Abstract
The pool size of the xanthophyll cycle pigment diadinoxanthin (DD) in the diatom Phaeodactylum tricornutum depends on illumination conditions during culture. Intermittent light caused a doubling of the DD pool without significant change in other pigment contents and photosynthetic parameters, including the photosystem II (PSII) antenna size. On exposure to high-light intensity, extensive de-epoxidation of DD to diatoxanthin (DT) rapidly caused a very strong quenching of the maximum chlorophyll fluorescence yield (Fm, PSII reaction centers closed), which was fully reversed in the dark. The non-photochemical quenching of the minimum fluorescence yield (Fo, PSII centers open) decreased the quantum efficiency of PSII proportionally. For both Fm and Fo, the non-photochemical quenching expressed as F/F′ − 1 (with F′ the quenched level) was proportional to the DT concentration. However, the quenching of Fo relative to that of Fm was much stronger than random quenching in a homogeneous antenna could explain, showing that the rate of photochemical excitation trapping was limited by energy transfer to the reaction center rather than by charge separation. The cells can increase not only the amount of DT they can produce, but also its efficiency in competing with the PSII reaction center for excitation. The combined effect allowed intermittent light grown cells to down-regulate PSII by 90% and virtually eliminated photoinhibition by saturating light. The unusually rapid and effective photoprotection by the xanthophyll cycle in diatoms may help to explain their dominance in turbulent waters.
Photosynthetic organisms have developed strategies to optimize light harvesting at low intensities while minimizing photoinhibitory damage due to excess energy at high-light intensity. From hours onwards, they regulate the quantity and composition of the light-harvesting complexes (LHCs) and of a number of other components of their photosynthetic machinery (for review, see Falkowski and Laroche, 1991; Anderson et al., 1995). On shorter time scales, they react to an unbalance between light intensity and photosynthetic capacity (e.g. due to a change in light intensity, temperature, or nutrient supply) by rapid structural modifications within the LHC of PSII (Horton et al., 1996; Bassi and Caffarri, 2000). These modifications lead to a decrease in the fluorescence yield designated as the non-photochemical quenching (NPQ) of chlorophyll (Chl) a fluorescence. NPQ is supposed to dissipate excess excitation energy through a harmless non-radiative pathway. The partitioning of absorbed energy between transfer to the reaction center and photoprotective non-radiative dissipation is controlled by the trans-thylakoid pH gradient (for recent review, see Müller et al., 2001) and by the reversible conversion of epoxidized to de-epoxidized forms of xanthophylls (the so-called xanthophyll cycle; Gilmore, 1997). The molecular mechanisms of photoprotection have mostly been studied in higher plants (Demmig-Adams and Adams, 1996). Several mutants of Arabidopsis and Chlamydomonas reinhardtii with modified violaxanthin (the epoxidated xanthophyll cycle pigment form) content and NPQ extent have been investigated and a specific role has been attributed to the PsbS protein (Niyogi, 1999; Müller et al., 2001). However, the field remains controversial due to the obvious complexity of photoprotective mechanisms. In this sense, the use of diatoms to study the role of xanthophylls in photoprotection may have important advantages.
Diatoms are well known to flourish in turbulent waters (Harris, 1986), where the amount of light available to the phytoplanktonic unicellular organisms is highly unpredictable. The deep vertical mixing continuously sweeps them up and down, exposing the cells to very large short-term changes in light intensity on a time scale of minutes to hours. The organization of the photosynthetic apparatus in diatoms differs in many respects from that of higher plants. The thylakoids are loosely appressed and organized in extended bands of three, and the PSI and PSII are not segregated in different domains (Pyszniak and Gibbs, 1992). The LHCs, which contain Chl a, Chl c, fucoxanthin, and the xanthophyll cycle pigment diadinoxanthin (DD; Brown, 1988), are equally distributed among appressed and nonappressed regions (Pyszniak and Gibbs, 1992) and there is no evidence of any state transitions (Owens, 1986). The LHCs subunits are made of several highly homologous proteins encoded by a multigene family (fucoxanthin Chl proteins; Bhaya and Grossman, 1993). The CP26 and CP29 subunits present in higher plants are not found in diatoms (Müller et al., 2001) and the existence of the PsbS protein has yet to be proven. The supramolecular organization of PSII within the membrane remains unknown. When the cells are suddenly exposed to high-light intensity, an NPQ is rapidly developed (Demers et al., 1991; Ting and Owens, 1993; Arsalane et al., 1994; Olaizola and Yamamoto, 1994; Olaizola et al., 1994; Casper-Lindley and Bjorkman, 1998). NPQ is associated with a xanthophyll cycle (the DD cycle), which differs from that of higher plants. The DD cycle converts the mono-epoxide carotenoid DD into the de-epoxide form diatoxanthin (DT) under high light, and DT back into DD under low light or in darkness (Arsalane et al., 1994; Casper-Lindley and Bjorkman, 1998).
In diatoms, the DD content can be modulated by the light regime to which culture is exposed (Willemoës and Monas, 1991; Arsalane et al., 1994; Casper-Lindley and Bjorkman, 1998; Mouget et al., 1999). We were able to define an intermittent light (IL) regime in which cells of the diatom Phaeodactylum tricornutum had an increased DD content but unchanged PSII antenna size. By comparing cells with either a low or high DD content, we studied the influence of the DD pool size on the DD cycle activity and NPQ formation under high light and investigated its functional role in protecting PSII from overexcitation. All results obtained demonstrate that a larger pool of DD leads to a more effective photoprotection.
RESULTS
Comparison of the Photosynthetic Apparatus of Continuous Light (CL) and IL Cells
P. tricornutum cells with different DD contents were obtained from cultures grown under different illumination regimes. CL cultures were grown using a 16-h-light/8-h-dark cycle. IL cultures were grown under a 5-min-light/55-min-dark cycle. In both cases, the light intensity was 40 μE m−2 s−1, which was weak enough not to induce any de-epoxidation of DD. The growth rate under IL illumination was reduced by a factor of 10, which is nearly accounted for by the 8-fold lower total amount of light supplied.
