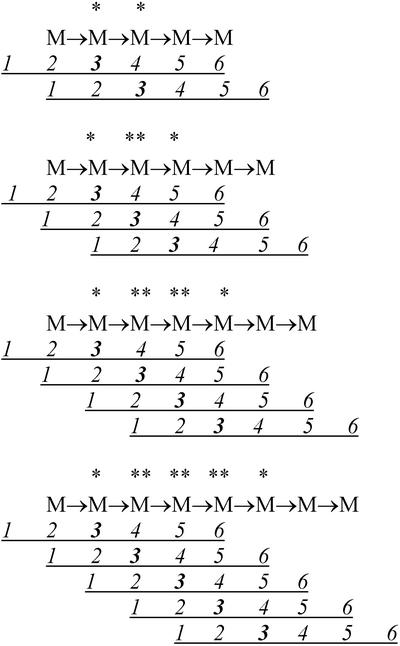Abstract
The current experimental model for galactomannan biosynthesis in membrane-bound enzyme systems from developing legume-seed endosperms involves functional interaction between a GDP-mannose (Man) mannan synthase and a UDP-galactose (Gal) galactosyltransferase. The transfer specificity of the galactosyltransferase to the elongating mannan chain is critical in regulating the distribution and the degree of Gal substitution of the mannan backbone of the primary biosynthetic product. Detergent solubilization of the galactosyltransferase of fenugreek (Trigonella foenum-graecum) with retention of activity permitted the partial purification of the enzyme and the cloning and sequencing of the corresponding cDNA with proof of functional identity. We now document the positional specificity of transfer of (14C)Gal from UDP-(14C)Gal to manno-oligosaccharide acceptors, chain lengths 5 to 8, catalyzed by the detergent-solubilized galactosyltransferase. Enzymatic fragmentation analyses of the labeled products showed that a single Gal residue was transferred per acceptor molecule, that the linkage was (1→6)-α, and that there was transfer to alternative Man residues within the acceptor molecules. Analysis of the relative frequencies of transfer to alternative Man residues within acceptor oligosaccharides of different chain length allowed the deduction of the substrate subsite recognition requirement of the galactosyltransferase. The enzyme has a principal recognition sequence of six Man residues, with transfer of Gal to the third Man residue from the nonreducing end of the sequence. These observations are incorporated into a refined model for enzyme interaction in galactomannan biosynthesis.
Galactomannans are the principal polysaccharide component of the walls of endosperm cells of legume seeds (Reid, 1985). Their structures are relatively simple, consisting of a linear (1→4)-β-linked d-mannan backbone with single-unit (1→6)-linked α-d-galactopyranosyl side chains. The Man-to-Gal (Man/Gal) ratio is variable between 1.1 (high Gal) and about 3.4 (low Gal), and is taxonomically significant (Reid and Meier, 1970; Bailey, 1971). The galactomannans are multifunctional molecules, serving as the molecular basis of a water imbibition and drought avoidance mechanism before and during germination, and then as a source of storage carbohydrate for the developing seedling (Reid and Bewley, 1979). When isolated, galactomannans are water soluble, with solution properties that have industrial applications. For example, the galactomannans from guar (Cyamopsis tetragonoloba) and locust bean (Ceratonia siliqua) are used in the food industry as viscosifiers, stabilizers, and gelling agents, alone and in combination with other polysaccharides (Dea and Morrison, 1975; Reid and Edwards, 1995). The Man/Gal ratio is an important determinant of commercial functionality, with high Man/Gal ratios (low Gal substitution) favoring valuable mixed-polymer interactions (Dea et al., 1977).
Galactomannan biosynthesis is catalyzed in vitro by membrane preparations isolated from the developing endosperms of fenugreek (Trigonella foenum-graecum; Man/Gal ratio in vivo = 1.1), guar (Man/Gal ratio = 1.6), and senna (Senna occidentalis; Man/Gal ratio = 3.3). The galactomannan molecule is assembled by the action of two membrane-bound glycosyltransferases. A GDP-Man-dependent mannan synthase (MS) catalyzes the successive transfer of Man residues from GDP-Man to an endogenous (presumably galactomannan) acceptor, thus elongating the mannan backbone. A galactomannan galactosyltransferase (GMGT) catalyzes the transfer of Gal residues from UDP-Gal to form the galactosyl side chains. Gal residues can be transferred only to an acceptor Man residue at or near the growing (nonreducing) end of an elongating backbone chain (Edwards et al., 1989). Transfer of Gal residues conforms to statistical rules whereby the probability of obtaining Gal substitution at the acceptor Man residue is determined by the existing states of substitution at the nearest neighbor and second nearest neighbor Man residues (toward the reducing terminus) of the elongating backbone. This is a second order Markov chain model. Furthermore, the maximum degrees of Gal substitution allowed by the deduced Markov probabilities for fenugreek, guar, and senna membranes are very close to those observed for the primary product of galactomannan biosynthesis in vivo (Reid et al., 1995). Thus, the biosynthesis of galactomannans requires a specific functional interaction between MS and GMGT, within which the transfer specificity of the GMGT is important in determining the statistical distribution of galactosyl residues along the mannan backbone and the Man/Gal ratio.
