Abstract
The rhythm of plasma melatonin originating from the pineal gland and driven by the circadian pacemaker located in the suprachiasmatic nucleus is closely associated with the circadian (approximately 24 h) variation in sleep propensity and sleep spindle activity in humans. We investigated the contribution of melatonin to variation in sleep propensity, structure, duration and EEG activity in a protocol in which sleep was scheduled to begin during the biological day, i.e. when endogenous melatonin concentrations are low. The two 14 day trials were conducted in an environmental scheduling facility. Each trial included two circadian phase assessments, baseline sleep and nine 16 h sleep opportunities (16.00–08.00 h) in near darkness. Eight healthy male volunteers (24.4 ± 4.4 years) without sleep complaints were recruited, and melatonin (1.5 mg) or placebo was administered at the start of the first eight 16 h sleep opportunities. During melatonin treatment, sleep in the first 8 h of the 16 h sleep opportunities was increased by 2 h. Sleep per 16 h was not significantly different and approached asymptotic values of 8.7 h in both conditions. The percentage of rapid eye movement (REM) sleep was not affected by melatonin, but the percentage of stage 2 sleep and sleep spindle activity increased, and the percentage of stage 3 sleep decreased. During the washout night, the melatonin-induced advance in sleep timing persisted, but was smaller than on the preceding treatment night and was consistent with the advance in the endogenous melatonin rhythm. These data demonstrate robust, direct sleep-facilitating and circadian effects of melatonin without concomitant changes in sleep duration, and support the use of melatonin in the treatment of sleep disorders in which the circadian melatonin rhythm is delayed relative to desired sleep time.
Human sleep is regulated by an interaction between circadian (∼24 h) and homeostatic processes (Dijk & Czeisler, 1995; Dijk et al. 1999). In mammals the circadian process is generated and maintained by an endogenous, self-sustaining pacemaker located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus (Klein et al. 1991). Circadian rhythms are synchronized to the environment mainly by light via a photoreceptor system in melanopsin-containing, light-sensitive ganglion cells (Berson et al. 2002). Disorders of circadian timing include delayed sleep phase syndrome and non-24 h sleep–wake disorder. Melatonin administration has, to some extent, proved effective in the treatment of such disorders (Arendt, 2000). However, the mechanisms by which melatonin exerts these effects are unclear.
The hypothesis that endogenous melatonin is involved in the physiological regulation of sleep is based largely on correlational evidence. The circadian rhythm of pineal melatonin synthesis and secretion is closely associated with the rhythm of sleep propensity in both sighted and blind individuals (Nakagawa et al. 1992; Dijk et al. 1997; Lockley et al. 1997; Wyatt et al. 1999; Wehr et al. 2001), and exogenous melatonin, especially at times of the day when endogenous levels are low, appears to facilitate sleep (Arendt et al. 1985; Dollins et al. 1994; Cajochen et al. 1996; Zhdanova et al. 1996; Hughes & Badia, 1997; Lavie, 1997; Stone et al. 2000), and influence sleep-stage-specific EEG characteristics (Dijk et al. 1995). Just prior to the onset of melatonin secretion is a time of the day referred to as the wake maintenance zone (Strogatz et al. 1986), or forbidden zone for sleep (Lavie, 1986), when sleep propensity is lowest (∼20.00–22.00 h). Coinciding with the nocturnal rise in endogenous melatonin, there is an abrupt transition from increased wakefulness or arousal to high sleep propensity (Lavie, 1986; Dijk et al. 1997, 1999). It has been suggested that melatonin gates the increase in nocturnal sleepiness by inhibiting circadian wakefulness-generating mechanisms (Lavie, 1997; Sack et al. 1997).
Appropriately timed melatonin administration to humans shifts the timing of endogenous circadian rhythms (Arendt et al. 1985; Sack et al. 1987; Deacon et al. 1994; Attenburrow et al. 1995; Deacon & Arendt, 1995; Lewy et al. 1998; Sharkey & Eastman, 2002; Rajaratnam et al. 2003). Melatonin appears to act on the circadian system via melatonin receptors at the SCN, although it is not clear which receptor subtype is responsible (von Gall et al. 2002). While it appears that sleep timing can also be shifted by exogenous melatonin (Arendt et al. 1985; Deacon & Arendt, 1995; Lavie, 1997), to date there are no conclusive polysomnographic data in this regard. Furthermore, the interaction between the direct, sleep-facilitating effects of melatonin and its circadian phase-shifting effects is poorly understood, and it is not clear if these effects are mediated by separate mechanisms.
Many previous studies examining the acute effects of melatonin on sleep and circadian rhythms have used protocols in which sleep propensity was assessed by self-report, actigraphy or from relatively short, polysomnographically recorded sleep episodes. To our knowledge there have been no studies that have examined the effects of chronic melatonin administration on polysomnographic sleep in a protocol in which longer term changes in sleep timing could be properly assessed. To establish whether or not melatonin could advance the timing of sleep, such that sleep occurred at a time normally associated with the wake maintenance zone, we devised a protocol in which the effects of melatonin were tested in healthy volunteers who were exposed to extended (16 h) sleep opportunities that began during the biological day (i.e. when endogenous melatonin levels are normally low). This protocol allowed us to examine simultaneously the effects of melatonin on sleep timing and the duration of sleep in the absence of constraints on sleep that are associated with protocols in which the sleep opportunity is only 8 h. Furthermore, the protocol avoided the problem of chronic sleep loss that is likely to occur in studies in which an 8 h sleep opportunity is shifted into the daytime over several days, as sleep under such conditions is likely to be disrupted due to the circadian drive for wakefulness (Dijk & Czeisler, 1995). On the basis of earlier work (Deacon & Arendt, 1995), it was hypothesized that early evening melatonin administration would cause an abrupt advance in the timing of sleep without affecting total sleep duration, because melatonin is not acting as a traditional hypnotic (Lancel & Steiger, 1999). It was also hypothesized that melatonin would promote sleep during the wake maintenance zone by a combination of its sleep-facilitating and circadian phase-shifting effects. It was predicted that EEG slow wave activity and slow wave sleep would be partially suppressed by exogenous melatonin, and that sleep spindle activity and stage 2 sleep would be increased (Dijk et al. 1995; Hughes & Badia, 1997; Stone et al. 2000). Finally, it was predicted that latency to sleep onset would be reduced by melatonin (Waldhauser et al. 1990; Zhdanova et al. 1995; Reid et al. 1996; Zhdanova et al. 1996; Hughes & Badia, 1997).
Methods
Participants
Participants were eight healthy, non-smoking, male volunteers (body mass index 23.8 ± 3.0 kg m−2; age 24.4 ± 4.4 years) who were recruited by one of the investigators via poster and newspaper advertisements. Two additional participants commenced the study, but one withdrew after completing the first of two trials, and one withdrew during the first trial. The final sample consisted of six Europeans, one Australasian and one South American. Females were not included as the protocol would have involved comparing circadian temperature parameters across the follicular and luteal phases of the menstrual cycle, which may have compromised the reliability of phase assessments (Kattapong et al. 1995; Baker et al. 2001). The sample size was determined on the basis of effect sizes observed in previous melatonin and sleep extension trials, carried out in controlled laboratory conditions, within the University of Surrey, QinetiQ Ltd and elsewhere (Wehr, 1991; Deacon & Arendt, 1995; Dijk et al. 1995; Stone et al. 2000). Exclusion criteria were chronic or recent acute medical illness, psychiatric or sleep disorders, drug or alcohol abuse, abnormal blood biochemistry or haematology, recent shiftwork or transmeridian travel, average daily caffeine consumption exceeding 300 mg, average daily alcohol consumption exceeding 90 mg, and abnormal sleep. These were assessed by self-report questionnaires, history-taking, medical examination, electrocardiography, biochemical and haematological screening tests and polysomnographic evaluation of sleep during a laboratory adaptation night.
