Abstract
Carotid baroreflex (CBR) function was examined in five men and three women (25 ± 1 years) using the variable-pressure neck collar technique at rest and during dynamic, one-legged knee extension exercise at 7 W and 25 W. The CBR exhibited control of leg vascular conductance (LVC) at rest and during exercise in both an exercising leg (EL) and a non-exercising leg (NEL) across a wide range of pressures from +40 Torr neck pressure (NP) to −80 Torr neck suction (NS). Specifically, increases in LVC (% change) in response to NS were no different across −20 to −80 Torr in either EL or NEL compared to rest, P > 0.05. However, CBR-mediated decreases in percentage LVC in response to NP were attenuated in EL at both 7 W (16 ± 1%) and 25 W (12 ± 1%) compared to rest (40 ± 3%; P < 0.05) as well as compared to responses in the NEL (36 ± 6% at 7 W and 36 ± 7% at 25 W; P < 0.05). This decrease in vascular responsiveness in EL was associated with a reduction in the gain of the percentage muscle sympathetic nerve activity (%MSNA)–%LVC relationship compared to rest (P < 0.05). Collectively, these data indicate that, despite a clear attenuation of the vascular response to MSNA in the exercising leg, CBR-mediated changes in mean arterial pressure were no different between rest and exercise.
During exercise, there is a resetting of the arterial baroreflex control of mean arterial pressure (MAP) (Potts et al. 1993), heart rate (HR) (Potts et al. 1993; Norton et al. 1999a) and muscle sympathetic nerve activity (MSNA) (Fadel et al. 2001). In experiments using the well-established variable-pressure neck collar technique, the percentage change in MSNA was similar over a wide range of carotid sinus pressures at rest and during 50% of peak oxygen uptake (V̇CO2peak) arm cycling concomitant with classic rightward and upward resetting of carotid baroreflex (CBR) control of MAP (Fadel et al. 2001). Subsequently, it has been demonstrated that CBR-mediated changes in MAP were primarily the result of changes in vascular conductance, as opposed to changes in cardiac output, in resting humans (Ogoh et al. 2002). Furthermore, the CBR becomes increasingly more reliant on changes in vascular conductance in contributing to changes in MAP during steady-state leg cycling at workload intensities eliciting heart rates as low as 90 beats min−1 compared to rest (Ogoh et al. 2003). Considering that the maximal flow capacity of the exercising human quadriceps exceeds 2.5 l min−1 kg−1 (Andersen & Saltin, 1985), it becomes imperative that a balance exist between the regulation of blood flow to the exercising muscles and the regulation of arterial blood pressure.
While it is clear that baroreflex control of blood pressure is preserved during exercise, CBR control of the vasculature supplying exercising skeletal muscle, and non-exercising skeletal muscle during exercise is incompletely understood. Recently, it has been reported that the CBR exhibits control of leg vascular conductance (LVC) at rest and during one-legged knee extension exercise (Keller et al. 2003). However, these findings were based on only one near-maximal change in neck pressure (NP) and neck suction (NS) without completing the full stimulus–response curve. Furthermore, the investigation provided no indices of MSNA from rest to exercise, or in response to NP, or NS, and therefore did not demonstrate the relationship between changes in MSNA and its effect on LVC. Although additional evidence of direct CBR control of the leg vasculature during exercise has recently been demonstrated by Wray et al. (2004a) using intermittent 5 s pulses of +40 Torr NP over 5 min, the functional range of response of LVC to carotid baroreceptor stimulation and its relationship to CBR-mediated changes in MSNA remains to be defined.
O'Leary et al. (1991) and Collins et al. (2001) demonstrated the importance of baroreflex control of hindlimb vasculature during treadmill exercise in the dog and concluded that changes in hindlimb vascular conductance contributed progressively more to changes in total vascular conductance in response to bilateral carotid artery occlusion (BCO). Collins et al. (2001) further indicated that the vasculature supplying inactive tissue (i.e. renal) contributed relatively less to BCO-induced changes in total vascular conductance during steady-state exercise with increasing workloads. However, in these investigations the contribution of non-exercising skeletal muscle to baroreflex-mediated changes in total vascular conductance during exercise was not addressed. Furthermore, a progressively greater contribution of the exercising skeletal muscle vasculature to total vascular conductance has not been demonstrated in humans.
Therefore, the aim of the current investigation was to examine CBR control of leg vasculature over a wide range of carotid sinus pressures at rest and during 7 W (light) and 25 W (moderate) trials of one-legged knee extension exercise. We further sought to examine the relationship between changes in MSNA and LVC at rest and during exercise in an exercising and non-exercising leg. We hypothesized that: (1) CBR control of LVC of the exercising leg and non-exercising leg would be reset during exercise in order to regulate the prevailing blood pressure, and (2) the relationship between CBR-mediated changes in MSNA and LVC would be altered in the exercising leg but not in the non-exercising leg.
Methods
Subjects
Five men and three women (age, 25 ± 1 years; height, 156 ± 9 cm; weight, 68 ± 1 kg; mean ± s.e.m.) voluntarily participated in the present investigation. Each subject provided written informed consent, and experiments were approved by the University of North Texas Health Science Center's Institutional Review Board, and performed in accordance with the Declaration of Helsinki. Prior to participation, all subjects were familiarized with the testing protocols. Subjects were healthy, non-smokers, free of known cardiovascular and respiratory disease, and were not using prescription or over-the-counter medications. Subjects were advised not to participate in any strenuous physical activity or alcohol consumption for 24 h before any of the scheduled experiments. Subjects were also asked to refrain from the consumption of caffeinated beverages for 12 h before any of the scheduled experiments. Each subject visited the laboratory on two separate occasions.