The successful detergent solubilization of fenugreek GMGT with retention of activity was instrumental in enabling the recent full molecular characterization of that enzyme (Edwards et al., 1999). The detergent-solubilized GMGT was no longer functionally associated with the MS, and had an absolute requirement for added acceptor substrate molecules to replace the nascent mannan chain. Galactomannans with low and, to a lesser extent, medium Gal substitution were acceptors, as were some unsubstituted (1→4)-β-linked manno-oligosaccharides. Only those manno-oligosaccharides with a degree of polymerization (DP) of 5 or more acted as acceptors, and acceptor efficiency increased up to DP 8. Larger oligosaccharides were acceptors, but their water solubility was limited (Edwards et al., 1999). We now report a detailed investigation of the specificity of transfer of Gal residues to manno-oligosaccharides DP 5 to 8, undertaken in the expectation that the results would facilitate the mapping of a recognition sequence within a mannan chain, and pinpoint the site of galactosyltransfer within that sequence.
RESULTS
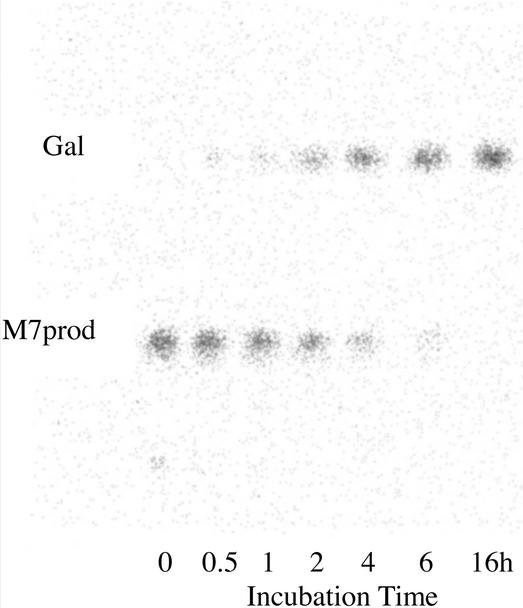
Successful solubilization of fenugreek GMGT was achieved using the detergents digitonin, Triton X-100, and NP-40. As the structures of Triton X-100 and NP-40 are closely similar and very different from that of digitonin (Neugebauer, 1994), all the experiments described below were performed with digitonin-solubilized and Triton X-100-solubilized enzymes. The products of the transfer reactions to the acceptor manno-oligosaccharides DP 5 to 8 (M5–M8) were prepared by incubating solubilized enzyme with UDP-(14C)Gal (0.8 mm) and acceptor oligosaccharide (1.0 mm). They were identified by thin-layer chromatography (TLC) and digital autoradiography in each case as a labeled product running as a compact radioactive zone with a TLC mobility slightly lower than that of the acceptor manno-oligosaccharide. They were purified by strip-loading TLC plates, followed by removal and elution of the appropriate labeled zone located by digital autoradiography. Each product was apparently homogeneous on rechromatography (Fig. 1, lane 0).
Figure 1.
α-Galactosidase-catalyzed release of labeled Gal from the purified product of incubating mannoheptaose (M7) with UDP-(14C)Gal in the presence of digitonin-solubilized fenugreek GMGT. The α-galactosidase was diluted to give a slow reaction. Samples of the α-galactosidase reaction mixture were removed at various times and subjected to TLC. The figure shows a digital autoradiogram of the TLC plate. M7prod, Purified transfer product.
