Abstract
In contrast to other extrahepatic malignancies many colorectal cancers can be cured even when there is metastatic spread to the liver. The diagnosis of liver metastases relies totally on imaging to decide which patients may be surgical candidates. The diagnostic value of ultrasound with contrast agents, multidetector CT and MR imaging with non-specific gadolinium chelates and liver-specific contrast agent is discussed. Nowadays MDCT is the mainstay of staging and follow-up of these patients, because it provides good coverage of the liver and the complete abdomen and the chest in one session. MR imaging has been shown to be superior to helical CT in the preoperative assessment of colorectal liver metastases. Large studies are needed to define the role of MDCT vs. MRI staging in patients referred for resection of liver metastases.
Keywords: Liver, metastasis, MRI, gadolinium, mangafodipir
Introduction
Metastastic disease to the liver is a very common clinical situation in oncology. The liver is one of the most common sites of metastatic spread of epithelial cancers, second only to regional lymph nodes. The true prevalence of metastatic disease is unknown, but approximately 20%–25% of patients with colorectal cancer have liver metastases at the time of diagnosis. Studies based on autopsy results showed that up to 70% of colon cancer patients have liver metastases at autopsy.
The early detection of liver metastases is of utmost importance in patients with cancer. In general, the presence of liver metastases indicates non-resectability of the primary tumour for oncologic reasons, except for tumour palliation (i.e. to relieve obstruction of the gastrointestinal tract). In these patients, chemotherapy is the method of choice. For a few malignancies, resection of liver metastases has been shown to improve the survival of the patients [1]. Colorectal cancer is one of a few malignant tumours in which the presence of limited synchronous liver metastases (i.e. occurring at the time of diagnosis of the primary tumour) or metachronous metastases (occurring after diagnosis of the primary tumour) warrants surgical resection. Exact knowledge of the number, size, and regional distribution of metastases is essential to determine their resectability. Based on the number and localization of the liver metastases and considering all other clinical parameters of the patient, only about 30% of colorectal patients with liver metastases may undergo resection. However, the 5-year survival of these patients is between 30% and 48% in comparison to a survival of less than 5% of patients with liver metastases not amenable to liver surgery [1–4].
It is the task of radiologic imaging to evaluate the liver to assess the presence or absence of liver metastases in surgical candidates and to evaluate the success of chemotherapy in others. Although transabdominal sonography is widely used to assess the liver, it has some limitations: it needs considerable operator expertise and often reveals equivocal results in patients with (chemotherapy-induced) fatty infiltration of the liver. These problem cases are then often referred for a computed tomography (CT) or magnetic resonance (MR) imaging examination. With the introduction of multidetector CT (MDCT) imaging, the use of CT in oncologic patients to ‘screen’ for lung, liver, and lymph node metastases in the body has dramatically increased. MR imaging is still limited in the anatomic coverage, although the recent introduction of multi-channel MR coils with wider coverage and the moving-table MR technique has re-established the ‘competitiveness’ of MR with MDCT with regard to patient throughput. One of the advantages of MR in liver imaging is the better soft tissue contrast, which reveals better characterization of focal liver lesions in question. The development of a liver-specific MR contrast agent has further improved the diagnostic yield of MRI in lesion detection and characterization.
In this review, the role of MR imaging with non-specific gadolinium chelates and liver-specific MR contrast agents is demonstrated. The CT and MR imaging features of liver metastases is presented. Emphasis is placed on the role of MRI in comparison to CT in the assessment of patients with extrahepatic cancer and limited liver metastases, who are surgical candidates.
Ultrasound
The development of ultrasound (US) contrast agents (SonoVue®, Bracco, Milan, Italy; Levovist®, Schering, Germany, etc.) has dramatically increased the potential of sonography in the assessment of focal liver lesions. The use of contrast agents allows perfusion mapping of focal lesions, thus enabling characterization of focal lesions. In the study of von Herbay et al., the use of contrast-enhanced US (CEUS) improved the sensitivity and specificity of US in the differentiation of malignant vs. benign from 78% to 100% and from 23% to 92%, respectively [5]. Bernatik et al. investigated the diagnostic yield of CEUS vs. helical CT in the detection of liver metastases. CEUS showed 97% of lesions seen by CT [6]. However, no histologic standard of reference was available to determine the true sensitivities of both methods. CEUS now has an established role in the evaluation of equivocal lesions seen at conventional US and in monitoring treatment response after local therapy of tumours. However, due to the limitations in the visualization of segmental distribution and 3D-shape of metastases, it is limited in the preoperative assessment of patients with colorectal liver metastases. However, contrast agents have improved the diagnostic yield of intraoperative US with an impact on surgical strategy [7].
