Abstract
Adaptations of skeletal muscle following exercise are accompanied by changes in gene expression, which can result in protection against subsequent potentially damaging exercise. One cellular signal activating these adaptations may be an increased production of reactive oxygen and nitrogen species (ROS). The aim of this study was to examine the effect of a short period of non-damaging contractions on the subsequent susceptibility of muscle to contraction-induced damage and to examine the changes in gene expression that occur following the initial contraction protocol. Comparisons with changes in gene expression in cultured myotubes following treatment with a non-damaging concentration of hydrogen peroxide (H2O2) were used to identify redox-sensitive genes whose expression may be modified by the increased ROS production during contractions. Hindlimb muscles of mice were subjected to a preconditioning, non-damaging isometric contraction protocol in vivo. After 4 or 12 h, extensor digitorum longus (EDL) and soleus muscles were removed and subjected to a (normally) damaging contraction protocol in vitro. Muscles were also analysed for changes in gene expression induced by the preconditioning protocol using cDNA expression techniques. In a parallel study, C2C12 myotubes were treated with a non-damaging concentration (100 μm) of H2O2 and, at 4 and 12 h following treatment, myotubes were treated with a damaging concentration of H2O2 (2 mm). Myotubes were analysed for changes in gene expression at 4 h following treatment with 100 μm H2O2 alone. Data demonstrate that a prior period of non-damaging contractile activity resulted in significant protection of EDL and soleus muscles against a normally damaging contraction protocol 4 h later. This protection was associated with significant changes in gene expression. Prior treatment of myotubes with a non-damaging concentration of H2O2 also resulted in significant protection against a damaging treatment, 4 and 12 h later. Comparison of changes in gene expression in both studies identified haem oxygenase-1 as the sole gene showing increased expression during adaptation in both instances suggesting that activation of this gene results from the increased ROS production during contractile activity and that it may play a role in protection of muscle cells against subsequent exposure to damaging activity.
Skeletal muscle adapts rapidly following exercise. This well-recognized process of adaptation is achieved by numerous structural and biochemical changes in the muscle cell and surrounding tissues (Goldspink, 1994) and results in significant protection against later, potentially damaging, exercise (Newham et al. 1987; Sacco & Jones, 1992; Koh & Brooks, 2001). Skeletal muscle adapts to even single bouts of unaccustomed exercise by up-regulation of a number of cytoprotective proteins (Ji, 1993; McArdle et al. 2001). Contracting skeletal muscle generates an increased amount of reactive oxygen and nitrogen species (ROS). Initial studies in this area used electron spin resonance techniques to demonstrate that skeletal muscle contained free radical species whose magnitude was increased by muscle activity (Davies et al. 1982; Jackson et al. 1985). Further studies have demonstrated that contracting diaphragm and limb muscles release increased amounts of superoxide anion (Reid et al. 1992; McArdle et al. 2001) and nitric oxide (Balon & Nadler, 1994) into the extracellular fluid and that hydroxyl radicals are formed from hydrogen peroxide (H2O2) also released from the muscle cells (O'Neill et al. 1996; McArdle et al. 2004). Many of the published studies in this area have concentrated on the potential role of ROS in damage to skeletal muscle, but it has become clear that these species are also produced during non-damaging contractions (McArdle et al. 2001) and may be a necessary signal for initiation of adaptive processes.
There is increasing evidence that exposure of cells to reactive oxygen species causes cells to respond by induction or repression of a wide variety of different genes. This appears to be due to modification of the intracellular redox balance influencing multiple signalling pathways and the activation of key transcription factors leading to a modulation of the expression of the genes controlled by these factors (Ammendola et al. 1995; Stortz & Polla, 1996; Jackson et al. 2002). This has been described in a number of different cell types (Nose et al. 1991; Wiese et al. 1995; Nakamura et al. 1997; Torti et al. 1998) and appears to be related to oxidation of key cysteine residues within the DNA-binding region of transcription factors (Stortz & Polla, 1996) or signalling molecules (Lander et al. 1996). A number of genes that can be differentially regulated by oxidative stress have been characterized and include early response genes, genes for proteins involved in antioxidant protection and genes for specific stress and heat shock proteins (HSPs; Applegate et al. 1991; Stortz & Polla, 1996; Jackson et al. 2002). Altered expression of some of these proteins has been assessed in muscle following exercise protocols. An acute bout of contractile activity (Ji, 1993; McArdle et al. 2001) or longer-term exercise training (Higuchi et al. 1985; Ji, 1993) resulted in increased activities of the antioxidant protective enzymes such as superoxide dismutase, catalase or glutathione peroxidase and an increase in the muscle content of HSPs (McArdle et al. 2001). Comparable data have been reported from studies in humans (Jenkins et al. 1984; Khassaf et al. 2001). We have previously hypothesized that the adaptive response of skeletal muscle to unaccustomed contractions is mediated (at least in part) by oxidants generated during contractile activity (McArdle et al. 2001; Khassaf et al. 2001, 2003).
Both cardiac and skeletal muscle are known to respond to short periods of (ischaemic) stress to produce an adaptive response that results in resistance to a subsequent (normally) damaging, prolonged ischaemic stress (Parratt, 1994; Bushell et al. 2002). This phenomenom is known as preconditioning. We hypothesize that, by analogy, a short period of non-damaging contractions would result in protection against a later (normally) damaging period of contractile activity and that this would be mediated by changes in muscle content of a variety of cytoprotective proteins through redox-regulated changes in gene expression. We also propose that this effect could be mimicked by exogenous addition of H2O2 to muscle cells in culture and would bring about common changes in gene expression. This could therefore be used as a tool to identify redox-sensitive, cytoprotective processes activated by an increased ROS production during contractile activity.
Methods
Mice and contraction protocols
Experiments were performed in accordance with UK Home Office guidelines under the UK Animals (Scientific Procedures) Act 1986. In order to assess the susceptibility of muscle to contraction-induced damage, C57Bl6 mice were killed by cervical dislocation and soleus or extensor digitorum longus (EDL) muscles were removed and subjected to a well-characterized damaging protocol of isometric contractions in vitro (McArdle et al. 1991; West-Jordan et al. 1991). This is referred to as the ‘damaging contraction protocol’ in the subsequent text. Adaptive responses and hence potential protection against the damaging contraction protocol were induced by either exposure of anaesthetized mice to hyperthermia in vivo or by a 15-min period of electrically stimulated contractions in vivo as previously reported (McArdle et al. 2001). This latter period of non-damaging contractions is termed the ‘preconditioning contraction protocol’ in subsequent text.
