Abstract
Many imaging modalities and scanning techniques, such as contrast enhanced CT, MRI and FDG-PET, are available for assessment of recurrent colorectal carcinoma. In addition, integrated PET/CT is becoming increasingly available. Intuitively, a synergistic combination of scanning characteristics sounds promising. However, the exact clinical value has not yet been fully established. The role of PET/CT image fusion must be weighed carefully against other available modalities. In this review we evaluate the potential of combined PET/CT in recurrent colorectal carcinoma. When available, PET/CT currently appears the diagnostic tool of choice. In the near future, combined PET/MRI may further enhance the diagnostic algorithm.
Keywords: PET, PET/CT, colorectal cancer, recurrence detection, restaging
Introduction
Early detection of recurrent colorectal carcinoma has become more important in the past decade, as the treatment options for localized disease have improved significantly. However, aggressive locoregional interventions (e.g. partial liver resections, radiofrequency ablation (RFA) of liver metastases, resections of pulmonary metastases) are as of yet considered futile in the presence of metastases elsewhere [1]. Therefore, detection of tumour sites throughout the body is needed with high sensitivity and specificity. For patient management with regard to invasive therapy, accurate information about the local extent of the tumour is also necessary.
Tumour visualization is traditionally performed using anatomical imaging techniques such as computed tomography (CT), ultrasound (US), and magnetic resonance imaging (MRI). Functional imaging may be of additional value. Visualization of metabolism with [18F]fluoro-deoxyglucose positron emission tomography (FDG-PET) is a valuable tool for detection of primary and recurrent colorectal cancer [2–4]. Tumour sites may be detected throughout the body with high contrast resolution. However, exact localization and demarcation of lesions with PET is hindered by its relatively low spatial resolution, and lack of anatomical reference.
The added value of simultaneous contemporaneous FDG-PET and CT has been demonstrated [5]. As a next step, the theoretical benefit of the joint capabilities of CT (anatomical reference) and FDG-PET (accurate tumour detection) have led to the practice of fusion of the images obtained by PET/CT. Although promising [6–8], the technique is relatively new and has limited availability. Furthermore, PET/CT image fusion may suffer from artefacts, and the exact clinical value has not yet been fully established. Therefore, the role of PET/CT image fusion must be weighed carefully against other more widely available modalities.
Integration of PET and CT
When considering the combination of PET and CT, different methods of fusion are available. The most prevailing approach today is ‘visual fusion’, where two scans are held side-by-side for comparison and correlation. Discrepancies between PET and CT may be resolved with this established technique. When further uncertainties persist, integration of the images can prove to be of additional value. But before attempting to integrate PET and CT images, some specific issues must be considered [9].
Scanning characteristics
Tissues appear differently on PET and on CT images. CT demonstrates anatomy with high spatial resolution, but with low contrast resolution for soft tissues. On the other hand, PET visualizes pathological sites with high contrast resolution, but spatial resolution is limited to 4–7 mm, and surrounding normal anatomical structures are hardly visualized. Due to these characteristics, discrepancies may exist between CT and PET images. Benign lesions may appear unequivocal on CT but may be negative on FDG-PET (e.g. cysts, haemangioma, scar tissue), while intensely FDG-positive lesions may be imperceptible on CT (e.g. local recurrence, liver metastasis). These characteristics complicate visual recognition and correlation. Furthermore, positional differences may exist between PET and CT because of repositioning and/or accidental voluntary motion. Organs may be displaced or changed in size (e.g. bowel motion, gastric emptying, bladder filling between PET and CT scanning). Also administration of furosemide may contribute to such discrepancies. The main problem is respiratory mismatch. PET is acquired during free breathing due to the duration of the scanning procedure (20–60 min), resulting in slightly blurred images in the upper abdomen. For correlation purposes, CT acquisition must be adapted to match these images by scanning during either free breathing or timed unforced expiration [9]. Failure to do this correctly will result in serious localization errors, as the diaphragm (including lower lung fields and upper abdominal organs such as the liver) will be relatively displaced.
Software fusion of PET and CT
When separate CT and PET images are available these may be integrated using specialized software. In such procedures certain preconditions need to be met. Identical positioning is a prerequisite. The issue of artefacts due to breathing motions needs to be addressed by breathing instructions. The time gap between scans must be limited, in order to avoid discrepancies due to disease progression (or regression) during the interval. Specific software and operator experience are needed. On the whole, the procedure is lengthy, logistically complex, and it has a serious risk of registration errors. Some authors do report adequate results using software fusion, even in the region of the liver [10], but others strongly disagree [11]. It must at least be accepted that the bladder region—and possibly the whole abdomen—has a limited accuracy in image registration.