Experimental protocol
Experimental day 1
Carotid baroreflex control of LVC was determined in each subject using the variable-pressure neck collar technique at rest and during all exercise trials on experimental day 1. After a period of resting NP/NS trials (∼1 h), subjects performed four trials of one-legged knee extension exercise in a randomized order. Subjects performed two bouts of 7 W workload exercise and two bouts of 25 W workload exercise at a kicking rate of 30 kicks per minute (kpm) using a modified cycle ergometer (Ergomedic 874 E, Monark) described by Saltin (1985). While kicking, the subjects were provided an audible cue via a metronome and verbally encouraged when necessary to maintain the consistency of each knee extension. The rate of kicking was set to 30 kpm to allow for adequate time in which the exercising leg was relaxed in order to optimize the integrity of the Doppler ultrasound measures during exercise. The effect of contraction rate on leg blood flow has previously been examined (Hoelting et al. 2001; Osada & Radegran, 2002). Osada & Radegran (2002) demonstrated that leg blood flow was linearly matched to total work rate, regardless of contraction frequency. Two exercise trials at each workload (7 W and 25 W) were performed for the collection of Doppler ultrasound measurements from an exercising leg (EL) and a non-exercising leg (NEL) during separate trials. The time of each exercise trial was limited to ∼25 min in order to eliminate the confounding effects of fatigue, or cardiovascular drift on CBR function (Norton et al. 1999b). Exercise trials were separated by a recovery period of ∼30 min to ensure the return of all measured cardiovascular variables to pre-exercise values. During periods of recovery, subjects remained in the semirecumbent seated position. On experimental days laboratory temperature was maintained between 24 and 25°C in an effort to minimize changes in skin blood flow.
Experimental day 2
Carotid baroreflex control of MSNA was determined in each subject using the variable-pressure neck collar technique at rest and during all exercise trials on experimental day 2. The resting and exercise protocols on experimental day 2 were identical to those of experimental day 1. However, only two randomized exercise trials, one at 7 W and one at 25 W, were performed for the collection of steady-state MSNA and CBR control of MSNA recordings. Microneurographic recordings of MSNA were successfully obtained in eight subjects at rest, in seven subjects during 7 W exercise and three subjects during 25 W exercise. The low success rate of maintaining MSNA recordings at the higher workload was primarily the consequence of movement artifact.
Measurements and procedures
All testing was performed with subjects in a semirecumbent ∼60 deg back-supported seated position, resulting in a ∼120 deg leg-to-torso angle to optimize one-legged exercise performance, as well as the Doppler ultrasound measurements. Cardiovascular variables were monitored beat-to-beat and recorded on a personal computer (PC) equipped with customized software (Necsuc3) that collects and records data (i.e. HR, MAP, chamber pressure) on each R-wave, as well as a second PC equipped with an on-line data acquisition program (DI-720, Dataq Instruments, Akron, OH, USA) for collection of HR, MSNA, arterial blood pressure and femoral blood velocity. Heart rate (HR) was monitored with a standard lead II electrocardiogram (ECG). The ECG signals were output to a monitor (model 78342A, Hewlett-Packard, Andover, MA, USA) interfaced with the PC. Arterial blood pressure (ABP) in seven subjects was measured using a Teflon catheter (18 gauge, 1.35 mm) placed in the femoral artery of the exercising leg connected to a pressure transducer (Maxxim Medical, Athens, TX, USA). In one subject, femoral artery catheterization was not performed due to an anatomical anomaly. In this subject, arterial blood pressure was obtained using finger-cuff photoplethysmography (Finapres, Ohmeda 2300) on the middle finger of the right hand positioned at heart level and calibrated to match the diastolic blood pressure achieved from brachial auscultation.
Leg blood flow
Leg blood flow was determined using pulsed Doppler ultrasound velocimetry using the product of the femoral artery mean blood velocity and diameter. Femoral blood velocity (FBV) was obtained using a Doppler unit (model MD6, D. E. Hokanson, Inc., Bellevue, WA, USA) with a bidirectional probe operating at a frequency of 5 MHz and calculated using the formula V = fa/(64.9cosØ), were fa is the audio frequency, Ø is the angle of insonation and V is the blood velocity in centimetres per second. The Doppler probe was placed on the skin over the common femoral artery distal to the inguinal ligament. The angle of the transducer crystal relative to the skin was ∼60 deg. Ultrasound imaging of femoral artery diameter was measured using a 2.5 MHz probe (model RT 6800, GE) at a site matching that at which velocity was measured. Average femoral artery diameter was determined at rest and during one-legged knee extension exercise in the EL and the NEL at minutes 5, 15 and 25 of each exercise trial. All ultrasound data of femoral arterial diameters were recorded onto VHS tape and further analysed using custom software. The femoral artery radius was determined for each subject at each condition using the formula: radius = diameter/2. All resting FBV and resting femoral artery diameter data were measured from one leg of each of the subjects (i.e. right or left) before any exercise trial was performed. Femoral artery diameter did not change as a result of 5 s pulses of either NP or NS and the following formula was used to calculate leg blood flow (LBF): LBF =π× radius2× FBV.
Muscle sympathetic nerve recordings
Postganglionic MSNA was recorded using standard microneurographic techniques (Vallbo et al. 1979). Briefly, a microelectrode was inserted into the peroneal nerve near the fibular head of the non-exercising leg (left leg in all subjects). The nerve signal was processed by a preamplifier and an amplifier (nerve traffic analyser model 662C-3, Department of Bioengineering, University of Iowa, Iowa City, IA, USA) with a total gain of 90 000. Amplified signals were band-pass filtered (700–2000 Hz), rectified and discriminated. Raw nerve signals were integrated by a resistance–capacitance circuit with a time constant of 0.1 s. Muscle sympathetic nerve recordings were recognized by their pulse-synchronous burst pattern and increased burst frequency with end-expiratory breath holds without any responses to arousal or skin stroking. These characteristics were used to discriminate between muscle and skin sympathetic nerve fibres while positioning the microelectrode. MSNA was expressed as burst frequency (bursts min−1) and total activity (burst frequency times mean burst amplitude) expressed in arbitrary units. While nerve recordings at the 25 W workload were few, recordings were attempted in all subjects. However, due to methodological limitations, only three were maintained during 25 W exercise.