Damaging contraction protocol in vitro
Mice were killed by cervical dislocation. EDL and soleus muscles were rapidly and carefully removed to prevent dissection damage, attached to glass holders and placed into 4 ml of oxygenated, mammalian Ringer solution at 37°C. After a 30 min stabilization period, the medium was replaced with fresh medium and muscles were electrically stimulated via platinum electrodes at 100 Hz for 0.5 s every 2 s at 30 V for a total time of 30 min as previously described (McArdle et al. 1991). Medium was removed and replaced every 30 min for a further 2 h. The removed samples of medium were analysed for total creatine kinase (CK) activity as a index of muscle damage (McArdle et al. 1991; Maglara et al. 2003). At the end of the experiment, muscles were removed and homogenized in ice-cooled 100 mm phosphate buffer. Homogenates were centrifuged (20 000 g, 10 min) at +4°C and supernatants were analysed for total muscle CK activity (McArdle et al. 1991; Maglara et al. 2003).
Preconditioning contraction protocol for induction of adaptations in skeletal muscle of mice in vivo
Adult mice were anaesthetized with sodium pentobarbital (10 mg (100 g body weight)−1, i.p.). Hindlimbs were fixed and the muscles were electrically stimulated to contract by surface electrodes placed around the upper limb and ankle to induce isometric contractile activity in vivo. Stimulation was for 15 min with square wave pulses of 0.1 ms. duration at 100 Hz and 70 V for 0.5 s. every 5 s. as previously described (McArdle et al. 2001). Control mice were anaesthetized without limb stimulation. Previous studies have demonstrated that this contraction protocol results in adaptive changes in specific muscle proteins (McArdle et al. 2001). This protocol is essentially non-damaging as it does not result in gross histological damage and no significant change in maximum force generation are detected in muscles at 3 h following this preconditioning contraction protocol compared with initial values (J. van der Meulen, personal communication). Mice were allowed to recover from anaesthetic and killed by cervical dislocation at 4 or 12 h following the end of the contraction protocol. The EDL and soleus muscles were removed and exposed to the damaging contraction protocol in vitro described above. Gastrocnemius muscles from the hindlimbs of mice were also rapidly dissected, frozen in liquid nitrogen and stored at −70°C for further analysis.
Exposure of mouse muscles to hyperthermia
Adult mice were anaesthetized with sodium pentobarbital (10 mg (100 g body weight)−1, i.p.), core temperature was monitored by insertion of a rectal thermocouple probe (RS Components, UK) and mice were wrapped in a heating blanket. Core temperature of mice was raised to 42°C and this was maintained for 15 min. Mice were allowed to recover from anaesthetic and, at 4 or 12 h following the end of the hyperthermia, killed by cervical dislocation and the EDL and soleus muscles carefully removed and exposed to the damaging contraction protocol in vitro described above.
Exposure of cultured skeletal muscle myotubes to H2O2
An immortalized mouse muscle cell line C2C12 (ATCC no. CRL1772) was grown in a humidified atmosphere of 95% air−5% CO2 at 37°C. Myoblasts were routinely maintained in Dulbecco's modified Eagles's medium (DMEM) supplemented with 12% fetal calf serum, glutamine, penicillin and streptomycin. At 80–90% confluency, cells were induced to differentiate into myotubes by maintenance in DMEM supplemented with 2% horse serum, glutamine, penicillin and streptomycin (Maglara et al. 2003). Myotubes were used in all subsequent experiments at 5 days post differentiation (Maglara et al. 2003).
Assessment of myotube viability following treatment with different concentrations of H2O2
In order to determine the effect of H2O2 on myotube viability, C2C12 myotubes were cultured in 96-well plates and exposed to 0.1, 1, 2, 3, 4 or 5 mm H2O2 for 30 min. A stock solution of H2O2 (30% w/w) was used for all experiments. The absolute concentration of this stock solution of H2O2 was determined spectrometrically by measurement of the absorbance at 240 nm using an extinction coefficient of 43.6 m−1 cm−1. H2O2 was diluted to the required concentration in Dulbecco's phosphate-buffered saline (DPBS) immediately prior to treatment of the myotubes. At this time, the medium was removed from the cells, DPBS containing H2O2 was added and the cells returned to the incubator for 30 min. Control cells were incubated with DPBS alone. After 30 min, the DPBS containing H2O2 was replaced with fresh DMEM containing 2% horse serum and the myotubes returned to the incubator for a defined time. The change in concentration of H2O2 in DPBS with time, in the absence of cells, was monitored colorimetrically using horseradish peroxidase and tetramethylbenzidine (TMB). Cell viability was assessed at 4 h following the end of the H2O2 treatment period using the tetrazolium salt (3-(4,5-dimethylthiazol-2yl)-2,5-diphenol tetrazolium bromide; MTT) assay (Gerlier & Thomasset, 1986). MTT was added to the culture medium to give a final concentration of 0.5 mg ml−1 and the myotubes were incubated for a further 3 h, at which time the medium was aspirated, 100 μl dimethyl sulphoxide (DMSO) was added and the absorbance at 540 nm was measured.
Preconditioning and damaging treatment of C2C12 myotubes with H2O2
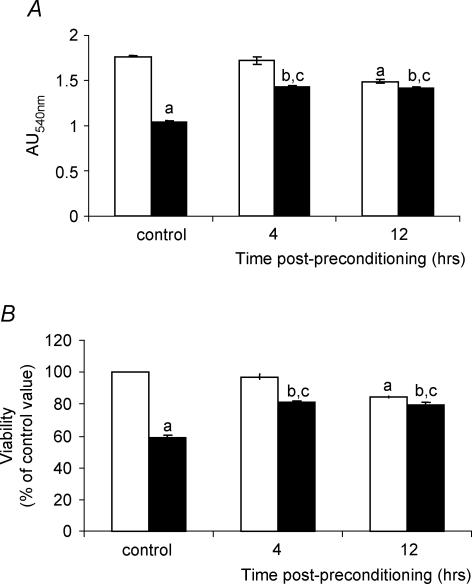
The H2O2 viability studies indicated that an initial concentration of 100 μm H2O2 was non-damaging at 4 h following treatment with only a minor effect on cell viability at 12 h following treatment (Fig. 3B). Preliminary studies suggested that this concentration also resulted in some adaptive changes in the myotubes. A concentration of 2 mm was found to result in an approximate 40% reduction in viability of myotubes at 4 h following treatment (Fig. 3B). Therefore an initial concentration of 100 μm H2O2 was used to ‘precondition’ the myotubes and the susceptibility of myotubes to damage was assessed 4 or 12 h later by challenge with 2 mm H2O2. Viability was measured using the MTT assay described above.
Figure 3. Viability of myotubes at different times following treatment with a preconditioning concentration (100 μm) of H2O2 prior to treatment with a damaging concentration (2 mm) of H2O2.
