Abstract
The value of ultrasound (US) in the diagnosis of prostate cancer has dramatically increased in the past decade. This is mainly related to the increasing incidence of prostate cancer, the most common cancer in men and one of the most important causes of death from cancer in men. The value of conventional gray-scale US for prostate cancer detection has been extensively investigated, and has shown a low sensitivity and specificity. Therefore conventional gray-scale US is mainly used by urologists for guiding systematic prostate biopsies. With the development of new US techniques, such as color and power Doppler US, and the introduction of US contrast agents, the role of US for prostate cancer detection has dramatically changed. Advances in US techniques were introduced to further increase the value of US contrast agents. Although most of these developments in US techniques, which use the interaction of the contrast agent with the transmitted US waves, are very sensitive for the detection of microbubbles, they are mostly unexplored, in particular for prostate applications. Early reports of contrast-enhanced US investigations of blood flow of the prostate have shown that contrast-enhanced US adds important information to the conventional gray-scale US technique. Furthermore, elastography or ‘strain imaging’ seems to have great potential in prostate cancer detection. Since these new advances in US are very sophisticated and need a long learning curve, radiologists, who are overall better trained with these new US techniques, will play a more important role in prostate cancer diagnosis. Current trends show that these new US techniques may allow for targeted biopsies and therefore replace the current ‘gold standard’ for prostate cancer detection—the systematic biopsy. Consequently the use of these new US techniques for the detection and clinical staging of prostate cancer is promising. However, future clinical trials will be needed to determine if the promise of these new US advances of the prostate evolves into clinical application.
Keywords: Ultrasound, color/power Doppler, contrast agent, elastography, prostate cancer
Introduction
The annual incidence of prostate cancer has increased dramatically over the past decade such that prostate cancer is now the most commonly diagnosed visceral malignancy in men [1]. Ninety-five percent of prostate cancer is diagnosed in men between 45 and 89 years of age, with a median age of diagnosis of 70 years. The prevalence of prostate cancer increases with age. After age 50, both the incidence and mortality increase at a near exponential rate. Men with a positive family history have a higher likelihood of developing the disease, often at a younger age, than their counterparts with no history of cancer in the family [2].
Approximately 50% of patients diagnosed with cancer develop metastatic, incurable disease [1]. Early diagnosis when the tumor is localized to the prostate is essential in order to provide definitive treatment and improve patient survival [2].
Three diagnostic tests are routinely used to detect malignancy in the prostate: prostate-specific antigen (PSA), digital rectal examination (DRE), and transrectal ultrasound (US) imaging [3]. The tests, however, lack both sensitivity and specificity, resulting in missed cancers as well as a large number of unnecessary biopsies.
Gray-scale ultrasound
Transrectal US of the prostate has revolutionized our ability to examine this organ [4]. Among the myriad of indications for US of the prostate, the most common indication is for evaluation of suspected prostate cancer. In this regard, transrectal US is usually performed in conjunction with needle biopsy of the prostate. The indication for prostate US and biopsy is either an abnormality on DRE or elevation in the serum PSA level. Occasionally, men undergo transrectal US owing to symptoms of bladder outlet obstruction or constitutional symptoms suggestive of metastatic prostate cancer. The utilization of serum assays for PSA and transrectal US guided needle biopsy has resulted, in general, in the diagnosis of prostate cancer at an earlier stage of presentation [5, 6].
Transrectal US provides excellent visualization of the prostate. Advantages of transrectal US guided biopsy include the ability to direct the biopsy needle precisely into regions of interest, or to provide uniform spatial separation of biopsy cores. For these reasons, most prostate biopsies are performed under US guidance [7, 8]. Transrectal US may identify non-palpable malignancies. However, few studies have compared the yield of digitally directed biopsy vs. those under transrectal US guidance. Weaver et al. performed biopsies under both US and digital guidance in 51 men with palpable prostatic abnormality [9]. They noted cancer in nine patients on digitally directed biopsy. In contrast, 23 men had cancer detected when biopsies were performed under US guidance. Each of the men who had a positive digitally guided biopsy also had cancer detected on the US guided procedure.
