Abstract
We used transcranial magnetic stimulation (TMS) in a paired pulse protocol to investigate interhemispheric interactions between the right dorsal premotor (dPM) and left primary motor cortex (M1) using interstimulus intervals of 4, 6, 8, 10, 12, 16 and 20 ms in ten healthy subjects. A conditioning stimulus over right dPM at an intensity of either 90 or 110% resting motor threshold (RMT) suppressed motor-evoked potentials (MEPs) evoked in the first dorsal interosseous (FDI) muscle by stimulation of left M1. Maximum effects occurred for interstimulus intervals (ISIs) of 8–10 ms. There was no effect if the conditioning stimulus was applied 2.5 cm lateral, anterior or medial to dPM. The effect differed from previously described M1 interhemispheric inhibition in that the threshold for the latter was greater than 90% RMT, whereas stimulation of the dPM at the same intensity led to significant inhibition. In addition, voluntary contraction of the left FDI (i.e. contralateral to the conditioning TMS) enhanced interhemispheric inhibition from right M1 but had no effect on the inhibition from right dPM. Finally, conditioning to right dPM at 90% RMT reduced short-interval intracortical inhibition (SICI; at ISI = 2 ms) in left M1 whilst there was no effect if the conditioning stimulus was applied to right M1. We conclude that conditioning TMS over dPM has effects that differ from the previous pattern of interhemispheric inhibition described between bilateral M1s. This may reflect the existence of commissural fibres between dPM and contralateral M1 that may play a role in bimanual coordination.
There have been many studies of interhemispheric interactions between the hand areas of the primary motor cortices (M1) using either paired pulse TMS protocols (Ferbert et al. 1992; Netz et al. 1995; Meyer et al. 1996; Di Lazzaro et al. 1999; Hanajima et al. 2001; Chen et al. 2003; Daskalakis et al. 2002) or by measuring the silent period induced by single-pulse TMS in pre-activated muscles of the ipsilateral hand (Meyer et al. 1995). Recordings of descending corticospinal volleys evoked by M1 stimulation (Di Lazzaro et al. 1999) as well as data from acallosal patients (Meyer et al. 1998) suggest that the majority of the effect is mediated by a transcallosal pathway between the hemispheres. Nevertheless, a smaller, spinal contribution may also occur (Gerloff et al. 1998).
There have been fewer studies of the interhemispheric connections of premotor areas despite the fact that premotor areas in the undamaged hemisphere may play a role in the recovery of function after hemispheric stroke (Johansen-Berg et al. 2002). Chouinard et al. (2003) applied repetitive TMS to dorsal premotor cortex (dPM) in a positron emission tomography (PET) experiment but did not report any changes in regional cerebral blood flow (rCBF) of the contralateral M1. In contrast, Mochizuki et al. (2004) found that a single TMS conditioning pulse over dPM suppressed surface motor-evoked potentials (MEPs) evoked from contralateral M1 at an interval of 150 ms. Clinical reports also suggest that activity in dPM may have bilateral effects. For example, some types of apraxia (Halsband et al. 1993) have been reported to result mainly from damage to left dPM, whereas bimanual coordination of finger movements has been reported to be associated mainly with activation of right dPM (Sadato et al. 1997). Studies in animals have confirmed the existence of direct commissural fibres from dPM to contralateral M1 (Jenny, 1979; Marconi et al. 2003).
In the present experiments we have investigated this possible interhemispheric interaction between dPM and contralateral M1 in more detail using paired pulse TMS. In particular we have tried to probe how dPM–M1 interaction may differ from the more familiar M1–M1 interaction.
Methods
Subjects
Ten healthy volunteers (seven men and three women, 21–49 years old) participated in this study. All subjects were right handed based on the Edinburgh Handedness Inventory (Oldfield, 1971). Written informed consent was obtained from all subjects. The experimental procedures used here were approved by the local Ethics Committee and were carried out in accordance with the Declaration of Helsinki.
Stimulation and recording
Motor-evoked potentials were recorded bilaterally from the first dorsal interosseous (FDI) muscles using 9 mm diameter, Ag–AgCl surface cup electrodes. The active electrode was placed over the muscle belly and the reference electrode over the metacarpophalangeal joint of the index finger. Responses were amplified with a Digitimer D360 amplifier (Digitimer Ltd, Welwyn Garden City, Herts, UK) through filters set at 20 Hz and 2 kHz, then recorded by a computer using SIGNAL software (Cambridge Electronic Devices, Cambridge, UK).
