Abstract
Vascular endothelial function is essential for maintenance of health of the vessel wall and for vasomotor control in both conduit and resistance vessels. These functions are due to the production of numerous autacoids, of which nitric oxide (NO) has been the most widely studied. Exercise training has been shown, in many animal and human studies, to augment endothelial, NO-dependent vasodilatation in both large and small vessels. The extent of the improvement in humans depends upon the muscle mass subjected to training; with forearm exercise, changes are restricted to the forearm vessels while lower body training can induce generalized benefit. Increased NO bioactivity with exercise training has been readily and consistently demonstrated in subjects with cardiovascular disease and risk factors, in whom antecedent endothelial dysfunction exists. These conditions may all be associated with increased oxygen free radicals which impact on NO synthase activity and with which NO reacts; repeated exercise and shear stress stimulation of NO bioactivity redresses this radical imbalance, hence leading to greater potential for autacoid bioavailability. Recent human studies also indicate that exercise training may improve endothelial function by up-regulating eNOS protein expression and phosphorylation. While improvement in NO vasodilator function has been less frequently found in healthy subjects, a higher level of training may lead to improvement. Regarding time course, studies indicate that short-term training increases NO bioactivity, which acts to homeostatically regulate the shear stress associated with exercise. Whilst the increase in NO bioactivity dissipates within weeks of training cessation, studies also indicate that if exercise is maintained, the short-term functional adaptation is succeeded by NO-dependent structural changes, leading to arterial remodelling and structural normalization of shear. Given the strong prognostic links between vascular structure, function and cardiovascular events, the implications of these findings are obvious, yet many unanswered questions remain, not only concerning the mechanisms responsible for NO bioactivity, the nature of the cellular effect and relevance of other autacoids, but also such practical questions as the optimal intensity, modality and volume of exercise training required in different populations.
Introduction
The endothelium produces numerous paracrine substances, including nitric oxide (NO), which help maintain the health of the vascular wall and regulate vasomotor function. Nitric oxide (NO) is a labile, lipid soluble gas synthesized in endothelial cells from the amino-acid l-arginine through the action of endothelial nitric oxide synthase (eNOS) (Palmer et al. 1988). It is released both basally and in response to pharmacological stimulation (Vallance et al. 1989). The likely physiological stimulus to endothelial NO production has been identified as increased flow through the vessel lumen (Pohl et al. 1986; Rubanyi et al. 1986), with acute NO-mediated vasodilatation tending to normalize shear stress (Hutcheson & Griffith, 1991; Koller & Kaley, 1991; Neibauer & Cooke, 1996; Dimmeler & Zeiher, 2003). These findings raised the possibility that NO may contribute to exercise hyperaemia, as exercise is associated with increased pulse pressure and pulsatility, and that repeated exposure to increased shear stress, as a result of exercise training, may improve the bioavailability of NO.
Exercise training is associated with reduction in primary (Hakin et al. 1999; Sesso et al. 2000; Myers et al. 2002) and secondary vascular events (Jolliffe et al. 2001), but the effect of exercise on conventional risk factors (Blair et al. 1984; Tran & Weltman, 1985; Holloszy et al. 1986; Blair, 1993; Williams, 1996; Malfattoo et al. 1998; Kelley, 1999; Smith et al. 1999; El-Sayed et al. 2000) cannot solely account for the magnitude of risk reduction, since the association with reduced mortality is independent of these risk factors (Dimmeler & Zeiher, 2003). Moreover, improvements in conduit and resistance vessel endothelial function can occur independent of changes in cardiovascular risk factors (Green et al. 2003). Direct shear-stress-mediated effects on the vascular endothelium may therefore provide a plausible explanation for the reduction in coronary events associated with exercise training. The purpose of this review is to summarize the extant and evolving evidence from human studies regarding the contribution of endothelium-derived NO to exercise hyperaemia and the impact of exercise training on NO vasodilator function.
How is the contribution of NO to exercise and training studied in humans?
In humans, the rapid half-life of NO and its various non-vascular sources have rendered direct assessment of endothelial production difficult (Vallance et al. 1995) but NO bioassay, dependent upon the vasodilator effect of NO, has become a practical surrogate for endothelial function assessment in vivo. After the elegant method of Vallance et al. (1989), intrabrachial or coronary infusion of locally active doses of inhibitors of eNOS, such as NG-monomethyl-l-arginine (l-NMMA), has been used to examine basal NO bioactivity in resting muscle or the contribution of NO to exercise hyperaemia. Using a similar approach, dose–response curves to intra-arterial infusion of endothelium-dependent NO vasodilators such as acetylcholine (ACh), the response to which is at least partly NO mediated (Newby et al. 1997), have been used to assay changes in the stimulated release of NO induced by exercise training. Similarly, sodium nitroprusside (SNP) infusion is commonly undertaken to assess endothelium-independent NO vasodilator function. Evaluating the responses to endothelium-dependent and -independent NO vasodilators provides information about the contribution to blood flow control of different components of the NO-dilator system (Benjamin et al. 1995). This methodological approach has typically been employed to provide an assessment of resistance vessel function in studies of the skeletal muscle vasculature (Shepherd, 1983), utilizing strain-gauge plethysmographic measurement of limb blood flow responses.
A less invasive method, that of flow-mediated dilatation (FMD), has been devised to assess conduit vessel NO-mediated endothelial function (Celermajer et al. 1992), particularly that of the brachial artery (Corretti et al. 2002). This method involves direct imaging of large artery dilator responses to shear-stress-induced FMD consequent to a brief period of limb ischaemia. Assuming the occluding cuff is placed distal to the scanned artery (Doshi et al. 2001) and that the period of ischaemia does not exceed 5 min (Mullen et al. 2001a), the increase in arterial diameter in response to this stimulus is almost exclusively mediated by NO (Joannides et al. 1995; Doshi et al. 2001), and FMD therefore provides an index of conduit artery endothelium-dependent NO function (Ganz & Vita, 2003). Sublingual nitroglycerine (GTN) administration is often undertaken to assess endothelium-independent NO vasodilator function in the same manner that SNP is used in invasive studies.
It is important to consider some of the limitations of each of the above approaches in the context of measurement of exercise hyperaemia and exercise training effects. Strain-gauge plethysmography reliably measures relative changes induced by pharmacological agents to a resting muscle bed (Joyner et al. 2001), but has several compelling limitations for measurement of exercise hyperaemia (Rowell, 1993); crucially, plethysmography provides data with poor temporal resolution and is highly sensitive to movement, necessitating measurement of ‘exercise’ flows from brief periods of rest following exercise bouts. Perhaps as a consequence, plethysmographic measures of exercise flows significantly underestimate those derived from thermodilution (Andersen & Saltin, 1985). Nonetheless, most of what we know about mechanisms responsible for exercise hyperaemia is derived from fundamental observations utilizing plethysmography (Joyner et al. 2001), and, importantly, studies of the effect of training on the vasculature, which typically involve pharmacological assay in resting vessel beds before and after the intervention, are not subject to the motion artefact limitations.
High resolution Doppler ultrasonography is becoming an increasingly common method to directly visualize and measure arterial diameter and blood flow through conduit arteries in humans, including during FMD studies (Radegran, 1997; Radegran & Saltin, 1999; Maiorana et al. 2001a; Green et al. 2002b). This method possesses the advantages of being non-invasive, provides absolute blood flow measures, greatly improves temporal resolution for measurement during exercise hyperaemia (Green et al. 2002c) and is potentially less affected by motion artefact than plethysmography. However, both arterial diameter and Doppler velocity measures are critically dependent upon image quality, which is operator dependent, and velocity is also importantly modulated by the insonation angle (Logason et al. 2001), which should be less than 60 deg. Furthermore, many groups fail to utilize edge-detection and wall-tracking software, which markedly improves the sensitivity of the technique and renders analysis less subjective (Woodman et al. 2001; Green et al. 2002c). In the context of the use of FMD in exercise training studies, Hijmering et al. (2002) recently demonstrated that acute changes in sympathetic outflow, similar in magnitude to those observed in ageing and disease and in response to training, can acutely blunt FMD by 50%. Whether this can be attributed to a decreased flow stimulus as a result of the downstream sympathetic vasoconstriction remains to be determined, but it warrants caution in the use of FMD in repeated measures studies in which the intervention may also modulate sympathetic tone. Finally, dose–response curves to FMD cannot be constructed at present, as the relationship between shear stress and the NO dependence of FMD has not been comprehensively assessed and appears complex (Doshi et al. 2001; Mullen et al. 2001b; Pyke et al. 2004).
In summary, all methods used to assess regional blood flow in vivo possess limitations and yet the oldest and arguably most compromised method, strain-gauge plethysmography, has yielded the greatest body of extant knowledge regarding the effects of acute and chronic exercise on the vasculature. Newer technologies will provide greater insight, but the approaches to which they are applied must in the first instance be physiologically sound. Several studies, including one which examined the effects of exercise training (Lind et al. 2000; Eskurza et al. 2001; Green et al. 2004), have demonstrated poor correlations between ACh and FMD measures derived in the same subjects and, although this may be indicative of differential NO contributions in resistance and conduit vessels, the possibility also exists that distinct physiological pathways are being interrogated.
Does endothelium-derived NO contribute to exercise hyperaemia in humans?
Skeletal muscle
Indirect evidence for a role of NO in skeletal muscle exercise hyperaemia is provided by the increased levels of plasma nitrite (Ambring et al. 1994) and urinary nitrate (Bode-Böger et al. 1994) in response to prolonged aerobic exercise, but these measures do not reliably reflect endothelium-derived NO. Therefore, despite technical difficulties associated with measuring blood flow during exercise, a number of investigators have attempted to determine NO involvement in the hyperaemic response to exercise in the peripheral circulation. In an early study, flow was measured using plethysmography during periods of inactivity immediately following two intensities of wrist flexor exercise and concurrent infusion of l-NMMA had no significant effect on blood flow (Wilson & Kapoor, 1993). Furthermore, a small reduction in exercise hyperaemia during static hand gripping following NO inhibition could be explained by changes in resting flow (Endo et al. 1994). However, both of these studies measured blood flow after the cessation of muscular contraction and blood flow during recovery may be under different control from that during exercise (Joyner & Dietz, 1997).
The role of NO in skeletal muscle vasodilatation may increase with the duration of exercise. While it is unlikely that NO plays a major role in the dilator response to a single muscle contraction (Brock et al. 1998), it may be important during prolonged exercise. l-NMMA infusion during intermittent forearm hand gripping at 15%, 30% and 45% of maximum grip strength induced a small, but significant, reduction in exercise-induced vasodilatation (Gilligan et al. 1994). Importantly, the greatest effect of l-NMMA was evident at the highest infusion rate of the drug, indicating that the dose of an antagonist may be critical during an exercise study, when increased blood flow effectively dilutes a constant dose delivery. Indeed, Katz et al. (1996) indicated that the effect of l-NMMA decreased with increasing intensity of exercise. In another study, l-NMMA infusion attenuated the blood flow response to prolonged forearm hand gripping by 20–30% (Dyke et al. 1995). Furthermore, Duffy et al. (1999a,b) recently confirmed a role for both NO and vasodilator prostanoids in mediating hyperaemia in response to forearm handgrip exercise. However, sequential inhibition of these two vasodilators had no additional effect on reducing blood flow over and above that induced by inhibition of one, highlighting the possibility that redundant mechanisms may contribute to blood flow control during continuous exercise. This was recently confirmed in a comprehensive study (Schrage et al. 2004) which used Doppler ultrasound of the brachial artery during prolonged forearm handgrip exercise at low intensity to demonstrate that NG-nitro-l-arginine methyl ester (l-NAME), a more potent NOS inhibitor than l-NMMA, reduced exercise blood flow by 20%, and that prostaglandin inhibition transiently decreased exercise hyperaemia, but that the combination of approaches was not additive. The authors concluded that both NO and prostaglandins contribute significantly to exercise hyperaemia, that their effects appear independent and that factors other than NO restore blood flow responses following PG inhibition (Schrage et al. 2004). This study also emphasized the importance of localized administration of blockers during, rather than prior to, contractions and reinforced the importance of redundancy in the control of exercise hyperaemia (Boushel & Kjær, 2004).
In the lower limbs, Hickner et al. (1997), using microdialysis probes, measured a reduction in blood flow of 50% following the infusion of l-NMMA during continuous dynamic exercise of the vastus lateralis. However this technique requires further validation and these results have not been supported by other studies of lower limb exercise. l-NMMA had no effect on blood flow measured using Doppler ultrasound during steady-state submaximal or peak voluntary knee extension exercise (Radegran & Saltin, 1999). Similarly, when blood flow to the lower limbs was determined using femoral thermodilution, l-NMMA did not reduce blood flow during supine cycling, despite decreasing glucose flux across the limb (Bradley et al. 1999). Leg exercise is associated with a high perfusion-to-muscle mass ratio, and flow may be increased over 10-fold. Possibly, this hyperaemia dilutes l-NMMA concentration to an inadequate blocking level. In a recent report, systemic intravenous infusion of l-NAME, a potent inhibitor of NO production, failed to reduce blood flow during submaximal leg extensor exercise, leading the authors to conclude that NO is not essential to exercise hyperaemia (Fransden et al. 2001). However, this interpretation may be considered controversial, since mean arterial pressure increased and vascular conductance was lower during l-NAME infusion. Furthermore, an increase in arterial pressure is likely to reduce sympathetic outflow during exercise (Scherrer et al. 1990), and this may mask a potential blood flow reduction due to eNOS inhibition (Sheriff et al. 2000). This possibility is supported by the recent findings of Schrage et al. (2004), who used localized exercise and infusions to avoid the confounding influences of reflex responses and observed a contribution of NO to exercise hyperaemia of similar magnitude to Sheriff et al. (2000).