Number of Gal Residues Transferred Per Acceptor Molecule
When the individual, purified, labeled reaction products were treated with the pure galactomannan-active α-galactosidase from germinated guar seeds (α-galactosidase II in McCleary, 1983; Edwards et al., 1989), there was always complete, smoothly time-dependent conversion to labeled Gal. No labeled intermediate compounds were detected, even when the amount of enzyme was adjusted to give a slow reaction, as illustrated in Figure 1. This demonstrated that only a single Gal residue was transferred per manno-oligosaccharide acceptor molecule and that the Gal was in the α-configuration. The labeled reaction products were treated also with the pure, structure-sensitive endo-(1→4)-β-mannanase from Aspergillus niger, the action of which has been described in detail by McCleary and Matheson (1983). The optimum substrate subsite-binding requirement of this enzyme is a stretch of five (1→4)-β-linked d-mannosyl residues, although mannotetraose is nonetheless hydrolyzed slowly. Gal substitution (as in galactomannans) at the Man residues occupying the second and/or the fourth position within the binding sequence prevents hydrolysis, with the result that only certain well-defined fragment oligosaccharides are released from galactomannans by the action of the enzyme (McCleary, 1979; McCleary and Matheson, 1983). The smallest of these allowed oligosaccharides are mannobiose (M2), mannotriose (M3), and the three Gal-substituted oligosaccharides M2G, M3G, and M5G2, the molecular structures of which are illustrated in Table I. On treatment of the labeled transfer products with this enzyme, the only radioactive end products of the reactions in each case were compounds that comigrated on TLC with M2G and M3G. This demonstrated that the transferred α-Gal residues were linked (1→6) as in galactomannans.
Specificity of Transfer to Manno-Oligosaccharide and Galactomannan Acceptors
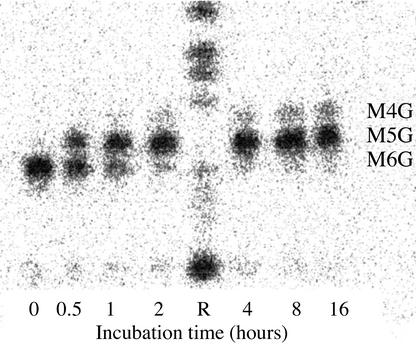
An enzymatic approach was used also to determine the positions of Gal substitution on the mannan backbones of the product (14C)Gal-labeled monogalactosyl-substituted manno-oligosaccharides M5G to M8G. A commercial exo-β-mannosidase preparation from snail was shown experimentally to be free of α-galactosidase contamination using p-nitrophenyl-α-galactoside and purified galactomannan oligosaccharides as substrates (data not shown). The action of the exo-β-mannosidase on model manno- and galactomanno-oligosaccharides demonstrated that it would catalyze the removal of Man residues successively from the nonreducing ends of these molecules until it approached within one Man residue of a Gal branch-point. Thus, mannohexaose was converted rapidly and completely to Man, whereas only one of the two unsubstituted Man residues in the galactomannan oligosaccharide M3G was susceptible to hydrolysis (Table I). When purified samples of the galactosyl-labeled products of GMGT action, M5G to M8G, were individually treated with the mannosidase and the progress of the reactions was followed by TLC and digital autoradiography, it was observed that each was converted to a “ladder” of labeled compounds with greater mobility than the starting compound (Fig. 2). On extended incubation, a stable pattern was reached, demonstrating that the remaining compounds were resistant to further β-mannosidase action. Given that each substrate oligosaccharide had a single Gal side chain, the proven ability of the TLC system to resolve galactomanan oligosaccharides differing in chain length by a single Man residue (Reid et al., 1995), and the observed action of the β-mannosidase on galactomannan oligosaccharides, each labeled compound surviving β-mannosidase digestion had to be a Gal-substituted manno-oligosaccharide with the Gal substituent attached to the Man residue second from the nonreducing end. In each case, more than one labeled compound survived β-mannosidase digestion. Thus, each of the products of GMGT-catalyzed transfer to a manno-oligosaccharide acceptor, M5G to M8G, must have been a mixture of structural isomers differing in the position of the single Gal substituent. Following the time course of the β-mannosidase digestions by TLC and digital autoradiography, as illustrated in Figure 2, allowed the chain lengths of the labeled end products of β-mannosidase action to be deduced, and hence the structures of all the positional isomers. Quantitative evaluation of the digital autoradiograms allowed the relative molar amounts of the isomers comprising M5G, M6G, M7G, and M8G to be calculated (Table II).
Figure 2.
β-Mannosidase digestion of a purified sample of labeled monogalactosylmannohexaose (M6G) formed by incubating mannohexaose with UDP-(14C)Gal in presence of Triton X-100-solubilized fenugreek GMGT. Samples of the β-mannosidase reaction mixture were taken at various times and subjected to TLC. The figure is a digital autoradiogram of the TLC plate. R, Reference standards of (14C)Man-labeled galactomannan oligosaccharides from endo-β-mannanase digestion of galactomannan biosynthesized in vitro (Reid et al., 1995).
