Abstract
The present study was conducted to evaluate the role of conventional protein kinase C (PKC) in calcium-evoked insulin secretion. In rat β cells transfected with green fluorescent protein-tagged PKC-α (PKC-α–EGFP), a depolarizing concentration of potassium induced transient elevation of cytoplasmic free calcium ([Ca2+]c), which was accompanied by transient translocation of PKC-α–EGFP from the cytosol to the plasma membrane. Potassium also induced transient translocation of PKC-θ–EGFP, the C1 domain of PKC-γ and PKC-ɛ–GFP. A high concentration of glucose induced repetitive elevation of [Ca2+]c and repetitive translocation of PKC-α–EGFP. Diazoxide completely blocked both elevation of [Ca2+]c and translocation of PKC-α–EGFP. We then studied the role of conventional PKC in calcium-evoked insulin secretion using rat islets. When islets were incubated for 10 min with high potassium, Gö-6976, an inhibitor of conventional PKC, and PKC-α pseudosubstrate fused to antennapedia peptide (Antp-PKC19–31) increased potassium induced secretion. Similarly, insulin release induced by high glucose for 10 min was enhanced by Gö-6976 and Antp-PKC19–31. However, when islets were stimulated for 60 min with high glucose, both Gö-6976 and Antp-PKC19–31 reduced glucose-induced insulin secretion. Similar results were obtained by transfection of dominant-negative PKC-α using adenovirus vector. Taken together, PKC-α is activated when cells are depolarized by a high concentration of potassium or glucose. Conventional PKC is inhibitory on depolarization-induced insulin secretion per se, but it also augments glucose-induced secretion.
Protein kinase C (PKC) is a family of related serine/threonine kinases (Nishizuka, 1992; Mellor & Parker, 1998). Ten isozymes have been identified and classified into three categories based on their structural differences in the regulatory domain: conventional PKC (PKC-α, PKC-βI, PKC-βII and PKC-γ), novel PKC (PKC-δ, PKC-ɛ, PKC-η and PKC-θ), and atypical PKC (PKC-ξ and PKC-λ). The C1 and C2 regions in the regulatory domain are responsible for diacylglycerol (DAG) and Ca2+ binding, respectively. DAG and Ca2+ activate the first category of PKCs that have both the C1 and C2 regions. The second category of the novel PKCs is also activated by DAG but in a Ca2+-independent manner because of the absence of the functional C2 region. The third category of the atypical PKCs can be activated by phosphoinositide-dependent kinase 1 in a Ca2+-independent manner (Nishizuka, 1992; Mellor & Parker, 1998). The C1 domain contains a Cys-rich motif, which is duplicated in most isozymes, and forms the DAG/phorbol ester binding site. This domain is immediately preceded by an autoinhibitory pseudosubstrate sequence. Individual isozymes have been implicated in many cellular responses including proliferation, apoptosis and secretion (Nishizuka, 1992; Mellor & Parker, 1998).
Multiple PKC isoforms are present in pancreatic β cells (Knutson & Hoenig, 1994; Tian et al. 1996; Yedovitzky et al. 1997), with the dominant classical Ca2+–DAG-sensitive isoform being PKC-α. Although the role of PKC in Ca2+-mobilizing, agonist-induced insulin release has been established, the possible role of PKC in nutrient-stimulated insulin secretion is still a matter of debate (Hii et al. 1987; Jones et al. 1991; Deeney et al. 1996; Harris et al. 1996; Yedovitzky et al. 1997; Zawalich & Zawalich, 2001; Carpenter et al. 2004). It is accepted that the function of PKC well correlates with its subcellular localization. In many tissues, activation of PKC is associated with translocation from the cytosol to the membrane-associated state. It was demonstrated that a stimulatory concentration of glucose promoted translocation of PKC-α from the cytosol to the plasma membrane in rat pancreatic islets (Deeney et al. 1996; Ganesan et al. 1990). These results suggest that PKC-α is activated by glucose. Nevertheless, the role of activated PKC is still controversial. For example, studies conducted on cells with down-regulated PKC activity suggested that PKC is not directly involved in insulin secretion promoted by glucose (Hii et al. 1987). However, it has been pointed out that ‘down-regulation of PKC’ is dependent on the type of isoform (Yaney et al. 2002), and ‘down-regulation’ also affects various cellular functions including the insulin content of the cells (Yaney et al. 2002). Therefore, the role of PKC, especially its isoform-specific role, should be considered carefully. In this regard, involvement of PKC in glucose-induced insulin secretion has been shown by a study using isoform-specific inhibitors of PKC translocation. Yedovitzky et al. (1997) reported that an inhibitor of PKC-α inhibited glucose-induced insulin secretion. Similarly, an inhibitor of PKC-ɛ inhibited glucose-induced insulin secretion from pancreatic islets (Yedovitzky et al. 1997). Their results clearly showed the isoform-specific role of PKC in glucose-induced insulin secretion but do not thoroughly explain the controversial data on the role of PKC in nutrient-stimulated insulin secretion (Hii et al. 1987; Jones et al. 1991; Persaud & Jones, 1995; Harris et al. 1996; Harris et al. 1999; Carpenter et al. 2004).
Recent advances in the use of green fluorescent protein (GFP) have allowed us to investigate the regulation of PKC activity in intact living cells by monitoring translocation of the GFP-tagged PKC (Oancea & Meyer, 1998; Almholt et al. 1999). Using this approach, we obtained a slightly different aspect of regulation of PKC in β cells: conventional PKC and novel PKC are activated by depolarization-evoked Ca2+ influx through voltage-dependent calcium channels (VDCCs) in insulinoma cells, INS-1 (Mogami et al. 2003). This raises an intriguing possibility that PKC in pancreatic β cells is activated by agents that depolarize the β cell membrane. Since β cells are excitable, many agents induce calcium influx in these cells. It is therefore crucial to determine whether or not these agents activate PKC as calcium-mobilizing agonists do, and if so, to determine the role of PKC in insulin secretion induced by these agents. The present study was conducted to investigate the role of calcium entry in the activation mechanism of PKC-α, a dominant conventional PKC isoform expressed in pancreatic β cells (Oancea & Meyer, 1998). The role of PKC-α in Ca2+-evoked insulin secretion was also evaluated.
Methods
Chemicals
Fura-2-AM was purchased from Sigma (St Louis, MO, USA). A PKC-α inhibitor peptide, antennapedia-PKC19–31 (Antp-PKC19–31) (RRMKW KKRFA RKGAL RQKNV), and a PKC-ɛ inhibitor peptide, antennapedia-PKC149–164 (Antp-PKC149–164) (RRMKW KKERM RPRKR QGAVR RRV), were synthesized by Takara Shuzo Co., Ltd (Tokyo, Japan). Both inhibitors are tandemly synthesized peptides comprising peptides derived from the third α-helix of the homeodomain of antennapedia (residues 52–58, known as penetratin) (Derossi et al. 1998; Fischer et al. 2000), and the PKC pseudosubstrate peptides (PKC-α residues 19–31 and PKC-ɛ residues 149–164) (Harris et al. 1999; Zoukhri et al. 1997). Antennapedia peptide (Antp), used as a control, was also synthesized by Takara Shuzo. PKC inhibitor Gö-6976 (12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole) was purchased from Calbiochem. Phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma. Polyclonal rabbit anti-MARCKS phosphoSer152/156 antibody was purchased from Chemicon International, Inc. (Temecula, CA, USA). Monoclonal mouse anti-PKC-α antibody cPKCα (H-7) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Polyclonal guinea pig anti-porcine insulin antibody was provided by Dr T. Matozaki (Gunma University, Maebashi, Japan). Alexa Fluor-labelled anti-mouse IgG and anti-guinea pig IgG antibodies were obtained from Molecular Probes (Eugene, OR, USA).
Plasmid construction
PKC-α–pEGFP, PKC-θ–pEGFP, pEGFP-N2 and pEGFP-N1 were obtained from Clontech Laboratory, Inc. (Palo Alto, CA, USA). MARCKS–GFP (Ohmori et al. 2000) and PKC-ɛ–GFP (Shirai et al. 1998) were kindly provided by Dr Naoaki Saito of Kobe University (Kobe, Japan). To obtain brighter fluorescence, the GFP in MARCKS–GFP was replaced with pEGFP-N2 as previously described (Mogami et al. 2003). A GFP-tagged C1 region of PKCγ (C1-GFP) was produced from a DNA clone of λCKRγ1, which was subcloned into an expression plasmid for mammalian cells, pTB701 (Ono et al. 1988), as previously described (Mogami et al. 2003). To obtain higher transfection efficiency and brighter fluorescence, the C1 was inserted into pEGFP-N1 vector. A cDNA fragment of PKCγ for the C1 region with an EcoRI site in the 5′ terminus and a BamHI in the 3′ terminus was produced by PCR using C1-GFP as a template. The sense and antisense primers used were 5′-ATCTTGAATTCGCCATGGTGAAGAGCCACAAGTTCACC-3′ and 5′-TCTTAGGATCCATGTCCACGCCGCAAAGGGAG-3′, respectively. The PCR product was verified by sequencing.
