Abstract
FDG-PET imaging has significantly altered the workup of the oncologic patient. With the introduction of combined FDG-PET/CT scanners, there has been a dramatic improvement in lesion detection, lesion characterization and accurate lesion localization. As attenuation correction can be attained with the CT images, PET exam times have been dramatically reduced, by as much as 50% in many instances. In this overview we briefly outline the advantages of CT/PET in oncology especially in diagnosis and clinical management of the common tumor types for which it is mainly used.
Keywords: Computed tomography (CT), positron emission tomography (PET), fluorine-18 fluorodeoxyglucose (FDG), head and neck neoplasms, lung carcinoma, lymphoma, colon cancer
Introduction
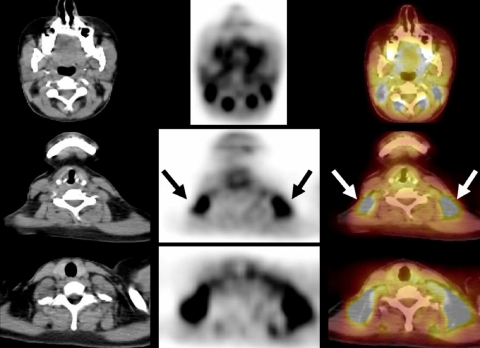
Fluorine-18 fluorodeoxyglucose ([18F]FDG) is currently the most common agent used in PET imaging. Normal tissues utilize glucose for energy and FDG is a glucose analog that is taken up by cells and then trapped intracellularly in this pathway. Most tumors tend to favor this pathway and therefore tend to appear more active (‘hotter’) than normal tissues. However, both benign and malignant processes can be FDG avid. Furthermore, normal physiologic uptake can simulate or obscure disease (i.e. brain uptake, muscle uptake after exercise, cardiac activity in the non-fasting state and uptake in brown fat (Fig. 1).
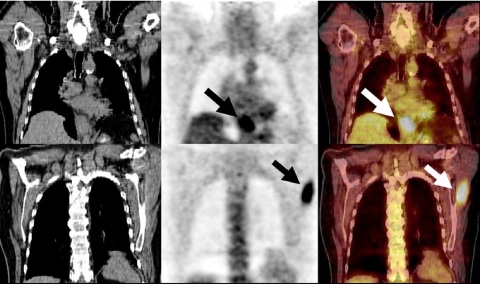
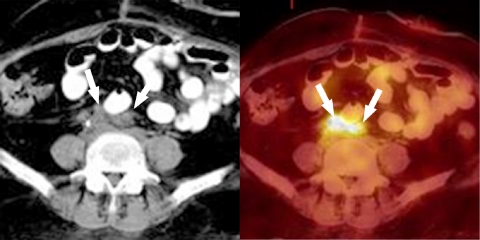
Figure 1.
A 27-year-old woman with diffuse large B cell NHL. An FDG-PET/CT study performed to assess response to chemotherapy demonstrated intense FDG activity in the lateral cervical and supraclavicular areas bilaterally. Axial PET/CT images showed that focal FDG activity corresponded to fat (arrows) and excluded the presence of lymph nodal disease.
Several cancers can be diagnosed and staged using [18F]FDG-PET with accuracies ranging from 80% to 98%. In addition, response to therapy assessment and prognostic information can be obtained from [18F]FDG-PET images. Although there was an already high performance for [18F]FDG-PET images, they lacked spatial resolution, even with attenuation correction schemes. Fusion of CT and PET images was developed with some success. But as these images were acquired separately and later fused, there were problems with accurate localization [1]. The development of a dedicated CT/PET system by Townsend et al. has overcome many of the problems that fusing CT and PET images has [1]. In addition acquisition times for PET/CT images are much shorter. PET/CT in general has improved reader confidence in lesion detection, localization, as well as characterization, leading to a marked reduction in indeterminate or equivocal findings. In addition, as the CT images are used for attenuation correction, acquisition times are much shorter, by up to 50% [2].
