Abstract
Although most clinical diagnostic imaging studies employ anatomic techniques such as computed tomography (CT) and magnetic resonance (MR) imaging, much of radiology research currently focuses on adapting these conventional methods to physiologic imaging as well as on introducing new techniques and probes for studying processes at the cellular and molecular levels in vivo, i.e. molecular imaging. Molecular imaging promises to provide new methods for the early detection of cancer and support for personalized cancer therapy. Although molecular imaging has been practiced in various incarnations for over 20 years in the context of nuclear medicine, other imaging modalities have only recently been applied to the noninvasive assessment of physiology and molecular events. Nevertheless, there has been sufficient experience with specifically targeted contrast agents and high-resolution techniques for MR imaging and other modalities that we must begin moving these new technologies from the laboratory to the clinic. This brief review outlines several of the more promising areas of pursuit in molecular imaging for oncology with an emphasis on those that show the most immediate likelihood for clinical translation.
Keywords: Molecular imaging, clinical translation
Introduction
As it relates to cancer, molecular imaging represents a group of methods to study the malignant phenotype noninvasively at high resolution with specific probes, contrast agents or MR pulse sequences with a view not only to understanding cancer biology but also to providing early diagnosis and support emerging cancer therapies. Molecular imaging is important to cancer research, diagnosis and therapy now because of the many new, specific therapies that may not be as indiscriminately cytotoxic as most have been to date. Much has been written about parallel progress in development of high-resolution, multimodality imaging devices [1–4] along with array-based techniques used to discover new targets for cancer imaging and therapy [5] and the ready availability of experimental models, many of which are genetically based and therefore provide unprecedented relevance to human cancer [6, 7]. Convergence of advances in those areas has provided interesting and in some cases spectacular imaging results in experimental models with some novel approaches beginning to find their way to the clinic.
Translational research is a somewhat nebulous term that attempts to describe the work that goes into bringing the most promising experimental therapies to the clinic after extensive testing in experimental models. In the US, the National Cancer Institute (NCI) has recognized the importance of this type of research and has invested substantial funding not only into the development of in vivo cellular and molecular imaging centers (ICMICs) but also into small animal imaging resource programs (SAIRPs), both of which involve translational research extensively. Because imaging agents are designed to be ‘tracers’ of physiology and therefore have no pharmacologic effect, they can be approved for human administration much more readily than most therapeutic agents. In the US, there has been a recent revision of the criteria needed to be met for an imaging agent to progress to the clinic, reflecting the general lack of toxicity of these agents, further promoting clinical translation. There are also other programs, such as the Development of Clinical Imaging Drug Enhancers (DCIDE) program at the NCI, that are beginning to hasten translation of new molecular imaging agents. The questions become: what are the promising areas in molecular imaging research on which to focus for near-term clinical translation? In light of the abundant, new targets and technologies, how do we know where to place our efforts? Small animal imaging can certainly help in validating or eliminating potential molecular imaging probes from the pool of available materials, however, small animal imaging itself is a time- and labor-intensive process such that only the most promising targets for the most useful indications should be pursued.
Cellular events and molecular pathways as imaging targets for cancer
Among the many possible targets for imaging cancer, those that have been the focus of the most intense research include angiogenesis [8, 9], apoptosis [10, 11], signal transduction [12–15], and study of protein interaction networks [16] as well as more conventional approaches to receptor- [17] or enzyme-based [18] and metabolic imaging [19–23]. Methods for imaging cellular trafficking are no longer experimental curiosity with the use of functionalized nanoparticles providing among the most compelling evidence for the use of MR in molecular imaging for clinical applications to cancer, arguably at sensitivities superior to those demonstrated for the radionuclide-based techniques [24–26].
Other targets that have been the subject of significant imaging inquiry include probing multidrug resistance [27–30] and gene delivery and expression [15, 31–33]. Among the most promising receptor-based targets are the prostate specific membrane antigen (PSMA) [18, 34], HER-2/neu [35], the vascular endothelium through the αvβ3 receptor using RGD peptides [8, 9, 36, 37] and steroid receptor proteins including the estrogen [38–40], progestin [41, 42] and androgen [43, 44] receptors. Promising metabolic techniques involve the use of [18F]fluorodeoxyglucose (FDG) for studying tumor glycolysis [45, 46] and therapeutic monitoring in clinical [47] and preclinical studies [48], radiolabeled choline analogs [49] for prostate cancer, and [18F]fluorothymidine (FLT) as well as other thymidine analogs as tumor proliferation agents, the uptake of which are dependent upon activity of the cell cycle [20]. Although an incomplete list, those entities were chosen because they have all been imaged clinically, have all proved useful and provide models for the translation of experimental molecular imaging agents (Table 1). Several illustrative examples are discussed in greater detail below.
Table 1.
A sampling of recently translated and near-term clinical molecular imaging probes and methods for cancer (promising preclinical techniques are depicted in bold type)
| Biology | Representative probe | Method | Reference |
|---|---|---|---|
| Angiogenesis | [18F]Galacto-RGD | PET a | [9] |
| Apoptosis | [99mTc]Annexin-V | SPECT b | [98] |
| Signal transduction | [124I]FIAU | PET | [14] |
| Protein interaction | Luciferin | Bioluminescence | [16] |
| Receptor/enzyme/transporter | [68Ga]F(ab) 2-Herceptin | PET | [72] |
| Metabolism | FLT | PET | [20] |
| Cell trafficking | Cy5.5-CLIO | Fluorescence/MR | [55] |
| Chemotherapy pharmacokinetics | 5-[18F]Fluorouracil | PET | [62] |
| Multidrug resistance | [99mTc]Sestamibi | SPECT | [63] |
| Hypoxia | [60Cu]ATSM | PET | [82] |
| Gene delivery/expression | Ormosil | Fluorescence | [32] |
aPositron emission tomography. bSingle photon emission computed tomography.