Multidetector-row CT
Helical and multidetector-row CT (MDCT) are the most commonly used imaging modalities for detection and characterization of hepatic metastases. Using a helical CT with a single detector row and a scanning speed of 0.8–1 s per rotation, it is impossible to scan the entire liver in a truly arterial phase, before contrast material inflow from the portal vein is encountered.
With the advent of four-row detector scanners in 1998, coverage of the liver within one breathhold of 10–14 s became feasible, which decreased the likelihood of motion artifacts due to breathing during scanning. Currently, 40–64-row MDCT scanners with 0.6 mm detector configuration are on the market. Rotation time has come down to 0.33 s with the latest generation. Therefore, the liver can be scanned with submillimetre collimation within one breathhold of not more than 2–3 s. Due to isotropic voxels, image reformation in any plane is possible without a loss of spatial resolution.
Several studies have assessed the value of using thin slices to improve detection of small metastases. In the study of Weg et al., 2.5 mm thick slices were significantly superior to 5, 7.5 and 10 mm thick slices [8]. In the study of Kopka et al., a slice thickness of 3.75 mm proved superior to 5 mm in terms of lesion characterization and superior to 7.5 mm in terms of detection and characterization [9]. When the slice thickness is decreased to 1 mm, no further improvement in lesion detection is seen, but there is a considerable increase in image noise with subsequent degradation of image quality [10]. Therefore a slice thickness of 2–4 mm is recommended for axial viewing. Not surprisingly, differences between imaging protocols were most prominent when small liver lesions (≤10 mm) were evaluated [9]. However, in addition to those 2–4 mm thick slices obtained for viewing, submillimetre slices are obtained for 3D-image reconstructions.
There has been an ongoing debate, how many scans are necessary for a CT examination of the liver. The value of an unenhanced scan lies primarily in the characterization of small lesions as being solid or cystic. However, in patients with colorectal cancer, liver metastases are calcified in 11% at initial presentation [11]. These lesions are much better seen on unenhanced scans than on portal-venous phase scans (Fig. 1). Arterial-phase scans are of great importance in the diagnosis of hypervascular metastases and in the differentiation between these lesions and haemangiomas, especially in case of early and completely enhancing haemangiomas. The increased temporal resolution of MDCT has led some investigators to add an early arterial phase to their protocols, which is only useful in patients with HCC, if ever [12]. Colorectal liver metastases are hypovascular in the vast majority, but arterial-phase scans may increase lesion conspicuity in a small number of cases (Fig. 2) [13]. Portal-venous phase scans are most reliable in detection of colorectal liver metastases, with a reported sensitivity of 85.1% for helical CT [14].
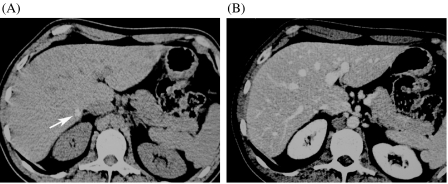
Figure 1.
Value of unenhanced CT in detection calcified metastases. (A) The unenhanced scan clearly depicts a small calcified metastasis (arrow), which turned out to be vital tumour at surgery. (B) The lesion is hardly seen in the portal-venous phase.
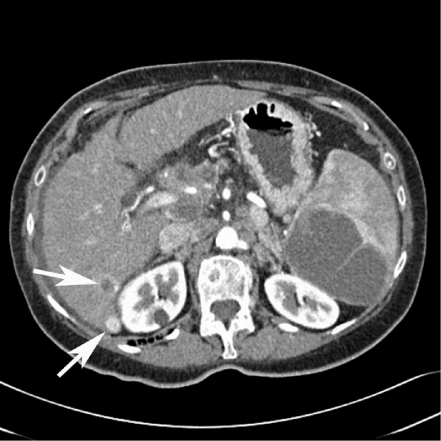
Figure 2.