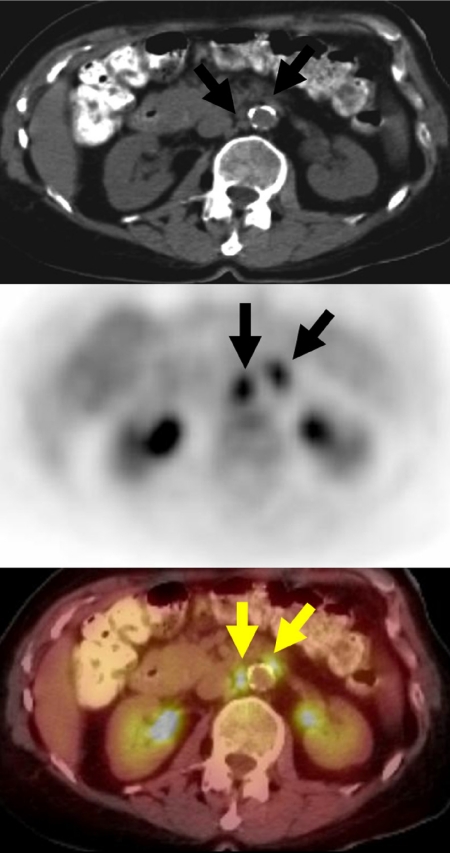
The added advantages of combined PET/CT images are its ability to overcome the limitations of size criteria used to suggest the presence of tumor deposits in normal sized lymph nodes (Fig. 2), and its ability to localize and characterize the functional aspect of an anatomic finding. Therefore combined CT/PET images offer the advantage of demonstrating metabolism and structure simultaneously [2–5].
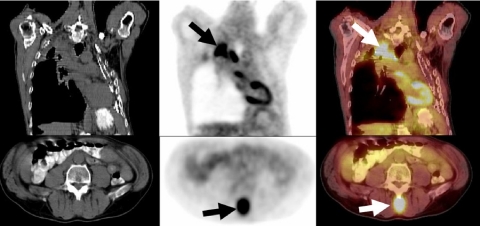
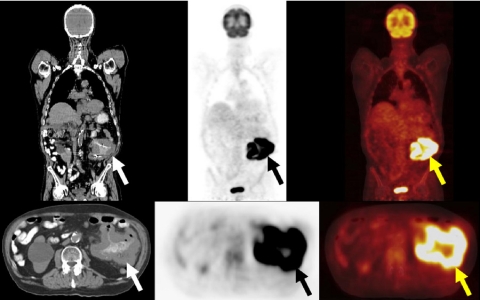
Figure 2.
A 60-year-old woman with a history of rectosigmoid colon cancer (T2N0M0), who underwent surgical resection 2 years previously, presented with progressively rising CEA levels. A restaging FDG-PET/CT study demonstrated intense FDG avidity in normal size inter-aortocaval and left para-aortic lymph nodes (arrows), due to metastatic retroperitoneal lymph nodes, unsuspected based on the initial diagnostic abdominal CT which showed normal sized retroperitoneal lymph nodes.
Added value of combined [18F]FDG-PET/CT over CT and [18F]FDG-PET as separate exams for diagnostic imaging and patient management in oncology
In a study of 204 patients, [18F]FDG-PET vs. [18F]FDG-PET/CT images were reviewed to determine if PET/CT was more advantageous than PET alone. In these 204 patients, 586 suspicious lesions were evaluated for change in lesion characterization, either definitely benign or malignant (Fig. 3), precise anatomic localization (Fig. 4), and retrospective detection on PET and CT. Combined [18F]FDG-PET/CT provided additional information in 99 patients (49%) at 178 sites (30%). [18F]PET/CT improved characterization of equivocal lesions as definitely benign at 10% of sites and as malignant in 6% of sites. Retrospective lesion detection on CT or PET images was achieved in 8% of patients (Fig. 5). Overall, the results of [18F]PET/CT had an impact on the management of 28 patients (14%). PET/CT obviated the need for further evaluation in five patients, guided further diagnostic procedures in seven and assisted in planning therapy for 16 patients [3].