Carotid baroreflex responsiveness
Carotid baroreflex control of MAP, LVC and MSNA were assessed at rest by applying single 5 s pulses of NP and NS ranging from +40 to −80 Torr as described by Potts et al. (1993). During screening of the subjects for this study, appropriate neck chamber placement was ensured by first fitting the subjects based on observed neck size, and then performing resting trials of NP and NS to determine directionally appropriate HR and MAP responses. In addition, when necessary, some subjects underwent ultrasound measures to ensure proper collar placement. Under resting conditions, NP/NS was applied during a 10–15 s breath hold at end-expiration, in order to minimize the respiratory modulation of HR and MAP (Eckberg et al. 1980). During exercise, NP/NS was applied without the presence of a breath hold. Breath holds were not performed during exercise in an effort to reduce the challenge to the subjects with multiple mental tasks (i.e. breath hold, maintenance of kicking frequency) to minimize the effect of complex central nervous system processing on the responses. While it possible that a discrepancy between resting and exercising conditions regarding the breath hold may alter CBR control of LBF, this is unlikely due to similar CBR control of MAP between rest and exercise. Furthermore, interpretation of the data during exercise by comparing the responses in EL and NEL, would account for any potential discrepancy. A minimum of 45 s was allowed to pass between each NP/NS trial to allow physiological variables to return to prestimulus values. Peak responses for MAP were determined as the greatest change over a 4 s period of time in response to the application of NP/NS and changes from each trial were averaged to provide a mean response for each subject. Changes in FBV were determined during the 4 s time period at which the peak MAP response occurred. An average FBV over the 4 s interval was used to assess peak FBV changes for each trial compared to an average FBV for the 4 s immediately preceding each NP/NS stimulus. A 4 s interval was chosen in an effort to minimize the effect of kicking frequency on the Doppler signal, and therefore, the contraction relative to the relaxation phase (30 kpm, 2 s kicking cycle). These changes were then averaged to provide a mean response for each NP and NS for each subject. Leg vascular conductance (LVC) was calculated using the following formula: LVC = LBF/MAP.
The MSNA responses to each NP and NS were determined over the 5 s period and presented as changes in total activity. At rest, the MSNA responses for each NP/NS were averaged to provide a mean response for each subject, which was expressed as a percentage change from the mean MSNA response obtained during a breath hold alone (i.e. control trial). During one-legged knee extension exercise, the average MSNA for each NP/NS was compared with the time interval immediately preceding the neck chamber stimulus. Estimated changes in carotid sinus pressure were calculated as the prestimulus MAP minus the chamber pressure. This estimated carotid sinus pressure (ECSP) was used in the representation of CBR control of MAP, LVC and MSNA. All NP and NS stimuli are presented in units of Torr (i.e. 40 Torr, −60 Torr, etc.), while arterial blood pressure and ECSP are presented in units of mmHg; for calculations of ECSP, 1 Torr is equal to 1 mmHg.
Data analyses
Stimulus–response curves for CBR control of MAP were fitted for each subject to a four-parameter logistic function described by Kent et al. (1972), using the following equation:
A1 is the MAP response range (maximum – minimum), A2 is the gain coefficient, A3 is the centring point (ECSP required to elicit equal pressor and depressor responses) and A4 is the minimum MAP response. The individual data were fitted to this model by non-linear least-squares regression which minimized the sum-of-squares error to predict a curve of ‘best fit’ for each data set. The gain of the CBR–MAP stimulus–response curve was derived from the first derivative of the logistic function of Kent et al. (1972), and the maximal gain (Gmax) was calculated as the gain at the centring point (A3). The threshold (i.e. point where no further increase in MAP occurred, despite reductions in ECSP), as well as the saturation (i.e. point where no further decrease in MAP occurred, despite increases in ECSP) were also determined. Carotid sinus pressure threshold and saturation (CSPthr and CSPsat) were calculated using the equations described by Chen & Chang (1991): CSPthr = −2.0/A2+A3 and CSPsat= 2.0/A2+A3. These calculations of threshold and saturation are the carotid sinus pressures at which MAP or HR are within 5% of their maximal or minimal responses. All parameters were averaged and presented as group means.
Changes in LVC were reported as both percentage changes and absolute changes from the respective baseline conditions. Previous reports have identified the relevance of using percentage changes in LVC as an index of changes in vascular responsiveness (i.e. vasoconstriction, vasodilatation) while absolute changes in conductance are indicative of the relative contribution of a given vascular bed (i.e. leg) to the total vascular system (Buckwalter & Clifford, 2001). CBR control of MSNA and LVC were unable to be modelled by the logistic function model of Kent et al. (1972) and therefore, estimates of CBR–MSNA and CBR–LVC reflex sensitivity at rest and during one-legged knee extension exercise were determined using linear regression analysis (Fadel et al. 2001). In addition, the relationship between CBR-mediated changes in MSNA and LVC (% and absolute changes) were also examined at rest and during exercise using linear regression analysis. These relationships were determined using the responses to a range of neck chamber pressures from +40 to −40 Torr so as to minimize any reductions in calculated gain within a given condition due to the extreme flatness of the CBR curve in response to increasingly hypertensive stimuli. Consequently, since responses to −60 and −80 Torr NS were not statistically different from −40 Torr NS, we did not include these points in our analyses to minimize the influence of this saturation effect on our overall gain estimates.
Statistical analyses
Comparisons of physiological variables, CBR–MAP stimulus–response parameters and CBR–LVC reflex sensitivity between rest and exercise were made using paired t tests. A two-way analysis of variance was used to determine significant differences in CBR control of %LVC between rest and exercise (7 W and 25 W) in the exercising and non-exercising leg. For comparisons of steady-state MSNA between rest and exercise at 7 W, a paired t test was used for the seven subjects for whom nerve recordings were maintained during exercise. Comparisons of steady-state MSNA at 25 W exercise were not made due to the low number of subjects for whom nerve recordings were maintained (n = 3). Statistical significance was set at P < 0.05. Values are means ± s.e.m.