The effect of 100 μm H2O2 alone is shown (□) and the effect of 2 mm H2O2 at specified times following the exposure to the preconditioning concentration (100 μm) (▪). aP < 0.05 compared with control untreated cells; bP < 0.05 compared with cells treated with 100 μm H2O2 alone; cP < 0.05 compared with cells treated with 2 mm H2O2 without any preconditioning. Data are presented as both the raw absorbance values from the MTT assay (Fig. 3A) and as the percentage viability compared with appropriate suitable control, untreated myotubes (Fig. 3B); n ≥ 20 in all cases.
Determination of stress gene expression in myotubes and gastrocnemius muscles
Total RNA was isolated from myotubes or gastrocnemius muscles using the Atlas Pure Total RNA labelling kit (BD Biosciences, Palo Alto, CA, USA). Briefly, myotubes were harvested and pelleted and gastrocnemius muscles were powdered under liquid nitrogen prior to phenol–chloroform extraction. Total RNA and DNA was precipitated with isopropanol and centrifuged (20 000 g, 10 min). The sample was washed with 80% ethanol, resuspended in RNase-free H2O and treated with DNAse I for 30 min at 37°C. The RNA content of samples was measured using the RiboGreen RNA quantification assay (Molecular Probes, Leiden, the Netherlands). The quality of total RNA was examined by electrophoresis of 1–2 μg RNA on a denaturing 1% agarose–formaldehyde gel containing ethidium bromide. cDNA probe synthesis was then undertaken by PCR incorporation of α32P[dATP] using Atlas Array specific primer set. Labelled cDNA was purified from unincorporated 32P by column chromatography.
The mouse stress array (Clontech, Palo Alto, CA, USA) comprising 140 mouse cDNAs, nine housekeeping control cDNAs and negative controls immobilized in duplicate on a nylon membrane were probed. This array contains cDNA for a number of stress response regulators and effectors, including proteins involved in DNA damage and repair, stress response proteins, chaperones and HSPs, oncogenes and tumour suppressors, kinase network members and proteins involved in xenobiotic metabolism. Filters were pre-hybridized at 68°C for 30 min using the ExpressHyb solution provided. Labelled cDNA probes were denatured by boiling for 2 min and stored on ice for 1 min. Probes were then added directly to the pre-hybridization solution. Hybridization was carried out overnight. Following removal of the probes, filters were washed three times in 2x saline–sodium citrate (SSC) buffer (150 mm sodium chloride, 15 mm sodium citrate) prepared from a stock 20x solution (Invitrogen, UK) containing 1% SDS at 68°C for 30 min, once in 0.1× SSC, 0.5% SDS at 68°C for 30 min and with a final wash in 2x SSC at room temperature for 5 min. Filters were exposed to a phosphor screen (Amersham International, Amersham, UK) for 24 h. The phosphor screen was scanned using a Biorad Personal Molecular Imager FX (Biorad, Hercules, CA, USA). Quantity one software (Biorad) was used to analyse the images. Microarray images were standardized by normalization to the ‘housekeeping’ genes that were detected on the array. There has been considerable discussion about the variability in mRNA data from arrays including strategies to eliminate false positive and negative changes in expression (Miller et al. 2001). In order to try to minimize the occurrence of these false positives and negatives, the data reported here were obtained from analysis of pooled RNA samples from three to four experiments (cell cultures or mouse muscle). Only data from genes that showed at least a two-fold change (increase or decrease) in expression are reported, in line with many recent publications using these technologies (e.g. see Chuang et al. 2002).
Analysis of haem oxygenase-1 expression in myotubes
mRNA content
mRNA from myotubes was obtained as above and analysed for haem oxygenase-1 (HO-1) mRNA by RT-PCR using the Access RT-RCR System (Promega, UK) in combination with primer pairs: 5′-ACACCTGAGGTCAAGCACAGGGTG-3′; 5′-GAGCGGTGTCTGGGATGAGCTAGT-3′ (SwissProt accession no. P14901).
Products were resolved on a 1.5% agarose gel and quantified using scanning densitometry. Data were normalized to β-actin mRNA levels and expressed as a percentage of control values.
Protein content
For analysis of HO-1 protein content, myotubes were pelleted and placed into 1% SDS containing protease inhibitors (McArdle et al. 2001). Following sonication, 50 μg soluble protein was subjected to one-dimensional SDS-PAGE through a 12% gel and transferred to a nitrocellulose membrane as previously described (McArdle et al. 2001). Non-specific binding was blocked by incubation of the membrane in PBS-Tween plus 5% dried milk (1 h at room temperature). Membranes were then probed with a monoclonal antibody against HO-1 (Stressgen, Canada). Antibody binding was visualized by enhanced chemiluminescence (ECL) following incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (McArdle et al. 2001).
Statistical analysis
Data were analysed by one-way analysis of variance with modified Bonferroni test where significance was indicated. P < 0.05 was considered significant. Data are expressed as the mean ±s.e.m.
Results
Intact muscle studies
Effect of preconditioning contraction protocol on release of CK activity following a damaging contraction protocol
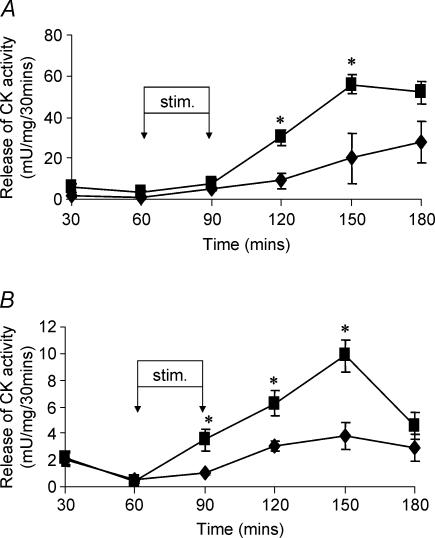
The in vitro damaging contraction protocol administered to EDL and soleus muscles from control adult mice resulted in a significant release of CK activity over the subsequent 120 min as previously reported (McArdle et al. 1991). The pattern of release of CK activity indicating that substantial damage to the isolated muscles had been induced by the contraction protocol is shown in Fig. 1. Prior exposure of the EDL and soleus muscles to the preconditioning protocol of in vivo isometric contractions 4 h prior to the in vitro protocol resulted in a significant protection against contraction-induced damage, shown by a reduction in the release of CK activity (Fig. 1A and B, summarized in Table 1). This protection was not apparent in muscles removed from mice at 12 h following the preconditioning protocol (Table 1). The preconditioning protocol had no effect on the maximum absolute force generated by the EDL and soleus muscles or on total muscle CK activity (data not shown in detail).
Figure 1. Contraction-induced release of CK activity.