Table II.
Deduction and quantitative estimation of structural isomers comprising M5G, M6G, M7G, and M8G formed in experiments using digitonin- and Triton X-100-solubilized fenugreek GMGT
| Compounds Surviving β-Mannosidase Action | Deduced Structure before β-Mannosidase Treatment | Relative Amount %
|
|
|---|---|---|---|
| Digitonin | TX-100 | ||
| se | |||
| M5G | |||
| MGMMM | MGMMM | 20 ± 3 | 26 ± 2 |
| MGMM | MMGMM | 80 ± 3 | 75 ± 2 |
| M6G | |||
| MGMMMM | MGMMMM | 10 ± 3 | 5 ± 5 |
| MGMMM | MMGMMM | 75 ± 4 | 83 ± 4 |
| MGMM | MMMGMM | 16 ± 0 | 12 ± 2 |
| M7G | |||
| MGMMMMM | MGMMMMM | 8 ± 4 | 3 ± 3 |
| MGMMMM | MMGMMMM | 41 ± 2 | 44 ± 4 |
| MGMMM | MMMGMMM | 43 ± 2 | 44 ± 3 |
| MGMM | MMMMGMM | 10 ± 2 | 9 ± 1 |
| M8G | |||
| MGMMMMMM | MGMMMMMM | 1 ± 1 | 3a |
| MGMMMMM | MMGMMMMM | 20 ± 4 | 20a |
| MGMMMM | MMMGMMMM | 34 ± 3 | 35a |
| MGMMM | MMMMGMMM | 30 ± 0 | 27a |
| MGMM | MMMMMGMM | 16 ± 5 | 15a |
M, Unsubstituted mannose residue; G, galactose-substituted mannose residue; TX-100, Triton X-100. Structures read from nonreducing to reducing end. Thus,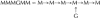 .
.
Single determination; otherwise, means of at least two independent experiments.
All of the deduced structures listed in Table II are potential substrates for the well-characterized endo-β-mannanase from A. niger. Thus, it was possible to apply the known action pattern of the enzyme (McCleary and Matheson, 1983; summarized above) to predict, for each isomer, which of the diagnostic galactomannan oligosaccharides M2G and M3G (structures in Table I) would be the labeled end product(s) from exhaustive digestion with this enzyme. This provided a means of confirming the structural deductions of Table II. Therefore, samples of M5G to M8G, formed using the digitonin-solubilized GMGT, were digested to completion with the endo-β-mannanase, and the labeled end products were identified and quantified by TLC and digital autoradiography. Experimental observations and theoretical predictions were found to be closely similar (Table III).
Table III.
Predicted and experimentally observed products of endo-β-mannanase digestion of labeled M5G, M6G, M7G and M8G formed using digitonin-solubilized fenugreek GMGT.
| Deduced Structures of Positional Isomers with Predicted Labeled Endo-β-Mannanase Products | Relative Proportions of Labeled M2G and M3G Released on Endo-β-Mannanase Digestion
|
|||
|---|---|---|---|---|
| Predicted | Experimentalb | |||
| M2G | M3G | M2G | M3G | |
| M5G | 20 ± 3 | 80 ± 3 | 24 ± 2 | 76 ± 2 |
| MGMMM → M2G | ||||
| MMGMM → M3G | ||||
| M6G | 26 ± 3 | 75 ± 4 | 25 ± 1 | 75 ± 1 |
| MGMMMM → M2G | ||||
| MMGMMM → M3G | ||||
| MMMGMM → M2G | ||||
| M7G | 61 ± 4 | 41 ± 2 | 57 ± 2 | 43 ± 2 |
| MGMMMMM → M2G | ||||
| MMGMMMM → M3G | ||||
| MMMGMMM → M2G | ||||
| MMMMGMM → M2G | ||||
| M8G | 73 ± 4 | 28 ± 4 | 68 ± 1 | 32 ± 1 |
| MGMMMMMM → M2G | ||||
| MMGMMMMM → M3G | ||||
| MMMGMMMM → M2G | ||||
| MMMMGMMM → M2G | ||||
| MMMMMGMM → M2G + M3Gb | ||||
Experimental data were obtained by digesting M5G to M8G completely with A. niger endo-β-mannanase, separating the digests by TLC and determining the relative proportions of the product oligosaccharides M2G and M3G by quantitative digital autoradiography. Predictions were made using the known specificity of the endo-β-mannanase (McCleary and Matheson, 1983; summarized in text) and the digitonin data from Table II.
