Abstract
Cells that express the NG2 proteoglycan are the largest proliferative progenitor population in the postnatal central nervous system (CNS). Although this entire population has long been considered to be oligodendrocyte progenitors, numerous NG2+ cells are present in the cerebral cortex, where relatively little myelination occurs, and also persist long after myelination is complete in the CNS. Several studies have alluded to the presence of distinct NG2+ cell subtypes based on marker expression, but no experimentally derived hypotheses about the physiological role of these subtypes has been proposed. In the current study, whole-cell patch-clamp data from acutely isolated slices demonstrate that subcortical white matter and cortical NG2+ cells display distinct membrane properties in addition to possessing differing K+- and Na+-channel expression profiles. A striking observation is that a subpopulation of cortical, but not white matter NG2+ cells, elicit depolarization-induced spikes that are akin to immature action potentials. Our data demonstrate that a population of cortical NG2+ cells display physiological properties that differ from their white matter counterparts.
Expression of the NG2 proteoglycan (Nishiyama et al. 1991) by cells of the central nervous system (CNS) has been widely employed as a criterion to identify oligodendrocyte progenitors (OPCs; for reviews see Dawson et al. 2000; Levine et al. 2001). However, it is clear that numerous NG2-expressing (NG2+) cells are located in grey matter areas of the brain, including the cerebral cortex (Reynolds & Hardy, 1997; Mallon et al. 2002; Yuan et al. 2002). These regions display relatively less myelination than white matter areas. Furthermore, NG2+ cells persist in the grey matter of the adult CNS long after myelination has terminated (Nishiyama et al. 1996; Reynolds & Hardy, 1997). Such observations have raised numerous questions regarding the physiological role of these NG2+ cells (Horner et al. 2002). Although it is clear that these cells are capable of differentiating into myelinating oligodendrocytes, whether this developmental program occurs for all NG2+ cells in the CNS throughout postnatal developmental and into adulthood is unknown. In fact, recent in vivo evidence has suggested that NG2+ cells in the postnatal CNS represent a heterogeneous cell population. NG2+ cells in the cerebral cortex can be segregated into two populations, based on proteolipid (PLP) promoter-driven enhanced green fluorescent protein (EGFP) expression (Mallon et al. 2002). NG2 immunoreactivity has been identified in a percentage of glial fibrillary acidic protein (GFAP)-EGFP+ hippocampal cells (Matthias et al. 2003). A subpopulation of hippocampal NG2+ cells also express the immature neuronal marker TOAD-64 (Belachew et al. 2003).
Although detailed functional analysis will undoubtedly shed further light on the possible heterogeneous nature of these cells, electrophysiological data have not been forthcoming and only a few reports have investigated the physiological properties of NG2+ cells in brain slices (Bergles et al. 2000; Diers-Fenger et al. 2001; Lin & Bergles, 2003; Matthias et al. 2003). In the current study, we utilized the 2′-3′-cyclic nucleotide 3′-phosphodiesterase (CNP)-EGFP transgenic mouse generated in our laboratory (Yuan et al. 2002) and focused on EGFP-expressing NG2+ cells in the subcortical white matter and cortical regions of the CNS. We investigated NG2+ progenitors shortly after birth at a developmental stage corresponding to maximal proliferation and expansion of these cells. Using the whole-cell patch-clamp technique we compared the expression of K+ and Na+ channels by these two NG2+ cell populations.
Methods
Materials
All reagents were from Sigma-Aldrich unless otherwise stated. All cell culture reagents were from InVitrogen (Carlsbad, CA, USA) or Hyclone (Logan, UT, USA). Antibodies used in the study and working dilutions are as follows: rabbit polyclonal anti-NG2 (1 : 500) and mouse monoclonal anti-NeuN (1 : 500) were from Chemicon (Temecula, CA, USA). Mouse monoclonal anti-CNP (1 : 500), anti-HuC/D (1 : 250), anti-TUJ1 (1 : 250) and anti-MBP (1 : 1000) were from Sternberger Monoclonals Inc. (Lutherville, MD, USA). Cy-5-, rhodamine- and Texas Red-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA, USA) and all were used at a dilution of 1 : 200.
CNP-EGFP transgenic mice
The CNP-EGFP transgenic mice were generated as previously described (Yuan et al. 2002), and animal procedures complied with the National Institutes of Health guidelines. In the present study, two separate CNP-EGFP transgenic lines were used (C1 and D1) and no significant differences in results were noted, therefore the data were pooled.
Electrophysiology
P5–P10 CNP-EGFP mice were anaesthetized with 4% isoflurane, decapitated and brains removed. Coronal slices 300 μm thick were prepared as previously described (Belachew et al. 2003). Following a 1 h recovery period, slices were transferred to a recording chamber and perfused with extracellular solution of the following composition (mm): NaCl 124, KCl 3, CaCl2 2.5, MgSO4 1.3, NaHCO3 26; NaHPO4 1.25; glucose 15; saturated with 95% O2–5% CO2 at room temperature. EGFP+ cells were identified as previously described (Yuan et al. 2002; Belachew et al. 2003). In the current study, EGFP+ cells in the white matter or in the cerebral cortices (between layer IV and the pial surface) were targeted. Patch electrodes had resistances between 4 and 8 MΩ, when filled with intracellular solution of the following composition (mm): potassium gluconate 130; NaCl 10; Mg-ATP 2; Na-GTP 0.3, Hepes 10; EGTA 0.6, 0.3% biocytin; adjusted to pH 7.2 and 275 mosmol l−1. The cells were voltage clamped at −80 mV and series resistances (which were typically 20–40 MΩ), input resistances and membrane capacitances were calculated from the capacitive transients and steady state currents resulting from a 5 mV test pulse filtered at 10 kHz following pipette capacitance compensation. Prior to whole-cell recording of currents, series resistances were compensated by at least 85%. In experiments to isolate sustained outward (IKDR), A-type transient (IKA) and inward-rectifying (IKir) K+-channel currents, the bathing solution contained 1 μm tetrodotoxin. Depolarizing voltage-step protocols to isolate IKDR and IKA were as previously described (Knutson et al. 1997). In brief, IKDR was elicited by a series of depolarizing voltage steps (voltage steps from −60 to 80 mV after a pre-pulse to −40 mV to inactivate IKA; 10 mV increments). IKA was isolated by digitally subtracting traces obtained as described above with traces obtained following a pre-pulse to −110 mV; voltage steps from −60 to 50 mV; 10 mV increments (note all values stated are prior to junction potential correction). Calculation of current densities and activation profile analysis with Boltzmann curve fitting were as previously described (Knutson et al. 1997; Chittajallu et al. 2002). IKir was isolated using a voltage-ramp protocol (−140 to 0 mV; 70 mV s−1) in the presence and absence of extracellular Cs+ (5 mm). Voltage-gated sodium channel currents (INa) were elicited by a series of depolarizing voltage steps (−60 to 30 mV; 10 mV increments; values stated are prior to junction potential correction) in the presence of 10 mm TEA and 1 mm 4-AP in the extracellular solution. To measure the ability of cells to generate action potentials (APs), EGFP+ cells were placed under current clamp and, from a resting potential of −80 mV, depolarizing current injection steps were applied (10–30 pA steps; 450 ms duration; maximum depolarization to approximately 0 mV) using a K+-based intracellular solution as described above. To measure AMPA receptor-mediated inward currents in cortical NG2+ cells, the intracellular solution comprised (mm): caesium methanesulphonate 135; NaCl 8; Mg-ATP 4; Na-GTP 0.3; Hepes 10; EGTA 0.6; 0.3% biocytin; adjusted to pH 7.2 and 275 mosmol l−1). The extracellular solution was as described above with the addition of 10 mm TEA; 1 mm 4-aminopyridine; 50 μm picrotoxin; 1 μm tetrodotoxin; 50 μm APV. Kainic acid, NBQX and cyclothiazide were added as described in Results to the extracellular solution. Continuous recording of the holding current was performed under voltage clamp (holding potential = −80 mV). To measure spontaneous AMPA-receptor mediated EPSCs, cells were voltage clamped at −80 mV and continuous current traces were obtained. Bicuculline (30 μm) was routinely present in the extracellular solution. After a baseline period was attained (1–2 min) either 1–2 μm pardaxin or 0.5–1 nm latrotoxin was added via the extracellular solution. In all the experiments an Axopatch 200B amplifier was used for voltage- and current-clamp (Axon Instruments; Union City, CA, USA). Data were filtered at 5 kHz and digitized at 10 kHz. All voltage measurements and steps were corrected for a junction potential offset. Offline analysis was performed using Clampfit (Axon Instruments).