Need for bi-phasic contrast-enhanced scan for detection of mixed vascularity metastatic adenocarcinoma: the arterial-phase scan demonstrates hypovascular and hypervascular metastases (arrows). Incidental note is made of a large metastasis in the spleen.
MR imaging
MR imaging is commonly used as the definitive imaging modality for the detection and characterization of liver lesions [15]. Use of MR units with a field strength of ≥1 T is preferable, and phased-array torso coils are now standard in body MR imaging. The standard MR imaging protocol should always include unenhanced T1- and T2-weighted and contrast-enhanced pulse sequences. In liver MR imaging a set of T1-weighted in-phase and opposed-phase gradient-recalled echo GRE images is acquired to assess the parenchyma for the presence of fatty infiltration or focal sparing of diffuse fatty infiltration. For T2-weighted imaging, the turbo-spin echo (TSE; or: fast spin echo, FSE) with fat suppression are preferred over the single-shot TSE pulse sequences, which lack inherent soft tissue contrast due to long echo trains. For detection of focal lesions a TE of approximately 80–100 ms is chosen. In addition, heavily T2-weighted pulse sequences with a TE of approximately 160–180 ms may help in differentiation between solid (metastasis, hepatocellular carcinoma (HCC), etc.) and non-solid lesions (e.g. haemangioma, cyst) (Figs 3 and 4) [16, 17]. After the acquisition of unenhanced pulse sequences, contrast-enhanced pulse sequences are always obtained.
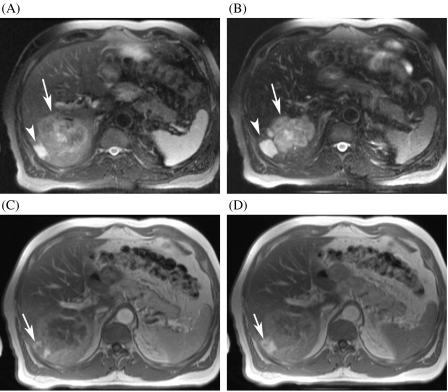
Figure 3.
Value of T2-weighted images and non-specific gadolinium chelates in lesion characterization in a patient with a history of haemangioma in segment 6. (A) The T2-weighted TSE image reveals a moderately hyperintense lesion, suggestive of a metastasis (arrow). There is a second small lesion adjacent to the metastasis, which is very hyperintense on T2-weighted images (arrowhead). (B) On the SPIO-enhanced image, there is better delineation of both lesions. (C), (D) The dynamic gadolinium-enhanced images in the arterial and the delayed phase show peripheral nodular enhancement with pooling in the smaller lesion, indicative of haemangioma (arrow). Patient had developed a colon cancer metastases close to this previously known haemangioma.
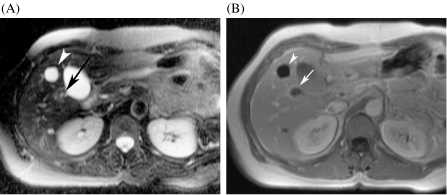
Figure 4.
Small metastasis and cyst: differentiation with T2-weighted TSE and non-specific gadolinium chelates. (A) The T2-weighted TSE image shows a small cyst, which is very bright (arrowhead). There is a second lesion, which is moderately hyperintense (arrow). (B) The gadolinium-enhanced T1-weighted GRE image shows lack of enhancement of the cyst (arrowhead). The other lesion displays a ring enhancement, which is suggestive of metastasis (arrow).
MR contrast agents
Nowadays, two different groups of MR contrast agents for liver imaging are available: First, the non-specific gadolinium chelates and second the liver-specific MR contrast agents. The latter group can be divided into two subgroups, the hepato-biliary contrast agents, and the reticulo-endothelial (or Kupffer cell) contrast agents.