In summary of the above results in the skeletal muscle circulation, the evidence for a role of NO in the hyperaemic response to prolonged exercise in the forearms, which have a relatively small muscle mass, is reasonably convincing. Because the increased blood flow in these studies was modest (3–6 times resting levels), the dilution of constant dose infusion of NO antagonists would be less than with leg exercise. Evidence is less compelling from studies involving the lower limbs. While this disparity may reflect regional differences between vascular beds (Green et al. 2004), it may depend upon greater dilution of NO antagonists during leg exercise. Further carefully designed studies will be required to clarify this issue. There is also evidence that redundancy of mechanisms of vasodilatation exist so that inhibition of one pathway may result in up-regulation of another (Boushel & Kjær, 2004; Schrage et al. 2004). A final problem concerns blood flow measurement techniques. For example, as summarized by Radegran & Saltin (1998), studies indicating an effect of l-NMMA have mostly been undertaken using plethysmography, necessitating measurement of flow during brief rest periods between contractions or exercise bouts. Flows measured using ultrasound/Doppler actually during exercise have, to date, not supported much role for NO during muscular contraction but have indicated an effect at rest and during recovery, at least in the lower limbs. Most of the existing data can therefore be rationalized if it is accepted that NO contributes to resting and postexercise hyperaemia, whilst the picture regarding NO contribution during exercise is possibly confounded by reflex effects of systemic doses of NO antagonists (Sheriff et al. 2000; Schrage et al. 2004). In the one recent study which used appropriately delivered potent NOS inhibition and measured flows during exercise using ultrasound Doppler, the magnitude of NO contribution was approximately 20%. The contribution of NO to exercise hyperaemia in humans therefore appears to be of around 20–30%, but redundancy exists and NO may not be obligatory for the hyperaemia observed during exercise.
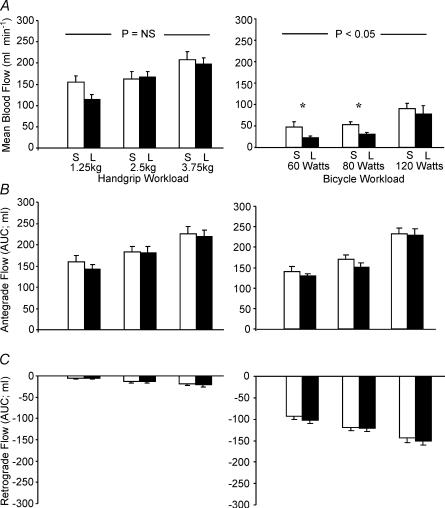
We believe one final point is germane, that the shear-stress-mediated effects of exercise, and consequent production and bioactivity of NO, differ qualitatively and quantitatively according to the exercise involved. In a recent study we examined brachial artery blood flow during lower limb exercise (Green et al. 2002b) and found that brachial artery infusion of l-NMMA decreased forearm blood flow during cycling at 60, 100 and 160 W, compared to the resting state. This finding suggests that systemic production of NO increases during exercise, even in vascular beds feeding metabolically inactive tissue. A subsequent study found that changes in HR alone in the absence of exercise were not associated with this phenomenon and, hence, it may be due to pulse pressure dynamics stimulating the endothelium throughout the circulation during exercise (Green et al. 2002a). When the contributions of NO to incremental handgrip and cycle exercise (see Fig. 1) were directly compared, mean flows were higher during handgrip, but the pattern of flow differed; handgrip exercise was associated with incremental increase in antegrade flow, whilst cycling induced a pattern of oscillatory antegrade systolic, retrograde diastolic flow in the resting upper limb which was amplified by exercise intensity (Fig. 1). Hence, whilst average forearm blood flow may be higher during handgrip exercise, the physiological stimulus for NO production may be paradoxically greater during cycling due to the pattern of oscillatory stretch on the endothelial cell membrane.
Figure 1. Effect of localized handgrip (left panels) and cycle ergometer (right panels) exercise on brachial artery haemodynamics.
Mean blood flow (A), antegrade (B) and retrograde (C) flows during exercise under saline (S; light bars) and l-NMMA (L; dark bars) infusion are displayed. l-NMMA significantly decreased flows under the cycle condition only (2-way ANOVA). Note that, although mean flows are lower during cycle exercise, the magnitude of antegrade flows are similar to handgrip exercise and there is substantial retrograde flow during cycling which is not evident during handgrip. This systolic antegrade, diastolic retrograde flow pattern and its accompanying oscillatory shear stress on the endothelium may explain the paradoxical greater contribution of NO during cycling than handgrip exercise, despite the greater mean flow during handgrip exercise. This difference in flow patterns may also resolve another conundrum in the literature, that exercise training studies involving localized handgrip training have not always produced significant improvement in NO bioactivity (Green et al. 1994, 1996a; Franke et al. 1998), whilst studies which have utilized typical ‘whole body’ exercise training regimes, predominantly involving lower limb exercise (cycling, running, etc.), have observed improvements in NO-mediated vasodilator capacity, even in the untrained upper limbs (Kingwell et al. 1997b; Maiorana et al. 2000, 2001a; Linke et al. 2001; Walsh et al. 2003a,b). An acute bout of cycle exercise may be a more potent stimulus to endothelial NO production in the forearm vasculature than a bout of localized handgrip exercise. (Green et al. in press).
Cardiac muscle
In contrast to the disparate findings in skeletal muscle, studies performed in the coronary circulation have generally indicated a role for NO in epicardial coronary vasodilatation during exercise. Experimental studies of coronary blood flows in humans rely upon increases in cardiac metabolism achieved by increasing ventricular rate using cardiac pacing. Indirect evidence for a role of NO in metabolic vasodilatation comes from a study showing an increase in by-products of NO in coronary sinus blood following pacing in subjects without coronary artery disease (CAD) risk factors (Minamino et al. 1998). No increase was evident in subjects with CAD risk factors but coronary sinus adenosine was elevated (Minamino et al. 1998), raising the possibility that adenosine production may be a compensatory mechanism maintaining myocardial blood flow in the presence of endothelial dysfunction. In other studies, l-NMMA abolished pacing-induced epicardial vessel dilatation, indicating that NO contributes to vasodilatation in these conduit vessels in patients free of CAD risk factors (Quyyumi et al. 1995a) and in those with angiographically normal coronary arteries (Tousoulis et al. 1997). l-NMMA also reduced pacing-induced dilatation of large epicardial arteries, but not of microvessels (Egashira et al. 1996). However, there have been negative studies in those with angiographically normal arteries, perhaps related to the shortcomings of angiography (Shiode et al. 1996; Nishikawa & Ogawa, 1997). In yet another study, NO was involved in pacing-induced hyperaemia in patients with risk factors for CAD, including some with mildly irregular arteries (Duffy et al. 1999a) despite risk factors leading to a reduction in vasodilatation (Quyyumi et al. 1995a), and an impaired response to stimulated NO release (Quyyumi et al. 1995b). In patients with CAD, NO production increased by pacing was abolished at the sites of stenoses (Tousoulis et al. 1997). In summary, there is strong evidence for a role of endothelium-derived NO in the metabolic vasodilatation of healthy coronary epicardial vessels, but NO-dependent vasodilatation decreases in the presence of risk factors and is absent or much reduced in grossly atherosclerotic vessels. Furthermore, in a recent review, Feigl and colleagues make the important point that, although the majority of studies indicate a decrease in epicardial coronary diameter during NO inhibition, coronary flows are not usually affected, indicating that downstream arteriolar dilatation adequately compensates for the decreased NO bioactivity (Tune et al. 2004). Hence, whilst NO inhibition doesn't steepen the relationship between coronary venous oxygen tension and myocardial oxygen consumption, indicating that it is not a metabolic vasodilator (Tune et al. 2002), it does play an important physiological role in epicardial dilatation, possibly as a result of ‘ascending vasodilatation’ via increased shear stress following downstream metabolic vasodilatation (Segal, 1992; Green et al. 1996b).
In summary, studies performed in humans indicate general agreement that NO contributes to resting and postexercise blood flows, although its contribution during exercise remains controversial. NO blockade reduces epicardial coronary diameters during pacing in the majority of studies, whereas the reduction in skeletal muscle blood flow during exercise is usually no greater than that at baseline (Radegran & Saltin, 1998; Bradley et al. 1999), suggesting that NO may not be an obligatory component of skeletal muscle exercise hyperaemia in humans. Despite the continuing controversy regarding the precise contribution of NO to exercise hyperaemia, regular exercise training is associated with increased vasodilator capacity (Sinoway et al. 1986, 1987; Green et al. 1996b) and longitudinal studies provide convincing evidence for the antiatherogenic effect of regular physical activity (Lee et al. 2000; Sesso et al. 2000; Myers et al. 2002). These phenomena may be due, in part, to up-regulation of endothelial function as a result of exercise training.
Does exercise training improve vascular NO function in healthy animals?
Insights into the time course of the effect of exercise training
Animal studies provide insight into the time course of effect of exercise training on NO vasodilator function. Short-term exercise-training in rats, of 2–4 weeks, increased endothelial NO synthesis in skeletal muscle arterioles and increased the vasodilator responses to ACh and l-arginine, but not to sodium nitroprusside (SNP), suggesting enhanced endothelium and NO-dependent function but unchanged smooth muscle cell sensitivity to NO (Sun et al. 1994). A subsequent study of similar duration also demonstrated augmented dilator response in rat muscle arterioles following training, which was partially abolished by l-NMMA infusion (Koller et al. 1995). Vascular structure was not obviously altered. These findings suggest that increased production of endothelial NO constitutes an initial phase in the adaptive response to exercise training. In large conduit vessels, improved endothelium-dependent vasodilatation has been observed after as few as 7 days of endurance training in pigs (McAllister & Laughlin, 1997). In another study, 4 weeks of training not only enhanced ACh-induced vasodilatation of the rat aorta but also increased eNOS protein levels in aortic tissue (Delp et al. 1993; Delp & Laughlin, 1997). In rabbits, 8 weeks of treadmill running increased ACh reactivity in the aorta and pulmonary arteries but not in the carotid artery, a vessel which is partly protected from dramatic changes in flow and shear stress (Chen & Li, 1993). Similarly, 4 weeks of daily exercise in rats improved flow-induced dilatation in skeletal muscle arteries, but not in mesenteric vessels, which are perhaps not subjected to the shear stress stimulus of exercise hyperaemia (Sun et al. 1998).
In contrast to short to moderate periods of exercise training, studies over a longer duration have not consistently shown augmented NO-related endothelial function. NO-dependent vasodilatation was unaltered after 16–20 weeks of training in pigs (McAllister et al. 1996) and 16 weeks in rats despite improved arterial compliance (Kingwell et al. 1997a). This seems to indicate that improvement in endothelium-dependent vasodilator responses in the periphery is a transient phenomenon that is lost with long-term training. Further, there is evidence that the expression of eNOS, initially increased by training, also exhibits this time dependence; daily exercise for 1 week resulted in an increased expression of eNOS protein in porcine pulmonary arteries and enhanced ACh-mediated relaxation (Johnson et al. 2001), whereas changes were not present after 16 weeks of exercise training (Johnson & Laughlin, 2000). Paralleling the time course of these changes, in various animal models, prolonged exercise training enlarges the diameter of arteries (Leon & Bloor, 1968; Wyatt & Mitchell, 1978; Kramsch et al. 1981; Lash & Bohlen, 1992); yet this vascular remodelling, in contrast to increased capillarization, also appears to be an endothelium- and NO-dependent phenomenon (Kamiya & Togawa, 1980; Langille & O'Donnell, 1986; Zarins et al. 1987; Gibbons & Dzau, 1994; Rudic et al. 1998; Prior et al. 2003), but one which partly supplants the acutely responsive vasodilator mechanisms.
In animal studies of the coronary circulation, early reports indicated that exercise training increased sensitivity to vasodilator agents in dogs (DiCarlo et al. 1989) and increased transport capacity in miniature swine (Laughlin et al. 1989). Evidence that exercise training enhances NO-mediated dilatation was found in a study of short-term, 7–10 days, treadmill exercise training in dogs (Wang et al. 1993). This intervention increased dilatation of the circumflex artery following both ACh infusion and reactive hyperaemia and infusion of nitro-l-arginine, an NO-synthase inhibitor, eliminated these responses. Training did not change the response to SNP. Evidence that eNOS gene expression is enhanced in the coronary vasculature by exercise training was first published by Sessa et al. (1994), who found that nitrite and NO production, and eNOS gene expression, were increased in coronary arterioles following 10 days of training in dogs. In a porcine model, 16 weeks of training increased eNOS mRNA (Woodman et al. 1997) and increased bradykinin-induced vasodilatation (Muller et al. 1994) in coronary resistance vessels, suggesting that enhanced NO and endothelium-dependent dilatation was persisting in these vessels. Clear evidence of withdrawal of this mechanism in the coronary circulation with longer term exercise training is not currently available. Additonally, however, 16–22 weeks of training augmented vasodilator responses to adenosine in the large epicardial arteries of pigs, even after removal of the endothelium. This one piece of evidence suggests that changes in vasomotor response in coronary arteries with longer-term training might be due, at least in part, to adaptations within smooth muscle (Oltman et al. 1992).
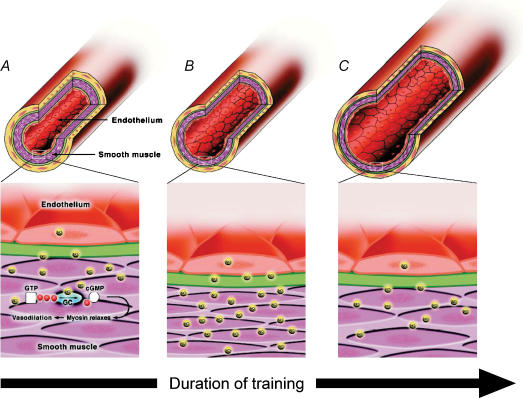
In summary, animal studies investigating both the peripheral and coronary vasculature suggest that short-term exercise training enhances eNOS, and NO production and bioactivity, producing a short-term buffer to the increased shear associated with exercise. After extended training, at least in the peripheral circulation, the increased production of NO and possibly other mediators induces structural changes in the vessels resulting in an increase in lumen diameter (Brown, 2003; Prior et al. 2003). Shear stress is hence ‘structurally’ normalized and endothelial NO activity returns towards initial levels (see Does exercise training modify vascular structure by a NO-dependent mechanism? and Figs 2 and 3).
Figure 2. Hypothesized response of arteries to increased flow and shear stress following varying durations of exercise training.