Results
Cardiovascular, haemodynamic and sympathetic responses to one-legged knee extension exercise
Cardiovascular and haemodynamic measurements obtained at rest and during exercise are presented in Table 1. During 7 W exercise, MAP was no different from rest while MAP during 25 W exercise was significantly increased compared to rest and 7 W exercise (P < 0.05). Muscle sympathetic nerve activity demonstrated a tendency to decrease during 7 W exercise compared to rest (29 ± 2 versus 24 ± 3 bursts min−1), but this difference did not reach statistical significance (P = 0.059). Likewise, in the three subjects for whom sympathetic nerve recordings were maintained during the 25 W exercise bout, no major changes in MSNA were observed (27 ± 6 versus 28 ± 4 bursts min−1 at rest). As expected, during one-legged knee extension exercise, LBF and LVC progressively increased in the exercising leg above rest during 7 W and 25 W exercise (P < 0.05). In contrast, LBF in NEL did not increase at 7 W, and only a minimal increase, albeit statistically significant, occurred at 25 W (P < 0.05). However, LVC did not change in NEL during exercise at 7 W or 25 W (P > 0.05).
Table 1.
Physiological responses to one-legged knee extension exercise
| Exercise | |||
|---|---|---|---|
| Rest | 7 W | 25 W | |
| HR (beats min−1) | 72 ± 4 | 81 ± 5* | 87 ± 5*† |
| MAP (mmHg) | 86 ± 4 | 87 ± 3 | 93 ± 3*† |
| LBF (ml min−1) | |||
| Exercising leg | 261 ± 55 | 717 ± 75* | 1185 ± 129*† |
| Non-exercising leg | 261 ± 55 | 254 ± 34 | 344 ± 33*† |
| LVC (ml min−1 mmHg−1) | |||
| Exercising leg | 3.1 ± 0.7 | 8.4 ± 1.0* | 12.8 ± 1.5*† |
| Non-exercising leg | 3.1 ± 0.7 | 3.0 ± 0.4 | 3.7 ± 0.4 |
Values are means ± s.e.m. HR, heart rate; MAP, mean arterial pressure; LBF, leg blood flow; LVC, leg vascular conductance.
Significantly different from rest (P < 0.05).
Significantly different from 7 W (P < 0.05).
Carotid baroreflex control of LVC
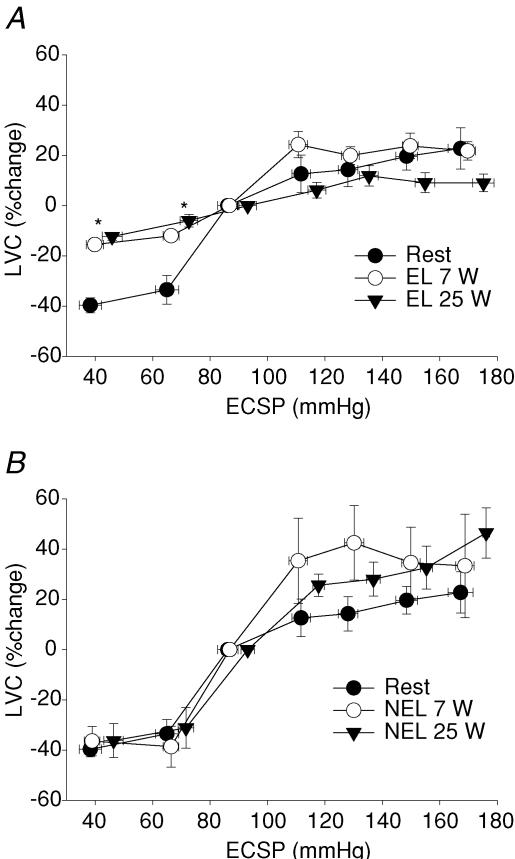
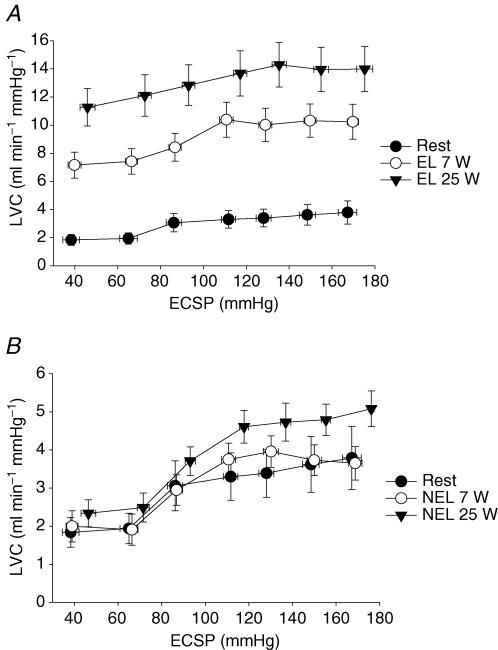
CBR-mediated changes in LVC are presented in Fig. 1 (percentage changes) and Fig. 2 (absolute changes). At rest, the application of NP resulted in decreases in LVC (−40 ± 3% to +40 Torr; −34 ± 6% to +20 Torr). During one-legged knee extension exercise at 7 W, the decreases in %LVC in the exercising leg in response to both +40 and +20 Torr NP (−16 ± 1% and −12 ± 1%, respectively) were attenuated compared to rest as well as compared to responses in NEL (−36 ± 6% and −39 ± 8%, respectively, P < 0.05). Interestingly, there was no difference between the attenuated changes in %LVC to NP of +40 and +20 Torr between exercise at 7 W and 25 W in the exercising leg (P > 0.05). Absolute changes in LVC in response to NP at rest and during exercise in both the exercising and non-exercising leg were not different across conditions (P > 0.05) (Fig. 2). The increases in LVC (percentage and absolute) in response to NS were no different across −20 to −80 Torr at rest and during exercise in either the exercising or non-exercising leg (P > 0.05). Estimated CBR–%LVC sensitivity (or gain) was significantly reduced in EL at 7 W and 25 W (0.54 ± 0.1 and 0.30 ± 0.1% mmHg−1) compared to rest (0.77 ± 0.1% mmHg−1).
