Abstract
Atypical protein kinase C (aPKC) and extracellular signal-regulated kinase (ERK) are emerging as important signalling molecules in the regulation of metabolism and gene expression in skeletal muscle. Exercise is known to increase activity of aPKC and ERK in skeletal muscle but the effect of exercise intensity hereon has not been studied. Furthermore, the relationship between activity and phosphorylation of the two enzymes during exercise is unknown. Nine healthy young men exercised for 30 min on a bicycle ergometer on two occasions. One occasion consisted of three consecutive 10 min bouts of 35, 60 and 85% of peak pulmonary oxygen uptake (V̇O2peak) and the second of one 30 min bout at 35% of V̇O2peak. Both trials also included 30 min recovery. Muscle biopsies were obtained from the vastus lateralis muscle before and after each exercise bout. Exercise increased muscle aPKC activity at 35% V̇O2peak, whereupon no further increase was observed at higher exercise intensities. Activation of aPKC was not accompanied by increased phosphorylation of aPKC Thr410/403. ERK1/2 activity increased in a similar pattern to aPKC, reaching maximal activity at 35% V̇O2peak, whereas ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 phosphorylation increased with increasing exercise intensity. Thus, aPKC and ERK1/2 activity in muscle during exercise did not correspond to phosphorylation of sites on aPKC or ERK1/2, respectively, which are considered important for their activation. It is concluded that assessment of aPKC and ERK1/2 activity in muscle using phosphospecific antibodies did not reflect direct activity measurements on immunoprecipitated enzyme in vitro. Thus, estimation of enzyme activity during exercise by use of phosphospecific antibodies should not be performed uncritically. In addition, increase in muscle activity of aPKC or ERK1/2 during exercise is not closely related to energy demands of the muscle but may serve other regulatory or permissive functions in muscle.
Exercise leads to marked changes in muscle metabolism and gene expression. The signalling mechanisms behind these phenomena are only beginning to be unravelled. For example, the AMP-activated protein kinase (AMPK; Richter et al. 2003; Wojtaszewski et al. 2003; Hardie, 2004) and the mitogen-activated protein kinase (MAPK) signalling pathways (Widegren et al. 2001) have been implicated in acute as well as chronic adaptations to exercise. In addition, the protein kinase C (PKC) family is thought to be implicated in contraction-induced metabolic responses in skeletal muscle, especially glucose transport (Farese, 2002). The PKC family constitutes a group of multifunctional Ser/Thr protein kinases involved in metabolism, mitogenesis and gene expression (Farese, 2002; Saito et al. 2002). Depending on mode of activation, the PKC family is divided into three subgroups: conventional isoforms (α, β1, β2 and γ) are dependent on both Ca2+ and diacylglycerol (DAG) for stimulation of activity; novel isoforms (δ, ɛ, θ and η) are dependent on DAG; and the atypical isoforms (ζ, λ/ι, where PKCλ is the mouse homologue of human PKCι) that are independent of Ca2+ and DAG but are activated by phosphatidic acid and phosphatidylinositol-3,4,5-trisphosphate (PIP3; Saito et al. 2002). The aPKC isoforms have been implicated in the action of insulin on glucose uptake in several tissues, including skeletal muscle (Bandyopadhyay et al. 2001), where they stimulate translocation of GLUT4 to the sarcolemma (Bandyopadhyay et al. 1999; Standaert et al. 1999; Braiman et al. 2001), and they are activated by insulin in human skeletal muscle (Kim et al. 2003; Beeson et al. 2003). Earlier studies have suggested the involvement of PKC also in contraction-induced glucose uptake (Richter et al. 1987; Cleland et al. 1989, 1990). Furthermore, treatment with the PKC inhibitor calphostin C led to decreased contraction-induced glucose uptake (Wojtaszewski et al. 1998; Ihlemann et al. 1999). However, lack of inhibitor-specificity precluded identification of the responsible isoforms.
Recently we showed that in endurance trained human subjects aPKC activity in skeletal muscle was increased at rest and the increase in activity during exercise of 80% of maximal aerobic capacity was similar to the increase in untrained subjects (Nielsen et al. 2003). Moreover, it was found that aPKC activity correlated with ERK activity both at rest and during exercise (Nielsen et al. 2003), suggesting coregulation of ERK and aPKC activation during exercise. This finding is in line with previous studies in rat muscle in which aPKC was found to be activated downstream of ERK when AMPK and ERK were activated with AICAR (Chen et al. 2002). Recently, Perrini et al. (2004) showed that one-legged cycling exercise increased muscle membrane abundance and phosphorylation on Thr410/403 residues of the aPKC equally in the active and the resting leg, which suggests that humoral factors may be important in activation of aPKC in skeletal muscle during exercise. Moreover, increased muscle membrane abundance and phosphorylation of the atypical PKC persisted 15 min into recovery (Perrini et al. 2004). These investigators also demonstrated no translocation or changes in phosphorylation of the novel PKC isoforms in muscle during exercise. Neither are the conventional PKC isoforms apparently activated by exercise (A. Rose and M. Hargreaves, personal communication). These observations, along with the previous indications of aPKC activation during exercise (Beeson et al. 2003; Nielsen et al. 2003), implicate aPKCs as the main PKC isoforms activated during exercise. However, the effect of exercise intensity on aPKC activity has not been studied in rodents or in humans.