Preparation of rat pancreatic β cells
Male Wistar rats (200–250 g) were obtained from Imai Animal Company (Saitama, Japan). Rats were anaesthesized by using diethylether and pancreatic islets were isolated by digestion with collagenase (Wako Pure Chemical Industries, Tokyo, Japan) (Lacy & Kostianovsky, 1967). Islets were picked up by inspection under microscopy. The experimental protocols were approved by the Ethical Committee of the Institute for Molecular and Cellular Regulation, Gunma University. For measurement of PKC translocation, islets were dispersed into single cells by digestion with Dispase (Sanko Junyaku, Tokyo, Japan). Cells were plated onto glass coverslips coated with 1 mg ml−1 concanavalin A (type V; Sigma). To identify β cells, cells were stimulated with 11 mm glucose. Cells were considered to be β cells when cytoplasmic calcium was elevated in response to 11 mm glucose.
Cell culture and transfection
Cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 10 mm glucose, 10% fetal bovine serum, 2 mml-glutamine and 1 mm sodium pyruvate in a humidified incubator (95% air, 5% CO2) at 37°C. The concentration of glucose was lowered to 3 mm for 5–6 h before experiments. PKC-α–EGFP, PKC-θ–EGFP (Clontech), C1-EGFP, PKC-ɛ–GFP and MARCKS–EGFP were transfected into the cells by lipofection using TransIT™-LT1 (Mirus, Madison, WI, USA). In some experiments, EGFP-N2 vector was also transfected into the cells using the same method. The transfection efficiency for pEGFP-N2 vector was about 10%, while the transfection efficiency for PKC-α–EGFP, PKC-θ–EGFP, C1-EGFP, PKC-ɛ–GFP and MARCKS–EGFP was about 5%, 3%, 3%, 4% and 5%, respectively. Measurement of PKC translocation was performed 1 or 2 days after the transfection.
Transfection using adenoviral vector
Adenoviruses containing the dominant-negative mutant of PKC-α (dn-PKC-α) (Tsuru et al. 2002) were kindly provided by Dr Y. Oka of Tohoku University (Sendai, Japan). The cDNA encoding dn-PKC-α was point-mutated by substituting methionine for lysine in the ATP-binding site in its catalytic domain (Bandyopadhyay et al. 1999). Fresh isolated islets were incubated with RPMI-1640 containing the adenovirus for 1 h at 37°C, and the islets were then cultured in RPMI-1640 containing 10 mm glucose and 10% serum. Experiments were performed 24 h after the infection. Adenoviruses were applied at 5 × 107 plaque-forming units (pfu) per 100 islets, which is about 500 pfu per cell. The multiplicity of infection (MOI) used neither affected glucose transport activity in 3T3-L1 cells (Tsuru et al. 2002) nor insulin secretion in islets (see Results) compared with non-infected cells. Islets infected with the AdeX-LacZ virus and naive islets were used as a control. LacZ expression was observed in > 80% of cells of islets at multiplicity of infection (MOI) of 5 × 107 pfu per 100 islets 24 h after infection. Immunostaining using PKC-α antibody was also carried out to verify the expression of dn-PKC-α in the core islets (described below).
Solutions
The standard extracellular solution contained (mm): NaCl 140, KCl 5, MgCl2 1, CaCl2 2.5, glucose 3, and Hepes/NaOH 10 (pH 7.4), and 0.1% bovine serum albumin. The solution for membrane depolarization contained (mm): NaCl 105, KCl 40, MgCl2 1, CaCl2 2.5, glucose 3, Hepes/NaOH 10 (pH 7.4), and 0.1% bovine serum albumin. In some experiments, CaCl2 was not included (Ca2+-free solution). Cells, placed on a glass coverslip attached to an open perifusion chamber, were continuously perifused from a gravity-fed system. In all imaging experiments, the chemicals were added at the appropriate concentration together with the perifusion solution. Experiments were performed in the standard extracellular solution at room temperature, unless otherwise stated.
Imaging experiments
Fluorescence images were captured using a Nikon Diaphot inverted microscope (60×, water immersion objective, Olympus) equipped with a cooled (−50°C) coupled charge device digital camera (ORCA-II, Hamamatsu Photonics, Hamamatsu, Japan), and recorded and analysed on a Aqua Cosmos imaging station (Hamamatsu Photonics). The excitation light source was a 150 W xenon lamp with a polychrome I monochromator (T.I.L.L. Photonics GmbH, Planeg, Germany). GFP/EGFP fluorescence was excited at 488 nm for high time resolution of GFP/EGFP-tagged PKCs digital imaging. Relative fluorescence changes in intensity of the GFP/EGFP-tagged PKCs at the region of interest placed in the cytosol of the cell excluding the nucleus (ratio PKC-Cyt), and on the plasma membrane of the cell (ratio PKC-PM) were measured and analysed as markers of translocation. The value of each fluorescence intensity (F) was normalized to the initial value (F0), so that relative fluorescence change was referred to as the ratio F/F0. For simultaneous measurement of relative fluorescence changes in intensity of the GFP/EGFP-tagged PKCs in the cytosol and cytoplasmic free calcium concentration ([Ca2+]c), GFP/EGFP fluorescence was excited at 488 nm, while fura-2 fluorescence was excited at alternating 340 and 380 nm. The Mirror Cassette A7807 was purchased from Hamamatsu Photonics for the simultaneous measurement. A short pass filter of 330–495 nm was used to reduce background fluorescence in the light pass between a diachronic mirror of 505 nm and an emission filter of 535/45 nm band pass. The cells expressing GFP/EGFP-tagged PKC were loaded with 2 μm fura-2 acetoxymethyl ester (fura-2/AM) in the standard extracellular solution for 30 min at room temperature. The cells were washed twice and used within 2 h (Grynkiewicz et al. 1985). Images were acquired at a rate of 0.2 s−1. For population analysis of [Ca2+]c, 2000 islets from four rats were isolated and dispersed into single cells. Dispersed islet cells were loaded with fura-2 as mentioned above. The islet cells were suspended to a final concentration of 1 × 105 cells per 1.5 ml in the extracellular solution and fluorescence was measured with a fluorescence spectrophotometer. The fura-2 ratio was calibrated using exposure to 10 μm ionomycin and 10 mm Ca2+ or 10 mm EGTA in the fura-2-loaded cells without transfection of the GFP/EGFP-tagged PKCs. [Ca2+]c was calibrated after exposure to 10% Triton X-100 and 500 mm EDTA. A dissociation constant of 150 nm for Ca2+ and fura-2 at room temperature was used.
Immunohistochemistry
To detect the expression of the PKC-α in the islets infected with/without adenoviruses containing the domain-negative mutant of PKC-α (dn-PKC-α) (Tsuru et al. 2002), double staining was performed using cryosection. The islets were isolated and transfected with adenoviruses containing dn-PKC-α as described above. The islets were collected and fixed for 4 h at 4°C in 4% paraformaldehyde/phosphate-buffered saline (PBS), washed in PBS, and embedded in a Tissue-Tek OCT compound (Sakura Finechemicals, Tokyo, Japan) and frozen in liquid nitrogen. Frozen sections (7 μm) were cut with a Jung CM 3000 cryostat (Leica Corp., Vienna, Austria), mounted on poly-lysine coated slides and stored at −80°C. The islets were permeabilized, and non-specific binding sites were blocked with normal donkey serum (5%). The sections were incubated with a mixture of monoclonal mouse anti-PKC-α antibody cPKC-α (H-7) (1 : 100, Santa Cruz Biotechnology, CA, USA) and polyclonal guinea pig anti-porcine insulin antibody (1 : 1000) overnight at 4°C. After washing with PBS, the islets were incubated with Alexa Fluor 488 conjugated anti-guinea pig IgG (1 : 500 dilution) and Alexa Fluor 568-conjugated anti-mouse IgG (1 : 1000 dilution) for 1 h at room temperature and then washed with PBS and coverslipped with PermaFluor Aqueous Mountant (Immunon Shandon, Pittsburgh, PA, USA).
Detection of phosphorylated MARCKS
To determine the optimal concentration for PKC inhibitors, measurement of the extent of PKC substrate MARCKS phosphorylation (Ohmori et al. 2000) was carried out. Fresh isolated or dn-PKC-α transfected islets (50 islets for each condition) were stimulated with test agents for 10 min. Incubations were terminated by removal of the incubation buffer and addition of 1 ml of ice-cold PBS. Whole cell lysates were prepared after removal of PBS by resuspension in 100 μl Laemmli sample buffer, subjected to SDS-PAGE using 10% gels and transferred to a polyvinylidene difluoride membrane (Millipore). The membrane was blocked with solution containing 5% bovine serum albumin, 10 mm Tris/HCl (pH 7.4) and 154 mm NaCl for 1 h at room temperature. The blocked membrane was incubated with anti-MARCKS phosphoSer152/156 antibody (1 : 500, Chemicon International) overnight at 4°C. The membrane was washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. Following an extensive wash, the blots were visualized by using an ECL Western Blotting System (Amersham Biosciences).