Contraction-induced release of CK activity from isolated EDL (A) or soleus (B) muscles following either no pre-treatment (▪) or stimulation 4 h previously (♦). *P < 0.05 compared with values for pre-stimulated muscles at the same time point; n = 4.
Table 1.
Effect of differing preconditioning protocols on relative creatine kinase activity released over 2 h from isolated EDL and soleus muscles in response to damaging contractile activity
| Time following preconditioning stress | ||||
|---|---|---|---|---|
| 4 h | 12 h | |||
| Preconditioning protocol | EDL muscles (%) | Soleus muscles (%) | EDL muscles (%) | Soleus muscles (%) |
| No preconditioning | 100 | 100 | 100 | 100 |
| 15 min contraction protocol in vivo | 47 ± 36 | 55 ± 26 | 100 ± 18 | 124 ± 29 |
| 15 min hyperthermia in vivo | 97 ± 10 | 82 ± 7 | 96 ± 17 | 81 ± 17 |
Effect of prior hyperthermia on release of CK activity following a damaging contraction protocol
Previous data have indicated that in EDL and soleus muscles, the preconditioning protocol leads to an increase in both the content of heat shock proteins 60 and 70 (HSP60 and HSP70) and the activities of superoxide dismutase (SOD) and catalase (McArdle et al. 2001). To examine the effect of a rise in HSP content on the susceptibility to contraction-induced damage, mice were subjected to a period of hyperthermia at 4 or 12 h prior to exposure of muscles to the damaging contraction protocol. A 15 min period of hyperthermia has previously been shown to result in a significant increase in the content of HSPs in soleus and EDL muscles at 4 h, peaking at 12–18 h post hyperthermia (McArdle & Jackson, 1996). The prior hyperthermia and induced elevation in HSP content did not appear to influence the release of CK activity from either EDL or soleus muscles following damaging contractile protocol (Table 1). Muscle hyperthermia also had no significant effect on the total CK content of muscles at any time point (data not shown in detail).
Cell culture studies
Effect of H2O2 on viability of myotubes.
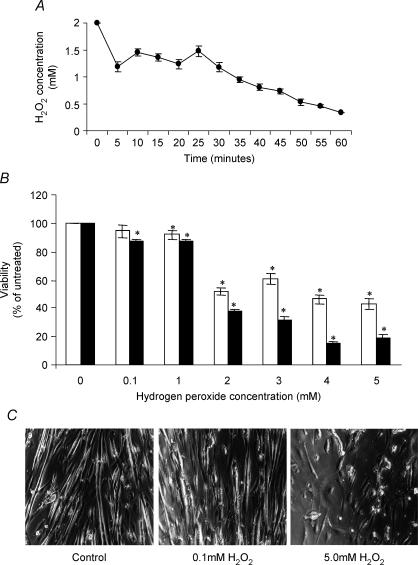
The effects of H2O2 treatment on muscle adaptation and susceptibility to H2O2-mediated damage were assessed using a cultured myotube model. Myotubes were found to be highly resistant to H2O2-induced damage. This appeared to be in part due to the rapid decline in H2O2 concentration in DPBS at 37°C which by 30 min was reduced to approximately 40% of its initial value (Fig. 2A). At initial concentrations of up to 1 mm no significant decrease in cell viability was detectable at 4 h after exposure (Fig. 2B) although some loss of viability was detected after 12 h. Concentrations of 2 mm and above were found to induce a ∼40% reduction in myotube viability at 4 h postexposure that had increased significantly by 12 h postexposure (Fig. 2B). Myotube death, as assessed by the MTT assay, was accompanied by a shortening of myotube length followed by cells lifting from the plate (Fig. 2C). In all subsequent studies 100 μm was used as the preconditioning concentration and 2 mm H2O2 was used to damage myotubes.
Figure 2. The effect of H2O2 treatment on the viability of C2C12 myotubes.
A, change in absolute concentration of H2O2 with time during incubation in Dulbecco's phosphate-buffered saline. The initial concentration of H2O2 was 2 mm. B, viability of 5-day-old cultured myotubes measured at either 4 (□) or 12 (▪) hours following exposure to different concentrations of H2O2 for 30 min *P < 0.05 compared to untreated cells; n = 12. C, light microscopy of control untreated 5-day-old myotubes and myotubes at 4 h following treatment with 0.1 or 2 mm H2O2 for 30 min.
Effect of pre-treatment with 100 μm H2O2 on susceptibility of myotubes to H2O2-induced loss of cell viability
Exposure of myotubes to 2 mm H2O2 induced a ∼40% loss of viability at 4 h following treatment (Fig. 3). Pre-treatment of cultures with 100 μm H2O2 at 4 or 12 h previously resulted in a significant protection against this loss of viability (Fig. 3). For clarity, data are presented as both the raw absorbance values from the MTT assay (Fig. 3A) and as the percentage viability compared to control untreated myotubes (Fig. 3B).
Gene expression studies
Effect of H2O2 on gene expression in myotubes.
Expression of 41 genes, out of 140 immobilized on the array, was detected in the control untreated myotubes. Table 2 shows those genes in which the mRNA levels changed by more than two-fold when compared with untreated cells at 4 h following treatment with 100 μm H2O2. Only 22 genes showed a greater than two-fold regulation and only one, haem oxygenase-1 showed differential up-regulation of expression at 4 h following treatment. The full data set from these cDNA array studies is presented as supplementary material to this paper on the Journal of Physiology website and has been deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), data sets GSM28693 and GSM 28694.
Table 2.