Integrated PET/CT scanning
A so-called hybrid scanner consists of separate CT and PET scanners placed in line, which acquire scans consecutively without repositioning of the patient. Fusion of images obtained by these two modalities is often referred to as ‘hardware fusion’, although this term ought to be reserved for situations where multiple images are acquired by a single detector system at the same time. ‘Hardware’ PET/CT fusion as currently available reduces (but not fully eliminates) many of the above-mentioned positioning problems, but the need for an adequate breathing protocol remains. Other problems such as bladder filling and bowel motion are reduced to acceptable levels. When compensating for all sources of errors, a fusion error below 10 mm is generally achievable in the abdomen [12]. In specific cases this accuracy may not be reached, for example when a patient is not able to comply with breathing instructions. This source of error is important when considering the liver, as the result may be misplacement of liver lesions in the lung or vice versa, albeit in a low percentage of scans [13].
When using a hybrid PET/CT scanner, the CT images can be used for attenuation correction of the PET images. Although convenient, as the total scanning time can be reduced by ±35%, any artefact in the CT images may cause secondary artefacts in the PET images. Examples of such artefacts are false-positive hotspots related to attenuating metal such as prosthesis or clips [14], and hotspots related to intravenous/oral contrast [15]. Further discrepancies between the PET images and the CT images may result from bowel movement [16], or when the patient accidentally moved between the two scans.
Balancing the benefits
Integration of PET and CT can provide synergistic benefit regardless of the technique applied. Hybrid PET/CT is more expensive than software fusion, but it delivers a fast, logistically easy and more reliable image correlation. A definitive advantage of hybrid PET/CT is that visual fusion and software fusion may be impossible or inadequate when demanded ad hoc [11]. In the case of unexpected findings, integrated PET/CT scanning will provide adequate images, while software image fusion is likely to result in suboptimal results.
Interpretation
While fused PET/CT images do appear straightforward, the above-mentioned characteristics indicate that the images may not be easy to interpret. The true benefit of integrated PET/CT not only depends on integration of images, but also on the integration of expert opinions. Therefore, it is strongly advised that joint reading sessions take place with the radiologist and nuclear medicine physician with the appropriate clinical input from clinical oncologists and/or surgeons.
PET/CT in detection of recurrent colorectal carcinoma
In the follow-up of colorectal carcinoma, or in suspected recurrence (e.g. detectable CEA level, residual or newly formed tissues), the clinically relevant questions to be answered include: where are the potentially malignant tissues localized, is a specific lesion malignant or not, and what is the local extent of a specific lesion? An important role of imaging is to guide the rational use of additional invasive diagnostic procedures (e.g. liver biopsy, colonoscopy, etc.). A second role is demarcation of lesions to guide locoregional therapy. The role of PET/CT in relation to other imaging modalities depends on the indications for the procedure.
Local recurrence
CT is not very accurate for early detection of local recurrence of colorectal carcinoma, due to the distorted local anatomy after operation. Selzner et al. demonstrated a sensitivity of only 53% for CT, and a much better sensitivity for FDG-PET of 93% [7]. Such excellent sensitivity in detection of local recurrence also applies in the evaluation after external beam therapy [17]. The lack of anatomical reference hampers exact localization and evaluation of the extent of local pathology on PET alone. Since these data are essential when considering therapeutic intervention such as re-excision or irradiation, PET/CT may be of great value. An example of local recurrence detection and localization is provided in Fig. 1. Therefore, for the detection and evaluation of local recurrence, it is advised to perform PET/CT when available rather than PET alone.
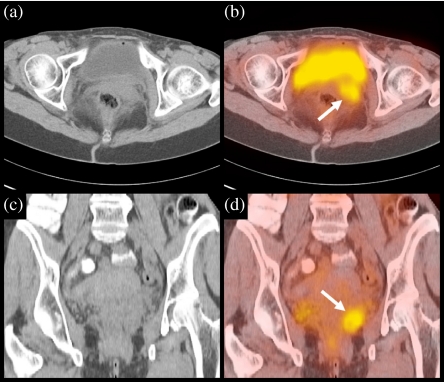
Figure 1.