Measurement of insulin secretion
Insulin secretion from pancreatic islets was measured in a static incubation system as previously described (Yamada et al. 2002). Fresh isolated islets for each experimental group (5 sized-matched islets per group) were pre-incubated in Krebs–Ringer–bicarbonate (KRB) buffer containing 2.7 mm glucose at 37°C in a humidified incubator. The solution was then replaced with basal solution alone or KRB buffer containing 20 mm glucose or 40 mm KCl. When 40 mm K+ was used, the concentration of NaCl in the solution was reduced to 105 mm. The PKC inhibitor Gö-6976 was added directly with secretion solution while Antp-PKC19–31 and Antp-PKC149–164 were added 1 h prior to the insulin secretion experiment. Short-term (10 min) and long-term (60 min) stimulations were performed in a water bath at 37°C. The stimulation time was carefully adjusted to avoid the influence of time loss on the solution changing and sample collection. The experiments were terminated by withdrawal of the supernatant solution, which was then placed in an ice bath. Samples were kept at −20°C until insulin assay was performed. Insulin concentration was determined using a time resolved immunofluorometric assay system as previously described (Mashima et al. 1996). All samples were assayed in duplicate. Regarding the concentration of PKC inhibitors used, we tested the effect of various doses of inhibitors by measuring MARCKS phosphorylation and used the minimal concentration which induced the maximal effect.
Electrophysiological measurement
We used a perforated whole-cell mode patch clamp for analysis of the voltage-dependent calcium channel (VDCC) in β-cells. All data were obtained using a computer-based amplifier system (EPC-9) controlled by E9 screen software (HEKA, Lambrecht, Germany), as previously described (Mogami et al. 1995). The cell membrane was perforated using the antibiotic amphotericin B (Sigma). Amphotericin B was dissolved in dimethyl sulfoxide (DMSO, 6 mg per 100 μl) and then diluted in the pipette solution as described below to a final concentration of 240 μg ml−1. After formation of gigaohm seals, perforation began within 5 min and stabilized within 15–30 min. With a series resistance of < 20 MΩ and size of the current, the voltage error would be < 4 mV, and hence was ignored. The typical value of membrane capacitance after achieving electrical access was about 5 pF. Membrane potential was changed from a holding potential of −60 to 60 mV using a sequence of voltage steps (10 mV; 300 ms) in cells before and 3 min after the addition of 1 μm Gö-6976. The extracellular solution for measurement of the VDCC contained (mm): NaCl 120, KCl 5, tetraethylammonium chloride 20, MgCl2 1, CaCl2 2.5, glucose 3 and Hepes-NaOH 10 (pH 7.4). The patch electrode solution contained (mm): NaCl 10, CsCl 50, Cs2SO4 40, tetraethylammonium chloride 30, MgCl2 1, EGTA 1 and Hepes-CsOH 10 (pH 7.2).
Statistical analysis
Statistical analysis was done by one-way ANOVA. For comparison between the two groups, Student's unpaired t test was used. P < 0.05 was considered to be significant. Results are expressed as means ± s.e.m.
Results
Effect of depolarizing concentration of potassium on translocation of PKC
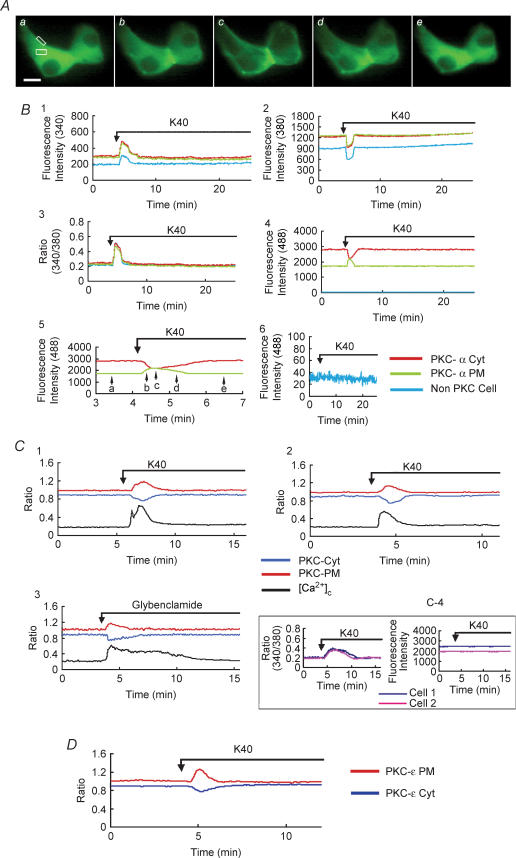
Our previous study showed that stimulation of calcium influx activated conventional PKC-α in INS-1 cells (Mogami et al. 2003). To examine whether or not calcium influx also induces translocation of PKC-α in pancreatic β cells, we simultaneously monitored relative changes in the fluorescence intensities of PKC-α–EGFP and [Ca2+]c in pancreatic β cells. Figure 1A and B shows the images of PKC-α–EGFP translocation and traces of relative changes in the fluorescence intensities of PKC-α–EGFP and [Ca2+]c under the stimulation of a high concentration of potassium. In a PKC-α–EGFP-expressing and fura-2-loaded β cell, administration of a depolarizing concentration of potassium (40 mm) evoked an immediate increase in [Ca2+]c due to Ca2+ influx through the opening of VDCCs. This was accompanied by a rapid and reversible increase in fluorescence of PKC-α–EGFP in the plasma membrane. Concomitant with this increase, fluorescence of PKC-α–EGFP in the cytosol decreased transiently. Hence, PKC-α–EGFP was translocated from the cytosol to the plasma membrane when [Ca2+]c elevation took place and was reversed when [Ca2+]c decreased. Of 324 cells examined, 296 showed translocation to the plasma membrane. The effect of the high concentration of potassium on translocation of PKC-α–EGFP was completely blocked by nifedipine, an inhibitor of voltage-dependent calcium channels (data not shown). The relative changes in the fluorescence intensities of PKC-α–EGFP in the cytosol (PKC-α-Cyt) and the plasma membrane (PKC-α-PM) were reciprocal (as plotted in Fig. 1B4 and 1B5). Therefore, in some of the following experiments, PKC-α-Cyt was monitored as a marker of PKC-EGFP translocation.
Figure 1. Effect of high concentration of K+ and glybenclamide on translocation of PKC.
A, images of PKC-α–EGFP translocation induced by 40 mm KCl at high time resolution (sample interval, 5 s). Panels a–e were taken at the times indicated by the arrows in B5. The scale bar represents 10 μm. The image is representative of 296 images from 18 independent experiments. B, the entire time course of simultaneous measurements of [Ca2+]c, PKC-α Cyt and PKC-α PM in islet cell transfected with PKC-α–EGFP and loaded with fura-2. B1, the fluorescence was excited at 340 nm. The fluorescence intensity in the regions of interest (white boxes in A) in a PKC-α–EGFP-expressing cell and a non-PKC-α–EGFP-expressing cell within the same monitor field (cell image not shown) was recorded. B2, the fluorescence was excited at 380 nm. The fluorescence intensity in the regions of interest was recorded. B3, the ratio of fluorescence 340/380 nm was recorded as the ratio of [Ca2+]c. B4, the fluorescence was excited at 488 nm. The fluorescence intensity in the regions of interest was recorded. The fluorescence in a non-PKC-α–EGFP-expressing cell was seen as almost a straight line on the X-axis indicating that the influence of fura-2 fluorescence on GFP fluorescence was negligible. B5, fluorescence changes shown in B4 with higher time resolution. B6, the fluorescence intensity was recorded in a non-PKC-α–EGFP-expressing cell loaded with fura-2 with excitation at 488 nm. No significant change was recorded during stimulation. The translocation of PKC-α–EGFP in PKC-α–EGFP-expressing cell not loaded with fura-2 was also monitored (data not shown). The time course of translocation is similar to the data shown in B5. Therefore, the crossover between fura-2 and EGFP images was negligible. C1, islet cells were transfected with PKC-θ–EGFP and loaded with fura-2. The cell was stimulated by 40 mm KCl and changes in the fluorescence of PKC-θ in cytosol (PKC-Cyt), PKC-θ in the plasma membrane (PKC-PM) and [Ca2+]c were monitored. The value of each EGFP fluorescence intensity (F) was normalized to the initial value (F0), so that the relative fluorescence change was referred to as the ratio F /F0. In order to distinguish the fluorescence ratio of PKC in the cytosol (PKC-Cyt) from the PKC in the plasma membrane (PKC-PM), the ratio of PKC-Cyt was modified as F /F0− 0.1 in the figure. The traces are representative of 73 traces from 9 independent experiments. C2, islet cells were transfected with C1-EGFP and loaded with fura-2. The cell was stimulated by 40 mm KCl and changes in the fluorescence from C1 in the cytosol (PKC-Cyt), plasma membrane (PKC-PM) and [Ca2+]c were monitored. Data are shown in the same way as described in C1. The traces are representative of 24 traces from 3 independent experiments. C3, islet cells were transfected with PKC-α–EGFP and loaded with fura-2. The cell was stimulated by 10 μm glybenclamide and changes in the fluorescence from PKC-α in the cytosol (PKC-Cyt), in the plasma membrane (PKC-PM) and [Ca2+]c were monitored. The traces are representative of 36 traces from 5 independent experiments. C4, islet cells were transfected with EGFP-N2 and loaded with fura-2. The cells were stimulated by 40 mm KCl and changes in the fluorescence from EGFP (right) in cytosol and [Ca2+]c (left) of two cells were monitored. [Ca2+]c was changed significantly after the stimulation of 40 mm KCl, while there were no apparent changes in the fluorescence from EGFP indicating that there is no apparent crossover between the fluorescence of fura-2 and EGFP. The traces are representative of 20 traces from 3 independent experiments. D, islet cells were transfected with PKC-ɛ–GFP. The cell was stimulated by 40 mm KCl.