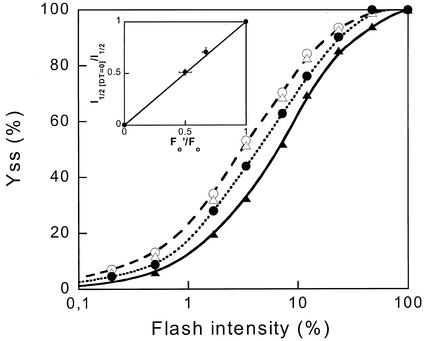
The DD content of IL cells was 2 times higher than that of CL cells (Table I). Otherwise, no significant differences were found in their pigment contents. Cell size and chloroplast ultrastructure were similar for the two types of cells. It is important to note that the PSII antenna size was the same in the two types of cells, in their dark-adapted state, as indicated by their equal fluorescence yield rise time upon illumination in the presence of DCMU (Table I). This was confirmed by the fact that equal flash intensities were required for one-half saturation of the steady-state O2 yield per flash with dark-adapted IL and CL cells.
Table I.
Pigment composition and PSII properties of the CL and IL cells
| Pigment/Parameter | CL Cells | IL Cells |
|---|---|---|
| DDb | 8.4 ± 1.1 | 15.4 ± 1.3 |
| Fucoxanthin | 68 ± 8.0 | 64 ± 4.4 |
| Chl c | 13.5 ± 1.4 | 12.0 ± 0.9 |
| β-Carotene | 7.6 ± 1.3 | 9.0 ± 0.7 |
| P700 | 0.16 ± 0.01 | 0.17 ± 0.01 |
| YSS (arbitrary units) | 29 ± 1.8 | 31 ± 1.2 |
| Fv/Fm | 0.77 ± 0.02 | 0.78 ± 0.01 |
| t1/2 of Fv with DCMU (ms) | 9 | 10 |
| I1/2 of YSS (%) | 3.8 | 4.0 |
| Isat (mE m−2 s−1) | 0.5 ± 0.05 | 0.5 ± 0.05 |
Pigment contentsa and PSII properties of P. tricornutum grown under CL and IL illumination are compared. YSS is the steady-state O2 yield per single-turnover flash, a measure of the concentration of active PSII reaction centers. Fv/Fm (Fv = Fm − Fo) is the quantum yield of excitation trapping by PSII. The one-half rise time of the fluorescence induction at a given light intensity in presence of 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea (DCMU) is a measure of the PSII antenna size. I1/2 of YSS is the flash intensity required for half-saturation of YSS, reciprocal measure of the PSII antenna size. Isat is the minimum intensity of continuous light that saturates the rate of O2 emission. Data (±sd) are the average of six measurements for pigment contents and three measurements for other parameters. All data except Isat refer to samples without DT and NPQ.
Pigment contents are given in mol per 100 mol Chl a. Total Chl a cell−1 was 0.38 ± 0.05 pg for CL cells and 0.35 ± 0.05 pg for IL cells.
The light intensity used for cell culture was weak enough to prevent any de-epoxidation of DD to DT.
NPQ Evaluated by Stern-Volmer Coefficients (SVo and SVm) in CL and IL Cells
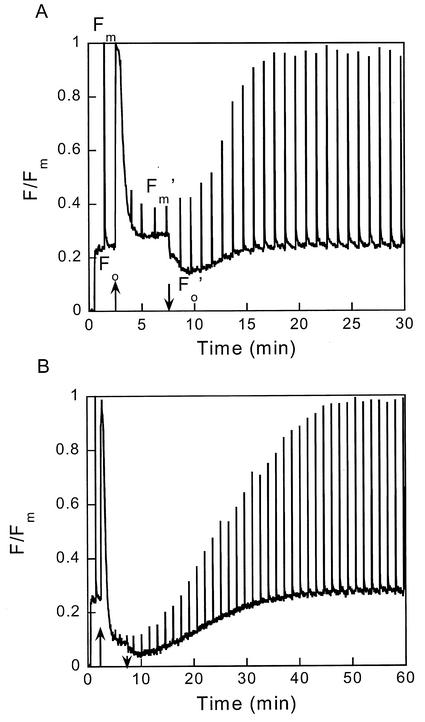
Figure 1 shows typical PAM fluorometer recordings of the Chl fluorescence yield changes induced by illumination of CL cells (A) and IL cells (B), as detected by a nonactinic modulated beam. The cells were illuminated for 5 min, indicated by up and down arrows, at an intensity of 450 μE m−2 s−1 that was just saturating for the rate of oxygen evolution. The spikes result from temporary accumulation of QA− (reduced primary acceptor of PS II) by short pulses of much stronger illumination, applied at regular intervals to probe the fluorescence yield in the absence of photochemical quenching. The data are normalized to the maximum fluorescence yield, Fm, reached when such a pulse is applied to dark-adapted cells that show only photochemical quenching and have a low fluorescence yield (Fo). During the 5-min illumination, a large NPQ rapidly developed, as shown by the lower maximum yields reached by the high intensity pulses (Fm′). This NPQ also affects the fluorescence yield of “open” PSII, as seen by the lower minimum yield (Fo′). We will use this value to indicate Fo′, but note that its real value might be even somewhat lower because the presence of some remaining QA− at this point in time cannot be ruled out. The minimum of Fo′ variations is reached in less than 2 min. Some NPQ may already have disappeared, but the corresponding Fm′ values show that NPQ relaxation is still small at this point. Recovery of the dark-adapted state took about 15 min in CL cells (Fig. 1A). In IL cells, NPQ induced by the same illumination was much more pronounced, appeared more rapidly, and disappeared more slowly than in CL cells (Fig. 1B).
Figure 1.
Fluorescence yield recordings by the pulse-amplitude-modulated (PAM) fluorometer of CL cells (A) and IL cells (B). After a few minutes of darkness (with the modulated detecting beam on), cells were illuminated for 5 min at 450 μE m−2 s−1 (between up and down arrows). At regular intervals, strong light pulses were fired to probe the fluorescence yield without photochemical quenching.
