the cell was now significantly reduced. In the same fashion, responses were obtained with 5, 10, 25 and 50 mm Hepes, all adjusted to pH 7.60. As the Hepes concentration increased, the size of the response decreased. The solution was then replaced with 0.5 mm Hepes, and the response was largely restored; the slight reduction in signal size compared to the initial condition may reflect incomplete removal of the 50 mm Hepes solution. Finally, moving the electrode to a position far removed from the cell (second asterisk) again resulted in a differential reading close to 0 μV.
Figure 3. Dependence of signal size as a function of the concentration of the pH buffer Hepes.
A, recordings from a single horizontal cell. Recordings were initially obtained with the cell bathed in 0.5 mm Hepes and the electrode tip 1 μm from the cell membrane. At the asterisk the electrode was moved 250 μm from the cell and a background reading obtained. Moving the electrode back close to the cell brought the signal to its original level. The solution in the dish was then replaced with solution containing different concentrations of Hepes while keeping the electrode in its original position. Breaks in the traces represent times when the solution was being replaced, which typically took 30 s to 1 min to accomplish. B, log of the electrode response plotted as a function of the log of the concentration of Hepes. Average responses obtained from eight cells using the same method of recording as in A. C, conversion of electrode response to H+ flux taking into account absorbtion of H+ by the pH buffer in the bath.
The dependence of the electrode response on the concentration of extracellular Hepes is shown for eight cells in Fig. 3B. The log of the response of the H+-selective electrode was found to follow linearly the log of the Hepes concentration over the range 2–50 mm Hepes, a finding expected from the physical binding characteristics of H+ ions to a pH buffer. Reducing the Hepes concentration further to 0.5 mm also resulted in an increase in the size of the response, but not as much as anticipated based on the responses observed with other Hepes concentrations. The reason for the departure of the point obtained with 0.5 mm Hepes is not yet clear, and because of this deviation, we conducted the remainder of our experiments with cells bathed in solutions containing 2 mm Hepes.
As described in detail in the Methods, the voltage values obtained using our H+-selective electrodes can be converted into H+ flux values, which are graphically depicted in Fig. 3C for the various Hepes concentrations examined. H+ flux refers to the concentration of free H+ ions per unit area and time that move across a given distance. The value for the flux of an ion depends upon the size of the detected signal, the distance over which the electrode moves, the diffusion constant for the ion, and the concentration of free ion in the bulk solution. In addition, for H+ ions the calculated flux will depend critically on the concentration of buffer in the extracellular solution and its rate of diffusion. This is because not all of the hydrogen ions released by the cell will remain in the free ionized state that can be detected by our H+ sensor. Rather, a significant fraction of H+ ions will bind to free Hepes molecules and thus will not be sensed by the H+-selective electrode. When voltage values are converted into H+ flux values, we find, as shown in Fig. 3C, that H+ flux from individual horizontal cells does not vary as a function of the Hepes concentration over the range 2 to 50 mm. Hence, the decrease in signal size observed as Hepes is increased from 2 to 50 mm can be accounted for solely on the basis of the increased pH-buffering capacity of the external solutions. This implies that the cellular mechanism responsible for the standing H+ gradient acts as a constant source of hydrogen ions near the cell membrane over this range of Hepes concentrations.
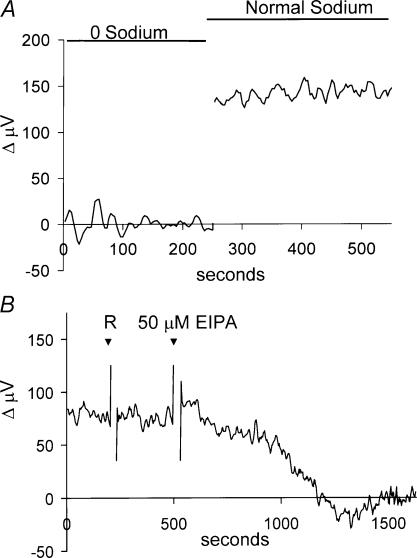
The standing H+ flux was critically dependent upon the presence of extracellular sodium. Figure 4A shows a recording from a single horizontal cell bathed initially in a solution in which all the extracellular sodium had been replaced by NMDG. During this time (indicated by the bar above the trace) the differential voltage trace was close to 0 μV. Replacing the 0 mm Na+ with a solution containing the normal 270 mm Na+ resulted in a restoration of the standing flux signal. In recordings from seven cells, the average signal was 9 ± 4 μV in 0 mm Na+ solution and 127 ± 21 μV in a solution containing the normal 270 mm Na+. Control experiments demonstrated that H+-selective electrodes retained their sensitivity to changes in pH in 0 mm Na+ solutions.
Figure 4. Effects of extracellular sodium and the Na+–H+ exchange blocker EIPA on H+ flux.
A, removing extracellular sodium abolished the standing H+ flux. The trace shows a recording from one cell first in a solution in which all sodium had been replaced by NMDG, then in normal extracellular solution containing 270 mm sodium. Full exchange of the solution was accomplished over 1 min. B, the Na+–H+ exchange inhibitor EIPA abolished the standing H+ flux. Initial application of 1 ml of skate Ringer solution (R) was without effect (the rapid ‘spike-like’ changes are artifacts of solution application). Subsequent application of 1 ml solution containing EIPA (final concentration 50 μm) eliminated the differentially recorded signal.
The dependence of the standing flux on extracellular sodium suggested that the signal might result from Na+–H+ exchange. We therefore examined the effects of 5-(N-ethyl-N-isopropyl) amiloride (EIPA), which blocks Na+–H+ exchange (Nguyen et al. 2000). Figure 4B shows a recording from one cell initially bathed in normal extracellular solution. The addition of 1 ml of the normal extracellular solution to the dish (marked ‘R’) did not alter the response (the rapid ‘spike-like’ changes are artifacts of solution application). A second application of 1 ml solution containing EIPA, resulting in a concentration of 50 μm, significantly reduced the standing H+ flux. EIPA reduced the differential signal by an average of 84.5% (n = 7), supporting the hypothesis that Na+–H+ ion exchanger activity contributes to the standing proton flux. In control experiments, the response of H+-selective electrodes to calibration solutions of pH 6.00, 7.00 and 8.00 was unaltered by addition of EIPA.
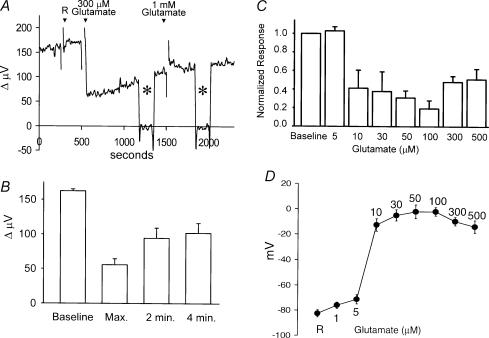
We next examined the effects of glutamate on H+ flux. Glutamate is believed to be the primary chemical means by which photoreceptors transmit messages to their postsynaptic targets, namely bipolar cells and horizontal cells (Copenhagen & Jahr, 1989; Ayoub & Dorst, 1998). Figure 5A shows the response of one horizontal cell to the application of glutamate. At 250 s after initiating the recording, 1 ml normal extracellular solution was added (‘R’ in the figure), and no change in signal was noted. At 500 s, 1 ml solution containing glutamate (final concentration, 300 μm) was applied. A decrease in the size of the signal was observed that, with time, slowly increased even though glutamate was still present. The electrode was moved to a background position, as shown by the asterisk, and no H+ gradient was detected at this location. Returning the electrode close to the cell restored the differential signal. At 1500 s, 1 ml solution containing additional glutamate was added (final concentration 1 mm); this resulted in little change in the response. The electrode was moved again to a background location (second asterisk) and again, the differential response was found to be close to zero at this control location. Finally, moving the electrode back to the original position next to the cell restored the measured signal. Figure 5B shows the averaged results to 300 μm glutamate from eight cells. The standing signal from this batch of cells was 162 ± 3 μV; addition of glutamate resulted in an initial decline in the signal from the H+ probe to 56 ± 9 μV (35% of the original response). After 2 min, the response had slowly increased to 94 ± 14 μV (58% of the original standing flux) despite the presence of glutamate in the bath; after 4 min of recording, the response was 102 ± 14 μV.