Two or three high-power Magstim 200 machines (Magstim Co., Whitland, Dyfed, UK) were used for the experiments. The conditioning stimulator was connected to a small custom-made figure-of-eight-shaped coil (external diameter 5.5 cm) in order to reduce the effective area of stimulation, and the test stimulator was connected to a standard, larger, figure-of-eight-shaped coil (external diameter 9 cm).
We used a randomized conditioning–test design similar to that reported previously (Ferbert et al. 1992; Hanajima et al. 2001). In short, various conditions (the test or conditioning stimulus given alone, or the test stimulus preceded by the conditioning stimulus at various interstimulus intervals (ISIs)) were intermixed randomly in one session, and two sessions were performed to study the complete time course of the effects. Interstimulus intervals between the conditioning and test stimulus were 4, 6, 8, 10, 12, 16 and 20 ms. Data were analysed off-line after the experiments. In each session 10 MEPs were collected for each condition in which both stimuli were given, and 12 MEPs for the control condition in which the test stimulus was given alone. The amplitude of each single MEP in each condition was measured in order to compare amplitudes of the control and conditioned MEPs in the same session. We calculated the ratio of the mean amplitude of the conditioned MEP to that of the control MEP for every ISI in each subject.
The conditioning TMS was delivered to one of five different locations over the right hemisphere: dPM, M1 hand area, dorsolateral prefrontal cortex (DLPFC), and (in a subset of subjects) either 2.5 cm medial or lateral to dPM. In all cases, the coil was orientated to induce medially directed current in the brain. M1 was defined as the ‘hot spot’ where stimulation evoked the largest MEP from the contralateral FDI muscle. Following previous reports, dPM was defined at 2.5 cm anterior and 1 cm medial site from M1 (Fink et al. 1997; Picard & Strick, 2001) and DLPFC at 5 cm anterior site from M1 (George et al. 1995; Epstein et al. 2002). The stimulus intensity was adjusted to be 90 or 110% of resting motor threshold (RMT) as defined for the M1 hand area of the same hemisphere. We defined the RMT as the lowest intensity that evoked five small responses (about 50 µV) in the contralateral FDI muscle in a series of ten stimuli when the subject kept the FDI muscles relaxed in both hands.
The test stimulus was given over the left M1 at random intervals every 4.5–5.5 s. The coil was fixed to induce medially directed current in the brain. The intensity was adjusted to evoke an MEP of approximately 1 mV peak to peak in the relaxed right FDI.
In most experiments the conditioning stimulus was applied over the right dPM and the test over the left M1. In an additional experiment, however, we investigated whether the effects were bidirectional by applying conditioning TMS to left dPM and testing right M1. In this experiment ISIs of 8 and 10 ms were investigated (the times of maximum interhemispheric effects) using a conditioning intensity of 90% RMT.
Effects of current direction on the conditioning effects of TMS
In eight subjects, a conditioning TMS pulse at 110% RMT was delivered over the right dPM or M1, using one of four different directions of induced currents: current directed anteriorly, posteriorly, medially or laterally in the brain. Each direction was investigated in a separate block of trials, with the order of the blocks randomized between subjects. ISIs between the conditioning and test stimulus were 6, 8 and 10 ms.
Effects of voluntary contraction on the conditioning effects of TMS
In eight subjects, we investigated whether the effect of a conditioning TMS pulse over the right hemisphere was modulated by tonic voluntary contraction of the left FDI muscle (i.e. contralateral to the conditioning hemisphere). Conditioning TMS at 110% RMT was delivered over right dPM or M1; the test TMS pulse was given over left M1. Subjects maintained a 20–30% maximal voluntary contraction (MVC) of the left FDI muscle whilst keeping the right FDI muscle relaxed during the experiment. The intensity of the test TMS was adjusted to evoke an MEP of approximately 1 mV peak to peak in the relaxed right FDI during contraction of the left FDI muscle. ISIs between conditioning and test stimuli were 6, 8 and 10 ms.