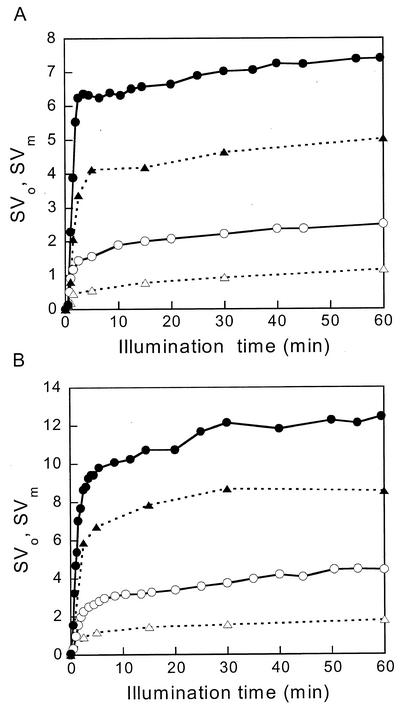
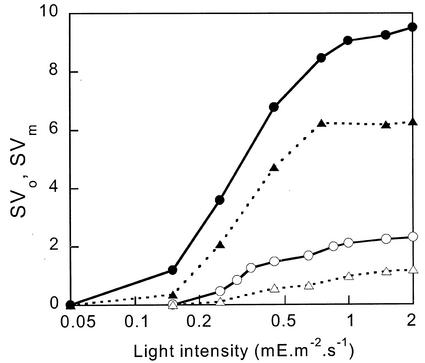
The extent of NPQ is usually quantified as F/F′ − 1, which should be proportional to the quencher concentration if a simple Stern-Volmer relation applies (see “Materials and Methods”). We will use the Stern-Volmer coefficients SVo and SVm to denote Fo/Fo′ − 1 and Fm/Fm′ − 1, respectively. The induction kinetics of SVo and SVm in the two types of cells is shown in Figure 2 for light intensities of 450 μE m−2 s−1 (A, just saturating light, Table I) and 2 mE m−2 s−1 (B, full sunlight, Long et al., 1994). The white and black symbols refer to CL and IL cells, respectively, and circles and triangles refer to SVo and SVm, respectively. In all conditions, the kinetics were similar: The major fast phase already visible in Figure 1 was followed by a much slower increase. Figure 3 shows SVo and SVm induced by 5 min of illumination as a function of light intensity. CL cells required a somewhat higher light intensity for NPQ induction than IL cells, but for both a large part of the maximum NPQ was already induced at a light intensity of 0.5 mE m−2 s−1 that was just saturating for oxygen evolution.
Figure 2.
NPQ as a function of illumination duration for two light intensities: 450 μE m−2 s−1 (A) and 2 mE m−2 s−1(B). Quenching of Fm (circles, SVm) and of Fo (triangles, SVo) in CL cells (white symbols) and IL cells (black symbols). The data were obtained from measurements as in Figure 1.
Figure 3.
NPQ as a function of light intensity for a fixed illumination duration of 5 min. SVm (circles) and SVo (triangles) for CL cells (white symbols) and IL cells (black symbols).
Correlation of NPQ and DT Accumulation
The kinetics of DT accumulation was similar to that of NPQ formation. No DT could be detected in dark-adapted cells. The amount of DT formed by de-epoxidation of DD results from the competition between epoxidation and de-epoxidation (activated by the proton gradient). At the onset of light, the first phase corresponds to the rapid transformation of DD to DT. The equilibrium reached at the end of the 5-min illumination depends on the light intensity: At low light, epoxidation competes efficiently with de-epoxidation, then de-epoxidation becomes more efficient with increasing light intensities and a quasi-steady state is reached when the equilibrium is strongly shifted in favor of DT.
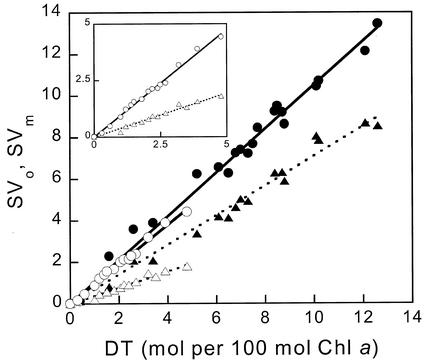
The maximum amount of DT formed, measured after 1 h of illumination at 2 mE m−2 s−1, was 5 and 12.5 mol of DT (100 mol of Chl a)−1 for CL and IL cells, respectively, corresponding to the de-epoxidation of 50% and 65% of the total DD pool. As shown in Figure 4, SVo and SVm were found to be proportional to the DT concentration for both types of cells. Although the regression coefficients for SVm were very similar (0.95 for CL and 1.04 for IL cells), those for SVo were quite different for the two types of cells: 0.39 for CL and 0.72 for IL cells. The ratio of SVo/SVm would have been equal to Fo/Fm (near 0.22 in both cell types) if the photochemical and non-photochemical quenchers were randomly competing for excitations in the same homogeneous pigment bed. The ratio SVo/SVm is, however, 0.41 for CL cells and 0.69 for IL cells. If the values used for Fo′ were slightly overestimated, even larger values of SVo would result after correction.
Figure 4.
Correlation of NPQ and DT concentration. SVm (circles) and SVo (triangles) for CL cells (white symbols, shown enlarged in the inset) and IL cells (black symbols). Linear regressions were: SVm(CL) = 0.95 [DT], SVm(IL) = 1.04 [DT], SVo(CL) = 0.39 [DT], and SVo(IL) = 0.72 [DT] with 0.98 < R2 <0.99. [DT]/[Chl a] was determined by HPLC on cells sampled at the end of the light treatment. The duration of light was varied from 30 s to 60 min and the light intensity from 0.05 to 2 mE m−2 s−1.