Changes in stress gene expression in myotubes and gastrocnemius muscle
| Fold change in expression at 4 h following preconditioning stress | |||
|---|---|---|---|
| Gene name | GenBank Acc. No. | C2C12 myotubes | Gastrocnemius muscle |
| 7,8-Dihydro-8-oxoguanine triphosphatase (8-oxo-dGTPase) | D49956 | Decreased to ND | NC |
| DNA-repair protein XRCC1 | U02887 | Decreased to ND | NC |
| ATP-dependent DNA helicase II 70 kDa subunit | M38700 | Decreased to ND | NC |
| RAD23 UV excision repair protein homologue A (RAD23A) | X92410 | Decreased to ND | Increased from ND |
| RAD23 UV excision repair protein homologue B (RAD23B) | X92411 | Decreased to ND | NC |
| General transcription factor IIHpolypeptide 1 62 kDa subunit | |||
| (TfIIH 62-kDa-subunit) | AJ002366 | NC | Decreased to ND |
| Growth arrest and DNA-damage-inducible protein 45 (GADD45) | L28177 | Decreased to ND | Decreased to ND |
| Cyclin-dependent kinase inhibitor 1 (CDKN1A) | U09507 | Decreased to ND | NC |
| Cyclophilin-40 | AA407024 | Decreased to ND | NC |
| Vimentin | X51438 | −2.93 | Increased from ND |
| T-complex protein 1 α subunit A (TCP1-α); | |||
| CCT-α (CCTA; CCT1) | D90344, M12899 | Decreased to ND | −2.02 |
| T-complex protein 1 β subunit (TCP1-β); CCT-β | Z31553 | Decreased to ND | NC |
| T-complex protein 1 δ subunit (TCP1-δ); CCT-δ | Z31554, L25913 | Decreased to ND | NC |
| T-complex protein 1 ɛ subunit (TCP1-ɛ); CCT-ɛ | Z31555 | Decreased to ND | − 2.27 |
| T-complex protein 1 γ subunit (TCP1-γ); CCT-γ | Z31556, L20509 | Decreased to ND | NC |
| T-complex protein 1 η subunit (TCP1-η); CCT-η | Z31399 | −2.63 | −2.22 |
| T-complex protein 1 Θ subunit (TCP1-Θ); CCT-Θ | Z37164 | NC | −2.23 |
| T-complex protein 1 ζ subunit (TCP1-ζ); CCT-ζ-1 | Z31557 | NC | Decreased to ND |
| Haem oxygenase 1 (HO-1) | M33203, X13356 | +2.01 | +2.58 |
| Calnexin precursor (CANX) | L18888, L23865 | NC | −2.03 |
| Probable protein disulphide isomerase ER-60 precursor (ERP60) | M73329 | Decreased to ND | NC |
| ERp72 endoplasmic reticulum stress protein | J05186 | NC | Decreased to ND |
| Calcium-binding protein 140-kDa (CAB140; CBP140) | S78797 | Decreased to ND | NC |
| Mitogen-activated protein kinase p38 (MAP kinase p38) | U10871 | Decreased to ND | NC |
| MAPKAPK-2; MAP kinase-activated protein kinase; MAPKAP kinase 2 | X76850 | Decreased to ND | Decreased to ND |
| Dual-specificity mitogen-activated protein kinase kinase 3 (MAPKK 3) | X93150 | Decreased to ND | NC |
| Transforming protein rhoB | X99963 | Decreased to ND | NC |
| Ubiquitous kinesin heavy chain (UKHC) | U86090 | NC | Decreased to ND |
| Multidrug resistance protein 2 (MDR2) | J03398 | NC | Decreased to ND |
| Nucleophosmin (NPM) | M33212 | NC | Decreased to ND |
| Adrenodoxin precursor; adrenal ferredoxin 1 (FDX1) | L29123 | NC | −2.17 |
| Cytochrome P450 1A1 (P450-P1) | M10021, K02588 | NC | Decreased to ND |
| Cytochrome P450 IIE1 (P450-J) | L11650 | NC | Decreased to ND |
| Cytochrome P450 IIF2 (P450-NAH-2) | M77497 | NC | Decreased to ND |
| Quinone oxidoreductase; NADH:quinone oxidoreductase | S70056 | NC | Decreased to ND |
| Microsomal UDP-glucuronyl transferase 1–2 precursor | S64760 | NC | Decreased to ND |
| Serum paraoxonase/arylesterase 3 (PON 3) | L76193 | NC | Decreased to ND |
ND, non-detectable. NC, no change or less than two-fold change in expression.
Effect of contractile activity on gene expression in adult mouse gastrocnemius.
Quiescent gastrocnemius muscles of adult mice were found to express mRNA from 46/140 genes immobilized on the array. Levels of expression from 23 of these 46 genes were found to change by over two-fold at 4 h post exercise (Table 2), but only three (haem oxygenase-1, RAD23 UV excision repair homologue A and vimentin) had an increased level of expression. The full data set from these cDNA array studies is presented as supplementary material to this paper on the Journal of Physiology website and has been deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), data sets GSM28695 and GSM 28696.
Haem oxygenase-1 expression in myotubes.
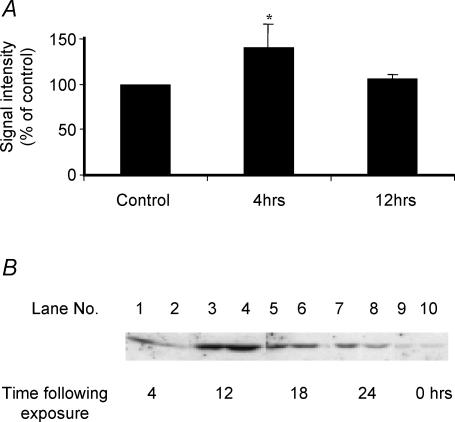
Haem oxygenase-1 (HO-1) was the only gene to show up-regulation in expression in the in vitro myotube and in vivo muscle models and in order to assess the validity of the array data, both mRNA and protein for HO-1 were subjected to more detailed analysis. RT-PCR of HO-1 mRNA revealed a significant increase at 4 h post H2O2 exposure in myotubes (Fig. 4A). Western blot analyses of HO-1 protein indicated an increased content of HO-1 protein at 4, 8, 12 and 24 h after H2O2 treatment with an apparent peak content at ∼12 h after treatment (Fig. 4B).
Figure 4. Haemoxygenase expression in C2C12 myotubes.
A, relative intensity of the RT-PCR band for HO-1 normalized to the expression of β-actin mRNA and presented as percentage of control values. *P < 0.05 compared with control untreated myotubes. B, typical Western blots of the HO-1 content of myotubes at 4 (lanes 1 and 2), 12 (lanes 3 and 4), 18 (lanes 5 and 6) and 24 h (lanes 7 and 8) following treatment for 30 min with 100 μm H2O2 in comparison with control untreated myotubes (lanes 9 and 10).
Discussion
Data indicate that prior exposure of skeletal muscle to a period of non-damaging contractile activity provides protection against a subsequent exposure to a normally damaging protocol of contractions. This protection was apparent at 4 h, but not at 12 h, following preconditioning. This reduced release of CK activity appears to represent a true reduction in susceptibility to contraction-induced damage as the pre-stimulation had no effect on total muscle CK activity or on the contractility of the muscle. The damage induced by the in vitro contraction protocol is associated with an increase in muscle free radical generation (Jackson et al. 1985) and a failure of muscle calcium homeostasis (Jones et al. 1984; McArdle et al. 1992). Furthermore, it can be modulated by antioxidants (Jackson et al. 1983) or substances influencing calcium handling (Jackson et al. 1984). We hypothesized that prior exposure of muscle to a short period of non-damaging contractile activity would up-regulate the activity of antioxidant protective enzymes and HSPs (McArdle et al. 2001) providing protection to the muscle tissue against future insults. However, the lack of induction of a protective effect by prior exposure to hyperthermia (Table 1) argues against a role for HSPs in the protection mechanism. It may be that the changes in gene expression following a stress are targeted to protect against damage induced by a similar stress. In addition, muscle temperature during the preconditioning contraction protocol does not increase significantly (McArdle et al. 2001) indicating that hyperthermia is not a factor activating changes in gene expression in this system. Thus it appears that the pattern of changes in gene expression following hyperthermia do not protect against contraction-induced muscle damage.