Figure 5. Glutamate-induced alterations in H+ flux.
A, responses obtained from a single horizontal cell. Application of 1 ml Ringer solution (R) did not alter measured H+ flux. A subsequent 1 ml application of the same solution containing glutamate (final concentration, 300 μm) produced a sharp decrease in H+ flux, which partially recovered with time. The electrode was moved, as indicated by the asterisks, to a background position 250 μm away from the cell. Addition of 1 mm glutamate to the bath had no additional effect. B, average responses to 300 μm glutamate obtained from eight cells showing the maximum alteration just after the application of glutamate and the response obtained 2 and 4 min later. C, dose-dependence of glutamate-induced alterations in measured H+ flux. Each bar is indicative of the response of six cells. Each cell was exposed to only one glutamate concentration. Values have been normalized to the responses obtained just prior to the application of glutamate. D, voltage changes induced by glutamate recorded in six separate horizontal cells obtained using sharp microelectrodes.
Figure 5C shows the dose dependence of the glutamate effect on H+ flux. In these experiments, one dose of glutamate was applied per cell, and the maximum decrease in H+ signal determined. Concentrations of glutamate of 10 μm and higher resulted in a significant decrease in H+ flux. This drop in H+ flux was quite sharp, with 10 μm glutamate producing a near maximal response. Figure 5D shows the voltage change induced in seven separate cells recorded using sharp intracellular microelectrodes; a steep change in voltage was noted when glutamate concentrations reached levels of 10 μm and higher. Thus, the change in H+ flux mirrored the change in voltage induced by glutamate. It is interesting to compare this dose–response relation with that of glutamate-induced electrical currents obtained from whole-cell voltage-clamped cells (Kreitzer et al. 2003). In the latter experiments, the whole-cell data were well fit by a Hill equation having a half-maximal concentration of 35 μm and a Hill coefficient of 3.2, indicating a very steep dose–response relationship.
Control experiments demonstrated that changes in H+ flux were not artifacts resulting from simple addition of glutamate to the bath. Self-referencing H+-selective microelectrodes were positioned several micrometres from the opening of a ‘H+ source’ pipette, which acted to continually leak H+ into the bath. In seven such trials, the measured proton flux was 82 ± 13 μV before and 78 ± 11 μV after the addition of 300 μm glutamate, indicating that glutamate addition by itself did not significantly alter measured H+ flux measured from an artificial H+ source.
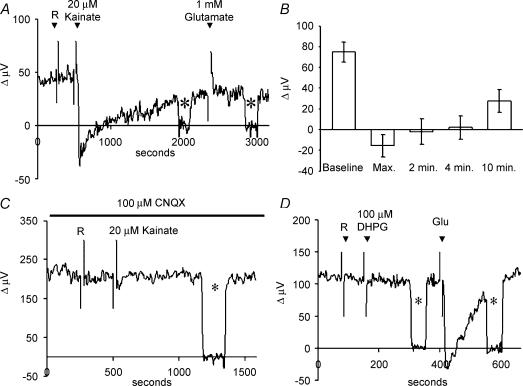
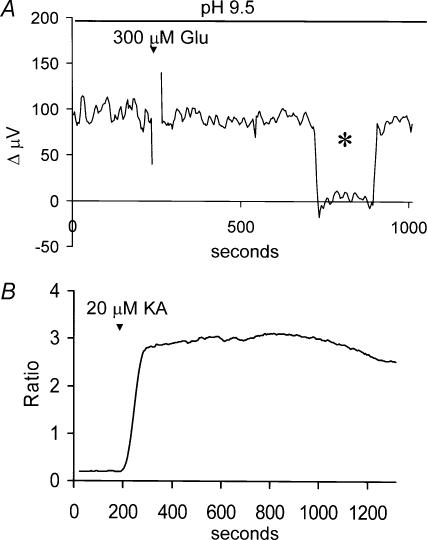
Figure 6 shows that the ionotropic glutamate receptor analogue kainate also altered H+-flux. Kainate activates AMPA/kainate glutamate receptors without causing the rapid and large desensitization typically induced by glutamate, and produces large inward currents in voltage-clamped skate horizontal cells (Kreitzer et al. 2003). Typical responses from a single cell are illustrated in Fig. 6A. The standing signal was not altered by 1 ml normal extracellular solution (R), but was substantially altered when kainate, at a final concentration of 20 μm, was added. Several aspects of the response to kainate are noteworthy. First, the peak response is seen to go below 0 μV. This indicates that the solution adjacent to the cell membrane is now more alkaline than the surrounding bath medium. Secondly, the H+ flux shows partial recovery, despite the continued presence of kainate. Third, the additional application of 1 mm glutamate produced no further change in H+ flux. Figure 6B shows averaged results from eight cells, confirming a maximal alteration of the response to a value below 0 μV, and the recovery of the H+ flux to about 50% of initial baseline level after about 10 min. Control experiments using a H+ source pipette confirmed that simple addition of kainate by itself did not alter the signal detected by the H+-selective electrodes.
Figure 6. Alterations in H+ flux induced by the ionotropic glutamate analogue kainate.
A, responses from a single cell showing the alteration in H+ flux that occurred upon the addition of 1 ml of Ringer solution alone (R), then with solution containing kainate (final concentration, 20 μm), and upon further addition of glutamate to a final concentration of 1 mm. Asterisks show recordings obtained with the electrode moved to a background location 250 μm away from the cell. B, average responses to kainate obtained from eight cells. C, responses from a single horizontal cell to 1 ml additions of normal saline solution and kainate (final concentration, 20 μm) in the presence of 100 μm CNQX. Asterisk again indicates recording obtained 250 μm from the cell. D, metabotropic glutamate receptor analogues did not alter H+ flux measured from horizontal cells. Responses obtained from a single cell to 1 ml normal extracellular solution and 1 ml solutions containing DHPG (final concentration, 100 μm) and 1 mm glutamate. Neither saline solution alone nor DHPG altered H+ flux, while glutamate produced a significant alteration in the measured signal.