There are several studies showing increased muscle ERK phosphorylation during exercise/muscle contraction (Goodyear et al. 1996; Aronson et al. 1997; Widegren et al. 2001; Williamson et al. 2003; Thompson et al. 2003), but only one study investigated the effect of two exercise intensities on phosphorylation of ERK (Widegren et al. 2000). Although phosphorylation of ERK is generally considered a prerequisite for its activation (Pelech & Sanghera, 1992; Seger & Krebs, 1995), it has in fact never been investigated in skeletal muscle whether phosphorylation of ERK is a good marker of ERK activity during exercise. If aPKC and/or ERK are involved in exercise-induced muscle glucose uptake, one might expect activities of aPKC and ERK to increase with exercise intensity and duration because glucose uptake increases with increasing exercise intensity and duration (for review see Richter, 1996). Furthermore, since both PKC and ERK may be involved in regulation of gene transcription (Widegren et al. 2001; Sakamoto & Goodyear, 2002), it is important to know how activity of these enzymes is regulated during and after exercise of various intensities in humans. Finally, since aPKC may be downstream of ERK in the enzyme cascade during exercise (Chen et al. 2002), it is interesting to study these two enzymes simultaneously. The aim of the present study, therefore, was to determine aPKC and ERK activities in human skeletal muscle during exercise of different intensities and recovery from exercise.
Methods
Nine healthy young men (26 ± 1 years old; mean ± s.e.m.) gave their written informed consent to participate in the study, which was approved by the Copenhagen Ethics Committee and conformed to the declaration of Helsinki. Body height and weight were 187 ± 2 cm and 85 ± 2 kg, respectively. The subjects participated in organized physical activity at the most once per week but used the bicycle for local transportation. One to two weeks before the experiments, peak pulmonary oxygen consumption was determined during an incremental cycling ergometer test (peak pulmonary oxygen uptake (V̇O2peak) = 53 ± 1 ml kg−1 min−1).
Subjects were investigated on two occasions, separated by 2–3 weeks. For the last 24 h before the first experiment subjects recorded intake of all food and drink. This food intake was then duplicated during the last 24 h before the second experiment. Experiments were performed in randomized order. On the morning of each experiment subjects arrived at the laboratory at 08.00 h, having abstained from food for the last 12 h and from caffeinated or alcoholic beverages as well as heavy physical activity for 48 h before the experiment. Subjects rested in the supine position for at least 30 min, after which four or two incisions (depending on the trial) for the biopsies were performed under local anaesthesia with 2 ml 2% lignocaine per incision. Incisions were placed in the mediolateral aspect of the vastus lateralis muscle 15–25 cm above the patella, at least 5 cm apart, and were placed equally in both legs. All biopsies were obtained from separate incisions. This was done to avoid erroneous activation of enzyme activity due to a previous biopsy having been taken through the same incision (Aronson et al. 1998). Five to ten miutes later a resting biopsy was obtained, after which the subject commenced exercise on a Monark cycle ergometer. In trial 1, subjects performed three 10 min sequential sessions at 35, 60 and 85% of their peak pulmonary oxygen consumption. After each 10 min session a muscle biopsy was obtained as quickly as possible with the subject still seated on the bicycle. The biopsy was frozen in liquid nitrogen within 5–10 s. In trial 2, a resting muscle biopsy was obtained and then subjects performed 30 min ergometer cycling at 35% of peak oxygen uptake, after which another muscle biopsy was obtained. In both trials a final muscle biopsy was obtained after 30 min rest in the supine position.
Preparation of muscle lysates
For studies of enzyme activity, approximately 30 mg of frozen muscle tissue was homogenized as previously described (Beeson et al. 2003). For determination of protein phosphorylation 20–30 mg muscle was homogenized in buffer (1:5 w/v) containing 50 mm Hepes, 150 mm sodium chloride, 20 mm sodium pyrophosphate, 20 mm glycerophosphate, 10 mm sodium fluoride, 2 mm sodium vanadate, 2 mm EDTA, 1% Nonidet P-40 (Sigma, St Louis, USA), 10% glycerol, 2 mm phenylmethylsulphonyl fluoride, 1 mm magnesium chloride, 1 mm calcium chloride, 10 μg ml−1 leupeptin, and 10 μg ml−1 aprotinin, pH 7.6. Homogenates were rotated end over end at 4°C for 60 min, after which they were centrifuged at 4°C for 60 min at 15 000g. The supernatants were harvested and total protein content was determined in the lysates by the BCA method (Pierce Chemical Company, IL, USA).
ERK activity
As described (Sajan et al. 1999), lysate protein (200–500 μg) was subjected to overnight immunoprecipitation at 4°C with mouse monoclonal anti-ERK2 antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Precipitates were collected on protein AG-agarose beads, washed, and incubated for 10 min at 30°C in 50 μl of buffer containing 25 mm glycerophosphate (pH 7.3), 0.5 mm dithiothreitol, 1.25 mm EGTA, 0.5 mm Na3VO4, 10 mm MgCl2, 1 mg ml−1 bovine serum albumin, 1 μm okadaic acid, 0.1 mm [γ-32P]ATP (PerkinElmer Life Sciences; 1500 000 d.p.m. nmol−1) and 50 μg of myelin basic protein (Sigma). After incubation, aliquots were spotted on p81 filter paper, washed and counted for 32P radioactivity. Blank values were obtained by substituting a non-immune antibody preparation for anti-ERK2 antibodies or by omitting myelin basic protein substrate (results were similar). As noted previously (Sajan et al. 1999), the Santa Cruz anti-ERK2 mouse monoclonal antibodies, although recognizing only ERK2 in Western analyses, precipitated ERK1 as well as ERK2, and the ratio of ERK2 to ERK1 in immunoprecipitates was approximately 1.4–1.5:1, as measured by Western blot analysis, using a Santa Cruz rabbit polyclonal antiserum that recognizes both ERK1 and ERK2.
