Abstract
Standardized CT-based criteria used for lymphoma staging and follow-up and the current role of FDG-PET are reviewed. The current CT-based International Workshop Criteria (IWC) still have the main advantage of representing standardized criteria allowing comparability of clinical trials in patients with lymphoma. However, functional imaging with integrated IWC and FDG-PET provide more accurate response assessment, and challenge the current paradigm. Although integration of FDG-PET in IWC requires validation in a prospective trial with a large number of patients, new long-term clinical and therapeutic trials probably need to be designed using these new and hopefully standardized functional criteria. This potentially could allow a more risk-adapted approach to the treatment of aggressive lymphoma: intensive (reinforced) therapies for non-responders vs. less intensive therapies for good responders with the main goal of improved clinical outcome.
Keywords: Lymphoma, CT analysis, Cheson’s criteria
Introduction
Since 1999, the International Workshop Criteria (IWC) have been widely accepted for response assessment in patients with non-Hodgkin’s lymphoma (NHL). IWC also ensure comparability among clinical trials [1]. Multi-agent chemotherapy regimen have transformed aggressive lymphoma from a fatal disease to a potentially curable one, but no more than half of all patients are cured [2]. More aggressive, but also potentially more toxic treatments are now available. At diagnosis, the therapeutic regimen will be chosen in part according to the International Prognostic Index, a well established predictor of outcome, including the performance status according to the Eastern Cooperative Oncology Group scale, the patient’s age, serum lactate dehydrogenase level, Ann Arbor stage (Table 1) (reflecting the anatomic extent and the tumoral mass primarily obtained by CT and bone marrow biopsy), and finally the number of extranodal sites [3]. In order to assess response to treatment, the IWC are primarily based on computed tomography (CT) although bone marrow biopsy (BMB) and clinical and biochemical information are also taken into account; IWC should also discriminate rapid responders to standard induction likely to show better and more durable response, from non-responders who could benefit from an early change of therapeutic orientation [4]. The inability of CT to differentiate between viable tumour, necrosis, or fibrosis in residual mass(es) in patients with otherwise complete clinical response has led to only CT-based size changes after treatment being considered to assign a response designation in the IWC [1]. Functional imaging, particularly using fluorodeoxyglucose positron emission tomography (FDG-PET) has been shown to provide additional metabolic information such as tumour viability in residual masses [5]. Gallium citrate and magnetic resonance (MR) imaging have also been used for this purpose in patients with lymphoma [6–8].
Table 1.
Ann Arbor staging classification of thoracic lymphoma
| Stage | Area of involvement |
|---|---|
| I | A single lymph node region or a single localised involvement of an extralymphatic site |
| II | Two or more lymph node regions on the same side of the diaphragm |
| III | Lymph node regions on both sides of the diaphragm |
| IV | Diffuse involvement of one or more extranodal organs with or without lymph node involvement |
| Additional qualifiers | |
| A | Absence of systemic symptoms |
| B | Presence of systemic symptoms |
| S | Involvement of spleen |
| E | Localised extralymphatic site involvement |
| H | Involvement of liver |
| M | Diffuse involvement of bone marrow |
International Workshop Criteria (IWC)
The standardized criteria for response assessment, proposed by Cheson and colleagues, include five designations [1, 9]:
(1) Complete response (CR) is the complete disappearance of all detectable evidence of disease on CT, and all disease-related symptoms, and normalization of biochemical abnormalities, and normal bone marrow biopsy (BMB). Previously involved nodes on CT more than 1.5 cm in their greatest axial diameter must regress to less than 1.5 cm, and previously measured nodes of 1.1–1.5 cm must decrease to less than 1 cm.
(2) CRu (uncertain) corresponds to CR criteria but with a residual mass more than 1.5 cm in greatest axial diameter that has regressed by more than 75%.
(3) Partial response (PR) is at least 50% reduction in the sum of the product of the greatest diameters (SPD) of the six largest nodes with no increase in the size of other nodes and no new sites of disease. Splenic and hepatic nodules must regress by at least 50% in the SPD. BMB is irrelevant for determination of PR.
(4) Stable disease (SD) is less than a PR but is not progressive disease. Progressive disease (PD) is more than 50% increase in the sum of the product of the greatest diameters of any previously abnormal node, or appearance of any new lesions during or at the end of therapy.
(5) Relapsed disease (RD) is the appearance of any new lesion or increase in size of more than 50% of previously involved sites or nodes in patients who achieved CR or CRu.