Mean (± se) of at least two independent experiments.
Assumed 50% of each.
DISCUSSION
The detergent-solubilized GMGT from the developing fenugreek seed endosperm catalyzes the transfer of Gal residues from UDP-Gal to (1→4)-β-linked manno-oligosaccharides with chain lengths comprising five or more Man residues. The efficiency of transfer is chain length dependent, mannopentaose (M5) being a very inefficient acceptor relative to the oligosaccharides of DP 6 and greater. There is a small increase in acceptor efficiency from M6 to M8, beyond which acceptor solubility becomes limiting due to self-association of the mannan chains (Edwards et al., 1999). Under the same experimental conditions (1.0 mm acceptor, 0.8 mm UDP-Gal), using manno-oligosaccharide acceptors M5 to M8, we have demonstrated that a single galactosyl residue is transferred per acceptor molecule, and that the linkage formed is (1→6)-α, as in galactomannans. We have further demonstrated that each of the four product monogalactosylmanno-oligosaccharides, M5G to M8G, is a mixture of isomers differing in the position of attachment of the galactosyl residue to the mannan backbone, and in each case, we have deduced the structures and relative molar proportions of the structural isomers (Table II).
Inspection of Table II reveals that galactosyltransfer to M5, M6, M7, and M8 does not occur randomly. In the case of M6, most of the Gal transferred (approximately 80%) is to the third mannosyl residue from the nonreducing end of the acceptor, with minor amounts of transfer to the neighboring residues at the second and fourth positions. M7 becomes substituted predominantly at residues 3 and 4 from the nonreducing end (together accounting for approximately 80% of Gal transferred), with lesser amounts at residues 2 and 5. M8 accepts Gal predominantly at residues 3, 4, and 5 (approximately 80% of Gal transferred), with the remainder at residues 2 and 6. The much lower amounts of Gal transferred to M5 are exclusively to residues 2 and 3 from the nonreducing end. The principal positions of Gal substitution observed for M6, M7, and M8 are consistent with the detergent-solubilized galactosyltransferase having a principal substrate recognition sequence comprising six Man residues, with transfer of Gal occurring to the third Man residue from the nonreducing end of the sequence. The occurrence of low level transfer to M5 and its positional specificity demonstrates that some transfer can occur if five of the six Man residues of the principal recognition sequence are present. This explains also the minor positions of galactosyltransfer to M6, M7, and M8. These arguments are illustrated in Figure 3 by fitting M5, M6, M7, and M8 in various ways to a model “enzyme” with six numbered binding sites for Man residues, catalysis occurring at site number 3. Major transfer is then seen to occur when sites 1 through 6 are all occupied by substrate mannosyl residues, and minor transfer when sites 1 through 5 or 2 through 6 are occupied. The quantitative distribution of galactosyltransfer to M5 indicates that galactosyltransfer is more efficient when sites 1 through 5 are occupied rather than sites 2 through 6 (Table II; Fig. 3). This conclusion is supported by the quantitative data for the positions of minor substitution on M6 through M8. In each case, greater amounts of transfer occur (Table II) when sites 1 through 5 are occupied rather than sites 2 through 6 (Fig. 3).
Figure 3.
Observed pattern of Gal substitution of M5, M6, M7, and M8 by detergent-solubilized fenugreek GMGT in relation to a hypothetical GMGT acceptor substrate subsite recognition sequence comprising six Man residues (numbered 1–6 from the nonreducing end of the sequence), with transfer occurring to the Man residue at site 3 of the recognition sequence. An asterisk indicates an observed position of minor substitution. A double asterisk indicates an observed position of major substitution.