Atypical PKC activity
PKCζ/λ/ι activity was measured as previously described (Standaert et al. 2002; Beeson et al. 2003). In brief, PKC-ζ/λ/ι was immunoprecipitated with a rabbit polyclonal antiserum (C-20, Santa Cruz Biotechnologies) that recognizes a common epitope in the COOH-termini of the atypical PKC isoforms ζ/λ/ι. Since PKCι is expressed in human skeletal muscle rather than PKCλ, which is expressed in rodent muscle (Farese, 2002), we will refer to aPKC as a common denominator of PKCζ/λ/ι. The antigen–antibody complexes were collected on Sepharose-AG beads, and incubated for 8 min at 30°C in 100 μl buffer containing 50 mmol l−1 TrisHCl (pH,7.5), 100 μmol l−1 Na3VO4, 100 μmol l−1 Na4P2O7, 1 mmol l−1 NaF, 100 μmol l−1 phenylmethylsulphonyl fluoride, 4 μg phosphatidylserine (Sigma), 50 μmol l−1 [γ-32P]ATP (NEN/Life Science Products), 5 mmol l−1 MgCl2, and, as substrate, 40 μmol l−1 serine analogue of the PKCɛ pseudosubstrate (BioSource). After incubation, 32P-labelled substrate was trapped on P-81 filter paper and counted in a liquid scintillation counter. As reported (Standaert et al. 2002) in our aPKC assays, aPKC recovery is partial (50–60%) using antiserum concentrations according to the supplier's instructions. Thus, with this limiting antibody concentration, equal amounts of aPKCs were immunoprecipitated from all samples, regardless of differences in lysate aPKC levels; aPKC activity values therefore reflect enzyme specific activity.
Western blot analyses
Muscle lysate proteins were resolved by SDS-PAGE (7.5%), transferred (semidry) to a polivinyldi-fluoride (PVDF) membrane (Immobilon Transfer Membrane, Millipore, Glostrup, Denmark) and incubated with primary antibodies. These were: anti-PKCζ/λ/ι (PKCζ[C-20], Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; phospho-PKCζ/λ[Thr410/403] (Cell Signalling Technology, Beverly, MA, USA); and anti-ERK1/2 and anti-phospho-ERK1 Thr202/Tyr204-phos/ERK2 Thr185/Tyr187-phos (both from Cell Signalling Technology). Because the sequence around Thr410/403 is conserved in all of the aPKC isoforms (Farese, 2002) the phospho-PKCζ/λ[Thr410/403] antibody from Cell Signalling Technologies will detect any of the aPKCs when phosphorylated on the phospho-Thr410/403 residues. Membranes were incubated with horseradish peroxidase-conjugated secondary antibody (DakoCytomation, Glostrup, Denmark). The immunoreactive bands were visualized with enhanced chemiluminescense (ECL+, Amersham Pharmacia Biotech Ltd, Little Chalfont, UK) and detected and quantified using a CCD-image sensor and 1D software (Image Station, E440CF, Kodak). Protein content was expressed in arbitrary units (a.u.) relative to a human skeletal muscle standard. Protein phosphorylation was expressed relative to protein content in each sample.
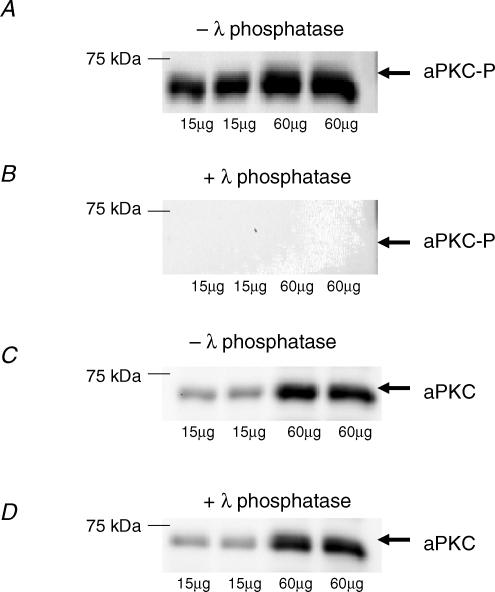
The phospho-specificity of the phospho-aPKC-Thr410/403 antibody was tested by incubation of PVDF membranes in a dephosphorylation buffer before immunoblotting and visualization. Human muscle lysate proteins were resolved by SDS-PAGE and, after semidry transfer to a PVDF membrane, the membrane was treated for 2 h at 37°C with a dephosphorylation buffer (50 mm Tris-HCl, 0.1 mm Na2EDTA, 5 mm dithiothreitol, 0.01% Brij 35 and 2 mm MnCl2; pH 7.5) either with or without 500 U ml−1 lambda protein phosphatase (λPPase, New England BioLabs, Hitchin, UK). As can be seen in Fig. 1, phosphatase treatment completely removed the signal on the membrane for the phospho-aPKC-Thr410/403, whereas the native protein was unaffected by phosphatase treatment.
Figure 1. Phosphospecificity of aPKC-antibody.
A shows blots of lysates prepared from human vastus lateralis muscle probed with the antibody against atypical PKC (aPKC) phosphorylated on Thr410/403. Same sample was loaded 4 times in 2 different amounts. B shows complete absence of signal after treatment for 2 h of the membrane with 500 U ml−1 λ-protein phosphatase before probing with the aPKC Thr410/403 phosphospecific antibody. C and D show blots for aPKC in its nonphosphorylated form without (C) and with (D) phosphatase treatment. This signal was unaffected by phosphatase treatment.
Statistics
Data are expressed as means ± s.e.m. Comparison between different time points in either trial was done by one-way ANOVA for repeated measures. If a significant time effect emerged the ANOVA was followed by a multiple comparison test (Student–Newman–Keuls method). Comparisons between the same time points in the two trials were made by two-way ANOVA for repeated measures followed by a multiple comparison test (Student–Newman–Keuls method in case significant effects of time, treatment or interactions were detected). P < 0.05 was considered statistically significant.