Further evidence supporting a role for ionotropic glutamate receptors came from experiments examining the effects of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), an antagonist of ionotropic AMPA/kainate receptors (Yang & Wu, 1989). CNQX (100 μm) completely abolished the changes in H+ flux induced by 20 μm kainate (Fig. 6C); average H+-electrode responses from seven cells were 127 ± 20 μV in solution containing 100 μm CNQX, and 123 ± 21 μV after application of 100 μm CNQX applied with 20 μm kainate. CNQX also abolished alterations in H+ signals induced by 100 μm glutamate: H+ recordings from seven cells averaged 107 ± 20 μV in solutions containing 100 μm CNQX and 104 ± 19 μV after addition of 100 μm glutamate with 100 μm CNQX. Control experiments showed that the same cells responded to glutamate with the typical change in H+ flux when CNQX was not present.
Metabotropic glutamate receptor analogues were without effect on H+ measurements from horizontal cells. Figure 6D shows the lack of response obtained from one cell upon addition of 100 μm (S)-3,5-dihydroxyphenylglycine (DHPG), which is a group 1 metabotropic glutamate receptor agonist (Caramelo et al. 1999), despite the ability of glutamate to induce a significant alteration in proton flux in the same cell. In seven cells tested, H+-electrode measurements were 130 ± 27 μV before and 129 ± 28 μV after addition of DHPG. l-(+)-2-Amino-4-phosphonobutyric acid (l-AP4), which is a group 3 metabotropic glutamate receptor agonist (Caramelo et al. 1999), and ammonium pyrrolidinedithiocarbamate (APDC), a group 2 agonist (Schoepp et al. 1996), were similarly without effect, with responses from seven cells being 97% and 96% of control values, respectively. In all cases, control experiments conducted on the same cells showed that they were still responsive to applications of 1 mm glutamate.
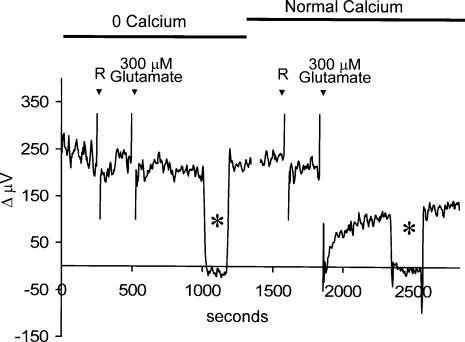
Like the glutamate receptors present in horizontal cells of a wide variety of other species, the ionotropic glutamate receptors of skate horizontal cells are permeable to calcium: glutamate, kainate and AMPA induce an increase in calcium when cells are voltage clamped at −70 mV and when the calcium channel blocker nifedipine is present (Kreitzer et al. 2003). In the present experiments, glutamate will also depolarize cells, which will in turn promote the opening of voltage-gated calcium channels (Malchow et al. 1990), leading to a further increase in intracellular calcium. The increase in calcium through glutamate- and voltage-gated calcium channels will also promote calcium-induced calcium release, further elevating intracellular calcium levels (Haugh-Scheidt et al. 1995). One compensatory mechanism used by many cells to regulate intracellular calcium is a plasmalemma Ca2+–H+-ATPase. This pump exchanges hydrogen ions from the extracellular fluid for calcium ions extruded from the interior of the cell (Schwiening et al. 1993; Salvador et al. 1998). This mechanism could account for the alkalinization we have observed adjacent to the cell membrane upon application of glutamate. To test this hypothesis, we bathed cells in a solution in which the normal 4 mm calcium was replaced by 4 mm magnesium. Figure 7 shows recordings obtained from a single horizontal cell under this condition. A significant standing H+ flux was detected from the cell even in the absence of extracellular calcium. However, application of 300 μm glutamate was now completely without effect on H+ flux. The 0 mm calcium solution was then replaced with a solution containing 4 mm calcium. While the application of 1 ml more of this normal extracellular solution was without effect, 300 μm glutamate now induced the typical alteration in H+ flux. In eight cells tested, 300 μm glutamate was unable to alter H+ flux in cells bathed in 0 mm calcium (98 ± 5% of control levels), but, in the same cells, induced a 67% decrease in H+ flux when 4 mm calcium was present.
Figure 7. Alteration of H+ flux by glutamate requires the presence of extracellular calcium.
Recording from a single cell bathed initially in a solution in which the 4 mm calcium normally present had been replaced by 4 mm magnesium. The first ‘R’ denotes application of 1 ml skate Ringer solution containing nominally 0 mm calcium. The addition of 300 μm glutamate now produced no change in H+ flux from the cell. At about 1250 s, the recording was halted and the solution in the dish exchanged for one containing normal 4 mm calcium. At the second ‘R’, 1 ml of normal skate Ringer solution was added to the dish; this was followed by a 1 ml drop of glutamate (final concentration, 300 μm), which produced the typical change in H+ flux expected. Asterisks denote recordings from background locations 250 μm away from the cell.
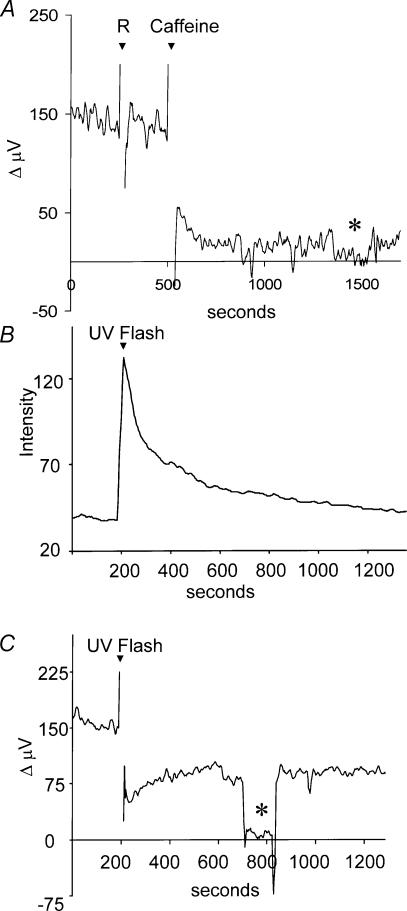
Caffeine causes the release of calcium from internal stores within horizontal cells, increasing intracellular calcium (Haugh-Scheidt et al. 1995). Figure 8A shows that 5 mm caffeine led to a significant reduction in the H+ signal from a single cell; the average H+ flux obtained from eight cells was reduced by 79 ± 8%. Control experiments performed using H+ source pipettes (no cell present) demonstrated that caffeine did not interfere with the ability of the H+ electrode to detect changes in H+ ion gradients.
Figure 8. Effects of caffeine and release of caged calcium on H+ flux.
A, effects of caffeine on H+ flux. Recording from a single cell in normal extracellular solution. This solution (1 ml) did not disturb the H+gradient, but 5 mm caffeine promoted a significant change in the measured signal. B, increase in fluorescence of the calcium indicator dye Oregon Green in a cell containing the caged calcium compound NP-EGTA. At 200 s into the recording, the cell was stimulated with a bright UV light stimulus for 100 ms. Y axis is in arbitrary units. C, self-referencing H+ recording made simultaneously from the same cell. The same ultraviolet flash resulted in a decrease in H+ flux. The asterisk denotes the differential response obtained when the electrode was placed at a background location 250 μm away from the cell membrane.