Main limitations of CT-based IWC
At staging, assessment of nodal involvement by CT is limited by its low specificity in the case of small nodes, especially less than 1.5 cm in diameter [9]. Spleen involvement by CT is based on an enlarged spleen and/or hypodense nodules. However, spleen size can vary with age and other associated diseases. Detection of spleen nodules will depend upon CT technical parameters including contrast injection rate and timing. When isolated, a spleen nodule can be difficult to characterize by CT alone. In young patients, when upper range measurements of the normal thymus according to age are obtained, involvement by lymphoma can be difficult to assess with certainty.
Extranodal sites of involvement can be difficult to detect by CT. Bone marrow involvement is usually not identified unless a lytic bone lesion is present. CT appearance of a small pulmonary nodule can be non-specific. Subtle changes in gastrointestinal wall thickness can be difficult to assess on CT [10, 11].
At CT follow-up of nodal masses, a residual mass is present in approximately 40% of NHL patients treated by chemotherapy and/or radiation. This led to the concept of ‘complete remission uncertain’ (CRu) which reflects the unknown significance of persistent CT abnormalities in patients who otherwise seem to be in CR. Previous studies showing that only 10%–20% of such patients have evidence of disease in these residual masses, considerably limits the value of CT for prediction of clinical outcome of NHL [11–13]. Due to its good spatial resolution, CT is also able to detect nodes less than 1 cm but these nodes are considered normal although residual disease cannot theoretically be ruled out.
In the case of extranodal involvement, residual masses can also be seen in cases of gastrointestinal involvement; size changes of wall thickness are also difficult to estimate [11]. In the case of a lytic bone involvement, bone remodelling is usually delayed with no ad integrum recovery. Pulmonary involvement can be difficult to assess during treatment due to drug-related and/or infectious complications.
Although IWC have been useful to standardize response to treatment, functional imaging has been shown to improve detection of sites of involvement, and to provide metabolic tissue characterization [14].
The emergence of fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in the clinical armamentarium and its increasing availability have recently provided an alternative to gallium-citrate scan and MR imaging, which were previously employed to detect active disease.
Integrated IWC and FDG-PET
Compared to CT staging, Moog et al. have reported more accurate staging by FDG-PET in NHL [15]; 8% of patients were up-staged at diagnosis because of the identification of additional disease sites. After treatment, several studies have demonstrated that persistence of an increased glycolytic activity on FDG-PET was associated with a high relapse rate, while the latter was low in the case of a negative scan [5, 16]. Only one recent report has shown that a response classification based on integration of FDG-PET results into IWC should provide a more accurate response assessment than IWC alone in patients with lymphoma. In a retrospective study of 54 patients with aggressive NHL, Juweid et al. showed that only 61% had concordant response designations between integrated IWC including FDG-PET results vs. IWC alone [9]. The most pronounced discordance was observed in the CRu by IWC designation, in which all CRu patients were reclassified as CR in case of no FDG uptake or PR in case of FDG uptake. All patients reclassified as CR remained progression free at a median of more than 32 months. An example of a residual mass detected by CT but with no FDG uptake on PET is shown in Fig. 1. The other major discordance was found in the PR by IWC designation in which half the patients were reclassified as CR by integrated IWC and FDG-PET. All but one of these reclassified CR patients remained without evidence of disease progression at a median of more than 32 months. In contrast, only two of nine patients with concordant PR designation by IWC and integrated FDG-PET, and IWC alone were progression free at follow-up. Based on the Kaplan–Meier method, this study demonstrated that integrated IWC and FDG-PET was a statistically significant independent predictor for progression-free survival.