A word on instrumentation and modality choice
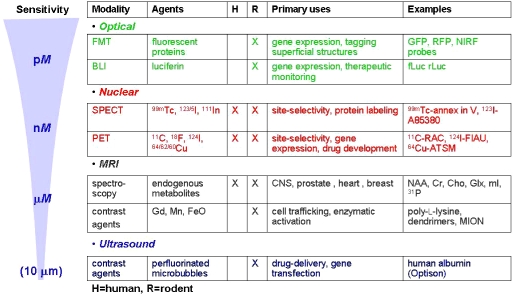
Because molecular imaging is a biology-driven enterprise, workers in this field are generally less interested in applying a specific imaging modality than at uncovering a particular biological process, which may require complementary modalities. Fig. 1 summarizes the most commonly used molecular imaging modalities with respect to their relative sensitivities. The imaging modalities must be thought of as complementary in that while some, such as MR spectroscopy, may have a built-in correlative anatomic mode, i.e. MR imaging, and therefore provide high spatial resolution, that technique is of considerably less sensitivity than, for example, positron emission tomography (PET). On the other hand, the use of superparamagnetic iron oxide (SPIO) particles for cellular trafficking has enabled the visualization of a single cell using a clinical magnet [26, 50]. That fact appears to contradict the sensitivity scale shown in Fig. 1. Nevertheless, the radionuclide-based techniques have been used for many years to study specific molecular species, and remain supremely translatable. As we gain more experience with experimental models, we continue to learn the strengths and weaknesses of the modalities for particular oncologic indications, leading to certain generalizations. For example, MR-based nanoparticles are proving useful for cell trafficking studies, both clinically [25] (Fig. 2) and preclinically [51, 52], while radionuclide- and optically-based molecular-genetic reporter systems, although useful experimentally, have not yet enjoyed much clinical exposure. On the other hand, for receptor- or enzyme-based imaging or for studying the pharmacokinetic disposition of chemotherapeutic agents, the radionuclide-based techniques predominate. MR- and optically-based, activatable probes have proved useful experimentally [53–56] but have not yet achieved clinical translation. Of course the development of nanodiagnostics, which are often engineered to provide multimodality imaging, will soon add a new dimension to clinical molecular imaging as the translation of such agents is being vigorously pursued [57, 58].
Figure 1.
Modalities for molecular imaging.
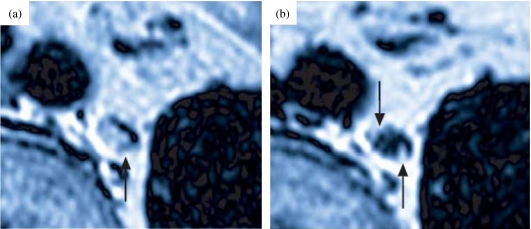
Figure 2.
Use of a lymphotropic MR contrast agent (iron oxide-containing nanoparticle) in a patient with prostate cancer. Arrows indicate micrometastases, i.e. where nanoparticles are excluded from lymph node uptake (from reference [25]).
Translational molecular imaging: examples
Radiolabeled chemotherapeutic agents and monitoring chemotherapy
A number of groups, most notably that of Aboagye et al. at the Hammersmith Hospital, have worked to provide positron-emitting analogs of chemotherapeutic agents such as paclitaxel [59, 60], fluorouracil [61] and others, in an effort to assess the pharmacokinetic profile of these agents in specific patients as well as studies in modulation of pharmacokinetics with other enhancing drugs [62]. Perhaps one of the most important applications of this technology is to determine whether a patient will be a candidate for the corresponding chemotherapeutic agent based on the ability of the tumor to sequester the radiolabeled analog. Another key application will be to determine if the radiolabeled agent can be used for prediction of early cytostasis and cytotoxicity. A classic example of that application has been provided by Saleem et al. in which they showed how pharmacologic doses of eniluracil were able to improve the delivery of 5-[18F]fluorouracil to tumors quantitatively and noninvasively in human subjects [62]. Radiolabeled substrates for the multidrug resistance (MDR)-derived P-glycoprotein pump (Pgp), such as [99mTc]sestamibi, have been used to predict how patients may respond to therapy for lung cancer [63]. Radiolabeled taxanes have also been used to quantify the modulation of Pgp, a new strategy in enhancing cancer chemotherapy, in nonhuman primates [60]. Small animal PET imaging has been used in this context, such as the study by Leyton et al. in which FLT was used to measure the effects of cisplatin treatment of fibrosarcoma tumor-bearing mice [64]. That study found FLT-PET to be superior to FDG-PET for predicting early changes due to chemotherapy, lending support to the use of FLT-PET for early detection of chemotherapy-induced tumor metabolic changes. In this sense, small animal PET imaging can be used to aid in the translation not only of diagnostic but also new therapeutic agents or drugs, which are increasingly of the cytostatic variety. A cytostatic drug may not produce a decrease in tumor size readily detectable by anatomic measures, such as by CT diameter, the current clinical standard. A metabolic technique should be tailored to a new metabolic therapy, as shown for a recent study performed in an effort to provide information on 3-bromopyruvic acid as a putative treatment for sarcoma [48] (Fig. 3). Badly needed in conjunction with all of these animal PET studies is concurrent validation in vitro using histologic techniques that reflect the underlying biological changes that are expected with therapy, i.e. validation of mechanism.
Figure 3.
FDG-PET images of tumor-bearing rats before and after 12 days of therapy with 3-bromopyruvate (from reference [46]).