Resting membrane potentials were attained using cell-attached patch recordings as previously described (Verheugen et al. 1995, 1999; Wang et al. 2003). This approach is based on ascertaining the reversal potential of K+ currents through cell-attached patch to estimate Vm. The intracellular patch solution contained the estimated intracellular K+ concentration and was of the following composition (mm); KCl 150, CaCl2 2, MgCl2 1, Hepes 10; 0.3% biocytin; adjusted to pH 7.4 with KOH (final K+ concentration 155 mm) and osmolarity was 270–290 mosmol l−1. Extracellular solution was as described above. After cell-attached patch was attained, a voltage ramp protocol was applied to activate voltage-gated K+ channels (see supplemental Fig. 2A). Under the conditions of the experiment, the equilibrium potential for K+ (EK) across the patch is approximately 0 mV. Thus K+ currents will reverse when the pipette potential (Vpip) cancels out Vm, giving a direct quantitative measure for membrane potential of the cells (i.e. at K+ reversal, Vm + Vh = Ek∼0 mV). Note: in cell-attached patches Vh = −Vpip. The liquid junction potential was calculated at 3 mV and was corrected after the experiment. Following cell-attached patch recordings whole-cell access was gained to allow for filling of the cell with biocytin and subsequent immunocytochemistry to confirm NG2 expression (see below for details). Data were filtered at 2 kHz and digitized at 50 kHz.
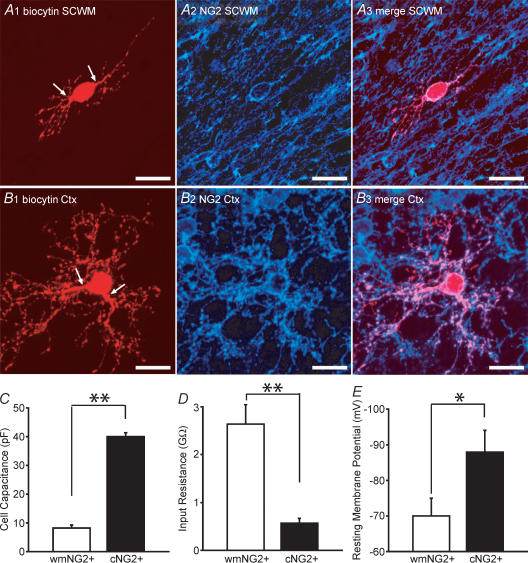
Figure 2. Distinct morphology and membrane properties of white matter NG2+ and cortical NG2+ cells.
A1, a low fluorescence intensity white matter (wm) EGFP+ cell after biocytin injection, followed by rhodamine conjugation. The cell has a bipolar morphology (arrows indicate processes). A2–A3, this cell was NG2+ and was morphologically representative of the EGFP+/white matter NG2+ cells identified. B1, cortical EGFP+ cell processed with the same protocol as in A1. Although the cell body is a similar size to the white matter NG2+, a distinct arborization of processes is apparent. B2–B3, this cell was also found to be NG2+. C and D, measurement of whole-cell capacitances, input resistances in white matter NG2+ (open bars; n = 28) and cortical NG2+ cells (filled bars; n = 40). E, resting membrane potential in white mater NG2+ (open bars; n = 7) and cortical NG2+ cells (filled bars; n = 10). Scale bars, 20 μm. All data was obtained from P5–P8 CNP-EGFP mice. *P < 0.05, **P < 0.01 Mann-Whitney U test.
Statistical analysis was performed with the Mann-Whitney U test and results were deemed significantly different if P < 0.05. *P < 0.05, **P < 0.01, ns, not significant (i.e. P > 0.05).
Immunocytochemistry
Following anaesthesia, perfusion with fixative (4% paraformaldheyde) and brain removal, 50 μm frozen coronal tissue sections were attained from P8 CNP-EGFP mice and immunostaining was performed as previously described (Yuan et al. 2002; Belachew et al. 2003). The procedures for immunocytochemistry following electrophysiological recordings were as follows: after careful withdrawal of the recording electrode from EGFP+ cells, slices were immediately fixed in 4% paraformaldehyde−15% picric acid solution for at least 24 h at 4°C. Slices were then washed 5 times for 10 min each in 0.1 m phosphate buffer, followed by a single wash with NaHCO3 buffer (50 mm NaHCO3, 150 mm NaCl, 0.08% NaN3, pH 8.3) for 20 min. After treatment with 2% Triton X-100/NaHCO3 buffer, the slices were incubated for 2 h with Rhodamine- or Texas Red-conjugated Avidin-D (1: 200) in 0.5% Triton X-100/NaHCO3 solution. Slices were further washed twice in PBS and then incubated with a blocking solution (20% goat serum in PBS). The slices were then incubated overnight with anti-NG2 at 4°C. Further washing of the slices (3 times for 15 min each) was followed by incubation with a Cy5-conjugated secondary antibody for 1 h at room temperature. The slices were then rinsed 3 times in PBS for 15 min, mounted and observed with confocal fluorescent microscopy.