Non-specific gadolinium chelates
The liver and liver-lesion enhancement patterns obtained with non-specific gadolinium chelates (extracellular contrast agents) are similar to those obtained with iodinated contrast agents used in CT. Several agents with similar properties are on the market, including gadopentetate dimeglumine (Schering, Berlin, Germany), Gd-DTPA-BMA (GE Healthcare, Oslo, Norway), Gd-DOTA (Guerbet, Aulnay-sous-Bois, France), and Gadoteridol (Bracco, Milan, Italy). The standard dosage of non-specific gadolinium chelates is 0.1 mmol /kg b.w. After i.v. bolus injection dynamic T1-weighted GRE sequences are obtained at least in the arterial phase, portal venous phase and equilibrium phase (3–5 min post). Colorectal liver metastases are typically hypovascular. In the arterial phase, they are often isointense or minimally hypointense; maximum lesion-to-liver contrast is reached in the portal-venous phase, when a ring enhancement is present (Fig. 4) [18]. The equilibrium phase is important, because it helps with lesion differentiation (e.g. haemangioma vs. metastasis). Haemangiomas show persistent pooling of contrast material during the equilibrium phase, whereas most metastases appear hypointense or centrally isointense with peripheral wash-out of contrast material (Fig. 3) [17].
Liver-specific contrast agents
Hepatobiliary agents
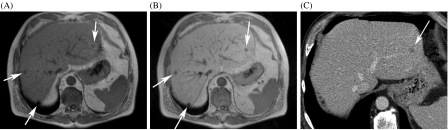
Hepatobiliary agents represent a heterogeneous group of paramagnetic molecules of which a fraction is taken up by hepatocytes and excreted into the bile. Mangafodipir trisodium (Teslascan®, GE Healthcare) is taken up by hepatocytes and results in signal intensity increase on T1-weighted images (a so-called ‘T1 enhancer’) [19], and a fraction is also taken up by the pancreas, which has been used for pancreatic MR imaging [20, 21]. Focal non-hepatocellular lesions (i.e. metastases) do not enhance post-contrast, resulting in improved lesion conspicuity (Fig. 5). Mangafodipir-enhanced MRI has been show to be superior to unenhanced MRI and helical CT for detection of liver metastases [21, 22].
Figure 5.
Comparison of mangafodipir (Teslascan®)-enhanced MRI and CT. (A) Unenhanced T1-weighted MRI shows some lesions in the liver (arrows). (B) There is much better delineation of the metastases on the mangafodipir-enhanced images. (C) On the contrast-enhanced MDCT, only one metastasis in the left lobe is seen. The other lesions were also not seen on adjacent slices.
Gd-BOPTA (Multihance®, Bracco) and Gd-EOB-DTPA (Primovist®, Schering) are hybrid contrast agents, which carry a lipophilic ligand [23]. After i.v. bolus injection these agents show biphasic liver enhancement with a rapid T1 enhancement of the liver similar to that seen with non-specific extracellular gadolinium agents. Then hepatic signal intensity continues to rise for 20–40 min (Gd-EOB-DTPA) and 60–90 min (Gd-BOPTA), reaching a plateau after about 2 h because of hepatocytic uptake. This results in increasing contrast between liver and non-hepatocellular tumours [24].
Reticuloendothelial agents
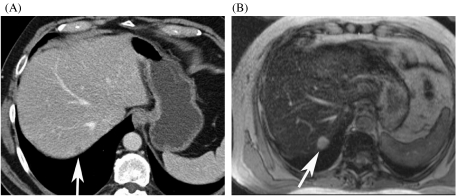
All reticuloendothelial system (RES) agents are superparamagnetic iron oxide-based contrast agents (SPIO). They are predominantly phagocytosed by the Kupffer cells in the liver and the spleen and cause local field inhomogeneities, which result in shortening of T2 relaxation times and decreased signal intensity of liver tissue. Currently, two SPIO agents (Endorem®, ferumoxide, Guerbet; Resovist®, SHU 555A, Schering) are available. SHU 555A (Resovist®) can be administered as an i.v. bolus and dynamic T1-weighted sequences can be obtained to assess tumour vascularization. SHU 555A has fewer side effects than ferumoxide (Endorem®). After SPIO administration, the liver parenchyma containing Kupffer cells shows a marked reduction in signal intensity on T2-weighted images, whereas liver metastases remain hyperintense on T2-weighted images. Thus, due to the decreased SI of normal liver and no signal loss of metastases, the lesion contrast is markedly improved on post-contrast T2-weighted images [25] (Fig. 6). RES agents are also useful in differentiation of metastases from focal liver lesions from benign hepatocellular lesions (such as focal nodular hyperplasia (FNH) or adenomas) and haemangiomas, because the latter show uptake of contrast material with subsequent signal intensity loss [25]. SPIO contrast agents have a predominant T2 effect, although there is also a T1 effect, which may be used for perfusion imaging of metastases.