Recent and near-term translation of receptor-based imaging agents
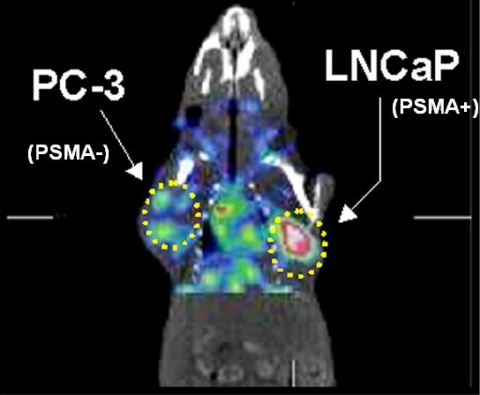
The prostate specific membrane antigen (PSMA) has been a target for molecular imaging for the last ten years using the monoclonal antibody ProstaScint ® [65]. Because of the inherent difficulty of antibody-based imaging, small molecule ligands for PSMA are being pursued actively [18, 66, 67]. Animal models reveal high tumor target selectivity, providing the impetus for translation of agents of this class to the clinic [18] (Fig. 4). Although the ultimate clinical success of those agents for prostate cancer remain to be seen, steroid receptor-based imaging has proved clinically useful in studies dating back nearly 20 years [38]. First, estrogen [38, 68] and more recently androgen receptor [44] imaging have been shown to provide biologically meaningful images in patients with receptor-positive breast and prostate cancer, respectively. HER-2/neu, a receptor up-regulated in certain forms of breast cancer, primarily due to amplification, has been a target for multimodality molecular imaging [69–71]. Artemov et al. have pursued an MR-based approach using avidin-biotin technology to image successfully HER-2/neu overexpressing tumors in an experimental model [69]. Although not yet ready for clinical translation, that study provided the proof-of-principle that receptor-based MR imaging in vivo was possible provided that appropriate signal amplification techniques are employed. On the same theme, Larson et al. have developed a positron-emitting anti-HER-2/neu antibody labeled with gallium-68. That construct has been used preclinically and is currently under assessment for clinical translation [72] (Fig. 5). The use of a positron-emitting analog has certain advantages over an MR-based agent in that it will be administered in subpharmacologic, i.e. ‘tracer’, doses, enabling a smoother path to clinical translation. In addition to early diagnosis or detection of metastatic disease, receptor-based imaging techniques can also be used to check the efficacy of receptor-based therapies. Although not performed to date, receptor occupancy studies, in analogy with those that are performed for neuropsychiatric drugs [17, 73], could be performed in oncology research as well. Notably both PSMA and HER-2/neu have been targets for the development of nanodiagnostics, illustrating the wide variety of potential new agents that can be developed once a suitable target is chosen [74, 75].
Figure 4.
SPECT-CT imaging of [125I]DCIT in an LNCaP and PC-3 tumor-bearing SCID mouse. The PSMA-expressing LNCaP tumor displays high uptake while the PSMA non-expressing PC-3 tumor shows minimal uptake (adapted from reference [18]).
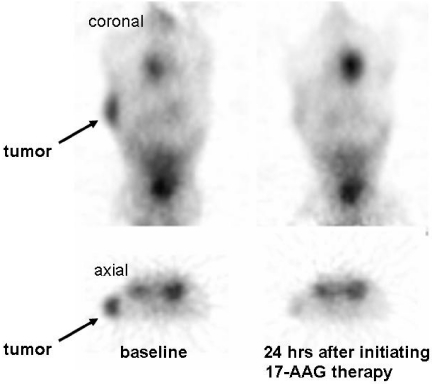
Figure 5.
MicroPET images obtained 3 h post injection with 68Ga-F(ab) 2-Herceptin in a mouse with a BT 474 breast tumor (images provided courtesy Steven Larson, Memorial Sloan Kettering Cancer Center). Note the early metabolic response to therapy with 17-allylamino-17-demethoxygeldanamycin (17-AAG).
Radiolabeled RGD peptides, directed toward the angiogenic marker, αvβ3 integrin receptor, have shown great utility in imaging experimental tumor models and are beginning to be used in the clinic [8, 9, 36, 37]. Much effort has been expended in pharmacokinetic optimization of these agents, including incorporation of a polyethylene glycol moiety and coupling with a long-lived positron emitter such as copper-64 [76]. The RGD peptides represent an excellent example of a class of compounds that are likely to find widespread clinical use in the near future for imaging a variety of cancers.
Metabolic imaging agents
FDG-PET imaging for cancer has been reviewed extensively elsewhere [19, 47, 77]. FDG is the only FDA-approved positron-emitting radiopharmaceutical in widespread clinical use and only for cancer and Alzheimer disease. As suggested above in the discussion of radiolabeled chemotherapeutic agents, perhaps the most important role for imaging in therapeutic monitoring is in the early prediction of patient outcome with a particular therapeutic agent. For example, FDG-PET was able to predict the outcome of patients with gastrointestinal stromal tumors treated with imatinib within several days of initiating treatment [78]. A similar result, indicating early prediction of tumor response to chemotherapy, has recently been shown in breast cancer using MR spectroscopy [79]. The article cited above by Leyton et al. suggests the improved utility of FLT over FDG for predicting tumor response [64]. FLT is the result of many years of development of other thymidine analogs for cancer imaging and is based on the fact that thymidine kinase, the enzyme of which FLT is a substrate, is regulated by the cell cycle, which often goes awry in cancer [80]. Because of the salutary metabolic characteristics and direct link of the mechanism of action to FLT uptake to cancer, FLT will soon gain widespread use and quite possibly displace FDG as the primary metabolic tumor imaging agent. Other promising metabolic cancer molecular imaging agents include the family of radiolabeled choline analogs for prostate cancer [49, 81] as well as radiolabeled analogs of ATSM for imaging tumor hypoxia [30, 82]. Agents of both of these classes recently entered the clinic and are beginning to demonstrate utility in cancer detection and monitoring. The metabolic imaging technique of magnetic resonance spectroscopy, which has been in clinical use for about 15 years, has been applied both to central nervous system malignancies as well as to prostate cancer to good advantage [22, 83–85]. In the former, it can be used to distinguish radiation necrosis versus recurrent neoplasm, an important problem in brain tumor imaging [86], and in the latter it can be used to direct biopsy toward the most malignant elements of a prostate tumor [87]. That indication is important because current prostate cancer biopsies are random and highly subject to sampling error.