Confocal immunofluorescence microscopy
Images were acquired using a Bio-Rad MRC-1024 confocal laser scanning microscope (CLSM) fitted with a krypton/argon laser (Center for Microscopy and Image Analysis, George Washington University, Washington DC, USA). Excitation via a 488 nm laser line and emission using a 522/35 filter were used to visualize EGFP fluorescence. Rhodamine and Texas Red fluorescence was visualized by excitation and emission via a 568 nm laser line and with a 605/32 filter, respectively. Finally Cy5 fluorescence was visualized by excitation and emission via a 647 nm laser line and with a 680/32 filter, respectively. All images were taken using a 40 × oil objective (NA = 1.35) or 60 × oil objective (NA = 1.4). Five to twenty sequential optical Z-sections (Z-step = 1 μm; total optical section = 5–20 μm) were acquired per objective field. Acquisition was achieved using the LaserSharp software (Bio-Rad Laboratories). Image analysis and preparation were performed using Confocal Assistant, analySIS software (Soft Imaging Systems) and with the Adobe suite of products.
Results
Correlation of EGFP fluorescence and NG2 expression in the subcortical white matter and cerebral cortex
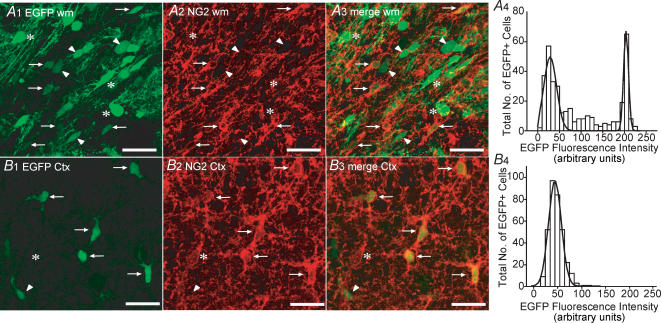
Numerous EGFP+ cells were easily identified in both the white matter and cerebral cortex of CNP-EGFP mice at P8 (Fig. 1A1 and B1). Under the confocal imaging parameters used, the majority of detectable EGFP fluorescence was confined to the cell body with the processes being more difficult to delineate (Fig. 1A1 and B1). In the white matter, many EGFP+ cell bodies were orientated in a manner that is polarized in the direction of the axonal fibres that run through this area (Fig. 1A1). In contrast, EGFP+ cell bodies in cortex displayed more heterogeneity with respect to shape and orientation (Fig. 1B1). Interestingly, Gaussian analysis of the frequency distribution histograms for EGFP fluorescence intensity in the white matter revealed two distinct fluorescent intensity peaks at 29 (width = 30) and 204 (width = 11) arbitrary fluorescence units (Fig. 1A4; 363 cells). We also noted that approximately 28% of the EGFP+ cells displayed a fluorescent intensity that lay between these two peaks. The EGFP+ cells with the higher fluorescent intensities had larger cell bodies with more visible processes that were aligned parallel to axonal fibres (Fig. 1A1; arrowheads). In contrast, only one discrete population of cells was noted with respect to EGFP fluorescent intensity in the cortex (Fig. 1B4; peak = 48; width = 26 arbitrary fluorescence units; 333 cells).
Figure 1. Co-localization of EGFP fluorescence and NG2 immunoreactivity in P8 subcortical white matter and cortex.
A1, confocal image (z = 18 μm) of white matter (wm) from a coronal slice of a P8 CNP-EGFP mouse. Note differences in EGFP fluorescent intensity (arrows/arrowheads versus asterisks). A2 and A3, immunostaining with anti-NG2 shows a proportion of white matter EGFP+ cells are positive for NG2 (arrows). A4, Gaussian analysis of EGFP+ fluorescent intensity frequency distribution. B1, confocal image (z = 18 μm) of cortex (Ctx) in the same coronal slice using identical imaging parameters as in panel A1. Cortical EGFP+ cells display a distinct morphology (see also Fig. 2 A and B) to those found in white matter. B2 and B3, the majority of cortical EGFP+ cells express NG2 (arrows; arrowhead indicates the single cortical EGFP+/NG2-negative cell found in this field). In addition we also noted a small percentage (1–2%) of NG2+ cells that do not express detectable EGFP levels (asterisk). B4, only one population of EGFP+ cells with respect to fluorescence intensity is seen in cortex. Data for the EGFP fluorescence intensity analysis (bin size = 10) was derived from 3 separate P8 CNP-EGFP mice. The total number of cells analysed in white matter and cortex was 363 and 333, respectively, from multiple microscopic fields (4–12). Scale bars, 50 μm.
From previous studies, it is clear that the vast majority of EGFP+ cells in the subventricular zone of P2 CNP-EGFP transgenic mice are positive for NG2 (Yuan et al. 2002; Belachew et al. 2003). In addition, a significant colocalization of EGFP fluorescence and NG2 expression was noted in the cerebral cortex at P30 (Yuan et al. 2002). However, an analysis of NG2 expression in EGFP+ cells in white matter and cerebral cortex at the developmental stage used in the present study (P5–P10) had not been previously performed. Therefore, we sought to determine to which extent EGFP fluorescence was a reliable indicator to identify NG2+ cells in a slice preparation under the experimental conditions employed in this study. In white matter, NG2 immunoreactivity was detected in cells that displayed lower EGFP intensities (see arrows in Fig. 1A1 and A3). The vast majority of cells with higher EGFP fluorescent intensities did not show NG2 immunoreactivity (Fig. 1A1 and A3), but expressed myelin basic and CNP proteins, indicative of a mature oligodendrocyte phenotype (supplemental Fig. 1). In cortex, the vast majority of EGFP+ cells expressed NG2 (see arrows in Figs 1B1 and B3), although a small percentage of EGFP+ cells were noted to be NG2-negative (1–2%; arrowhead in Fig. 1B1–B3). In light of a recent report (Mallon et al. 2002), it is also important to note that NG2+/EGFP-negative cells (asterisk in Fig. 1B1 and B3) could be observed, but these constituted only 1–2% of the total NG2+ cells.
We wanted to confirm that identification of NG2+ cells could be satisfactorily achieved for functional analysis at the single cell level. Therefore, we performed whole-cell voltage clamp on white matter and cortical EGFP+ cells before staining with anti-NG2. Inclusion of biocytin in the patch pipette followed by subsequent immunocytochemical analysis permitted an evaluation of the success rate. Furthermore, the morphology of the cells including their process arborization, which was not readily identified with EGFP fluorescence alone, could be more accurately visualized.
In accordance with our EGFP intensity data (Fig. 1A4 and B4), we only targeted white matter and cortical EGFP+ cells that had low levels of fluorescence, to ensure a higher chance of identifying NG2+ cells. This approach revealed that in white matter 28 out of 54 (52%) EGFP+ cells also expressed NG2. The low intensity EGFP+ cells that were NG2-negative did in fact express the O4 antigen and therefore represent a later stage of the oligodendrocyte lineage (authors' unpublished data). All the white matter NG2+ cells possessed very few processes that were short in length and were emanating from opposing poles of the cell body (Fig. 2A1). Thus, with our selection criteria, white matter NG2+ cells displayed a morphology that is akin to the classic bipolar morphology of OPCs.