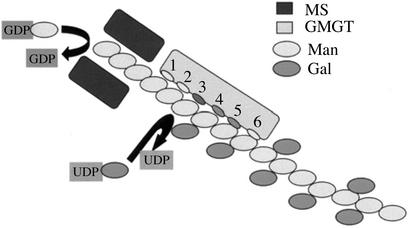
In membrane-bound enzyme systems catalyzing galactomannan biosynthesis, GMGT cannot act independently of MS (Edwards et al., 1989). The experimental data from such systems conform to a model (Reid et al., 1995) whereby MS catalyzes the elongation of the mannan backbone toward the nonreducing end and GMGT transfers Gal to an acceptor Man residue at or near the (elongating) nonreducing end of the backbone, and the statistical probability of obtaining Gal substitution at the acceptor residue is dependent upon the existing states of substitution at the nearest and second nearest neighbor mannosyl residues toward the reducing end of the backbone. The relevance of the two previous substitution events makes this a second order Markov chain model. Given the known tendency of unsubstituted (1→4)-β-linked mannan chains longer than about eight residues to self-associate, this functional interaction between MS and GMGT in membrane-bound systems can be explained reasonably only if the two enzymes are arranged in such a way that the nascent, unsubstituted mannan backbone emerging from the MS is presented to the GMGT before association between adjacent emerging mannan chains can occur. Such an arrangement is illustrated diagrammatically in Figure 4. In Figure 4, it is assumed that the six-site mannan chain recognition sequence deduced for the detergent-solubilized fenugreek GMGT (Fig. 3) is valid also in the membrane-bound state. Thus, sites 1 and 2 of the recognition sequence will be occupied by unsubstituted Man residues recently transferred by the MS, and the GMGT will catalyze the transfer of a galactosyl side chain to the acceptor Man residue occupying site 3. According to the second-order Markov chain model, the probability of this catalytic event will reflect the existing state of Gal substitution at the Man residues occupying sites 4 and 5. The experimental observation that galactomannan biosynthesis in vivo and in membrane-bound enzyme systems complies with a second order Markov chain model indicates that the state of Gal substitution of the Man residue occupying site 6 has a negligible influence on the probability of galactosyl transfer occurring at site 3. Although the model illustrated in Figure 4 is sufficient to explain the functional cooperativity between MS and GMGT in membrane-bound systems, it is far from complete or proven. The MS has not yet been purified and characterized, and the number of Man residues recognized by it is not known. It is also not known how many, if any (none shown in Fig. 4), Man residues intervene between the emergence of the nascent mannan chain from the MS and the beginning of the GMGT recognition sequence.
Figure 4.
A model for the interaction of MS and GMGT during galactomannan biosynthesis in fenugreek.
The model in Figure 4 invites the assumption that in membrane-bound systems, the GMGT must catalyze the repetitive substitution of the same nascent mannan chain—a type of action (“single chain”) that may be likened to “processivity” in main-chain synthesizing glycosyltransferases. Yet, our observations on the transfer specificity of the detergent-solubilized GMGT offer no evidence for single-chain action. Only a single Gal residue is transferred per acceptor molecule, even when the acceptor oligosaccharides are sufficiently long to accommodate repositioning of the acceptor relative to the recognition sequence. Detergent solubilization causes the processivity of an α-galacturonyltransferase involved in pectic homogalacturonan biosynthesis to be lost (Doong and Mohnen, 1998). Thus, it is possible that detergent solubilization may have disrupted any specific association between the MS and GMGT proteins that might impose single-chain action. Nonetheless, it is possible also that repetitive transfer by the same GMGT molecule may, although attractive in concept, not be a requirement for galactomannan biosynthesis, according to the model in Figure 4. An excess of GMGT molecules constrained by membrane anchoring (Edwards et al., 1999) to remain close to emerging mannan chains may be sufficient.
Independently of whether or not GMGT action is single chain, the model predicts that fenugreek GMGT is capable of catalyzing the transfer of a Gal residue to an acceptor Man residue adjacent to one already carrying a substituent. Our observation that only a single Gal residue is transferred per reacted manno-oligosaccharide acceptor molecule should not be taken as evidence that the detergent-solubilized GMGT is unable to do so. Under our experimental conditions (0.8 mm UDP-Gal donor and 1.0 mm manno-oligosaccharide acceptor), the extent of galactosyltransfer does not exceed 2%. Thus, there is always a large excess (at least 60-fold) of unsubstituted acceptor over monosubstituted product, making multiple substitution events very unlikely. Independent evidence that the detergent-solubilized GMGT can catalyze the transfer of Gal to a position adjacent to an existing Gal substituent is available from experiments where galactomannans are used as acceptor substrates for the solubilized GMGT (Edwards et al., 1999). When the labeled polysaccharide products are hydrolyzed using the A. niger endo-β-mannanase, the labeled oligosaccharide fragments always include M5G2 (structure in Table I) in which the two Gal substituents are adjacent (data not shown; similar data from expressed, soluble fenugreek GMGT illustrated in Edwards et al., 1999; Fig. 4). A further important prediction of the model in Figure 4 is that the observed conformity of galactomannan biosynthesis to a second order Markov chain model reflects GMGT specificity. Current work in our laboratory is aimed at testing this hypothesis by comparing the transfer properties in vitro of soluble, expressed GMGTs (Edwards et al., 1999) from fenugreek, guar, and senna.