Results
Pulmonary and cardiac responses to exercise
The subjects worked at 36 ± 1, 60 ± 1 and 86 ± 2% of V̇O2peak during each consecutive session in trial 1 and at 36 ± 1% of V̇O2peak at the end of trial 2. Heart rate was 107 ± 3, 142 ± 5 and 179 ± 4 beats min−1 at the end of each 10 min period during trial 1 and 106 ± 3 beats min−1 at the end of the 30 min in trial 2.
aPKC activity and phosphorylation
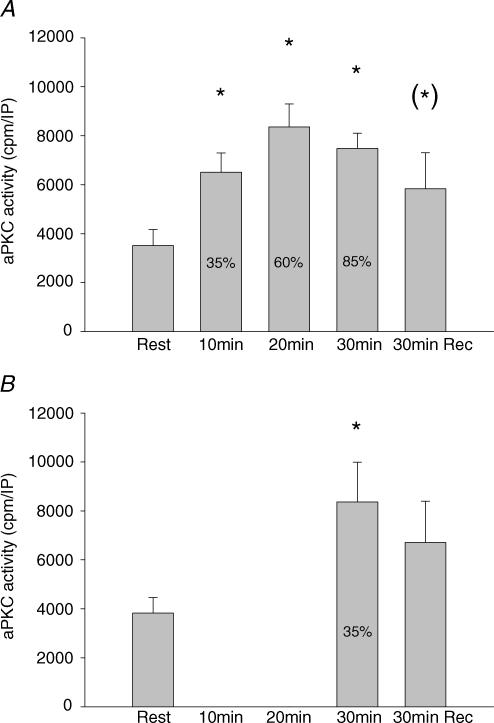
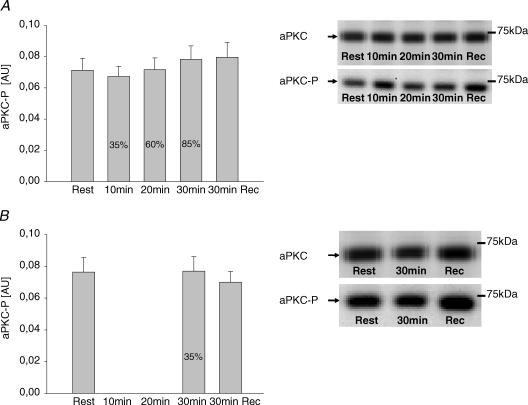
In trial 1, activity of atypical PKCs in vastus lateralis muscle increased significantly from rest to 35% V̇O2peak, whereupon no further significant increase occurred at the next two work loads (Fig. 2A). After 30 min of recovery, there was only a marginal elevation in activity (P < 0.1) compared with resting values (Fig. 2A). In trial 2, aPKC activity after 30 min exercise at 35% V̇O2peak had increased to the same level as during exercise in trial 1 (Fig. 2B) and returned to similar postexercise values to those in trial 1 (Fig. 2A and B). Phosphorylation of aPKC Thr410/403 did not change significantly with exercise in either trial (Fig. 3). aPKC protein abundance did not vary between time points or trials (mean data not shown but representative blots shown in Fig. 3). To further verify the lack of increased aPKC Thr410/403 phosphorylation during exercise, in the biopsies from a few subjects we first immunoprecipitated aPKC and then the phosphorylation status of PKC was evaluated by Western blotting with the aPKC Thr410/403 phosphospecific antibody. These preliminary data did not reveal any exercise-induced increase in PKC Thr410/403 phosphorylation during exercise (data not shown).
Figure 2. aPKC activity.
A, activity of aPKC in vastus lateralis muscle at rest, immediately after each 10 min sequential exercise bout of 35, 60 and 85% of V̇O2peak and after 30 min recovery. Values are means ± s.e.m. of 9 determinations. B, aPKC activity in vastus lateralis muscle at rest, after 30 min exercise at 35% of V̇O2peak and after 30 min recovery. Values are means ± s.e.m. of 9 determinations. *P < 0.05 compared to rest; (*)P < 0.1 compared to rest.
Figure 3. aPKC phosphorylation.
A, phosphorylation of aPKC Thr410/403 in vastus lateralis muscle at rest, immediately after each 10 min sequential exercise bout at 35, 60 and 85% of V̇O2peak and after 30 min recovery. Representative Western blots of both aPKC Thr410/403 phosphorylation and aPKC protein are shown in the inset. Values are means ± s.e.m. of 9 determinations and are expressed in arbitrary units relative to aPKC protein content in each sample. B, phosphorylation of aPKC Thr410/403 in vastus lateralis muscle at rest, after 30 min exercise at 35% of V̇O2peak and after 30 min recovery. Representative Western blots of both aPKC Thr410/403 phosphorylation and aPKC protein are also shown. Values are means ± s.e.m. of 9 determinations and are expressed in arbitrary units relative to aPKC protein content in each sample.