Decrease of the Quantum Efficiency of PSII by NPQ
Because NPQ dissipates part of the excitation energy, it is expected to decrease the quantum efficiency of PSII. This was verified by measurements of the amount of oxygen produced in short flashes as a function of flash energy (Fig. 5). The saturation curves of dark-adapted CL and IL cells (white symbols) were almost identical, confirming that the PSII antenna size was the same in both culture conditions. The flash illumination used, 20 single-turnover flashes in 10 s, corresponds to a very low continuous intensity and did not induce any NPQ. After exposure to high-light intensity, the saturation curves for flash-induced oxygen evolution were shifted to higher flash energies (black symbols). These measurements were taken 7 min after the light treatment (the time required for sedimentation on the electrode) and were compared with the fluorescence yield measured at the same time after illumination, which is already substantially less quenched by NPQ than 2 min after illumination (see Fig. 1). The remaining quenching of Fo at this time was equal to the decrease of the quantum yield of O2 evolution, as indicated by the reciprocal of the one-half-saturating flash intensity (inset, Fig. 5). This result is consistent with Equation 3 in “Materials and Methods” and may indicate that PSI fluorescence does not significantly contribute to Fo or is similarly quenched by NPQ.
Figure 5.
Steady-state O2 yield per flash (YSS) as a function of flash intensity. Dark-adapted (SVo = 0) CL cells (white circles) and IL cells (white triangles), CL cells at SVo = 0.5 (black circles) and IL cells at SVo = 1 (black triangles). SVo was measured simultaneously to YSS (after 7 min of darkness) with the PAM fluorometer. The intermediary curve correspond to CL cells with a maximal SVo value of 1.5 after cessation of illumination (15 min at 2,000 μE m−2 s−1) lowered to 0.5 min when the flash series was fired 7 min later. The curve to the right corresponded to IL cells with a maximal SVo value of 4 after cessation of illumination (5 min at 450 μE m−2 s−1) lowered to 1, when the flash sequence was fired. Inset, Relative reciprocal light intensity needed for one-half saturation of YSS (I1/2 [DT = 0]/I1/2) versus Fo′/Fo.
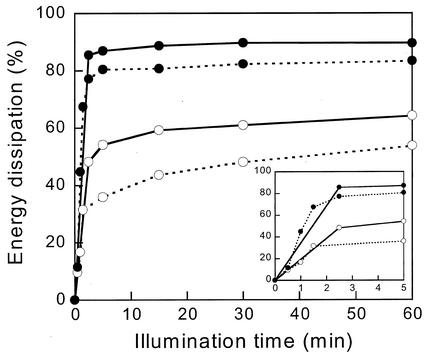
If NPQ decreases the quantum yields of fluorescence and O2 evolution equally also at stronger quenching, when the available flash energies were insufficient to determine the one-half-saturating intensity, the energy dissipation measured by 1 − Fo′/Fo equals the down-regulation of PSII by NPQ. Figure 6 shows the development of the energy dissipation in the antenna of open PSII centers for the two types of cells as a function of illumination time at 0.45 and at 2 mE m−2 s−1. The energy dissipation reached values up to 90% and was much higher for IL cells than for CL cells at both light intensities, especially after short illumination times (enlarged in the inset).
Figure 6.
Energy dissipation in the antenna of open PSII reaction centers (1 − Fo′/Fo) as a function of illumination duration. CL cells (white symbols) and IL cells (black symbols) at 450 μE m−2 s−1 (dashed lines) and 2 mE m−2 s−1 (black lines). Inset, First 5 min enlarged.
Protection by NPQ against Photoinhibition
Because CL and IL cells appeared to differ only in the capacity of energy dissipation by DT, their comparison provides a unique opportunity to verify the functional role of this process in protecting PSII against photoinhibition. Photoinhibition was evaluated by monitoring O2 evolution at the Clark electrode and measuring the time needed to reach the compensation point (Arsalane et al., 1994). As predicted by Arsalane et al. (1994), the larger the DD content, the longer time is needed to reach the compensation point (not shown).
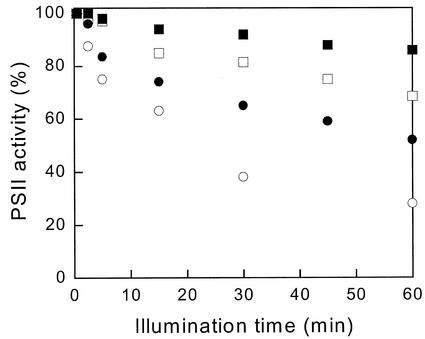
An additional method for evaluation of photoinhibition was used. Figure 7 shows the decrease of PSII activity measured as the steady-state O2 yield per flash (YSS) after illumination at 0.45 or 2 mE m−2 s−1 followed by 7 min of dark adaptation. Photoinhibition kinetics were faster for CL cells (white symbols) than for IL cells (black symbols), especially during the first 5 min. After 1 h of illumination, the remaining percentage of active PSII centers was lower for CL cells under the two light intensities. The difference between IL and CL cells is actually larger than Figure 7 suggests, because especially for IL cells the recovery from NPQ is much slower than 7 min (Fig. 1) and saturation of YSS could not be reached with the available flash energy. Because 85% or 90% of the energy was dissipated by NPQ after 1 h at 0.45 or 2 mE m−2 s−1, respectively (Fig. 6), the flash saturation curve for dark-adapted cells in Figure 5 indicates that only 87% or 79% saturation of YSS could be reached. The exact correction has not been done because combining the information from the different data in Figures 5 through 7 in this way seems too daring, but the corrections may obviously be large for IL cells. The correction would leave essentially no evidence for photoinhibition of IL cells at 0.45 mE at all. For CL cells, no such correction applies because NPQ largely disappeared in 7 min and even at 65% energy dissipation (Fig. 6), the flashes would still be more than 95% saturating (Fig. 5). The fast phase of photoinhibition seen during the first few minutes at high-light intensity correlates with the time required for accumulation of DT and NPQ and illustrates the importance of the photoprotective effect. Such a short delay in the buildup of the protection is unlikely to be of consequence even in the turbulent native environment of P. tricornutum.