Effects on short-interval intracortical inhibition (SICI)
In seven subjects, we investigated the effects of a conditioning TMS pulse over right dPM or M1 on SICI in the left M1 as evaluated using paired pulse TMS over M1 (Kujirai et al. 1993). We used three high-power Magstim 200 machines; the first conditioning TMS pulse (CS1) was delivered at 90% RMT to right dPM or M1; the second conditioning TMS pulse (CS2) was applied over left M1; and finally, the test TMS pulse (TS) was given over left M1. We set the intensity of CS2 to the relatively low value of 80% active motor threshold (AMT) to avoid floor or ceiling effects on the percentage SICI. We defined the AMT as the lowest intensity that evoked five small responses (about 100 µV) in a series of ten stimuli when the subject made a 5% MVC (about 50 µV) of the right FDI. The intensity of TS was adjusted to evoke an MEP of approximately 1 mV peak to peak in the relaxed right FDI. The ISI between CS1 and CS2 was fixed at 8 ms, whilst the ISI between CS2 and TS was fixed at 2 ms. Four conditions were intermixed randomly in one session (MEP1: TS alone, control; MEP2: CS2 and TS; MEP3: CS1 and TS; and MEP4: CS1, CS2 and TS). The ratio of MEP2:MEP1 is the usual measure of SICI. The ratio of MEP4:MEP3 indicates the amount of SICI in the presence of CS1.
Statistical analyses
We performed one-factor analysis of variance (ANOVA) to test whether a conditioning TMS pulse had an effect on the size of the test MEP at any of the ISIs tested. To investigate whether these effects were dependent on the site or orientation of the conditioning pulse, we used a two-factor ANOVA (factors: site or direction and ISI). In order to test whether contraction of muscles contralateral to the conditioning hemisphere had a different effect for conditioning stimuli applied to M1 versus dPM, we used a three-factor ANOVA (factors: site, contraction and ISI). If an ANOVA showed significant main effects, we performed post hoc analyses with Scheffe's method. To investigate the effects on SICI, we used Student's paired t test. The statistical significance was set at P = 0.05.
Results
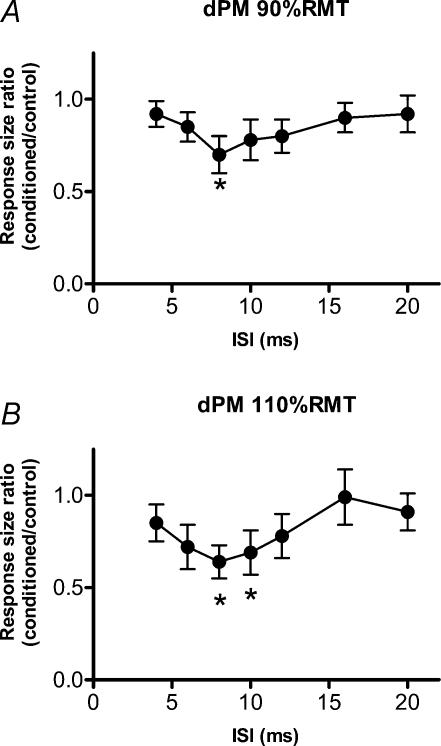
Figure 1 illustrates the time course of the effect of conditioning TMS pulses applied to right dPM on responses evoked in the FDI from left M1. Figure 1A plots data using a conditioning intensity of 90% RMT, whereas Fig. 1B shows data using 110% RMT. A one-factor ANOVA showed a significant main effect of ISI (Fig. 1A: F = 2.37, P = 0.031; and Fig. 1B: F = 2.38, P = 0.030) and post hoc analyses with Scheffe's method disclosed that TMS at 90% RMT had a significant inhibitory effect at an ISI of 8 ms and that TMS at 110% RMT had a significant inhibitory effect at ISIs of 8 and 10 ms.
Figure 1. Time course of the effects of conditioning TMS pulses applied to right dPM (A, 90% RMT; B, 110% RMT) on responses evoked in the right FDI from left M1.
n = 10. Significant inhibitory points are indicated by * (P < 0.05).