ERK1/2 activity and phosphorylation
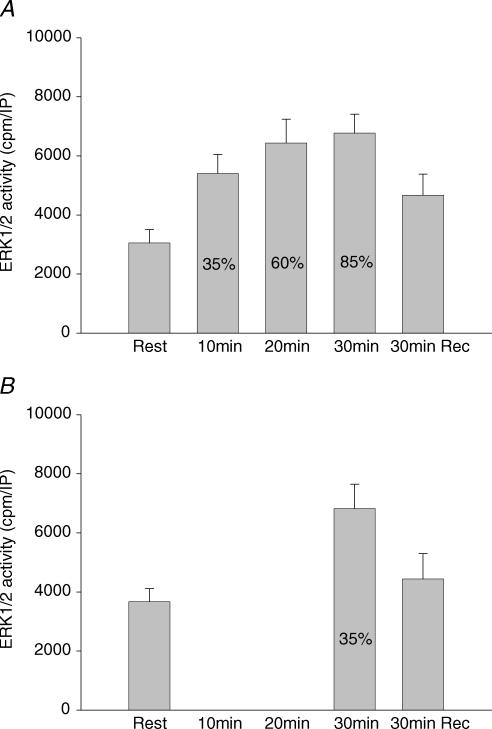
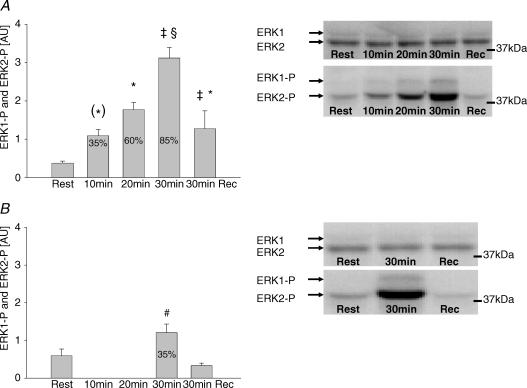
In trial 1, the activity of ERK1/2 in skeletal muscle increased significantly in response to exercise at 35% of V̇O2peak, whereupon no further significant increase occurred at the next two work loads (Fig. 4A). After 30 min recovery the ERK1/2 activity was no longer significantly higher than before exercise (Fig. 4A). In trial 2, ERK1/2 activity after 30 min exercise at 35% of V̇O2peak had increased to the same level as after exercise at 60 and 85% of V̇O2peak (Fig. 4B) and returned to levels not significantly different from pre-exercise values after 30 min recovery (Fig. 4B). Phosphorylation of ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 increased after 10 min at 35% of V̇O2peak and increased significantly further at 85% of V̇O2peak (Fig. 5A). During trial 2, the increase in ERK1/2 phosphorylation was similar to that after 10 min of trial 1, but was markedly lower than after exercise at 85% of V̇O2peak (Fig. 5A and B). These findings suggest that the marked increase in phosphorylation of ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 observed after exercise at 85% of V̇O2peak was due to differences in exercise intensity and not exercise duration. Furthermore, the marked difference in phosphorylation of ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 after 30 min of exercise in trials 1 and 2 was not accompanied by any difference in ERK1/2 activity. ERK1/2 protein levels were not different at any exercise level compared to rest (mean data not shown but representative blots shown in Fig. 5).
Figure 4. ERK1/2 activity.
A, activity of ERK1/2 in vastus lateralis muscle at rest, immediately after each 10 min sequential exercise bout of 35, 60 and 85% of V̇O2peak and after 30 min recovery. Values are means ± s.e.m. of 9 determinations. B, activity of ERK1/2 in vastus lateralis muscle at rest, after 30 min exercise at 35% of V̇O2peak and after 30 min recovery. Values are means ± s.e.m. of 9 determinations. *P < 0.05 compared to rest.
Figure 5. ERK1/2 phosphorylation.
A, phosphorylation of ERK1/2 in vastus lateralis muscle at rest, immediately after each 10 min sequential exercise bout of 35, 60 and 85% of V̇O2peak and after 30 min recovery. Representative Western blots of both ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 and ERK1/2 protein are also shown. Values are means ± s.e.m. of 9 determinations and are expressed in arbitrary units relative to ERK1/2 protein content in each sample. B, phosphorylation of ERK1/2 in vastus lateralis muscle at rest, after 30 min exercise at 35% V̇O2peak and after 30 min recovery. Representative Western blots of both phosphorylation of ERK1 Thr202/Tyr204, ERK2 Thr183/Tyr185 and ERK1/2 protein are also shown. Values are means ± s.e.m. of 9 determinations and are expressed in arbitrary units relative to ERK1/2 protein content in each sample. *P < 0.05 compared to rest; (*)P < 0.1 compared to rest; ‡P < 0.05 compared to other trial; #P < 0.05 compared to rest and recovery; and §P < 0.05 compared to rest, exercise at 35 and 60% of V̇O2peak and recovery.
Discussion
The main finding in the present study was an increase in activity of atypical protein kinase C (aPKC) as well as extracellular signal-regulated protein kinase 1/2 (ERK1/2) in human skeletal muscle after 10 min of exercise at 35% of V̇O2peak and that no further significant increases could be detected at higher intensities reaching 85% of V̇O2peak. For both enzymes there was a marked discrepancy between measured activity and phosphorylation of the enzyme on sites believed to be important for activity. Increased aPKC activity was not accompanied by increased aPKC Thr410/403 phosphorylation, whereas ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 phosphorylation increased markedly more than ERK activity during graded exercise. Furthermore, 30 min of low intensity exercise at 35% of V̇O2peak in trial 2 resulted in the same ERK1/2 activity as 30 min of graded exercise reaching 85% of V̇O2peak in trial 1, whereas ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 phosphorylation was 2- to 3-fold higher after graded exercise in trial 1 compared with trial 2. These data suggest that activity of muscle aPKC as well as ERK1/2 during exercise is not tightly linked to muscle metabolic stress and that mechanisms other than phosphorylation on Thr410/403 on aPKC and of Thr202/Tyr204 and Thr183/Tyr185 and ERK1 and 2, respectively, are of importance for increasing activity during exercise.