Figure 7.
Photoinhibition kinetics of PSII as a function of illumination duration. CL cells (white symbols) and IL cells (black symbols) at 450 μE m−2 s−1 (squares) and 2 mE m−2 s−1 (circles). After the light treatment, cells were allowed to settle on a rate electrode for 7 min in darkness. PSII activity was estimated by measurement of the steady-state O2 yield per flash after a sequence of twenty single-turnover flashes.
DISCUSSION
DD Enrichment and the Light-Induced DT Accumulation
P. tricornutum cultures in stationary phase show an inverse relation between growth rate and DD content (Arsalane et al., 1994). To slow down the growth rate and to keep the cells in the exponential phase of growth, we grew P. tricornutum under an IL regime. When P. tricornutum are grown under such a regime, the Chl and fucoxanthin content per cell do not change, whereas the size of the DD pool is increased, in striking contrast to the effect of intermittent illumination on higher plants, which leads to a drastic reduction of PSII antenna size. IL-grown peas (Pisum sativum) are devoid of all LHCII proteins, with the exception of CP26, one of the minor inner antenna Chl-binding proteins (Jahns and Junge, 1992). This way, a specific increase in DD content could be induced without significant change in the amounts of other pigments, PSI/PSII ratio, or PSII antenna size and quantum yield.
Upon exposure to high-light intensity, a substantial de-epoxidation of DD occurred, largely within a few minutes. This in agreement with DD cycle activity previously reported (Arsalane et al., 1994; Olaizola et al., 1994; Casper-Lindley and Bjorkman, 1998). The larger pool of DD in IL cells, and its more extensive de-epoxidation, led to much larger accumulation of DT during high-light illumination. The maximal DT accumulation is 5 mol of DT (100 mol of Chl a)−1 in CL cells and 12.5 mol of DT (100 mol of Chl a)−1 in IL cells. In higher plants, Ruban et al. (1999) have estimated that each LHCII monomer can bind at least one violaxanthin. Upon activation of the violaxanthin de-epoxidase, the highest de-epoxidation state was found for the main LHCII components and the lowest for CP29. Very little is known about the nature of the subunits of LHC in diatoms (Bhaya and Grossman, 1993) and it seems premature to speculate on their DT-binding sites at this stage.
Correlation of Light-Induced DT Accumulation and NPQ
The Stern-Volmer parameters SVo and SVm relate the NPQ on Fo and Fm to the quencher concentration (Bilger and Björkman, 1990; Gilmore and Yamamoto, 1991; Olaizola and Yamamoto, 1994). Random quenching in a homogenous antenna would produce a normalized fluorescence yield as depicted by Equation 1 (see “Materials and Methods”). For the quenching of Fm, DT is the only quencher present in the LHC of PSII, the photochemical quenching being totally suppressed. In that case, the slope of SVm is the same for IL and CL cells. (SVm)IL can reach values higher than (SVm)CL, the difference being only due to the difference in the maximal concentration of DT that can be formed (see Fig. 4). For the Fo level, the photochemical quencher (Q) is present (PSII centers are open) and the two types of quenchers compete for the trapping of the excitation. If the quenchers were randomly competing in a homogeneous antenna, the slope of SVo should be Fo/Fm × slope of SVm (see Eq. 2 in “Materials and Methods”). It is not the case: The observed SVo/SVm ratios were much larger than Fo/Fm, especially for IL cells. The discrepancy is far too large to be substantially affected by possible errors in the determination of the extent of photochemical quenching due to quenching by the plastoquinone pool or incomplete oxidation of QA− after illumination. Our results provide an additional indirect proof that excitation transfer to the open PSII reaction centers becomes rate limiting for trapping by open PSII reaction centers, causing a heterogeneity of the Chl fluorescence yield in the PSII antenna. The selective increase of SVo indicates that the Chls involved contribute a larger fraction of the emission at Fo than at Fm.
Functional Significance of Energy Dissipation by DT and Photoinhibition
Most studies on NPQ consider only the quenching of Fm. Although the quenching of Fm presumably helps to diminish singlet oxygen production in the antenna by reducing the yield of Chl triplet formation, the quantitative significance of this protective effect under physiological conditions is probably negligible. The triplet yield of Chl a is twice the fluorescence yield and in an aerobic environment its very fast transfer to carotenoids followed by intersystem crossing to the ground state is essential anytime. Only the reaction center Chl itself cannot be protected this way (van Gorkom and Schelvis, 1993), but at Fm the PSII reaction center does not quench fluorescence (Thielen and van Gorkom, 1981). Hence, the probability that the triplet state is formed there, rather than in the antenna, is as small as the fraction of Chl a contained in the reaction center. At Fo, on the other hand, most excitations cause a charge separation. If that cannot be stabilized by secondary electron transfer reactions due to saturation of electron transport, it will most likely recombine to the reaction center triplet state (van Gorkom, 1985, 1986). Any mechanism to avoid that would be physiologically significant and could bring a decisive advantage under continually and widely changing light intensity. Our results show that the xanthophyll cycle in P. tricornutum provides such a mechanism.
The quenching of Fo is a direct measure of the extent of down-regulation of PSII electron transport resulting from NPQ. We have shown that energy dissipation by DT equally decreases the quantum yield of O2 evolution and the quantum yield of fluorescence emission when the PSII centers are open. The corresponding effect in higher plants may be small (see Santabarbara et al., 2001), but our results clearly confirm the large decrease of PSII quantum efficiency by NPQ in P. tricornutum, reported by Koblizek et al. (2001). Moreover, this unusual capacity for down-regulation of PSII is adjusted to the illumination conditions during growth.