Figure 1. Carotid baroreflex (CBR)-mediated percentage changes in leg vascular conductance (LVC).
CBR-mediated percentage changes in LVC, determined with the variable-pressure neck collar technique, at rest and during one-legged knee extension exercise at 7 W and 25 W in an exercising leg (EL, A) and in a non-exercising leg (NEL, B). *Significantly different from rest, P < 0.05.
Figure 2. CBR-mediated changes in absolute LVC (ml min−1 mmHg−1).
CBR-mediated changes in absolute LVC, determined with the variable-pressure neck collar technique, at rest and during one-legged knee extension exercise at 7 W and 25 W in EL (A) and NEL (B). *Significantly different from rest, P < 0.05.
Carotid baroreflex control of MSNA
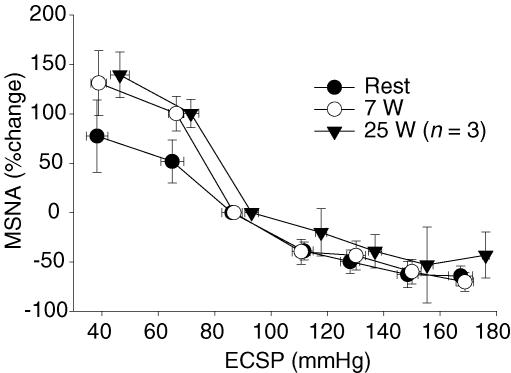
Figure 3 displays the stimulus–response relationship for CBR control of MSNA at rest and during 7 W and 25 W exercise. The percentage change in MSNA total activity in response to each NP/NS stimulus from +40 to −80 Torr was not different from rest to exercise at 7 W. Likewise, the estimated CBR–%MSNA sensitivity over an examined range of neck chamber pressures from +40 to −40 Torr were no different between rest and 7 W exercise (−1.72 ± 0.6 versus −2.88 ± 0.6% mmHg−1, respectively, P > 0.05).
Figure 3. CBR-mediated changes in muscle sympathetic nerve activity (MSNA).
CBR-mediated changes in MSNA, determined with the variable-pressure neck collar technique, at rest and during one-legged knee extension exercise at 7 W and 25 W in a non-exercising leg. Data are expressed as percentage change from baseline.
Reflex-mediated changes in MSNA and the transduction to changes in LVC
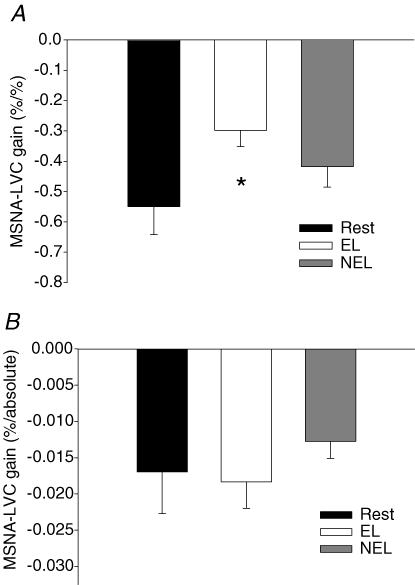
Statistical comparisons of reflex-mediated changes in MSNA and the transduction to changes in LVC were only performed between rest and 7 W exercise in EL and NEL. The gain of the relationship between changes in %MSNA and %LVC to a range of chamber pressures from +40 to −40 Torr was significantly reduced in EL compared to rest (see Fig. 4A, P < 0.05). However, the gain of the relationship between changes in %MSNA and absolute changes in LVC in EL was similar to rest (see Fig. 4B, P > 0.05). In NEL, the gain for changes in %MSNA and both percentage and absolute changes in LVC were no different compared to rest (P > 0.05).
Figure 4. Comparisons of CBR-mediated changes in MSNA and the transduction to changes in LVC between rest and 7 W exercise in EL and NEL.
A, group average gain of CBR-mediated changes in muscle sympathetic nerve activity (%MSNA) and percentage changes in leg vascular conductance (%LVC) at rest and during one-legged knee extension exercise (7 W). B, group average gain of CBR-mediated changes in %MSNA and absolute changes in LVC at rest and during one-legged knee extension exercise. Gains represent group average slopes for linear regressions of percentage changes in MSNA and changes in LVC across neck chamber pressures +40, +20, 0, −20 and −40 Torr. *Significantly different from rest, P < 0.05.
Carotid baroreflex control of MAP
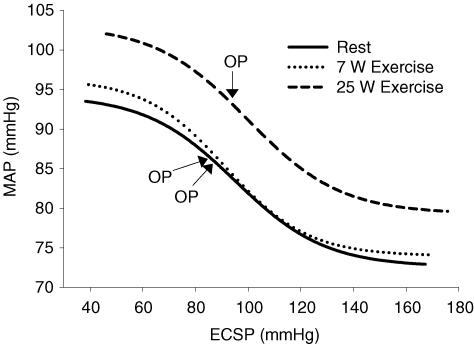
Figure 5 displays the stimulus–response curves for CBR control of MAP at rest and during 7 W and 25 W exercise. Table 2 illustrates CBR stimulus–response curve parameters for the logistic modelling. All parameters for the CBR–MAP function curves were similar for rest and exercise. However, the operating point (OP) for CBR control of MAP during 25 W exercise was shifted rightward and upward to the prevailing MAP of the exercise.
Figure 5. Carotid baroreflex–MAP stimulus–response curves.
CBR–MAP stimulus–response curves, determined with the variable-pressure neck collar technique, at rest and during one-legged knee extension exercise at 7 W and 25 W. Lines represent fitted logistic functions generated from the CBR–MAP curve parameters for the group. ECSP, estimated carotid sinus pressure; OP, operating point.
Table 2.