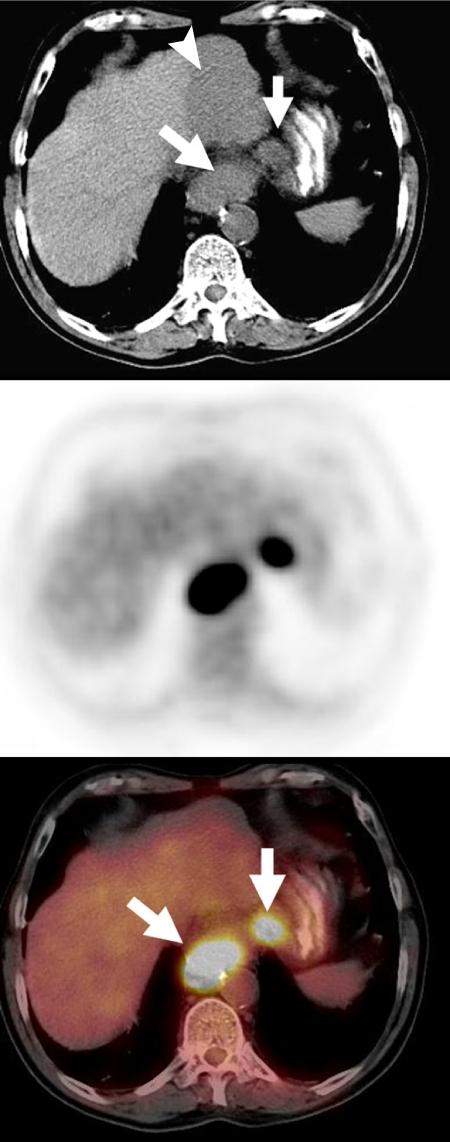
Figure 3.
A 67-year-old man diagnosed with esophageal cancer. Unenhanced CT scan showed a large GE junction tumor (arrow) and an enlarged lesser gastric curvature lymph node (arrow), as well as a large hypodense lesion in the left hepatic lobe (arrowhead). FDG-PET/CT showed intense metabolic activity in the primary tumor, confirmed regional metastatic lymphadenopathy in the lesser curvature lymph node and demonstrated lack of FDG avidity in the liver lesion, which was shown to be a large hepatic hemangioma by other imaging methods.
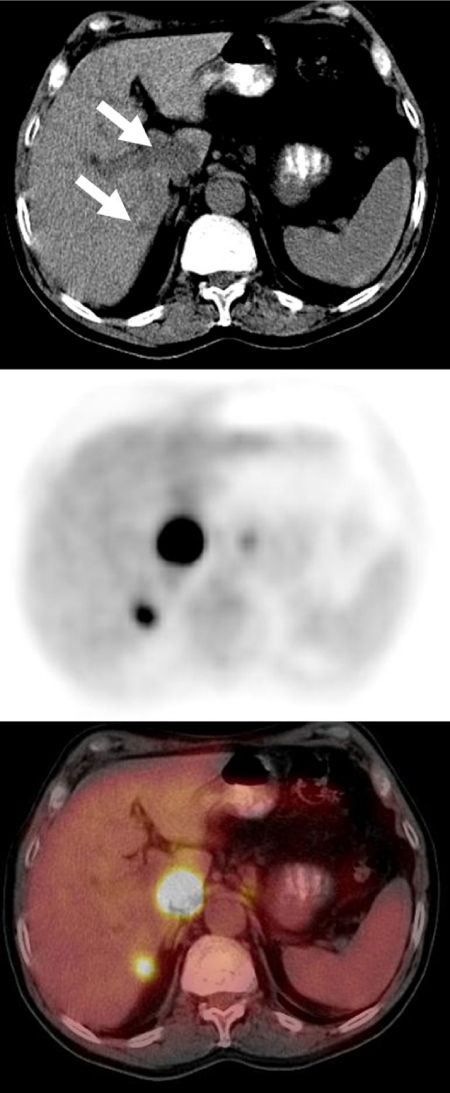
Figure 4.
A staging FDG-PET/CT scan in a patient with esophageal cancer demonstrated intense FDG avidity in two hypodense liver lesions (large lesion in the caudate lobe and a smaller lesion in the medial aspect of the posterior segment of the right hepatic lobe) consistent with liver metastases. The CT scan provided accurate anatomic localization of the abnormal FDG activity to the liver lesions, clearly excluding the right adrenal gland or paraaortic lymph nodes as sites of metastatic disease.
Figure 5.
A 70-year-old man presenting with dysphagia and weight loss was diagnosed with esophageal adenocarcinoma based on endoscopy. A staging FDG-PET/CT study demonstrated intense metabolic activity in a distal esophageal tumor (top row images arrow) and in the left scapular region (bottom row images arrow) consistent with unsuspected distant soft tissue metastasis (stage IV disease). Based on FDG-PET/CT results the clinical management of this patient was changed from surgical resection of the primary tumor to combined chemo-radiation therapy.