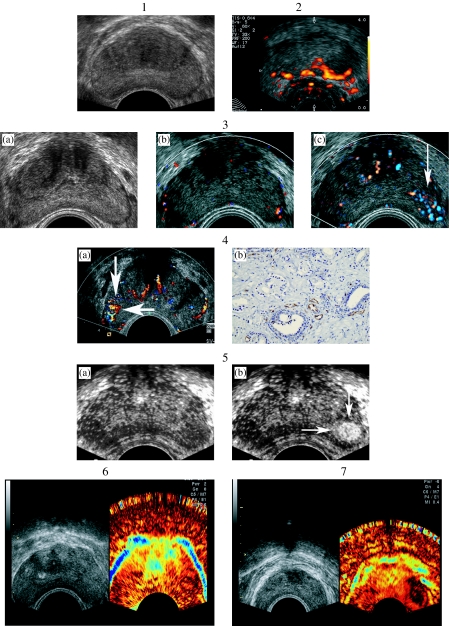
Although conventional gray-scale US does improve the yield of needle biopsy, this technique has only limited sensitivity and specificity for the detection of cancer. Specifically, transrectal US is limited by its inability to detect isoechoic tumors (Fig. 1(1)) and by the often heterogeneous appearance of the prostate. In order to improve the detection of cancer, additional Doppler and contrast-enhanced techniques may be used.
Figure 1.
(1) Transverse gray-scale US image of the prostate. The isoechoic cancer on the left side is not visible; however it was detected by systematic biopsy. Reprinted with permission: Radiologe 2005; 45: 544–51. (2) Power Doppler US of the prostate. A hypervascular area is seen on the left side, which proved to be prostate cancer. Reprinted with permission: Radiologe 2005; 45: 544–51. (3) Contrast-enhanced color Doppler US of the prostate. (a) Transverse gray-scale US image of the prostate shows no focal abnormality. (b) Transverse color Doppler US image of the prostate shows no focal hypervascularity. (c) Transverse contrast-enhanced color Doppler US image of the prostate shows focal hypervascularity on the left side, which has proved to be prostate cancer. Reprinted with permission: Radiologe 2005; 45: 544–51. (4) Contrast-enhanced color Doppler US of the prostate in comparison with MVD. (a) Transverse contrast-enhanced color Doppler US image of the prostate shows focal hypervascularity on the right side, which has proved to be prostate cancer. (b) Immunhistochemistry shows high MVD of this cancer. Reprinted with permission: Radiologe 2005; 45: 544–51. (5) Gray-scale harmonic imaging of the prostate: (a) Continuous image shows no focal enhancement. (b) Intermittent gray-scale harmonic US shows focal enhancement on the left side (Gleason 7 cancer). Reprinted with permission: Radiologe 2005; 45: 544–51. (6) Real-time elastography of the normal prostate. No suspicious lesion is visualized. (7) Real-time elastography of a patient with prostate cancer. No suspicious lesion is visualized on the gray-scale US, whereas real-time elastography shows a stiffer area—corresponding to the cancer area—on the left side.
Color Doppler ultrasound
Color Doppler US has been applied to evaluate the vascularity within the prostate and the surrounding structures [10–17]. The motivation behind the application of color Doppler US is to detect tumor neovascularity. Cancerous tissue generally grows more rapidly than normal tissue [18–21], and demonstrates increased blood flow, as compared to normal tissue and benign lesions. Color Doppler US may demonstrate an increased number of visualized vessels, as well as an increase in flow rate, size and irregularity of vessels within prostate cancer [22]. Three different flow patterns may be associated with prostate cancer: diffuse flow, focal flow and surrounding flow [10]. The most frequently identified flow pattern is diffuse flow within the lesion.
Early results have suggested that up to 85% of men with prostate cancers greater than 5 mm in size have visibly increased flow in the area of tumor involvement [10]. In addition, hypervascularity may be seen in patients with more difficult to identify, isoechoic and hyperechoic lesions. Unfortunately, subsequent studies suggested that the combined application of gray-scale and color Doppler US will still miss some cancers and is insufficient to preclude prostate biopsy.