Image fusion of contrast-enhanced CT and FDG-PET. The images show axial and coronal slices of CT (a, c) and PET (b, d) through the abdomen. On the CT images, the pelvic recurrence is difficult to appreciate due to extensive tissue masses. Within these masses, the PET image clearly shows a pathological lesion consistent with local recurrence of malignancy behind the bladder (white arrow). Image fusion with CT provided sufficient anatomical reference to guide a surgical approach.
Lymph node metastases
Abdominal lymph node metastases from colorectal carcinoma tend to be small. Many involved lymph nodes are below 1 cm in diameter, thus explaining the poor sensitivity of CT. Some of these small metastases can be detected by FDG-PET, albeit with a poor sensitivity of 29%, but with a high specificity of 88% [18]. Problems arise when a hotspot on PET may correlate with several anatomical structures including activity excreted in the urinary tract, blood vessels and bowel polyps, or be the result of physiological bowel uptake. In these cases, PET/CT can adequately identify a hotspot, and settle the diagnosis. Fig. 2 illustrates PET/CT localization of a pathological lymph node.
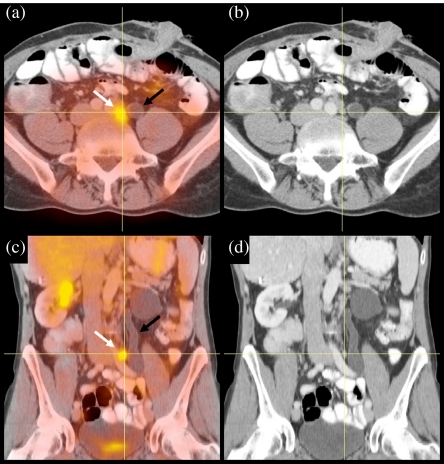
Figure 2.
Image fusion of contrast-enhanced CT and FDG-PET. The images show transverse slices (a, b) and coronal slices (c, d) through the abdomen of a patient who previously underwent primary resection of a sigmoid carcinoma. The PET image clearly showed a pathological lesion (white arrow), but the cause remained unclear as no clear lymph node was found, and the dilated ureter suggested another explanation (black arrow). Image fusion with CT could demonstrate correlation with a lymph node that was overlooked before.
Liver metastases
Ruers et al. demonstrated that FDG-PET as a stand-alone modality improves diagnostic work-up in patients with liver metastasis when added to conventional diagnostic imaging. Furthermore, it has an impact on and improves therapeutic management [4]. Integrated PET/CT can provide further value especially in the postoperatively distorted liver with scar tissue and artificial materials, where sensitivity and specificity are relatively low for both CT and MRI [19, 20]. After local ablative therapy, PET may detect recurrence of liver metastasis earlier than CT [3, 21], but correlation with CT is needed for more exact localization [8]. Conversely, CT may be false-positive at the rim of the lesions because of hyperperfusion after RFA, while FDG-PET remains reliable [22]. MRI using enhancement with manganese containing contrast may further improve detection of liver metastases and provide additional information on the nature of liver lesions [23]. Fig. 3 demonstrates that FDG-PET is not affected by scar tissue and artificial materials. For the detection of liver metastasis after hepatectomy a sensitivity of 100% and specificity of 89% was demonstrated for PET/CT, while the specificity of contrast enhanced CT dropped to 50% for this specific patient category [7]. An example of recurrent metastasis in the liver resection area, not recognized on CT and MRI but detected by FDG-PET and localized by image fusion, is shown in Fig. 4. For the evaluation of liver metastases, PET/CT appears to be the technique of choice.
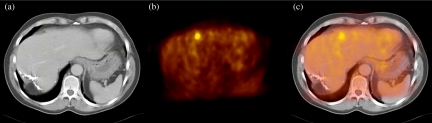
Figure 3.
Image fusion of contrast-enhanced CT and FDG-PET. The images show transverse slices of the abdomen through the liver, from CT (a), PET (b) and PET/CT (c). PET shows a clear metastasis in the ventral border of the liver that is hardly visible on CT, indicating the high sensitivity of FDG-PET, but also illustrating the need for correlation with anatomical imaging. Furthermore, the image illustrates that FDG-PET is unaffected by the extensive residual changes and surgical clips posterior in the right liver lobe, after partial liver resection.