In contrast, cortical NG2+ cells possessed one or two thick proximal processes and clearly displayed an extensive network of finer branches (Figs 2B1, 5F1 and 5G1). As expected from our NG2 staining in cerebral cortical tissue sections (Fig. 1B), a large proportion of EGFP+ cells that were injected with biocytin during physiological analysis were found to be NG2+ (40 out of 42 cells; 95%). The relatively complex morphology of cortical NG2+ cells, as compared with their white matter counterpart, described in this study is in close agreement with previous reports (Nishiyama et al. 1996; Reynolds & Hardy, 1997; Levine et al. 2001). In all cells that were successfully imaged using this approach, no evidence for dye-coupling of NG2+ cells was noted.
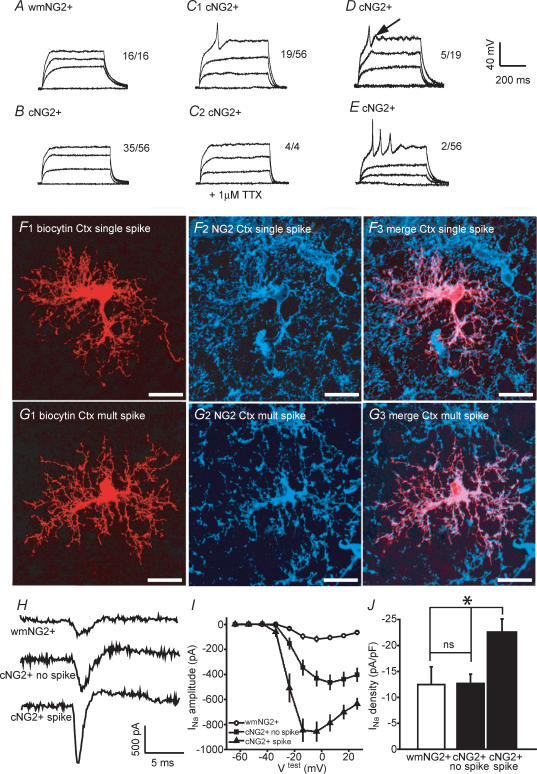
Figure 5. A subpopulation of cortical NG2+, but not white matter NG2+ cells, display TTX-sensitive spikes in response to depolarizing current pulses.
A, representative current clamp traces from a single white matter (wm) NG2+ cell in response to membrane depolarization (current steps in single example were 30, 60 and 90 pA; total no. of cells tested, 16). B and C, from a total of 56 cortical (c) NG2+ cells tested, 35 showed no membrane response (see B for single example; current steps were 15, 30 and 45 pA), and 19 displayed a single spike occurring at the beginning of the test pulse (see C1 for single example; current steps were 10, 20 and 30 pA). In all 4 of these 19 cells tested, the spike was abolished by 1 μm TTX (see C2 for single example; current steps were 10, 20 and 30 pA). D, 5 out of the 19 spike-producing cortical NG2+ cells also clearly displayed an after-depolarization (see arrow; current steps were 15, 30 and 45 pA). E, single example of a cortical NG2+ cell that displayed multiple spikes (2 cells were found with this response; current steps were 10, 20 and 30 pA). In all cells, current injections were in 10–30 pA steps for 450 ms duration with a maximum depolarization to approximately 0 mV. F and G, morphology and confirmation of NG2+ expression in single and multiple spiking cells, respectively. H, single trace examples of INa in a white matter NG2+ cell (top trace), a cortical NG2+ cell not displaying a spike (middle trace) and a cortical NG2+ cell with spike (bottom trace) I, voltage–current relationships of INa for white matter NG2+ cells (open circle; n = 5), and for cortical NG2+ cells without and with spike (filled circles and filled triangles; n = 10 and 9, respectively). The average uncompensated series resistance for recordings from the three cell groups was not significantly different (33 ± 4, 36 ± 2, 35 ± 3 MΩ, respectively). Note that prior to recordings series resistance compensation of at least 85% was performed. J, corresponding pooled INa densities (n = 5–10). All data were obtained from P5–P8 CNP-EGFP mice. Scale bars, 20 μm *P < 0.05, ns, not significant (i.e. P > 0.05), Mann-Whitney U test.
NG2+ cells in subcortical white matter and in cerebral cortex display distinct passive and resting membrane properties
The whole-cell capacitance of white matter NG2+ cells (8 ± 1 pF, n = 28) was approximately 5-fold and significantly lower than that measured for cortical NG2+ cells (40 ± 1 pF, n = 40; Fig. 2C; P < 0.01). This correlated with the marked morphological differences observed between these populations of cells (see Fig. 2A and B). We also noted significant higher input resistance values in white matter NG2+ cells than in their cortical counterparts (Fig. 2D; P < 0.01). As a consequence, white matter NG2+ cells displayed very minimal passive currents than cortical NG2+ cells during depolarizing or hyperpolarizing voltage steps (data not shown).
We then wanted to compare the resting membrane potential between white matter and cortical NG2+ cells. Significant errors are present in measuring resting membrane potential using a zero-current approach in whole-cell current clamp in high input resistance cells such as the white matter NG2+ cells (Fig. 2D) that are caused by a significant shunting by the seal conductance. Thus, this would lead to an estimation of the membrane potential that is more depolarized than the true value (Pongracz et al. 1991). Therefore, we determined resting membrane potential by measuring the reversal potential of potassium currents in cell attached patch recordings, as previously described for lymphocytes, interneurones and neuronal progenitors (Verheugen et al. 1995, 1999; Wang et al. 2003), which circumvents these technical considerations. Note, that this method contains two assumptions. Firstly, that the leak current can be extrapolated in a linear manner within the range of the voltage ramp protocol. Secondly, that the intracellular K+ concentration is the same in all cells tested. However, as calculated by Verheugen et al. (1999), an error of 15 mm in the intracellular K+ concentration would result in a subsequent error of less than 3 mV in the membrane potential measurement.
White matter NG2+ cells possessed a mean resting membrane potential of −70 ± 5 mV (n = 7; Fig. 2E; for single example trace see supplemental Fig. 2). In comparison, cortical NG2+ cells possessed a more hyperpolarized resting membrane potential of −87 ± 5 mV (n = 10; Fig. 2E; for single example trace see supplemental Fig. 2), which was significantly different than that found in white matter NG2+ cells (P < 0.05). Thus, NG2+ cells in the two areas described and at the same age possessed distinct passive and resting membrane properties illustrating a previously unidentified heterogeneity.