Power Doppler ultrasound
Power Doppler US, an amplitude-based technique for the detection of flow, is more sensitive to slow flow and is less angle-dependent than color Doppler US. This newer technique has been less commonly applied to the assessment of prostate tumor vascularity, and there are few papers addressing its use [23]. However, early studies have suggested that power Doppler US may be useful in detection of prostate cancer (Fig. 1(2)).
In a recent study Halpern et al. assessed the value of gray-scale, color and power Doppler US for detection of prostatic cancer. They investigated 251 patients prior to biopsy. Each biopsy site was prospectively scored for gray-scale abnormality and Doppler flow. Cancer was detected in 211 biopsy sites from 85 patients. Overall agreement between sonographic findings and biopsy results as measured with the kappa statistic was minimally superior to chance (kappa=0.12 for gray-scale, kappa=0.11 for color Doppler, kappa=0.09 for power Doppler). Among patients with at least one positive biopsy for cancer, foci of increased power Doppler flow were 4.7 times more likely to contain cancer than adjacent tissues without flow. They concluded that power Doppler may be useful for targeted biopsies when the number of biopsy passes must be limited [24], but that there is no substantial advantage of power Doppler over color Doppler. Other investigators suggest that although Doppler flow patterns may correlate with microvascular density, Doppler imaging does not provide sufficient sensitivity to preclude biopsy [25].
Contrast enhanced ultrasound imaging
Recently developed US contrast agents can improve the detection of low-volume blood flow by increasing the signal-to-noise ratio [26–29]. Intravascular contrast agents allow a more complete delineation of the neovascular anatomy, by enhancing the signal strength from small vessels. Furthermore, these agents may be used to time the transit of an injected bolus. Unlike radiographic contrast media, which diffuse into the tissue and may obscure smaller vessels, microbubble echo-enhancing agents are confined to the vascular lumen, where they persist until they dissolve. Contrast agents are made of gas bubbles small enough to cross through capillary beds [29]. They have two important acoustic properties. First, they are many times more reflective than blood, thus improving flow detection. Second, their vibrations generate higher harmonics to a much greater degree than surrounding tissues. The half-life of contrast agents is dependent on bubble construction. Bubbles can be free or encapsuled in soft or hard shells. The duration of enhancement after injection may last from a few seconds to many minutes, depending on the bubble type.
In a previous study we examined the use of contrast-enhanced color Doppler US in 72 patients identified by PSA screening. Using a quantitative scale to characterize the degree of vascularity, the technique had a sensitivity of 53%, specificity of 72%, and a positive predictive value (PPV) of 70% in distinguishing prostate cancer from benign lesions [30].
Bree et al. reported the potential use of contrast-enhanced color Doppler to increase the diagnostic yield in a group of 17 patients with normal gray-scale transrectal US and elevated PSA values. Correlation of biopsy sites with color Doppler US abnormalities revealed a sensitivity of 54%, a specificity of 78%, a PPV of 61%, and a negative predictive value (NPV) of 72% for the detection of prostate cancer. Three of the cases with a positive contrast-enhanced biopsy site had negative transrectal US random biopsy within the previous year [31].
Bogers et al. evaluated contrast-enhanced 3D imaging of the prostate vasculature with power Doppler. 3D power Doppler images were obtained before and after intravenous administration of 2.5 g of Levovist™ (Schering, Berlin, Germany). Subsequently, random and/or directed trans-rectal US-guided biopsies were performed. Prostate vasculature was judged with respect to symmetry and vessel distribution. Eighteen patients with a suspicion of prostate cancer either because of an elevated PSA (greater than 4.0ng/ml; Tandem-R-assay) or an abnormal DRE were included in the study. Prostate cancer was detected in 13 patients. Vascular anatomy was judged abnormal in unenhanced images in six cases, of which five proved to be malignant. Enhanced images were considered suspicious for malignancy in 12 cases, including one benign and 11 malignant biopsy results. Sensitivity of enhanced images was 85% (specificity 80%) compared with 38% for unenhanced images (specificity 80%) and 77% for conventional gray-scale transrectal US (specificity 60%). Among six patients who showed no B-mode abnormalities, vascular patterns were judged abnormal in four cases, of which three were malignant. Based on these findings they concluded that contrast-enhanced 3D power Doppler angiography is feasible in patients with suspicion of prostate cancer who are scheduled for prostate biopsies [32].