Finally, we note an intriguing observation: After 1 h at 2 mE m−2 s−1, CL cells lost up to 70% of their O2-evolving PSII reaction centers, and yet no deviation from the proportionality between SVo, SVm, and DT is seen in the inset of Figure 4. Apparently, there is nothing special about the fluorescence yield, including the variable fluorescence, of this “inactivated PSII.” This indicates that these diatoms are not photoinhibited in the classical sense and, in fact, Olaizola et al. (1994) already mentioned the absence of D1 photodamage in P. tricornutum. Evidence for the induction of an electron transport cycle around PSII that competes with oxygen evolution at high-light intensity will be presented elsewhere (Lavaud et al., 2002).
The phenomena reported here are probably not restricted to P. tricornutum. Other marine planktonic diatoms like Chaetoceros muelleri (Olaizola et al., 1994), Thalassiosira pseudonana (Demers et al., 1991), Haslea ostrearia (Mouget et al., 1999), and freshwater planktonic diatoms like Nitzschia palea (Willemoës and Monas, 1991) are able to synthesize large amounts of de-epoxidizable xanthophylls. It seems likely that the large and adjustable capacity of DT accumulation, and the fast and efficient photoprotection by the associated NPQ, are general properties of planktonic diatoms and play an important role in their successful adaptation to the strongly fluctuating light intensity in their natural habitat (Harris, 1986).
MATERIALS AND METHODS
Cultivation and Preparation of Cells
Phaeodactylum tricornutum Böhlin cells were grown photoautotrophically in sterile natural seawater F/2 medium (Guillard and Ryther, 1962). Cultures of 300 mL were incubated at 18°C in airlifts continuously flushed with sterile air. They were illuminated at a light intensity of 40 μE m−2 s−1 with white fluorescent tubes (Claude, Blanc Industrie, France) with a 16-h-light/8-h-dark cycle for CL cells or with 5-min-light/55-min-dark cycle for IL cells. Cells were harvested during the exponential phase of growth, centrifuged at 3,000g for 10 min, and resuspended in their culture medium to a final Chl a concentration of 10 μg Chl a mL−1. The algae were continuously stirred at 18°C under low CL for CL cells and under IL for IL cells until measurement.
Pigment Contents
Pigment analyses were performed by HPLC as previously described (Arsalane et al., 1994). Cells collected from the PAM fluorometer vial (see below) were frozen in liquid nitrogen. Pigments were extracted with a methanol:acetone (70:30 [v/v]) solution. The extinction coefficients used were the same as in Berkaloff et al. (1990) for Chl and as Johansen et al. (1974) for DD and DT. Cell counts were performed with a Thoma hematocymeter, using the public domain NIH Image program (National Institutes of Health, Rockville, MD).
PSI Reaction Center (P700) Concentration
P700 was determined as described by Newman and Sherman (1978). Cells were first adjusted to the same Chl a concentration and then broken by sonication and kept on ice. The light-induced absorption decrease at 700 nm was measured with a DW-2 Aminco spectrophotometer (American Instrument Co., Olis Incorporated, Bogart, GA) in dual-beam mode (reference at 730 nm) in the presence of 6 mm sodium ascorbate and 300 to 600 μm methylviologen; 30 μm DCMU was added to prevent P700+ reduction by linear electron flow. Stock solutions of sodium ascorbate (0.2 m in distilled water, Sigma, St. Louis), methylviologen (Sigma, 10 mm in distilled water), and DCMU (Sigma, 10 mm in absolute ethanol) were freshly prepared.
PSII Antenna Size Assessed by Fluorescence Emission Induction in the Presence of DCMU
PSII Chl fluorescence induction kinetics of dark-adapted cells were measured at room temperature in a laboratory-built continuous fluorometer. The setup and the data acquisition were described previously by Ritz et al. (1999). Samples were adjusted to a final concentration of 4 μg Chl a mL−1 and were dark adapted for 20 min before the experiment. Twenty micromolar DCMU was added 5 min before the end of the dark period.
O2 Concentration and Photosynthetic Light Response Curves
O2 concentration was measured with a DW1-Clark electrode (Hansatech Ltd., King's Lynn, UK) at 18°C. White light of adjustable intensity (measured with a PAR-sensor, LI-COR, Lincoln, NE) was provided by KL-1500 quartz iodine lamp (Schott, Mainz, Germany). Cell culture samples were dark adapted for 20 min before measurement. Photosynthetic light-response curves were obtained by illuminating a 2-mL sample during 5 min at various intensities. A new sample was used for each intensity.
Chl Fluorescence Yield Measurements
The Clark electrode set-up was modified to allow simultaneous measurement of oxygen concentration and Chl fluorescence by a PAM-101 fluorometer (Walz, Effeltrich, Germany) as described previously (Ritz et al., 1999). The fluorescence excited by a very weak (nonactinic) modulated 650 nm of light was measured. After 20 min of dark adaptation, continuous actinic light of adjustable intensity was switched on. Six hundred-millisecond pulses of white light (4 mE m−2 s−1) were admitted by an electronic shutter (Uniblitz, Vincent, Rochester, NY, opening time 2 ms) placed in front of a KL-1500 quartz iodine lamp continuously switched on to monitor the NPQ evolution. The average fluorescence (acquisition time 33 μs) measured during the last 400 ms of the pulse was taken as Fm or Fm′. Data were recorded with a microcomputer through a 12-bit analog-digital interface and the system was driven by homemade software (Arsalane et al., 1993). For each experiment, 2 mL of cell suspension was used. Sodium bicarbonate (Labosi, Elancourt, France) was added at a concentration of 4 mm from a freshly prepared 0.2 m stock solution in distilled water to prevent any limitation of the photosynthetic rate by carbon supply.
Standard fluorescence nomenclature was used (van Kooten and Snel, 1990). Fo and Fm are defined as the fluorescence yield of dark-adapted cells and the maximum fluorescence reached in such cells during a saturating pulse of white light, respectively. The quantum yield of excitation trapping by PSII is the ratio Fv/Fm, where Fv is the variable part of the fluorescence emission and is equal to Fm − Fo. NPQ is quantified as F/F′ − 1, where F′ is the fluorescence yield in the presence of quenching (Bilger and Björkman, 1990; Gilmore and Yamamoto, 1991). We use the Stern-Volmer parameters SVo and SVm to indicate NPQ of Fo and NPQ of Fm, respectively. The fluorescence yields Fo, Fm, Fo′, and Fm′ are indicated in Figure 1A.