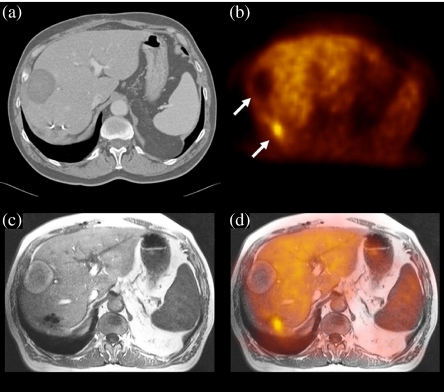
Figure 4.
Software image fusion of CT, MRI and FDG-PET. The images show transverse slices through the liver of a patient who underwent prior RFA treatment (upper arrow) and liver resection for liver metastasis of colon carcinoma. Both CT (a) and MRI (c) are difficult to interpret in the region of the surgical clips. The PET image (b) clearly shows a recurrent liver metastasis (lower arrow), which could be localized only after image fusion with MRI (d). This permitted guided locoregional therapy.
Extrahepatic metastases
Whole body imaging as a standard procedure is a major benefit of FDG-PET, thus providing information on extrahepatic metastases, which has a direct impact on patient management. Lai et al. demonstrated that 29% of patients with liver metastases appeared inoperable because FDG-PET detected extrahepatic metastases [2]. In recurrent colorectal carcinoma, most extrahepatic distant metastases will be pulmonary. Detection of these metastases is of particular importance as surgical intervention may still be possible, by combining liver surgery with resection of a limited number of pulmonary lesions [24, 25]. Both CT and FDG-PET have demonstrated high sensitivity for pulmonary lesions, but PET may be particularly helpful in discriminating benign from malignant lesions [26]. FDG-PET has also demonstrated added value in detection of other extrahepatic distant metastases such as bone metastases [27]. In unexpected extrahepatic lesions detected by PET, exact localization may be very hard without correlative anatomical imaging as provided by PET/CT. This also applies to the detection of unexpected second primaries, which may occur in approximately 1% of cases [28].
Lesion characterization
Regardless of the type of lesion as seen on imaging, differentiation of benign from malignant disease is always a challenge. Both CT and FDG-PET can contribute to the final diagnosis, but a combination of both modalities delivers the strongest diagnostic tool [29, 30]. Given this asset, we consider PET/CT the best option when atypical lesions need to be characterized at the highest possible level of accuracy, especially in cases where a definitive diagnosis through pathology cannot be obtained.
Future developments
The true clinical value of FDG-PET—and the added value of the application of PET/CT scanners—should ideally be clarified by prospective clinical trials. But a true comparison between separately acquired PET and CT images, visual fusion, software fusion, and integrated PET/CT images can hardly be achieved, as this implies the acquisition of multiple scans with a high cumulative radiation burden to the patient. As a result of the rather limited scientific evidence, the current choices for implementation of FDG-PET in diagnostic strategies appear rather random, and large variations exist among institutes. This also applies to the application of hybrid PET/CT scanning for various specific questions. Nevertheless, scientific evidence about the diagnostic values of PET and PET/CT are increasing rapidly, and eagerly awaited.
New PET tracers
Besides visualization of glucose metabolism with FDG, PET scanning may be applied for in vivo non-invasive evaluation of other tissue characteristics using tracers other than FDG. For example, DNA synthesis activity may be quantitatively assessed using [18F]fluoro-deoxy-L-thymidine (FLT), as a reflection of cell proliferation and tumour growth [31]. The exact clinical applicability of FLT, as well as several other tracers currently under investigation, is at present even less clear than the utility of FDG-PET. It is to be expected that many new tracers will accumulate selectively in pathological lesions, and will show poor or no normal tissue activity. These images may therefore be uninterpretable without integration of PET and CT.
Integration of PET and MRI
The combination of PET and CT is not the only possibility, nor is it a perfect solution. On theoretical grounds it is preferable to combine PET with (functional) MRI, for better soft tissue evaluation with a relatively low radiation burden. An excellent example of the application of PET/MRI fusion is accurate delineation of malignant lesions in the liver, to allow optimally guided locoregional therapeutic intervention. The PET/MRI fusion procedure is already possible when using software fusion; an example is shown in Fig. 4. It is expected that integrated PET/MRI scanners will become clinically available in the next 5 years.
Conclusions
The combination of PET and CT is currently proving itself as a valuable tool in the diagnostic strategy for detection of recurrent colorectal carcinoma, especially in the field of staging before surgical re-interventions. This has an impact on diagnosis and choice of therapy. The application of separate PET and CT is not to be considered ‘second class’, when visually correlated adequately. Although unbiased supporting literature is currently limited, hardware integrated PET/CT using a hybrid scanner does seem to be able to improve diagnostic accuracy over correlated stand-alone PET and CT in several specific cases. As software image fusion is prone to error, this technique should be used with caution and should be reserved for specific applications.