A more recent analyses by the same group suggested that 3D contrast-enhanced power Doppler US is a better diagnostic tool than the DRE, PSA level, gray-scale US or power Doppler US alone. The most suitable diagnostic predictor for prostate cancer was a combination of 3D contrast-enhanced power Doppler US and PSA level [33].
We reported the value of contrast enhanced color Doppler in a prospective study in 90 [34], and 230 male screening volunteers [35], and found that targeted biopsies based on contrast enhanced color Doppler detected as many cancers as systematic biopsies, with fewer than half the number of biopsy cores (Fig. 1(3)). Sedelaar et al. demonstrated the correlation between microvessel density (MVD) and three-dimensional contrast enhanced power Doppler imaging [36]. In all patients, the enhanced side of the prostate was correlated with a higher MVD count. Concerning the MVD and the color pixel density, we found similar results using contrast-enhanced color Doppler US using the US contrast agent Levovist™ (Schering, Berlin, Germany) [37] (Fig. 1(4)).
A new approach to contrast-enhanced imaging of the prostate is phase or pulse inversion technology to exploit the nonlinear behavior of the bubbles in order to differentiate blood flow echoes from background tissue with high contrast, and spatial resolution. Albrecht et al. demonstrated this technique in 39 human subjects. Gray-scale enhancement of vessels and organ parenchyma was seen in all cases. Enhancement occurred from both flowing and stationary microbubbles. Flow-independent enhancement of tissue represents a major advance in contrast-enhanced US with many potential applications especially in tumor imaging [38].
In a preliminary study of 26 subjects Halpern et al. investigated the usefulness of contrast-enhanced US to depict vascularity in the prostate. Continuous gray-scale and intermittent gray-scale imaging were performed at the fundamental frequency. Phase inversion gray-scale imaging was available only for the final few subjects in the study. US findings were correlated with sextant biopsy results. After the administration of the contrast material Imagent™ (Alliance Pharmaceutical Corp. San Diego, CA), gray-scale and Doppler images revealed visible enhancement (p<0.05). Using intermittent imaging, they found focal enhancement in two isoechoic tumors that were not visible on baseline images. No definite focal area of enhancement was identified in any patient without cancer. Furthermore contrast-enhanced images revealed obvious enhancement of transient hemorrhage in the biopsy tracts of three patients. They concluded that gray-scale as well as Doppler enhancement of the prostate can be seen on US images after the administration of an i.v. contrast agent, and that contrast-enhanced intermittent US of the prostate may be useful for the selective enhancement of malignant prostatic tissue [39].
Halpern et al. have recently completed a prospective study of contrast-enhanced transrectal US in 60 patients who underwent sextant biopsy of the prostate. All examinations were performed with the Sonoline Elegra (Siemens Medical Systems, Ultrasound Group, Issaquah, WA) using gray-scale harmonic imaging. Each subject was evaluated with conventional gray-scale, harmonic gray-scale and power Doppler US prior to contrast infusion. The evaluation was repeated during intravenous infusion of Definity™ (DuPont Pharmaceuticals, Billerica, MA). Gray-scale imaging was performed in continuous mode and with intermittent imaging using interscan delay times of 0.5 s, 1.0 s, 2.0 s and 5.0 s. Each biopsy was scored prospectively as benign or malignant on baseline imaging, again during contrast-enhanced transrectal US. Prostate cancer was present in 37 biopsy sites from 20 subjects. Baseline imaging demonstrated cancer in 14 sites from 11 subjects. Contrast-enhanced transrectal US showed cancer in 24 sites from 15 subjects. Each of the five subjects whose prostate cancer was not detected by contrast-enhanced transrectal US had only a single positive biopsy core with a Gleason score of six or less. The improvement in sensitivity from 38% at baseline to 65% with contrast was significant (p<0.004) (Fig. 1(5)). Specificity was not significantly different at baseline (83%) and during contrast imaging (80%). These results suggest that contrast-enhanced transrectal US may improve sensitivity for prostate cancer detection without substantial loss of specificity [40].