There is evidence that muscles subjected to the preconditioning protocol used here produce increased amounts of ROS (McArdle et al. 2001, 2004) and that supplementation with antioxidants abolishes the increased production of HSPs following exercise in humans (Khassaf et al. 2003). This suggests that this increased ROS production may act as a signal for some aspects of the stress response in muscle. To examine the potential role of reactive oxygen species in the protection provided by the preconditioning contraction protocol, we undertook parallel studies to examine the possibility that mild exposure of muscle cells to a model oxidant (H2O2) would induce adaptive responses to protect against subsequent (normally) damaging exposure to the oxidant. We reasoned that comparison of the changes in gene expression that occur in muscle cells following treatment with H2O2 with those in muscle following contractile activity would help identify ROS-mediated changes in gene expression in the intact muscle system in vivo.
H2O2 was chosen as the model oxidant as it has been used in many other studies (for recent examples see Matoba et al. 2000; Chuang et al. 2002; Jaramillo & Olivier, 2002) and there is evidence that H2O2 is released during contractile activity in skeletal muscle (O'Neill et al. 1996; McArdle et al. 2004). H2O2 is generated in mitochondria by dismutation of superoxide and by oxidation of specific molecules such as biogenic amines (Cadenas & Davies, 2000), and within the cytosol by dismutation of superoxide. Our preliminary data indicated that myotubes were relatively resistant to H2O2-induced toxicity and maintained viability at concentrations that would damage many other cell types. Thus, a concentration of 2 mm was necessary to produce ∼40% damage to myotubes at 4 h following treatment. Preliminary data also indicated that myotubes respond to treatment with a non-damaging concentration of H2O2 (100 μm) by up-regulation of cellular HSP25 indicating that this dose and duration of exposure (30 min) was appropriate to stimulate adaptive changes in gene expression in myotubes. Exposure of myotubes to 100 μm H2O2 resulted in a significant protection against subsequent exposure to 2 mm H2O2 at 4 and 12 h after treatment with 100 μm H2O2 (Fig. 3). We therefore used 4 h after exposure for the study of mRNA profiles.
The changes in gene expression that followed exposure of myotubes to 100 μm H2O2 were relatively modest with 19 of the 140 genes on the array showing significant changes 4 h postexposure (Table 2). The major response to H2O2 involved a reduced expression of 18 of the expressed mRNA although the expression of HO-1 was increased. In growing or replicating cells, this concentration of H2O2 has been reported to lead to a temporary growth arrest and prolongation of the cell cycle (Wiese et al. 1995) and the reduction in expression of 18 mRNA species may reflect a temporary reduction in growth in the differentiated multinucleated mytotubes. Standardization of stress-targeted arrays under these conditions may be problematic. If a substantial number of the genes present on the array all change in the same direction (e.g. down-regulated) then the use of a global standardization would be inappropriate. The data obtained were analysed by various methods including global standardization, standardization to one ‘housekeeping’ gene and standardization to multiple ‘housekeeping’ genes and generally comparable data were obtained regardless of the method of standardization suggesting no gross down- or up-regulation of multiple genes had occurred after treatment of myotubes with H2O2 or in intact muscle after contractile activity. The data presented have been normalized to the expression of the ‘housekeeping’ genes expressed (n = 6–9 genes) on the array.
Some previous data have indicated that exposure of myotubes to a much higher concentration of H2O2 (2 mm) resulted in up-regulation of mRNA for four antioxidant enzymes (catalase, GPx1, SOD1 and SOD2; Franco et al. 1999). We consider it likely that higher concentrations of H2O2 than the 100 μm used in the current study would have additionally induced changes in the expression of these antioxidant genes and other genes, but our data indicated that the concentration of 2 mm used by Franco et al. (1999), caused substantial loss of cell viability in the myotubes (Fig. 2). Our experiments were designed to identify those genes regulated by the non-damaging concentration of the oxidant that preconditioned the muscle cells against subsequent exposure to damaging concentrations.
The preconditioning contraction protocol that was used on intact muscle has previously been shown to lead to an increase in the free radical signal seen on electron spin resonance examination of skeletal muscle (Jackson et al. 1985), the release of an increased amount of superoxide (McArdle et al. 2001) plus an increase in hydroxyl radical activity in the interstitial fluid of contracting skeletal muscle (McArdle et al. 2004). In addition there is evidence for a transient and reversible oxidation of muscle protein thiols with subsequent increases in SOD and catalase activities and HSP60 and 70 contents (McArdle et al. 2001). In the light of this, we predicted that the contraction protocol would lead to altered expression of multiple genes, at least partly as a response to the oxidative stress induced by contractions. Twenty-three mRNA species showed a significant change in expression at 4 h post contractions (Table 2).
A comparison of the pattern of changes in the mRNA for the genes modified by contractile activity with the changes in expression of the same genes following H2O2 exposure of myotubes reveals that changes in only six of the genes show approximately the same pattern with the different stresses (Table 2). It can therefore be argued that changes in the expression of these genes may represent part of an oxidant-mediated response to contractile activity. These genes (GADD45, HO-1, MAP kinase-activated protein kinase, T complex protein 1 α, ɛ and η) respond to H2O2 in muscle cells and are modulated in a similar manner by contractile activity. Only one of these (HO-1) showed up-regulation following contractile activity. Data from both the PCR and the Western blot analyses of peroxide-treated myotubes (Fig. 4) alongside published data showing HO-1 was increased in skeletal muscle following contractile activity (Pilegaard et al. 2000) support a role for HO-1 in this response. A number of genes are present on the arrays that would be predicted to have been increased in expression by the oxidative stress of contractile activity, including catalase, Sod2 (MnSOD) and HSP60, but this was not seen. We have previously reported an increase in protein level of HSP60 and activities of catalase and SOD in soleus muscles at various time points following the preconditioning contraction protocol (McArdle et al. 2001). It may be that the changes in mRNA expression for these genes occurred at later time points or that control of absolute levels/activities of these proteins is not at the RNA level. However, previously obtained data do not support the hypothesis that changes in the expression of these proteins underly the mechanism responsible for protection against damage. These include the observations that catalase and SOD activities were not elevated at 4 h following the contraction protocol (McArdle et al. 2001) at a time when protection against damage was seen. Also, we previously reported that no significant elevation of HSP60 was seen in the EDL muscles at this time point (McArdle et al. 2001), whereas here we show significant protection against subsequent damage in the EDL muscle.