MATERIALS AND METHODS
Chemicals
(14C)Gal-labeled UDP-Gal was purchased from DuPont-NEN (Stevenage, UK), (1→4)-β-linked manno-oligosaccharides was obtained from Megazyme International (Bray, Ireland), and Triton X-100 was purchased from Roche (Lewes, East Sussex, UK). Other specialized chemicals were from Sigma (Poole, Dorset, UK). General laboratory chemicals were at least of analytical quality.
Hydrolytic Enzymes
The preparations of Aspergillus niger endo-β-mannanase and guar (Cyamopsis tetragonoloba) α-galactosidase were those described in Edwards et al. (1989). Snail β-mannosidase was purchased from Sigma (product no. M9400). Received as a suspension (total activity, 5 units) in ammonium sulfate, the enzyme was dialyzed against 50 mm ammonium acetate, pH 4.0. The dialyzed solution was diluted to 1.0 mL and was stored frozen.
Membrane Preparation and Detergent Solubilization of Fenugreek (Trigonella foenum-graecum) GMGT
These procedures have been described elsewhere (Edwards et al., 1999). The solubilized enzyme preparations were used without further dilution and were stored frozen at −70°C.
General Methods
TLC separations were carried out as before (Reid et al., 1995). Dried plates were subjected to digital autoradiography (Digital Autoradiograph; EG&G Berthold, Milton Keynes, UK). This allowed for the precise localization of radioactive zones on plates, and the quantitative evaluation of the relative amounts of radioactivity in separated zones.
Preparation, Isolation, and Purification of Labeled Products of GMGT-Catalyzed Transfer of 14C-Gal from UDP-(14C)Gal to Manno-Oligosaccharides
The reaction mixture for the galactosyltransfer reaction (total volume of 1.0 mL) contained 0.5 mL of detergent-solubilized fenugreek GMGT (in 1% [w/v] by volume detergent, 50 mm Tris-HCl buffer, pH 7.5, and 1.0 mm EDTA) and the following components at the final concentrations indicated: UDP-(14C)Gal, 0.8 mm (specific radioactivity adjusted in each experiment, usually to give 360,000 dpm 100 μL−1); manno-oligosaccharide, 1.0 mm; MgSO4, 5 mm; and MnCl2, 10 mm. The mixture was incubated at 30°C for 2 h, heated at 100°C for 4 min, cooled, and then largely freed of unreacted UDP-Gal using small spin columns packed with diethylaminoethyl-cellulose as described earlier (Edwards et al., 1999). The pooled column effluents were separated by TLC (strip-loaded onto three 20- × 20-cm plates) and the air-dried plates were subjected to digital autoradiography. Unreacted manno-oligosaccharide was detected by spraying (Edwards et al., 1989) side strips. In each case, the labeled product of transfer from Gal-labeled UDP-Gal to the manno-oligosaccharide ran as a compact radioactive zone with a TLC mobility less than that of the parent oligosaccharide. Each labeled product was recovered from the plates by removal of the corresponding area of silica gel and elution with 2.5 mL of water (1, 1, and 0.5 mL for 30 min each at room temperature with intermediate centrifugation at 12,000g). The eluates were adjusted to the desired concentration by dilution or by freeze-drying and redissolution in the appropriate volume of water. The solutions were stored frozen. The recovered products accounted for 1% to 2% of Gal transfer from UDP-Gal.
Treatment of Labeled Transfer Products with Guar α-Galactosidase
Reaction mixtures (usually 150 μL) contained approximately 2,000 dpm of labeled transfer product (approximately 3 μm transferred Gal), ammonium acetate (50 mm, pH 5.0), and guar α-galactosidase, diluted to give complete conversion to labeled Gal over 4 to 6 h. The progress of the reaction was monitored by TLC and digital autoradiography over 24 h.
Treatment of Labeled Monogalactosyl Manno-Oligosaccharides M5G to M8G with Snail β-Mannosidase
Reaction mixtures (usually 240 μL) contained labeled oligosaccharide (approximately 6,000 dpm; 5.6 μm), ammonium acetate (25 mm, pH 4.0), and snail β-mannosidase at one-half stock concentration. Reaction progress was monitored over 24 h by TLC and digital autoradiography.