The following formalism was used to relate the Stern-Volmer equations with the amounts of photochemical (Q for quinones) and non-photochemical (DT) quenchers. The decay rate of the excited state of Chl a molecules in PSII is:
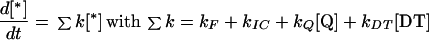 |
where kF is the rate sum of the constant of fluorescence emission, kIC is the sum of the rate constant of intersystem crossing to the Chl a triplet state and the rate constant of internal conversion to the ground state, kQ [Q] is the rate constant of photochemical quenching, and kDT [DT] is the rate constant of NPQ.
The fluorescence yield is:
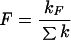 |
In the absence of NPQ, Q is the only quencher and
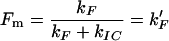 |
when Q = 0 and when Q is at its maximal concentration
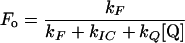 |
then,
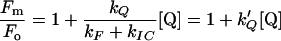 |
In the presence of the quencher DT, if Q = 0,
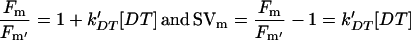 |
1 |
In the presence of both quenchers,
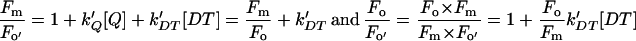 |
Then,
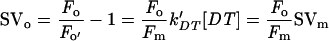 |
2 |
O2 Yield per Flash
The relative O2 yield produced per flash during a sequence of single-turnover saturating flashes at a frequency of 2 Hz was measured with a rate electrode described by (Lemasson and Etienne, 1975). Short (5-μs) saturating flashes were produced by a Strobotac (General Radio Co., Concord, MA). O2 evolution was monitored at 18°C. For the control sequences, cells were first dark adapted during 20 min and then deposited on the electrode. Both control and illuminated samples taken from the PAM fluorometer were allowed to settle on the electrode for 7 min in darkness before measurement. The steady-state O2 yield per flash (YSS) was taken to be the average yield of the last four flashes of a series of 20 flashes when the classical four-step oscillations due to the S states cycle (Kok et al., 1970) was almost damped. YSS was used to assess the relative concentration of O2-evolving PSII reaction centers. For the saturation curves of YSS, the intensity of the flashes was varied with neutral density filters. Samples were taken from the PAM fluorometer after illumination and deposited on the electrode. For each flash intensity, a new sample was used. In parallel, fluorescence yield was measured with the PAM setup at the same time after illumination.
The reciprocal of the one-half-saturating flash intensity as a measure of the antenna size was expressed as follows. The quantum yield of photochemistry is given by the equation:
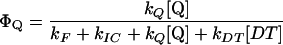 |
and should be inversely proportional to the amount of light needed to activate a given fraction of the reaction centers, so that:
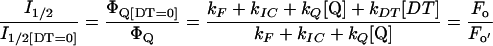 |
3 |
with I1/2 the light intensity needed for one-half saturation of YSS in the presence of DT and I1/2 [DT = 0] the light intensity needed for one-half saturation of YSS in absence of DT.
ACKNOWLEDGMENTS
We thank Prof. Jean-Claude Duval for experimental assistance and helpful discussions, Christiane Lichtlé for electron microscopic photographs, and Dr. Krishna Niyogi and Andrew Pascal for critical reading of the manuscript.
Footnotes
This work was supported by the French Ministry of National Education, Research, and Technology (grant to J.L.) and by the Ecole Normale Supérieure (invited professorship to H.J.v.G.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002014.
LITERATURE CITED
- Anderson JM, Chow WS, Park Y-I. The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res. 1995;46:129–139. doi: 10.1007/BF00020423. [DOI] [PubMed] [Google Scholar]
- Arsalane W, Paresys G, Duval J-C, Wilhelm C, Conrad R, Büchel C. A new fluorometric device to measure the in vivo chlorophyll a fluorescence yield in microalgae and its use as a herbicide monitor. Eur J Phycol. 1993;28:247–252. [Google Scholar]
- Arsalane W, Rousseau B, Duval J-C. Influence of the pool size of the xanthophyll cycle on the effects of light stress in a diatom: competition between photoprotection and photoinhibition. Photochem Photobiol. 1994;60:237–243. [Google Scholar]
- Bassi R, Caffarri S. LHC proteins and the regulation of photosynthetic light harvesting function by xanthophylls. Photosynth Res. 2000;64:243–256. doi: 10.1023/A:1006409506272. [DOI] [PubMed] [Google Scholar]
- Berkaloff C, Caron L, Rousseau B. Subunit organization of PSI particles from brown algae and diatoms: polypeptide and pigments analysis. Photosynth Res. 1990;23:181–193. doi: 10.1007/BF00035009. [DOI] [PubMed] [Google Scholar]
- Bhaya D, Grossman AR. Characterization of gene clusters encoding the fucoxanthin chlorophyll proteins of the diatom Phaeodactylum tricornutum. Nucleic Acids Res. 1993;21:4458–4466. doi: 10.1093/nar/21.19.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger W, Björkman O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res. 1990;25:175–185. doi: 10.1007/BF00033159. [DOI] [PubMed] [Google Scholar]
- Brown JS. Photosynthetic pigment organization in diatoms (Bacillariophyceae) J Phycol. 1988;24:96–102. [Google Scholar]
- Casper-Lindley C, Bjorkman O. Fluorescence quenching in four unicellular algae with different light-harvesting and xanthophyll-cycle pigments. Photosynth Res. 1998;56:277–289. [Google Scholar]
- Demers S, Roy S, Gagnon R, Vignault C. Rapid light-induced changes in cell fluorescence and in xanthophyll-cycle pigments of Alexandrium excavatum (Dynophyceae) and Thalassiosira pseudonana (Bacillariophyceae): a photo-protection mechanism. Mar Ecol Prog Ser. 1991;76:185–193. [Google Scholar]