Recent and near-term translation of novel molecular imaging agents and methods
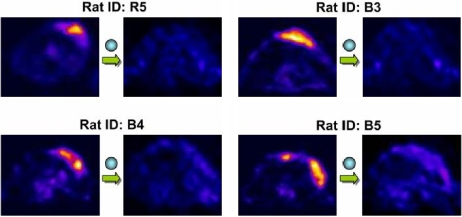
The abovementioned examples tend to focus on radionuclide-based techniques, however MR-based methods are also proving useful. After more than ten years of meticulous preclinical optimization, Weissleder et al. have shown the ability for iron oxide nanoparticles to differentiate benign from malignant lymph nodes with prostate cancer [25]. One significant facet of that study was that lesions smaller than what could be detected by PET, touted to be the much more sensitive technique (1 million-fold), were detectable. Also, significant about that work is that it represents the first practical clinical application of nanotechnology to imaging in that the particles used to generate contrast were engineered, primarily inorganic, substances. Similar technology has also been used recently to delineate the margins of gliomas, using a multifunctional reporter for intraoperative management [88]. Other multifunctional nanoparticles have also been developed using antibody-based approaches as well as for the visualization of angiogenesis with αvβ3 as the target, in analogy to the RGD peptides [89]. Although nanoparticle technology suffers from the relatively large size of the resultant imaging agents, suggesting that they may be limited to intravascular applications, that is not necessarily the case as they may be linked to various peptides that promote internalization [90] or may be introduced to the cells of interest, such as in tracking studies using MR, through ex vivo techniques such as microelectroporation [91]. Another, new MR-based molecular imaging technique that will find clinical use shortly is amide proton transfer imaging (APTI). APTI obviates the use of exogenously administered contrast media and is performed in analogy to magnetization transfer imaging for visualization of proteins within malignant tissue [92] (Fig. 6) [93]. While the MR-based techniques may suffer, in many cases, from less sensitivity than the radionuclide-based methods, they do have the advantage of ready clinical translation, if no exogenous contrast is administered, as in the case of APTI. So far the only clinical example of molecular-genetic imaging of cancer is the study performed by Jacobs et al., now nearly 4 years old, in which patients undergoing ganciclovir therapy were imaged with a radiolabeled nucleoside analog [94].
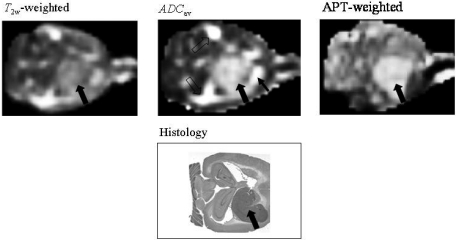
Figure 6.
Amide proton transfer imaging (APTI) in 9L tumors in rat. Both conventional (T2-weighted and apparent diffusion coefficient (ADC) maps) and APTI are shown. Note that the hyperintensity within the peritumoral tissue (small arrow) and cerebrospinal fluid (open arrows) in the ADC map become normal with APTI, adding to the clearer contour of the tumor (large arrow) on the latter images. APTI provides clearer tumor contour than the T2-weighted image as well (adapted from reference [92]).
Hurdles to overcome for translational molecular imaging
Although molecular imaging is a relatively new field, there is a sense that clinical translation of molecular imaging agents is not happening sufficiently rapidly. Although the scientific hurdles, such as cell penetration of imaging agents, appropriate pharmacokinetic profiles, etc. are being steadily overcome, regulatory hurdles persist. The US Food and Drug Administration (FDA) has recently recommended a relaxation in what were relatively stringent requirements for toxicity testing of new imaging agents, many of which can now be assessed using a microdosing protocol. However, increased burden has been placed on individual laboratories for assuring good manufacturing practice (GMP) through heavy documentation of each step en route to the clinical examination. As discussed above, the DCIDE program at the NCI provides funds for toxicology and a supportive staff for the dissemination of promising new molecular imaging agents, however, despite these measures, few new molecular imaging agents have gained widespread clinical use over the last several years.
Clinical translation: the cautionary tail of Apomate ™
As stated at the outset, one of the most promising targets for molecular imaging in cancer is the process of apoptosis, which is the mechanism by which many chemotherapeutic agents produce their tumoricidal effect. Accordingly, Theseus Imaging Corporation (a wholly owned subsidiary of North American Scientific, Inc., Chatsworth, CA) invested significant manpower and funds into the clinical development of an early biomarker for cancer therapy based on apoptosis imaging technology using single photon emission computed tomography (SPECT) of annexin V [95, 96]. Because the imaging agent was a recombinant DNA-derived protein, the US FDA required extensive preclinical testing to assure the lack of immunogenicity of the protein and required an assay to be in place during the clinical trial for further assurance that there would be no deleterious immune-mediated effects [97]. The development of those assays is challenging, time-consuming and caused a delay in obtaining approval for clinical apoptosis imaging in the US. Furthermore, the results of the first phase II trial were difficult to interpret, largely based on the design of the trial that combined broad based cytotoxic agents such as Taxol and Cisplatin, as well as steroids to counterbalance the side-effects of the chemotherapy. The results proved of great scientific interest, particularly with baseline imaging, and in some cases produced the counterintuitive result that patients who responded to conventional chemotherapy tended to demonstrate lower uptake of the radiolabeled annexin relative to the baseline scan. Those results suggest that the tumor environment studied by this imaging agent was much more complex than expected and included possibly lymphocytes and phagocytic cells in addition to tumor cells. Taken together, these findings indicate that a straightforward clinical protocol, in which patients were chosen at the outset based on a more targeted pro-apoptotic therapy, such as radiation therapy, would have possibly provided more readily interpretable data [98] (Fig. 7). Therefore, regulatory hurdles surrounding a challenging initial product such as those that are protein-based, and, in retrospect, a suboptimal phase II imaging trial design, may have significantly limited the dissemination of clinical apoptosis imaging.
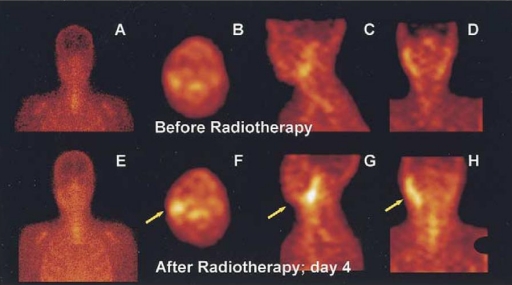
Figure 7.