K+ channel expression by white matter and cortical NG2+ cells
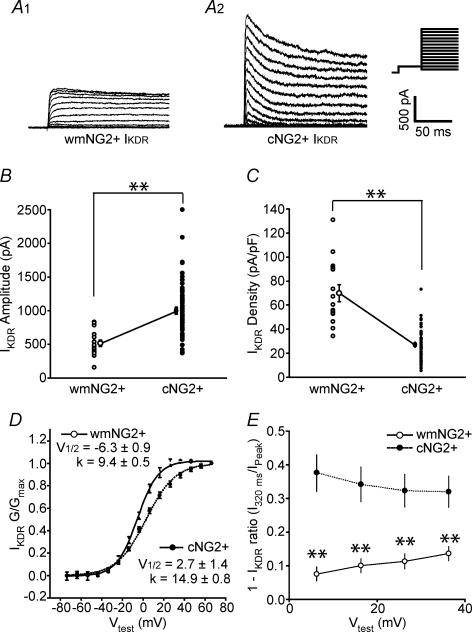
Although white matter and cortical NG2+ cells both expressed IKDR (Fig. 3A1 and A2), the mean amplitude was approximately 2-fold higher in cortical NG2+ cells (test potential = +50 mV; Fig. 3B; P < 0.01). However, taking into account the differences in cell size of the two NG2+ cell populations as determined by the cell capacitance (Fig. 2C), this actually translated to a significantly lower mean IKDR density in cortical NG2+ cells (Fig. 3C; P < 0.01). In addition, we also noted significant differences in the calculated half-activation and slope factors following Boltzmann curve analysis of the conductance–voltage relationship of IKDR (Fig. 3D; P < 0.01). However, we must note that although these values were found to be significantly different, it is unclear whether this relates to a true distinction between white matter and cortical NG2+ cells, since even after series resistance compensation a residual resistance of approximately 5 MΩ is present. This could result in a leftward hyperpolarizing shift of the cortical NG2+ activation curve due to the relative difference in IKDR magnitude between white matter and cortical NG2+ cells.
Figure 3. Expression of IKDR by white matter NG2+ and cortical NG2+ cells.
A1 and A2, representative examples of IKDR activation from a white matter (wm) NG2+ and cortical (c) NG2+ cell in response to a series of depolarizing voltage steps from −60 mV to 80 mV after a pre-pulse to −40 mV to inactivate IKA; 10 mV increments; holding potential was −80 mV (see inset; note all values are prior to junction potential correction) B, pooled data of IKDR amplitude measured at steady state (180 ms after a test pulse to +50 mV). C, pooled data of IKDR density. In B and C, open and filled circles are data from all white matter NG2+ (n = 22) and cortical NG2+ (n = 74) cells, respectively. Circles with s.e.m. are the corresponding mean values. The average uncompensated series resistance for recordings from the two cell groups was not significantly different (33 ± 2 and 35 ± 2 MΩ for white matter NG2+ and cortical NG2+ cells, respectively). Note that prior to recordings series resistance compensation of at least 85% was performed. D, activation–conductance profiles and single Boltzmann curve fits for IKDR from white matter NG2+ (open circles; continuous line; n = 16) and cortical NG2+ cells (filled circles; dotted line; n = 24). Calculated half-activation and slope values were significantly different between the two groups (P < 0.01; Mann-Whitney U test). E, inactivation of IKDR during varying test pulses was measured. Open and filled circles indicate white matter NG2+ and cortical NG2+ cells, respectively (data are from 16 white matter NG2+ and 24 cortical NG2+ cells). All data were obtained from P5–P8 CNP-EGFP mice. **P < 0.01 Mann-Whitney U test.
The extent of inactivation of IKDR during the test pulse (200 ms) was also distinct over a range of test pulses analysed (see Fig. 3A and E; P < 0.01 at all test pulses). The extent of inactivation of IKDR during depolarizing test pulses can be influenced by the identities of Kv subunits that are assembled in these K+ channels. For example the presence of certain Kv1α- or Kv1β-subunits in the channel complex can increase the extent of IKDR inactivation (Rettig et al. 1994; Morales et al. 1996). From these data, it is plausible that the subunit identities of the channels underlying IKDR differ between white matter and cortical NG2+ cells. Although it has been shown that modulation or regulation of certain Kv1α subunits are functionally more relevant in the processes of cell proliferation (Kotecha & Schlichter, 1999; MacFarlane & Sontheimer, 2000; Chittajallu et al. 2002), we cannot rule out the possibility that the differences observed may be related to distinct roles of IKDR in white matter and cortical NG2+ cells.
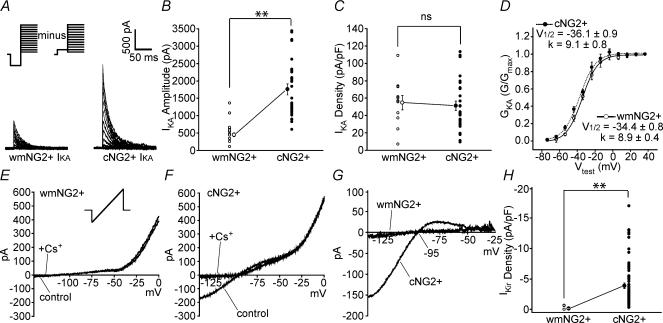
As with IKDR, both white matter and cortical cells possess a significant IKA (Fig. 4A; P < 0.01). Although, the IKA amplitude is higher in the latter group (Fig. 4B), IKA density and activation profiles were essentially the same between both groups (Fig. 4C and D; P > 0.05). The role of IKA in cells of the oligodendrocyte lineage is still uncertain, but its functional expression is down-regulated in OPCs in a manner similar to IKDR, as the cells become less proliferative and attain a more mature phenotype (Sontheimer et al. 1989; Kettenmann et al. 1991). However, our data show that the cortical NG2+ cells, although displaying a lower IKDR density than white matter NG2+ cells, did not display a lower IKA density, i.e. a marked difference in IKDR/IKA ratio was noted between the two NG2+ cell populations. Therefore, cortical NG2+ cells appear to maintain IKA expression differently than do white matter NG2+ cells and cells of the oligodendrocyte lineage (Knutson et al. 1997; Chittajallu et al. 2002; Yuan et al. 2002).
Figure 4. Expression of IKA and IKir by white matter NG2+ and cortical NG2+ cells.