- Demmig-Adams B, Adams WW. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- Falkowski PG, Laroche J. Acclimation to spectral irradiance in algae. J Phycol. 1991;27:8–14. [Google Scholar]
- Gilmore AM. Mechanistics aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant. 1997;99:197–209. [Google Scholar]
- Gilmore AM, Yamamoto HY. Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol. 1991;96:635–643. doi: 10.1104/pp.96.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard RRR, Ryther JH. Studies of marin planktonic diatoms: I. C. nana (Hustedt) and D. confervacea (Cleve) Gran Can J Microbiol. 1962;8:229–238. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Harris GP. Phytoplankton Ecology: Structure, Function, and Fluctuation. London: Chapman & Hall; 1986. [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Jahns P, Junge W. Thylakoids from pea seedlings grown under intermittent light: biochemical and flash-spectrophotometric properties. Biochemistry. 1992;31:7390–7397. doi: 10.1021/bi00147a025. [DOI] [PubMed] [Google Scholar]
- Johansen JE, Wa S, Liaaen-Jensen S, Haxo FT. Carotenoids of the Dinophyceae. Phytochemistry. 1974;13:2261–2271. [Google Scholar]
- Koblizek M, Kaftan D, Nebdal L. On the relationship between the non-photochemical quenching of the chlorophyll fluorescence and photosystem II light harvesting efficiency. A repetitive flash fluorescence induction study. Photosynth Res. 2001;68:141–152. doi: 10.1023/A:1011830015167. [DOI] [PubMed] [Google Scholar]
- Kok B, Forbush B, McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970;11:457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Lavaud J, van Gorkom HJ, Etienne A-L (2002) Photosystem II electron transfer cycle and chlororespiration in planktonic diatoms. Photosynth Res (in press) [DOI] [PubMed]
- Lemasson C, Etienne A-L. Photo-inactivation of system II centers by carbonyl cyanide m-chlorophenylhydrazone in Chlorella pyrenoidosa. Biochim Biophys Acta. 1975;408:135–142. doi: 10.1016/0005-2728(75)90005-5. [DOI] [PubMed] [Google Scholar]
- Long S, Humphries S, Falkowski P. Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:633–662. [Google Scholar]
- Mouget J-L, Tremblin G, Morant-Manceau A, Morançais M, Robert J-M. Long-term photoacclimation of Haslea ostrearia (Bacillariophyta): effect of irradiance on growth rates, pigment content and photosynthesis. Eur J Phycol. 1999;34:109–115. [Google Scholar]
- Müller P, Li X-P, Niyogi KK. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ, Sherman LA. Isolation and characterization of photosystem I and II membrane particles from the blue-green alga, Synechococcus cedrorum. Biochim Biophys Acta. 1978;503:343–361. doi: 10.1016/0005-2728(78)90193-7. [DOI] [PubMed] [Google Scholar]
- Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- Olaizola M, Laroche J, Kolber Z, Falkowski PG. Non-photochemical fluorescence quenching and the diadinoxanthin cycle in a marine diatom. Photosynth Res. 1994;41:357–370. doi: 10.1007/BF00019413. [DOI] [PubMed] [Google Scholar]
- Olaizola M, Yamamoto HY. Short-term response of the diadinoxanthin cycle and fluorescence yield to high irradiance in Chaetoceros muelleri (Bacillariophyceae) J Phycol. 1994;30:606–612. [Google Scholar]
- Owens TG. Light-harvesting function in the diatom Phaeodactylum tricornutum: II. Distribution of excitation energy between the photosystems. Plant Physiol. 1986;80:739–746. doi: 10.1104/pp.80.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyszniak AM, Gibbs SP. Immunocytochemical localisation of photosystem I and the fucoxanthin-chlorophyll a/c light-harvesting complex in the diatom Phaeodactylum tricornutum. Protoplasma. 1992;166:208–217. [Google Scholar]
- Ritz M, Neverov KV, Etienne A-L. ΔpH-dependent fluorescence quenching and its photoprotective role in the unicellular red alga Rhodella violacea. Photosynthetica. 1999;37:267–280. [Google Scholar]
- Ruban AV, Lee PJ, Wentworth M, Young AJ, Horton P. Determination of the stoichiometry and strength of binding of xanthophylls to the photosystem II light harvesting complexes. J Biol Chem. 1999;274:10458–10465. doi: 10.1074/jbc.274.15.10458. [DOI] [PubMed] [Google Scholar]
- Santabarbara S, Barbato R, Zuccheli G, Garlasch FM, Jennings RC. The quenching of photosystem II fluorescence does not protect the D1 protein against light induced degradation in thylakoids. FEBS Lett. 2001;505:159–162. doi: 10.1016/s0014-5793(01)02796-x. [DOI] [PubMed] [Google Scholar]
- Thielen APGM, van Gorkom HJ. Energy transfer and quantum yield in photosystem II. Biochim Biophys Acta. 1981;637:439–446. [Google Scholar]
- Ting CS, Owens TG. Photochemical and non-photochemical fluorescence quenching processes in the diatom Pheaodactylum tricornutum. Plant Physiol. 1993;101:1323–1330. doi: 10.1104/pp.101.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorkom HJ. Electron transfer in photosystem II. Photosynth Res. 1985;6:97–112. doi: 10.1007/BF00032785. [DOI] [PubMed] [Google Scholar]
- van Gorkom HJ. Photochemistry of photosynthetic reaction centers. Bioelectroch Bioeng. 1986;16:77–87. [Google Scholar]
- van Gorkom HJ, Schelvis JPM. Kok's oxygen clock: What makes it tick? The structure of P680 and consequences of its oxidizing power. Photosynth Res. 1993;38:297–301. doi: 10.1007/BF00046753. [DOI] [PubMed] [Google Scholar]
- van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- Willemoës M, Monas E. Relationship between growth irradiance and the xanthophyll cycle pool in the diatom Nitzschia palea. Physiol Plant. 1991;83:449–456. [Google Scholar]