We have recently shown activation of aPKC in human (Beeson et al. 2003; Nielsen et al. 2003) and mouse (Chen et al. 2002) skeletal muscle during exercise at a constant intensity. In rodent muscle aPKC has been suggested to be downstream in the enzyme cascade of ERK when 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) stimulation was used to activate 5′AMP-activated protein kinase (AMPK), aPKC and ERK (Chen et al. 2002), and the roughly parallel increase in ERK and aPKC activity found in the present study is in accordance with the view that ERK also may activate aPKC during exercise. How this may be accomplished is not known, but activation of aPKC by the kinase ERK would seem to involve phosphorylation of aPKC. Nevertheless, exercise-induced activation of aPKC activity did not involve an increase in phosphorylation of aPKC Thr410/403, suggesting that phosphorylation of this site is not the mechanism behind the exercise-induced increase in aPKC activity measured in muscle lysate. The inability to show increased phosphorylation of aPKC Thr410/403 was not due to lack of phosphospecificity of the antibody used, since the signal for the phosphorylated form of aPKC Thr410/403 was completely removed by incubation of the blotting membrane with phosphatase (Fig. 1A and B), whereas the native protein was unaffected by this treatment (Fig. 1C and D). The lack of increase in phosphorylation of aPKC Thr410/403 with exercise is not entirely surprising, since phosphorylation of this site reflects activity of 3-phosphoinositide-dependent kinase-1 (PDK1), which is generally believed to be controlled by phosphoinositide 3-kinase (PI3-kinase), the activity of which is not increased with exercise (Goodyear et al. 1995; Wojtaszewski et al. 1996). Previous studies showing increased activity of aPKC during exercise have not investigated the phosphorylation status of aPKC Thr410/403 (Chen et al. 2002; Beeson et al. 2003; Nielsen et al. 2003), but phosphorylation of aPKC Thr410 in the activation loop has been shown to be necessary for activation of aPKC by insulin (Bandyopadhyay et al. 1999). However, an exercise-induced increased membrane content and decreased cytosolic content of phosphorylated PKC Thr410/403 of skeletal muscle has recently been reported (Perrini et al. 2004), suggesting that translocation of phosphorylated aPKC may be a mechanism to activate aPKC during exercise.
Other possible mechanisms for activating aPKC during exercise include activation through phospholipase D. Phospholipase D has been implicated in aPKC activation (Chen et al. 2002) and glucose transport (Kristiansen et al. 2001; Kristiansen & Richter, 2002) in rodent skeletal muscle. The product of the phospholipase D reaction is phosphatidic acid and it has been shown that phosphatidic acid increases aPKC activity in vitro (Chen et al. 2002). Furthermore, phosphatidic acid concentrations were found to increase with muscle contractions in rat skeletal muscle (Cleland et al. 1989), suggesting that aPKC may be activated during exercise by phosphatidic acid production in muscle. Whether the effect of phosphatidic acid on aPKC activity is due to phosphorylation of aPKC is not understood, but the fact that phosphatidic acid was found to affect aPKC activity measured in immunoprecipitates suggests that phosphatidic acid covalently affects aPKC but not necessarily via phosphorylation of the Thr410/403 residues. Perhaps a phosphatidic acid-induced allosteric relief of the pseudosubstrate site from the catalytic domain potentiates autophosphorylation of other critical threonine residues (e.g. Thr560) within the catalytic domain of aPKC.
ERK activity is usually evaluated by measuring phosphorylation of ERK on tyrosine and threonine residues, since activation of ERK1 and 2 by the upstream kinase MAP kinase kinase or ERK kinase (MEK) requires phosphorylation on both residues (Pelech & Sanghera, 1992; Seger & Krebs, 1995). However, ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 phosphorylation increased 5-fold during graded exercise from rest to exercise at 85% of V̇O2peak, whereas ERK activity only increased 2-fold, and the maximum increase was already reached at 35% of V̇O2peak. In addition, 30 min of graded exercise reaching 85% of V̇O2peak in trial 1 resulted in the same ERK1/2 activity as 30 min of low intensity exercise at 35% of V̇O2peak in trial 2, whereas ERK1/2 phosphorylation was 2- to 3-fold higher after graded exercise in trial 1 compared to trial 2. To our knowledge a direct comparison between increases in ERK activity and phosphorylation during graded exercise has not previously been performed. Our data indicate that during exercise phosphorylation of ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 does not linearly reflect, and may in fact exceed, activity of ERK1/2 measured in immunoprecipitates. In fact, phosphorylation of ERK reflects activity of the upstream kinase MEK rather than activity of ERK itself. However, whether activity measured in immunoprecipitates in vitro or estimation of activity in vivo by use of phosphospecific antibodies towards ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 gives the best estimate of actual activity in the muscle during contractions is not easily resolved. Since activity measured in vitro does not reflect allosteric activation but rather covalent modifications of the enzyme such as phosphorylation, it would a priori be expected that in vitro measured enzyme activity and enzyme phosphorylation should basically change in a similar manner although not necessarily in a linear fashion. However, our findings suggest that ERK1/2 activity is dependent upon other factors in addition to phosphorylation of ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 and that estimation of ERK1/2 activity by use of phosphospecific antibodies should be interpreted cautiously.
In the present study it was surprising that aPKC and ERK activity did not significantly increase when exercise intensity was increased from 35 to 60 and 85% of V̇O2peak. This suggests that there is no tight linkage between muscle metabolic stress and aPKC or ERK activity in contrast to, for instance, AMPK activity, which clearly is dependent upon exercise intensity (Wojtaszewski et al. 2000). During exercise the motor units recruited during light exercise are primarily the smaller ones innervating slow-twitch fibres and when exercise intensity increases, larger motor units innervating fast-twitch fibres are also recruited (Milner-Brown et al. 1973). aPKC is expressed at a higher level in rat soleus (slow-twitch fibres) than gastrocnemius muscle (primarily fast-twitch fibres; Given et al. 1998). Thus preferential recruitment of slow-twitch fibres at the low intensity might to some extent explain the apparent maximal activation already observed at the lowest exercise intensity, although recruitment of additional fast-twitch fibres during higher exercise intensities would be expected to increase aPKC activity further in a muscle lysate prepared from a mixture of slow- and fast-twitch fibres.