The largest benefit from integration of PET and CT images depends on the integration of knowledge. This implies joint consensus reading by a multidisciplinary team. This will be of even greater importance when new PET tracers and new MRI applications enter the clinical field.
With the increasing availability of integrated PET/CT scanners, it is to be expected that clinical use and experience will rapidly expand. However, a critical review of indications and added value of these techniques are a prerequisite for rational application and maximum diagnostic yield.
References
- 1.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703–10. [PMC free article] [PubMed] [Google Scholar]
- 2.Lai DT, Fulham M, Stephen MS, et al. The role of whole-body positron emission tomography with [18F]fluorodeoxyglucose in identifying operable colorectal cancer metastases to the liver. Arch Surg. 1996;131:703–7. doi: 10.1001/archsurg.1996.01430190025007. [DOI] [PubMed] [Google Scholar]
- 3.Langenhoff BS, Oyen WJ, Jager GJ, et al. Efficacy of fluorine-18-deoxyglucose positron emission tomography in detecting tumor recurrence after local ablative therapy for liver metastases: a prospective study. J Clin Oncol. 2002;20:4453–8. doi: 10.1200/JCO.2002.12.134. [DOI] [PubMed] [Google Scholar]
- 4.Ruers TJ, Langenhoff BS, Neeleman N, et al. Value of positron emission tomography with [F-18]fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol. 2002;20:388–95. doi: 10.1200/JCO.2002.20.2.388. [DOI] [PubMed] [Google Scholar]
- 5.Wiering B, Ruers TJ, Oyen WJ. Role of FDG-PET in the diagnosis and treatment of colorectal liver metastases. Expert Rev Anticancer Ther. 2004;4:607–13. doi: 10.1586/14737140.4.4.607. [DOI] [PubMed] [Google Scholar]
- 6.Kamel IR, Cohade C, Neyman E, Fishman EK, Wahl RL. Incremental value of CT in PET/CT of patients with colorectal carcinoma. Abdom Imaging. 2004;29:663–8. doi: 10.1007/s00261-003-0163-2. [DOI] [PubMed] [Google Scholar]
- 7.Selzner M, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg. 2004;240:1027–34. doi: 10.1097/01.sla.0000146145.69835.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Veit P, Antoch G, Stergar H, Bockisch A, Forsting M, Kuehl H, Detection of residual tumor after radiofrequency ablation of liver metastasis with dual-modality PET/CT: initial results. Eur Radiol 2005; E-pub ahead of print
- 9.Vogel WV, Oyen WJ, Barentsz JO, Kaanders JH, Corstens FH. PET/CT: panacea, redundancy, or something in between? J Nucl Med. 2004;45((Suppl 1)):S15–24. [PubMed] [Google Scholar]
- 10.van Dalen JA, Vogel W, Huisman HJ, Oyen WJ, Jager GJ, Karssemeijer N. Accuracy of rigid CT-FDG-PET image registration of the liver. Phys Med Biol. 2004;49:5393–405. doi: 10.1088/0031-9155/49/23/014. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Czernin J, Allen-Auerbach MS, et al. Comparison between 18F-FDG PET, in-line PET/CT, and software fusion for restaging of recurrent colorectal cancer. J Nucl Med. 2005;46:587–95. [PubMed] [Google Scholar]
- 12.Nakamoto Y, Tatsumi M, Cohade C, Osman M, Marshall LT, Wahl RL. Accuracy of image fusion of normal upper abdominal organs visualized with PET/CT. Eur J Nucl Med Mol Imaging. 2003;30:597–602. doi: 10.1007/s00259-002-1080-2. [DOI] [PubMed] [Google Scholar]
- 13.Osman MM, Cohade C, Nakamoto Y, Marshall LT, Leal JP, Wahl RL. Clinically significant inaccurate localization of lesions with PET/CT: frequency in 300 patients. J Nucl Med. 2003;44:240–3. [PubMed] [Google Scholar]
- 14.Goerres GW, Ziegler SI, Burger C, Berthold T, Von Schulthess GK, Buck A. Artifacts at PET and PET/CT caused by metallic hip prosthetic material. Radiology. 2003;226:577–84. doi: 10.1148/radiol.2262012141. [DOI] [PubMed] [Google Scholar]