To determine if an increase in [Ca2+]c induced hydrolysis of polyphosphoinositides by activating phospholipase C, we measured translocation of novel PKC, which is activated by DAG. To this end, first we transfected cells with a novel PKC, PKC-θ–EGFP. Since PKC-θ is not expressed in β cells (Knutson & Hoenig, 1994; Tian et al. 1996; Yedovitzky et al. 1997), we used PKC-θ–EGFP as a probe to monitor the production of DAG. Figure 1C1 shows the traces of relative changes in the fluorescence intensities of PKC-θ–EGFP and [Ca2+]c under the stimulation of a high concentration of potassium. In PKC-θ–EGFP-expressing and fura-2-loaded pancreatic β cells, a depolarizing concentration of potassium evoked an immediate increase in [Ca2+]c and a rapid and reversible translocation of PKC-θ–EGFP. Of 81 cells examined, 73 showed the translocation to the plasma membrane. To further prove that an increase in [Ca2+]c induced hydrolysis of polyphosphoinositides and generated DAG, we measured translocation of the C1 domain of PKCγ (C1-EGFP). Figure 1C2 shows the traces of relative changes in the fluorescence intensities of C1-EGFP and [Ca2+]c under the stimulation of a high concentration of potassium. In the C1-EGFP-expressing and fura-2-loaded pancreatic β cell, a depolarizing concentration of potassium evoked an immediate increase in [Ca2+]c and a rapid and reversible translocation of C1-EGFP. Of 28 cells examined, 24 showed the translocation to the plasma membrane. To further confirm that the production of DAG was due to the activation of phospholipase C, we examined the effect of an inhibitor of phospholipase C, U-73122. The inhibitor blocked the translocation of PKC-α–EGFP induced by the depolarizing concentration of potassium (data not shown).
We also examined the effect of glybenclamide, an inhibitor of the KATP channel, on the translocation of PKC-α. As shown in Fig. 1C3, glybenclamide increased [Ca2+]c, which was accompanied by translocation of PKC-α from the cytosol to the plasma membrane. Of 54 cells examined, 36 cells showed the translocation to the plasma membrane.
A recent report on the role of PKC isoforms participating in the glucose-induced insulin secretion indicates that PKC-ɛ is the most important candidate (Mendez et al. 2003). We therefore monitored translocation of PKC-ɛ–GFP in β cells. As shown in Fig. 1D, in PKC-ɛ–GFP-expressing pancreatic β cells, a depolarizing concentration of potassium evoked a rapid and reversible translocation of PKC-ɛ–GFP, with the time course similar to the translocation of PKC-θ and the C1 domain.
Effect of glucose on translocation of PKC-α–EGFP in pancreatic β cells
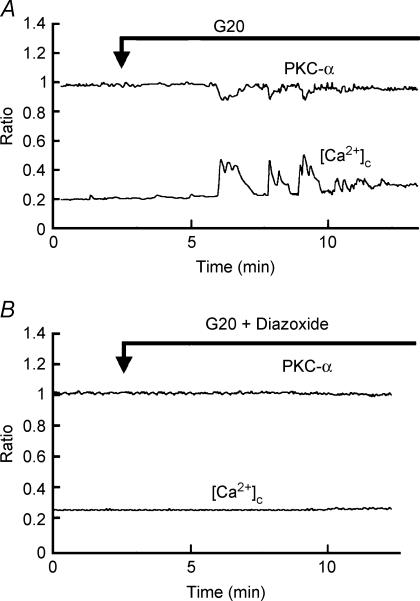
Figure 2A shows the traces of relative changes in the fluorescence intensities of PKC-α–EGFP and [Ca2+]c under the stimulation of high glucose (20 mm) in PKC-α–EGFP-expressing and fura-2-loaded pancreatic β-cells. Upon perifusion of β cells in standard extracellular solution containing 3 mm glucose, no apparent changes in the fluorescence intensities of PKC-α–EGFP and [Ca2+]c were observed. After the elevation of extracellular glucose concentration from 3 mm to 20 mm, an increase in the ratio of fura-2 fluorescence was observed after a 3–5 min interval. In PKC-α–EGFP-expressing β cells, fluorescence of PKC-α–EGFP in the cytosol was decreased following the increase in [Ca2+]c. With the decrease in [Ca2+]c, the translocation of PKC-α–EGFP in β cells was reversed. Repetitive translocation was observed with the oscillation of [Ca2+]c. Of 54 cells examined, 32 showed the translocation. Figure 2B shows the traces of relative changes in the fluorescence intensities of PKC-α–EGFP and [Ca2+]c induced by high glucose in the presence of the KATP channel opener diazoxide (200 μm). In the presence of diazoxide, no apparent changes were observed in [Ca2+]c in 18 cells examined, nor was translocation of PKC-α–EGFP observed in PKC-α–EGFP-expressing cells.
Figure 2. Effect of glucose on translocation of PKC-α.
Islet cells were transfected with PKC-α–EGFP and loaded with fura-2. The cell was stimulated by 20 mm glucose in the presence (B) or absence (A) of 200 μm diazoxide. Changes in the fluorescence from PKC-α and [Ca2+]c were monitored. The traces are representative of 32 from 4 independent experiments.
Effects of inhibitors of PKC-α on calcium-evoked insulin secretion
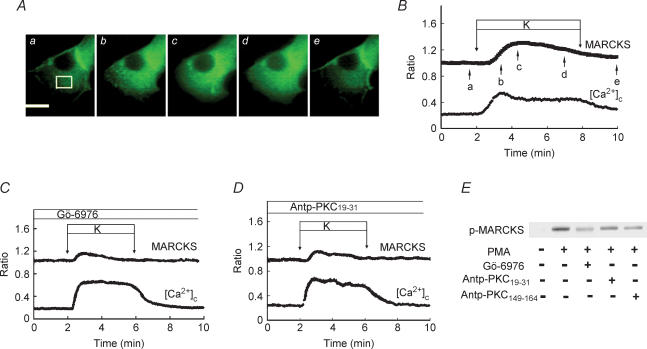
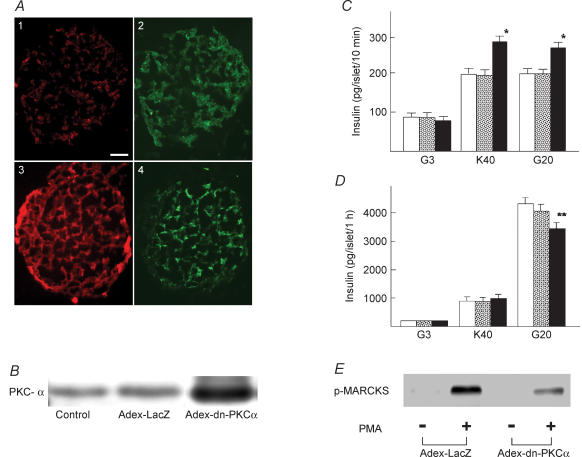
To evaluate the role of PKC-α on calcium-evoked insulin secretion, we measured insulin secretion from isolated rat islets in the presence of various agents which block the activity of PKC-α. We first employed Gö-6976, an inhibitor of conventional PKC (Martiny-Baron et al. 1993). To assess calcium-evoked insulin secretion, we incubated islets for 10 min in the presence of a depolarizing concentration of potassium or 20 mm glucose. As shown in Fig. 3A, 40 mm potassium induced an approximately 2-fold increase in insulin secretion (n = 8). Gö-6976 significantly enhanced potassium-induced insulin secretion (n = 10). Likewise, Gö-6976 significantly increased glucose-induced secretion. To confirm that Gö-6976 inhibited the activity of conventional PKC, we monitored translocation of MARCKS (myristoylated alanine-rich C-kinase substrate), a PKC substrate that translocates from the plasma membrane to the cytosol upon phosphorylation (Ohmori et al. 2000). As shown in Fig. 4C, Gö-6976 inhibited translocation of MARCKS compared with its normal translocation (P < 0.01) as shown in Fig. 4B (n = 22). The peak value was significantly (P < 0.05) lower in Gö-6976-treated cells. We then used pseudosubstrate peptide for PKC-α (Harris et al. 1999). We introduced the pseudosubstrate peptide into β cells using a peptide sequence corresponding to the third α-helix of the homeodomain of the antennapedia (Derossi et al. 1998, Fischer et al. 2000). The use of antennapedia-derived peptide (Antp-PKC19–31) enables loading of the pseudosubstrate peptide into intact cells (Derossi et al. 1998; Fischer et al. 2000). As shown in Fig. 4A and B, a high concentration of potassium induced translocation of MARCKS from the plasma membrane to the cytosol, indicating that MARCKS was phosphorylated by the depolarizing concentration of potassium. As shown in Fig. 4D, Antp-PKC19–31 inhibited translocation of MARCKS (n = 15). The peak value was significantly (P < 0.05) lower in Antp-PKC19–31-treated cells. To further confirm that Gö-6976 and Antp-PKC19–31 inhibited the activity of conventional PKC, we measured the phosphorylation of MARCKS using immunoblotting. As shown in Fig. 4E, both Gö-6976 and Antp-PKC19–31 inhibited phosphorylation of MARCKS. Preliminary data examining the MARCKS phosphorylation using inhibitors indicated that the minimal concentrations for Gö-6976 and Antp-PKC19–31 that induced the maximal inhibitory effect were 1 μm and 75 μm, respectively. Similar to the results for Gö-6976, short-term insulin secretion induced by a high concentration of glucose was slightly but significantly greater in the presence of Antp-PKC19–31 (Fig. 3B) (n = 10). Likewise, Antp-PKC19–31 enhanced short-term insulin secretion induced by potassium (n = 10).