[18F]FDG-PET and [18F]FDG-CT/PET have also been shown to be very useful in guiding the most suitable lesion to biopsy as well as its most viable portion [6, 7]. CT/PET is also useful in radiotherapy treatment planning, and in one study gross tumor volume (GTV) delineation was increased by 25% or more in 17% of head and neck and lung cancers respectively and 33% of pelvic tumors. GTV was reduced by 25% or less in 33% of head and neck cancers, 67% of lung cancers and 19% of pelvic cancers. Overall treatment volume was altered in 56% of patients using CT/PET [6].
Head and neck cancer
In a study of 68 patients with 157 sites of uptake, with [18F]FDG-PET alone, 71 malignant, 45 benign and 39 equivocal lesions were demonstrated (Table 1). With [18F]FDG-PET/CT, there was a decrease in equivocal lesions of 53%, and accuracy improved from 90% to 96%. Six proven malignancies were missed with PET and only one with PET/CT. In addition accurate anatomic localization was achieved in 100 lesions and overall management was impacted in 18% [8].
Table 1.
| PET finding | PET/CT finding |
||
|---|---|---|---|
| Benign | Equivocal | Malignant | |
| Benign (N=45) | 41 | 2 | 2 |
| Equivocal (N=39) | 21 | 13 | 5 |
| Malignant (N=71) | 1 | 2 | 68 |
| Total (N=155) | 63 | 17 | 75 |
Adapted from Schoder et al. [8].
Lung cancer
Forty-nine consecutive cases of non-small cell lung cancer (NSCLC) underwent CT and [18F]FDG-PET and [18F]FDG-PET/CT. Surgical proof was obtained in 40/49 patients. [18F]FDG-PET/CT provided additional data in 20/49 (41%) patients (Table 2). It demonstrated accurate localization of metastatic lymph nodes in nine, accurate determination of chest wall involvement in three, mediastinal invasion in three, and the presence of distant metastases in two instances (Fig. 6). It was also helpful in distinguishing tumor from atelectasis/inflammation in seven cases [9].
Table 2.
| No. of patients (%) |
|||
|---|---|---|---|
| Correct | Correct but equivocal | Incorrect | |
| CT alone | 23 (58) | 8 (20) | 9 (22) |
| PET alone | 16 (40) | 16 (40) | 8 (20) |
| Visual correlation of | |||
| PET and CT | 26 (65) | 5 (12) | 9 (22) |
| Integrated PET/CT | 35 (88) | 4 (10) | 1 (2) |
Adapted from Lardinois et al. [9].
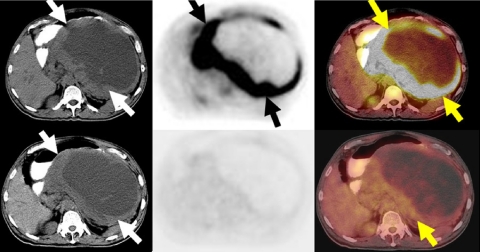
Figure 6.
A 60-year-old man with a history of left lung bronchogenic carcinoma, prior left pneumectomy 5 years previously, presented with cough and weight loss. A restaging FDG-PET/CT study to evaluate for recurrent disease demonstrated intense FDG avidity in a large right upper lobe mass (arrows) and in metastatic mediastinal lymph nodes. The study also revealed an intensely FDG avid soft tissue mass associated with a destructive L5 spinous process consistent with metastatic deposit (arrow).
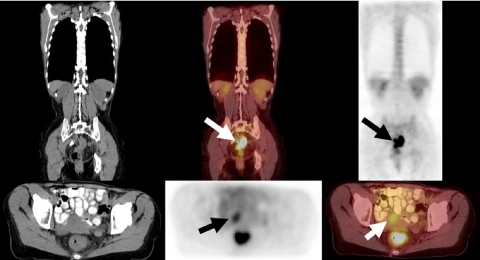
Colon cancer
In a retrospective study of 45 patients with colon cancer, comparing [18F]FDG-PET/CT with [18F]FDG-PET alone, there was a decrease in the number of equivocal readings by 50% for [18F]FDG-PET/CT. In addition, lesion characterization increased by 30%, and accurate localization by 25% (Tables 3 and 4). Overall staging accuracy improved from 78% to 89% on a patient-by-patient analysis (Fig. 7) [10, 11].