SPECT imaging of 99mTc-annexin V in a patient with follicular lymphoma. Note high uptake in tumor-bearing lymph nodes after radiation therapy (from reference [98]).
Perspective
Conventional clinical molecular imaging, i.e. with radionuclide-based probes, has been practiced for many years and is a growing field with the development of ever more selective receptor-, enzyme- and transporter-based imaging agents. We are beginning to see the first applications of MR- and optically-based clinical molecular imaging, particularly for cell trafficking studies and intraoperative guidance. One of the great promises of molecular imaging research, i.e. molecular-genetic imaging, will gain clinical use in parallel with the acceptance of gene therapy, which has proved challenging, and as more sensitive, biocompatible reporter-probe combinations are discovered. Regulatory hurdles remain prominent for translation of the most novel agents, but microdosing protocols are being adopted for some and programs such as DCIDE will continue to shepherd the most promising new contrast agents and probes to the clinic.
Acknowledgments
I thank Jean-Luc Vanderheyden for discussion and CA92871 for financial support.
References
- 1.Pomper MG. Molecular imaging: an overview. Acad Radiol. 2001;8:1141–53. doi: 10.1016/S1076-6332(03)80728-6. [DOI] [PubMed] [Google Scholar]
- 2.Chatziioannou AF. Molecular imaging of small animals with dedicated PET tomographs. Eur J Nucl Med Mol Imaging. 2002;29:98–114. doi: 10.1007/s00259-001-0683-3. [DOI] [PubMed] [Google Scholar]
- 3.Pomper MG. Can small animal imaging accelerate drug development? J Cell Biochem Suppl. 2002;39:211–20. doi: 10.1002/jcb.10443. [DOI] [PubMed] [Google Scholar]
- 4.Doubrovin M, Serganova I, Mayer-Kuckuk P, Ponomarev V, Blasberg RG. Multimodality in vivo molecular-genetic imaging. Bioconjug Chem. 2004;15:1376–88. doi: 10.1021/bc0498572. [DOI] [PubMed] [Google Scholar]
- 5.Guccione S, Yang YS, Shi G, Lee DY, Li KC, Bednarski MD. Functional genomics guided with MR imaging: mouse tumor model study. Radiology. 2003;228:560–8. doi: 10.1148/radiol.2282020907. [DOI] [PubMed] [Google Scholar]
- 6.Holland EC. Mouse models of human cancer as tools in drug development. Cancer Cell. 2004;6:197–8. doi: 10.1016/j.ccr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Hu X, Holland EC. Applications of mouse glioma models in preclinical trials. Mutat Res. 2005;576:54–65. doi: 10.1016/j.mrfmmm.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Haubner RH, Wester HJ, Weber WA, Schwaiger M. Radiotracer-based strategies to image angiogenesis. Q J Nucl Med. 2003;47:189–99. [PubMed] [Google Scholar]
- 9.Haubner R, Weber WA, Beer AJ, et al. Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med. 2005;2:e70. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blankenberg F, Mari C, Strauss HW. Imaging cell death in vivo. Q J Nucl Med. 2003;47:337–48. [PubMed] [Google Scholar]
- 11.Takei T, Kuge Y, Zhao S, et al. Enhanced apoptotic reaction correlates with suppressed tumor glucose utilization after cytotoxic chemotherapy: use of 99mTc-Annexin V, 18F-FDG, and histologic evaluation. J Nucl Med. 2005;46:794–9. [PubMed] [Google Scholar]
- 12.Tjuvajev JG, Stockhammer G, Desai R, et al. Imaging the expression of transfected genes in vivo. Cancer Res. 1995;55:6126–6132. [PubMed] [Google Scholar]
- 13.Gambhir SS, Herschman HR, Cherry SR, et al. Imaging transgene expression with radionuclide imaging technologies. Neoplasia. 2000;2:118–38. doi: 10.1038/sj.neo.7900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doubrovin M, Ponomarev V, Beresten T, et al. Imaging transcriptional regulation of p53-dependent genes with positron emission tomography in vivo. Proc Natl Acad Sci USA. 2001;98:9300–5. doi: 10.1073/pnas.161091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blasberg RG, Tjuvajev JG. Molecular-genetic imaging: current and future perspectives. J Clin Invest. 2003;111:1620–9. doi: 10.1172/JCI18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luker GD, Pica CM, Song J, Luker KE, Piwnica-Worms D. Imaging 26S proteasome activity and inhibition in living mice. Nat Med. 2003;9:969–73. doi: 10.1038/nm894. [DOI] [PubMed] [Google Scholar]
- 17.Wong DF, Pomper MG. Predicting the success of a radiopharmaceutical for in vivo imaging of central nervous system neuroreceptor systems. Mol Imaging Biol. 2003;5:350–62. doi: 10.1016/j.mibio.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Foss CA, Mease RC, Fan H, et al. Radiolabeled small molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin Cancer Res. 2005;11:4022–8. doi: 10.1158/1078-0432.CCR-04-2690. [DOI] [PubMed] [Google Scholar]
- 19.Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med. 2005;46:983–95. [PubMed] [Google Scholar]
- 20.Mankoff DA, Shields AF, Krohn KA. PET imaging of cellular proliferation. Radiol Clin North Am. 2005;43:153–67. doi: 10.1016/j.rcl.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA. Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology. 2003;227:529–37. doi: 10.1148/radiol.2272020483. [DOI] [PubMed] [Google Scholar]
- 22.Nelson SJ. Multivoxel magnetic resonance spectroscopy of brain tumors. Mol Cancer Ther. 2003;2:497–507. [PubMed] [Google Scholar]