The effects of caffeine on extracellular H+ flux were relatively long lasting, while changes in intracellular calcium induced by caffeine are typically transient (Haugh-Scheidt et al. 1995). Caffeine might well have additional effects unrelated to its ability to increase calcium, which might also result in alteration of H+ flux. To avoid these potential complications, we also examined changes in H+ ion flux upon liberation of caged calcium within the cell. Cells were loaded with NP-EGTA, and intracellular calcium release effected by shining UV light onto the cell; increases in intracellular calcium were monitored in the same cells using the calcium indicator dye Oregon Green. Figure 8B shows the increase in Oregon Green fluorescence that was induced in one cell following a 100-ms exposure to UV light. Fluorescence rapidly increased, representing a rise in intracellular calcium that then declined with time. Figure 8C shows the extracellular H+ response recorded simultaneously from the same cell. At the time of UV stimulation and release of caged calcium, a marked decrease in H+ flux was observed. In four cells tested, the UV flash liberating intracellular calcium, led to an average peak decrease in proton flux of 53 ± 9%; after 300 s, the H+ flux had returned to 93 ± 18% of pre-stimulation values. In control experiments using cells filled with Oregon Green and NP-EGTA, but not pre-loaded with calcium, the UV flash resulted in a peak reduction of H+ flux of only 8 ± 3% (n = 7). Cells loaded with Oregon Green but not with NP-EGTA showed no increases in intracellular calcium or changes in extracellular H+ flux upon stimulation with UV light.
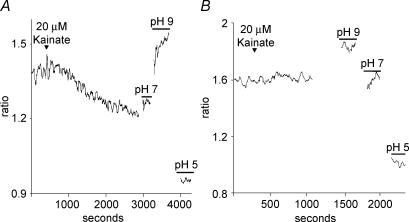
Bathing cells in highly alkaline solutions inhibits plasma membrane Ca2+-H+-ATPase activity (Benham et al. 1992; Schwiening et al. 1993). If the effects of glutamate depend upon the functioning of this pump, then the ability of glutamate to alter H+ flux should be abolished when cells are bathed in such a solution. Figure 9 shows that this was indeed the case. Figure 9A shows a recording made from a cell placed in a solution adjusted to pH 9.50. While a standing H+ flux could be detected, the application of 300 μm glutamate was without effect. In six cells tested in this alkaline solution, the H+ signal was 95 ± 4 μV before addition of glutamate and 97 ± 7 μV after addition of 300 μm glutamate. Control experiments demonstrated that H+-selective electrodes continued to respond with Nernstian changes in voltage to alterations in pH in these alkaline conditions. In addition, activation of glutamate receptors still resulted in increases in intracellular calcium when cells were maintained in a solution of pH 9.50. Figure 9B shows the fluorescent signal from a cell filled with Fura-2 and bathed in a solution at pH 9.50. Kainate produced a marked increase in the ratio of 334/380 fluorescence, indicating that activation of glutamate receptors still induced increases in intracellular calcium in horizontal cells bathed in this alkaline solution.
Figure 9. Alkalinization of the external solution abolished the ability of glutamate to alter H+ flux from horizontal cells.
A, recording from one cell bathed in a solution adjusted to have an extracellular pH of 9.5. Application of 300 μm glutamate now failed to alter H+ flux. B, measurement of fura-2 fluorescence to examine calcium increases in horizontal cells when bathed in Ringer solution at pH 9.5. Addition of kainate at 20 μm produced a sizable increase in the ratio of 334 nm/380 nm fluorescence, indicating a significant increase in intracellular calcium.
The differential signal recorded by the H+ sensors depends not only on the value for H+ flux, but also on the background free H+ concentration. That is, a given flux of H+ will produce a smaller voltage signal across the H+-selective microelectrode when the background level of H+ ions is high, as it is when the extracellular pH is 7.60 as compared to 9.50. Thus, although the standing differential voltages recorded at control pH (7.60) and at a pH of 9.50 were of similar magnitude, they actually represent a decrease of H+ flux in the more alkaline condition of approximately two orders of magnitude. However this phenomenon makes the lack of effect of glutamate on H+ flux in alkaline Ringer solution all the more striking, as our sensors are also two orders of magnitude more sensitive to changes in H+ flux in this solution.
Activation of the plasmalemma Ca2+–H+-ATPase by glutamate- and kainate-mediated increases in intracellular calcium should acidify the cell interior (cf. Schwiening & Willoughby, 2002). Moreover, this acidification should be abolished when the cells are bathed in a solution containing nominally 0 mm calcium. Figure 10 shows data obtained using the intracellular pH indicator dye BCECF and demonstrates that this was indeed the case. Figure 10A shows the ratio of fluorescence obtained when cells were stimulated alternately with 488 nm and 460 nm light. Upon the application of kainate (20 μm), a steady drop in the ratio of fluorescence was observed, indicating acidification of the cell interior. Kainate was unable to alter intracellular pH from a second horizontal cell bathed in a solution in which magnesium had replaced all of the normally added calcium (Fig. 10B). In seven cells, kainate induced an acidification of the cell by 0.5 ± 0.1 pH units in normal extracellular solution, but failed to produce a significant alteration in intracellular pH when the solution lacked extracellular calcium.
Figure 10. Kainate acidifies the intracellular milieu of skate horizontal cells. Intracellular pH levels were monitored using the pH indicator dye BCECF.
A, kainate (20 μm) produced a decrease in the ratio of fluorescence induced by stimulation with 488 and 460 nm light when cells were bathed in normal extracellular solution containing 4 mm calcium. Responses labelled pH 7, 9 and 5 are calibrations performed on the same cell bathed in nigericin. B, recording from a different horizontal cell showing that kainate does not alter intracellular pH when the solution lacks extracellular calcium.
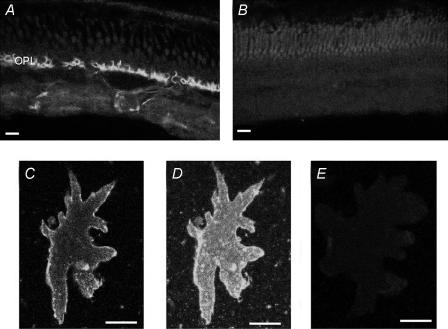
Finally, we attempted to detect the presence of the plasma membrane Ca2+-ATPase using immunocytochemical techniques. We used a commercial antibody, 5F10, originally developed against the Ca2+-ATPase present in mouse. Figure 11A shows fluorescence observed in a transverse section through a skate retina using this antibody. Intense staining was detected at the outer plexiform (synaptic) layer (OPL) with lighter but still obvious staining detectable within the inner plexiform layer. Figure 11B shows the fluorescence signal present in a control section in which the primary antibody was omitted; light fluorescence at the level of the outer segments of photoreceptors was all that could be detected. Figure 11C shows a confocal optical slice through an isolated external horizontal cell; labelling is readily apparent along the plasma membrane of the cell, with very little fluorescence detected within the cell interior. A stack of optical sections of the same cell is shown in Fig. 11D, and shows that labelling was also evident on the flat surface of the cell and not restricted to the lateral edges. Figure 11E shows lack of labelling in a different isolated horizontal cell with the primary antibody omitted.
Figure 11. Immunocytochemical localization of the plasmalemma Ca2+–H+-ATPase protein in isolated retina and isolated horizontal cells.