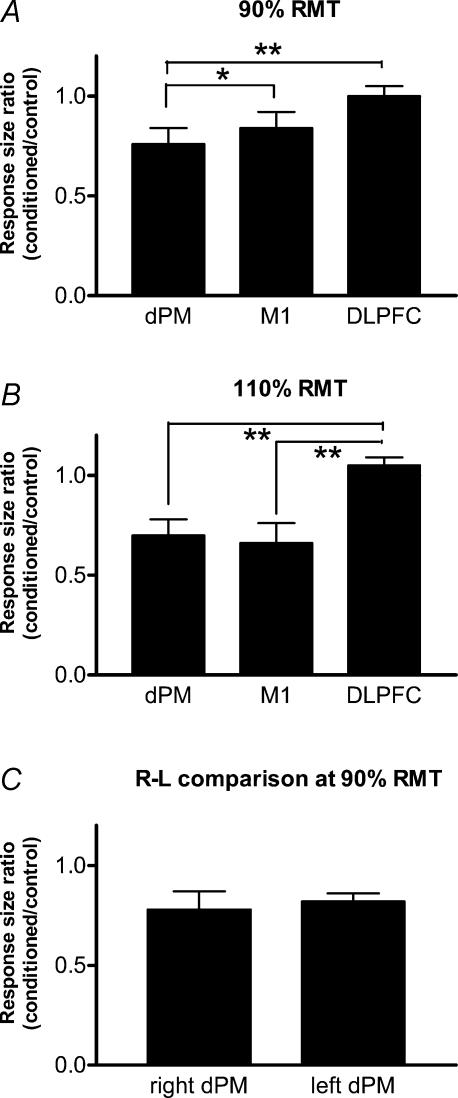
Figure 2A and B compare the mean amount of interhemispheric suppression of MEPs at ISIs of 8 and 10 ms following stimulation of dPM, M1 and DLPFC in the same ten subjects as in Fig. 1. Figure 2A shows data with a conditioning intensity of 90% RMT and Fig. 2B with a conditioning intensity of 110% RMT. A one-factor ANOVA showed a significant effect of site of stimulation in both cases (Fig. 2A: F = 5.78, P = 0.008; and Fig. 2B: F = 10.21, P < 0.001). Post hoc analysis with Scheffe's method revealed that at 90% RMT, dPM produced more suppression than either M1 or DLPFC, whereas at 110% RMT, the suppression after stimulation of M1 and dPM was equal and differed from the effect of conditioning over DLPFC (see Fig. 2A and B).
Figure 2. Comparisons of the effect of conditioning TMS over dPM, M1 and DLPFC at an intensity of 90% RMT (A, n = 10) or 110% RMT (B, n = 10) and a comparison of the effects of right–left conditioning TMS over dPM on the contralateral M1 (C, n = 8).
Significant differences are indicated by * (P < 0.05) or ** (P < 0.01).
Figure 2C compares the effect in eight subjects of right–left conditioning (i.e. conditioning stimulus to right dPM, test stimulus to left M1) and left–right conditioning after TMS of dPM at 90% RMT. Data are averages of effects at ISI 8 and 10 ms, and show no significant differences between the two directions (ANOVA: F = 0.28, P = 0.61).
With regard to MEPs evoked by conditioning TMS, only TMS over M1 at 110% RMT induced a definite MEP (mean ± s.e.m.: 1.27 ± 0.29 mV) in the contralateral FDI muscle. Conditioning over dPM at 90 or 110% RMT evoked no sign of contralateral MEPs.
Effects of changing the direction of current induced by the conditioning stimulus in the brain
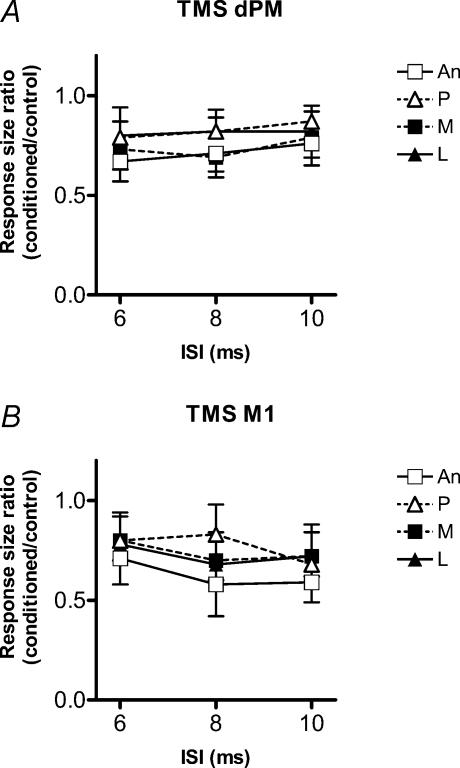
In eight subjects we compared the effectiveness of four orthogonal directions of induced current on the amount of MEP suppression produced by conditioning pulses over dPM (Fig. 3A) or M1 (Fig. 3B). The intensity of the conditioning TMS was adjusted to be 110% RMT. A two-factor ANOVA (with main factors of direction and ISI) showed no significant effects at either location (for dPM: direction, F = 1.86, P = 0.14; ISI, F = 0.60, P = 0.55; direction *ISI, F = 0.09, P = 0.99; and for M1: direction, F = 1.43, P = 0.24; ISI, F = 1.80, P = 0.17; direction *ISI, F = 0.33, P = 0.92). As reported by others (Meyer et al. 1996; Chen et al. 2003) there was a tendency for anteriorly directed current in the brain to produce a greater interhemispheric suppression after conditioning over M1, particularly at an ISI of 8 ms. However, this was not significant (P = 0.08, Student's paired t test comparison between anteriorly and posteriorly directed current).