In conclusion, in human skeletal muscle exercise-induced activation of aPKC was not accompanied by changes in aPKC Thr410/403 phosphorylation, and ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 phosphorylation did not reflect ERK1/2 activity measured in vitro. Thus, assessment of enzyme activity using phosphospecific antibodies did not reflect direct activity measurements on immunoprecipitated enzyme in vitro. In addition, the present study showed activation of aPKC and ERK1/2 during 10 min exercise at a low exercise intensity requiring 35% of V̇O2peak. Surprisingly, increasing exercise intensity to 85% of V̇O2peak did not elicit any further increase in activities, indicating that activity of these enzymes during exercise is not closely related to energy demands of the muscle, but may serve other regulatory or permissive functions in muscle.
Acknowledgments
This study was supported by a Research & Technological Development Project (QLG1-CT-2001-01488) funded by the European Commission and by grant no. 504-14 from the Danish National Research Foundation, The Copenhagen Muscle Research Centre, The Novo-Nordisk Research Foundation, The Danish Medical Research Foundation and the Media and Grants Secretariat of the Danish Ministry of Culture, and the Danish Diabetes Association. R. V. Farese was supported by funds from the Department of Veterans Affairs Merit Program, National Institute of Health Research Grant 2RO1-DK-38079–09A1 and a research grant from the American Diabetes Association.
References
- Aronson D, Violan MA, Dusfresne SD, Zangen D, Fielding RA, Goodyear LJ. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J Clin Invest. 1997;99:1251–1257. doi: 10.1172/JCI119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson D, Wojtaszewski JF, Thorell A, Nygren J, Zangen D, Richter EA, Ljungqvist O, Fielding RA, Goodyear LJ. Extracellular-regulated protein kinase cascades are activated in response to injury in human skeletal muscle. Am J Physiol. 1998;275:C555–C561. doi: 10.1152/ajpcell.1998.275.2.C555. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Quon MJ, Reed BC, Dikic I, Farese RV. Glucose activates protein kinase C zeta/lambda through proline-rich tyrosine kinase-2, extracellular signal-regulated kinase, and phospholipase D: a novel mechanism for activating glucose transporter translocation. J Biol Chem. 2001;276:35537–35545. doi: 10.1074/jbc.M106042200. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Standaert ML, Sajan MP, Karnitz LM, Cong L, Quon MJ, Farese RV. Dependence of insulin-stimulated glucose transporter 4 translocation on 3-phosphoinositide-dependent protein kinase-1 and its target threonine-410 in the activation loop of protein kinase C-{zeta} Mol Endocrinol. 1999;13:1766–1772. doi: 10.1210/mend.13.10.0364. [DOI] [PubMed] [Google Scholar]
- Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4) 3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson SR. Activation of protein kinase C zeta induces serine phosphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle. Mol Cell Biol. 2001;21:7852–7861. doi: 10.1128/MCB.21.22.7852-7861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV, Jr, Farese RV. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and aminoimidazole-4-carboxamide-1-beta-D-riboside (AICAR)-stimulated glucose transport. J Biol Chem. 2002;277:23554–23562. doi: 10.1074/jbc.M201152200. [DOI] [PubMed] [Google Scholar]
- Cleland PJ, Abel K, Rattigan S, Clark M. Long-term treatment of isolated rat soleus muscle with phorbol ester leads to loss of contraction-induced glucose transport. Biochem J. 1990;267:659–663. doi: 10.1042/bj2670659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland PJ, Appleby G, Rattigan S, Clark M. Exercise-induced translocation of protein kinase C and production of diacylglycerol and phosphatidic acid in rat skeletal muscle in vivo. J Biol Chem. 1989;264:17704–17711. [PubMed] [Google Scholar]
- Farese RV. Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. Am J Physiol. 2002;283:E1–E11. doi: 10.1152/ajpendo.00045.2002. [DOI] [PubMed] [Google Scholar]
- Given MB, Jie O, Zhao X, Giles TD, Greenberg SS. Protein kinase C isozymes in skeletal muscles during the early stage of genetic and streptozocin diabetes. Proc Soc Exp Biol Med. 1998;218:382–389. doi: 10.3181/00379727-218-44308. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Chang PY, Sherwood DJ, Dufresne SD, Moller DE. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol. 1996;271:E403–E408. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Giorgino F, Balon TW, Condorelli G, Smith RJ. Effects of contractile activity on tyrosine phosphoproteins and PI 3-kinase activity in rat skeletal muscle. Am J Physiol. 1995;268:E987–E995. doi: 10.1152/ajpendo.1995.268.5.E987. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36:28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- Ihlemann J, Galbo H, Ploug T. Calphostin C is an inhibitor of contraction, but not insulin-stimulated glucose transport, in skeletal muscle. Acta Physiol Scand. 1999;167:69–75. doi: 10.1046/j.1365-201x.1999.00591.x. 10.1046/j.1365-201x.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- Kim YB, Kotani K, Ciaraldi TP, Henry RR, Kahn BB. Insulin-stimulated protein kinase C{lambda}/{zeta} activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes. 2003;52:1935–1942. doi: 10.2337/diabetes.52.8.1935. [DOI] [PubMed] [Google Scholar]