A, staining pattern of fluorescence in a transverse section through the skate retina. Intense staining is distinctly visible at the level of the outer synaptic layer; lighter staining is evident within the inner synaptic layer. B, control section showing florescence seen in the absence of the primary antibody. C, confocal optical section through a single external horizontal cell stained for the plasmalemma Ca2+–H+-ATPase. Note the bright staining observed along the edges of the cell membrane. D, optical stack showing complete staining pattern for the same horizontal cell as shown in C. Note the intense staining on the cell membrane surface. E, control photomicrograph of a horizontal cell in which the primary antibody was omitted during the staining procedure. Scale bars are 10 μm for A and B, 35 μm for C–E.
Discussion
This work demonstrates the capability of self-referencing H+-selective microelectrodes to detect H+ fluxes from single isolated retinal horizontal cells. Our results reveal a standing H+ flux from quiescent cells, and implicate a Na+–H+ exchanger that contributes to this flux. Our work also demonstrates modulation of H+ flux by glutamate, which is believed to be the neurotransmitter released by photoreceptors. The effects of glutamate are calcium-dependent and probably reflect the activation of the plasmalemma Ca2+–H+-ATPase that acts to lower intracellular calcium levels and reduce the concentration of free H+ ions adjacent to the cell membrane.
Several factors support the contention that the measured signal is a reflection of the H+ ion gradient. First, the resin used in these experiments has a high degree of selectivity for H+ as compared to other ions; indeed, it is reported to be more than 109 times more sensitive to H+ ions than to sodium or potassium (Fluka, 1991). In addition, the size of the differential signal varied as a function of the concentration of the pH buffer Hepes. Over the range of 2–50 mm Hepes, the log of the electrical signal varied as a linear function of the log of the Hepes concentration. This is expected, as higher extracellular concentrations of Hepes would tend to act to limit free H+ ions near the plasma membrane of the cells. It is unlikely that extracellular voltage fields associated with cellular currents contribute to the observed signals, as such fields are usually in the nanovolt range and below the sensitivity of ion-selective self-referencing probes (Kuhtreiber & Jaffe, 1990; Smith et al. 1999). Electrical potentials arising from local boundary conditions associated with membrane surface charges (McLaughlin et al. 1971, 1981) are also unlikely to be the source of the signals we have detected. Such fields typically drop away with the Debye length and do not extend into the medium by more than tens of angstroms (cf. Cevc, 1990; Hille, 1992). Our sensors were located at least 1 μm away from the cell surface and, as Fig. 2 indicates, the measured gradient extended many tens of micrometres away from the cell.
The standing H+ flux in unstimulated horizontal cells appears to be mediated largely by activity of Na+–H+ exchange. The H+ flux is greatly reduced when extracellular sodium is replaced by NMDG, and the flux is also significantly diminished by the Na+–H+ exchange blocker EIPA. The present experiments were conducted without bicarbonate, to ensure that the buffering power of the extracellular solution remained relatively constant throughout the duration of the experiments. This also makes it likely that bicarbonate-related mechanisms that might normally be employed by skate retinal neurones to regulate pH are unlikely to have been detected in the current experiments. H+ fluxes could also potentially derive from carbon dioxide released by the cell, with emitted CO2 converted ultimately to bicarbonate ions and H+ ions. However, if this were the case, the H+ flux would not have been abolished by removal of sodium or addition of EIPA.
Glutamate-induced alterations in H+ ion flux were mimicked by the ionotropic glutamate analogues kainate and AMPA, implicating activation of ionotropic receptors in modulation of H+ flux from skate horizontal cells. Kainate also acidified the interior of the cell when measurements of intracellular pH were made using the fluorescent pH indicator dye BCECF. In addition, the effects of glutamate and kainate on H+ ion flux were abolished by addition of the ionotropic glutamate receptor antagonist CNQX, and were completely unaltered upon the addition of metabotropic glutamate receptor agonists. These data, taken with past experiments showing that NMDA receptors are not present on skate horizontal cells (Kreitzer et al. 2003), lead us to conclude that the change in H+ ion flux is mediated by the activation of AMPA/kainate-type ionotropic glutamate receptors.
Glutamate-induced modulation of H+ flux was dependent on the presence of extracellular calcium. The photolytic release of caged calcium also resulted in a decrease in H+ flux. Taken together, these data suggest that calcium is a key regulator of the observed changes in H+ flux, and suggest that the plasma membrane Ca2+-H+- ATPase (PMCA), which shuttles Ca2+ ions out of the cell in exchange for extracellular H+ ions (Hao et al. 1994; Salvador et al. 1998), may be involved in this phenomenon. The ratio of calcium:hydrogen ion exchange has been reported to be 1:2, 1:1, or to vary between the two depending on experimental conditions (cf. Waldeck et al. 1998). Differences in the precise stoichiometry of the pump will certainly have significant impact on its function, altering its potential electrogenicity and the rates and maximal amounts of calcium extruded into, and H+ uptake from, the extracellular space. Nonetheless, for all stoichiometries thus far reported, activation of the PMCA pump will result in a flux of H+ ions into the cell and a net decrease in the concentration of free H+ ions along the extracellular face of the cell.
Glutamate binding to AMPA/kainate-type receptors is likely to result in an increase in intracellular calcium through three mechanisms. (1) The AMPA/kainate receptors present on skate horizontal cells are themselves permeable to calcium (Kreitzer et al. 2003), permitting a direct influx of calcium into the cell through these receptors. (2) Glutamate will also depolarize the cells, leading to activation of l-type voltage-gated calcium channels and consequent influx of calcium through this pathway. (3) Finally, the increase in calcium through the above two pathways will also result in calcium-induced calcium release from intracellular stores (Haugh-Scheidt et al. 1995). Our data suggest that the increase in intracellular calcium brought about by glutamate activates PMCA pumps which work towards restoring the resting intracellular calcium concentration. The observation that the glutamate analogue kainate transiently alkalinizes the extracellular solution near the cell membrane strongly suggests the activation of a mechanism that transports H+ ions from the extracellular milieu into the cell. The alkalinization induced by kainate cannot be accounted for by simple reduction in activity of Na+–H+ exchange. Even complete shut down of this exchanger would reduce H+ flux at most to zero, not to the transiently negative value we have found. Activation of PMCA activity is also suggested by the intracellular acidification induced by kainate, and the fact that the kainate-induced changes in both extracellular and intracellular levels of pH require the presence of extracellular calcium. Moreover, the changes in H+ flux induced by glutamate and its analogues are abolished when cells are bathed in a solution adjusted to a pH of 9.5, which has been shown to shut down the activity of the PMCA in other cells (Benham et al. 1992; Schwiening et al. 1993). Despite the inability of glutamate and its analogues to alter H+ ion flux in this alkaline condition, these compounds still evoked increases in intracellular calcium, indicating that the cells were still responsive to neurotransmitter receptor activation. The extent to which calcium influx through glutamate-gated channels, calcium influx through voltage-gated channels, and release from calcium stores contribute to the total amount of intracellular calcium and subsequent decreases in extracellular H+ levels via activation of the plasmalemma Ca2+–H+-ATPase is not yet clear. Furthermore, the three pathways involved in promoting increases in intracellular calcium are likely to be highly interdependent. Recent findings have suggested a close spatial relationship between the AMPA/kainate receptors and at least a portion of the calcium available for release from intracellular stores (Kreitzer et al. 2003).