Elastography
Elastography or strain imaging was first described in 1991 by Ophir et al. [41]. The phenomenon is based on the fact that the backscattered US signal changes its local characteristic pattern only to a comparably small extent if the insonified tissue is slightly compressed and decompressed (i.e. approximately up to 2%) during the examination. A high internal correlation is maintained within local regions of interest. However, time or space differences between local regions of interest under different compression ratios change with differences in compressibility of the insonified tissue. Time differences between two local regions of interest within two subsequent images recorded under different compression ratios can be calculated for each pixel of the images. Time differences are not absolute but relative values since the compressibility of local tissue regions always depends on the surrounding tissue and the applied compression force.
In a pilot study at our institution, patients with clinically localized prostate cancer who underwent radical prostatectomy were examined prospectively. Prior to surgery these patients were examined with conventional gray-scale US as well as with real-time elastography. Areas suspicious for prostate cancer were depicted. After surgery the histological specimens were compared with the transverse US images and with elastography findings. Thirty-two foci of prostate cancer were present at pathologic evaluation, with multiple foci of cancer in 13 of the 15 glands. Real-time elastography detected 28 of 32 cancer foci (sensitivity 88%). Four sites were false-positive with no pathological abnormality. The by patient analysis demonstrated that real-time elastography detected at least one cancer foci in each of the 15 patients. Therefore we concluded that real-time elastography of the prostate is a sensitive new imaging modality for the detection of prostate cancer [42] (Fig. 1(6) and Fig. 1(7)).
In a recent study by Konig et al., who evaluated elastography for biopsy guidance for prostate cancer detection, after imaging with conventional gray-scale US in conjunction with real-time elastography 404 men underwent systematic sextant biopsy. Prostate cancer was found in 151 of 404 cases (37.4%). In 127 of 151 cases (84.1%), prostate cancer was detected using real-time elastography as an additional diagnostic feature. They concluded that it is possible to detect prostate cancer with a high degree of sensitivity using real-time elastography in conjunction with conventional diagnostic methods for guided prostate biopsies [43].
Future perspectives
Although the detection of prostate cancer with contrast-enhanced transrectal US may be improved relative to baseline transrectal US, substantial uncertainty remains in the interpretation of contrast-enhanced transrectal US images. In a recent study, 16% (59/360) of contrast-enhanced transrectal US images were rated as indeterminate with respect to vascular enhancement [40]. Future studies of contrast-enhanced transrectal US should investigate new techniques to optimize the signal from contrast agents in the prostate, and to maximize the difference in signal between benign and malignant tissues. Halpern et al. reported greater enhancement with intermittent imaging and bolus administration of contrast. New imaging techniques may be developed to reduce bubble destruction during imaging. Since the prostate is generally evaluated with a frequency in the range of 5.0–7.5 MHz, newer bubble agents that resonate at higher frequencies may provide better signals. Alternatively, harmonic imaging at lower frequencies or with subharmonics may be useful with current contrast agents [44, 45].
Quantification of color Doppler US information is generally based upon a classification or subjective estimation by the examiner [46]. Both these approaches are highly user dependent. A system for objective evaluation was presented by Cosgrove et al. [47], who introduced a method of color pixel and vessel counting. This “manual” method, however, is cumbersome and does not distinguish pixels with different flow velocity. Flow velocity has been shown to be helpful in the differentiation of benign and malignant lesions in continuous-wave and duplex US studies. A computerized method for the evaluation of color Doppler US images has been developed to quantify color pixels in a selected region of interest with reconstruction of local flow velocities [36]. Huber et al. [48] improved the value of this technique by using a microbubble based US contrast agent, which allows additional assessment of enhancement kinetics. After microbubble contrast agent injection, breast carcinomas and benign lesions behave differently in degree, onset, and duration of Doppler US enhancement. Thus, time intensity curves may also be useful as another objective measure to differentiate benign from malignant prostatic tissue.
Exact assessment of the elasticity of the tissue with new computer-assisted techniques may allow for better differentiation of benign from malignant tissue using real-time elastography.