In the untrained vessel (left panel), basal release of NO causes subjacent smooth muscle cell vasodilatation which acts to homeostatically regulate wall shear. In response to medium-term exercise training (middle panel), acute increase in shear stress, associated with repetitive exposure to increased flow during bouts of exercise, stimulates increased endothelial NO production and consequent vasodilatation. Up-regulation of the NO-dilator system, including eNOS expression, occurs to buffer increased shear stress. Following long-term exercise training (right panel), structural adaptation occurs, possibly in part due to NO-mediated remodelling, resulting in chronic increase in vessel calibre which ‘structurally normalises’ shear stress. NO function returns towards baseline levels. Figure based on Maiorana et al. (2003). Permission granted by Adis International Ltd.
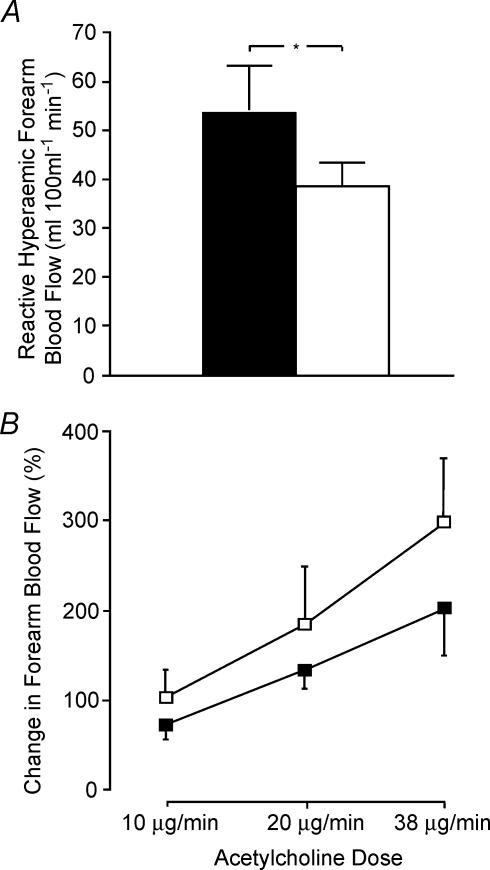
Figure 3. Reactive hyperaemic forearm blood flow (A) and change in forearm blood flow response to three doses of acetylcholine (ACh; B) in the preferred (▪) and non-preferred (□) forearms of elite single-handed tennis players (*P < 0.05) (Green et al. 1996a).
Peak reactive hyperaemia provides an index of resistance vessel structure and the higher preferred limb data indicates that chronic or recurrent episodic changes in blood flow induce arterial remodelling in humans which enhances vasodilator capacity. Responses to ACh, which provide an index of stimulated endothelium-dependent activity, did not differ between the limbs, indicating that chronic training is not associated with altered endothelium-dependent vascular responses. This study supports the schema presented in Fig. 2, that NO production and bioactivity initially produce a short-term buffer to the increased shear associated with exercise. Following extended training, arterial remodelling results in an increase in lumen diameter (Brown, 2003; Prior et al. 2003) which ‘structurally’ normalizes shear and endothelial NO activity returns towards initial levels. Figure based on Green et al. (1996a). Permission granted by the American Physiological Society.
Can normal endothelial function be enhanced by exercise training?
Does localized exercise training improve vascular NO function in healthy humans?
The first investigation to elucidate mechanisms associated with blood flow changes following exercise training was conducted in young males (Green et al. 1994). Four weeks of handgrip training reduced minimum vascular resistance following an ischaemic stimulus in the trained limb but not the untrained contralateral arm, nor in sedentary controls. However, neither the forearm blood flow response to the endothelium-dependent vasodilator methacholine chloride nor to SNP was altered following training. It was concluded that either NO did not contribute to enhanced vasodilatation in the forearm, or that the relatively brief period of conditioning and low stimulus intensity were insufficient to generate a detectable bioassay response in young fit healthy subjects. To examine the latter hypothesis, a subsequent study examined the effect of long-term repetitive conditioning, using local ACh and SNP infusion into the preferred and non-preferred limbs of elite tennis players (Green et al. 1996a). We observed enhanced forearm and wrist girth, grip strength and forearm volume in the preferred limbs and significantly higher peak vasodilator capacity, indicating a unilateral structural vascular enlargement (Fig. 3). However, neither l-NMMA, ACh nor SNP dose–response curves were significantly different between limbs, indicating no difference in either basal or stimulated NO dilator function. Similar findings were evident in studies comparing involved and control arms following recovery from forearm immobilization after casting (Green et al. 1997).
These studies suggest that forearm muscle training, that is, localized exercise of a small muscle group, does not influence endothelial function in apparently healthy subjects, despite demonstrable increased capacity for peak blood flow. The lack of benefit may have several explanations, one being that the muscle mass recruited or haemodynamics associated with handgrip studies may not induce shear-stress-mediated improvement in NO function (see Fig. 1). Alternatively, the vasculature remodelling evident in these studies may structurally normalize shear stress and negate the requirement for NO functional adaptations (see Insights into the time course of the effect of exercise training). Finally, it may be that exercise training of healthy subjects with normal endothelial function does not induce benefits in terms of the NO-vasodilator system, whereas benefit may be possible in those with antecedent endothelial dysfunction (see Can endothelial dysfunction be improved by exercise training?).
Does whole body exercise exert a generalized effect on NO vasodilator function?
Exercise involving large muscle groups of the legs is likely to produce systemic changes in vascular haemodynamics not apparent with localized exercise of the upper limb (see Fig. 1). In normal subjects, Kingwell et al. (1997b) examined the effect of 4 weeks of cycling on NO bioactivity in the forearm. Following training, l-NMMA-induced vasoconstriction was increased, suggesting enhanced basal NO function. Contrarily, there was no significant effect of training on the response to ACh or SNP, suggesting that stimulated endothelium-dependent and -independent function were unaltered. However, following a 10 week programme of daily aerobic and anaerobic exercise training in young military recruits, FMD of the brachial artery was significantly improved while GTN-mediated vasodilatation was not (Clarkson et al. 1999). This study also involved predominantly lower limb exercise, indicating that the improvement in endothelial function was likely to be systemic in nature although, despite an intensive training regime, the change in FMD was relatively small, increasing from 2.2 to 3.9%. Even the post-training FMD was lower than commonly reported in sedentary healthy subjects, the explanation for which remains unclear as the baseline vessel diameters were not substantially different from those in other studies (Neunteufl et al. 1997; Maiorana et al. 2001a; Walsh et al. 2003a; Walsh et al. 2003b).
Recently, in a randomised cross-over designed study of combined aerobic and resistance exercise in healthy middle-aged men, training did not significantly affect endothelium-dependent or -independent function (Maiorana et al. 2001b). This finding is in contrast to similar training studies in chronic heart failure (CHF) (Maiorana et al. 2000; Linke et al. 2001) and type 2 diabetic subjects (Maiorana et al. 2001a). NO-related endothelial function is impaired in these conditions and it is therefore probable that depressed endothelial function is more capable of augmentation by moderate exercise training than is the well preserved function of healthy subjects (see Can endothelial dysfunction be improved by exercise training?), which may, however, be improved by more intense training (Clarkson et al. 1999). This theory is supported by findings that regular physical activity may partially reduce the age-related decline in NO-related endothelial function (Yasue et al. 1990; Gerhard et al. 1996), possibly by reducing the impact of oxidative stress (DeSouza et al. 2000; Taddei et al. 2000).
In summary, localized exercise training, at least of the forearm, does not improve NO vasodilator function in healthy subjects and the evidence for benefit from larger muscle group exercise is relatively modest. It appears that a beneficial effect of exercise training in those with a priori normal vascular function may require a high volume of the exercise, if indeed it is possible at all.
Can endothelial dysfunction be improved by exercise training?
Numerous conditions relevant to cardiovascular health are associated with depressed endothelial function, including impaired NO dilator system activity. The potential for beneficial impact of exercise on the endothelium has assumed particular relevance because endothelial dysfunction, characterized by decreased NO-vasodilator activity, appears to be an important early and integral manifestation of vascular disease. Evidence for this includes the following: (i) endothelial dysfunction occurs initially at coronary branch points as do atherosclerotic plaques (McLenachan et al. 1990), (ii) endothelial dysfunction precedes and predicts the development of atherosclerotic disease in normal coronary arteries post-cardiac transplant (Fish et al. 1988), (iii) endothelial dysfunction is evident in children and adolescents with risk factors for future cardiovascular disease (Celermajer et al. 1992, 1993a,b, 1994, 1996; Watts et al. 2004a,b), (iv) interventions of known benefit in terms of cardiovascular risk also improve NO-mediated endothelial function (O'Driscoll et al. 1997a,b, 1999), (v) coronary (Al Suwaidi et al. 2000; Schachinger et al. 2000; Heitzer et al. 2001; Hollenberg et al. 2001) and peripheral (Neunteufl et al. 2000; Kuvin et al. 2001; Perticone et al. 2001b; Gokce et al. 2002a) vascular endothelial dysfunction predict cardiovascular events, and (vi) improvement in endothelial function improves prognosis (Modena et al. 2002).
The mechanisms proposed to explain depressed NO activity in subjects with cardiovascular disease and risk factors include a loss of endothelial synthesis of NO due to inadequate substrate or cofactors or abnormalities in enzymatic activity. Alternatively, a reduction in NO bioavailability may occur due to quenching by superoxide anions associated with an abnormal redox state (Ohara et al. 1993; Berliner et al. 1995; Kojda & Harrison, 1999). However, the mechanisms are not well defined and probably depend upon the specific aetiology. In the majority of subject groups the abnormality appears localized to the endothelium; responses to endothelium-independent NO donors are typically not impaired. The exception may be those subjects with advanced vascular disease, where the abnormality may spread to involve the vascular smooth muscle component of the NO dilator system (Nakamura et al. 1996).
The detailed effects of exercise training in various populations with endothelial dysfunction are summarized in Table 1 and fully reviewed elsewhere (Maiorana et al. 2003). Briefly, exercise training of both localized muscle groups (Hornig et al. 1996; Katz et al. 1997; Hambrecht et al. 2000b) and whole body exercise, predominantly of the lower limbs (Hambrecht et al. 1998, 2000c, 2003; Higashi et al. 1999a; Maiorana et al. 2000, 2001a; Linke et al. 2001; Gokce et al. 2002b; Schmidt et al. 2002; Gielen et al. 2003; Walsh et al. 2003a,b) are associated with improvement in measures of NO vasodilator function in subjects with cardiovascular risk factors and disease. Indeed, the consistency of the published data indicating that exercise training improves endothelial function in heterogeneous groups in whom it is initially depressed is remarkable, and contrasts with training studies of subjects with normal endothelial function. This strongly suggests that subjects with impaired endothelial function may be more amenable to improvement in NO function as a result of training than healthy subjects. Also, as mentioned above, the beneficial effects of exercise are generalized if the exercise involves large muscle groups; lower limb exercise improves upper limb conduit and resistance vessel function (Linke et al. 2001; Maiorana et al. 2001a; Schmidt et al. 2002; Walsh et al. 2003a,b). Several other findings, which provide specific physiological insight, can be gleaned from studies of patients with endothelial dysfunction.
Table 1.