The addition of 5 mm caffeine, known to cause the release of calcium from intracellular stores in skate horizontal cells (Haugh-Scheidt et al. 1995), also led to a marked alteration in H+ flux, lowering the free H+ concentration around the external face of the cell. The effects of caffeine on H+ flux were typically long lasting, while changes in intracellular calcium induced by caffeine are relatively transient. It is possible that caffeine might exert additional effects on H+ flux unrelated to its ability to increase intracellular calcium levels. These effects could potentially be manifested at multiple sites, including both the Ca2+–H+-ATPase and the Na+–H+ exchanger.
The dose–response relationship for the effects of glutamate on H+ flux has a particularly sharp onset and an almost ‘all-or-none’ characteristic. The dose–response relationship for glutamate-induced changes in voltage from these cells followed a very similar pattern. Our H+ flux measurements were obtained from cells that were not voltage-clamped. Depolarization of the cells by glutamate will thus affect voltage-sensitive conductances, most notably turning on the l-type calcium conductance and decreasing a hyperpolarization-activated inward rectifier. Skate horizontal cells possess a depolarization-activated and highly transient ‘A’ current, but lack a large contribution from the delayed rectifying potassium current that is present in many neurones. The persistent depolarization induced by the continued presence of glutamate along with the activation of the l-type calcium conductance is thus likely to lock the cells in a depolarized state (cf. Lasater et al. 1984), and this is indeed the behaviour we have observed when examining voltage changes of the cells using sharp electrodes (Fig. 5D). In this state, a significant sharp and prolonged influx of calcium can be expected. The sharp onset of both the change in H+ flux and the large depolarization strongly implicate the voltage-gated calcium channels as a key source of calcium influx leading to activation of the Ca2+–H+-ATPase and consequent alteration in extracellular proton flux. Attempts to confirm this hypothesis by blocking voltage-gated calcium channels with nifedipine were stymied by findings in control experiments that 100 μm nifedipine interfered with the ability of our H+ sensors to detect changes in extracellular pH. Attempts to block calcium entry through voltage-gated calcium channels with extracellular cobalt also proved to be problematic. Although 4 mm cobalt had minimal influence on our H+ sensors, it interfered with the ability of glutamate to activate and open the glutamate-gated receptors as indicated by greatly reduced inward currents elicited by glutamate in voltage-clamped horizontal cells. Thus, at present, we believe that calcium influx through voltage-gated channels is a critical component of the response, but cannot rule out some contribution of calcium entry directly through the glutamate-gated channels themselves.
The initial rapid decrease in H+ flux induced by glutamate was typically followed by a gradual increase, eventually returning to about half the original baseline flux despite the continued presence of glutamate in the bath. We do not yet understand the mechanism of this time-dependent change in response, and a number of possibilities exist. The l-type calcium conductance of the horizontal cells activated by depolarization may inactivate slowly as a function of time, as has been seen for other l-type channels (Soldatov et al. 1997; Zuhlke et al. 2000). Another possibility stems from the transient nature of the rise in calcium that results from calcium-activated calcium release (Haugh-Scheidt et al. 1995). It also may be that the plasmalemma Ca2+–H+-ATPase pump itself may alter its activity as a function with time. Additional possibilities, such as activation of Na+–Ca2+ exchange, also exist.
The activation of ionotropic glutamate receptors will permit the flux not only of Ca2+ but also of Na+ into the cell. It is thus possible that enough Na+ could enter the cell to either reduce or reverse the Na+ gradient, and thus potentially alter activity of a Na+–H+ exchanger. Several of our findings argue against this hypothesis. First, the effects of glutamate on H+ flux are abolished when extracellular Ca2+ is removed from the extracellular bath. Under this condition, Na+ would still be expected to permeate readily into the cell, yet there is no change in H+ flux. Secondly, glutamate should also permit Na+ influx when the extracellular solution is made alkaline. Despite this, the effect of glutamate on H+ flux is abolished when cells are bathed in solution at pH 9.5, again consistent with the hypothesis that the plasmalemma Ca2+–H+-ATPase mediates the decrease in H+ flux upon the addition of glutamate.
Light stimulation causes transient alterations in extracellular pH surrounding cells in the intact retina (cf. Borgula et al. 1989; Oakley & Wen, 1989). These observations, along with the potent ability of protons to shut down voltage-gated calcium channels of photoreceptors (Barnes et al. 1993), has led to the hypothesis that H+ ions may act as the feedback neurotransmitter used by horizontal cells to create the surround portion of the centre–surround receptive field. In this hypothesis, H+ extruded by horizontal cells would act to decrease neurotransmitter release from photoreceptor synaptic terminals by closing voltage-gated calcium channels of the photoreceptors (cf. Kamermans & Spekreijse, 1999). Recent recordings from cone photoreceptors of the newt by Hirasawa & Kaneko (2003) lends further experimental support to this hypothesis: increases in extracellular pH buffers increased cone calcium currents and blocked alterations in cone calcium currents by surround illumination. However, our data argue strongly against this notion, at least for the retina of the skate. The release of an inhibitory substance from horizontal cells that feeds back onto photoreceptors to provide lateral inhibition should be greatest when the cells are depolarized by glutamate released by the photoreceptor terminals. However, we have found that glutamate decreases the concentration of free H+ ions adjacent to the extracellular face of horizontal cells. Thus, glutamate would probably lead to a weaker feedback inhibition by H+ ions on the calcium channels of photoreceptors, precisely the opposite of what would be expected if H+ ions were acting as the inhibitory feedback neurotransmitter onto photoreceptor synaptic terminals. In this context, three important considerations need to be noted. First, our conclusion assumes that Na+–H+ transporters and PMCA pumps are distributed at the intact synapse in the same fashion as in the isolated cells we have examined. A heterogeneous distribution of these two types of proton transporting molecules in horizontal cells in the intact retina could significantly alter their potential contribution to changes of extracellular pH at the synapse. Second is the fact that the skate possesses an all-rod retina (Szamier & Ripps, 1983). While there is considerable evidence for inhibitory feedback from horizontal cells to cone photoreceptors in several species (e.g. Baylor et al. 1971; Kamermans et al. 1989; Wu, 1992), there is little evidence for feedback from horizontal cells onto rod synaptic pedicles. It may well be that feedforward inhibition from horizontal cells directly onto the dendrites of bipolar cells is the primary means by which the surround portion of the centre–surround response of bipolar cells is established in rod pathways. Alterations in extracellular pH by horizontal cells might have a role in such feedforward inhibition; however, a given change in extracellular pH would then have to have opposite effects on the ON and OFF subclasses of bipolar cells in the retina. Finally, the present experiments were conducted with 2 mm of the pH buffer Hepes in the bath; no bicarbonate was added to the solutions. This experimental condition was chosen to permit reasonable control of the pH of the solution while still permitting changes in extracellular pH at the face of the horizontal cell membrane to be readily detected. In this condition, contributions from bicarbonate-dependent mechanisms are likely to be reduced or absent. Previous data (Haugh-Scheidt & Ripps, 1998) have suggested a role for bicarbonate-dependent mechanisms in the regulation of internal pH of skate horizontal cells, and it is thus possible that pH changes in the intact retina of the skate may differ from those reported on the isolated cells studied here.