A, representative examples of IKA activation from a white matter (wm) NG2+ (left panel) and cortical (c) NG2+ (right panel) cell in response to a series of depolarizing voltage steps (IKA was isolated by digitally subtracting traces obtained as described in Fig. 3 from traces following a pre-pulse to −110 mV; voltage steps from −60 mV to 80 mV; 10 mV increments (see inset; note all values are prior to junction potential correction). B, individual and pooled data points (with s.e.m.) of IKA peak amplitude. C, corresponding IKA densities (n = 10 and 28 for white matter NG2+ and cortical NG2+ cells, respectively). The average uncompensated series resistance for recordings from the two cell groups was not significantly different (32 ± 3 and 35 ± 3 MΩ for white matter NG2+ and cortical NG2+ cells, respectively). Note that prior to recordings series resistance compensation of at least 85% was performed. D, activation–conductance profiles and single Boltzmann curve fit for IKA from white matter NG2+ (n = 9) and cortical NG2+ cells (n = 15). Calculated half-activation and slope values were not significantly different (P > 0.05). Notation of open and filled circles is the same as in Fig. 3. E and F, representative single current traces in response to a ramp protocol (see inset) in the absence (control) and presence of 5 mm extracellular Cs+ (+Cs+; 1 min after application) from a white matter NG2+ and cortical NG2+ cell, respectively. G, isolation of Cs+-sensitive component by subtraction of traces in E and F. H, pooled data for the IKir density in white matter NG2+ (n = 8; open circles) and cortical NG2+ cells (n = 65; filled circles). Corresponding mean values are shown with s.e.m. The average uncompensated series resistance for the two populations of cells was not significantly different (30 ± 4 and 32 ± 2 MΩ for white matter NG2+ and cortical NG2+ cells, respectively). Note that prior to recordings series resistance compensation of at least 85% was performed. All data were obtained from P5–P8 CNP-EGFP mice. **P < 0.01, ns, not significant (P > 0.05) Mann-Whitney U test.
Finally, we investigated the expression of an inward-rectifying K+ current by white matter and cortical NG2+ cells (Fig. 4E–H). In all white matter NG2+ cells tested, none showed an appreciable Cs+-sensitive current at hyperpolarizing potentials (Fig. 4E, G and H), in good agreement with a previous report (Diers-Fenger et al. 2001). In contrast, cortical NG2+ cells expressed this current to varying degrees (Fig. 4F–H; P < 0.01 when compared with that seen in white matter NG2+ cells). The reversal of the Cs+-sensitive current component (−95 mV; Fig. 4G) was very close to the calculated EK (−97 mV), and therefore we propose that the majority of this current is representative of IKir with minimal involvement of Ih, which has a reversal potential at more positive values to EK (Halliwell & Adams, 1982; Maccaferri et al. 1993). Expression of IKir is of importance in the events leading to maturation of oligodendrocyte lineage cells (Neusch et al. 2001), and it is clear that IKir is up-regulated in post-mitotic cells of the oligodendrocyte lineage (Sontheimer et al. 1989; Knutson et al. 1997; Yuan et al. 2002). IKir in glial cells has been implicated in several functions, which include setting and/or stabilizing the resting membrane potential and removing extracellular K+. In white matter, it appears that IKir is not highly expressed at the NG2+ stage of the lineage (also see Diers-Fenger et al. 2002), but is found at later and more mature developmental stages (Kettenmann et al. 1991; Sontheimer et al. 1989; Gipson & Bordey, 2002). In cortex, significant IKir is already detectable at the NG2+ stage. The physiological correlate underlying these differences is unclear, but these data in cortical NG2+ cells emphasize a distinct ion channel expression profile to that previously noted during oligodendrocyte lineage cell maturation.
NG2+ cells in cerebral cortex are heterogeneous with respect to INa expression and excitable membrane properties
Depolarizing current injections resulted in only passive membrane responses in all of the white matter NG2+ cells (n = 16; Fig. 5A) and in a proportion of the cortical NG2+ cells tested (35 out of 56 cells; Fig. 5B). However, in the remainder of cortical NG2+ cells, a single spike was apparent (19 out of 56 cells; Fig. 5C1; a single example of the morphology and NG2 expression by a single spiking cell is shown in Fig. 5F). In all cortical NG2+ cells that displayed this phenomenon, the depolarizing peak of the spike was positive to 0 mV and was followed by a pronounced hyperpolarization (Fig. 5C1). Furthermore, in 5 out of these 19 cells tested, a detectable after-depolarization was noted (see arrow in Fig. 5D). The nature of these spikes was very different compared with that of a typical action potential found in mature neurones. In cortical NG2+ cells, the spikes were activated at much more depolarized membrane potentials (mean threshold = −26 ± 3 mV; n = 19), the peak of the spike did not closely reach ENa (overshoot = 14 ± 6 mV; n = 19), and the duration of the spike was relatively long (width of spike at half-maximal amplitude = 5.4 ± 6 ms; n = 19). However, in all the cells tested (n = 4), the spike was completely abolished by TTX, indicating the involvement of Na+ channels (Fig. 5C2). The distinct ability of some cortical NG2+ cells to elicit a spike under our experimental conditions, a phenomenon that is never observed in white matter NG2+ cells, demonstrates the existence of functional heterogeneity.
Interestingly, in 2 out of 56 cortical NG2+ cells tested, we did observe more than one spike, although the overshoot consistently decreased with each subsequent spike ending in a cessation of excitability prior to the end of the depolarizing step (450 ms; Fig. 5E; a single example of the morphology and NG2 expression by a multiple spiking cell is shown in Fig. 5G).
We subsequently investigated voltage-gated Na+ channel expression in white matter and cortical NG2+ cells (Fig. 5H–J). All three populations of cells possessed a depolarization-induced INa albeit to differing extents (Fig. 5H and I). White matter NG2+ cells showed very small INa on average (Fig. 5H and I), consistent with a previous report (Diers-Fenger et al. 2002). In contrast, the INa amplitude in cortical cells was higher (Fig. 5H and I). However, when the data were divided between non-spiking and spiking cortical NG2+ cells, a difference in INa amplitude was apparent (Fig. 5H middle and bottom panels, and 5I). When INa density was calculated, the white matter NG2+ and non-spiking cortical NG2+ cells displayed very similar values (P > 0.05) with a much higher INa density noted in spiking cortical NG2+ cells (Fig. 5J; P < 0.05 for white matter versus spiking cortical NG2+ cells and non-spiking cortical versus spiking cortical NG2+ cells.). Thus, this relative increase of INa is likely to represent the underlying cause for the ability of a subpopulation of cortical NG2+ cells to generate spikes.