Experimental protocol
We used a sound-attenuated, light-, temperature- and humidity-controlled environmental scheduling facility, consisting of four individual bedrooms, communal kitchen and living areas, showers and lavatories (QinetiQ Ltd, Farnborough, Hampshire, UK). Participants gave written informed consent to the procedures. The protocol was approved by the Defence Evaluation Research Agency Centre for Human Sciences Ethics Committee (Farnborough, Hampshire, UK), and the study conformed to the standards set out by the Declaration of Helsinki. Ambient light was supplied by ceiling-mounted, broad-spectrum fluorescent tubes (Osram Biolux, Langley, Berkshire, UK).
The study used a double-blind, placebo-controlled, repeated measures design, and was conducted between November 2000 and August 2001. Participants took part in two separate trials in which either melatonin (1.5 mg, surge-sustained release; Rajaratnam et al. 2003) or placebo was administered for 8 consecutive days in gelatine capsules presented in coded plastic containers. The two trials were separated by at least 14 days. Treatment order was counterbalanced. An individual who was not involved with recruitment, data collection or data analysis, and who did not have any contact with participants or knowledge of their identity, determined the order of treatment administration according to participant codes, such that four participants received melatonin in the first trial and four received placebo. After treatment order was determined, participants were allocated their codes according to the order in which they were recruited to the study.
Prior to attending the laboratory, participants were instructed to abstain from caffeine for 7 days and from alcohol for 24 h. They maintained a controlled sleep–wake schedule (sleep 23.00–07.00 h) for 10 days, which was verified by wrist actigraphy (Actiwatch-L, Cambridge Neurotechnology, Cambridge, UK).
Each laboratory trial commenced at 16.00 h on day (D)1 and was concluded at 09.00 h on D14. Meals, light levels and sleep times were controlled during the trials. During scheduled wake episodes (except constant routines), ambient light was 147.6 ± 4.3 lx (mean ± s.e.m.) measured at the horizontal angle of gaze. During constant routines and sleep opportunities, ambient light was 2.4 ± 0.1 lx. After a 8.25 h baseline sleep opportunity from 23.00 h on D1, a 29 h constant routine was imposed to assess circadian rhythms (Duffy & Dijk, 2002). During the constant routine, participants were instructed to remain awake and semirecumbent and received identical nutrients every 2 h. A 9 day extended sleep opportunity protocol then followed, with participants confined to their beds with no recreational materials such as books or television from 16.00 h to 08.00 h. Melatonin or placebo was administered at 16.00 h for the first 8 days (D3–10), followed by a ‘washout night’ on D11 when placebo was administered in both trials. After the last extended sleep opportunity, a second 29 h constant routine was imposed commencing at 08.00 h on D12. The trial ended after a 16 h recovery sleep episode. The protocol has been previously described (Rajaratnam et al. 2003).
Sleep recording and analysis
During all scheduled sleep opportunities and constant routines, vigilance state was monitored by polysomnography (EEG, EOG, submental EMG and a two-lead EKG) using Embla 16-channel digital sleep recorders (Flaga hf., Iceland). EEG signals were derived from the O1–A2, O2–A1, C4–A1 and C3–A2 positions, with a high-pass filter at 0.5 Hz and a low-pass filter at 45 Hz. Hormone, actigraphy, alertness and mood data that were collected during this protocol are reported elsewhere (Rajaratnam et al. 2003).
Polysomnographic recordings were scored in 30 s epochs according to standard criteria (Rechtschaffen & Kales, 1968) by an experienced, experimentally naive sleep technician. Sleep records were excluded from analyses if more than 2.5% of data were missing. In addition, two sleep records from one participant were excluded due to a minor elevation of core body temperature and headache during 2 days of the study. A total of 11 sleep records (6.3%) were excluded.
EEG power spectra were calculated per 256 samples and were averaged per 30 s epoch using 50% overlap between consecutive 256 sample windows (Somnologica Science, Flaga hf., Iceland). Here we report data on delta (or slow-wave) activity in non-rapid eye movement (NREM) sleep, which corresponds to power density in the 0.75–4.50 Hz range, and sigma activity in NREM sleep, which is power density in the 12.0–13.59 Hz range. EEG activity in these frequency ranges appears to be most influenced by melatonin (Dijk et al. 1995). Epochs with artefacts were manually identified and excluded from the spectral analysis. To reduce the between-subjects variability in delta and sigma activity, these data were expressed as a percentage of each participant's baseline value (Dijk et al. 1997), deemed as the mean level of delta or sigma activity for the first night (D1).
Total sleep time (h, TST) was defined as the amount of time in which the participant was asleep, including all sleep stages. Sleep efficiency was defined as the TST divided by the chosen interval, and expressed as a percentage. To evaluate the effects of melatonin on the distribution of sleep during the treatment, the 16 h sleep opportunity was divided into two separate 8 h intervals. The percentage of wakefulness, delta and sigma activity expressed as percentages of baseline were analysed by factors treatment and interval. For the analysis of melatonin effects on sleep, the first sleep episode after the initial constant routine (i.e. D3) was excluded because sleep during this episode was affected by the sleep deprivation associated with the constant routine.
Data were analysed by repeated measures analysis of variance (ANOVA), or paired samples t test. Significant effects (P < 0.05) revealed by ANOVA were subjected to post hoc paired sample t tests. To examine possible associations between the sleep efficiency profile on the washout night (D11) and the observed shift in the endogenous melatonin rhythm (Rajaratnam et al. 2003), cross-correlation analyses over fourteen 1 h lags were performed (Cajochen et al. 1999). Statistical analyses were performed using SAS version 8.0 (SAS Institute Inc., Cary, NC, USA) or SPSS version 11.0 (SPSS Inc., Chicago, IL, USA).
Results
Sleep profiles for one representative participant during all sleep opportunities for the placebo and melatonin trials are shown in Fig. 1. Clear differences between the melatonin and placebo trials are observed in the distribution of sleep during the 16 h sleep opportunities (D4–10).
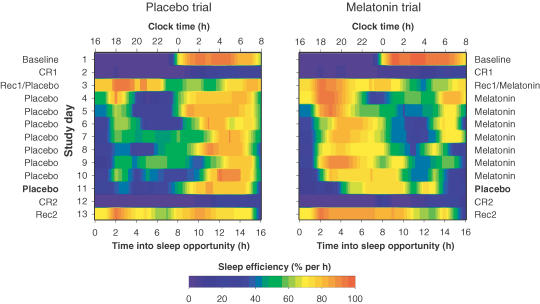
Figure 1. Sleep profiles for one representative participant.