Figure 3. Effect of PKC inhibitors on short-term insulin secretion.
A, islets were incubated for 10 min with 40 mm KCl or 20 mm glucose in the presence (filled column) or absence (open column) of 1 μm Gö-6976. B, islets were incubated for 10 min with 40 mm KCl or 20 mm glucose in the presence of 75 μm Antp-PKC19–31 (filled column) or 75 μm Antp (stippled column) or absence of either of them (open column). Values are means ± s.e.m. for 8–10 experiments. *P < 0.05 versus without inhibitor.
Figure 4. Effect of PKC inhibitors on phosphorylation of MARCKS.
A, images of MARCKS–EGFP translocation induced by 40 mm KCl at high time resolution (sample interval, 5 s). Panels a–e were taken at the times indicated by the arrows in B. The bar represents 10 μm. The images are representative of 22 images from 3 independent experiments. B, time course of MARCKS–EGFP translocation induced by 40 mm KCl. Changes in the fluorescence of EGFP and [Ca2+]c in the cytosol (white box in A) were monitored. C, islet cells were transfected with MARCKS–EGFP and stimulated by 40 mm KCl in the presence of 1 μm Gö-6976. Gö-6976 was added 3 min before the stimulation. Changes in the fluorescence of EGFP in the cytosol and [Ca2+]c were monitored. The trace is an average of 29 traces from 3 independent experiments. D, islet cells were transfected with MARCKS–EGFP and stimulated by 40 mm KCl in the presence of 75 μm Antp-PKC19–31. Antp-PKC19–31 was added 1 h before the stimulation. Changes in the fluorescence of EGFP in the cytosol and [Ca2+]c were monitored. The trace is an average of 15 traces from 3 independent experiments. E, effects of PKC inhibitors on PKC substrate phosphorylation using freshly isolated pancreatic islets. Batches of islets were incubated for 10 min with 3 mm glucose and 500 nm PMA in the presence or absence of either 1 μm Gö-6976 or 75 μm Antp-PKC19–31 (PKC-α inhibitor) or 75 μm Antp-PKC149–164 (PKC-ɛ inhibitor). Extracts (equivalent to 10 islets per lane) were subjected to SDS-PAGE using 10% gels. Protein bands were detected by immunoblotting using polyclonal rabbit anti-MARCKS phosphoSer152/156 antibody. The figure is a representative immunoblot from two independent experiments.
Glucose induces sustained insulin secretion. Previous studies have shown that glucose-induced secretion is inhibited by inhibitors of PKC (Yedovitzky et al. 1997; Zawalich & Zawalich, 2001). We therefore examined the effect of Gö-6976 and Antp-PKC19–31 on long-term insulin secretion evoked by a high concentration of glucose. In contrast to the enhancement of the short-term insulin secretion, long-term insulin secretion induced by a high concentration of glucose was significantly reduced by Gö-6976 and pseudosubstrate peptide (Fig. 5A and B). Note that Gö-6976 and Antp-PKC19–31 significantly increased potassium-induced insulin secretion when islets were incubated for 60 min (Fig. 5A and B) (n = 10).
Figure 5. Effect of PKC inhibitors on long-term insulin secretion.
A, islets were incubated for 60 min with 40 mm KCl or 20 mm glucose in the presence (filled column) or absence (open column) of 1 μm Gö-6976. B, islets were incubated for 60 min with 40 mm KCl or 20 mm glucose in the presence (filled column) of 75 μm Antp-PKC19–31 or 75 μm Antp (stippled column) or absence of either of them (open column). C, islets were incubated for 60 min with 40 mm KCl or 20 mm glucose in the presence of 75 μm Antp-PKC149–164 (filled column) or 75 μm Antp (stippled column) or absence of either of them (open column). Values are the means ± s. e.m. for 10 experiments. *P < 0.01 versus no inhibitor.
PKC-ɛ–GFP translocated from cytosol to the plasma membrane under the stimulation of a high concentration of potassium. We therefore used antennapedia combined pseudosubstrate peptide for PKC-ɛ (Antp-PKC149–164) to assess the effect of PKC-ɛ in potassium-induced insulin secretion. As shown in Fig. 5C, although Antp-PKC149–164 did not significantly affect the insulin secretion induced by depolarizing potassium, it markedly inhibited the long-term insulin secretion induced by high glucose (n = 10). The effect of Antp-PKC149–164 on phosphorylation of MARCKS was also detected (Fig. 4E).
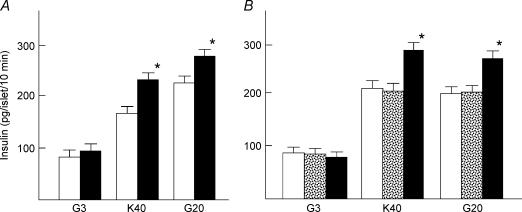
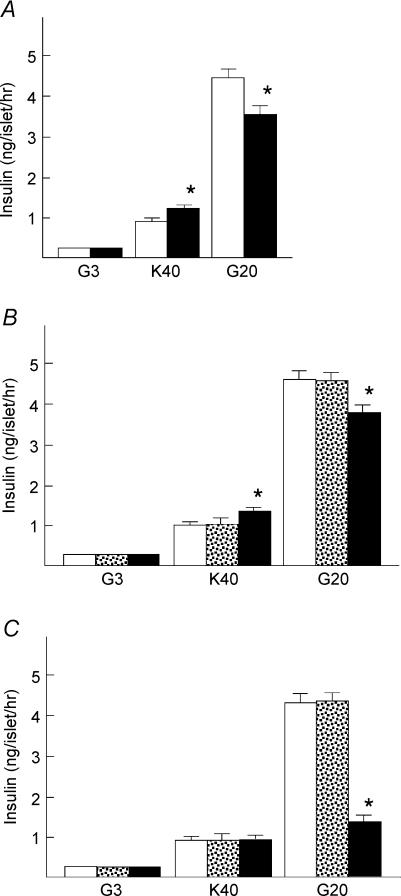
To further confirm the role of PKC-α in calcium-induced insulin secretion, we transfected islet cells with the dn-PKC-α gene (Tsuru et al. 2002) using adenovirus vector. We used three methods to confirm the efficiency of transfection. First, islets were transfected with Adex-LacZ. Islet cells were then dispersed and the expression of LacZ was estimated by LacZ staining. More than 80% of the islet cells were positive for LacZ (data not shown). Second, islets were transfected with Adex-dn-PKC-α, and PKC-α expression was measured by immunostaining of the cryosections (Fig. 6A). Overexpression of PKC-α was detected in the periphery and core of the islet (n = 40). Third, islets were transfected with Adex-dn-PKC-α or Adex-LacZ. Immunoblotting was then carried out to detect the transfection efficiency (Fig. 6B). Densitometric analysis showed that PKC-α expression was approximately 8-fold in Adex-dn-PKC-α-tranfected islets. Short-term insulin secretion induced by 40 mm potassium and 20 mm glucose was measured in islets transfected with either LacZ or dn-PKC-α. As shown in Fig. 6C, insulin secretion induced by 40 mm potassium was significantly higher in dn-PKC-α-transfected islets compared to LacZ-transfected or naive islets (n = 10). Similarly, short-term insulin secretion induced by 20 mm glucose was significantly higher in dn-PKC-α-transfected islets (n = 10). In contrast to short-term incubation, dn-PKC-α inhibited long-term insulin secretion induced by 20 mm glucose (Fig. 6D) (n = 10). The effect of dn-PKC-α on phosphorylation of MARCKS was also determined (Fig. 6E).