Cortical NG2+ cells express functional AMPA receptors
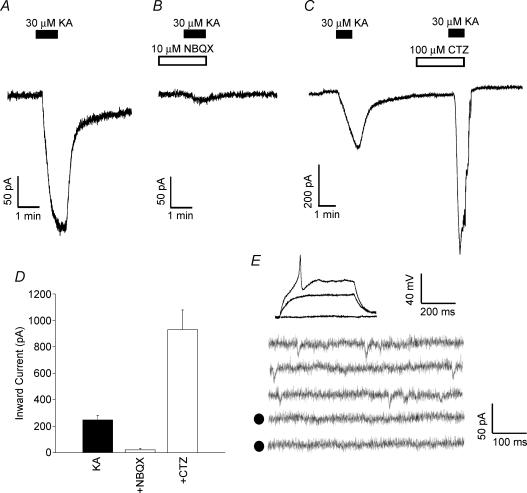
We tested the functional expression of AMPA receptors by cortical NG2+ cells by monitoring changes in holding current during voltage-clamp experiments upon bath application of kainate. In all cells tested, brief application of kainate (30 μm for 1 min) elicited a marked inward current that was reversible upon washout (n = 9; Fig. 6A). This response was virtually abolished in the presence of 10 μm NBQX (n = 4; Fig. 6B and D) and substantially potentiated in the presence of 100 μm cyclothiazide (CTZ; n = 4; Fig. 6C and D). These data indicate that kainate, acting via AMPA receptors, was responsible for the observed effect and illustrates the presence of functional AMPA receptors in cortical NG2+ cells. We also investigated the presence of spontaneous AMPA receptor-mediated responses in spiking cortical NG2+ cells. Under our experimental conditions (Cs+-based intracellular solution; holding potential = −80 mV; in the presence of 30 μm bicuculline and 2 nm latrotoxin or 2 μm pardaxin) we only observed spontaneous NBQX-sensitive inward currents in 2 out of the 36 spiking cortical NG2+ cells tested (Fig. 6E).
Figure 6. Cortical NG2+ cells express functional AMPA receptor channels but rarely display spontaneous AMPA receptor activation.
Cortical NG2 cells were voltage clamped at −80 mV and the holding current was continuously monitored. The extracellular solution was supplemented with 10 mm TEA, 1 mm 4-aminopyridine, 50 μm picrotoxin, 1 μm tetrodotoxin and 50 μm APV. A, brief application of 30 μm kainate (KA; 1 min) elicited an inward current which returned to 95% of control value following washout. B, the kainite-induced inward current was severely attenuated in the presence of 10 μm NBQX (added 1 min prior to and during the 1 min KA application). C, 100 μm cyclothiazide (CTZ; added 2 min prior to and during the KA application) markedly increased the KA-induced inward current. D, pooled data for inward currents induced by KA (n = 9) in the presence of NBQX (n = 4) and CTZ (n = 4). Data are means ± s.e.m. E, 2 out of 36 spiking cortical NG2+ cells displayed spontaneous inward currents following application of pardaxin (2 μm). This panel displays one of these cells. Top panel shows the spike response to depolarizing current injection under current clamp (30 and 60 pA injections). The bottom panel shows continuous traces under voltage clamp (holding potential −80 mV) following a 10 min application of 2 μm pardaxin. Note the traces are not consecutive. The traces denoted by filled circles are in the additional presence of 10 μm NBQX (10 min after application).
Discussion
Direct identification of NG2+ cells and hence their functional analysis in acutely isolated slices is challenging. This has been highlighted by Diers-Fenger et al. (2001) who reported that, in the corpus callosum of wild-type P5 mice, only 5 out of 68 cells from which patch-clamp recordings were performed were found to be AN2+/NG2+. A major advantage of transgenic methodologies in which EGFP fluorescence is driven by cell promoters, lies in the ability to directly identify and perform physiological analysis of neural cell types in acutely isolated slices. The data presented in this study highlight the value of employing the CNP-EGFP mouse to permit a functional analysis of NG2+ cells. In addition, this experimental approach maintains the cellular environment to a greater extent than in vitro studies. The direct functional comparison performed in this study indicates that a population of cortical NG2+ cells displays ion channel expression profiles that differ from the bipolar NG2+ cells found in the white matter. Furthermore, some cortical NG2+ cells display TTX-sensitive spikes upon depolarization – a phenomenon that is never observed in white matter.
Recently, cortical NG2+ cells have also been identified using a transgenic approach in which EGFP expression is driven by the proteolipid (PLP) promoter (Mallon et al. 2002). However, not all cortical NG2+ cells expressed EGFP and the authors conclude that two populations of cortical NG2+ cells exist, which display differential expression of the PLP gene. However, it is unclear as to the physiological correlate underlying this distinction. In the CNP-EGFP mouse, more than 98% of the cortical EGFP+ cells express NG2 at the developmental stage examined in the present study, thus allowing for a functional analysis of virtually all cortical NG2+ cells. These data, taken together, suggest that both PLP-EGFP+/NG2+ and PLP-EGFP-negative/NG2+ cells identified in the PLP-EGFP mouse must express CNP-promotor activity. However, to date, no published functional data are available examining the ability of PLP-EGFP+/NG2+ and PLP-EGFP-negative/NG2+ cells to display immature action potentials upon membrane depolarization. It remains to be determined whether the two cortical NG2+ populations identified in the current study (non-spiking and spiking) correlate to the two populations noted based on PLP-promoter activity.
It has been demonstrated that hippocampal NG2+ cells can receive functional inputs via ‘classical synapses’ (Bergles et al. 2000; Lin & Bergles, 2003). We have provided evidence that all spiking cortical NG2+ cells tested possess functional AMPA receptors and their activation causes a marked inward current in these cells. However, very few had detectable spontaneous AMPA-receptor mediated currents, suggesting that synaptic innervation is minimal. These observations, in conjunction with the hyperpolarized RMP and the high threshold level seen in spiking cortical NG2+ cells, suggest that the vast majority of the spiking NG2+ cells (at the developmental stage of our recordings) do not receive a physiological synaptic stimulus that would be sufficient to elicit the single spike.
What is the physiological role of the subpopulation of cortical NG2+ cells that can elicit TTX-sensitive spikes? This functional observation is neither characteristic of glial cells, nor reminiscent of mature neurones. Do these cells represent a cell type that possesses mixed neuronal/glial properties (Stallcup, 2002)? It has been proposed that a subpopulation of NG2+ cells that persist into adulthood, although being termed adult oligodendrocyte progenitors, in fact belong to a 5th cellular population within the CNS that is distinguishable from neurones, astrocyte, oligodendrocytes and microglia (Butt et al. 2002; Berry et al. 2002). Although, to date, no physiological analysis of adult cortical NG2+ cells is available, it is possible that the spiking cortical NG2+ cells described in the current study could in fact be the perinatal equivalent of the cells that are present within the adult CNS.
An alternative possibility is that white matter and cortical NG2+ cells represent different developmental stages of the same cell type. Although this cannot be excluded for every NG2+ cell investigated, certain physiological properties of a population of cortical NG2+ cells (i.e. differing IKDR/IKA ratio, presence of a significant IKir and TTX-sensitive spikes) were never observed in developing white matter NG2+ cells. In addition, transplanted NG2+ cells into the lateral ventricles of mice have been shown to migrate and give rise to a neuronal population in grey matter areas such as the hippocampus (Aguirre et al. 2004). However, cells derived from the graft that migrated to the white matter only gave rise to oligodendrocytes (Aguirre et al. 2004). We propose that such differing cellular fates may be due to the distinct cellular environments in white versus grey matter regions and are responsible for the differing physiological properties observed.