In summary, the application of the new advances in US for the detection and clinical staging of prostate cancer is promising. Technical improvements in US equipment will allow improved detection of smaller, low flow vessels. 3D/4D displays may allow improved detection of areas of flow asymmetry. Quantification of enhancement will provide an objective grading system. These new advances require excellent trained investigators. Overall, radiologists are better trained in the application of these new US techniques and should therefore play a more important role in the diagnosis of prostate cancer in the future.
References
- 1.American Cancer Society . Atlanta, GA: American Cancer Society; 2004. Cancer Facts and Figures. [Google Scholar]
- 2.Carter HB, Coffey DS. The prostate: an increasing medical problem. Prostate. 1990;16:39–48. doi: 10.1002/pros.2990160105. [DOI] [PubMed] [Google Scholar]
- 3.Scardino PT, Weaver R, Hudson MA. Early detection of prostate cancer. Human Pathol. 1992;23:211–22. doi: 10.1016/0046-8177(92)90102-9. [DOI] [PubMed] [Google Scholar]
- 4.Rifkin MD. 2nd edn. Philadelphia, PA: Lippincott-Raven; 1997. Ultrasound of the Prostate. [Google Scholar]
- 5.Rifkin MD. Prostate cancer: the diagnostic dilemma and the place of imaging in detection and staging. World J Urol. 1998;16:76–80. doi: 10.1007/s003450050029. [DOI] [PubMed] [Google Scholar]
- 6.Bartsch G, Egender G, Hubscher H, Rohr H. Sonometrics of the prostate. J Urol. 1982;127:1119–21. doi: 10.1016/s0022-5347(17)54259-7. [DOI] [PubMed] [Google Scholar]
- 7.Rifkin MD, Alexander AA, Pisarchick J, Matteucci T. Palpable masses in the prostate: superior accuracy of US-guided biopsy compared with accuracy of digitally guided biopsy. Radiology. 1991;179:41–7. doi: 10.1148/radiology.179.1.2006301. [DOI] [PubMed] [Google Scholar]
- 8.Spencer JA, Alexander AA, Gomella L, Matteucci T, Goldberg BB. Ultrasound-guided four quadrant biopsy of the prostate: efficacy in the diagnosis of isoechoic cancer. Clin Radiol. 1994;49:711–4. doi: 10.1016/s0009-9260(05)82667-4. [DOI] [PubMed] [Google Scholar]
- 9.Weaver RP, Noble MJ, Weigel JW. Correlation of ultrasound guided and digitally guided directed transrectal biopsies of palpable prostatic abnormalities. J Urol. 1991;145:516–8. doi: 10.1016/s0022-5347(17)38384-2. [DOI] [PubMed] [Google Scholar]
- 10.Rifkin MD, Sudakoff GS, Alexander AA. Prostate: techniques, results, and potential applications of color Doppler US scanning. Radiology. 1993;186:509–13. doi: 10.1148/radiology.186.2.7678467. [DOI] [PubMed] [Google Scholar]
- 11.Kelly IM, Lees WR, Rickards D. Prostate cancer and the role of color Doppler US. Radiology. 1993;189:153–6. doi: 10.1148/radiology.189.1.7690489. [DOI] [PubMed] [Google Scholar]
- 12.Newman JS, Bree RL, Rubin JM. Prostate cancer: diagnosis with color Doppler sonography with histologic correlation of each biopsy site. Radiology. 1995;195:86–90. doi: 10.1148/radiology.195.1.7534429. [DOI] [PubMed] [Google Scholar]
- 13.Littrup PJ, Klein RM, Gross ML, Sparschu RA, Segel MC, Zingas AP. Color Doppler of the prostate: histologic and racial correlations. Radiology. 1995;197:365. (abstract). [Google Scholar]
- 14.Ismail M, Petersen RO, Alexander AA, Newschaffer C, Gomella LG. Color Doppler imaging in predicting the biologic behavior of prostate cancer: correlation with disease-free survival. Urology. 1997;50:906–12. doi: 10.1016/S0090-4295(97)00403-2. [DOI] [PubMed] [Google Scholar]