Average carotid baroreflex–MAP function curve parameters
| Exercise | |||
|---|---|---|---|
| Rest | 7 W | 25 W | |
| Threshold (mmHg) | 65 ± 6 | 61 ± 4 | 59 ± 7 |
| Saturation (mmHg) | 125 ± 5 | 122 ± 7 | 144 ± 7† |
| Operating point (mmHg) | 86 ± 4 | 87 ± 3 | 93 ± 3*† |
| Centring point (mmHg) | 95 ± 4 | 92 ± 4 | 101 ± 4† |
| Gmax (mmHg mmHg−1) | −0.39 ± 0.05 | −0.39 ± 0.04 | −0.034 ± 0.03 |
| Response range (mmHg) | 22 ± 3 | 23 ± 2 | 27 ± 2* |
| Minimum MAP response (mmHg) | 73 ± 4 | 74 ± 3 | 77 ± 3 |
Values are means ± s.e.m. Gmax, the maximal gain.
Significantly different from rest (P < 0.05).
Significantly different from 7 W.
Discussion
There are several new findings from the present investigation. First, carotid baroreflex control of LVC was present across a wide range of carotid sinus pressures at rest and during exercise in both exercising and non-exercising skeletal muscle. However, estimated CBR–%LVC sensitivity was significantly reduced in the exercising leg. Second, the relationship between CBR-mediated changes in MSNA and the consequent changes in %LVC was attenuated in the exercising leg compared to rest. Third, the attenuated vascular responsiveness in the exercising leg to hypotensive stimuli (NP) was not different for 7 W and 25 W. Thus, the degree of attenuation in exercising muscle did not seem to be affected by exercise intensity, which may allow for continued increases in perfusion of the exercising muscle without completely eliminating CBR control of LVC. Lastly, despite the attenuated percentage change in LVC in the exercising leg, the CBR-mediated decreases in absolute LVC were similar in the exercising and non-exercising leg. This suggests that even though blood flow is elevated 3- to 4-fold in the exercising leg during low to moderate knee extensor exercise the overall absolute contribution of the exercising and non-exercising leg to CBR-mediated changes in total vascular conductance are equivalent. Collectively these data demonstrate that, despite the modulation of sympathetic responsiveness in exercising skeletal muscle, carotid baroreflex control of LVC is maintained during exercise.
Carotid baroreflex control of LVC at rest and during exercise
In the current investigation, we have demonstrated for the first time CBR control of the leg vasculature across a range of carotid sinus pressures at rest and during one-legged knee extension exercise at 7 W and 25 W in an exercising and non-exercising leg. Thus, similar to previous reports on CBR control of HR, MAP and MSNA, it is now clear that the CBR maintains its ability to modulate LVC around the prevailing blood pressure during exercise. However, the ability of the CBR to modulate LVC is attenuated by exercise, an effect that is specific to the active muscle bed. In response to hypotensive stimuli (+40 and +20 Torr NP), CBR-induced decreases in %LVC were attenuated during both 7 W and 25 W in the exercising leg compared to rest and the non-exercising leg.
These findings using graded CBR-mediated sympathetic activation are in agreement with several animal (Thomas et al. 1994; Buckwalter et al. 2001; Ruble et al. 2002) and human investigations (Tschakovsky et al. 2002; Rosenmeier et al. 2003; Wray et al. 2004b) that have demonstrated the ability of muscle contraction to modulate pharmacologically induced sympathetic vasoconstriction (i.e. functional sympatholysis). Likewise, Keller et al. (2003) had previously demonstrated an attenuated decrease in %LVC in response to NP (+40 Torr) during exercise. However, these investigators used only one near-maximal change in NP along with one hypertensive NS (−60 Torr) without completing the full stimulus–response curve.
An interesting aspect from our findings using light to moderate knee extension exercise is that the degree of attenuation in exercising muscle did not seem to be affected by exercise intensity in that the responses to both +40 and +20 Torr NP were attenuated to a similar degree during both 7 W and 25 W exercise. This finding differs from previous reports which have indicated an intensity-dependent attenuation of sympathetic vasoconstriction to endogenous noradrenaline (norepinephrine) release in the human forearm (Tschakovsky et al. 2002) and to selective α-adrenergic vasoconstrictors in the dog hindlimb (Buckwalter et al. 2001). It is likely that these differing results between studies are due to variations in vascular beds being studied, exercise protocols employed, and/or sympathetic stimuli utilized. Nevertheless, we speculate that the lack of a further attenuation with the increasing exercise intensity to 25 W allows for increases in perfusion of the exercising muscle with increases in exercise intensity without completely eliminating CBR control of LVC. Clearly, further studies incorporating larger muscle mass exercise and using higher intensities are needed to explore such speculation.
In response to neck suction (NS), the carotid baroreflex induced increases in both %LVC and absolute LVC over a range of neck chamber pressures from −20 to −80 Torr at rest and during exercise in both the exercising and non-exercising leg. However, in contrast to NP, there were no significant differences in CBR-mediated increases in %LVC in the exercising leg at 7 W or at 25 W in comparison to rest. These findings indicate that vascular responsiveness to CBR-induced withdrawal of MSNA is unchanged during light to moderate exercise. This proposed discrepancy between NP and NS is most likely to be due to the active and passive nature of the end-organ response. The result of NP is an increase in MSNA directed to the vasculature causing some degree of active vasoconstriction which is then modulated by the influence of functional sympatholysis (Remensnyder et al. 1962; Hansen et al. 2000; Buckwalter & Clifford, 2001; Buckwalter et al. 2001; Ruble et al. 2002; Dinenno & Joyner, 2003; Joyner & Thomas, 2003; Wray et al. 2004b). On the other hand, NS causes a withdrawal of MSNA which leads to a passive dilatation of the vasculature. In the present investigation, this effect of NS to cause passive dilatation was not influenced by functional sympatholysis during light to moderate workload exercise. However, Strange et al. (1990), using repetitive pulses of NS, demonstrated that as exercise intensity increased to relatively high workloads, CBR-mediated increases in LVC were diminished during leg cycling. Thus, it is plausible that during high intensity exercise competitive vasoconstrictor influences which are not present during exercise at light or moderate workloads (i.e. angiotensin II, circulating noradrenaline, etc.) modulate the vascular response to baroreflex-mediated withdrawal of MSNA.