- 23.Kurhanewicz J, Swanson MG, Nelson SJ, Vigneron DB. Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer. J Magn Reson Imaging. 2002;16:451–63. doi: 10.1002/jmri.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enochs WS, Harsh G, Hochberg F, Weissleder R. Improved delineation of human brain tumors on MR images using a long-circulating, superparamagnetic iron oxide agent. J Magn Reson Imaging. 1999;9:228–32. doi: 10.1002/(sici)1522-2586(199902)9:2<228::aid-jmri12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 25.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–9. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 26.Heyn C, Bowen CV, Rutt BK, Foster PJ. Detection threshold of single SPIO-labeled cells with FIESTA. Magn Reson Med. 2005;53:312–20. doi: 10.1002/mrm.20356. [DOI] [PubMed] [Google Scholar]
- 27.Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol. 2000;12:450–8. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Sharma V, Prior JL, Belinsky MG, Kruh GD, Piwnica-Worms D. Characterization of a 67Ga/ 68Ga radiopharmaceutical for SPECT and PET of MDR1 P-glycoprotein transport activity in vivo: validation in multidrug-resistant tumors and at the blood-brain barrier. J Nucl Med. 2005;46:354–64. [PubMed] [Google Scholar]
- 29.Serganova I, Doubrovin M, Vider J, et al. Molecular imaging of temporal dynamics and spatial heterogeneity of hypoxia-inducible factor-1 signal transduction activity in tumors in living mice. Cancer Res. 2004;64:6101–8. doi: 10.1158/0008-5472.CAN-04-0842. [DOI] [PubMed] [Google Scholar]
- 30.O’Donoghue JA, Zanzonico P, Pugachev A, et al. Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N 4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int J Radiat Oncol Biol Phys. 2005;61:1493–502. doi: 10.1016/j.ijrobp.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 31.Blasberg RG, Gelovani J. Molecular-genetic imaging: a nuclear medicine-based perspective. Mol Imaging. 2002;1:280–300. doi: 10.1162/15353500200202127. [DOI] [PubMed] [Google Scholar]
- 32.Bharali DJ, Klejbor I, Stachowiak EK, et al. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci USA. 2005;102:11539–44. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolar J, Osborn M, Bell S, et al. Real-time in vivo imaging of stem cells following transgenesis by transposition. Mol Ther. 2005;12:42–8. doi: 10.1016/j.ymthe.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Sodee DB, Nelson AD, Faulhaber PF, Maclennan GT, Resnick MI, Bakale G. Update on fused capromab pendetide imaging of prostate cancer. Clin Prostate Cancer. 2005;3:230–8. doi: 10.3816/cgc.2005.n.004. [DOI] [PubMed] [Google Scholar]
- 35.Artemov D, Mori N, Ravi R, Bhujwalla ZM. Magnetic resonance molecular imaging of the HER-2/neu receptor. Cancer Res. 2003;63:2723–7. [PubMed] [Google Scholar]
- 36.Haubner R, Wester HJ, Weber WA, et al. Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781–5. [PubMed] [Google Scholar]
- 37.Chen X, Sievers E, Hou Y, et al. Integrin alpha v beta 3-targeted imaging of lung cancer. Neoplasia. 2005;7:271–9. doi: 10.1593/neo.04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169:45–8. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 39.Pomper MG, VanBrocklin H, Thieme AM, et al. 11 beta-methoxy-, 11 beta-ethyl- and 17 alpha-ethynyl-substituted 16 alpha-fluoroestradiols: receptor-based imaging agents with enhanced uptake efficiency and selectivity. J Med Chem. 1990;33:3143–55. doi: 10.1021/jm00174a009. [DOI] [PubMed] [Google Scholar]
- 40.Dehdashti F, Flanagan FL, Mortimer JE, Katzenellenbogen JA, Welch MJ, Siegel BA. Positron emission tomographic assessment of ‘metabolic flare’ to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med. 1999;26:51–6. doi: 10.1007/s002590050359. [DOI] [PubMed] [Google Scholar]
- 41.Pomper MG, Katzenellenbogen JA, Welch MJ, Brodack JW, Mathias CJ. 21-[18F]fluoro-16 alpha-ethyl-19-norprogesterone: synthesis and target tissue selective uptake of a progestin receptor based radiotracer for positron emission tomography. J Med Chem. 1988;31:1360–3. doi: 10.1021/jm00402a019. [DOI] [PubMed] [Google Scholar]
- 42.Pomper MG, Pinney KG, Carlson KE, et al. Target tissue uptake selectivity of three fluorine-substituted progestins: potential imaging agents for receptor-positive breast tumors. Int J Rad Appl Instrum B. 1990;17:309–19. doi: 10.1016/0883-2897(90)90058-9. [DOI] [PubMed] [Google Scholar]
- 43.Liu A, Carlson KE, Katzenellenbogen JA. Synthesis of high affinity fluorine-substituted ligands for the androgen receptor. Potential agents for imaging prostatic cancer by positron emission tomography. J Med Chem. 1992;35:2113–29. doi: 10.1021/jm00089a024. [DOI] [PubMed] [Google Scholar]
- 44.Larson SM, Morris M, Gunther I, et al. Tumor localization of 16beta- 18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366–73. [PubMed] [Google Scholar]
- 45.Pauwels EK, Sturm EJ, Bombardieri E, Cleton FJ, Stokkel MP. Positron-emission tomography with [18F]fluorodeoxyglucose. Part I. Biochemical uptake mechanism and its implication for clinical studies. J Cancer Res Clin Oncol. 2000;126:549–59. doi: 10.1007/PL00008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ak I, Stokkel MP, Pauwels EK. Positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose in oncology. Part II. The clinical value in detecting and staging primary tumours. J Cancer Res Clin Oncol. 2000;126:560–74. doi: 10.1007/PL00008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am. 2005;43:189–204. doi: 10.1016/j.rcl.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 48. Ko YH, Smith BL, Wang Y, Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun 2004 (in press)