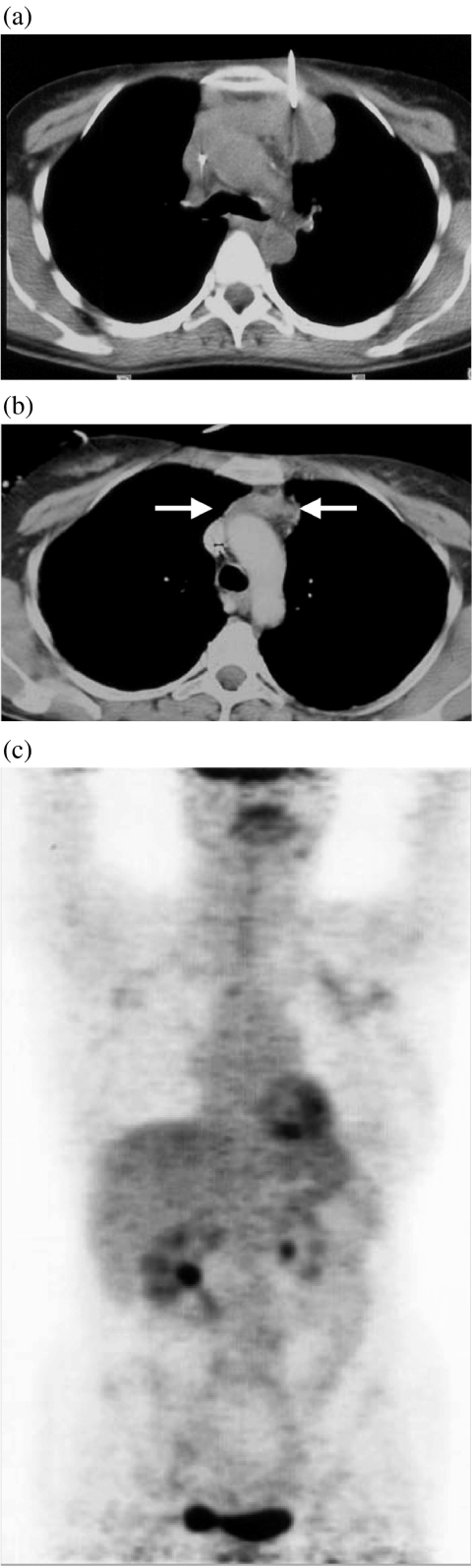
Figure 1.
A 32-year-old woman with aggressive non-Hodgkin’s lymphoma. (a) Unenhanced CT scan of the thorax performed during percutaneous biopsy showing a 7×4 cm large anterior mediastinal mass. (b) Based on sole CT follow-up findings, the patient was classified as complete response (uncertain) (CRu) according to IWC after 3 months of treatment with a 3×2 cm large residual mass consistent with ≥75% decrease in size (arrows). (c) FDG-PET performed 3 days after CT did not show any significant residual uptake within the mass allowing the reclassification of the response to treatment as complete response (CR).
Using FDG-PET results at follow-up will substantially increase the proportion of CR designation by IWC, and probably cancel the CRu designation. It will also show two distinct subgroups within the PR by IWC: a subgroup of PET-positive patients with poor outcome and another of PET-negative patients with excellent outcome. The biological explanation is likely related to the fact that the residual CT abnormalities represent necrosis and/or fibrosis in the majority of the PET-negative patients, whereas the abnormalities represent active tumour in the majority of the PET-positive patients.
One must, however, take into account that owing to its high spatial resolution, CT can identify extranodal involvement sometimes not apparent on PET, thus triggering the need for combined PET/CT, and for combined radiological and nuclear medicine workout of image interpretation, as illustrated by Fig. 2.
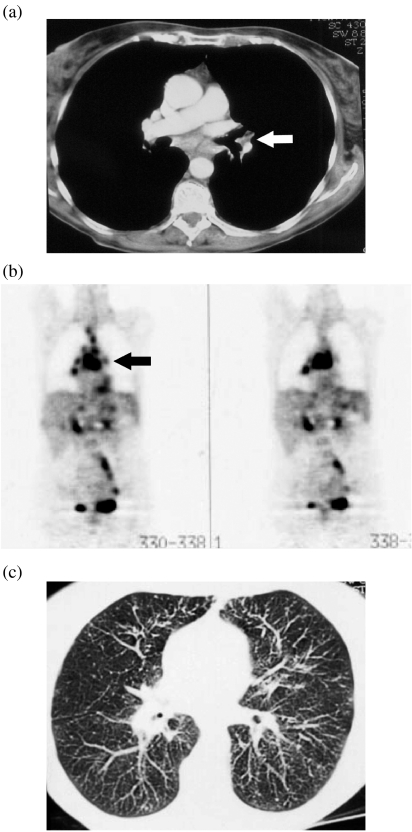
Figure 2.
A 25-year-old male patient with aggressive non-Hodgkin’s lymphoma. (a) Enhanced CT scan of the thorax showed a small 1 cm large left hilar node (arrow), and multiple subcareneal enlarged lymph nodes. (b) FDG-PET showed increased uptake of all nodes detected by CT including the centimetric hilar node (arrow). However, FDG-PET failed to identify multiple disseminated lung nodules consistent with pulmonary involvement as confirmed by the disappearance of these CT findings after completion of the first line of treatment.