Figure 3. Comparisons of four orthogonal directions of conditioning TMS (110% RMT) over dPM (A) or M1 (B).
n = 8. Current in the brain was directed in four directions: anteriorly (An), posteriorly (P), medially (M) and laterally (L). A two-factor ANOVA (with main factors of direction and ISI) showed no significant main effect.
Medio-lateral mapping of dPM effects
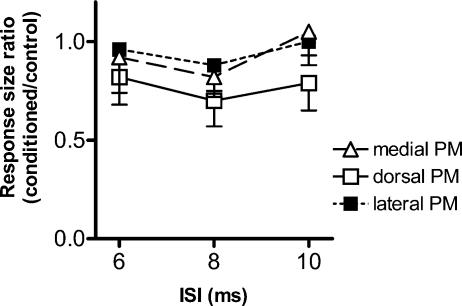
Figure 4 compares the effectiveness of conditioning stimuli at 110% RMT given to dPM and to sites 2.5 cm medial or lateral to that point, across ISIs of 6, 8 and 10 ms. A two-factor ANOVA (with main factors of conditioning site and ISI) showed a main effect of site (site, F = 4.30, P = 0.018: ISI, F = 2.74, P = 0.072; site*ISI, F = 0.22, P = 0.93). Post hoc analysis with Scheffe's method showed that conditioning at dPM produced larger suppression than conditioning at either of the other two sites. The conditioning stimulus evoked no contralateral MEP at any of these sites.
Figure 4. A comparison of the effects of conditioning TMS (110% RMT) given to dorsal PM, and to sites 2.5 cm medial (medial PM) or lateral (lateral PM) to that point.
n = 8. A two-factor ANOVA (with main factors of site and ISI) and post hoc analysis showed that dorsal PM produced larger suppression than conditioning at either of the other two sites.
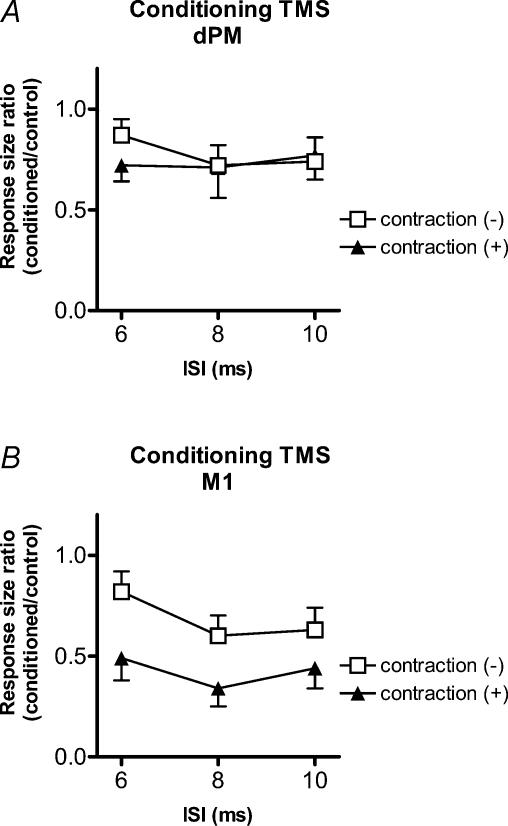
Effects of voluntary contraction on the conditioning effects of TMS over right dPM or M1
Figure 5 compares the effects of tonic contraction of the left FDI versus rest on the effectiveness of conditioning stimuli (110% RMT) applied over right dPM (Fig. 5A) or M1 (Fig. 5B). A three-factor ANOVA (with main factors of site of conditioning TMS, ISI and contraction level) revealed a significant site*contraction interaction (F = 4.45, P = 0.038). Post hoc analyses with Scheffe's method disclosed that this was due to the fact that conditioning TMS over right M1 had a larger suppressive effect if it was applied during contraction of left FDI, whereas the level of contraction had no effect on the conditioning effect of dPM stimulation.
Figure 5. Comparisons of the effects of tonic contraction of the left FDI versus rest on the effectiveness of conditioning stimuli (110% RMT) applied over right dPM (A) or M1 (B).
n = 8. A three-factor ANOVA and post hoc analyses disclosed that conditioning TMS over right M1 had a larger suppressive effect if it was applied during contraction of left FDI, whereas the level of contraction had no effect on the conditioning effect of dPM stimulation.