Table 3.
| Lesion location score | Number of lesions |
% change: PET/CT | |
|---|---|---|---|
| PET | PET/CT | vs. PET | |
| Definite location | 92 | 115 | 25 |
| Uncertain location | 28 | 14 | |
| Probable location | 14 | 5 | |
| Total | 42 | 19 | −55 |
Adapted from Cohade et al. [11].
Table 4.
| Lesion characterization | Number of lesions |
% change: PET/CT | |
|---|---|---|---|
| PET | PET/CT | vs. PET | |
| Definite lesions | |||
| Definitely benign | 0 | 15 | |
| Definitely malignant | 84 | 94 | |
| Total | 84 | 109 | 30 |
| Probably lesions | |||
| Probably benign | 19 | 9 | |
| Equivocal | 12 | 9 | |
| Probably malignant | 19 | 7 | |
| Total | 50 | 25 | −50 |
Adapted from Cohade et al. [11].
Figure 7.
A 57-year-old woman presented with pain and constipation and colonoscopy revealed an obstructing rectal mass. A staging FDG-PET/CT demonstrated intense FDG avidity in a circumscribed mass-like thickening of the proximal rectum (arrows in top row images) and a focus of mild metabolic activity anterior to the rectum (bottom row arrow) which was not avid as the rectal malignancy. This was located within the uterus as seen on CT images (bottom row), and was subsequently shown to be a uterine fibroid on other imaging studies.
In another study of 65 patients with suspected or known colorectal carcinoma, sensitivity and specificity for PET/CT was 96% and 97% compared to 77% and 89% for PET alone. Similarly the sensitivity and specificity for metastases detection were 95% and 98% for PET/CT compared to 66% and 79% for PET alone [12–14].
Lymphoma
Overall staging specificity for both Hodgkin’s disease (HD) and non-Hodgkin’s lymphoma (NHL) using PET is superior to CT (Fig. 8(a), (b)). PET usually has a specificity that is about 15% higher than that of CT for primary staging of lymphoma [15].
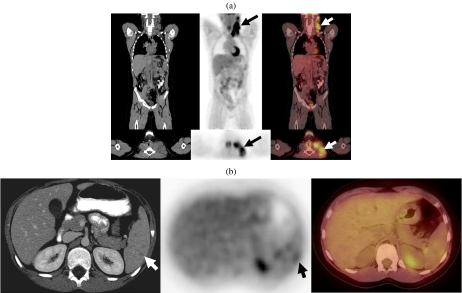
Figure 8.
(a) (Top row) A 9-year-old boy presenting with bulky left cervical lymphadenopathy was diagnosed with HD based on biopsy. A staging FDG-PET/CT study revealed intense metabolic activity in a left lateral cervical conglomerate lymph node enlargement extending from the mastoid to the supraclavicular fossa and the thoracic inlet (arrows). No other foci of abnormal FDG activity are present in the thorax, abdomen or pelvis. (b) (Bottom row) A diagnostic CT scan demonstrated several small hypodense splenic lesions (arrows). A PET-CT study showed minimal FDG activity associated with the splenic lesions (arrow), significantly less than the activity in the enlarged cervical lymph nodes. This was subsequently shown to be due to non-neoplastic cause.
In a study of 27 patients who presented for restaging of lymphoma, PET and CT were compared with PET/CT for staging accuracy. On a patient-based evaluation, CT had a sensitivity of 78%, PET 86%, and PET/CT 93% (Table 5). Using a region-based analysis for lymph node involvement, CT had a sensitivity of 61%, PET 78%, and PET/CT 96%. FDG-PET/CT is accurate in restaging lymphoma and has advantages over CT and FDG-PET alone [16].
Table 5.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | |
| CT | 61 | 89 | 54 | 92 | 84 |
| FDG-PET | 78 | 98 | 90 | 96 | 95 |
| FDG-PET and CT | 91 | 99 | 96 | 98 | 98 |
| FDG-PET/CT | 96 | 99 | 96 | 99 | 99 |
Adapted from Freudenberg et al. [16].