- Kristiansen S, Nielsen JN, Bourgoin S, Klip A, Franco M, Richter EA. GLUT-4 translocation in skeletal muscle studied with a cell-free assay: involvement of phospholipase D. Am J Physiol. 2001;281:E608–E618. doi: 10.1152/ajpendo.2001.281.3.E608. [DOI] [PubMed] [Google Scholar]
- Kristiansen S, Richter EA. GLUT4-containing vesicles are released from membranes by phospholipase D cleavage of a GPI anchor. Am J Physiol. 2002;283:E374–E382. doi: 10.1152/ajpendo.00441.2001. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H, Stein R, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973;230:359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JN, Frosig C, Sajan MP, Miura A, Standaert ML, Graham DA, Wojtaszewski JF, Farese RV, Richter EA. Increased atypical PKC activity in endurance-trained human skeletal muscle. Biochem Biophys Res Commun. 2003;312:1147–1153. doi: 10.1016/j.bbrc.2003.11.041. 10.1016/j.bbrc.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Pelech SL, Sanghera JS. MAP kinases: charting the regulatory pathways. Science. 1992;257:1355–1356. doi: 10.1126/science.1382311. [DOI] [PubMed] [Google Scholar]
- Perrini S, Henriksson J, Zierath JR, Widegren U. Exercise-induced protein kinase C isoform-specific activation in human skeletal muscle. Diabetes. 2004;53:21–24. doi: 10.2337/diabetes.53.1.21. [DOI] [PubMed] [Google Scholar]
- Richter EA. Glucose utilization. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 912–951. [Google Scholar]
- Richter EA, Cleland PJF, Rattigan S, Clark MG. Contraction-associated translocation of protein kinase C in rat skeletal muscle. FEBS Lett. 1987;217:232–236. doi: 10.1016/0014-5793(87)80669-5. 10.1016/0014-5793(87)80669-5. [DOI] [PubMed] [Google Scholar]
- Richter EA, Nielsen JN, Jorgensen SB, Frosig C, Wojtaszewski JF. Signalling to glucose transport in skeletal muscle during exercise. Acta Physiol Scand. 2003;178:329–335. doi: 10.1046/j.1365-201X.2003.01153.x. 10.1046/j.1365-201X.2003.01153.x. [DOI] [PubMed] [Google Scholar]
- Saito N, Kikkawa U, Nishizuka Y. The family of protein kinase C and membrane lipid mediators. J Diabetes Complications. 2002;16:4–8. doi: 10.1016/s1056-8727(01)00200-8. 10.1016/S1056-8727(01)00200-8. [DOI] [PubMed] [Google Scholar]
- Sajan MP, Standaert ML, Bandyopadhyay G, Quon MJ, Burke TR, Jr, Farese RV. Protein kinase C-zeta and phosphoinositide-dependent protein kinase-1 are required for insulin-induced activation of ERK in rat adipocytes. J Biol Chem. 1999;274:30495–30500. doi: 10.1074/jbc.274.43.30495. 10.1074/jbc.274.43.30495. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goodyear LJ. Invited review: intracellular signaling in contracting skeletal muscle. J Appl Physiol. 2002;93:369–383. doi: 10.1152/japplphysiol.00167.2002. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Standaert ML, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, Sajan MP, Cenni V, Sirri A, Moscat J, Toker A, Farese RV. Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J Biol Chem. 1999;274:25308–25316. doi: 10.1074/jbc.274.36.25308. 10.1074/jbc.274.36.25308. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Ortmeyer HK, Sajan MP, Kanoh Y, Bandyopadhyay G, Hansen BC, Farese RV. Skeletal muscle insulin resistance in obesity-associated type 2 diabetes in monkeys is linked to a defect in insulin activation of protein kinase C-zeta/lambda/iota. Diabetes. 2002;51:2936–2943. doi: 10.2337/diabetes.51.10.2936. [DOI] [PubMed] [Google Scholar]
- Thompson HS, Maynard EB, Morales ER, Scordilis SP. Exercise-induced HSP27, HSP70 and MAPK responses in human skeletal muscle. Acta Physiol Scand. 2003;178:61–72. doi: 10.1046/j.1365-201X.2003.01112.x. [DOI] [PubMed] [Google Scholar]
- Widegren U, Ryder JW, Zierath JR. Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol Scand. 2001;172:227–238. doi: 10.1046/j.1365-201x.2001.00855.x. 10.1046/j.1365-201x.2001.00855.x. [DOI] [PubMed] [Google Scholar]
- Widegren U, Wretman C, Lionikas A, Hedin G, Henriksson J. Influence of exercise intensity on ERK/MAP kinase signalling in human skeletal muscle. Pflugers Arch. 2000;441:317–322. doi: 10.1007/s004240000417. 10.1007/s004240000417. [DOI] [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547:977–987. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JFP, Hansen BF, Ursø B, Richter EA. Wortmannin inhibits both insulin- and contraction-stimulated glucose uptake and transport in rat skeletal muscle. J Appl Physiol. 1996;81:1501–1509. doi: 10.1152/jappl.1996.81.4.1501. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JFP, Laustsen JL, Richter EA. Contraction- and hypoxia-stimulated glucose transport in skeletal muscle is affected differently by wortmannin. Evidence for different signalling mechanisms. Biochim Biophys Acta. 1998;1340:396–404. doi: 10.1016/s0304-4165(98)00011-7. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen JN, Jorgensen SB, Frosig C, Birk JB, Richter EA. Transgenic models – a scientific tool to understand exercise-induced metabolism: the regulatory role of AMPK (5′-AMP-activated protein kinase) in glucose transport and glycogen synthase activity in skeletal muscle. Biochem Soc Trans. 2003;31:1290–1294. doi: 10.1042/bst0311290. [DOI] [PubMed] [Google Scholar]