- 15.Dizendorf E, Hany TF, Buck A, Von Schulthess GK, Burger C. Cause and magnitude of the error induced by oral CT contrast agent in CT-based attenuation correction of PET emission studies. J Nucl Med. 2003;44:732–8. [PubMed] [Google Scholar]
- 16.Nakamoto Y, Chin BB, Cohade C, Osman M, Tatsumi M, Wahl RL. PET/CT: artifacts caused by bowel motion. Nucl Med Commun. 2004;25:221–5. doi: 10.1097/00006231-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Moore HG, Akhurst T, Larson SM, Minsky BD, Mazumdar M, Guillem JG. A case-controlled study of 18-fluorodeoxyglucose positron emission tomography in the detection of pelvic recurrence in previously irradiated rectal cancer patients. J Am Coll Surg. 2003;197:22–8. doi: 10.1016/S1072-7515(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 18.Kantorova I, Lipska L, Belohlavek O, Visokai V, Trubac M, Schneiderova M. Routine (18)F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med. 2003;44:1784–8. [PubMed] [Google Scholar]
- 19.Dromain C, de Baere T, Elias D, et al. Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow-up. Radiology. 2002;223:255–62. doi: 10.1148/radiol.2231010780. [DOI] [PubMed] [Google Scholar]
- 20.Lim HK, Choi D, Lee WJ, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221:447–54. doi: 10.1148/radiol.2212010446. [DOI] [PubMed] [Google Scholar]
- 21.Blokhuis TJ, van der Schaaf MC, van den Tol MP, Comans EF, Manoliu RA, van der Sijp JRM. Results of radio frequency ablation of primary and secondary liver tumors: long-term follow-up with computed tomography and positron emission tomography-18F-deoxyfluoroglucose scanning. Scand J Gastroenterol. 2004;Suppl:93–7. doi: 10.1080/00855920410014623. [DOI] [PubMed] [Google Scholar]
- 22.Antoch G, Vogt FM, Veit P, et al. Assessment of liver tissue after radiofrequency ablation: findings with different imaging procedures. J Nucl Med. 2005;46:520–5. [PubMed] [Google Scholar]
- 23.Reimer P, Schneider G, Schima W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur Radiol. 2004;14:559–78. doi: 10.1007/s00330-004-2236-1. [DOI] [PubMed] [Google Scholar]
- 24.King J, Glenn D, Clark W, et al. Percutaneous radiofrequency ablation of pulmonary metastases in patients with colorectal cancer. Br J Surg. 2004;91:217–3. doi: 10.1002/bjs.4392. [DOI] [PubMed] [Google Scholar]
- 25.Steinke K, Glenn D, King J, et al. Percutaneous imaging-guided radiofrequency ablation in patients with colorectal pulmonary metastases: 1-year follow-up. Ann Surg Oncol. 2004;11:207–12. doi: 10.1245/aso.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Lowe VJ, Fletcher JW, Gobar L, et al. Prospective investigation of positron emission tomography in lung nodules. J Clin Oncol. 1998;16:1075–84. doi: 10.1200/JCO.1998.16.3.1075. [DOI] [PubMed] [Google Scholar]
- 27.Bohdiewicz PJ, Wong CY, Kondas D, Gaskill M, Dworkin HJ. High predictive value of F-18 FDG PET patterns of the spine for metastases or benign lesions with good agreement between readers. Clin Nucl Med. 2003;28:966–70. doi: 10.1097/01.rlu.0000099806.96580.db. [DOI] [PubMed] [Google Scholar]
- 28.Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med. 2005;46:752–7. [PubMed] [Google Scholar]
- 29.Antoch G, Freudenberg LS, Beyer T, Bockisch A, Debatin JF. To enhance or not to enhance? 18F-FDG and CT contrast agents in dual-modality 18F-FDG PET/CT. J Nucl Med. 2004;45((Suppl 1)):S56–65. [PubMed] [Google Scholar]
- 30.Cohade C, Osman M, Leal J, Wahl RL. Direct comparison of (18)F-FDG PET and PET/CT in patients with colorectal carcinoma. J Nucl Med. 2003;44:1797–1803. [PubMed] [Google Scholar]
- 31.Francis DL, Freeman A, Visvikis D, et al. In vivo imaging of cellular proliferation in colorectal cancer using positron emission tomography. Gut. 2003;52:1602–6. doi: 10.1136/gut.52.11.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]