Melanoma
In a prospective study of 106 patients with Stage III disease, unsuspected metastases were detected in 15%. The overall sensitivity and specificity for lesion detection were 92% and 90% and FDG-PET changed overall management in 22% of patients (Fig. 9) [17]. In another study of 84 patients with recurrent melanoma, FDG-PET had a therapeutic impact in 26%: upstaging one-third and downstaging two-thirds of cases [18].
Figure 9.
A 78-year-old man with right temple melanoma (Breslow depth 2.28 mm), after wide local excision 6 years previously, presented with anemia and a left upper quadrant mass. A restaging FDG-PET/CT study demonstrated an intensely FDG avid mass associated with thickened jejunal loops of bowel (arrows). Biopsy and subsequent surgery diagnosed metastatic melanoma involving the proximal jejunum.
Impact of [18F]FDG-PET on management patients scheduled for radiation therapy
Two hundred and two patients with a variety of malignancies, who were scheduled to undergo radiotherapy (RT), underwent a PET scan using [18F]FDG. This included 55 head and neck tumors, 28 gynecologic tumors, 28 breast cancers, 26 lung cancers, 24 lymphomas, 18 tumors of the gastrointestinal tract and 23 other cancers. In 55/202 (27%) of patients, the PET results changed the patients’ management. In 9% of cases treatment was canceled due to detection of previously undetected distant metastases, detection of new lymph node metastases, presence of residual tumor, and exclusion of active disease. In 20/202 (10%) of patients, the PET results changed the intention of therapy (curative or palliative). Radiotherapy dose was altered in 12% and therapy volume was altered in 6%. This study shows that PET has a major impact on the management of patients who are scheduled to undergo radiotherapy [19].
FDG-PET and monitoring response to therapy
Conventional anatomical imaging with CT and MRI are limited in assessing response to therapy for various reasons:
(a) inability to distinguish between residual fibrosis and residual active tumor;
(b) anatomical regression usually lags behind metabolic cell death.
The advantages of FDG-PET are that metabolic change usually precedes anatomic regression, and residual masses can be characterized as metabolically active tumor or residual fibrosis (Figs 10 and 11).
Figure 10.
A 58-year-old man with large gastrointestinal stromal tumor (GIST). Staging PET/CT study demonstrated an extensive primary tumor displaying a peripheral rim of intense metabolic activity (top row) [arrows] surrounding a central photopenic core consistent with massive central tumor necrosis. The patient was treated with Gleevec (bottom row) [arrows] with excellent therapeutic response, as documented by follow-up PET/CT at 2 weeks, showing resolution of the metabolic activity in the tumor with only mild activity.
Figure 11.
A 50-year-old woman presented elevated creatinine and new right ureteral obstruction. She was previously treated for B-cell lymphoma with eight cycles of chemotherapy. CT scans showed abnormal soft tissue density (arrows) in the retroperitoneum around the aortic bifurcation. Residual active disease vs. fibrosis from previously treated lymphoma could not be distinguished. On PET scan there is intense activity (arrows) corresponding to the site of abnormal retroperitoneal soft tissue confirming the presence of residual active disease.
FDG-PET and prognostic implications
Fifteen patients with HD and 17 with aggressive histology NHL with residual masses following therapy underwent [18-F]FDG-PET imaging. Eight of nine patients who were PET positive relapsed. Of 23 patients with PET negative scans only to relapsed (8.7%) (Table 6). Both these patients had aggressive NHL histology and none of the 11 HD patients with PET negative scans relapsed. [18-F]FDG-PET imaging can differentiate between residual masses that are fibrotic and those containing viable tumor (Fig. 11) [20].
Table 6.
| PET | Type | Number of | Residual/relapse | Continued remission |
|---|---|---|---|---|
| patients | (%) | (%) | ||
| Positive | HD | 4 | 3 | 1 |
| NHL | 5 | 5 | 0 | |
| Total | 9 | 8 (89) | 1 (11) | |
| Negative | HD | 11 | 0 | 11 |
| NHL | 12 | 2 | 10 | |
| Total | 23 | 2 (9) | 21 (91) | |
Adapted from Mikhaeel et al. [20].
Conclusion
As FDG-PET/CT is now being accepted as a valid and useful clinical tool in oncology, major insurance carriers are willing to cover its cost. Its initial use in a limited number of malignancies is now being expanded and it may become the standard and possibly the single imaging modality that will be used to detect, stage cancer and monitor its response to therapy. As FDG has limitations in the diagnosis of some malignancies such as those involving the genitourinary tract, as well as for detecting hypoxia, etc., various new tracers are being developed which will enhance the existing value of PET imaging.