- 49.Price DT, Coleman RE, Liao RP, Robertson CN, Polascik TJ, DeGrado TR. Comparison of [18F]fluorocholine and [18F]fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J Urol. 2002;168:273–80. [PubMed] [Google Scholar]
- 50.Foster-Gareau P, Heyn C, Alejski A, Rutt BK. Imaging single mammalian cells with a 1.5 T clinical MRI scanner. Magn Reson Med. 2003;49:968–71. doi: 10.1002/mrm.10417. [DOI] [PubMed] [Google Scholar]
- 51.Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002;22:899–907. doi: 10.1097/00004647-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Bulte JW, Douglas T, Witwer B, et al. Monitoring stem cell therapy in vivo using magnetodendrimers as a new class of cellular MR contrast agents. Acad Radiol. 2002;9:S332–5. doi: 10.1016/s1076-6332(03)80221-0. [DOI] [PubMed] [Google Scholar]
- 53.Louie AY, Huber MM, Ahrens ET, et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18:321–5. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 54.Li WH, Parigi G, Fragai M, Luchinat C, Meade TJ. Mechanistic studies of a calcium-dependent MRI contrast agent. Inorg Chem. 2002;41:4018–24. doi: 10.1021/ic0200390. [DOI] [PubMed] [Google Scholar]
- 55.Kircher MF, Weissleder R, Josephson L. A dual fluorochrome probe for imaging proteases. Bioconjug Chem. 2004;15:242–8. doi: 10.1021/bc034151d. [DOI] [PubMed] [Google Scholar]
- 56.Shah K, Weissleder R. Molecular optical imaging: applications leading to the development of present day therapeutics. NeuroRx. 2005;2:215–25. doi: 10.1602/neurorx.2.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morawski AM, Winter PM, Yu X, et al. Quantitative ‘magnetic resonance immunohistochemistry’ with ligand-targeted (19)F nanoparticles. Magn Reson Med. 2004;52:1255–62. doi: 10.1002/mrm.20287. [DOI] [PubMed] [Google Scholar]
- 58.Schmieder AH, Winter PM, Caruthers SD, et al. Molecular MR imaging of melanoma angiogenesis with alphanubeta3-targeted paramagnetic nanoparticles. Magn Reson Med. 2005;53:621–7. doi: 10.1002/mrm.20391. [DOI] [PubMed] [Google Scholar]
- 59.Ravert HT, Klecker RW, Collins JM, et al. Radiosynthesis of [11C]paclitaxel. J Label Compd Radiopharm. 2002;45:471–7. [Google Scholar]
- 60.Kurdziel KA, Kiesewetter DO, Carson RE, Eckelman WC, Herscovitch P. Biodistribution, radiation dose estimates, and in vivo Pgp modulation studies of 18F-paclitaxel in nonhuman primates. J Nucl Med. 2003;44:1330–9. [PubMed] [Google Scholar]
- 61.Gupta N, Price PM, Aboagye EO. PET for in vivo pharmacokinetic and pharmacodynamic measurements. Eur J Cancer. 2002;38:2094. doi: 10.1016/s0959-8049(02)00413-6. [DOI] [PubMed] [Google Scholar]
- 62.Saleem A, Yap J, Osman S, et al. Modulation of fluorouracil tissue pharmacokinetics by eniluracil: in-vivo imaging of drug action. Lancet. 2000;355:2125–31. doi: 10.1016/s0140-6736(00)02380-1. [DOI] [PubMed] [Google Scholar]
- 63.Schillaci O, Spanu A, Madeddu G. [(99m)Tc]sestamibi and [(99m)Tc]tetrofosmin in oncology: SPET and fusion imaging in lung cancer, malignant lymphomas and brain tumors. Q J Nucl Med Mol Imaging. 2005;49:133–44. [PubMed] [Google Scholar]
- 64.Leyton J, Latigo JR, Perumal M, Dhaliwal H, He Q, Aboagye EO. Early detection of tumor response to chemotherapy by 3 ′-deoxy-3 ′-[18F]fluorothymidine positron emission tomography: the effect of cisplatin on a fibrosarcoma tumor model in vivo. Cancer Res. 2005;65:4202–10. doi: 10.1158/0008-5472.CAN-04-4008. [DOI] [PubMed] [Google Scholar]
- 65.Wilkinson S, Chodak G. The role of 111indium-capromab pendetide imaging for assessing biochemical failure after radical prostatectomy. J Urol. 2004;172:133–6. doi: 10.1097/01.ju.0000132138.02846.08. [DOI] [PubMed] [Google Scholar]
- 66.Ding P, Miller MJ, Chen Y, Helquist P, Oliver AJ, Wiest O. Syntheses of conformationally constricted molecules as potential NAALADase/PSMA inhibitors. Org Lett. 2004;6:1805–8. doi: 10.1021/ol049473r. [DOI] [PubMed] [Google Scholar]
- 67.Grohs DC, Maison W. Synthesis of modular dipeptide mimetics on the basis of diazabicycloalkanes and derivatives thereof with sulphur containing side chains. Amino Acids. 2005 doi: 10.1007/s00726-005-0182-0. [DOI] [PubMed] [Google Scholar]
- 68.McGuire AH, Dehdashti F, Siegel BA, et al. Positron tomographic assessment of 16 alpha-[18F] fluoro-17 beta-estradiol uptake in metastatic breast carcinoma. J Nucl Med. 1991;32:1526–31. [PubMed] [Google Scholar]
- 69.Artemov D, Mori N, Okollie B, Bhujwalla ZM. MR molecular imaging of the Her-2/neu receptor in breast cancer cells using targeted iron oxide nanoparticles. Magn Reson Med. 2003;49:403–8. doi: 10.1002/mrm.10406. [DOI] [PubMed] [Google Scholar]
- 70.Olafsen T, Kenanova VE, Sundaresan G, et al. Optimizing radiolabeled engineered anti-p185HER2 antibody fragments for in vivo imaging. Cancer Res. 2005;65:5907–16. doi: 10.1158/0008-5472.CAN-04-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steffen AC, Wikman M, Tolmachev V, et al. In vitro characterization of a bivalent anti-HER-2 affibody with potential for radionuclide-based diagnostics. Cancer Biother Radiopharm. 2005;20:239–48. doi: 10.1089/cbr.2005.20.239. [DOI] [PubMed] [Google Scholar]
- 72.Smith-Jones PM, Solit DB, Akhurst T, Afroze F, Rosen N, Larson SM. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol. 2004;22:701–6. doi: 10.1038/nbt968. [DOI] [PubMed] [Google Scholar]