Summary of exercise training studies of conduit and/or resistance vessel function in healthy humans and subjects with cardiovascular disease and risk factors
| Study | Methods | Exercise training | Effect of NO-mediated vasodilator function | NO improvement? |
|---|---|---|---|---|
| Normal subjects: | ||||
| Localized exercise | ||||
| Green et al. 1994 | Healthy young controls. VOP | Handgrip, 4 weeks, 3 × weekly | No difference in ACh, SNP or l-NMMA responses after training. Peak reactive hyperaemia improved | ↔ |
| Franke et al. 1998 | Young healthy subjects. VOP | Handgrip, 4 weeks, 3 × weekly | Response to exercise increased. No change in ACh + exercise responses | ↔ |
| Bank et al. 1998 | Controls for study of CHF patients. VOP | Handgrip, 30 min, 4–6 times/week | ACh improved after training. No change SNP | ↑ |
| Whole body exercise | ||||
| Kingwell et al. 1997b | Male sedentary 4 week cycle training. VOP | Cycling, 4 weeks, 3 × weekly, 30 min | Training ↑l-NMMA response at 2 μmol only. No difference ACh or SNP | ↔a |
| First suggestion that leg training improves forearm responses | ↑b | |||
| Bergholm et al. 1999 | Fit young males. VOP | Running. High intensity 4 × 1 h running session, weekly | ACh and l-NMMA ↓ after training. No change SNP. Effect may be due to ↓ antioxidant concentrations | ↓ |
| Clarkson et al. 1999 | Healthy army recruits Brachial D/U | Running, 10 weeks Daily 3 mile run + strength sessions High volume exercise | FMD increased from 2.2 to 3.9%. No change GTN | ↑ |
| Higashi et al. 1999a | Middle-aged controls for study of hypertensives. VOP | Walking, 30 mins, 5–7/week, 12 weeks | ACh response increased. No change in isosorbide dinitrate | ↑ |
| DeSouza et al. 2000 | Normal middle aged sedentary. VOP | Walking, 3 months home basedNo control group or period | Training improved ACh responses. No changes in SNP responses | ↑ |
| Maiorana et al. 2001b | Normals. Middle-aged. VOP | Circuit training, 8 weeks, 3 × week | No changes ACh or SNP or l-NMMA | ↔ |
| Goto et al. 2003 | Normal young males. VOP | Cycle exercise, 30 min, 5–7 × weekly, 12 weeks | Moderate intensity improved ACh responses. l-NMMA abolished this improvement | ↔c |
| 25%, 50% and 75% groups | No improvement in low or high intensity groups | |||
| Oxidant stress increased, high intensity group | ↑d | |||
| ↔e | ||||
| CHF | ||||
| Localized exercise | ||||
| Hornig et al. 1996 | Radial artery D/U | Handgrip, 4 weeks training, daily for 30 min | Training improved FMD, especially the l-NMMA sensitive component | ↑ |
| Katz et al. 1997 | VOP | Handgrip, 30 min daily, 8 weeks | Yes ACh ↑. GTN no change | ↑ |
| Bank et al. 1998 | VOP | Handgrip, 30 min, 4-6 times/weeks | No improvement in ACh responses after training. No change SNP | ↔ |
| Hambrecht et al. 2000b | Radial D/U | Handgrip, 6 × daily, 4 weeks | ACh dilatation increased, l-arginine potentiated the effect. No change in GTN | ↑ |
| Whole body exercise | ||||
| Hambrecht et al. 1998 | Doppler flow velocity + angiography of femoral artery | Cycle training, 6 months, 6 × day, 10 min, 80% HRmax | ACh mediated blood flow response increased. l-NMMA response also increased. No change GTN. | ↑ |
| Maiorana et al. 2000 | VOP. | Circuit training, 8 weeks, 3× week | ACh increased. SNP response also significantly improved. | ↑ |
| Linke et al. 2001 | Radial artery D/U. | 4 weeks cycle training, 6 × day, 10 min, 80% HRmax | Leg training improved ACh and FMD responses in the forearm. No change in GTN. | ↑ |
| CAD | ||||
| Whole body exercise | ||||
| Hambrecht et al. 2000c | Coronary bed. Flow wire/Doppler | Cycle training, 6 × day, 10 min, 4 weeks 80% HRmax | ACh responses and peak flow velocity improved. | ↑ |
| Gielen et al. 2003 | Coronary bed Intravascular ultrasound/angiography | Home based follow-up to above study | ACh responses improved, improved flow and velocity to ACh. No changes in response to GTN. | ↑ |
| Bicycle exercise, 20 min daily, 5 months | ||||
| Gokce et al. 2002b | Brachial and tibial D/U | Leg exercise, 40 min, 3x weekly, 10 weeks | FMD increased significantly in the tibial artery, with increase (NS) also in the brachial. No changes in GTN responses. | ↑ |
| Walsh et al. 2003a | Brachial D/U | Circuit training, 8 weeks, 3 × week | FMD improved. No change in GTN | ↑ |
| Hambrecht et al. 2003 | Coronary bed Intravascular ultrasound/angiography | Cycle or rowing, 3 × day, 10 min, 4 weeks | Peak flow velocity response to ACh increased eNOS expression and phosphorylation increased in coronary artery samples. | ↑ |
| Hypertensives | ||||
| Whole body exercise | ||||
| Higashi et al. 1999a | VOP | Walking, 30 mins, 5-7/week, 12 weeks | ACh increase sig in hypertensives. No difference isosorbide dinitrate LNNMA abolished training enhancement in ACh response. | ↑ |
| Higashi et al. 1999b | VOP | Walking, 30 mins, 5-7/week, 12 weeks | Max RHBF response increased only in hypertensives l-NMMA abolished thins enhancement | ↑ |
| Hypercholesterolaemia | ||||
| Whole body exercise | ||||
| Lewis et al. 1999 | Hyperchols not medicated. VOP | Cycle training, 4 weeks, 3 × 30 min/week | l-NMMA responses greater after training at 4 μmol. No effect of training on ACh or SNP responses | ↑f |
| Walsh et al. 2003b | Hyperchols on and off medication.VOP and Brachial D/U | Circuit training, 8 weeks, 3 × week | ACh and FMD responses improved in overall and in medicated subjects. No change SNP or GTN. l-NMMA improved in unmedicated subjects. | ↑h |
| Obesity | ||||
| Whole body exercise | ||||
| Sciacqua et al. 2003 | Adults. VOP | Walking, 30 min daily, 3 × weekly, 10–16 weeks | In those who lost weight: improved response to upper dose of AChNo change in SNP responses | ↑ |
| Watts et al. 2004a | Adolescents. Brachial D/U | Circuit training, 8 weeks, 3 × week | FMD increased. Improvement not related to change in adiposity | ↑ |
| Watts et al. 2004b | Children. Brachial D/U | Structured play/games, 1 h, 3 × week | FMD increased | ↑ |
| Woo et al. 2004 | Overweight children. Brachial D/U | Diet + exercise versus diet alone design | At 6 weeks, both group improved FMD | ↑ |
| Diabetes | ||||
| Whole body exercise | ||||
| Lavrencic et al. 2000 | Polymetabolic syndrome. Brachial D/U | Bicycle exercise, 3 × weekly, 12 weeks | FMD increased. No change in GTN responses | ↑ |
| Maiorana et al. 2001a | Type 2 diabetics. VOP and Brachial D/U | Circuit training, 8 weeks, 3 × week | ACh and FMD increased. No change in SNP or GTN | ↑ |
| Fuchsjager-Mayrl et al. 2002 | Type 1 diabetes. Brachial D/U | Cycle exercise, 1 h, 2–3× weekly, 4 months | FMD increased significantly. No significant change in GTN, despite trend | ↑ |
VOP, venous occlusion plethysmography; D/U, Doppler ultrasound arterial assessment; l-NMMA, NG-monomethyl-l-arginine: ACh, acetylcholine, an endothelium-dependent NO vasodilator; SNP, sodium nitroprusside, an endothelium-independent NO vasodilator; FMD, flow-mediated vasodilatation, an endothelium- and NO-dependent vasodilator stimulus; GTN, glyceryl trinitrate, an endothelium-independent vasodilator; CHF, chronic heart failure; CAD, coronary artery disease; ↑, improvement in NO vasodilator function; ↓, deterioration in NO vasodilator function; ↔, no change in NO vasodilator function. aStimulated function; bbasal function; clow intensity; dmoderate intensity; ehigh intensity; fbasal function; gstimulated function; hbasal function in unmedicated subjects; istimulated function in unmedicated subjects; jmedicated subjects.
Does improved vascular function relate to improved functional capacity?
The majority of exercise training studies in humans have been undertaken in patients with chronic heart failure, probably due to the well established endothelial dysfunction evident in this group (Treasure et al. 1990; Kubo et al. 1991; Drexler et al. 1992; Katz et al. 1992, 1993) and the clinical significance of peripheral abnormalities which limit V̇O2peak (Wilson et al. 1984; Kraemer et al. 1993). These studies indicate that exercise training, predominantly of the lower limbs, improves NO-mediated vasodilator function in the vasculature of the exercised muscle (Hambrecht et al. 1998) and also in the untrained upper limb (Maiorana et al. 2000; Linke et al. 2001). Increases in endothelium-dependent vasodilatation in vessels supplying the trained musculature were significantly correlated with changes in functional capacity (Hambrecht et al. 1998) and a small increase in stroke volume (Hambrecht et al. 2000a). These studies in CHF subjects therefore suggest that improved NO-mediated vascular function as a result of training may improve cardiac function and V̇O2peak, possibly by improving vascular compliance and decreasing afterload.
Hypercholesterolaemia is associated with impaired endothelium-dependent vascular relaxation (Creager et al. 1990; Vita et al. 1990; Drexler et al. 1991; Chowienczyk et al. 1992; Casino et al. 1993; Sorensen et al. 1994), which, in mice, correlates with reduced aerobic capacity (Maxwell et al. 1998). Exercise training in hypercholesterolaemic mice partially reverses endothelial dysfunction, and this is correlated with improved aerobic capacity (Niebauer et al. 1999). These findings raise the question of whether exercise training alone, or in combination with lipid lowering, may improve NO vasodilator function and functional capacity in humans. Cycle training in unmedicated subjects enhanced basal NO bioactivity but did not alter ACh-stimulated vasodilatation nor smooth muscle sensitivity to SNP in the forearm (Lewis et al. 1999) and, in a randomised, cross-over design study, 8 weeks of training also failed to alter endothelial function in unmedicated subjects (Walsh et al. 2003b). Conversely, FMD and ACh responses improved in subjects taking 3-hydroxy-3-methylglutaryl CoA (HMGCoA) reductase inhibitor lipid-lowering therapy (Walsh et al. 2003b). This disparity may be due to the longer duration and initially greater severity of hypercholesterolaemia in the treated subjects and their depression of NO-related vasodilatation compared to the untreated and normal subjects, again consistent with the greater potential for improving depressed compared to normal function. Alternatively, as HMGCoA reductase inhibitors and exercise training have both been reported to increase eNOS activity (Griffin et al. 2001; Bates et al. 2002), and perhaps both affect the oxidant state (Powers et al. 1999; Carneado et al. 2002), there is a hypothetical possibility of synergistic benefit from the two interventions (Dimmeler & Zeiher, 2003).
In summary, it appears that improvement in NO vasodilator function is associated with improved functional capacity in patients with CHF, due perhaps to enhanced coronary perfusion or decreased afterload. This is important clinically, as V̇O2peak strongly correlates with prognosis in CHF (Mancini et al. 1991; Myers et al. 1998). Whether exercise training contributes to improved V̇O2peak in other groups such as subjects with hypercholesterolaemia, hypertension or diabetes remains to be seen, but positive evidence has emerged in animals (Maxwell et al. 1998; Niebauer et al. 1999) and a possible synergism may exist between pharmacological approaches known to improve NO function, such as statins and ACE inhibitors, and shear-stress-mediated exercise training effects. Future studies will need to be specifically designed to address this possibility.
Is improvement in NO function with training related to training-induced improvement in risk factors?
Cardiovascular risk factors such as hypercholesterolaemia (Creager et al. 1990; Vita et al. 1990; Drexler et al. 1991; Chowienczyk et al. 1992; Casino et al. 1993; Sorensen et al. 1994), hypertension (Panza et al. 1990; Brush et al. 1992; Treasure et al. 1992; Egashira et al. 1995; Higashi et al. 1997), diabetes (McVeigh et al. 1992; Karusa et al. 1995; Ting et al. 1996; Watts et al. 1996; Williams et al. 1996; O'Driscoll et al. 1999) and obesity (Arcaro et al. 1999; Perticone et al. 2001a) are associated with impaired NO-vasodilator function and treatment with pharmacological agents can reverse this (O'Driscoll et al. 1997a,b, 1999; Widlansky et al. 2003). These findings raise the possibility that exercise training may improve NO-mediated vasodilator function via its effects on cardiovascular risk factors (Blair et al. 1984; Tran & Weltman, 1985; Holloszy et al. 1986; Blair, 1993; Williams, 1996; Malfattoo et al. 1998; Kelley, 1999; Smith et al. 1999; El-Sayed et al. 2000).
Improvement in basal (Lewis et al. 1999) and stimulated (Walsh et al. 2003b) endothelial function with exercise training have been reported in the absence of a decrease in serum lipids, and reduction in lipid levels are not necessarily associated with improved endothelial function (Jodoin et al. 1999). Exercise-induced improvements in endothelial function in hypercholesterolaemia therefore appear to result from mechanisms independent of the effect of exercise training on serum cholesterol. In hypertensive subjects, 12 weeks of daily walking increased forearm reactive hyperaemia (Higashi et al. 1999b) and the blood flow response to ACh (Higashi et al. 1999a), the latter effect abolished by l-NMMA, indicating that the improvement following training was due to augmented bioactivity of NO. There was a reduction in blood pressure as a result of exercise training in this study, but it did not correlate with improved endothelial function indicating that the vascular function benefit is likely to be independent of the pressure lowering effect of training. The effect of exercise training on endothelial function in type 2 diabetes also appears to be independent of changes in glucose tolerance. Diabetic rats with impaired aortic endothelial function were randomised to sedentary, exercise trained and food restricted groups (Sakamoto et al. 1998). Both exercise training and food restriction reduced plasma glucose and insulin, and increased insulin sensitivity, but only exercise training improved endothelium-dependent relaxation to histamine and increased urinary excretion of nitrite. Similarly, in humans, exercise training enhanced endothelium-dependent reactivity to ACh and FMD in forearm resistance and conduit vessels but these changes were not significantly related to changes in fasting blood glucose or glycated haemoglobin, suggesting that improved endothelial function may occur independently of glycaemic control (Maiorana et al. 2001a).
Similar results were evident in obese children and adolescents; improvement in FMD with training was not associated with changes in lipid fractions, haemodynamic variables or glycaemic control (Watts et al. 2004a; Watts et al. 2004b). Indeed, improvements in endothelial function occurred independent of changes in body weight and BMI, lending some support to studies in overweight and obese adults which have demonstrated that low cardiovascular fitness may be a more important predictor of cardiovascular mortality than BMI (Barlow et al. 1995; Blair et al. 2001; Farrell et al. 2002).
In summary, improvement in cardiovascular risk factors does not appear to be essential for improvement in NO vasodilator function. Indeed, in a pooled analysis of 75 subjects with diverse risk factors who undertook a similar training regimen, training did not significantly alter plasma lipids, blood pressure, blood glucose, waist: hip or BMI, despite significant improvement in both conduit and resistance vessel NO vasodilator function, and there were no correlations between changes in any risk factor variables and the improvements in vascular function. At a minimum, these data indicate that the beneficial effects of relatively short-term exercise training on vascular function are not solely mediated by the effects of exercise on cardiovascular risk factors (Green et al. 2003).
What mechanisms are responsible for the beneficial effect of exercise training on NO vasodilator function?
It seems likely that a direct shear-stress-related mechanism is largely responsible for the beneficial effects of repeated exercise, but it is also known that NO reacts with oxygen free radicals and that the latter can quench NO bioactivity. It is possible that the repeated induction of NOS activity with exercise training prolongs the half-life of NO by reducing its degradation by free radicals in these conditions (Fukai et al. 2000) or by directly decreasing free radical production (Adams et al. 2002). In this context, Ennezat et al. (2001) recently observed markedly increased expression of genes encoding antioxidant enzymes in skeletal muscle, in the absence of changes in eNOS levels, in patients with CHF following training. This is obviously an area requiring much further study.
In the most recent of their impressive series of studies on exercise training on vascular function, Hambrecht et al. (2003) examined the effects of 4 weeks of cycle ergometer exercise on ACh responses in the left internal mammary artery of patients with stable CAD and in matched controls. Training increased peak flow velocity responses to ACh by 56% and flow-mediated dilatation responses to adenosine by 150% while no changes were evident in the untrained control group. A unique aspect of this study was that within 36 h of the final training session and 24 h of the repeat vascular function assessments, subjects underwent coronary bypass surgery from which a section of the internal mammary artery was harvested and used for in vitro ACh and SNP assessment, immunohistochemistry, eNOS mRNA isolation and protein quantification. These cross-sectional analyses revealed significantly higher eNOS mRNA and protein expression in the trained subjects compared to controls and substantially higher eNOS phosphorylation which correlated with in vivo ACh-mediated vasodilator capacity. These unique human data reveal that exercise training improves endothelial function in vivo by up-regulating eNOS protein expression and by increased phosphorylation of this enzyme. These effects are consistent with a shear-stress mechanism for enhanced NO bioactivity with training, although reduced degradation of NO by oxidant species cannot be ruled out as a contributing mechanism (Fukai et al. 2000).