Sleep opportunities are shown for the placebo (left side) and melatonin (right side) trials, as a function of clock time (h). Study days are presented beneath each other. For each day, sleep stages (REM, wake, stage 1, stage 2, stage 3, stage 4) are shown in the upper panel, and delta activity (power density in the 0.75–4.5 Hz band) is shown in the lower panel. On the constant routine days (D2 and D12), participants were instructed to remain awake, and therefore sleep profiles on these days are not presented. The onset of endogenous melatonin secretion (the time when plasma melatonin levels increased beyond 25% of the peak nocturnal level) is indicated as an open circle for each constant routine (redrawn with permission from Rajaratnam et al. 2003). Baseline sleep (D1) was similar in the two treatment trials. Sleep during the 16 h sleep opportunity following the first constant routine was well consolidated in both the placebo and the melatonin trials (D3). During the subsequent 16 h sleep opportunities (D4 to D10) clear differences between the two conditions emerged. In the placebo trial, the 16 h sleep opportunities were often characterized by relatively rapid onset to sleep, followed by long periods of wakefulness in the initial 8 h (i.e. 16.00 h to 00.00 h). This in turn was followed by consolidated sleep in the second 8 h (i.e. from 00.00 h to 08.00 h). In the melatonin trial, the longest consolidated bout of sleep was present in the first part of the sleep opportunity and more wakefulness was observed in the second part. It is noted that on several occasions, sleep was not initiated immediately after melatonin administration (e.g. D7 to D10). During the washout night on D11, when placebo was administered in both trials, sleep onset occurred earlier in the melatonin trial than in the placebo trial. Wakefulness was present in the second half of the sleep opportunity in the melatonin trial, but not in the placebo trial. Following the second constant routine, sleep was well consolidated in both trials and delta activity declined progressively in the course of the sleep episode, as it did in most other episodes of consolidated sleep.
Baseline sleep characteristics
The first sleep opportunity was used to determine baseline sleep characteristics, that is, prior to melatonin treatment and the extension of sleep opportunity. No significant differences were observed in baseline sleep characteristics between the treatment trials (Table 1). On the baseline day of each treatment trial, sleep was relatively consolidated (Figs 1 and 2), and although mean sleep efficiencies were slightly different between the two groups (85.5% versus 90.9% for placebo and melatonin trials, respectively) no significant difference was observed (Table 1). Latencies to sleep onset were within the normal range.
Table 1.
Effects of melatonin on sleep parameters during baseline and extended sleep opportunity nights
| Baseline (D1) | 1st half of sleep opportunity (D4–10) | 2nd half of sleep opportunity (D4–10) | Total sleep opportunity (D4–10) | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Melatonin | Placebo | Melatonin | Placebo | Melatonin | Placebo | Melatonin | |
| Total sleep time (TST) (h) | 7.07 (0.38) | 7.51 (0.12) | 3.37* (0.24) | 5.37 (0.20) | 5.57* (0.36) | 3.96 (0.45) | 8.94 (0.22) | 9.33 (0.37) |
| Sleep efficiency (%) | 85.51 (4.61) | 90.90 (1.50) | 42.31* (2.96) | 67.52 (2.53) | 69.61* (4.56) | 49.56 (5.67) | 56.00 (1.39) | 58.52 (2.33) |
| Stage 1 sleep duration (h) | 0.89 (0.11) | 0.87 (0.15) | 0.42* (0.03) | 0.64 (0.05) | 0.84* (0.09) | 0.57 (0.07) | 1.26 (0.10) | 1.21 (0.10) |
| Stage 2 sleep duration (h) | 3.86 (0.23) | 4.24 (0.18) | 1.83* (0.16) | 3.08 (0.12) | 2.94* (0.21) | 2.09 (0.30) | 4.78 (0.16) | 5.17 (0.30) |
| Stage 3 sleep duration (h) | 0.52 (0.07) | 0.43 (0.06) | 0.36 (0.03) | 0.43 (0.06) | 0.25 (0.05) | 0.18 (0.05) | 0.61 (0.05) | 0.61 (0.09) |
| Stage 4 sleep duration (h) | 0.26 (0.10) | 0.23 (0.09) | 0.19 (0.06) | 0.24 (0.07) | 0.06 (0.04) | 0.04 (0.01) | 0.25 (0.08) | 0.27 (0.08) |
| REM sleep duration (h) | 1.53 (0.23) | 1.73 (0.14) | 0.57* (0.08) | 0.99 (0.08) | 1.47* (0.09) | 1.07 (0.11) | 2.04 (0.10) | 2.07 (0.11) |
| Stage 1 sleep, %TST | 12.74 (1.74) | 11.61 (1.92) | 12.95 (1.10) | 12.12 (1.12) | 15.37 (1.30) | 14.65 (1.59) | 14.13 (1.03) | 13.01 (1.07) |
| Stage 2 sleep, %TST | 55.20 (3.11) | 56.53 (2.24) | 53.74* (1.47) | 57.31 (1.50) | 52.43 (1.81) | 51.21 (1.78) | 53.38 (1.49) | 55.13 (1.24) |
| Stage 3 sleep, %TST | 7.40 (1.01) | 5.77 (0.84) | 11.13* (1.21) | 7.93 (0.96) | 4.38 (0.80) | 5.11 (1.12) | 6.91 (0.60) | 6.72 (0.97) |
| Stage 4 sleep, %TST | 3.57 (1.39) | 3.12 (1.25) | 6.28 (2.00) | 4.46 (1.21) | 0.92 (0.53) | 1.02 (0.37) | 2.73 (0.83) | 3.04 (0.81) |
| REM sleep, %TST | 21.09 (2.30) | 22.98 (1.83) | 15.90 (1.35) | 18.17 (0.95) | 26.89 (1.26) | 28.02 (1.51) | 22.86 (1.02) | 22.10 (0.87) |
| REM : NREM sleep ratio | 0.27 (0.04) | 0.30 (0.03) | 0.20 (0.02) | 0.23 (0.01) | 0.38 (0.02) | 0.44 (0.06) | 0.30 (0.02) | 0.29 (0.01) |
| Latency to stage 1 (min) | 24.06 (12.46) | 16.56 (4.15) | 81.36 (23.13) | 54.34 (12.56) | ||||
| Latency to stage 2 (min) | 29.44 (12.23) | 23.38 (4.89) | 98.72 (29.26) | 62.97 (13.90) | ||||
| Latency to first REM (min) | 99.75 (21.97) | 95.38 (15.08) | 65.68 (5.86) | 75.18 (4.88) | ||||
| Duration of first REM (min) | 22.50 (5.09) | 17.00 (3.72) | 15.51 (2.10) | 15.91 (1.34) | ||||
Mean sleep parameters (with standard error of mean in parentheses) during the baseline night (D1), during the first and second half of the treatment nights (8 h) and across the entire sleep opportunity (16 h) on treatment nights. For each participant, mean sleep parameters were calculated across the treatment period excluding the first treatment night (D3) due to the influence of the extended period of wakefulness associated with the preceding constant routine. The 16 h sleep opportunity was divided into two equal parts: 16.00 h to 00.00 h, and 00.00 h to 08.00 h.
Significant differences (P < 0.05) between the melatonin trial and the corresponding data for the placebo trial.
Figure 2. Sleep efficiency profiles in the melatonin and placebo trials.