Figure 6.
Comparison of SHU 555A (Resovist®)-enhanced MRI and MDCT. (A) On the contrast-enhanced MDCT, a metastasis is only faintly seen (arrow). (B) The SHU 555A-enhanced T2-weighted image depicts a 1.5 cm metastasis in segment 7.
Detection of liver metastases: which imaging modality?
In a time of limited resources in health care, there has been considerable debate which imaging modality offers the best non-invasive examination of the liver, offering both detection and characterization of local liver lesions. The use of multiple diagnostic modalities is both costly and time-consuming.
A meta-analysis has compared the diagnostic value of US, CT, MRI and PET in the detection of gastrointestinal cancer metastases derived from studies published in the literature [26]. Surprisingly, this meta-analysis found that FDG-PET (with CT) is the most sensitive method for detection of metastases, with a mean weighted sensitivity of 90%–92% [26]. However, several studies in this analysis assessed metastases per lesion, which yields lower sensitivities than studies assessing metastatic load per patient. Seventy-three percent of MR studies in this analysis used per-lesion analysis, whereas only 22% of PET studies did so. The reliance on a per-patient analysis in most of the PET studies is likely to inflate the sensitivity of this method (e.g. detection of only one of four metastases present would be considered a correct positive diagnosis). So, inhomogeneities of the studies analysed make it difficult to draw conclusions [26].
Accordingly, the issue of when to use which imaging method is still not solved. The answer likely depends on local equipment, availability, and operator expertise. MDCT scanning is well established and is often the first choice for a ‘screening’ liver examination at many institutions. The MDCT technique has improved small lesion detection by reducing respiration-related artifacts. Shortened scan time of MDCT enables exact multiphase scanning of the chest and abdomen with improved lesion characterization, but increases the radiation exposure on the other hand. MDCT has the big advantage of ‘one-stop-shopping’ in imaging of the liver and extrahepatic disease (both abdominal and thoracic). This ensures that MDCT will continue to have an important role in staging and screening.
Several studies have reported MRI to be more sensitive and more specific than dynamic CT and helical CT. Ferumoxide-enhanced MRI has been shown to detect more, especially small, metastatic lesions than contrast-enhanced CT [27]. Small lesions, which are detected at a greater frequency with this technique, are particularly difficult to characterize exactly. Gadolinium-enhanced MRI may be helpful in characterization of these lesions, particularly for small haemangiomas, cysts, and biliary hamartomas. Liver-specific MR contrast agents are helpful in the differentiation between FNH and hypervascular liver metastases. The wide array of MR pulse sequences and MR contrast agents available makes MRI the most powerful tool for non-invasive lesion characterization [15].
Preoperative assessment of surgical candidates
The majority of liver metastases are non-resectable because of extrahepatic disease or extensive liver involvement. With increasing surgical expertise in liver resection, indications for resection of limited metastatic disease have expanded in recent years. To prevent unnecessary laparatomies in patients referred for surgery, meticulous preoperative assessment of metastatic liver involvement should be performed [27].
The ideal preoperative imaging modality should combine (1) high sensitivity and (2) high specificity, with a low false-positive rate for metastases detection and characterization. It should provide (3) precise anatomic information of the tumour location in relation to the major anatomic structures. In most oncologic centres, contrast-enhanced CT and/or MRI are the mainstay of preoperative staging in patients with liver tumours. However, in the study of Zacherl et al., helical CT either showed either false-positive and false-negative diagnoses in 42% of patients referred for surgery [28]. In comparison to preoperative staging the surgical strategy was changed by intraoperative US in 22.8% [28].
Recently, two prospective studies on the use of CT and MRI in surgical candidates with colorectal liver metastases have been reported [22, 29]. Mann et al. compared mangafodipir-enhanced MRI helical CT in preoperative assessment of liver metastases for resectability [22]. He found MRI to be more sensitive than helical CT in the preoperative assessment of the resectability of hepatic lesions (Fig. 5). MRI detected significantly more lesions than helical CT (sensitivity 83% vs. 61%), but intraoperative US detected a few subcentimetre metastases not seen by MRI. The extent of metastatic disease was under- or overestimated in only 10% of patients by mangafodipir-enhanced MRI [22]. Van Etten et al. found the ferumoxide-enhanced MRI technique at least as accurate as CT during arterioportography (CTAP) in preoperative assessment of colorectal liver metastases [29]. Both methods were equivalent in 81% of patients, and CTAP showed more lesions in another 11%. However, this influenced further management in only 2%. In 8%, ferumoxide-enhanced MRI showed more lesions than CTAP, and this influenced the clinical decision in 4%, rendering these patients with widespread disease non-resectable [29]. Up to now no studies comparing MRI with MDCT have been performed.