Figure 6. Effect of transfection of dn-PKC-α on insulin secretion.
A, detection on PKC-α expression in cultured pancreatic islets with or without Adex-dn-PKC-α transfection. Islets sections were double-stained with antibodies against PKC-α (red) and insulin (green). The Adex-dn-PKC-α expression (A3) detected was apparently larger than the endogenous PKC-α (A1) both in the periphery and in the core while there is no apparent changes in insulin expression in the control islet (A2, the same islet as in A1) and Adex-dn-PKC-α-expressing islet (A4, the same islet as in A3). Magnification × 200. The figure is representative of 40 islets detected from three independent experiments. B, detection of PKC-α in islet.The expression of PKC-α was determined by immunoblotting in control islets, and islets transfected with Adex-LacZ or Adex-dn-PKC-α. The results are representative of two experiments. C, 10 min insulin secretion from dn-PKC-α transfected islets. Islets were transfected with Adex-LacZ (open column) or Adex-dn-PKC-α (filled column) or without adenovirus transfection (stippled column). After 24 h incubation, the medium was changed to normal extracellular solution containing 3 mm glucose (G3) or 40 mm KCl (K40) or 20 mm glucose (G20). Values are the means ± s.e.m. for 10 experiments. D, 1 h insulin secretion from dn-PKC-α transfected islets. Islets were transfected with Adex-LacZ (open column) or Adex-dn-PKC-α (filled column) or without adenovirus transfection (stippled column). Islets were then incubated for 1 h with 3 mm glucose (G3), 40 mm KCl (K40) or 20 mm glucose (G20). Values are the means ± s.e.m. for 10 experiments. E, effect of dn-PKC-α on MARCKS phosphorylation. Islets transfected by dn-PKC-α or LacZ were incubated for 10 min in the presence or absence of 500 nm PMA. MARCKS phosphorylation (p-MARCKS) was determined by immunoblotting. The figure is a representative immunoblot from two independent experiments.*P < 0.05 versus Adex-LacZ, **P < 0.01 versus Adex-LacZ.
Effect of inhibitor of PKC-α on potassium-induced increase in cytoplasmic free calcium concentration
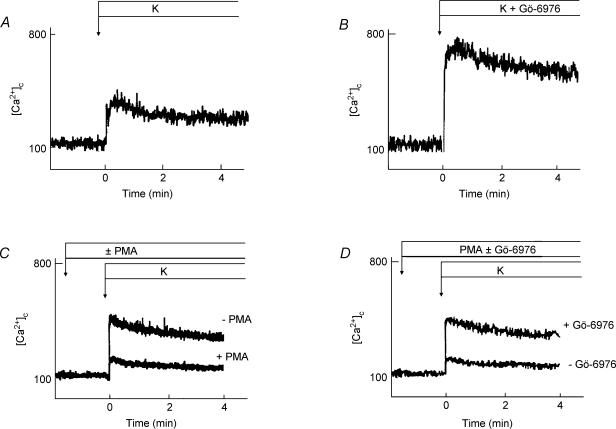
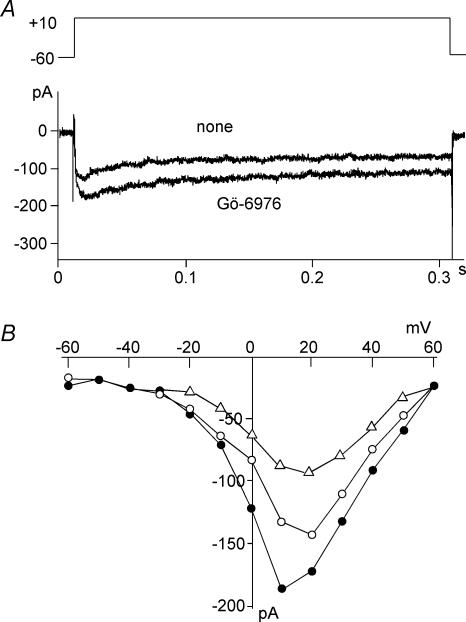
We then examined whether or not PKC inhibitors affected potassium-induced elevation of [Ca2+]c in β cells. To analyse this quantitatively, we measured [Ca2+]c by population analysis. Dispersed islet cells were loaded with fura-2, and changes in the fluorescence were measured by fluorescence spectrophotometry. As shown in Fig. 7, potassium induced a rapid increase in [Ca2+]c. This potassium-induced calcium transient was markedly increased by Gö-6976 (Fig. 7B) (n = 5). A similar result was obtained by Antp-PKC19–31 (data not shown). The effect of a PKC activator, PMA, on potassium-induced elevation of [Ca2+]c in β cells was also tested. PMA (500 nm) decreased potassium-induced increase in [Ca2+]c (Fig. 7C). The peak value in the presence of PMA was 40% of that in the absence of PMA (P < 0.05). This effect of PMA was almost reversed by adding Gö-6976 (Fig. 7D). We then addressed whether or not PKC inhibitor affected calcium influx through a voltage-dependent calcium channel. As shown in Fig. 8, Gö-6976 increased the calcium current upon depolarization of the plasma membrane. The effect of Gö-6976 was most evident at the membrane potential of +10 mV (n = 5), where the calcium current was approximately 140 ± 5.6% of that in the absence of Gö-6976. Conversely, the calcium current was inhibited by PMA (Fig. 8B).
Figure 7. Effect of Gö-6976 on K+-induced elevation of [Ca2+]c.
Population analysis of [Ca2+]c was carried out to detect the effect of Gö-6976 on K+-induced elevation of [Ca2+]c. Islets were dispersed into single cells and were loaded with fura-2. Cells were stimulated by 40 mm KCl in the presence (B) or absence (A) of 1 μm Gö-6976. Effect of PMA on K+-induced elevation of [Ca2+]c was also determined. Cells were stimulated by 40 mm KCl in the presence or absence of 500 nm PMA (C) and with 500 nm PMA and 40 mm KCl in the presence and absence of 1 μm Gö-6976 (D). Changes in [Ca2+]c were monitored. The results are representative of five experiments.
Figure 8. Effect of Gö-6976 on voltage-dependent calcium channel current.
Perforated whole-cell patch clamp was performed in an isolated β cell. Voltage-dependent calcium channel current was measured in the presence or absence of 1 μm Gö-6976. A, membrane potential was changed from a holding potential of −60 mV to 10 mV in a cell before and 3 min after the addition of 1 μm Gö-6976. B, voltage-dependent calcium current was measured by various voltage jumps, and the current–voltage relationship was measured in cells before (○) and 3 min after the addition of 1 μm Gö-6976 (•) or 500 nm PMA (▵). Results are representative of five experiments.
Discussion
In the first part of the study, we investigated whether or not calcium influx through VDCCs activated PKC in isolated pancreatic β cells. As shown in Fig. 1B, a depolarizing concentration of potassium induced a transient increase in [Ca2+]c, which was accompanied by translocation of PKC-α. It is evident that, as in INS-1 cells (Mogami et al. 2003), calcium influx through VDCCs induces translocation of PKC-α in β cells. The results are in agreement with a recent report by Pinton et al. (2002) in which a high concentration of potassium induced translocation of PKC-βII in β cells. We reported previously (Mogami et al. 2003) that translocation of PKC-α is followed by phosphorylation of the PKC substrate protein, MARCKS. Similar results were obtained in β cells as shown in Fig. 4. Therefore, calcium influx through VDCCs activates PKC-α in β cells. Calcium-mediated activation of PKC-α may be at least partly due to an increase in the content of DAG, a sensitivity modulator of conventional PKC. We measured translocation of PKC-θ, C1 domain and PKC-ɛ, to monitor the production of DAG. As shown in Fig. 1C and D, a depolarizing concentration of potassium also induced a transient translocation of PKC-θ, C1 domain and PKC-ɛ. This is in agreement with our previous report that in INS-1 cells, depolarization induced translocation of PKC-θ, the C1-domain of the PKC-γ and pleckstrin homology-domain, an indicator of inositol trisphosphate production (Mogami et al. 2003). Hence, DAG may be generated by depolarization through activation of calcium-sensitive phospholipase C. Translocation of PKC-α was also induced by glybenclamide and a high concentration of glucose. These observations together with the observation that diazoxide prevented glucose-induced translocation of PKC-α support the notion that calcium influx through VDCCs is critical in activating PKC-α in β cells. It has been reported that a high concentration of glucose stimulates de novo formation of DAG (Peter-Riesch et al. 1988). Since diazoxide almost completely blocked glucose-induced translocation of PKC-α, the contribution of de novo formation of DAG is not significant. In any case, calcium influx through VDCCs activates PKC-α in isolated β cells. It is reasonable to speculate that calcium influx also activates PKC-α in β cells in intact islets.