The expression of only one of the genes present on the stress array (HO-1) shows a precise association with protection in both the myotube and in vivo model systems. HO-1 is a strong candidate to play a key role in protecting skeletal muscle against damage due to increased generation of oxidants and has been reported to perform this action in other cell types (Barreiro et al. 2002; Lee et al. 2003; Hirai et al. 2003). HO-1 expression is induced by a wide variety of stimuli, many of which are known to cause oxidative stress (Morse & Choi, 2002). The manner by which an increased content of this protein can be cytoprotective is the subject of considerable research. A major function of the protein is to catabolize haem to generate bilirubin, and carbon monoxide and free iron, all of which have been postulated to play direct or indirect roles in the cytoprotective effects of HO-1 (Morse & Choi, 2002). We have attempted to further examine the possible role of this protein in protection of skeletal muscle by looking for potential effects of inhibitors of HO-1 activity (Yee et al. 1996; Schaaf et al. 2002), but unfortunately these compounds induced damage to muscle cells and hence experiments were not informative. Further studies with mice overexpressing or lacking HO-1 expression appear warranted.
In conclusion, we have shown that in mouse muscle cells both contractile activity and H2O2 stress induce adaptive responses that protect against subsequent exposure to contractile activity or oxidant stress. Furthermore there is a small cohort of skeletal muscle ‘stress’ genes that respond to contractile activity and to oxidative stress of which HO-1 may be particularly important.
Supplementary Material
Acknowledgments
The authors would like to thank the Biotechnology and Biological Sciences Research Council, the Wellcome Trust, Research into Ageing and the University of Liverpool Research Development Fund for financial support.
Supplementary material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2004.069914http://jp.physoc.org/cgi/content/full/jphysiol.2004.069914/DC1 and contains supplementary material entitled: Determination of stress gene expression in myotubes following exposure to hydrogen peroxide; Determination of stress gene expression in mouse gastrocnemius muscle following demanding isometric contractions; and Array data in tabular form.
This material can also be found at:http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp526/tjp526sm.htm
References
- Ammendola R, Fiore F, Esposito F, Caserta G, Mesuraca M, Russo T, Cimino F. Differentially expressed mRNAs as a consequence of oxidative stress in intact cells. FEBS Lett. 1995;371:209–213. doi: 10.1016/0014-5793(95)00871-6. 10.1016/0014-5793(95)00871-6. [DOI] [PubMed] [Google Scholar]
- Applegate LA, Luscher P, Tyrrell RM. Induction of haem oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–978. [PubMed] [Google Scholar]
- Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Comtois AS, Mohammed S, Lands LC, Hussain SN. Role of haem oxygenases in sepsis-induced diaphragmatic contractile dysfunction and oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2002;283:L476–L484. doi: 10.1152/ajplung.00495.2001. [DOI] [PubMed] [Google Scholar]
- Bushell AJ, Klenerman L, Taylor S, Davies H, Grierson I, Helliwell TR, Jackson MJ. Ischaemic preconditioning of skeletal muscle. 1. Protection against the structural changes induced by ischaemia/reperfusion injury. J Bone Joint Surg. 2002;84-B:1184–1188. doi: 10.1302/0301-620x.84b8.9361. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Chuang YY, Chen Y, Gadisetti Chandramouli VR, Cook JA, Coffin D, Tsai MH, DeGraff W, Yan H, Zhao S, Russo A, Liu ET, Mitchell JB. Gene expression after treatment with hydrogen peroxide, menadione, or t-butyl hydroperoxide in breast cancer cells. Cancer Res. 2002;62:6246–6254. [PubMed] [Google Scholar]
- Davies KJA, Quintanilla AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med. 1999;27:1122–1132. doi: 10.1016/s0891-5849(99)00166-5. 10.1016/S0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Cellular and molecular aspects of adaptation in skeletal muscle. In: Komi PV, editor. Strength and Power in Sport. Oxford, UK: Blackwell Science; 1994. pp. 211–229. [Google Scholar]
- Higuchi M, Cartier LJ, Chen M, Holloszy JO. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol. 1985;40:281–286. doi: 10.1093/geronj/40.3.281. [DOI] [PubMed] [Google Scholar]
- Hirai H, Kubo H, Yamaya M, Nakayama K, Numasaki M, Kobayashi S, Suzuki S, Shibahara S, Sasaki H. Microsatellite polymorphism in haem oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood. 2003;102:1619–1621. doi: 10.1182/blood-2002-12-3733. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Edwards RHT, Symons MCR. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta. 1985;847:185–190. doi: 10.1016/0167-4889(85)90019-9. 10.1016/0167-4889(85)90019-9. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Jones DA, Edwards RHT. Vitamin E and skeletal muscle. Ciba Found Symp. 1983;101:224–239. doi: 10.1002/9780470720820.ch14. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Jones DA, Edwards RH. Experimental skeletal muscle damage: the nature of the calcium-activated degenerative processes. Eur J Clin Invest. 1984;14:369–374. doi: 10.1111/j.1365-2362.1984.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Papa S, Bolanos J, Bruckdorfer R, Carlsen H, Elliott RM, et al. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. 10.1016/S0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- Jaramillo M, Olivier M. Hydrogen peroxide induces murine macrophage chemokine gene transcription via extracellular signal-regulated kinase-and cyclic adenosine 5′-monophosphate (cAMP) -dependent pathways: involvement of NF-kappa B, activator protein 1, and cAMP response element binding protein. J Immunol. 2002;169:7026–7038. doi: 10.4049/jimmunol.169.12.7026. [DOI] [PubMed] [Google Scholar]
- Jenkins RR, Friedland R, Howald H. The relationship of oxygen uptake to superoxide dismutase and catalase activity in human skeletal muscle. Int J Sports Med. 1984;5:11–14. doi: 10.1055/s-2008-1025872. [DOI] [PubMed] [Google Scholar]
- Ji LL. Antioxidant enzyme response to exercise and aging. Med Sci Sports Exerc. 1993;25:225–231. [PubMed] [Google Scholar]