- 15.Ismail M, Gomella LG, Alexander AA. Color Doppler sonography of the prostate. Tech Urol. 1997;3:140–6. [PubMed] [Google Scholar]
- 16.Alexander AA. To color Doppler image the prostate or not: that is the question. Radiology. 1995;195:11–3. doi: 10.1148/radiology.195.1.7534428. [DOI] [PubMed] [Google Scholar]
- 17.Rifkin MD, Alexander AA, Helinek TG, Merton DA. Color Doppler as an adjunct to prostate ultrasound. Scand J Urol Nephrol Suppl. 1991;137:85–9. [PubMed] [Google Scholar]
- 18.Sillman F, Boyce J, Fruchter R. The significance of atypical vessels and neovascularization in cervical neoplasia. Am J Obstet Gynecol. 1981;139:154–9. doi: 10.1016/0002-9378(81)90438-5. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava A, Laidler P, Davies RP, et al. The prognostic significance of tumor vascularity in intermediate-thickness (0.76–4.0 mm thick) skin melanoma. A quantitative histologic study. Am J Pathol. 1988;133:419–23. [PMC free article] [PubMed] [Google Scholar]
- 20.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 21.Brawer MK, Deering RE, Brown M, et al. Predictors of pathologic stage in prostatic carcinoma. The role of neovascularity. Cancer. 1994;73:678–7. doi: 10.1002/1097-0142(19940201)73:3<678::aid-cncr2820730329>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Fleischer AC, Rodgers WH, Rao BK, et al. Assessment of ovarian tumor vascularity with transvaginal color Doppler sonography. J Ultrasound Med. 1991;10:563–8. doi: 10.7863/jum.1991.10.10.563. [DOI] [PubMed] [Google Scholar]
- 23.Cho JY, Kim SH, Lee SE. Diffuse prostatic lesions: role of color and power Doppler ultrasonography. J Ultrasound Med. 1998;17:283–7. doi: 10.7863/jum.1998.17.5.283. [DOI] [PubMed] [Google Scholar]
- 24.Halpern EJ, Sirup SE. Using gray-scale and color and power Doppler sonography to detect prostatic cancer. AJR. 2000;174:623–7. doi: 10.2214/ajr.174.3.1740623. [DOI] [PubMed] [Google Scholar]
- 25.Louvar E, Littrup PJ, Goldstein A, et al. Correlation of color Doppler flow in the prostate with tissue microvascularity. Cancer. 1998;83:135–40. [PubMed] [Google Scholar]
- 26.Kedar RP, Cosgrove D, McCready VR, et al. Microbubble contrast agent for color Doppler US: effect on breast masses. Work in progress. Radiology. 1996;198:679–86. doi: 10.1148/radiology.198.3.8628854. [DOI] [PubMed] [Google Scholar]
- 27.Forsberg F, Merton DA, Liu JB, et al. Clinical applications of ultrasound contrast agents. Ultrasonics. 1998;36:695–701. doi: 10.1016/s0041-624x(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 28.Forsberg E, Liu JB, Bums PN, et al. Artifacts in ultrasonic contrast agent studies. J Ultrasound Med. 1994;13:357–65. doi: 10.7863/jum.1994.13.5.357. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg BB, Liu JB, Forsberg F. Ultrasound contrast agents: a review. Ultrasound Med Biol. 1994;20:319–33. doi: 10.1016/0301-5629(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 30.Frauscher E, Helweg G, Gotwald TF, et al. The value of contrast-enhanced color Doppler ultrasonography in the diagnosis of prostate cancer. Radiology. 1998;209:417. (abstract). [Google Scholar]
- 31.Bree RL, DeDreu SE. Contrast enhanced color Doppler of the prostate as an adjunct to gray-scale identification of cancer prior to biopsy. Radiology. 1998;209:418. [Google Scholar]
- 32.Bogers HA, Sedelaar JP, Beerlage HP, et al. Contrast-enhanced three-dimensional power Doppler angiography of the human prostate: correlation with biopsy outcome. Urology. 1999;54:97–104. doi: 10.1016/s0090-4295(99)00040-0. [DOI] [PubMed] [Google Scholar]