Mean sleep efficiency profiles in the placebo (left side) and melatonin (right side) trials. Sleep efficiency is calculated across participants (n = 8) in 1 h intervals for each sleep opportunity. Data are represented as colour contour plots according to the legend presented in the lower panel, with lowest (0%) sleep efficiency indicated in dark purple and highest sleep efficiency (100%) indicated in dark orange. Consecutive study days are presented beneath each other. Clock time (h) and hours into the sleep opportunity are shown on the horizontal axis. For each day, important study events and treatments are indicated (baseline; CR1, constant routine 1; Rec1, recovery sleep 1; placebo or melatonin treatment administration; CR2, constant routine 2; Rec2, recovery sleep 2). In the placebo trial, sleep efficiency was generally low in the initial part of the 16 h sleep episode, i.e. 16.00 h to midnight, though D4 to D11. The major bout of sleep, as indicated by high sleep efficiency, occurred at a similar phase throughout the protocol, i.e. from about midnight to 08.00 h, with some day-to-day variability. In contrast, in the melatonin trial, the major sleep bout was substantially advanced in time compared to placebo, and occurred during the first half of the sleep opportunity. This change in distribution was found to persist on D11, when both groups received placebo, although the advance in the period of high sleep efficiency on D11 is less pronounced than when melatonin was administered on D10.
Sleep duration and structure
Sleep duration and structure data were averaged for the melatonin and placebo treatment periods (D4–10) in order to examine the effects of melatonin during extension of the sleep opportunity. The mean TST in the placebo trial was 8.9 h and in the melatonin trial was 9.3 h (Table 1). Melatonin treatment did not significantly affect TST over the entire sleep opportunity.
During melatonin treatment, no significant changes in sleep structure across the extended sleep opportunity were observed (Table 1). However, in the first half of the sleep opportunity, NREM sleep stages 1 and 2 were significantly longer after melatonin compared to placebo (stage 1: P < 0.001, 95% confidence interval (CI) 0.1–0.3; stage 2: P < 0.01, 95% CI 0.9–1.6), and in the second half, the amount of time spent in these stages was significantly less after melatonin compared to placebo (stage 1: P < 0.05, 95% CI 0.1–0.5; stage 2: P < 0.05, 95% CI 0.2–1.5). As with the duration of sleep stages 1 and 2, REM sleep duration was significantly greater during the first half of the sleep opportunity after melatonin compared to placebo (P < 0.01, 95% CI 0.2–0.6). In the second half, REM sleep duration was lower after melatonin compared to placebo (P < 0.05, 95% CI 0.1–0.7). The amount of time spent in stages 3 and 4 of NREM sleep was not significantly affected by melatonin.
To examine the acute effects of melatonin on sleep structure whilst taking into account the change in the distribution of sleep, the percentage of each sleep stage was examined as a function of TST in the first half of the sleep opportunity (16.00–00.00 h) compared to the second half (00.00–08.00 h). Stage 2 as a percentage of TST was found to increase in the first half of the sleep opportunity after melatonin (P < 0.05, 95% CI 0.7–6.4), and decrease in the second half after melatonin (P < 0.05, 95% CI 0.2–1.5). Melatonin treatment decreased the stage 3 percentage in the first half of the sleep opportunity (P < 0.05, 95% CI 0.5–5.9), but did not significantly affect it in the second half. When expressed as a percentage of TST, NREM sleep stages 1 and 4 and REM sleep were not significantly affected by melatonin treatment.
Sleep timing
Mean sleep latencies were greater in the placebo trial; however, these differences did not reach statistical significance (Table 1). Latency to the first REM episode was longer in the melatonin trial, but again this difference was not significant. However, the timing or distribution of sleep within the 16 h sleep opportunity was substantially altered by melatonin (Fig. 2). To examine changes in the distribution of sleep during melatonin treatment, TST in the first and second halves of the sleep opportunity were compared. In the placebo trial, participants slept on average for 3.4 h during the first half, whereas in the melatonin trial they slept on average for 5.4 h (P < 0.01, 95% CI 1.3–2.7). All participants (8/8) slept more during the first half in the melatonin trial compared with the placebo trial. In contrast, during the second half of the sleep opportunity participants slept on average 1.6 h less during melatonin treatment compared to placebo (P < 0.05, 95% CI 0.4–2.8).
The effects of melatonin treatment on sleep timing were more closely examined by calculating mean sleep efficiency per hour (Fig. 2) and conversely percentage wakefulness per hour (Fig. 3A). When averaged across D4–10, percentage wakefulness was greater than 50% for the first half of the sleep opportunity but decreased substantially in the second half in the placebo trial (Fig. 3A). The wakefulness profile in the melatonin trial showed an approximately inverse pattern with higher levels of wakefulness in the second half of the sleep opportunity. ANOVA revealed a significant treatment by interval interaction (P < 0.01), and paired sample t tests confirmed that wakefulness during the first half of the sleep opportunity was significantly greater in the placebo compared to melatonin trials (P < 0.01, 95% CI 16.6–33.8), and wakefulness in the second half of the sleep opportunity was significantly greater for melatonin compared to placebo (P < 0.05, 95% CI 5.1–35.0).
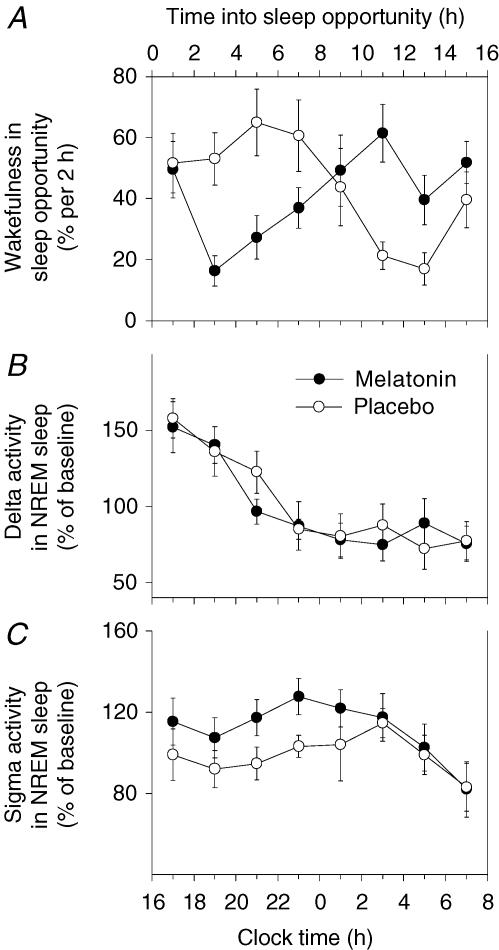
Figure 3. Wakefulness, delta activity and sigma activity.
Time course of mean wakefulness (% per 2 h; A), mean delta activity in NREM sleep (power density in the 0.75–4.5 Hz band) expressed as a percentage of baseline (D1; B), and mean sigma activity in NREM sleep (power density in the 12.0–13.59 Hz band) expressed as a percentage of baseline (D1; C) (n = 8). Standard error of the mean (s.e.m.) is shown. Data points have been calculated across the treatment administration period (D4 to D10). D3 was excluded due to the influence of the extended period of wakefulness associated with the initial constant routine. During the placebo trial, wakefulness was greater during the first half of the sleep opportunity compared to the second half. In contrast, the opposite pattern is observed for wakefulness during the melatonin trial. The time course of delta activity is similar for the melatonin and placebo trials, showing a decrease as a function of the number of hours into the sleep opportunity. Sigma activity is increased in the first half of the sleep opportunity after melatonin treatment, but is not different to placebo in the second half of the sleep opportunity.