Is there an optimal volume of exercise training for vascular effects?
Two studies raise the possibility that there may be an optimal threshold of training, beyond which exercise may not improve endothelial function. In the only published study which has demonstrated a deleterious effect of exercise training on vascular function in humans, Bergholm et al. (1999) reported that 3 months of high intensity running reduced endothelium-dependent function but not endothelium-independent function. The degree of endothelial dysfunction following training was significantly correlated with a decrease in serum uric acid and was most severe in subjects with the greatest improvement in V̇O2max. The authors postulated that the training-induced decrease in circulating antioxidant levels may adversely affect endothelial function in the highly trained or overtrained state. Goto et al. (2003) recently studied the effects of 12 weeks of exercise undertaken at low (25% V̇O2max), moderate (50% V̇O2max) and high (75% V̇O2max) intensity in young healthy men. ACh-mediated forearm vasodilatation improved in the moderate intensity group only, and this occurred in the absence of changes in blood-borne measures of oxidative stress. In contrast, ACh responses did not improve, nor did they deteriorate, in the high intensity group in whom increased oxidative stress was evident. Taken together, these intriguing studies suggest that low intensity exercise may fall below the threshold for improvement in NO-related vascular function, that moderate intensity exercise enhances endothelial NO bioavailability, whilst any improvement in vascular function as a result of enhanced NO production following high-intensity exercise may be abolished through scavenging by free radicals generated at high intensities. These findings parallel recent data on the effects of exercise intensity on cardiovascular disease prevention (Manson et al. 2002; Tanasescu et al. 2002).
Does exercise training modify vascular structure by a NO-dependent mechanism?
It has been known for many years that exercise training is associated with structural vascular enlargement, for example autopsy and angiographic studies demonstrate enlarged coronary arteries in athletes (Currens & White, 1961; Pellicia et al. 1990) and physically fit individuals (Rose et al. 1967; Mann et al. 1972; Hildick-Smith et al. 2000), indicating that physical conditioning may induce a change in arterial calibre (i.e. ‘arterial remodelling’). Similarly, cross-sectional studies have consistently reported enlargement of both skeletal muscle conduit (Zeppilli et al. 1995; Schmidt-Trucksass et al. 2000; Dinenno et al. 2001; Huonker et al. 2003) and resistance (Sinoway et al. 1986; Green et al. 1996a) vessels in athletes relative to matched controls. Exercise training studies support these findings, with increases in resistance vessel structure (Sinoway et al. 1987; Green et al. 1994) and resting conduit vessel diameter (Miyachi & Iemitsu Okutsu, 1998; Dinenno et al. 2001) reported following exercise training in healthy men. Findings from animal studies regarding epicardial and resistance coronary vasculature concur (Brown, 2003).
A link between chronic changes in flow and modification of vascular structure is supported by a classic study which examined rabbit carotid arteries after unilateral ligation-mediated chronic decreases in flow (Langille & O'Donnell, 1986). The diameter of the ligated vessel exposed to a 70% reduction in flow for a period of 2 weeks was significantly smaller than the contralateral control vessel. In addition, this change in vascular structure was dependent upon the presence of the endothelium, indicating that changes in vessel structure secondary to chronic changes in flow may be dependent upon the release of a labile factor from endothelial cells. A similar conclusion was derived from an earlier study which found that shear stress was autoregulated after initial perturbation by an arteriovenous fistula (Kamiya & Togawa, 1980). More recent studies confirm that the stimulus to arterial remodelling is shear stress (Tuttle et al. 2001) and that vessels enlarge to homeostatically regulate wall shear in a NO-dependent manner (Tronc et al. 1996).
The above data are consistent with the evolving hypothesis that exercise training induces structural enlargement of conduit vessels which is dependent upon shear-stress-mediated NO release and may be an adaptive response which acts to mitigate the increases in transmural pressure and wall stress brought about by repeated exercise bouts (Rodbard, 1975; Zamir, 1977; Guyton & Hartley, 1985; Langille et al. 1989; Tronc et al. 1996; Lloyd et al. 2001; Prior et al. 2003). This structural remodelling and consequent normalization of shear obviates the need for ongoing functional dilatation, including enhanced NO dilator system function (Brown, 2003). In contrast, microvascular angiogenesis, whilst endothelium (and VEGF) dependent, appears to occur primarily in responses to hypoxia rather than shear stress and NO is not obligatory (Lloyd et al. 2001; Prior et al. 2003). Indeed, evidence is emerging that the contribution of substances such as NO to exercise training-mediated adaptations in vascular structure and function may be vessel calibre dependent with larger vessels, which are exposed to higher shear stress forces, possessing greater capacity for NO production (Laughlin et al. 2003a,b; Green et al. 2004). Given that acute bouts of exercise induce changes in local haemodynamic conditions and shear stress which differ according to branch order of the vascular tree, it is likely that the NO-mediated contribution to vascular adaptations differs at distinct loci.
Collectively, the above data from both animal and human studies of exercise training suggest that functional and structural adaptations of the vasculature to exercise training alter with training duration and intensity and the vessel beds involved. Brown, in an excellent review of coronary remodelling in response to exercise, concluded that vascular remodelling differs depending on the size and location of vessels along the vascular tree; capillaries and small arterioles appear to undergo longitudinal network extension whilst resistance and conduit arteries circumferentially expand (Brown, 2003). In humans, this might be considered a hypothesis to fit the current data; more evidence is required regarding the time course of changes in resistance, conduit and microvessels, particularly in the coronary circulation, and concerning functional mechanisms aside from those which are NO dependent.
What is the effect of exercise cessation and detraining on NO function?
Localized and systemic exercise-induced changes in endothelial function are quickly lost following the cessation of relatively short duration training. Six weeks after handgrip training was ceased in patients with CHF, radial artery diameters had returned to pre-training size (Hornig et al. 1996). Similarly, endothelial function returned to baseline 8 weeks following cessation of training in patients with CHF (Maiorana et al. 2000), CAD (Walsh et al. 2003a), hypercholesterolaemia (Walsh et al. 2003b) and type 2 diabetes (Maiorana et al. 2001a) and in obese adolescents (Watts et al. 2004a,b), all of whom had undergone an initial 8 weeks of training. This is consistent with the time course of deconditioning associated with other physiological adaptations to exercise training. These data suggest therefore that an exercise programme, at some level as yet undetermined, would be necessary to maintain vascular benefits in these extremely common and important conditions for which an exercise programme should be prescribed. Gielen et al. (2003) recently found that a 5-month period of moderate volume home-based exercise maintained a proportion, but not the entire benefit, of a preceding intensive hospital based programme. To date, no data exist on the effect of detraining on endothelial function in healthy subjects.
Summary and conclusions
In an excellent recent review for this journal, Booth et al. (2002) suggested that humans possess a genome which was selected during an era of obligatory physical activity, whereas contemporary Western lifestyle is characteristically sedentary. In this context, habitual exercise restores perturbed homeostatic mechanisms towards the normal physiological range. Analysis of the studies presented in this review reveal data consistent with this hypothesis. The majority of those studies undertaken in subjects with a priori impaired NO-related vasodilator function produced improvement in conduit or resistance vessel function, whereas studies in healthy normal subjects have been less consistent. That is, depressed endothelial function is more amenable to improvement by exercise training than is ‘normal’ endothelial function in the young and healthy, who might need a higher intensity or volume of exercise training for benefit to be apparent. This raises the intriguing possibility of a threshold effect for training on endothelial function which may be determined by age, fitness or pathological state. Studies are now beginning to emerge regarding the optimal modality and intensity of exercise for improvement in vascular structure and function and intriguing relationships between these factors, endothelial function and oxidative stress are becoming apparent.
Endothelial function may improve after as few as 7 days in trained animals (Wang et al. 1993; McAllister & Laughlin, 1997). In healthy humans, 4 weeks of training improved basal NO-related endothelial function in healthy subjects (Kingwell et al. 1997b). However, studies that have identified improved stimulated endothelium-dependent vasodilatation have prescribed at least 10 weeks of training in normal subjects (Clarkson et al. 1999; DeSouza et al. 2000). Pathological states associated with depressed NO bioactivity may respond more rapidly to training, with endothelial function improving after as little as 4 weeks (Hornig et al. 1996; Hambrecht et al. 2000c), but returning rapidly to baseline following the cessation of training (Hornig et al. 1996; Maiorana et al. 2000, 2001a). Conditions in which exercise training can improve NO-dependent endothelial vasodilator function include CAD, type 2 diabetes, obesity, hypercholesterolaemia, hypertension and CHF. In the latter, peripheral abnormalities typically limit V̇O2peak and improvement in vasodilator function correlates with changes in functional capacity and may improve cardiac function by decreasing afterload. Few studies have investigated the relationship between improvements in vasodilator function and functional capacity in other conditions.
It is likely that exercise training improves NO vasodilator function by both direct and indirect means. Conventional risk factors impair NO-mediated endothelial function and beneficial training effects on risk factor profiles are widely accepted, although the evidence is less convincing in the secondary prevention setting in which pharmacotherapy has a greater impact. Nonetheless, exercise training consistently improves NO function in patients with cardiovascular risk factors and disease, including those on optimal medical regimes and in the absence of risk factor modulation. These findings strongly implicate a direct effect of exercise on the vasculature, mediated through intermittent increases in endothelial shear stress. This effect may, in turn, be mediated through up-regulation of eNOS or decreased free radical degradation of NO, with evidence beginning to emerge for both possibilities (Fukai et al. 2000; Dimmeler & Zeiher, 2003; Hambrecht et al. 2003). The possibility that synergistic benefits accrue from combined pharmacological and exercise-mediated shear stress strategies remains to be investigated in humans.
Vascular remodelling with increase in vessel diameter occurs with prolonged exercise (Leon & Bloor, 1968; Kramsch et al. 1981; Miyachi et al. 2001; Prior et al. 2003). This process teleologically constitutes a longer term mechanism for reducing shear stress on a more sustained basis, allowing NO bioactivity to return towards pre-training levels. This hypothetical schema, which promotes shear stress as a homeostatically regulated variable, explains the observation that improvement in NO-related vasodilatation is observed in short to medium term studies, whereas longer term training studies, associated with arterial remodelling, have not usually reported improved endothelial function. Whilst the mechanisms responsible for mediating vascular structural change remain largely undefined, there is strong evidence that NO plays an important role in arterial remodelling (Kamiya & Togawa, 1980; Langille & O'Donnell, 1986; Zarins et al. 1987; Gibbons & Dzau, 1994; Tronc et al. 1996; Rudic et al. 1998; Prior et al. 2003). These findings reinforce the conclusion that future questions regarding the physiological effects of exercise training on the endothelium will be best resolved if studies consider the direct effects of various interventions on the vessel wall.