In summary, contrast-enhanced multi-phasic MDCT is a robust and accurate technique to assess liver and extrahepatic disease in patients with colorectal cancer. In patients with limited metastatic disease to the liver, MR imaging enhanced with liver-specific contrast agents is recommended for preoperative assessment.
References
- 1.Lodge JPA. Modern surgery for liver metastases. Cancer Imaging. 2000;1:77–9. doi: 10.1102/1470-7330/00/010077+09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rees M, John TG. Current status of surgery in colorectal metastases to the liver. Hepato-Gastroenterology. 2001;48:341–4. [PubMed] [Google Scholar]
- 3.Scheele J, Stangl R, Altendorf-Hofman A. Hepatic metastases from colorectal cancer: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–6. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 4.Stangl R, Altendorf-Hofman A, Charnely R, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–10. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 5.von Herbay A, Vogt C, Willers R, Haussinger D. Real-time imaging with the sonographic contrast agent SonoVue: differentiation between benign and malignant hepatic lesions. J Ultrasound Med. 2004;23:1557–68. doi: 10.7863/jum.2004.23.12.1557. [DOI] [PubMed] [Google Scholar]
- 6.Bernatik T, Strobel D, Hahn EG, Becker D. Detection of liver metastases: comparison of contrast-enhanced wide-band harmonic imaging with conventional ultrasonography. J Ultrasound Med. 2001;20:509–15. doi: 10.7863/jum.2001.20.5.509. [DOI] [PubMed] [Google Scholar]
- 7.Torzilli G. Contrast-enhanced intraoperative ultrasonography in surgery for liver tumors. Eur J Radiol. 2004;51((Suppl)):S25–29. doi: 10.1016/j.ejrad.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Weg N, Scheer MR, Gabor MP. Liver lesions: improved detection with dual-detector-array CT and routine 2.5-mm thin collimation. Radiology. 1998;209:417–26. doi: 10.1148/radiology.209.2.9807568. [DOI] [PubMed] [Google Scholar]
- 9.Kopka L, Grabbe E. Biphasische Leberdiagnostik mit der Mehrzeilendetektor-Spiral-CT. Radiologe. 1999;39:971–8. doi: 10.1007/s001170050590. [DOI] [PubMed] [Google Scholar]
- 10.Kulinna C, Helmberger T, Kessler M, Reiser M. Verbesserung der Diagnostik von Lebermetastasen mit der Multi-Detektor-CT. Radiologe. 2001;41:16–23. doi: 10.1007/s001170050923. [DOI] [PubMed] [Google Scholar]
- 11.Hale HL, Husband JE, Gossios K, Norman AR, Cunningham D. CT of calcified liver metastases in colorectal cancer. Clin Radiol. 1998;53:735–41. doi: 10.1016/s0009-9260(98)80315-2. [DOI] [PubMed] [Google Scholar]
- 12.Foley WD, Mallisee TA, Hohenwalter MD, Wilson CR, Quirz FA, Taylor AJ. Multiphase hepatic CT with a multirow detector CT scanner. AJR. 2000;175:679–85. doi: 10.2214/ajr.175.3.1750679. [DOI] [PubMed] [Google Scholar]
- 13.Ch’en IY, Katz DS, Jeffrey RB, et al. Do arterial phase helical CT improve detection or characterization of colorectal liver metastases? J Comput Assist Tomogr. 1997;21:391–7. doi: 10.1097/00004728-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Valls C, Andia E, Sanchez A, et al. Hepatic metastases from colorectal cancer: preoperative detection and assessment of resectability with helical CT. Radiology. 2001;218:55–60. doi: 10.1148/radiology.218.1.r01dc1155. [DOI] [PubMed] [Google Scholar]
- 15.Robinson PJ. Indeterminate liver lesions in cancer. Cancer Imaging. 2002;2:130–2. DOI: 10.1102/1470-7330.2002.0010. [Google Scholar]