We then addressed whether or not PKC-α modulates insulin secretion from pancreatic islets. We measured short-term insulin secretion induced by 40 mm potassium and 20 mm glucose to monitor calcium-evoked secretion. We employed three approaches to inhibit the activity of conventional PKC. Gö-6976 is a relatively specific inhibitor of conventional PKC (Martiny-Baron et al. 1993). Antp-PKC19–31 inhibits the activity of PKC-α (Harris et al. 1999). However, because of the sequence similarity, it probably inhibits PKC-βII as well, which is also expressed in β cells albeit in a small amount (Knutson & Hoenig, 1994, Tian et al. 1996). dn-PKC-α blocks the activity of PKC-α and possibly PKC-βII. Collectively, three different inhibitors block PKC-α and possibly PKC-βII by different mechanisms. In the present study, we confirmed the inhibitory effect of these inhibitors by measuring translocation and the phosphorylation extent of MARCKS, a PKC substrate which translocates when phosphorylated by PKC (Ohmori et al. 2000). Although the inhibition of MARCKS translocation and phosphorylation was not complete, this may have been due to the inability of these inhibitors to block novel PKC, presumably PKC-ɛ, since a PKC-ɛ inhibitor, Antp-PKC149–164, also inhibited phosphorylation of MARCKS to a certain extent. To our knowledge, no study has addressed whether inhibitors of PKC affect calcium-evoked insulin secretion. The results were rather unexpected. All three of the inhibitors increased short-term insulin secretion induced by a depolarizing concentration of potassium and glucose. Conventional PKC, PKC-α and possibly PKC-βII may be inhibitory on calcium-evoked insulin secretion. It should be noted that we observed the net effect of inhibitors on insulin secretion. Thus, it is possible that conventional PKC modulates insulin secretion by acting on multiple steps, both stimulatory and inhibitory, with inhibitory action being dominant. Although the present results are rather surprising, they are in line with the observation made by Zawalich & Zawalich (2001). They showed that Gö-6976 enhanced the first phase of the glucose-induced insulin secretion in a perifusion system (Zawalich & Zawalich, 2001). Given that the first phase of glucose-induced insulin secretion is largely dependent on the elevation of [Ca2+]c (Aizawa et al. 2002; Henquin, 2000), their results suggest that conventional PKC is inhibitory on calcium-evoked insulin secretion. To increase insulin secretion, PKC inhibitors augmented the elevation of [Ca2+]c induced by potassium. Conventional PKC may function to inhibit secretion by reducing the calcium signal. PKC inhibitors enhanced potassium-induced increase in [Ca2+]c. This is at least partly due to the augmentation of calcium influx since the inhibitor increased the calcium current through VDCCs. Rane & Dunlop (1986) reported previously that protein kinase C activators 1,2-oleoylacetylglycerol and the phorbol ester 12-deoxyphorbol 13-isobutyrate decreased the current through VDCCs in neurones. This is in agreement with our observation that PMA decreased calcium influx stimulated by high potassium. In addition, Smallwood et al. (1988). reported that the plasma membrane calcium pump is activated by protein kinase C. Therefore, it is also possible that conventional PKC activates the calcium pump and reduces the calcium signal. Presumably, activation of the calcium pump accelerates termination of the calcium transient and thereby is effective in reducing calcium toxicity. It may be favourable to generate repetitive calcium transient, calcium oscillation.
Recently, Carpenter et al. (2004) reported that PKC-α was activated but not required during glucose-induced insulin secretion, using a cPKC inhibitor and islets overexpressing kinase-dead PKC-α. It should be noted, however, that in their preparation of islets, insulin secretory response was not good. For example, a high concentration of glucose induced an 8-fold increase in freshly isolated islets and only a 3-fold increase in islets infected with adenovirus vector. On the other hand, we showed that glucose induced a more than 20-fold increase in insulin secretion even in adenovirus infected islets. Their islet preparation may not be good enough to detect a relatively small effect of PKC inhibitors. We transfected dn-PKC-α using a relatively high titre of adenovirus (500 MOI). Weber et al. (1997) reported that infection at 1000 MOI induced apoptosis in β cells whereas infection at 100 MOI did not. On the other hand, Diraison et al. (2004) reported that infection at 100 MOI reduced the viability of cultured β cells. In our experimental system, however, infection with adenovirus did not alter the insulin secretory responses. Therefore, islets transfected with dn-PKC-α may be suitable for the assessment of the function of PKC. Our results are in good agreement with those of Zawalich & Zawalich (2001), who also showed that Gö-6967 inhibits the second phase of the glucose-induced secretion. The amount of insulin released during the second phase is much greater than that of the first phase. Once again, their results are in good agreement with ours showing that long-term insulin secretion is inhibited by the inhibitors of conventional PKC. Our results also agree with the report by Yedovitzky et al. (1997) in which a specific inhibitor of PKC-α translocation attenuated glucose-induced insulin secretion. Despite the potentiation of the calcium influx signal, inhibitors of conventional PKC induced an approximately 20% decrease in insulin secretion when islets were incubated for 60 min. Hence, positive regulation by conventional PKC may be greater in the second phase of the glucose-induced insulin secretion. It is likely that conventional PKC plays some role in the ATP-sensitive potassium channel-independent mechanism (Aizawa et al. 2002) or in the amplifying mechanism (Henquin, 2000) activated by glucose. Presumably, conventional PKC phosphorylates some proteins, which may be involved in the amplifying mechanism. It is possible that PKC regulates recruitment of secretory granules to the plasma membrane and supplies the granules to exocytotic machinery. Long-term glucose-induced insulin secretion was inhibited to a greater extent by a PKC-ɛ inhibitor, indicating a more important role of the novel type PKC, PKC-ɛ, in the ATP-sensitive potassium channel-independent mechanism (Aizawa et al. 2002) or in the amplifying mechanism (Henquin, 2000) activated by glucose. In contrast, Antp-PKC149–164 did not affect potassium-induced secretion. Presumably, unlike PKC-α, PKC-ɛ may not affect calcium entry or extrusion. Rather, PKC-ɛ may regulate mainly the exocytotic machinery. In addition, involvement of PKC is less in the potassium effect compared with the glucose effect. In fact, potassium induces a single rise in [Ca2+]c and the activation of PKC is only once, whereas glucose induces calcium oscillation, and activation of PKC occurs many times synchronizing with calcium oscillation. In any event, identification of the substrate for individual PKC is necessary to elucidate the precise mechanism.
The present results indicate that conventional PKC acts as a positive and negative regulator of insulin secretion induced by glucose. This may be one reason for the controversial data in the literature for the role of PKC in insulin secretion. Besides conventional PKC, novel PKC is also translocated to the plasma membrane by calcium influx and, specifically, PKC-ɛ is also involved in the second phase of insulin secretion (Yedovitzky et al. 1997). The role of PKCs in insulin secretion should be further re-evaluated by appropriate approaches.
Acknowledgments
The authors are grateful to Ms Mayumi Odagiri for secretarial assistance. The present study was supported by a Grant-in-Aid from the Ministry of Science, Education, Sports and Culture of Japan.