A related question concerns the magnitude of the pH changes observed here and likely to occur within the synaptic cavity where photoreceptors, horizontal cells, and bipolar cells contact one another. The changes in extracellular pH we have observed are small; a 100 μV signal reflects a change of about 0.002 pH units. However, in our recording configuration, hydrogen ions can readily and rapidly diffuse away into the vast sink of extracellular fluid surrounding the cells. The situation in the intact physiological system is likely to be quite different. The invaginating synapse created by the synaptic terminals of photoreceptors tends to encapsulate the processes of horizontal cells and bipolar cells and has a very limited extracellular space (cf. Mariani, 1984; Hidaka et al. 1986). This restricted and small volume is an environment in which small changes in the amount of H+ ions could have a dramatic effect on extracellular pH, acting to magnify the effects exerted by pH regulatory mechanisms of horizontal cells. Indeed, recent evidence suggests that the small change in extracellular pH resulting from the fusion of photoreceptor vesicles with the plasma membrane is sufficient to induce a temporary inhibition of neurotransmitter release, due to blockade of photoreceptor calcium channels by H+ ions (DeVries, 2001). This latter observation leads us to speculate that glutamate-induced decreases in H+ flux from horizontal cells might aid in the reduction of the increased levels of acidity associated with synaptic release of glutamate from photoreceptors. This would promote additional release of glutamate by permitting the opening of photoreceptor calcium channels and also potentially relieve H+ ion block of postsynatpic glutamate receptors. In both cases, the effects of glutamate on postsynaptic elements would be augmented. Thus, the glutamate-induced alteration in H+ flux from horizontal cells has the potential to act as a mechanism to enhance the effects of glutamate within the outer plexiform layer of the retina.
Acknowledgments
We thank Mark Messerli for help with calculations to convert microvolt values to H+ flux values, Naomi Rosenkranz and Jill Bodily for preparation of primary cell cultures, Jill Bodily for conducting control experiments with nifedipine, Richard Sanger and Robert Lewis for expert computer and hardware assistance, Marek Mori for expert machining, Rudy Rottenfusser (Zeiss Corp) for loan of equipment, Shayan Sartipi and Zaya Tun for help with voltage recordings and Adrienne Boutwell for help with preparing figures. This work was supported by the National Science Foundation (009–1281 and DBI-9605155), National Eye Institute (EYO9411), National Center for Research Resources (P41 RR01395), National Institute for Deafness and Communication Disorders (DC04215 and DC03828), an unrestricted grant from Research to Prevent Blindness to the University of Illinois at Chicago Department Ophthalmology, a Sigma Xi GIAR Award, a UIC Provost Award and a Fellowship from the Grass Foundation.
References
- Ammann D, Oesch U, Buhrer T, Simon W. Design of ionophores for ion-selective microsensors. Can J Physiol Pharmacol. 1987;65:879–884. doi: 10.1139/y87-141. [DOI] [PubMed] [Google Scholar]
- Arif I, Newman IA, Keenylside N. Proton flux measurements from tissues in buffered solution. Plant Cell Environ. 1995;18:1319–1324. [Google Scholar]
- Ayoub GS, Dorst K. Imaging of glutamate release from the goldfish retinal slice. Vision Res. 1998;38:2909–2912. doi: 10.1016/s0042-6989(98)00103-5. 10.1016/S0042-6989(98)00103-5. [DOI] [PubMed] [Google Scholar]
- Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci U S A. 1993;90:10081–10085. [Google Scholar]
- Baylor DA, Fuortes MG, O'Bryan PM. Receptive fields of cones in the retina of the turtle. J Physiol. 1971;214:265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Evans ML, McBain CJ. Ca2+ efflux mechanisms following depolarization evoked calcium transients in cultured rat sensory neurones. J Physiol. 1992;455:567–583. doi: 10.1113/jphysiol.1992.sp019316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgula GA, Karwoski CJ, Steinberg RH. Light-evoked changes in extracellular pH in frog retina. Vision Res. 1989;29:1069–1077. doi: 10.1016/0042-6989(89)90054-0. [DOI] [PubMed] [Google Scholar]
- Caramelo OL, Santos PF, Carvalho AP, Duarte CB. Metabotropic glutamate receptors modulate [3H]acetylcholine release from cultured amacrine-like neurons. J Neurosci Res. 1999;58:505–514. doi: 10.1002/(sici)1097-4547(19991115)58:4<505::aid-jnr4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Cevc G. Membrane electrostatics. Biochim Biophys Acta. 1990;1031:311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:2909–2912. doi: 10.1152/physrev.00010.2003. 10.1016/S0042-6989(98)00103-5. [DOI] [PubMed] [Google Scholar]
- Coles JA, Marcaggi P, Vega C, Cotillon N. Effects of photoreceptor metabolism on interstitial and glial cell pH in bee retina: evidence of a role for NH4+ J Physiol. 1996;495:305–318. doi: 10.1113/jphysiol.1996.sp021595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen DR, Jahr CE. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989;341:536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Rose CR. pH regulation and proton signalling by glial cells. Prog Neurobiol. 1996;48:73–103. doi: 10.1016/0301-0082(95)00039-9. 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Demarest JR, Morgan JLM. Effect of pH buffers on proton secretion from gastric oxyntic cells measured with vibrating ion-selective microelectrodes. Biol Bull. 1995;189:219–220. doi: 10.1086/BBLv189n2p219. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. 10.1016/S0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC. A circadian clock regulates the pH of the fish retina. J Physiol. 2000;522:77–82. doi: 10.1111/j.1469-7793.2000.0077m.x. 10.1111/j.1469-7793.2000.0077m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC. Circadian clock regulation of pH in the rabbit retina. J Neurosci. 2001;21:2897–2902. doi: 10.1523/JNEUROSCI.21-08-02897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluka . Selectophore, Ionophores for Ion-selective Electrodes and Optrodes. Ronkonkoma NY: Fluka Chemie AG; 1991. [Google Scholar]
- Gatto C, Milanick MA. Inhibition of the red blood cell calcium pump by eosin and other florescein analogues. Am J Physiol. 1993;264:C1577–C1586. doi: 10.1152/ajpcell.1993.264.6.C1577. [DOI] [PubMed] [Google Scholar]
- Hao L, Rigaud JL, Inesi G. Ca2+/H+ countertransport and electrogenicity in proteoliposomes containing erythrocyte plasma membrane Ca-ATPase and exogenous lipids. J Biol Chem. 1994;269:14268–14275. [PubMed] [Google Scholar]
- Harsanyi K, Mangel SC. Modulation of cone to horizontal cell transmission by calcium and pH in the fish retina. Vis Neurosci. 1993;10:81–91. doi: 10.1017/s0952523800003242. [DOI] [PubMed] [Google Scholar]