- 16.Schima W, Saini S, Echeverri JA, Hahn PF, Harisinghani M, Mueller PR. T2-weighted MR imaging for characterization of focal liver lesions: conventional spin-echo vs. fast spin-echo. Radiology. 1997;202:389–93. doi: 10.1148/radiology.202.2.9015063. [DOI] [PubMed] [Google Scholar]
- 17.Bennett GL, Petersein A, Mayo-Smith WW, Hahn PF, Schima W, Saini S. Addition of gadolinium chelates to heavily T2-weighted MR imaging: limited role in differentiating hepatic hemangiomas from metastases. AJR. 2000;174:477–85. doi: 10.2214/ajr.174.2.1740477. [DOI] [PubMed] [Google Scholar]
- 18.Hamm B, Mahfouz A-E, Taupitz M, et al. Liver metastases: improved detection with dynamic gadolinium-enhanced MR imaging? Radiology. 1997;202:677–82. doi: 10.1148/radiology.202.3.9051015. [DOI] [PubMed] [Google Scholar]
- 19.Elizondo G, Fretz CJ, Stark DD, et al. Preclinical evaluation of Mn-DPDP: new paramagnetic hepatobiliary contrast agent for MR imaging. Radiology. 1991;178:73–8. doi: 10.1148/radiology.178.1.1898538. [DOI] [PubMed] [Google Scholar]
- 20.Gehl H-B, Urhahn R, Bohndorf K, et al. Mn-DPDP in MR imaging of pancreatic adenocarcinoma: initial clinical experience. Radiology. 1993;186:795–8. doi: 10.1148/radiology.186.3.8430190. [DOI] [PubMed] [Google Scholar]
- 21.Schima W, Függer R, Schober E, et al. Diagnosis and staging of pancreatic cancer: comparison of mangafodipir-enhanced MRI and contrast-enhanced helical hydro-CT. AJR. 2002;179:717–24. doi: 10.2214/ajr.179.3.1790717. [DOI] [PubMed] [Google Scholar]
- 22.Mann GN, Mary HF, Lai LL, Wagman LD. Clinical and cost effectiveness of a new hepatocellular MRI contrast agent, mangafodipir trisodium, in the preoperative assessment of liver resectability. Ann Surg Oncol. 2001;8:573–9. doi: 10.1007/s10434-001-0573-8. [DOI] [PubMed] [Google Scholar]
- 23.Hamm B, Staks T, Muehler A, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195:785–92. doi: 10.1148/radiology.195.3.7754011. [DOI] [PubMed] [Google Scholar]
- 24.Caudana R, Morana G, Pirovano GP, et al. Focal malignant hepatic lesions: MR imaging enhanced with gadolinium benzyloxypropionictetra-acetate (BOPTA)-preliminary results of phase II clinical application. Radiology. 1996;199:513–20. doi: 10.1148/radiology.199.2.8668804. [DOI] [PubMed] [Google Scholar]
- 25.Reimer P, Jähnke N, Fiebich M, et al. Hepatic lesion detection and characterization: value of nonenhanced MR imaging, superparamagnetic iron oxide-enhanced MR imaging, and spiral CT-ROC analysis. Radiology. 2000;217:152–8. doi: 10.1148/radiology.217.1.r00oc31152. [DOI] [PubMed] [Google Scholar]
- 26.Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748–56. doi: 10.1148/radiol.2243011362. [DOI] [PubMed] [Google Scholar]
- 27.Robinson PJ. The early detection of liver metastases. Cancer Imaging. 2002;2:113–115. DOI: 10.1102/1470-7330.2002.0009. [Google Scholar]
- 28.Zacherl J, Scheuba C, Imhof M, et al. Current value of intraoperative sonography during surgery for hepatic neoplasms. World J Surg. 2002;26:550–4. doi: 10.1007/s00268-001-0266-2. [DOI] [PubMed] [Google Scholar]
- 29.van Etten B, van der Sijp JRM, Kruyt RH, Oudkerk M, van der Holt B, Wiggers T. Ferumoxide-enhanced magnetic resonance imaging technique in pre operative assessment for colorectal liver metastases. Eur J Surg Oncol. 2002;28:645–51. doi: 10.1053/ejso.2001.1251. [DOI] [PubMed] [Google Scholar]