Tranduction of changes in MSNA to changes in LVC
In the present investigation, the percentage changes in MSNA to a given neck chamber pressure were not different between rest and 7 W exercise confirming the preservation of CBR control of MSNA during exercise initially identified by Fadel et al. (2001). The CBR control of MSNA at 25 W exercise also appeared to be similar to rest; however, caution should be taken when interpreting these data because nerve recordings at this workload could only be maintained in a limited number of subjects. More importantly, by directly measuring MSNA and LVC in the same subjects, we have demonstrated for the first time the static relationship between CBR-mediated changes in MSNA and the consequent changes in LVC at rest and during one-legged knee extension exercise in an exercising and non-exercising leg. Although the CBR control of MSNA was maintained during exercise, the relationship between CBR-mediated changes in MSNA and %LVC was attenuated in the exercising leg compared to rest and a non-exercising leg. These data demonstrate that the attenuated responses in the exercising leg are primarily the consequence of alterations in vascular responsiveness without any major effects on the actual sympathetic stimulus.
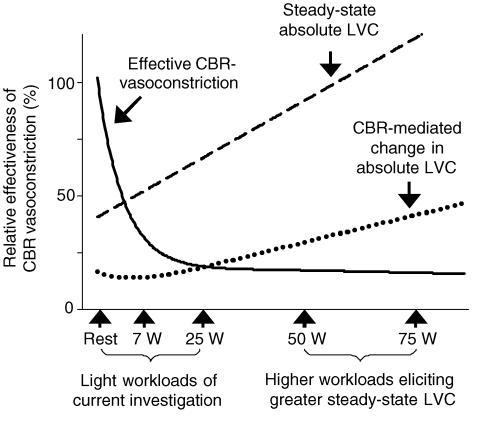
Interestingly, despite this effect of exercise to attenuate sympathetic responsiveness (i.e. functional sympatholysis) in the exercising leg, the gain between percentage changes in MSNA and absolute changes in LVC were no different in the exercising leg. This occurred despite a 3- to 4-fold increase in steady-state LVC of the exercising leg. Thus, at the low to moderate workloads employed in the current study a balance must exist between baroreflex-mediated changes in the conductance of a given vascular bed and the influence of contraction-induced attenuation of sympathetically mediated vasoconstriction such that the overall absolute contribution of exercising and non-exercising vascular beds are equivalent. However, even though it was clear that the absolute change in LVC in response to NP in the exercising leg was not different at rest or at 7 W exercise, there was a trend for a greater change in absolute LVC at 25 W exercise in the current investigation. Thus, as exercise intensity increases the absolute change in LVC may be greater in the exercising muscle even though the percentage change in LVC is attenuated. A proposed model of baroreflex control of the vasculature in an exercising leg and absolute changes in leg vascular conductance at higher workloads is presented in Fig. 6. This model takes into account several studies in dogs which demonstrate that with increasing exercise intensity, the changes in hindlimb vasculature of an exercising dog to bilateral carotid artery occlusion becomes significantly greater during exercise in a workload-dependent manner (O'Leary et al. 1991; Collins et al. 2001). Thus, a balance must exist between baroreflex-mediated changes in conductance of a given vascular bed and the influence of exercise-induced attenuation of sympathetic vasoconstriction. It is likely that this balance allows for continued increases in perfusion of the exercising muscle in conjunction with a maintained ability of the baroreflex to control vascular conductance and thus, blood pressure.
Figure 6. Proposed hypothetical model demonstrating the relationship between predicted carotid baroreflex control of LVC in an exercising leg and increasing steady-state LVC.
Note that with a proposed plateau of effective CBR vasoconstriction (i.e. sympatholysis) in an exercising leg, the absolute changes beyond a hypothetical threshold steady-state leg conductance become progressively larger. Therefore, the exercising leg may contribute more to CBR-mediated changes in arterial blood pressure with increasing steady-state leg conductance. Effective CBR vasoconstriction (continuous line), relative effectiveness of CBR-mediated vasoconstrictor stimuli derived from %LVC with the predicted plateau of the influence of functional sympatholysis. Steady-state LVC (dashed line), steady-state absolute leg vascular conductance; CBR-mediated change in LVC (dotted line), absolute change in leg vascular conductance in response to CBR hypotension (i.e. neck pressure).
Potential limitations
In this investigation, measures of MSNA were collected from the non-exercising leg. While it has been demonstrated that MSNA is global throughout the body during exercise (Seals & Victor, 1991), it remains possible that the activity between vascular beds is different. Another limitation in this investigation was our inability to model CBR–MSNA and CBR–LVC relationships using the logistic function model described by Kent et al. (1972). However, while it is apparent that this logistic function can be used to describe CBR control of MAP, HR and R–R interval, the use of this logistic function to describe CBR control of MSNA and LVC appears unsuitable in small subject populations, which is likely due to the large variability of individual responses to NP/NS for these specific variables. Fadel et al. (2001) compared CBR-mediated changes in MSNA between rest and exercise as the percentage change in MSNA from the respective baseline sympathetic outflow. We too have chosen to report changes in CBR control of MSNA in a similar fashion. Also, we identified potential changes in CBR control of LVC in a similar fashion to MSNA in that both percentage changes and absolute changes in LVC in response to each NP/NS stimulus were compared during rest and exercise. In terms of physiological significance, we used percentage changes in LVC as an index of vascular responsiveness (i.e. vasoconstriction) and absolute changes as an index of the contribution of the leg vasculature to baroreflex control of blood pressure (Buckwalter & Clifford, 2001). Another methodological limitation involves the lack of our ability when using Doppler ultrasound measures of leg blood flow to distinguish the supply of other tissue in the leg (i.e. skin, bone, inactive skeletal muscle, etc.). However, with respect to CBR-mediated changes in LVC, it is likely that any modulation during exercise would primarily originate in the active skeletal muscle.