Delta and sigma activity
Delta activity in NREM sleep (% baseline) showed a similar time course after melatonin and placebo (Fig. 3B). In both trials, delta activity decreased approximately equally throughout the sleep opportunity. ANOVA revealed a significant main effect of sleep opportunity half, confirming the decrease in delta activity from the first to the second half (P < 0.01, 95% CI 27.0–58.4). However, there was no significant effect of treatment or treatment by sleep opportunity half interaction. In contrast, sigma activity in NREM sleep was increased by melatonin during the first half of the sleep opportunity, but did not appear to differ in the second half (Fig. 3C). ANOVA revealed no significant main effects of treatment or interval, but showed a treatment by interval interaction (P < 0.01). Paired sample t tests confirmed that sigma activity was higher after melatonin compared to placebo in the first half of the sleep opportunity (P < 0.05, 95% CI 1.9–33.8) but was not significantly different in the second half.
Dynamics of TST, REM sleep and NREM sleep during protocol
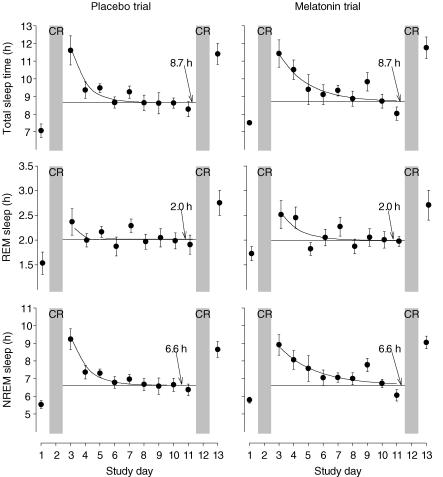
This protocol involved extension of the sleep opportunity and also sleep deprivation associated with constant routines. We examined the dynamics of TST, REM sleep and NREM sleep in the course of the 14 day protocol (Fig. 4) to assess the effects of melatonin on the homeostatic regulation of sleep. TST, NREM sleep and REM sleep were all higher in the sleep episodes immediately following the constant routines and declined during subsequent sleep episodes. Asymptotic values were estimated to be 8.7 h for TST, 2.0 h for REM sleep and 6.6 h for NREM sleep. These values were identical for the melatonin and placebo trials.
Figure 4. Total sleep time, REM sleep and NREM sleep.
Time course of total sleep time (TST; h), REM sleep (h) and NREM sleep (h) during the melatonin and placebo trials quantified with an exponential decaying function fitted to mean data (n = 8) using a non-linear regression procedure. For TST, the function used was TSTday = TST0× e−day/t+ TST∞. TST∞ represents the value of TST when ‘day’ approaches ∞; TST0 is the hypothetical intercept with the ordinate if TST0 were 0; t is the time constant of the exponential decaying function. The parts of the study involving sleep deprivation (i.e. the constant routines, CR) are indicated with light grey shading. The horizontal asymptote is shown with a horizontal line, and the asymptote value is indicated for each function. Asymptotic values were estimated to be 8.7 h for TST (95% CI: melatonin 7.7–9.7, placebo 8.3–9.1), 2.0 h for REM sleep (95% CI: melatonin 1.8–2.2, placebo 1.9–2.2) and 6.6 h for NREM sleep (95% CI: melatonin 5.5–7.8, placebo 6.4–6.9). These values were identical for the melatonin and placebo trials.
Separation of direct and circadian effects of melatonin on sleep
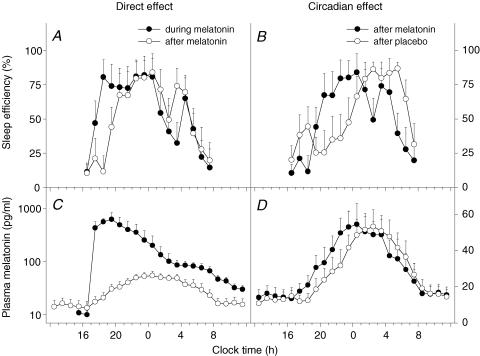
To investigate the direct, sleep-facilitating effects of melatonin, the mean sleep efficiency profile (in 1 h intervals) during the last day of melatonin treatment (D10) was compared to the profile during the washout night (D11) (Fig. 5A). This analysis is based on the assumption that circadian phase differences between these 2 days are minor because 1 day of placebo treatment is unlikely to undo the phase-shifting effects of 8 days of melatonin treatment. Substantially higher sleep efficiency values were observed during melatonin treatment for the first 3 h of the sleep opportunity. Sleep efficiency was found to be significantly greater during melatonin treatment at 18.00 h (2 h post-treatment) compared to the corresponding time on the washout night (P < 0.01, 95% CI 34.5–103.6).
Figure 5. Separation of direct and circadian effects of melatonin.
Mean sleep efficiency levels (% per hour; A and B, n = 8) and mean plasma melatonin levels (C and D n = 8). Standard error of the mean (s.e.m.) is shown for sleep efficiency and plasma melatonin data. The direct, sleep-facilitating effect of melatonin (A) is illustrated by a comparison between sleep efficiency profiles on the last day of melatonin treatment (D10, •) and sleep efficiency on the washout day (D11, ○). Increased sleep efficiency is observed for the first 2–3 h during melatonin treatment. The circadian effect of melatonin on sleep (B) is illustrated by a comparison between sleep efficiency levels on the washout day (D11), which was the day after melatonin (•) or placebo (○). On D11, placebo was administered to all participants. A shift in the distribution of sleep can be observed after melatonin treatment, with the major bout of sleep occurring earlier in the sleep opportunity. On the corresponding day after placebo, the major bout of sleep occurred later in the sleep opportunity, although an initial rise in sleep efficiency is noted at around the commencement of the sleep opportunity. Mean plasma melatonin profiles are presented (C) to illustrate the difference in circulating melatonin levels during melatonin treatment (•) compared to the levels after treatment had stopped (○) (reproduced and re-analysed with permission from Rajaratnam et al. 2003). Note that during melatonin treatment, plasma melatonin levels remained elevated for the duration of the 16 h sleep opportunity. Plasma melatonin levels during the second constant routine (i.e. after the treatment period) are presented (D) to illustrate the melatonin-induced advance in timing of the endogenous melatonin profile (•) compared to placebo (○) (reproduced with permission from Rajaratnam et al. 2003).
The mean sleep efficiency profile on the washout night of the melatonin trial (D11) was compared to the corresponding night of the placebo trial to examine the circadian (or phase-shifting) effects of melatonin (Fig. 5B). The sleep profile was phase-advanced after melatonin compared to placebo. Sleep efficiency was found to be significantly higher after melatonin treatment at 20.00 h (P < 0.05, 95% CI 2.8–80.9) and 22.00 h (P < 0.05, 95% CI 3.6–84.2), and was significantly lower after melatonin treatment at 02.00 h (P < 0.05, 95% CI 4.5–69.5), 04.00 h (P < 0.01, 95% CI 5.6–22.6) and 05.00 h (P < 0.01, 95% CI 18.8–76.2) compared to placebo.
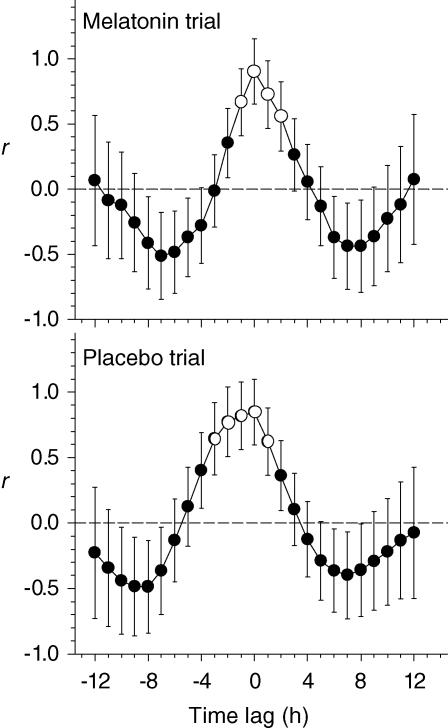
To examine the possible association between the shifts in sleep timing and in the endogenous melatonin rhythm, sleep efficiency data from the washout night were cross-correlated with plasma melatonin levels from the second constant routine in each treatment trial (Fig. 5D). The highest correlation obtained in both trials was for 0 h lag (Fig. 6). These analyses indicate that the profile of sleep propensity was advanced by an amount approximately equal to the advance observed in plasma melatonin, which we previously reported was 5.3 h and 2.4 h for the melatonin and placebo trials, respectively (Rajaratnam et al. 2003).