Conclusion
Current CT-based IWC still has the main advantage of representing standardized criteria allowing comparability of clinical trials in patients with lymphoma. However, functional imaging with integrated IWC and FDG-PET provide more accurate response assessment, and challenge the current paradigm. As FDG-PET combined with visual correlation with a contrast-enhanced CT accurately predicts progression-free survival, fused FDG-PET and CT images acquired during the same examination will yield similar or superior results. Although integration of FDG-PET in IWC requires validation in a prospective trial with a large number of patients, new long-term clinical and therapeutic trials probably need to be designed using these new and hopefully standardized functional criteria. This potentially could allow a more risk-adapted approach to treatment of aggressive lymphoma: intensive (reinforced) therapies for non-responders vs. less intensive therapies for good responders with the main goal of improved clinical outcome.
References
- 1.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 2.Vose JM. Current approaches to the management of non-Hodgkin’s lymphoma. Semin Oncol. 1998;25:483–91. [PubMed] [Google Scholar]
- 3.Shipp M, Harrington D, Anderson J, et al. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 4. Haioun C, Itti E, Rahmouni A, [ 18F]Fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood 2005; prepublished online April 28, 2005 (DOI 10.1182/blood-2005-01-0272)
- 5.Jerusalem G, Beguin Y, Fassotte MF, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin’s disease and non-Hodgkin’s lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood. 1999;94:429–33. [PubMed] [Google Scholar]
- 6.Israel O, Mor M, Epelbaum R, et al. Clinical pretreatment risk factors and Ga-67 scintigraphy early during treatment for prediction of outcome of patients with aggressive non-Hodgkin lymphoma. Cancer. 2002;94:873–8. [PubMed] [Google Scholar]
- 7.Rahmouni A, Divine M, Lepage E, et al. Mediastinal lymphoma: quantitative changes in gadolinium enhancement at MR imaging after treatment. Radiology. 2001;219:621–8. doi: 10.1148/radiology.219.3.r01jn06621. [DOI] [PubMed] [Google Scholar]
- 8.Janicek M, Kaplan W, Neuberg D, Canellos GP, Shulman LN, Shipp MA. Early restaging gallium scans predict outcome in poor-prognosis patients with aggressive non-Hodgkin’s lymphoma treated with high-dose CHOP chemotherapy. J Clin Oncol. 1997;15:1631–7. doi: 10.1200/JCO.1997.15.4.1631. [DOI] [PubMed] [Google Scholar]
- 9.Juweid ME, Wiseman GA, Vose JM, et al. Response assessment of aggressive non-Hodgkin’s lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005;23:4652–61. doi: 10.1200/JCO.2005.01.891. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Xiu Y, Potenta S, et al. 18F-FDG PET for evaluation of the treatment response in patients with gastrointestinal tract lymphomas. J Nucl Med. 2004;45:1796–1803. [PubMed] [Google Scholar]
- 11.Surbone A, Longo DL, DeVita Jr VT, et al. Residual abdominal masses in aggressive non-Hodgkin’s lymphoma after combination chemotherapy: significance and management. J Clin Oncol. 1988;6:1832–7. doi: 10.1200/JCO.1988.6.12.1832. [DOI] [PubMed] [Google Scholar]
- 12.Fuks JZ, Aisner J, Wiernik PH. Restaging laparotomy in the management of the non-Hodgkin lymphomas. Med Pediatr Oncol. 1982;10:429–38. doi: 10.1002/mpo.2950100502. [DOI] [PubMed] [Google Scholar]
- 13.Coiffier B, Lepage E. Prognosis of aggressive lymphomas: a study of five prognostic models with patients included in the LNH-84 regimen. Blood. 1989;74:558–64. [PubMed] [Google Scholar]
- 14.Juweid ME, Cheson BD. Role of positron emission tomography in lymphoma. J Clin Oncol. 2005;23:4577–80. doi: 10.1200/JCO.2005.01.904. [DOI] [PubMed] [Google Scholar]
- 15.Moog F, Bangerter M, Diederichs CG, et al. Extranodal malignant lymphoma: detection with FDG PET versus CT. Radiology. 1998;206:475–81. doi: 10.1148/radiology.206.2.9457202. [DOI] [PubMed] [Google Scholar]
- 16.Mikhaeel NG, Timothy AR, O’Doherty MJ, Hain S, Maisey MN. 18-FDG-PET as a prognostic indicator in the treatment of aggressive non-Hodgkin’s lymphoma—comparison with CT. Leuk Lymphoma. 2000;39:543–53. doi: 10.3109/10428190009113384. [DOI] [PubMed] [Google Scholar]