In summary, we have demonstrated that carotid baroreflex control of LVC was present across a wide range of carotid sinus pressures both at rest and during exercise. Furthermore, the relationship between CBR-mediated changes in MSNA and the consequent changes in %LVC was attenuated in the exercising leg compared to rest, indicating an exercise-induced attenuation of sympathetic vasoconstriction in the active muscle bed. However, despite the clear attenuation of the vascular response to MSNA in the exercising leg, CBR-mediated changes in mean arterial pressure were no different between rest and exercise. These data indicate that an exquisite balance exists between baroreflex-mediated changes in conductance of a given vascular bed and the influence of exercise-induced attenuation of sympathetic vasoconstriction. It is likely that this balance allows for continued increases in perfusion of the exercising muscle in conjunction with a maintained ability of the baroreflex to control vascular conductance and thus, blood pressure.
Acknowledgments
The authors thank Lisa Marquez for secretarial support, Jill Kurschner for technical assistance and all of the subjects who participated in the study for their cooperation. This study was supported in part by National Heart, Lung and Blood Institute Grant HL-045547. This research was submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy for D. Melvin Keller.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle? Exerc Sport Sci Rev. 2001;29:159–163. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates alpha-adrenergic-receptor responsiveness in skeletal muscle vasculature. J Appl Physiol. 2001;90:172–178. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- Chen HI, Chang KC. Assessment of threshold and saturation pressure in the baroreflex function curve: a new mathematical analysis. Jpn J Physiol. 1991;41:861–877. doi: 10.2170/jjphysiol.41.861. [DOI] [PubMed] [Google Scholar]
- Collins HL, Augustyniak RA, Ansorge EJ, O'Leary DS. Carotid baroreflex pressor responses at rest and during exercise: cardiac output vs. regional vasoconstriction. Am J Physiol Heart Circ Physiol. 2001;280:H642–H648. doi: 10.1152/ajpheart.2001.280.2.H642. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol. 1980;304:489–502. doi: 10.1113/jphysiol.1980.sp013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, et al. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2001;280:H1383–H1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sander M, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in exercising skeletal muscle. Acta Physiol Scand. 2000;168:489–503. doi: 10.1046/j.1365-201x.2000.00701.x. 10.1046/j.1365-201x.2000.00701.x. [DOI] [PubMed] [Google Scholar]
- Hoelting BD, Scheuermann BW, Barstow TJ. Effect of contraction frequency on leg blood flow during knee extension exercise in humans. J Appl Physiol. 2001;91:671–679. doi: 10.1152/jappl.2001.91.2.671. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Thomas GD. Having it both ways? Vasoconstriction in contracting muscles. J Physiol. 2003;550:333. doi: 10.1113/jphysiol.2003.044628. 10.1113/jphysiol.2003.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Wasmund WL, Wray DW, Ogoh S, Fadel PJ, Smith ML, et al. Carotid baroreflex control of leg vascular conductance at rest and during exercise. J Appl Physiol. 2003;94:542–548. doi: 10.1152/japplphysiol.00817.2002. [DOI] [PubMed] [Google Scholar]
- Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- Norton KH, Boushel R, Strange S, Saltin B, Raven PB. Resetting of the carotid arterial baroreflex during dynamic exercise in humans. J Appl Physiol. 1999a;87:332–338. doi: 10.1152/jappl.1999.87.1.332. [DOI] [PubMed] [Google Scholar]
- Norton KH, Gallagher KM, Smith SA, Querry RG, Welch-O'Connor RM, Raven PB. Carotid baroreflex function during prolonged exercise. J Appl Physiol. 1999b;87:339–347. doi: 10.1152/jappl.1999.87.1.339. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Monteiro F, Wasmund WL, Raven PB. Haemodynamic changes during neck pressure and suction in seated and supine positions. J Physiol. 2002;540:707–716. doi: 10.1113/jphysiol.2001.013259. 10.1113/jphysiol.2001.013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, et al. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol. 2003;550:317–324. doi: 10.1113/jphysiol.2003.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DS, Rowell LB, Scher AM. Baroreflex-induced vasoconstriction in active skeletal muscle of conscious dogs. Am J Physiol. 1991;260:H37–H41. doi: 10.1152/ajpheart.1991.260.1.H37. [DOI] [PubMed] [Google Scholar]
- Osada T, Radegran G. Femoral artery inflow in relation to external and total work rate at different knee extensor contraction rates. J Appl Physiol. 2002;92:1325–1330. doi: 10.1152/japplphysiol.00848.2001. [DOI] [PubMed] [Google Scholar]
- Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol. 1993;265:H1928–H1938. doi: 10.1152/ajpheart.1993.265.6.H1928. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruble SB, Valic Z, Buckwalter JB, Tschakovsky ME, Clifford PS. Attenuated vascular responsiveness to noradrenaline release during dynamic exercise in dogs. J Physiol. 2002;541:637–644. doi: 10.1113/jphysiol.2001.014738. 10.1113/jphysiol.2001.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B. Hemodynamic adaptations to exercise. Am J Cardiol. 1985;55:42D–47D. doi: 10.1016/0002-9149(85)91054-9. 10.1016/0002-9149(85)91054-9. [DOI] [PubMed] [Google Scholar]
- Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev. 1991;19:313–349. [PubMed] [Google Scholar]
- Strange S, Rowell LB, Christensen NJ, Saltin B. Cardiovascular responses to carotid sinus baroreceptor stimulation during moderate to severe exercise in man. Acta Physiol Scand. 1990;138:145–153. doi: 10.1111/j.1748-1716.1990.tb08826.x. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of alpha 2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Wray D, Fadel P, Keller D, Ogoh S, Sander M, Raven P, et al. Dynamic carotid baroreflex control of the peripheral circulation during exercise in humans. J Physiol. 2004a;559:675–684. doi: 10.1113/jphysiol.2004.066183. 10.1113/jphysiol.2004.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition of α-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol. 2004b;555:545–563. doi: 10.1113/jphysiol.2003.054650. 10.1113/jphysiol.2003.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]