Figure 6. Relationship between the sleep efficiency profile and the plasma melatonin rhythm.
Mean cross-correlation coefficients, where the observations from one data series are correlated with another series at various lags and leads. Sleep efficiency data from the washout night are compared to plasma melatonin levels during the second constant routine, for the melatonin trial (upper panel) and the placebo trial (lower panel). Plasma melatonin data were taken with permission from Rajaratnam et al. (2003). The horizontal dashed line represents a correlation coefficient (r) of 0. Time lag (h) is shown on the horizontal axis. Data points represented by open circles indicate time lags with significant correlation coefficients (P < 0.05). Maximal correlation coefficients obtained for both trials were at 0 h lag.
Discussion
Using an extended sleep opportunity protocol, the present study demonstrates unequivocally that exogenous melatonin can advance the timing of human sleep, without significantly affecting TST. To our knowledge, this is the first demonstration of the phase-shifting effects of melatonin on polysomnographically recorded sleep with concurrent assessment of melatonin effects on homeostatic sleep regulation, in the absence of the confounding effects of sleep restriction.
When the sleep opportunity commenced during the day, sleep could be initiated in both the placebo and melatonin trials, in accordance with nap studies that have demonstrated that young people can initiate sleep at this time of day, even when sleep pressure is relatively low (Dijk et al. 1987). The finding that sleep latency was not significantly reduced by melatonin is somewhat surprising, as the majority of previous studies report that melatonin decreased sleep latency (Waldhauser et al. 1990; Zhdanova et al. 1995; Reid et al. 1996; Zhdanova et al. 1996; Hughes & Badia, 1997). However, clear effects of melatonin were observed on the temporal placement of the primary sleep bout within the sleep opportunity. In the placebo trial the initial sleep bout was typically followed by a prolonged period of wakefulness in the late evening, a time which has been previously described as the wake maintenance zone (Strogatz et al. 1986), and the ‘forbidden zone’ for sleep (Lavie, 1986). In contrast, during the melatonin trial, the corresponding time was characterized by a relatively long, consolidated sleep bout.
According to current models of sleep–wake regulation, the wake maintenance zone is thought to be a function of the circadian peak in arousal which promotes wakefulness at this time (Dijk & Czeisler, 1995). Lesion studies in squirrel monkeys indicate that the SCN are primarily responsible for this wakefulness-generating mechanism (Edgar et al. 1993). The wake maintenance zone occurs close to the onset of melatonin secretion, and the abrupt rise in nocturnal sleep propensity coincides with, or occurs shortly after, the rise of melatonin (Dijk & Czeisler, 1994). It is speculated that melatonin contributes to this abrupt change by inhibiting the wake-promoting signal from the SCN, and thereby opening the sleep gate (Lavie, 1986, 1997; Shochat et al. 1998; Jin et al. 2003). The present findings support this claim by showing that (artificially) increased melatonin levels during the early evening effectively ‘silenced’ and shifted the wake maintenance zone (Sack et al. 1997).
When the sleep propensity profile was examined 24 h after the last melatonin treatment (i.e. during the washout night, D11), the redistribution in sleep timing was found to persist, albeit to a lesser extent than during melatonin administration (e.g. D10). These data clearly demonstrate that the observed changes in the distribution of sleep during melatonin treatment were caused by a combination of the direct, sleep-promoting effects of melatonin and the circadian or phase-shifting effects. In a previous report on this study, we demonstrated that melatonin administration at the beginning of the sleep opportunity (16.00 h) phase advanced endogenous melatonin and cortisol rhythms (Rajaratnam et al. 2003). Here we report that melatonin essentially phase-advanced sleep by an amount approximately equal to the shift in the endogenous melatonin rhythm. In addition, melatonin directly facilitated sleep for approximately 3 h post-administration.
Although melatonin exerted both direct and circadian effects on sleep, there was no change in TST or duration of REM sleep and NREM sleep. These data indicate that unlike classic hypnotic drugs (Lancel & Steiger, 1999), melatonin facilitates sleep rather than inducing it. Furthermore, as sleep duration is known to be regulated by an interaction between circadian and homeostatic factors (Dijk & Czeisler, 1995; Dijk et al. 1999), our finding that TST was unaffected even though melatonin advanced the circadian system suggests that sleep need (as reflected by TST) was not altered. By exposing participants to extended sleep opportunities (16 h) in very dim light with enforced bedrest, we were able to assess how much sleep an individual would obtain in such an environment. It should be noted that if the duration of the sleep opportunity had been limited to the standard 8 h, a significant change in TST would probably be observed, due to a combination of the phase-shifting effects of melatonin on sleep and its direct, sleep-facilitating effects.
To reduce interindividual differences in sleep duration and the duration of endogenous circadian rhythms (Aeschbach et al. 2003), we excluded potential subjects with short (< 7 h) or long (> 10 h) habitual sleep duration. We estimate that after recovery from the sleep deprivation associated with the first constant routine, TST in our sample was 8.7 h in both the melatonin and placebo trials (Fig. 4). Therefore, despite the 16 h available for sleep, and the fact that participants reported anecdotally that they were highly motivated to sleep to relieve boredom, participants were only able to obtain on average approximately 8.7 h of sleep. REM sleep duration was 2.0 h under these conditions, and NREM sleep was 6.6 h. In a previous sleep extension study, TST after 4 weeks of exposure to a 14:10 h sleep–wake cycle was 8.2 h (Wehr et al. 1993). The slight discrepancy between findings of the previous study and ours may be explained by the imposition of an initial constant routine in the present study, differences in the duration of exposure to the extended sleep opportunities (9 days versus 4 weeks), and differences in duration of the sleep opportunity (16 h versus 14 h).
When sleep structure was assessed across the entire 16 h sleep opportunity, no changes were observed during melatonin treatment. Corresponding with the melatonin-induced change in the distribution of sleep, NREM stages 1 and 2 sleep and REM sleep were found to be greater in the first half of the sleep opportunity after melatonin treatment. The selective increase in these sleep stages by melatonin has previously been reported (Nave et al. 1996; Cajochen et al. 1997, 1998; Hughes & Badia, 1997; Stone et al. 2000). When the relative proportion of time spent in each sleep stage was examined as a function of the amount of sleep in each half of the sleep opportunity, stage 2 sleep percentage was increased by melatonin and stage 3 sleep percentage was decreased in the first half of the sleep opportunity. Again, these findings are consistent with a previous report that melatonin administration during the daytime increases visually scored stage 2 sleep and decreases slow wave sleep (Hughes & Badia, 1997).