References
- Adams V, Hambrecht R, Erbs S. Impact of physical exercise training on the expression of NAD(P)H-oxidase and angiotensin-II receptors in the left mammarial artery of patients with coronary artery disease. Circulation. 2002;106:II-354. abstract. [Google Scholar]
- Al Suwaidi J, Hamasaki S, Higano S, Nishimura R, Holmes D, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- Ambring A, Benthin G, Petersson A-S, Jungersten L, Wennmalm Å. Indirect evidence of increased expression of NO synthase in marathon runners, and upregulation of NO synthase activity during running. Circulation. 1994;90:I-137. abstract. [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro G, Zamboni M, Rossi L, Turcato E, Covi G, Armellini F, Bosello O, Lechi A. Body fat distribution predicts degree of endothelial dysfunction in uncomplicated obesity. Int J Obesity. 1999;23:9936–9942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- Bank AJ, Shammas RA, Mullen K, Chuang PP. Effects of short-term forearm exercise training on resistance vessel endothelial function in normal subjects and patients with heart failure. J Card Fail. 1998;4:193–201. doi: 10.1016/s1071-9164(98)80006-7. [DOI] [PubMed] [Google Scholar]
- Barlow CE, Kohl HW, Gibbons LW, Blair SN. Physical fitness, mortality and obesity. Int J Obes. 1995;19:S41–S44. [PubMed] [Google Scholar]
- Bates K, Ruggeroli C, Goldman S, Gaballa M. Simvastatin restores endothelial NO-mediated vasorelaxation in large arteries after myocardial infarction. Am J Physiol. 2002;283:H768–H775. doi: 10.1152/ajpheart.00826.2001. [DOI] [PubMed] [Google Scholar]
- Benjamin N, Calver A, Collier J, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension. 1995;25:918–923. doi: 10.1161/01.hyp.25.5.918. [DOI] [PubMed] [Google Scholar]
- Bergholm R, Makimattila S, Valkonen N, Liu M, Lahdenpera S, Taskinen M, Sovijarvi A, Malmberg P, Yki-Jarvinen H. Intense physical training decreases circulating antioxidants and endothelium-dependent vasodilation in vivo. Atherosclerosis. 1999;145:141–149. doi: 10.1016/s0021-9150(99)00089-1. [DOI] [PubMed] [Google Scholar]
- Berliner JA, Navab M, Fogelman AB. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- Blair S. Evidence for success of exercise in weight loss and control. Ann Intern Med. 1993;119:702–706. doi: 10.7326/0003-4819-119-7_part_2-199310011-00015. [DOI] [PubMed] [Google Scholar]
- Blair SN, Cheng YJ, Holder JS. Is physical activity or physical fitness more important in defining health benefits. Med Sci Sports Exerc. 2001;33:S379–S399. doi: 10.1097/00005768-200106001-00007. [DOI] [PubMed] [Google Scholar]
- Blair S, Goodyear N, Gibbons L. Physical activity and incidence of hypertension in healthy normotensive men and women. JAMA. 1984;252:487–490. [PubMed] [Google Scholar]
- Bode-Böger SM, Böger RH, Scroder EP, Frölich JC. Exercise increases systemic nitric oxide production in men. J Cardiovasc Risk. 1994;1:173–178. [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Spangenburg EE. Exercise and gene expression: physiological regulation of the human genome through physical activity. J Physiol. 2002;543:399–411. doi: 10.1113/jphysiol.2002.019265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R, Kjær M. Redundancy reflects versatility of blood flow regulation mechanisms. J Physiol. 2004;557:346. doi: 10.1113/jphysiol.2004.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SJ, Kingwell BA, McConnell GK. Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes. 1999;48:1815–1821. doi: 10.2337/diabetes.48.9.1815. [DOI] [PubMed] [Google Scholar]
- Brock RW, Tschakovsky ME, Shoemaker JK, Halliwell JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. Am J Physiol. 1998;85:2249–2254. doi: 10.1152/jappl.1998.85.6.2249. [DOI] [PubMed] [Google Scholar]
- Brown MD. Exercise and coronary vascular remodelling in the healthy heart. Exp Biol Med. 2003;88:645–658. doi: 10.1113/eph8802618. [DOI] [PubMed] [Google Scholar]
- Brush JE, Faxon DP, Salmon S, Jacobs AK, Ryan TJ. Abnormal endothelium-dependent coronary vasomotion in hypertensive patients. J Am Coll Cardiol. 1992;19:809–815. doi: 10.1016/0735-1097(92)90522-o. [DOI] [PubMed] [Google Scholar]
- Carneado J, Alvarez de Sotomayor M, Perez-Guerrero C, Jimenez L, Herrera M, Pamies E, Martin-Sanz M, Stiefel P, Miranda M, Bravo L, EM Simvastatin improves endothelial function in spontaneously hypertensive rats through a superoxide dismutase mediated antioxidant effect. J Hypertens. 2002;20:429–437. doi: 10.1097/00004872-200203000-00018. [DOI] [PubMed] [Google Scholar]
- Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. Role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Georgakopoulos D, Buller C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993b;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen K, Ryalls M, Robinson J, Thomas O, Leonard JV, Deanfield JE. Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not their heterozygous parents. J Am Coll Cardiol. 1993a;22:854–858. doi: 10.1016/0735-1097(93)90203-d. [DOI] [PubMed] [Google Scholar]
- Chen H, Li H-T. Physical conditioning can modulate endothelium-dependent vasorelaxation in rabbits. Arterioscler Thromb Vasc Biol. 1993;13:852–856. doi: 10.1161/01.atv.13.6.852. [DOI] [PubMed] [Google Scholar]
- Chowienczyk PJ, Watts GF, Cockcroft JR, Ritter JM. Impaired endothelium-dependent vasodilation of forearm resistance vessels in hypercholesterolaemia. Lancet. 1992;340:1430–1432. doi: 10.1016/0140-6736(92)92621-l. [DOI] [PubMed] [Google Scholar]
- Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33:1379–1385. doi: 10.1016/s0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer DS, Charbonneau F, Creager MA, Deanfield J, Drexer H, Gerhard-Herman M, Herrington D, Vallance P, Vogel R. Guidelines for the ultrasound assessment of flow-mediated vasodilation of the brachial artery. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Creager MA, Cooke JP, Mendelhson ME, Gallagher SJ, Colemen SM, Loscalzo J, Dzau VJ. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currens JH, White PD. Half century of running: clinical, physiological and autopsy findings in the case of Clarence De Mar, ‘Mr. Marathoner’. N Engl J Med. 1961;265:988–993. doi: 10.1056/NEJM196111162652006. [DOI] [PubMed] [Google Scholar]
- Delp MD, Laughlin MH. Time course of enhanced endothelium-mediated dilation in aorta of trained rats. Med Sci Sports Exerc. 1997;29:1454–1461. doi: 10.1097/00005768-199711000-00011. [DOI] [PubMed] [Google Scholar]
- Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol. 1993;75:1354–1363. doi: 10.1152/jappl.1993.75.3.1354. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger C, Dinenno F, Monahan M, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- DiCarlo SE, Blair RW, Bishop VS, Stone HL. Daily exercise enhances coronary resistance vessel sensitivity to pharmacological activation. J Appl Physiol. 1989;66:421–428. doi: 10.1152/jappl.1989.66.1.421. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Exercise and cardiovascular health. Get active to AKTivate your endothelial nitric oxide synthase. Circulation. 2003;107:3118–3120. doi: 10.1161/01.CIR.0000074244.82874.A0. editorial. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR. Regular endurance exercise induces expansively arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534:287–295. doi: 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi SN, Naka KK, Payne N, Jones CJH, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci. 2001;101:629–635. [PubMed] [Google Scholar]
- Drexler H, Hayoz D, Munzel T, Hornig B, Just H, Brunner HR, Zelis R. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69:1596–1601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- Drexler H, Zeiher K, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by L-arginine. Lancet. 1991;338:1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation. 1999a;100:1951–1957. doi: 10.1161/01.cir.100.19.1951. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Gishel N, Tran BT, Harper RW, Meredith IT. Relative contribution of vasodilator prostanoids and NO to metabolic vasodilation in the human forearm. Am J Physiol. 1999b;45:H663–H670. doi: 10.1152/ajpheart.1999.276.2.H663. [DOI] [PubMed] [Google Scholar]
- Dyke CK, Proctor DN, Deitz NM, Joyner MJ. Role of nitric oxide in exercise hyperemia during prolonged rhythmic handgripping in humans. J Physiol. 1995;488:259–265. doi: 10.1113/jphysiol.1995.sp020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira K, Katsuda Y, Mohri M, Kuga T, Tagawa T, Kubota T, Hirakawa Y, Takeshita A. Role of endothelium-derived nitric-oxide in coronary vasodilation induced by pacing tachycardia in humans. Circ Res. 1996;79:331–335. doi: 10.1161/01.res.79.2.331. [DOI] [PubMed] [Google Scholar]
- Egashira K, Suzuki S, Hirooka Y, Kai H, Sugimachi M, Imaizumi T, Takeshita A. Impaired endothelium-dependent vasodilation in large epicardial and resistance arteries in patients with essential hypertension: different responses to acetylcholine and substance P. Hypertension. 1995;25:201–206. doi: 10.1161/01.hyp.25.2.201. [DOI] [PubMed] [Google Scholar]
- El-Sayed MS, Sale C, Jones PG, Chester M. Blood hemostasis in exercise and training. Med Sci Sports Exerc. 2000;32:918–925. doi: 10.1097/00005768-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Endo T, Imaizumi T, Tagawa T, Shiramoto M, Ando S, Takeshita A. Role of nitric oxide in exercise-induced vasodilation of the forearm. Circulation. 1994;90:2886–2890. doi: 10.1161/01.cir.90.6.2886. [DOI] [PubMed] [Google Scholar]
- Ennezat PV, Malendowicz SL, Testa M, Colombo PC, Cohen-Solal A, Evans T, LeJemtel TH. Physical training in patients with chronic heart failure enhances the expression of genes encoding antioxidative enzymes. J Am Coll Cardiol. 2001;38:194–198. doi: 10.1016/s0735-1097(01)01321-3. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacologic versus flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol. 2001;88:1067–1069. doi: 10.1016/s0002-9149(01)01997-x. [DOI] [PubMed] [Google Scholar]
- Farrell SW, Braun L, Barlow CE, Cheng YJ, Blair SN. The relation of body mass index, cardiorespiratory fitness, and all-cause mortality in women. Obes Res. 2002;10:417–423. doi: 10.1038/oby.2002.58. [DOI] [PubMed] [Google Scholar]
- Fish RD, Nabel EG, Selwyn AP. Responses of coronary arteries of cardiac transplant patients to acetylcholine. J Clin Invest. 1988;80:1808–1811. doi: 10.1172/JCI113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WD, Stephens GM, Schmid PG. Effects of intense exercise training on endothelium-dependent exercise induced vasodilatation. Clin Physiol. 1998;18:521–528. doi: 10.1046/j.1365-2281.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- Fransden U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with, NG-nitro-L-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsjager-Mayrl G, Pleiner J, Weisinger GF, Seider AE, Quittan M, Nuhr MJ, Francesconi C, Seit H-P, Francesconi M, Schmetterer L, Wolszt M. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care. 2002;25:1795–1801. doi: 10.2337/diacare.25.10.1795. [DOI] [PubMed] [Google Scholar]
- Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz P, Vita JA. Testing endothelial vasomotor function. Nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Roddy M-A, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- Gibbons G, Dzau V. The emerging concept of vascular remodelling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- Gielen S, Erbs S, Linke A, Mobius-Winkler S, Schuler G, Hambrecht R. Home-based versus hospital-based exercise programs in patients with coronary artery disease: effects on coronary vasomotion. Am Heart J. 2003;145:e3–6. doi: 10.1067/mhj.2003.58a. [DOI] [PubMed] [Google Scholar]
- Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR, Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation. 1994;90:2853–2858. doi: 10.1161/01.cir.90.6.2853. [DOI] [PubMed] [Google Scholar]
- Gokce N, Keaney JF, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002a;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- Gokce N, Vita J, Bader D, Sherman D, Hunter L, Holbrook M, O'Malley C, Keaney J, Balady G. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. J Am Coll Cardiol. 2002b;90:124–127. doi: 10.1016/s0002-9149(02)02433-5. [DOI] [PubMed] [Google Scholar]
- Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans. Role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530–535. doi: 10.1161/01.CIR.0000080893.55729.28. [DOI] [PubMed] [Google Scholar]
- Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G, Walsh JH. Incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol. doi: 10.1113/jphysiol.2004.075929. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Cable NT, Fox C, Rankin JM, Taylor RR. Modification of forearm resistance vessels by exercise training in young men. J Appl Physiol. 1994;77:1829–1833. doi: 10.1152/jappl.1994.77.4.1829. [DOI] [PubMed] [Google Scholar]
- Green D, Cheetham C, Henderson C, Weerasooriya R, O'Driscoll G. Effect of cardiac pacing on forearm vascular function. Am J Physiol. 2002a;283:H1354–H1360. doi: 10.1152/ajpheart.00050.2002. [DOI] [PubMed] [Google Scholar]
- Green D, Cheetham C, Mavaddat L, Watts K, Best M, Taylor R, O'Driscoll G. Effect of lower limb exercise on forearm vascular function: Contribution of nitric oxide. Am J Physiol. 2002b;283:H899–H907. doi: 10.1152/ajpheart.00049.2002. [DOI] [PubMed] [Google Scholar]
- Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol. 2002c;93:361–368. doi: 10.1152/japplphysiol.00051.2002. [DOI] [PubMed] [Google Scholar]
- Green DJ, Fowler DT, O'Driscoll JG, Blanksby BA, Taylor RR. Endothelium-derived nitric oxide activity in forearm vessels of tennis players. J Appl Physiol. 1996a;81:943–948. doi: 10.1152/jappl.1996.81.2.943. [DOI] [PubMed] [Google Scholar]
- Green DJ, O'Driscoll JG, Blanksby BA, Taylor RR. Control of skeletal muscle blood flow during dynamic exercise. Sports Med. 1996b;21:119–146. doi: 10.2165/00007256-199621020-00004. [DOI] [PubMed] [Google Scholar]
- Green DJ, O'Driscoll JG, Blanksby BA, Taylor RR. Effect of casting on forearm resistance vessels in young men. Med Sci Sports Exerc. 1997;29:1325–1331. doi: 10.1097/00005768-199710000-00008. [DOI] [PubMed] [Google Scholar]
- Green DJ, Walsh JH, Maiorana A, Best M, Taylor RR, O'Driscoll JG. Exercise-induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: a pooled analysis of diverse patient populations. Am J Physiol. 2003;285:H2679–H2687. doi: 10.1152/ajpheart.00519.2003. [DOI] [PubMed] [Google Scholar]
- Green DJ, Walsh JH, Maiorana AJ, Burke V, Taylor RR, O'Driscoll G. Comparison of resistance and conduit vessel nitric oxide-mediated vascular function in vivo: Effects of exercise training. J Appl Physiol. 2004;97:749–755. doi: 10.1152/japplphysiol.00109.2004. [DOI] [PubMed] [Google Scholar]