Treatment of Labeled Monogalactosyl Manno-Oligosaccharides M5G to M8G with A. niger Endo-β-Mannanase
Reaction mixtures (usually 200 μL) contained labeled oligosaccharide (approximately 6,000 dpm; 6.7 μm), ammonium acetate (50 mm, pH 5.0), and endo-β-mannanase at 1/250 stock concentration. Reaction progress was monitored over 24 h by TLC and digital autoradiography.
Table 1.
Products of snail exo-β-d-mannosidase action on manno- and galactomanno-oligosaccharides
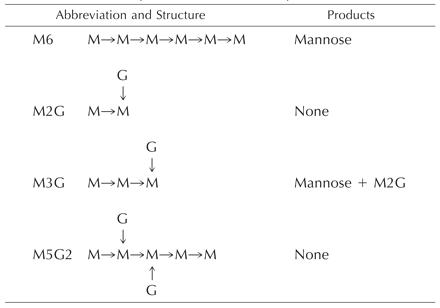 |
All mannosyl linkages are (1→4)-β, and galactosyl linkages are (1→6)-α. M, Mannosyl residue. G, Galactosyl residue.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002592.
LITERATURE CITED
- Bailey RW. Polysaccharides in the leguminosae. In: Harborne JB, Boulter D, Turner BL, editors. Chemotaxonomy of the Leguminosae. London: Academic Press; 1971. pp. 503–541. [Google Scholar]
- Dea ICM, Morrison A. Chemistry and interactions of seed galactomannans. Adv Carbohydr Chem Biochem. 1975;31:241–312. [Google Scholar]
- Dea ICM, Morris ER, Rees DA, Welsh EJ, Barnes HA, Price J. Associations of like and unlike polysaccharides: mechanism and specificity in galactomannans, interacting bacterial polysaccharides and related systems. Carbohydr Res. 1977;57:249–272. [Google Scholar]
- Doong RL, Mohnen D. Solubilization and characterization of a galacturonosyltransferase that synthesizes the pectic polysaccharide homogalacturonan. Plant J. 1998;13:363–374. [Google Scholar]
- Edwards M, Bulpin PV, Dea ICM, Reid JSG. Biosynthesis of legume seed galactomannans in vitro. Planta. 1989;178:41–51. doi: 10.1007/BF00392525. [DOI] [PubMed] [Google Scholar]
- Edwards ME, Dickson CA, Chengappa S, Sidebottom C, Gidley MJ, Reid JSG. Molecular characterisation of a membrane-bound galactosyltransferase of plant cell wall matrix polysaccharide biosynthesis. Plant J. 1999;19:691–697. doi: 10.1046/j.1365-313x.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- McCleary BV. Modes of action of β-d-mannanase enzymes of diverse origin on legume seed galactomannans. Phytochemistry. 1979;18:757–763. [Google Scholar]
- McCleary BV. Enzymic interactions in the hydrolysis of galactomannan in germinating guar: the role of exo-β-mannanase. Phytochemistry. 1983;22:649–658. [Google Scholar]
- McCleary BV, Matheson NK. Action patterns and substrate-binding requirements of β-mannanase with mannosaccharides and mannan-type polysaccharides. Carbohydr Res. 1983;119:191–219. [Google Scholar]
- Neugebauer J. A Guide to the Properties and Uses of Detergents in Biology and Biochemistry. Ed 5. San Diego: Calbiochem-Novabiochem; 1994. [Google Scholar]
- Reid JSG. Cell wall storage carbohydrates in seeds: biochemistry of the seed “gums” and “hemicelluloses.”. Adv Bot Res. 1985;11:125–155. [Google Scholar]
- Reid JSG, Bewley JD. A dual role for the endosperm and its galactomannan reserves in the germinative physiology of fenugreek (Trigonella foenum-graecum L.), an endospermic leguminous seed. Planta. 1979;147:145–150. doi: 10.1007/BF00389515. [DOI] [PubMed] [Google Scholar]
- Reid JSG, Edwards M. Galactomannans and other cell wall storage polysaccharides in seeds. In: Stephen AM, editor. Food Polysaccharides and Their Applications. New York: Marcel Dekker; 1995. pp. 155–186. [Google Scholar]
- Reid JSG, Edwards M, Gidley MJ, Clark AH. Enzyme specificity in galactomannan biosynthesis. Planta. 1995;195:489–495. [Google Scholar]
- Reid JSG, Meier H. Chemotaxonomic aspects of the reserve galactomannan in leguminous seeds. Z Pflanzenphysiol. 1970;62:89–92. [Google Scholar]