- Jones DA, Jackson MJ, McPhail G, Edwards RHT. Experimental mouse muscle damage: the importance of external calcium. Clin Sci. 1984;66:317–322. doi: 10.1042/cs0660317. [DOI] [PubMed] [Google Scholar]
- Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol. 2001;90:1031–1035. doi: 10.1152/jappl.2001.90.3.1031. [DOI] [PubMed] [Google Scholar]
- Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol. 2003;549:645–652. doi: 10.1113/jphysiol.2003.040303. 10.1113/jphysiol.2003.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TJ, Brooks SV. Lengthening contractions are not required to induce protection from contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2001;281:R155–R161. doi: 10.1152/ajpregu.2001.281.1.R155. [DOI] [PubMed] [Google Scholar]
- Lander HM, Jacovina AT, Davis RJ, Tauras JM. Differential activation of mitogen-activated protein kinases by nitric oxide-related species. J Biol Chem. 1996;271:19705–19709. doi: 10.1074/jbc.271.33.19705. 10.1074/jbc.271.33.19705. [DOI] [PubMed] [Google Scholar]
- Lee HT, Xu H, Ota-Setlik A, Emala CW. Oxidant preconditioning protects human proximal tubular cells against lethal oxidant injury via p38 MAPK and heme oxygenase-1. Am J Nephrol. 2003;23:324–333. doi: 10.1159/000072914. 10.1159/000072914. [DOI] [PubMed] [Google Scholar]
- McArdle A, Edwards RHT, Jackson MJ. Effects of contractile activity on muscle damage in the dystrophin-deficient mdx mouse. Clin Sci. 1991;80:367–371. doi: 10.1042/cs0800367. [DOI] [PubMed] [Google Scholar]
- McArdle A, Edwards RH, Jackson MJ. Accumulation of calcium by normal and dystrophin-deficient mouse muscle during contractile activity in vitro. Clin Sci. 1992;82:455–459. doi: 10.1042/cs0820455. [DOI] [PubMed] [Google Scholar]
- McArdle A, Jackson MJ. Heat shock protein 70 expression in skeletal muscle. Biochem Soc Trans. 1996;24:485S. doi: 10.1042/bst024485s. [DOI] [PubMed] [Google Scholar]
- McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol. 2001;280:C621–C627. doi: 10.1152/ajpcell.2001.280.3.C621. [DOI] [PubMed] [Google Scholar]
- McArdle A, van Der Meulen J, Close GL, Pattwell D, Van Remmen H, Huang TT, Richardson AG, Epstein CJ, Faulkner JA, Jackson MJ. The role of mitochondrial superoxide dismutase in contraction-induced generation of reactive oxygen species in skeletal muscle extracellular space. Am J Physiol Cell Physiol. 2004;286:C1152–C1158. doi: 10.1152/ajpcell.00322.2003. [DOI] [PubMed] [Google Scholar]
- Maglara AA, Vasilaki A, Jackson MJ, McArdle A. Damage to developing mouse skeletal muscle myotubes in culture: protective effect of heat shock proteins. J Physiol. 2003;548:837–846. doi: 10.1113/jphysiol.2002.034520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Galecki A, Shmookler-Reis RJ. Interpretation, design and analysis of gene array expression experiments. J Gerontol A Biol Sci Med Sci. 2001;56:B327–B330. doi: 10.1093/gerona/56.2.b52. [DOI] [PubMed] [Google Scholar]
- Morse D, Choi AM. Heme oxygenase-1: the ‘emerging molecule’ has arrived. Am J Respir Cell Mol Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- Newham DJ, Jones DA, Clarkson PM. Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol. 1987;63:1381–386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- Nose K, Shibanuma M, Kikuchi K, Kageyama H, Sakiyama S, Kuroki T. Transcriptional activation of early-response genes by hydrogen peroxide in a mouse osteoblastic cell line. Eur J Biochem. 1991;201:99–106. doi: 10.1111/j.1432-1033.1991.tb16261.x. [DOI] [PubMed] [Google Scholar]
- O'Neill CA, Stebbins CL, Bonigut S, Halliwell B, Longhurst JC. Production of hydroxyl radicals in contracting skeletal muscle of cats. J Appl Physiol. 1996;81:1197–1206. doi: 10.1152/jappl.1996.81.3.1197. [DOI] [PubMed] [Google Scholar]
- Parratt RJ. Protection of the heart by ischaemic preconditioning: mechanisms and possibilities for pharmacological exploitation. Trends Pharmacol Sci. 1994;15:19–25. doi: 10.1016/0165-6147(94)90129-5. 10.1016/0165-6147(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- Sacco P, Jones DA. The protective effect of damaging eccentric exercise against repeated bouts of exercise in the mouse tibialis anterior muscle. Exp Physiol. 1992;77:757–760. doi: 10.1113/expphysiol.1992.sp003642. [DOI] [PubMed] [Google Scholar]
- Schaaf GJ, Maas RF, de Groene EM, Fink-Gremmels J. Management of oxidative stress by heme oxygenase-1 in cisplatin-induced toxicity in renal tubular cells. Free Radic Res. 2002;36:835–843. doi: 10.1080/1071576021000005267. 10.1080/1071576021000005267. [DOI] [PubMed] [Google Scholar]
- Stortz G, Polla BS. Transcriptional regulators of oxidative stress – inducible genes in prokaryotes and eukaryotes. In: Feige U, Morimoto R II, Yahara I, Polla BS, editors. Stress Inducible Cellular Responses. Basel: Birkhauser-Verlag; 1996. pp. 239–254. [DOI] [PubMed] [Google Scholar]
- Torti SV, Akimoto H, Lin K, Billingham ME, Torti FM. Selective inhibition of muscle gene expression by oxidative stress in cardiac cells. J Mol Cell Cardiol. 1998;30:1173–1180. doi: 10.1006/jmcc.1998.0681. 10.1006/jmcc.1998.0681. [DOI] [PubMed] [Google Scholar]
- West-Jordan JA, Martin PA, Abraham RJ, Edwards RH, Jackson MJ. Energy metabolism during damaging contractile activity in isolated skeletal muscle: a 31P-NMR study. Clin Chim Acta. 1991;203:119–134. doi: 10.1016/0009-8981(91)90284-j. 10.1016/0009-8981(91)90284-J. [DOI] [PubMed] [Google Scholar]
- Wiese AG, Pacifici RE, Davies KJ. Transient adaptation of oxidative stress in mammalian cells. Arch Biochem Biophys. 1995;318:231–240. doi: 10.1006/abbi.1995.1225. 10.1006/abbi.1995.1225. [DOI] [PubMed] [Google Scholar]
- Yee EL, Pitt BR, Billiar TR, Kim YM. Effect of nitric oxide on heme metabolism in pulmonary artery endothelial cells. Am J Physiol. 1996;271:L512–L518. doi: 10.1152/ajplung.1996.271.4.L512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2004.069914http://jp.physoc.org/cgi/content/full/jphysiol.2004.069914/DC1 and contains supplementary material entitled: Determination of stress gene expression in myotubes following exposure to hydrogen peroxide; Determination of stress gene expression in mouse gastrocnemius muscle following demanding isometric contractions; and Array data in tabular form.
This material can also be found at:http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp526/tjp526sm.htm