Effects on SICI
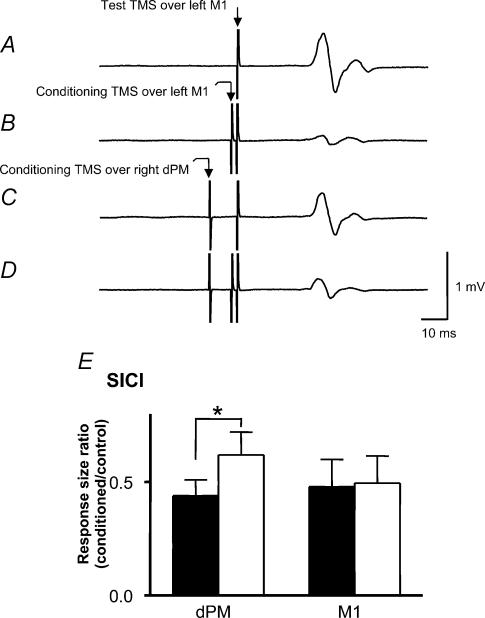
These experiments investigated the effect of conditioning stimulation over right dPM (90% RMT) on SICI in the left M1. Figure 6A–D illustrates typical raw data in one subject. Typical SICI can be seen in left M1 by comparing Fig. 6A and B. The test MEP was suppressed to 16% of its control size (from 0.86 to 0.14 mV). A conditioning stimulus over dPM given 10 ms before the test pulse to left M1 produced a small suppression of the MEP (0.63 mV). However, the same stimulus had a much larger effect on the level of SICI. Comparison of Fig. 6C and D shows that the percentage SICI had declined to about 48% of control size (from 0.63 to 0.30 mV). In other words, conditioning TMS over right dPM reduced the amount of SICI in left M1. Figure 6E compares the effect of conditioning stimuli applied over right dPM with right M1. Conditioning stimuli over right M1 had no effect on SICI in left M1 (P = 0.7), whereas conditioning stimuli over right dPM reduced SICI in left M1 (P = 0.006).
Figure 6.
A–D, traces show average MEPs from right FDI muscle in a single subject for analysis of the conditioning TMS effect (dPM, 90% RMT) on short-interval intracortical inhibition (SICI). A, a response to the test TMS pulse over left M1 (control wave). B, a preceding conditioning TMS over left M1 at 2 ms before test stimulus leads to inhibition of the test pulse (16% of control wave, usual SICI; trace B/A). C, a conditioning TMS over right dPM 10 ms before the test stimulus leads to a small inhibition of the test pulse (conditioned control wave). D, a response in the presence of conditioning TMS over right dPM 8 ms before a conditioning TMS over left M1; the conditioned SICI (trace D/C) was decreased (48% of conditioned control wave). E, comparisons of the effect on SICI of conditioning stimuli applied over right dPM or M1. Filled bars are usual SICI and open bars are conditioned SICI. Conditioning stimuli over right M1 had no effect on SICI in left M1 (P = 0.7), whereas conditioning stimuli over right dPM reduced SICI in left M1 (*P = 0.006).
Discussion
The present results show that conditioning TMS over dPM can suppress MEPs evoked by stimulation of the contralateral M1 if the ISI is about 8–10 ms. The effect is spatially specific, and differs from previously described interhemispheric effects of conditioning over M1 in three ways: threshold, effect of voluntary contraction and effects on SICI. We conclude that stimulation of dPM activates a specific set of connections that affect the excitability of responses evoked from the contralateral M1. These may use a transcallosal pathway similar to the previously described interhemispheric interaction between right and left M1.