- Griffin K, Woodman C, Price E, Laughlin M, JLP Endothelium-mediated relaxation of porcine collateral-dependent arterioles is improved by exercise training. Circulation. 2001;103:2839–2844. doi: 10.1161/hc3601.094274. [DOI] [PubMed] [Google Scholar]
- Guyton JR, Hartley CJ. Flow restriction of one carotid artery in juvenile rats inhibits growth of arterial diameter. Am J Physiol. 1985;248:H540–H546. doi: 10.1152/ajpheart.1985.248.4.H540. [DOI] [PubMed] [Google Scholar]
- Hakin AA, Curb JD, Petrovich H, Rodriguez BL, Yano K, Ross GW, White LR, RDA Effects of walking on coronary heart disease in elderly men: the Honolulu Heart Program. Circulation. 1999;100:9–13. doi: 10.1161/01.cir.100.1.9. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Adams V, Erbs S, Linke a Krankel N, Shu Y, Baither Y, Geilen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Gielen S, Linke A, Fiehn E, Jiangtao Y, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure. JAMA. 2000a;283:3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Hilbrich L, Erbs S, Gielen S, Fiehn E, Schoene N, Schuler G. Correction of endothelial dysfunction in chronic heart failure: additional effects of exercise training and oral L-arginine supplementation. J Am Coll Cardiol. 2000b;35:706–713. doi: 10.1016/s0735-1097(99)00602-6. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Wolf A, Geilen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000c;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- Hickner R, Fisher J, Ehsani A, Kohrt W. Role of nitric oxide in skeletal muscle blood flow at rest and during dynamic exercise in humans. Am J Physiol. 1997;273:H405–H410. doi: 10.1152/ajpheart.1997.273.1.H405. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Oshima T, Ozono R, Matsuura H, Kajiyama G. Aging and severity of hypertension attenuate endothelium-dependent renal vascular relaxation in humans. Hypertension. 1997;30:252–258. doi: 10.1161/01.hyp.30.2.252. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects. Circulation. 1999a;100:1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Sasaki N, Nakaagawa K, Ueda T, Yoshimizu A, Kurisu S, Matsuura H, Kajiyama G, Oshima T. Daily aerobic exercise improves reactive hyperaemia in patients with essential hypertension. Hypertension. 1999b;33:591–597. doi: 10.1161/01.hyp.33.1.591. [DOI] [PubMed] [Google Scholar]
- Hijmering ML, Stroes ESGIO, Iijhoek J, Hutten BA, Blankestijn PJ, Rebelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- Hildick-Smith DJ, Johnson PJ, Wisbey CR, Winter EM, Shapiro LM. Coronary flow reserve is supranormal in endurance athletes: an adenosine transthoracici echocardiographic study. Heart. 2000;84:383–390. doi: 10.1136/heart.84.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg S, Klein L, Parrillo J, Scherer M, Burns D, Tamburro P, Oberoi M, Johnson MR, Costanzo MR. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091–3096. doi: 10.1161/hc5001.100796. [DOI] [PubMed] [Google Scholar]
- Holloszy J, Schultz J, Kusnierkiewicz J, Hagberg JM, Ehsani AA. Effects of exercise on glucose tolerance and insulin resistance. Acta Med Scand Supplement. 1986;711:55–65. doi: 10.1111/j.0954-6820.1986.tb08932.x. [DOI] [PubMed] [Google Scholar]
- Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93:210–214. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- Huonker M, Schmid A, Schmid-Truckass A, Grathwohl D, Keul J. Size and blood flow of central and peripheral arteries in highly trained able-bodied and disabled athletes. J Appl Physiol. 2003;95:685–691. doi: 10.1152/japplphysiol.00710.2001. [DOI] [PubMed] [Google Scholar]
- Hutten BA, Blankestijn PJ, Hutcheson IR, Griffith TM. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. Am J Physiol. 1991;261:H257–H262. doi: 10.1152/ajpheart.1991.261.1.H257. [DOI] [PubMed] [Google Scholar]
- Joannides R, Haefeli WE, Linder L, Richard V, Bakkali E, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- Jodoin I, Bussieres LM, Tardif JC, Juneau M. Effect of a short-term primary prevention program on endothelium-dependent vasodilation in adults at risk for atherosclerosis. Can J Cardiol. 1999;15:83–89. [PubMed] [Google Scholar]
- Johnson LR, Laughlin MH. Chronic exercise training does not alter pulmonary vasorelaxation in normal pigs. J Appl Physiol. 2000;88:2008–2016. doi: 10.1152/jappl.2000.88.6.2008. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Rush JWE, Turk JR, Price EM, Laughlin MH. Short-term exercise training increases ACh-induced relaxation and eNOS protein in porcine pulmonary arteries. J Appl Physiol. 2001;90:1102–1110. doi: 10.1152/jappl.2001.90.3.1102. [DOI] [PubMed] [Google Scholar]
- Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-Based Rehabilitation For Coronary Heart Disease. Chichester, UK: Cochrane Library, John Wiley and Sons Ltd; 2001. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol. 1997;83:1785–1796. doi: 10.1152/jappl.1997.83.6.1785. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM, Shephard JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in humans limbs. J Appl Physiol. 2001;91:2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980;239:H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- Karusa C, Socul H, Altan VM. Effects of non-insulin dependent diabetes mellitus on the reactivity of human internal mammary artery and human saphenous vein. Life Sci. 1995;57:103–112. doi: 10.1016/0024-3205(95)00251-z. [DOI] [PubMed] [Google Scholar]
- Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvoa M, Solomon S, Nikolic SD, Forman R, LeJemtel TH. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol. 1992;19:918–925. doi: 10.1016/0735-1097(92)90271-n. [DOI] [PubMed] [Google Scholar]
- Katz SD, Krum H, Kahn T, Knecht M. Exercise-induced vasodilation in forearm circulation of normal subjects and patients with congestive heart failure: Role of endothlium-derived nitric oxide. J Am Coll Cardiol. 1996;28:585–590. doi: 10.1016/0735-1097(96)00204-5. [DOI] [PubMed] [Google Scholar]
- Katz SD, Schwarz M, Yuen J, LeJemtel TH. Impaired acetylcholine-mediated vasodilation in patients with congestive heart failure. Circulation. 1993;88:55–61. doi: 10.1161/01.cir.88.1.55. [DOI] [PubMed] [Google Scholar]
- Katz SD, Yuen J, Bijou R, Lejemtel TH. Training improves endothelium-dependent vasodilation in resistance vessels of patients with heart failure. J Appl Physiol. 1997;82:1488–1492. doi: 10.1152/jappl.1997.82.5.1488. [DOI] [PubMed] [Google Scholar]
- Kelley G. Aerobic exercise and resting blood pressure among women: a meta-analysis of randomised controlled trials. Prev Med. 1999;28:264–275. doi: 10.1006/pmed.1998.0417. [DOI] [PubMed] [Google Scholar]
- Kingwell B, Arnold P, Jennings G, Dart A. Spontaneous running increases aortic compliance in Wistar-Kyoto rats. Cardiovasc Res. 1997a;35:132–137. doi: 10.1016/s0008-6363(97)00079-5. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Sherrard B, Jennings GL, Dart AM. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol. 1997b;272:H1070–H1077. doi: 10.1152/ajpheart.1997.272.3.H1070. [DOI] [PubMed] [Google Scholar]
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Koller A, Huang A, Sun D, Kaley G. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles. Circ Res. 1995;76:544–550. doi: 10.1161/01.res.76.4.544. [DOI] [PubMed] [Google Scholar]
- Koller A, Kaley G. Endothelial regulation of wall shear stress and blood flow in skeletal muscle microcirculation. Am J Physiol. 1991;260:H862–H868. doi: 10.1152/ajpheart.1991.260.3.H862. [DOI] [PubMed] [Google Scholar]
- Kraemer MD, Kubo SH, Rector TS, Brunsvold N, Bank AJ. Pulmonary and peripheral vascular factors are important determinants of peak exercise oxygen uptake in patients with heart failure. J Am Coll Cardiol. 1993;21:641–648. doi: 10.1016/0735-1097(93)90096-j. [DOI] [PubMed] [Google Scholar]
- Kramsch DM, Aspen AJ, Abramowitz BM, Kreimendahl T, Hood WB. Reduction of coronary atherosclerosis by moderate conditioning exercise in monkeys on an atherogenic diet. N Engl J Med. 1981;305:1483–1489. doi: 10.1056/NEJM198112173052501. [DOI] [PubMed] [Google Scholar]
- Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- Kuvin J, Patel R, Sliney K, Pandain NG, Rand WM, Udelson JE, Karas RH. Peripheral vascular endothelial function testing as a non-invasive assessment of endothelial function. J Am Coll Cardiol. 2001;38:1843–1849. doi: 10.1016/s0735-1097(01)01657-6. [DOI] [PubMed] [Google Scholar]
- Langille BL, Bendeck MP, Keeley FW. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol. 1989;256:H931–H939. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Nature. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Lash J, Bohlen H. Functional adaptations of rat skeletal muscle arterioles to aerobic exercise training. J Appl Physiol. 1992;72:2052–2062. doi: 10.1152/jappl.1992.72.6.2052. [DOI] [PubMed] [Google Scholar]
- Laughlin M, Overholser K, Bhatte M. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol. 1989;67:1140–1149. doi: 10.1152/jappl.1989.67.3.1140. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Rubin LJ, Rush JW, Price EM, Schrage WG, Woodman CR. Short-term training enhances endothelium-dependent dilation of coronary arteries, not arterioles. J Appl Physiol. 2003a;94:234–244. doi: 10.1152/japplphysiol.00246.2002. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Turk JR, Schrage WG, Woodman CR, Price EM. Influence of coronary artery diameter on eNOS protein content. Am J Physiol. 2003b;284:H1307–H1312. doi: 10.1152/ajpheart.00792.2002. [DOI] [PubMed] [Google Scholar]
- Lavrencic A, Salobir BG, Keber I. Physical training improves flow-mediated dilation in patients with polymetabolic syndrome. Arterioscler Thromb Vasc Biol. 2000;19:2782–2787. doi: 10.1161/01.atv.20.2.551. [DOI] [PubMed] [Google Scholar]
- Lee I, Sesso H, Paffenbarger RS. Physical activity and and coronary heart disease risk in men; does the duration of exercise episodes predict risk. Circulation. 2000;102:981–986. doi: 10.1161/01.cir.102.9.981. [DOI] [PubMed] [Google Scholar]
- Leon AS, Bloor CM. Effects of exercise and its cessation on the heart and its blood supply. J Appl Physiol. 1968;24:485–490. doi: 10.1152/jappl.1968.24.4.485. [DOI] [PubMed] [Google Scholar]
- Lewis TV, Dart AM, Chin-Dusting JPF, Kingwell BA. Exercise training increases basal nitric oxide production from the forearm in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 1999;19:2782–2787. doi: 10.1161/01.atv.19.11.2782. [DOI] [PubMed] [Google Scholar]
- Lind L, Hall J, Larson A, Annuk M, Fellstrom B, Lithell H. Evaluation of endothelium-dependent vasodilation in the human forearm peripheral circulation. Clin Physiol. 2000;20:440–448. doi: 10.1046/j.1365-2281.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Linke A, Schoene N, Gielen S, Hofer J, Erbs S, Schuler G, Hambrecht R. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol. 2001;37:392–397. doi: 10.1016/s0735-1097(00)01108-6. [DOI] [PubMed] [Google Scholar]
- Lloyd PG, Yang HT, Terjung RL. Arteriogenesis and angiogenesis in rat ischemic hindlimbs: role of nitric oxide. Am J Physiol. 2001;281:H2528–H2538. doi: 10.1152/ajpheart.2001.281.6.H2528. [DOI] [PubMed] [Google Scholar]
- Logason K, Barlin T, Jonsson ML, Bostrom A, Hardemark HG, Karacagil S. The importance of Doppler angle of insonation on differentiation between 50 and 69% and 70–99% carotid artery stenosis. Eur J Of Vasc Endovasc Surg. 2001;21:311–313. doi: 10.1053/ejvs.2001.1331. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001a;38:860–866. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O'Driscoll G, Dembo L, Cheetham C, Goodman C, Taylor R, Green D. Effect of aerobic and resistance exercise training on vascular function in heart failure. Am J Physiol. 2000;279:H1999–H2005. doi: 10.1152/ajpheart.2000.279.4.H1999. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O'Driscoll G, Dembo L, Goodman C, Taylor R, Green D. Exercise training, vascular function, and functional capacity in middle-aged subjects. Med Sci Sports Exerc. 2001b;33:2022–2028. doi: 10.1097/00005768-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O'Driscoll GJ, Taylor RR, Green DJ. Exercise and the nitric oxide vasodilator system. Sports Med. 2003;33:1013–1015. doi: 10.2165/00007256-200333140-00001. [DOI] [PubMed] [Google Scholar]
- Malfattoo G, Facchini M, Sala L, Branzi G, Bragato R, Leonetti G. Effects of cardiac rehabilitation and beta-blocker therapy on heart-rate variability after first acute myocardial infaction. Am J Cardiol. 1998;81:834–840. doi: 10.1016/s0002-9149(98)00021-6. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- Mann GV, Spoerry A, Gray M, Jarashow D. Atherosclerosis in the Masi. Am J Epidemiol. 1972;95:26–37. doi: 10.1093/oxfordjournals.aje.a121365. [DOI] [PubMed] [Google Scholar]
- Manson JE, Greenlan P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Sisovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- Maxwell AJ, Schauble E, Berstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation. 1998;98:369–374. doi: 10.1161/01.cir.98.4.369. [DOI] [PubMed] [Google Scholar]
- McAllister RM, Kimani JK, Webster JL, Parker JL, Laughlin MH. Effects of exercise training on peripheral and visceral arteries in swine. J Appl Physiol. 1996;80:216–215. doi: 10.1152/jappl.1996.80.1.216. [DOI] [PubMed] [Google Scholar]
- McAllister RM, Laughlin MH. Short-term exercise training alters responses of porcine femoral and brachial arteries. J Appl Physiol. 1997;82:1438–1444. doi: 10.1152/jappl.1997.82.5.1438. [DOI] [PubMed] [Google Scholar]
- McLenachan JM, Vita J, Fish DR, Treasure CB, Cox DA, Ganz P, Selwyn AP. Early evidence of endothelial vasodilator dysfunction at coronary branch points. Circulation. 1990;82:1169–1173. doi: 10.1161/01.cir.82.4.1169. [DOI] [PubMed] [Google Scholar]
- McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- Minamino T, Kitakaze M, Matsumura Y, Nishida K, Kato Y, Hasimura K, Matsu-ura Y, Funaya H, Sato H, Kuzuya T, Hori M. Impact of coronary risk factors on contribution of nitric oxide and adenosine to metabolic coronary vasodilation in humans. J Am Coll Cardiol. 1998;31:1274–1279. doi: 10.1016/s0735-1097(98)00095-3. [DOI] [PubMed] [Google Scholar]
- Miyachi M, Iemitsu M, Okutsu M, Onodera S. Effects of endurance training on the size and blood flow of the arterial conductance vessels in humans. Acta Physiol Scand. 1998;163:13–16. doi: 10.1046/j.1365-201x.1998.0337f.x. [DOI] [PubMed] [Google Scholar]
- Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S. Effects of one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol. 2001;90:2439–2444. doi: 10.1152/jappl.2001.90.6.2439. [DOI] [PubMed] [Google Scholar]
- Modena M, Bonetti L, Coppi F, Buris F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo. Circ Res. 2001a;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo. Circ Res. 2001b;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- Muller JD, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation. 1994;89:2308–2314. doi: 10.1161/01.cir.89.5.2308. [DOI] [PubMed] [Google Scholar]