- Haugh-Scheidt L, Malchow RP, Ripps H. GABA transport and calcium dynamics in horizontal cells from the skate retina. J Physiol. 1995;488:565–576. doi: 10.1113/jphysiol.1995.sp020990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh-Scheidt L, Ripps H. pH regulation in horizontal cells of the skate retina. Exp Eye Res. 1998;66:449–463. doi: 10.1006/exer.1997.0441. 10.1006/exer.1997.0441. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Christensen BN, Naka K. The synaptic ultrastructure in the outer plexiform layer of the catfish retina: a three-dimensional study with HVEM and conventional EM of Golgi-impregnated bipolar and horizontal cells. J Comp Neurol. 1986;247:181–199. doi: 10.1002/cne.902470205. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland MA: Sinauer Associates Inc.; 1992. Modifiers of gating. [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ currents. J Gen Physiol. 2003;122:657–671. doi: 10.1085/jgp.200308863. 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M, Spekreijse H. The feedback pathway from horizontal cells to cones. A mini review with a look ahead. Vision Res. 1999;39:2449–2468. doi: 10.1016/s0042-6989(99)00043-7. 10.1016/S0042-6989(99)00043-7. [DOI] [PubMed] [Google Scholar]
- Kamermans M, van Dijk BW, Spekreijse H, Zweypfenning RC. Lateral feedback from monophasic horizontal cells to cones in carp retina. I. Experiments. J GenPhysiol. 1989;93:681–694. doi: 10.1085/jgp.93.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J. Signal transmission at the photoreceptor synapse. Role of calcium ions and protons. Ann N Y Acad Sci. 1991;635:468–470. doi: 10.1111/j.1749-6632.1991.tb36529.x. [DOI] [PubMed] [Google Scholar]
- Kreitzer MA, Andersen KA, Malchow RP. Glutamate modulation of GABA transport in retinal horizontal cells of the skate. J Physiol. 2003;546:717–731. doi: 10.1113/jphysiol.2002.034421. 10.1113/jphysiol.2002.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhtreiber WM, Jaffe LF. Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J Cell Biol. 1990;110:1565–1573. doi: 10.1083/jcb.110.5.1565. 10.1083/jcb.110.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM, Dowling JE, Ripps H. Pharmacological properties of isolated horizontal and bipolar cells from the skate retina. J Neurosci. 1984;4:1966–1975. doi: 10.1523/JNEUROSCI.04-08-01966.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Mulrine N, Gresalfi T, Vaio G, McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981;77:445–473. doi: 10.1085/jgp.77.4.445. 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SG, Szabo G, Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971;58:667–687. doi: 10.1085/jgp.58.6.667. 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow RP, Qian HH, Ripps H, Dowling JE. Structural and functional properties of two types of horizontal cell in the skate retina. J Gen Physiol. 1990;95:177–198. doi: 10.1085/jgp.95.1.177. 10.1085/jgp.95.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani AP. The neuronal organization of the outer plexiform layer of the primate retina. Int Rev Cytol. 1984;86:285–320. doi: 10.1016/s0074-7696(08)60181-3. [DOI] [PubMed] [Google Scholar]
- Nguyen HV, Shull GE, Melvin JE. Muscarinic receptor-induced acidification in sublingual mucous acinar cells: loss of pH recovery in Na+–H+ exchanger-1 deficient mice. J Physiol. 2000;523:139–146. doi: 10.1111/j.1469-7793.2000.t01-2-00139.x. 10.1111/j.1469-7793.2000.t01-2-00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B, II, Wen R. Extracellular pH in the isolated retina of the toad in darkness and during illumination. J Physiol. 1989;419:353–378. doi: 10.1113/jphysiol.1989.sp017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador JM, Inesi G, Rigaud JL, Mata AM. Ca2+ transport by reconstituted synaptosomal ATPase is associated with H+ countertransport and net charge displacement. J Biol Chem. 1998;273:18230–18234. doi: 10.1074/jbc.273.29.18230. 10.1074/jbc.273.29.18230. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Johnson BG, Monn JA. (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid-induced increases in cyclic AMP formation in the neonatal rat hippocampus are mediated by a synergistic interaction between phosphoinositide- and inhibitory cyclic AMP-coupled mGluRs. J Neurochem. 1996;66:1981–1985. doi: 10.1046/j.1471-4159.1996.66051981.x. [DOI] [PubMed] [Google Scholar]
- Schwiening C, Kennedy HJ, Thomas RC. Calcium-hydrogen exchange by the plasma membrane Ca-ATPase of voltage-clamped snail neurons. Proc R Soc Lond B. 1993;253:285–289. doi: 10.1098/rspb.1993.0115. [DOI] [PubMed] [Google Scholar]
- Schwiening CJ, Willoughby D. Depolarization-induced pH microdomains and their relationship to calcium transients in isolated snail neurones. J Physiol. 2002;538:371–382. doi: 10.1113/jphysiol.2001.013055. 10.1113/jphysiol.2001.013055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJS, Hammar K, Porterfield DM, Sanger RH, Trimarchi JR. Self-referencing, non-invasive, ion selective electrode for single cell detection of trans-plasma membrane calcium flux. Microsc Res Tech. 1999;46:398–417. doi: 10.1002/(SICI)1097-0029(19990915)46:6<398::AID-JEMT8>3.0.CO;2-H. 10.1002/(SICI)1097-0029(19990915)46:6<398::AID-JEMT8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Smith PJS, Sanger RH, Jaffe LF. The vibrating Ca2+ electrode: a new technique for detecting plasma membrane regions of Ca2+ influx and efflux. Methods Cell Biol. 1994;40:115–134. doi: 10.1016/s0091-679x(08)61112-7. [DOI] [PubMed] [Google Scholar]
- Smith PJS, Trimarchi J. Noninvasive measurement of hydrogen and potassium ion flux from single cells and epithelial structures. Am J Physiol Cell. 2001;280:C1–C11. doi: 10.1152/ajpcell.2001.280.1.C1. Physiol. [DOI] [PubMed] [Google Scholar]
- Soldatov NM, Zuhlke RD, Bouron A, Reuter H. Molecular structures involved in L-type calcium channel inactivation. Role of the carboxyl-terminal region encoded by exons 40–42 in alpha1C subunit in the kinetics and Ca2+ dependence of inactivation. J Biol Chem. 1997;272:3560–3566. doi: 10.1074/jbc.272.6.3560. 10.1074/jbc.272.6.3560. [DOI] [PubMed] [Google Scholar]
- Szamier RB, Ripps H. The visual cells of the skate retina: structure, histochemistry, and disc-shedding properties. J Comp Neurol. 1983;215:51–62. doi: 10.1002/cne.902150105. [DOI] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–3953. doi: 10.1016/s0042-6989(96)00142-3. 10.1016/S0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Voipio J, Pasternak M, MacLeod K. Ion-selective microelectrodes. In: Ogden D, editor. Microelectrode Techniques. The Plymouth Workshop Handbook. Cambridge: The Company of Biologists Ltd; 1994. pp. 275–316. [Google Scholar]
- Waldeck AR, van Dam K, Berden J, Kuchel PW. A non-equilibrium thermodynamics model of reconstituted Ca(2+)-ATPase. Eur Biophys J. 1998;27:255–262. doi: 10.1007/s002490050132. 10.1007/s002490050132. [DOI] [PubMed] [Google Scholar]
- Wu SM. Feedback connections and operation of the outer plexiform layer of the retina. Curr Opin Neurobiol. 1992;2:462–468. doi: 10.1016/0959-4388(92)90181-j. 10.1016/0959-4388(92)90181-J. [DOI] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Effects of CNQX, APB, PDA, and kynurenate on horizontal cells of the tiger salamander retina. Vis Neurosci. 1989;3:207–212. doi: 10.1017/s0952523800009962. [DOI] [PubMed] [Google Scholar]
- Zuhlke RD, Pitt GS, Tsien RW, Reuter H. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the α1C subunit. J Biol Chem. 2000;275:21121–21129. doi: 10.1074/jbc.M002986200. 10.1074/jbc.M002986200. [DOI] [PubMed] [Google Scholar]