Comparison of the interhemispheric effects of conditioning over dPM and M1
Conditioning stimuli over dPM suppressed MEPs evoked from contralateral M1 at ISIs of 8 and 10 ms. These are similar timings to those that have previously been shown to give interhemispheric inhibition between the hand areas of the left and right M1 (Ferbert et al. 1992). Since the distance between dPM and M1 in this study was less than 30 mm, it seems possible that, despite using a small (5.5 cm) figure-of-eight TMS coil, some of the effects we observed were due to spread of the conditioning stimulus to M1. However, three features of the results suggest that dPM and M1 evoke effects through different mechanisms. First, conditioning stimuli of 90% RMT produced MEP suppression if applied over dPM but had no effect if applied over M1. As noted previously by others (Ferbert et al. 1992; Hanajima et al. 2001; De Gennaro et al. 2004), in our subjects M1 interhemispheric inhibition only occurred above motor threshold with conditioning intensities of 110% RMT. The second difference between the effect of conditioning stimuli over dPM and M1 is that whereas tonic voluntary contraction of the left FDI increased M1 interhemispheric suppression (see also Ferbert et al. 1992), it had no effect on conditioning from right dPM. The former difference is thought to result from increased excitability in transcallosal connections during voluntary contraction of one hand whilst the other remains at rest. The fact that voluntary contraction of one hand has no influence on interhemispheric effects from dPM might be compatible with a role of dPM in coordination of both hands during bilateral movements (Sadato et al. 1997). Finally, conditioning TMS over right dPM (90% RMT) reduced SICI in the left M1, but conditioning TMS over right M1 at the same intensity did not. Interestingly, Daskalakis et al. (2002) noted that M1 interhemispheric suppression also could reduce SICI in the contralateral M1, but they used much higher stimulus intensities than we did (75% stimulator output, which would be approx. 180% RMT in the present subjects), suggesting involvement of a different mechanism.
Although Ferbert et al. (1992) showed that the M1 interhemispheric effects were spatially specific to the M1 hand area, the lower threshold for interhemispheric effects from dPM, together with its proximity to M1 makes it likely that some M1 interhemispheric effects measured at high stimulus intensities may well be contaminated with effects from spread of the stimulus to dPM. This could pose a particular problem, for example, in studies of hemispheric stroke patients, who might have a high threshold for M1 activation due to local damage, but in whom rostral dPM cortex is relatively intact. Precisely why the threshold for dPM is lower than for M1 is not clear. As suggested previously for effects of repetitive TMS (rTMS) (Gerschlager et al. 2001), this may be because M1 is located in the anterior bank of the central sulcus, but dPM is located on the surface of the frontal lobe (Picard & Strick, 2001). The difference in distance from the head surface to the cortical target may explain the difference of thresholds.
Is the effect of dPM conditioning exerted on the contralateral M1 cortex, and by what pathway?
Two main questions arise. First, does conditioning over dPM affect MEPs because it affects the excitability of the M1 cortex itself, or are the effects on MEPs due to activation of subcortical projections from dPM that suppress MEPs at a spinal level? Second, even if the effects are cortical, are they due to activation of a direct pathway from dPM to contralateral M1, or are the effects from dPM conducted via projections to the adjacent M1 in the same hemisphere, and then secondarily transmitted to the opposite M1? (Conversely, are they due to transcallosal transfer first to the contralateral dPM and secondarily to M1 in the same hemisphere?)
As outlined in the Introduction, M1 interhemispheric effects are thought to be primarily due to activity in a transcallosal pathway that leads to changes in the excitability of the M1 hand area. Given the similar latency to onset, the present results from conditioning over dPM are compatible with a similar direct pathway from dPM to M1. Indeed, given the fact that paired pulse TMS experiments suggest that the conduction time from dPM to the ipsilateral M1 is no less than 4 ms (Civardi et al. 2001), then the latency of MEP suppression from dPM conditioning would be too short to allow conduction through relay involving projections from dPM to M1 within the same hemisphere. Such an explanation would also be compatible with the existence of commissural fibres from dPM to contralateral premotor cortex, M1 and the supplementary motor area (SMA; Marconi et al. 2003) in monkeys.
Nevertheless, the possibility remains that some of the effects of dPM conditioning could be mediated at the spinal level rather than at the cortex. However, this cannot explain the conditioning effects on SICI since SICI is thought to reflect interactions between circuits within the cortex, rather than at a subcortical level (Kujirai et al. 1993; Di Lazzaro et al. 1998).
The effect of changing the direction of current flow in the conditioning pulse
Meyer et al. (1996) reported that M1 interhemispheric interactions were sensitive to the direction of current in the conditioning pulse, being most effective with anteriorly directed current in the brain. A more recent study by Chen et al. (2003) found no significant effect of current direction, although there was a tendency to support the original findings of Meyer et al. (1996). In the present study, we found similar results for conditioning stimulation over M1. Although there was a tendency for interhemispheric suppression to be maximal with anteriorly directed current, this was not significant. There was also no significant effect of current direction on stimulation of dPM. We conclude that the neurones responsible for the interhemispheric effects observed here do not have a preferential orientation with respect to the surface of the brain, unlike those that contribute to the MEP evoked by TMS of the hand area.