- 73.Meyer JH, Wilson AA, Ginovart N, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–9. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- 74.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 75.Wu X, Liu H, Liu J, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–6. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 76.Chen X, Hou Y, Tohme M, et al. Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor alphavbeta3-integrin expression. J Nucl Med. 2004;45:1776–83. [PubMed] [Google Scholar]
- 77.Jerusalem G, Hustinx R, Beguin Y, Fillet G. PET scan imaging in oncology. Eur J Cancer. 2003;39:1525–34. doi: 10.1016/s0959-8049(03)00374-5. [DOI] [PubMed] [Google Scholar]
- 78.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 79.Meisamy S, Bolan PJ, Baker EH, et al. Adding in vivo quantitative 1H MR spectroscopy to improve diagnostic accuracy of breast MR imaging: preliminary results of observer performance study at 4.0 T. Radiology. 2005;236:465–75. doi: 10.1148/radiol.2362040836. [DOI] [PubMed] [Google Scholar]
- 80.Aboagye EO. Positron emission tomography imaging of small animals in anticancer drug development. Mol Imaging Biol. 2005;7:53–8. doi: 10.1007/s11307-005-0886-2. [DOI] [PubMed] [Google Scholar]
- 81.de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. Visualization of prostate cancer with 11C-choline positron emission tomography. Eur Urol. 2002;42:18–23. doi: 10.1016/s0302-2838(02)00129-x. [DOI] [PubMed] [Google Scholar]
- 82.Dehdashti F, Grigsby PW, Mintun MA, Lewis JS, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: relationship to therapeutic response—a preliminary report. Int J Radiat Oncol Biol Phys. 2003;55:1233–8. doi: 10.1016/s0360-3016(02)04477-2. [DOI] [PubMed] [Google Scholar]
- 83.Fulham MJ, Bizzi A, Dietz MJ, et al. Mapping of brain tumor metabolites with proton MR spectroscopic imaging: clinical relevance. Radiology. 1992;185:675–86. doi: 10.1148/radiology.185.3.1438744. [DOI] [PubMed] [Google Scholar]
- 84.Lin A, Ross BD, Harris K, Wong W. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx. 2005;2:197–214. doi: 10.1602/neurorx.2.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pucar D, Koutcher JA, Shah A, et al. Preliminary assessment of magnetic resonance spectroscopic imaging in predicting treatment outcome in patients with prostate cancer at high risk for relapse. Clin Prostate Cancer. 2004;3:174–81. doi: 10.3816/cgc.2004.n.028. [DOI] [PubMed] [Google Scholar]
- 86.Lee MC, Pirzkall A, McKnight TR, Nelson SJ. 1H-MRSI of radiation effects in normal-appearing white matter: dose-dependence and impact on automated spectral classification. J Magn Reson Imaging. 2004;19:379–88. doi: 10.1002/jmri.20017. [DOI] [PubMed] [Google Scholar]
- 87.Pouliot J, Kim Y, Lessard E, Hsu IC, Vigneron DB, Kurhanewicz J. Inverse planning for HDR prostate brachytherapy used to boost dominant intraprostatic lesions defined by magnetic resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys. 2004;59:1196–207. doi: 10.1016/j.ijrobp.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 88.Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003;63:8122–5. [PubMed] [Google Scholar]
- 89.Chen X, Conti PS, Moats RA. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in brain tumor xenografts. Cancer Res. 2004;64:8009–14. doi: 10.1158/0008-5472.CAN-04-1956. [DOI] [PubMed] [Google Scholar]
- 90.Santra S, Yang H, Stanley JT, et al. Rapid and effective labeling of brain tissue using TAT-conjugated CdS: Mn/ZnS quantum dots. Chem Commun (Camb) 2005:3144–46. doi: 10.1039/b503234b. [DOI] [PubMed] [Google Scholar]
- 91.Walczak P, Kedziorek DA, Gilad AA, Lin S, Bulte JWM. American Academy of Nanomedicine, 1st Annual Meeting. Baltimore, MD: 2005. Magnetoeletroporation: improved nanoparticle labeling of stem cells for use in magnetic resonance imaging. [Google Scholar]
- 92.Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50:1120–6. doi: 10.1002/mrm.10651. [DOI] [PubMed] [Google Scholar]
- 93.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PC. Suppression of lipid artifacts in amide proton transfer imaging. Magn Reson Med. 2005;54:222–5. doi: 10.1002/mrm.20530. [DOI] [PubMed] [Google Scholar]
- 94.Jacobs A, Voges J, Reszka R, et al. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001;358:727–9. doi: 10.1016/s0140-6736(01)05904-9. [DOI] [PubMed] [Google Scholar]
- 95.Vermeersch H, Loose D, Lahorte C, et al. 99mTc-HYNIC Annexin-V imaging of primary head and neck carcinoma. Nucl Med Commun. 2004;25:259–63. doi: 10.1097/00006231-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Green AM, Steinmetz ND. Correlation of biodistribution changes of 99mTc-Hynic-rh-Annexin V before and after the initial dose of chemotherapy for non-small-cell lung cancer with subsequent patient response. Eur J Nucl Med Mol Imaging. 2004;31((Suppl 2)):S347. [Google Scholar]
- 97.Boersma HH, Stolk LM, Kenis H, et al. Development of a magnetic bead assay (AnnexNeutral) to measure neutralizing antibodies against Annexin A5 in the plasma of patients undergoing 99mTc-Annexin A5 imaging. Eur J Nucl Med Mol Imaging. 2004;31((Suppl 2)):S265. [Google Scholar]
- 98.Haas RL, de Jong D, Valdes Olmos RA, et al. In vivo imaging of radiation-induced apoptosis in follicular lymphoma patients. Int J Radiat Oncol Biol Phys. 2004;59:782–7. doi: 10.1016/j.ijrobp.2003.11.017. [DOI] [PubMed] [Google Scholar]