Daytime effects of melatonin on sleep-stage-specific EEG characteristics have been demonstrated (Dijk et al. 1995; Nave et al. 1996). Here we report that melatonin administered during extended sleep opportunities did not influence delta activity, but did increase sigma activity in NREM sleep during the first half of the sleep opportunity. Sigma represents the frequency range of sleep spindles. Low-frequency spindle activity shows a marked circadian variation, reaching its maximum just prior to the peak in endogenous melatonin synthesis (Dijk et al. 1997). The present findings lend further support to the proposition that melatonin may be involved in the circadian regulation of sleep spindle activity (Dijk et al. 1995).
The physiological mechanisms by which melatonin alters sleep and circadian timing in humans are presently unclear. Rodent studies provide evidence that the phase-shifting effects of melatonin are mediated by melatonin receptors in the SCN (Cassone et al. 1986; Vanecek et al. 1987; Kilduff et al. 1992; McArthur et al. 1997; Dubocovich et al. 1998; Hunt et al. 2001). Melatonin receptors have also been identified in human SCN (Dubocovich, 1995) and cerebellar cortex (Al-Ghoul et al. 1998). As for the sleep-facilitating effects of melatonin, the mechanisms have not been elucidated. It may be that these effects are related to the acute inhibition of SCN multiple unit activity (MUA) observed after melatonin administration in vitro. This inhibitory effect of melatonin on MUA appears to be mediated primarily by the Mel1a receptor (von Gall et al. 2002). Alternatively, the sleep-facilitating effects of melatonin may be related to a hypothermic response mediated by peripheral vasodilatation (Krauchi et al. 1997). Posture is believed to play an important role in the hypothermic and sleep-facilitating effects of melatonin. It is likely that enforced recumbency (and very dim light) contributed to the effects observed in the present study (Krauchi et al. 1997).
The mean peak plasma melatonin concentration achieved after melatonin was administered in this study was 626 pg ml−1, which was approximately 10 times higher than the endogenous peak levels for these individuals. Whether this could be considered a pharmacological or a physiological treatment is a matter for discussion, and will depend on the concentration of melatonin at its target sites. In sheep studied in the absence of exogenous melatonin, endogenous melatonin concentrations in cisterna magna are approximately 20 times higher than in plasma (Skinner & Malpaux, 1999). The relationship between plasma melatonin concentration and melatonin concentration in cisterna magna during melatonin administration is presently unclear, but it may very well be that under such conditions plasma concentrations are more similar to concentrations at target sites.
We have demonstrated that melatonin administration (1.5 mg) can advance the timing of sleep and endogenous circadian rhythms (Rajaratnam et al. 2003) and facilitate sleep during the wake maintenance zone, without significantly affecting sleep duration. These findings suggest that endogenous melatonin is involved in the circadian regulation of sleep propensity in humans. The findings also have practical relevance for the use of melatonin in the treatment of circadian rhythm sleep disorders, such as delayed sleep phase syndrome (Dahlitz et al. 1991), and in other situations in which the endogenous sleep propensity rhythm is delayed relative to the preferred time for sleep (Arendt, 2000).
Acknowledgments
We thank the study participants for their time and effort. We also thank Ms S. Ratnasingam, Dr J. Wright and Dr R. Stott for nursing and medical supervision, QinetiQ Ltd staff (in particular Ms K. Robertson and Mr S. Foster) for technical and other assistance, and Ms B. Thorleifsdottir and Ms K. Robertson for assistance with data analysis. The study was supported by a joint grant from the Medical Research Council and the Ministry of Defence (G9810584) and funding from Stockgrand Ltd (University of Surrey, UK).
References
- Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab. 2003;88:26–30. doi: 10.1210/jc.2002-020827. 10.1210/jc.2002-020827. [DOI] [PubMed] [Google Scholar]
- Al-Ghoul WM, Herman MD, Dubocovich ML. Melatonin receptor subtype expression in human cerebellum. Neuroreport. 1998;9:4063–4068. doi: 10.1097/00001756-199812210-00011. [DOI] [PubMed] [Google Scholar]
- Arendt J. Melatonin, circadian rhythms, and sleep. N Engl J Med. 2000;343:1114–1116. doi: 10.1056/NEJM200010123431510. 10.1056/NEJM200010123431510. [DOI] [PubMed] [Google Scholar]
- Arendt J, Bojkowski C, Folkard S, Franey C, Marks V, Minors D, Waterhouse J, Wever RA, Wildgruber C, Wright J. Some effects of melatonin and the control of its secretion in humans. In: Evered D, Clark S, editors. Photoperiodism, Melatonin and the Pineal. Vol. 117. London: Pitman; 1985. pp. 266–283. Ciba Foundation Symposium. [DOI] [PubMed] [Google Scholar]
- Attenburrow MEJ, Dowling BA, Sargent PA, Sharpley AL, Cowen PJ. Melatonin phase advances circadian rhythm. Psychopharmacology (Berl) 1995;121:503–505. doi: 10.1007/BF02246501. [DOI] [PubMed] [Google Scholar]
- Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530:565–574. doi: 10.1111/j.1469-7793.2001.0565k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;46:R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Kraeuchi K, Von Arx MA, Moeri D, Graw P, Wirz-Justice A. Daytime melatonin administration enhances sleepiness and theta/alpha activity in the waking EEG. Neurosci Lett. 1996;207:209–213. doi: 10.1016/0304-3940(96)12517-9. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Krauchi K, Danilenko KV, Wirz-Justice A. Evening administration of melatonin and bright light: interactions on the EEG during sleep and wakefulness. J Sleep Res. 1998;7:145–157. doi: 10.1046/j.1365-2869.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Krauchi K, Mori D, Graw P, Wirz-Justice A. Melatonin and S-20098 increase REM sleep and wake-up propensity without modifying NREM sleep homeostasis. Am J Physiol. 1997;272:R1189–R1196. doi: 10.1152/ajpregu.1997.272.4.R1189. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Chesworth MJ, Armstrong SM. Entrainment of rat circadian rhythms by daily injection of melatonin depends upon the hypothalamic suprachiasmatic nuclei. Physiol Behav. 1986;36:1111–1121. doi: 10.1016/0031-9384(86)90488-9. [DOI] [PubMed] [Google Scholar]
- Dahlitz MJ, Alvarez B, Vignau J, English J, Arendt J, Parkes JD. Delayed sleep–phase syndrome: response to melatonin. Lancet. 1991;337:1121–1124. doi: 10.1016/0140-6736(91)92787-3. 10.1016/0140-6736(91)92787-3. [DOI] [PubMed] [Google Scholar]
- Deacon S, Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 1995;688:77–85. doi: 10.1016/0006-8993(95)96872-i. 10.1016/0006-8993(95)96872-I. [DOI] [PubMed] [Google Scholar]