Mapping the effective location of conditioning effects from dPM
Two separate experiments showed that dPM produced clearer interhemispheric effects, at least at the ISIs tested here, than stimulation over DLPFC or over points 2.5 cm lateral or medial to dPM. Lack of an effect from DLPFC onto contralateral M1 is not surprising since there is no evidence for direct connections between these two sites in primates or humans. The site 2.5 cm medial to dPM would be located around the borderline between SMA and dPM and would again be unexpected to have a major impact on contralateral M1, since experiments in monkeys show only a sparse projection from SMA to the contralateral M1 (Liu et al. 2002). The site 2.5 cm lateral to dPM is located over ventral premotor cortex (vPM). There is no evidence that dPM is connected to contralateral M1 more strongly than vPM in animal studies or human neuroimaging studies, so we might have expected this lateral site to lead to effects on contralateral M1. Lack of an effect might, for example, reflect differences in the threshold for TMS of vPM and dPM. Alternatively, our paired pulse design may test different neurophysiological circuits from those employed by previous methods.
We have demonstrated that TMS over dPM has suppressive effects on the MEPs evoked from contralateral M1 at ISIs of 8–10 ms. We suggest that this may reflect activation of interhemispheric cortico-cortical pathways between dPM and the contralateral M1 as described in monkeys. It is possible that these interhemispheric commissural fibres have a role in bimanual coordination.
References
- Chen R, Yung D, Li J. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Van Der Werf YD, Leonard G, Paus T. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol. 2003;90:1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Bertini M, Pauri F, Cristiani R, Curcio G, Ferrara M, et al. Callosal effects of transcranial magnetic stimulation (TMS): the influence of gender and stimulus parameters. Neurosci Res. 2004;48:129–137. doi: 10.1016/j.neures.2003.10.004. 10.1016/j.neures.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, et al. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res. 1999;124:520–524. doi: 10.1007/s002210050648. 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, et al. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibition circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Sekino M, Yamaguchi K, Kamiya S, Ueno S. Asymmetries of prefrontal cortex in human episodic memory: effects of transcranial magnetic stimulation on learning abstract patterns. Neurosci Lett. 2002;320:5–8. doi: 10.1016/s0304-3940(01)02573-3. 10.1016/S0304-3940(01)02573-3. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RSJ, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6:1853–1856. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol. 1998;510:249–259. doi: 10.1111/j.1469-7793.1998.249bz.x. 10.1111/j.1469-7793.1998.249bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Halsband U, Ito N, Tanji J, Freund HJ. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain. 1993;116:243–266. doi: 10.1093/brain/116.1.243. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, et al. Interhemispheric facilitation of the hand motor area in humans. J Physiol. 2001;531:849–859. doi: 10.1111/j.1469-7793.2001.0849h.x. 10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny AB. Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol. 1979;188:137–146. doi: 10.1002/cne.901880111. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MFS, Bogdanovic MD, Kischka U, Wimalaratna S, Mattews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Febert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Morel A, Wannier T, Rouiller EM. Origins of callosal projections to the supplementary motor area (SMA): a direct comparison between pre-SMA and SMA-proper in macaque monkeys. J Comp Neurol. 2002;443:71–85. doi: 10.1002/cne.10087. 10.1002/cne.10087. [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Giannetti S, Molinari M, Caminiti R. Callosal connections of dorso-lateral premotor cortex. Eur J Neurosci. 2003;18:775–788. doi: 10.1046/j.1460-9568.2003.02807.x. 10.1046/j.1460-9568.2003.02807.x. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Kuehn A, Roericht S. Influence of the direction of induced currents on callosally and corticospinally mediated electromyographic responses following magnetic motor cortex stimulation in man. J Physiol. 1996;497:34–35. P. [Google Scholar]
- Meyer BU, Röricht S, Gräfin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Röricht S, Woiciechowsky C. Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol. 1998;43:360–369. doi: 10.1002/ana.410430314. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Terao Y, Okabe S, Furubayashi T, Arai N, Iwata NK, et al. Effects of motor cortical stimulation on the excitability of contralateral motor and sensory cortices. Exp Brain Res. 2004;158:519–526. doi: 10.1007/s00221-004-1923-0. [DOI] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Hömberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995;104:527–533. doi: 10.1007/BF00231987. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. 10.1016/S0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y. Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci. 1997;17:9667–9674. doi: 10.1523/JNEUROSCI.17-24-09667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]