- 33. Unal D, Sedelaar JP, Aarnink RG, Three-dimensional contrast-enhanced power Doppler ultrasonography and conventional examination methods: the value of diagnostic predictors of prostate cancer. BJU Int (in press)
- 34.Frauscher F, Klauser A, Halpern EJ, Horninger W, Bartsch G. Detection of prostate cancer with a microbubble ultrasound contrast agent. Lancet. 2001;357:1849–50. doi: 10.1016/s0140-6736(00)04970-9. [DOI] [PubMed] [Google Scholar]
- 35.Frauscher F, Klauser A, Volgger H, et al. Comparison of contrast enhanced color Doppler targeted biopsy with conventional systematic biopsy: impact on prostate cancer detection. J Urol. 2002;167:1648–52. [PubMed] [Google Scholar]
- 36.Sedelaar JP, Van Leenders GJ, Hulsbergen-Van De Kaa CA, et al. Microvessel density: correlation between contrast ultrasonography and histology of prostate cancer. Eur Urol. 2001;40:285–93. doi: 10.1159/000049788. [DOI] [PubMed] [Google Scholar]
- 37.Strohmeyer D, Frauscher F, Klauser A, et al. Contrast-enhanced transrectal color Doppler ultrasonography (TRCDUS) for assessment of angiogenesis in prostate cancer. Anticancer Res. 2001;21:2907–13. [PubMed] [Google Scholar]
- 38.Albrecht T, Hoffmann CW, Schettler S, et al. B-mode enhancement at phase-inversion US with air-based microbubble contrast agent: initial experience in humans. Radiology. 2000;216:273–8. doi: 10.1148/radiology.216.1.r00jl27273. [DOI] [PubMed] [Google Scholar]
- 39.Halpern EJ, Verkh L, Forsberg F, et al. Initial experience with contrast-enhanced sonography of the prostate. AJR. 2000;174:1757–580. doi: 10.2214/ajr.174.6.1741575. [DOI] [PubMed] [Google Scholar]
- 40.Halpern EJ, Rosenberg M, Gomolla LG. Contrast enhanced sonography of the prostate. Radiology. 2001;219:219–25. doi: 10.1148/radiology.219.1.r01ap21219. [DOI] [PubMed] [Google Scholar]
- 41.Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–34. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 42.Klauser A, Koppelstaetter F, Berger AP, Horninger W, Lorenz A, Frauscher F. Real-time elastography for prostate cancer detection: preliminary experience. Radiology. 2003;229((Suppl)):1395. (abstract). [Google Scholar]
- 43.Konig K, Scheipers U, Pesavento A, Lorenz A, Ermert H, Senge T. Initial experiences with real-time elastography guided biopsies of the prostate. J Urol. 2005;174:115–17. doi: 10.1097/01.ju.0000162043.72294.4a. [DOI] [PubMed] [Google Scholar]
- 44.Shi WT, Forsberg F, Hall AL, et al. Subharmonic imaging with contrast agents: initial results. Ultrason Imaging. 1999;21:79–94. doi: 10.1177/016173469902100201. [DOI] [PubMed] [Google Scholar]
- 45.Forsberg F, Shi WT, Goldberg BB. Subharmonic imaging of contrast agents. Ultrasonic. 2000;38:93–8. doi: 10.1016/s0041-624x(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 46.Huber S, Delorme S, Knopp MV, et al. Breast tumors: computer-assisted quantitative assessment with color Doppler US. Radiology. 1994;192:797–801. doi: 10.1148/radiology.192.3.8058950. [DOI] [PubMed] [Google Scholar]
- 47.Cosgrove DO, Bamber JC, Davey JB, et al. Color Doppler signals from breast tumors. Work in progress. Radiology. 1990;176:175–80. doi: 10.1148/radiology.176.1.2191364. [DOI] [PubMed] [Google Scholar]
- 48.Huber S, Helbich T, Kettenbach J, et al. Effects of a microbubble contrast agent on breast tumors: computer-assisted quantitative assessment with color Doppler US-early experience. Radiology. 1998;208:485–9. doi: 10.1148/radiology.208.2.9680580. [DOI] [PubMed] [Google Scholar]