References
- 1.Townsend DW, Carney JPJ, Yap JT, et al. PET/CT today and tomorrow. J Nucl Med. 2004;45:S4–13. [PubMed] [Google Scholar]
- 2.Czernin J, Schelbert H. PET/CT imaging: facts, opinions, hopes and questions. J Nucl Med. 2004;45:S1–3. [Google Scholar]
- 3.Bar-Shalom R, Yefremov N, Guralnik L, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44:1200–9. [PubMed] [Google Scholar]
- 4.Gambhir SS, Czernin J, Schwimmer J, Silverman DHS, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42:1–93. [PubMed] [Google Scholar]
- 5.Wahl RL. Why nearly all PET of abdominal and pelvic cancers will be performed as PET/CT. J Nucl Med. 2004;45:S82–95. [PubMed] [Google Scholar]
- 6.Ciernik IF, Dizendorf E, Baumert BG, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys. 2003;57:853–63. doi: 10.1016/s0360-3016(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 7.Bradley JD, Perez CA, Dehdashti F, et al. Implementing biologic target volumes in radiation treatment planning for non-small cell lung cancer. J Nucl Med. 2004;45:S96–101. [PubMed] [Google Scholar]
- 8.Schoder H, Yeung HHWD, Gone M, et al. Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. Radiology. 2004;231:65–72. doi: 10.1148/radiol.2311030271. [DOI] [PubMed] [Google Scholar]
- 9.Lardinois D, Weder W, Hany TF, et al. Staging non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–7. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 10.Schöder H, Larson SM, Yeung HWD. PET/CT in oncology: integration into clinical management of lymphoma, melanoma, and gastrointestinal malignancies. J Nucl Med. 2004;45:S72–81. [PubMed] [Google Scholar]
- 11.Cohade C, Osman M, Leal J, Wahl R. Direct comparison of (18)F-FDG PET/CT in patients with colorectal carcinoma. J Nucl Med. 2003;44:1797–1803. [PubMed] [Google Scholar]
- 12.Shiepers C. PET/CT in colorectal cancer. J Nucl Med. 2003;44:1804–5. [PubMed] [Google Scholar]
- 13.Francis DL, Visvikis D, Bomanji JB, et al. The impact of FDG PET/CT in colorectal cancer: an outcome study (abstract) J Nucl Med. 2003;44:26P. [Google Scholar]
- 14.Berger I, Goerres GW, von Schulthess GK, et al. PET/CT: diagnostic improvement in recurrent colorectal carcinoma compared to PET alone. Radiology. 2002;225((suppl P)):242. [Google Scholar]
- 15.Schiepers C, Filmont J-E, Czernin J. PET for staging Hodgkin’s disease and non-Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging. 2003;30:S82–8. doi: 10.1007/s00259-003-1165-6. [DOI] [PubMed] [Google Scholar]
- 16.Freudenberg LS, Antoch G, Shutt P, et al. FDGPET/CT in restaging of patients with lymphoma. Eur J Nucl Med Mol Imaging. 2004;31:325–9. doi: 10.1007/s00259-003-1375-y. [DOI] [PubMed] [Google Scholar]
- 17.Tyler DS, Onaitis M, Kherani A, et al. Positron emission tomography screening in malignant melanoma: clinical utility in patients with Stage III disease. Cancer. 2000;89:1019–25. [PubMed] [Google Scholar]
- 18.Stas M, Stroobants S, Dupont P, et al. 18-FDG PET scan in the staging of recurrent melanoma: additional value and therapeutic impact. Melanoma Res. 2002;12:479–90. doi: 10.1097/00008390-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Dizendorf EV, Baumert BG, von Schulthess GK, et al. Impact of whole body 18F-FDGPET on staging and managing patients for radiation therapy. J Nucl Med. 2003;44:24–9. [PubMed] [Google Scholar]
- 20.Mikhaeel NG, Timothy AR, Hain SF, et al. 18-FDG PET for the assessment of residual masses on CT following therapy for lymphomas. Ann Oncol. 2000;11:S147–50. [PubMed] [Google Scholar]