- Deacon S, English J, Arendt J. Acute phase-shifting effects of melatonin associated with suppression of core body temperature in humans. Neurosci Lett. 1994;178:32–34. doi: 10.1016/0304-3940(94)90282-8. 10.1016/0304-3940(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207–219. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Roth C, Landolt HP, Werth E, Aeppli M, Achermann P, Borbely AA. Melatonin effect on daytime sleep in men: Suppression of EEG low frequency activity and enhancement of spindle frequency activity. Neurosci Lett. 1995;201:13–16. doi: 10.1016/0304-3940(95)12118-n. 10.1016/0304-3940(95)12118-N. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol. 1997;505:851–858. doi: 10.1111/j.1469-7793.1997.851ba.x. 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci U S A. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin receptors: Are there multiple subtypes? Trends Pharmacol Sci. 1995;16:50–56. doi: 10.1016/s0165-6147(00)88978-6. 10.1016/S0165-6147(00)88978-6. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Yun K, Alghoul WM, Benloucif S, Masana MI. Selective Mt2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–1220. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: Evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20:124–131. [PubMed] [Google Scholar]
- Hunt AE, Al-Ghoul WM, Gillette MU, Dubocovich ML. Activation of MT(2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol. 2001;280:C110–C118. doi: 10.1152/ajpcell.2001.280.1.C110. [DOI] [PubMed] [Google Scholar]
- Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, Weaver DR. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003;23:1054–1060. doi: 10.1128/MCB.23.3.1054-1060.2003. 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattapong KR, Fogg LF, Eastman CI. Effect of sex, menstrual cycle phase, and oral contraceptive use on circadian temperature rhythms. Chronobiol Int. 1995;12:257–266. [Google Scholar]
- Kilduff TS, Landel HB, Nagy GS, Sutin EL, Dement WC, Heller HC. Melatonin influences fos expression in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1992;16:47–56. doi: 10.1016/0169-328x(92)90192-e. 10.1016/0169-328X(92)90192-E. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus. The Minds Clock. New York, Oxford: Oxford University Press; 1991. [Google Scholar]
- Krauchi K, Cajochen C, Wirz-Justice A. A relationship between heat loss and sleepiness: effects of postural change and melatonin administration. J Appl Physiol. 1997;83:134–139. doi: 10.1152/jappl.1997.83.1.134. [DOI] [PubMed] [Google Scholar]
- Lancel M, Steiger A. Sleep and its modulation by drugs that affect GABAA receptor function. Angew Chem Int Ed Engl. 1999;38:2853–2864. doi: 10.1002/(sici)1521-3773(19991004)38:19<2852::aid-anie2852>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lavie P. Ultrashort sleep-waking schedule. III. ‘Gates’ and ‘forbidden zones’ for sleep. Electroencephalogr Clin Neurophysiol. 1986;63:414–425. doi: 10.1016/0013-4694(86)90123-9. 10.1016/0013-4694(86)90123-9. [DOI] [PubMed] [Google Scholar]
- Lavie P. Melatonin: Role in gating nocturnal rise in sleep propensity. J Biol Rhythms. 1997;12:657–665. doi: 10.1177/074873049701200622. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Tabandeh H, Bird AC, Defrance R, Arendt J. Relationship between napping and melatonin in the blind. J Biol Rhythms. 1997;12:16–25. doi: 10.1177/074873049701200104. [DOI] [PubMed] [Google Scholar]
- McArthur AJ, Hunt AE, Gillette MU. Melatonin and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dawn and dusk. Endocrinology. 1997;138:627–634. doi: 10.1210/endo.138.2.4925. 10.1210/en.138.2.627. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Sack RL, Lewy AJ. Sleep propensity free-runs with the temperature, melatonin and cortisol rhythms in a totally blind person. Sleep. 1992;15:330–336. doi: 10.1093/sleep/15.4.330. [DOI] [PubMed] [Google Scholar]
- Nave R, Herer P, Haimov I, Shlitner A, Lavie P. Hypnotic and hypothermic effects of melatonin on daytime sleep in humans: Lack of antagonism by flumazenil. Neurosci Lett. 1996;214:123–126. doi: 10.1016/0304-3940(96)12899-8. 10.1016/0304-3940(96)12899-8. [DOI] [PubMed] [Google Scholar]
- Rajaratnam SMW, Dijk DJ, Middleton B, Stone BM, Arendt J. Melatonin phase-shifts human circadian rhythms with no evidence of changes in the duration of endogenous melatonin secretion or the 24- h production of reproductive hormones. J Clin Endocrinol Metab. 2003;88:4303–4309. doi: 10.1210/jc.2003-030460. 10.1210/jc.2003-030460. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human SubJects. Washington DC: US Government Printing Office; 1968. [Google Scholar]
- Reid K, Van den Heuvel C, Dawson D. Day-time melatonin administration: effects on core temperature and sleep onset latency. J Sleep Res. 1996;5:150–154. doi: 10.1046/j.1365-2869.1996.t01-1-00006.x. 10.1046/j.1365-2869.1996.t01-1-00006.x. [DOI] [PubMed] [Google Scholar]
- Sack RL, Hughes RJ, Edgar DM, Lewy AJ. Sleep promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms. Sleep. 1997;20:908–915. doi: 10.1093/sleep/20.10.908. [DOI] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ, Hoban TM. Free-running melatonin rhythms in blind people: phase shifts with melatonin and triazolam administration. In: Rensing L, an der Heiden U, Mackey MC, editors. Temporal Disorder in Human Oscillatory Systems. Heidelberg: Springer-Verlag; 1987. pp. 219–224. [Google Scholar]
- Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol Regul Integr Comp Physiol. 2002;282:R454–R463. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat T, Haimov I, Lavie P. Melatonin – the key to the gate of sleep. Ann Med. 1998;30:109–114. doi: 10.3109/07853899808999392. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Malpaux B. High melatonin concentrations in third ventricular cerebrospinal fluid are not due to Galen vein blood recirculating through the choroid plexus. Endocrinology. 1999;140:4399–4405. doi: 10.1210/endo.140.10.7074. 10.1210/en.140.10.4399. [DOI] [PubMed] [Google Scholar]
- Stone BM, Turner C, Mills SL, Nicholson AN. Hypnotic activity of melatonin. Sleep. 2000;23:663–669. [PubMed] [Google Scholar]
- Strogatz SH, Kronauer RE, Czeisler CA. Circadian regulation dominates homeostatic control of sleep length and prior wake length in humans. Sleep. 1986;9:353–364. doi: 10.1093/sleep/9.2.353. [DOI] [PubMed] [Google Scholar]
- Vanecek J, Pavlik A, Illnerova H. Hypothalamic melatonin receptor sites revealed by autoradiography. Brain Res. 1987;435:359–362. doi: 10.1016/0006-8993(87)91625-8. 10.1016/0006-8993(87)91625-8. [DOI] [PubMed] [Google Scholar]
- von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151–162. doi: 10.1007/s00441-002-0581-4. 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Saletu B, Trinchard-Lugan I. Sleep laboratory investigations on hypnotic properties of melatonin. Psychopharmacology (Berl) 1990;100:222–226. doi: 10.1007/BF02244410. [DOI] [PubMed] [Google Scholar]
- Wehr TA. The durations of human melatonin secretion and sleep respond to changes in daylength (photoperiod) J Clin Endocrinol Metab. 1991;73:1276–1280. doi: 10.1210/jcem-73-6-1276. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Aeschbach D, Duncan WC., Jr Evidence for a biological dawn and dusk in the human circadian timing system. J Physiol. 2001;535:937–951. doi: 10.1111/j.1469-7793.2001.t01-1-00937.x. 10.1111/j.1469-7793.2001.t01-1-00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol. 1993;265:R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk D-J. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Wurtman RJ, Lynch HJ, Ives JR, Dollins AB, Morabito C, Matheson JK, Schomer DL. Sleep-inducing effects of low doses of melatonin ingested in the evening. Clin Pharmacol Ther. 1995;57:552–558. doi: 10.1016/0009-9236(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Wurtman RJ, Morabito C, Piotrovska VR, Lynch HJ. Effects of low oral doses of melatonin, given 2–4 hours before habitual bedtime, on sleep in normal young humans. Sleep. 1996;19:423–431. doi: 10.1093/sleep/19.5.423. [DOI] [PubMed] [Google Scholar]