References
- Aizawa T, Sato Y, Komatsu M. Importance of nonionic signals for glucose-induced biphasic insulin secretion. Diabetes. 2002;51:S96–S98. doi: 10.2337/diabetes.51.2007.s96. [DOI] [PubMed] [Google Scholar]
- Almholt K, Arkhammar PO, Thastrup O, Tullin S. Simultaneous visualization of the translocation of protein kinase Cα-green fluorescent protein hybrids and intracellular calcium concentrations. Biochem J. 1999;337:211–218. 10.1042/0264-6021:3370211. [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay G, Standaert ML, Kikkawa U, Ono Y, Moscat J, Farese RV. Effects of transiently expressed atypical (zeta, lambda), conventional (alpha, beta) and novel (delta, epsilon) protein kinase C isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes: specific interchangeable effects of protein kinase C-zeta and C-lambda. Biochem J. 1999;337:461–470. [PMC free article] [PubMed] [Google Scholar]
- Carpenter L, Mitchell CJ, Xu ZZ, Poronnik P, Both GW, Biden TJ. PKC-alpha is activated but not required during glucose-induced insulin secretion from rat pancreatic islets. Diabetes. 2004;53:53–60. doi: 10.2337/diabetes.53.1.53. [DOI] [PubMed] [Google Scholar]
- Deeney JT, Cunningham BA, Chheda S, Bokvist K, Juntti-Berggren L, Lam K, Korchak HM, Corkey BE, Berggren PO. Reversible Ca2+-dependent translocation of protein kinase C and glucose-induced insulin release. J Biol Chem. 1996;271:18154–18160. doi: 10.1074/jbc.271.30.18154. [DOI] [PubMed] [Google Scholar]
- Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. 10.1016/S0962-8924(97)01214-2. [PubMed] [Google Scholar]
- Diraison F, Patton L, Ferre P, Fouffele F, Briscoe CP, Leclerc I, Rutter GA. Over-expression of sterol-regulatory-element-binding protein-1c (SREBP1c) in rat pancreatic islets induces lipogenesis and decreases glucose-stimulated insulin release. Biochem J. 2004;378:769–778. doi: 10.1042/BJ20031277. 10.1042/BJ20031277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer PM, Zhelev NZ, Wang S, Melville JE, Fahraeus R, Lane DP. Structure-activity relationship of truncated and substituted analogues of the intracellular delivery vector Penetratin. J Pept Res. 2000;55:163–172. doi: 10.1034/j.1399-3011.2000.00163.x. 10.1034/j.1399-3011.2000.00163.x. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Calle R, Zawalich K, Smallwood JI, Zawalich WS, Rasmussen H. Glucose-induced translocation of protein kinase C in rat pancreatic islets. Proc Natl Acad Sci U S A. 1990;87:9893–9897. doi: 10.1073/pnas.87.24.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Harris TE, Persaud SJ, Jones PM. Atypical isoforms of PKC and insulin secretion from pancreatic β cells: evidence using Gö 6976 and Ro-31–8220 as PKC inhibitors. Biochem Biophys Res Commun. 1996;227:672–676. doi: 10.1006/bbrc.1996.1567. 10.1006/bbrc.1996.1567. [DOI] [PubMed] [Google Scholar]
- Harris TE, Persaud SJ, Jones PM. Pseudosubstrate peptide inhibitors of β-cell protein kinases: altered selectivity after myristoylation. Mol Cell Endocrinol. 1999;155:61–68. doi: 10.1016/s0303-7207(99)00114-8. 10.1016/S0303-7207(99)00114-8. [DOI] [PubMed] [Google Scholar]
- Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- Hii CS, Jones PM, Persaud SJ, Howell SL. A re-assessment of the role of protein kinase C in glucose-stimulated insulin secretion. Biochem J. 1987;246:489–493. doi: 10.1042/bj2460489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PM, Persaud SJ, Howell SL. Protein kinase C and the regulation of insulin secretion from pancreatic β cells. J Mol Endocrinol. 1991;6:121–127. doi: 10.1677/jme.0.0060121. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Hoenig M. Identification and subcellular characterization of protein kinase C isoforms in insulinoma β-cells and whole islets. Endocrinology. 1994;135:881–886. doi: 10.1210/endo.135.3.8070382. 10.1210/en.135.3.881. [DOI] [PubMed] [Google Scholar]
- Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö-6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- Mashima H, Ohnishi H, Wakabayashi K, Mine T, Miyagawa J, Hanafusa T, Seno M, Yamada H, Kojima I. Betacellulin and activin A coordinately convert amylase-secreting pancreatic AR42J cells into insulin-secreting cells. J Clin Invest. 1996;97:1647–1654. doi: 10.1172/JCI118591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez CF, Leibiger IB, Leibiger B, Hoy M, Gromada J, Berggren PO, Bertorello AM. Rapid association of protein kinase C-ɛ with insulin granules is essential for insulin exocytosis. J Biol Chem. 2003;278:44753–44757. doi: 10.1074/jbc.M308664200. 10.1074/jbc.M308664200. [DOI] [PubMed] [Google Scholar]
- Mogami H, Kanzaki M, Nobusawa R, Zhang YQ, Furukawa M, Kojima I. Modulation of adenosine triphosphate-sensitive potassium channel and voltage-dependent calcium channel by activin A in HIT-T15 cells. Endocrinology. 1995;136:2960–2966. doi: 10.1210/endo.136.7.7789321. 10.1210/en.136.7.2960. [DOI] [PubMed] [Google Scholar]
- Mogami H, Zhang H, Suzuki Y, Urano T, Saito N, Kojima I, Petersen OH. Decoding of short-lived Ca2+ influx signals into long-term substrate phosphorylation through activation of two distinct classes of protein kinase C. J Biol Chem. 2003;278:9896–9904. doi: 10.1074/jbc.M210653200. 10.1074/jbc.M210653200. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95:307–318. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- Ohmori S, Sakai N, Shirai Y, Yamamoto H, Miyamoto E, Shimizu N, Saito N. Importance of protein kinase C targeting for the phosphorylation of its substrate, myristoylated alanine-rich C-kinase substrate. J Biol Chem. 2000;275:26449–26457. doi: 10.1074/jbc.M003588200. 10.1074/jbc.M003588200. [DOI] [PubMed] [Google Scholar]
- Ono Y, Fujii T, Igarashi K, Kikkawa U, Ogita K, Nishizuka Y. Nucleotide sequences of cDNAs for alpha and gamma subspecies of rat brain protein kinase C. Nucl Acids Res. 1988;16:5199–5200. doi: 10.1093/nar/16.11.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud SJ, Jones PM. Inhibition of glucose-stimulated insulin secretion by Ro 31-8220, a protein kinase C inhibitor. Endocrine. 1995;3:285–289. doi: 10.1007/BF03021407. [DOI] [PubMed] [Google Scholar]
- Peter-Riesch B, Fathi M, Schlegel W, Wollheim CB. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J Clin Invest. 1988;81:1154–1161. doi: 10.1172/JCI113430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Tsuboi T, Ainscow EK, Pozzan T, Rizzuto R, Rutter GA. Dynamics of glucose-induced membrane recruitment of protein kinase C βII in living pancreatic islet β-cells. J Biol Chem. 2002;277:37702–37710. doi: 10.1074/jbc.M204478200. 10.1074/jbc.M204478200. [DOI] [PubMed] [Google Scholar]
- Rane SG, Dunlop K. Kinase C activator 1,2-oleoylacetylglycerol attenuates voltage-dependent calcium current in sensory neurons. Proc Natl Acad Sci U S A. 1986;83:184–188. doi: 10.1073/pnas.83.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai Y, Kashiwagi K, Yagi K, Sakai N, Saito N. Distinct effects of fatty acids on translocation of gamma- and epsilon-subspecies of protein kinase C. J Cell Biol. 1998;143:511–521. doi: 10.1083/jcb.143.2.511. 10.1083/jcb.143.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood JI, Gugi B, Rasmussen H. Regulation of erythrocyte Ca2+ pump activity by protein kinase C. J Biol Chem. 1988;263:2195–2202. [PubMed] [Google Scholar]
- Tian YM, Urquidi V, Ashcroft SJ. Protein kinase C in beta-cells: expression of multiple isoforms an involvement in cholinergic stimulation of insulin secretion. Mol Cell Endocrinol. 1996;119:185–193. doi: 10.1016/0303-7207(96)03811-7. 10.1016/0303-7207(96)03811-7. [DOI] [PubMed] [Google Scholar]
- Tsuru M, Katagiri H, Asano T, Yamada T, Ohno S, Ogihara T, Oka Y. Role of PKC isoforms in glucose transport in 3T3-L1 adipocytes: insignificance of atypical PKC. Am J Physiol. 2002;283:E338–E345. doi: 10.1152/ajpendo.00457.2001. [DOI] [PubMed] [Google Scholar]
- Weber M, Deng S, Kucher T, Shaked A, Ketchum RJ, Brayman KL. Adenovirus transfection of isolated pancreatic islets: a study of programmed cell death and islet function. J Surg Res. 1997;69:23–32. doi: 10.1006/jsre.1997.4995. 10.1006/jsre.1997.4995. [DOI] [PubMed] [Google Scholar]
- Yamada S, Komatsu M, Sato Y, Yamauchi K, Kojima I, Aizawa T, Hashizume K. Time-dependent stimulation of insulin exocytosis by cAMP in the rat islet β cell. Endocrinology. 2002;143:4203–4209. doi: 10.1210/en.2002-220368. 10.1210/en.2002-220368. [DOI] [PubMed] [Google Scholar]
- Yaney GC, Fairbanks JM, Deeney JT, Korchak HM, Tornheim K, Corkey BE. Potentiation of insulin secretion by phorbol esters is mediated by PKCα and nPKC isoforms. Am J Physiol. 2002;283:E880–E888. doi: 10.1152/ajpendo.00474.2001. [DOI] [PubMed] [Google Scholar]
- Yedovitzky M, Mochly-Rosen D, Johnson JA, Gray MO, Ron D, Abramovitch E, Cerasi E, Nesher R. Translocation inhibitors define specificity of protein kinase C isoenzymes in pancreatic β-cells. J Biol Chem. 1997;272:1417–1420. doi: 10.1074/jbc.272.3.1417. 10.1074/jbc.272.3.1417. [DOI] [PubMed] [Google Scholar]
- Zawalich WS, Zawalich KC. Effects of protein kinase C inhibitors on insulin secretory responses from rodent pancreatic islets. Mol Cell Endocrinol. 2001;177:95–105. doi: 10.1016/s0303-7207(01)00422-1. 10.1016/S0303-7207(01)00422-1. [DOI] [PubMed] [Google Scholar]
- Zoukhri D, Hodges RR, Sergheraert C, Toker A, Dartt DA. Lacrimal gland PKC isoforms are differentially involved in agonist-induced protein secretion. Am J Physiol. 1997;272:C263–C269. doi: 10.1152/ajpcell.1997.272.1.C263. [DOI] [PubMed] [Google Scholar]