- Myers J, Gullestad L, Vagelos R, Do D, Bellin D, Ross H, Fowler MB. Clinical, hemodynamic, and cardiopulmonary exercise test determinants of survival in patients referred for evaluation of heart failure. Ann Intern Med. 1998;129:286–293. doi: 10.7326/0003-4819-129-4-199808150-00004. [DOI] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Yoshida H, Arakawa N, Mizunuma Y, Makita S, Hiramori K. Endothelium-dependent vasodilation is not selectively impaired in patients with chronic heart failure secondary to valvular heart disease and congenital heart disease. Eur Heart J. 1996;17:1875–1881. doi: 10.1093/oxfordjournals.eurheartj.a014806. [DOI] [PubMed] [Google Scholar]
- Neibauer J, Cooke JP. Cardiovascular effects of exercise: Role of endothelial shear stress. J Am Coll Cardiol. 1996;28:1652–1660. doi: 10.1016/S0735-1097(96)00393-2. [DOI] [PubMed] [Google Scholar]
- Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- Neunteufl T, Katzenschlager R, Hassan A, Klaar U, Schwarzacher S, Glogar D, Bauer P, Weidinger F. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129:111–118. doi: 10.1016/s0021-9150(96)06018-2. [DOI] [PubMed] [Google Scholar]
- Newby DE, Boon NA, Webb DJ. Comparison of forearm vasodilatation to substance P and acetylcholine: contribution of nitric oxide. Clin Sci. 1997;92:133–138. doi: 10.1042/cs0920133. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Maxwell A, Lin P, Tsao P, Kosek J, Bernstein D, Cooke J. Impaired aerobic capacity in hypercholesterolemic mice: partial reversal by exercise training. Am J Physiol. 1999;276:H1346–H1354. doi: 10.1152/ajpheart.1999.276.4.H1346. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Ogawa S. Importance of nitric oxide in the coronary artery at rest and during pacing in humans. J Am Coll Cardiol. 1997;29:85–92. doi: 10.1016/s0735-1097(96)00429-9. [DOI] [PubMed] [Google Scholar]
- O'Driscoll JG, Green DJ, Maiorana A, Stanton K, Colreavy F, Taylor RR. Improvement in endothelial function by ACE inhibition in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1999;33:1506–1511. doi: 10.1016/s0735-1097(99)00065-0. [DOI] [PubMed] [Google Scholar]
- O'Driscoll G, Green D, Rankin J, Stanton K, Taylor R. Improvement in endothelial function by angiotensin converting enzyme inhibition in insulin-dependent diabetes mellitus. J Clin Invest. 1997a;100:678–684. doi: 10.1172/JCI119580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll G, Green D, Taylor R. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997b;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltman C, Parker J, Adams H, Laughlin M. Effects of exercise training on vasomotor reactivity of porcine coronary arteries. Am J Physiol. 1992;263:H372–H382. doi: 10.1152/ajpheart.1992.263.2.H372. [DOI] [PubMed] [Google Scholar]
- Palmer RMJ, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988;153:1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Panza J, Quyyumi A, Brush J, Epstein S. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- Pellicia A, Spataro A, Granata J, Biffi A, Caselli G, Alabiso A. Coronary arteries in physiological hypertrophy: echocardiographic evidence of increased proximal size in elite athletes. Int J Sports Med. 1990;11:120–126. doi: 10.1055/s-2007-1024775. [DOI] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioloi PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001a;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001b;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Powers S, Ji L, Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc. 1999;31:987–997. doi: 10.1097/00005768-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Prior BM, Lloyd PG, Yang HT, Terjung RL. Exercise-induced vascular remodelling. Ex Sports Sci Rev. 2003;31:26–33. doi: 10.1097/00003677-200301000-00006. [DOI] [PubMed] [Google Scholar]
- Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004;97:499–508. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO. Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995a;92:320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- Quyyumi AA, Dakak N, Andrews NP, Hussain S, Arora S, Gilligan DM, Panza J, Cannon RC. Nitric oxide activity in the human coronary circulation. J Clin Invest. 1995b;95:1747–1755. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Muscle blood flow at the onset of dynamic exercise in humans. Am J Physiol. 1998;274:H314–H322. doi: 10.1152/ajpheart.1998.274.1.H314. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Rodbard S. Vascular caliber. Cardiology. 1975;60:4–49. doi: 10.1159/000169701. [DOI] [PubMed] [Google Scholar]
- Rose G, Prineas RJ, Mitchell JRA. Myocardial infarction and the intrinsic calibre of coronary arteries. Br Heart J. 1967;29:548–552. doi: 10.1136/hrt.29.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Rudic R, Sheseley E, Maeda N, Smithies O, Segal S, Sessa W. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S, Kazushi M, Niwa Y, Ohnaka M, Nakaya Y, Mizuno A, Kuwajima M, Shima K. Effect of exercise training and food restriction on endothelium-dependent relaxation in the Otsuka Long-Evans Tokushima Fatty Rat, a model of spontaneous NIDDM. Diabetes. 1998;47:82–86. doi: 10.2337/diab.47.1.82. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Pryor SL, Bertocci LA, Victor RG. Arterial baroreflex buffering of sympathetic activation during exercise-induced elevations in arterial pressure. J Clin Invest. 1990;86:1855–1861. doi: 10.1172/JCI114916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Pleiner J, Bayerle-Eder M, Wiesinger GF, Rodler S, Quittan M, Mayer G, Wolzt M. Regular physical exercise improves endothelial function in heart transplant recipients. Clin Transplant. 2002;16:137–143. doi: 10.1034/j.1399-0012.2002.1o100.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Trucksass A, Schmid A, Brunner C, Scherer N, Zach G, Keul J, Huonker M. Arterial properties of the carotid and femoral artery in endurance-trained and paraplegic subjects. J Appl Physiol. 2000;89:1959–1963. doi: 10.1152/jappl.2000.89.5.1956. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciacqua A, Candigliota M, Ceravolo R, Scozzafava A, Sinopoli F, Corsonello A, Sesti G, Perticone F. Weight loss in combination with physical activity improves endothelial dysfunction in human obesity. Diabetes Care. 2003;26:1673–1678. doi: 10.2337/diacare.26.6.1673. [DOI] [PubMed] [Google Scholar]
- Segal SS. Communication among endothelial and smooth muscle cells coordinates blood flow control during exercise. News Physiol Sci. 1992;7:152–156. [Google Scholar]
- Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide synthase production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- Sesso H, Paffenbarger R, Lee I. Physical activity and coronary heart disease in men: The Harvard Alumni Health Study. Circulation. 2000;102:975–980. doi: 10.1161/01.cir.102.9.975. [DOI] [PubMed] [Google Scholar]
- Shepherd JT. Circulation to skeletal muscle. In: Shepherd JT, Abboud FM, Geiger SR, editors. Handbook of Physiology, section 2, The Cardiovascular System, Peripheral Circulation and Organ Blood Flow. part 1. III. Bethesda, MDUSA: American Physiological Society; 1983. pp. 319–370. [Google Scholar]
- Sheriff DD, Nelson CD, Sundermann RK. Does autonomic blockade reveal a potent contribution of nitric oxide to locomotion-induced vasodilation. Am J Physiol. 2000;279:H726–H732. doi: 10.1152/ajpheart.2000.279.2.H726. [DOI] [PubMed] [Google Scholar]
- Shiode N, Morishima N, Nakayama K, Yamagata T, Matsuura H, Kajiyama G. Flow-mediated vasodilation of human epicardial coronary arteries: effect of inhibition of nitric oxide synthesis. J Am Coll Cardiol. 1996;27:304–310. doi: 10.1016/0735-1097(95)00465-3. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Musch TI, Minotti JR, Zelis R. Enhanced maximal metabolic vasodilation in the dominant forearms of tennis players. J Appl Physiol. 1986;61:673–678. doi: 10.1152/jappl.1986.61.2.673. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Shenberger J, Wilson J, McLaughlin D, Musch T, Zelis R. A 30-day forearm work protocol increases maximal forearm blood flow. J Appl Physiol. 1987;62:1063–1067. doi: 10.1152/jappl.1987.62.3.1063. [DOI] [PubMed] [Google Scholar]
- Smith J, Dykes R, Douglas J, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischaemic heart disease. JAMA. 1999;281:1722–1727. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest. 1994;93:50–55. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Huang A, Koller A, Kaley G. Short-term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol. 1994;76:2241–2247. doi: 10.1152/jappl.1994.76.5.2241. [DOI] [PubMed] [Google Scholar]
- Sun D, Huang A, Koller A, Kaley G. Adaptation of flow-induced dilation of arterioles to daily exercise. Microvasc Res. 1998;56:54–61. doi: 10.1006/mvre.1998.2083. [DOI] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousoulis D, Tentolouris C, Crake T, Toutouzas P, Davies G. Basal and flow-mediated nitric oxide production by atheromatous coronary arteries. J Am Coll Cardiol. 1997;29:1256–1262. doi: 10.1016/s0735-1097(97)00046-6. [DOI] [PubMed] [Google Scholar]
- Tran Z, Weltman A. Differential effects of exercise on serum lipid and lipoprotein levels seen with changes in body weight: a meta-analysis. JAMA. 1985;254:919–924. [PubMed] [Google Scholar]
- Treasure C, Manoukian S, Klein J, Vita J, Nabel E, Renwick G, Selwyn A, Alexander R, Ganz P. Epicardial coronary artery responses to acetylcholine are impaired in hypertensive patients. Circ Res. 1992;71:776–781. doi: 10.1161/01.res.71.4.776. [DOI] [PubMed] [Google Scholar]
- Treasure CB, Vita JA, Cox DA. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. 1990;81:772–779. doi: 10.1161/01.cir.81.3.772. [DOI] [PubMed] [Google Scholar]
- Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996;16:1256–1262. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol. 2004;97:404–415. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- Tune JD, Richmond KN, Gorman MW, Feigl EO. Control of coronary blood flow during exercise. Exp Biol Med. 2002;227:238–250. doi: 10.1177/153537020222700404. [DOI] [PubMed] [Google Scholar]
- Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences artery remodelling, wall dimension, cell density and eNOS expression. Am J Physiol. 2001;281:H1380–H1389. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Vallance P, Patton S, Bhagat K, MacAllister R, Radomski M, Moncada S, Malinski T. Direct measurement of nitric oxide in human beings. Lancet. 1995;346:153–154. doi: 10.1016/s0140-6736(95)91211-8. [DOI] [PubMed] [Google Scholar]
- Vita J, Treasure C, Nabel E, McLenachan J, Fish R, Yeung A, Vekstein V, Selwyn A, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- Walsh JH, Best M, Maiorana AJ, Taylor RR, O'Driscoll GJ, Green DJ. Exercise improves conduit vessel endothelial function in CAD patients. J Appl Physiol. 2003a;285:20–25. doi: 10.1152/japplphysiol.00012.2003. [DOI] [PubMed] [Google Scholar]
- Walsh JH, Yong G, Cheetham C, Watts GF, O'Driscoll GJ, Taylor RR, Green DJ. Effect of exercise training on conduit and resistance vessel function in medicated and unmedicated hypercholesterolaemic patients. Eur Heart J. 2003b;24:1681–1689. doi: 10.1016/s0195-668x(03)00384-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Wolin MS, Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res. 1993;73:829–838. doi: 10.1161/01.res.73.5.829. [DOI] [PubMed] [Google Scholar]
- Watts K, Beye P, Siafarikas A, Davis E, Jones T, O'Driscoll G, Green DJ. Exercise training normalises vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol. 2004a;43:1823–1827. doi: 10.1016/j.jacc.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Watts K, Beye P, Siafarikas A, O'Driscoll G, Jones TW, Davis EA, Green DJ. Exercise training in obese children. J Pediatrics. 2004b;144:620–625. doi: 10.1016/j.jpeds.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Watts G, O'Brien S, Silvester W, Millar J. Impaired endothelium-dependent and independent dilation of forearm resistance vessels in men with diet-treated non-insulin-dependent diabetes: role of dyslipidemia. Clin Sci. 1996;91:567–573. doi: 10.1042/cs0910567. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Gocke N, Keaney JF, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Williams P. High-density lipoprotein cholesterol and other risk factors for coronary heart disease in female runners. N Eng J Med. 1996;334:1298–1303. doi: 10.1056/NEJM199605163342004. [DOI] [PubMed] [Google Scholar]
- Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Kapoor S. Contribution of endothelium-derived relaxing factor to exercise-induced vasodilation in humans. J Appl Physiol. 1993;75:2740–2744. doi: 10.1152/jappl.1993.75.6.2740. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Martin JL, Schwartz D, Ferraro N. Exercise intolerance in patients with chronic heart failure: Role of impaired nutritive flow to skeletal muscle. Circulation. 1984;69:1079–1087. doi: 10.1161/01.cir.69.6.1079. [DOI] [PubMed] [Google Scholar]
- Woo KS, Chook PYuCW, Sung RTY, Qiao M, Leung SSF, Lam CWK, Metreweli C, Celermajer DS. Effects of diet and exercise on obesity-related vascular dysfunction in children. J Am Coll Cardiol. 2004;109:1981–1986. doi: 10.1161/01.CIR.0000126599.47470.BE. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Muller JM, Laughlin MH. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am J Physiol. 1997;273:H1–H5. doi: 10.1152/ajpheart.1997.273.6.H2575. [DOI] [PubMed] [Google Scholar]
- Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Green D. Improved analysis of brachial artery ultrasound images using a novel edge-detection software system. J Appl Physiol. 2001;91:929–937. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- Wyatt HL, Mitchell J. Influences of physical conditioning and deconditioning on coronary vasculature of dogs. J Appl Physiol. 1978;45:619–625. doi: 10.1152/jappl.1978.45.4.619. [DOI] [PubMed] [Google Scholar]
- Yasue H, Matsuyama K, Matsuyama K, Okumura K, Morikami Y, Ogawa H. Responses of angiographically normal human coronary arteries to intracoronary injection of acetylcholine by age and segment. Possible role of early coronary atherosclerosis. Circulation. 1990;81:482–490. doi: 10.1161/01.cir.81.2.482. [DOI] [PubMed] [Google Scholar]
- Zamir M. Shear stress forces and blood vessel radii in the cardiovascular system. J General Physiol. 1977;69:449–461. doi: 10.1085/jgp.69.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarins CK, Zatina MA, Giddens DP, Nu DN, Glagov S. Shear stress regulation of artery lumen diameter in experimental atherosclerosis. J Vasc Surg. 1987;5:413–420. [PubMed] [Google Scholar]
- Zeppilli P, Vanniculli R, Dello Russso A, Palmieri V, Cameli S, Corsetti S, Pietrangeli L. Echocardiographic size of conductance vessels in athletes and sedentary people. Int J Sports Med. 1995;16:38–44. doi: 10.1055/s-2007-972961. [DOI] [PubMed] [Google Scholar]