Finally, cortical NG2+ cells may have the capability of developing into mature neurones. This possibility is based on a number of previous observations from independent laboratories. Although the immature neuronal identity of cortical NG2+ cells in vivo remains speculative, a population of hippocampal NG2+ cells expresses the immature neuronal marker TOAD-64 (Belachew et al. 2003). In addition, we have noted that approximately 10% of acutely isolated cortical NG2+ cells (isolated by fluorescence-activated cell sorting; FACS) also express the immature neuronal markers TUJ1 and HuC/D (supplemental Fig. 3). We have also previously shown that NG2+ cells in vitro can generate neurospheres, are multipotent and can give rise to both oligodendrocytes and mature neurones (Belachew et al. 2003). Thus NG2+ cells have the potential to differentiate towards a neuronal identity under appropriate culture conditions. In addition, a number of recent reports have suggested that progenitor cells previously thought to be exclusively part of the oligodendrocyte cell lineage can in fact give rise to neurones both in culture and in vivo (Kondo & Raff, 2000; Nunes et al. 2003; Aguirre et al. 2004; Goldman, 2004). Finally, a population of basal forebrain stem cells has been described as multipotent and can generate both inhibitory neurones and oligodendrocytes, suggesting the presence of a common progenitor for these neural cell types (He et al. 2001).
It is accepted that postnatal neurogenesis occurs in the hippocampus and olfactory bulb (Gage, 2002). In fact, recent evidence has indicated a lineage relationship between NG2+ cells and mature neuronal progeny in the postnatal hippocampus, suggesting that some novel neurones are generated from these progenitors in this region (Belachew et al. 2003; Aguirre et al. 2004). Furthermore, NG2+ cells that are grafted into the lateral ventricles of a host mouse brain are able to migrate and differentiate into functional inhibitory interneurones in the hippocampus (Aguirre et al. 2004). Taking these results together, the possibility that NG2+ cells can also give rise to a neuronal population is hard to ignore.
The question remains whether such a lineage relationship is also found in the cortex. The presence of postnatal cortical neurogenesis under physiological conditions remains a highly controversial issue, due to conflicting reports in the literature (Rakic, 2002). However, it has been demonstrated that neurogenesis in the cortex occurs following various experimentally induced CNS injuries (Magavi et al. 2000; Braun et al. 2002). The developmental origin of these newly born neurones remains unknown. Since it is well accepted that NG2+ cells are the largest population of postnatal progenitors in the CNS and their presence persists into adulthood (Dawson et al. 2000; Levine et al. 2001), it is possible that spiking cortical NG2+ cells under certain pathophysiological conditions could be triggered into neuronal maturation.
Our data clearly show that a very small percentage (2 out of 56 cells tested) of cortical NG2+ cells can elicit multiple spikes in response to depolarization (see Fig. 5E and G). Interestingly, the threshold to elicit this response was approximately −40 mV (cf. −26 mV for single spike cortical NG2+ cells). Also, the initial spike elicited by these two cells, consistently possessed a larger overshoot (mean overshoot = 37 mV) and shorter duration (width of spike at half-maximal amplitude = 1.5 ms) than those in single spike cortical NG2+ cells. Although by no means indicative of a mature neurone, these trend changes in spike characteristics (i.e. from Fig. 5C1 to E) have been previously described during endogenous neurogenesis (Gao & Ziskind-Conhaim, 1998; Mienville et al. 1999; Picken Bahrey & Moody, 2003), and also in the course of neuronal differentiation and maturation of transplanted embryonic stem cells (Benninger et al. 2003). However, does this represent a possible maturation of NG2+ cells into a neuronal population? Two reasons can be put forward to explain why multiple spiking NG2+ cells are very rare in the cortex. Firstly, it is possible that neuronal maturation of single spiking NG2+ cells does not occur under physiological conditions but may require a pathological stimulus as described above. Secondly, NG2+ expression could be markedly down-regulated prior to attainment of fully mature neuronal characteristics (see Belachew et al. 2003). In fact, no detectable NeuN expression was found in acutely FACS-purified cortical NG2+ cells (supplemental Fig. 3).
To summarize, we have successfully employed a transgenic mouse to physiologically characterize cells expressing the NG2 proteoglycan in two differing brain regions. Our data highlight the existence of a cortical NG2+ cell population that has characteristics which are not entirely typical of oligodendrocyte progenitors. These cells could represent a previously functionally uncharacterized class of CNS cell or, in light of recent findings, could be a population of neuronal progenitors. Future studies will address whether this population continues to exist into adulthood and under what biological circumstances, if any, these cells could possibly contribute to a cortical neuronal population.
Supplementary Material
Acknowledgments
We are grateful to L.J. Chew for breeding and maintenance of the CNP-EGFP mice. We thank W. King (The William and Shirley Howard Hematopoetic Stem Cell Laboratory, CRI, CNMC) and R. Ruffner (Center for Microscopy and Image Analysis, George Washington University School of Medicine) for assistance with FACS sorting and acquisition of confocal images, respectively. We also thank S. Belachew and T. Haydar for valuable discussion. This work was supported by NIH R01NS045702 (V.G), by the Wadsworth Foundation and by NIH MRDDRC P30HD40677.
Supplementary material
The online version of this paper can be accessed at: DOI:10.1113/jphysiol.2004.074252 http://jp.physoc.org/cgi/content/full/jphysiol.2004.074252/DC1 and contains supplementary material consisting of three figures with the following titles:
Supplementary Fig. 1. Expression of myelin basic and 2′,3′-cyclic nucleotide 3′-phospodiesterase proteins in cells displaying high EGFP intensity in white matter
Supplementary Fig. 2. Non-invasive cell-attached measurements of the resting membrane potential in white matter and cortical NG2+ cells
Supplementary Fig. 3. NG2+/EGFP+ cells FACS-purified from the P8 cerebral cortex express immature neuronal markers
References
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger F, Beck H, Werning M, Tucker KL, Brustle O, Scheffler B. Functional integration of embryonic stem cell-derived neurons in hippocampal slice cultures. J Neurosci. 2003;23:7075–7083. doi: 10.1523/JNEUROSCI.23-18-07075.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Berry M, Hubbard P, Butt AM. Cytology and lineage of NG2-positive glia. J Neurocytol. 2002;31:457–467. doi: 10.1023/a:1025735513560. [DOI] [PubMed] [Google Scholar]
- Braun H, Schafer K, Hollt V. Beta III tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J Neurotrauma. 2002;19:975–983. doi: 10.1089/089771502320317122. [DOI] [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Chen Y, Wang H, Yuan X, Ghiani CA, Heckman T, et al. Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc Natl Acad Sci U S A. 2002;99:2350–2355. doi: 10.1073/pnas.042698399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.3.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J. AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia. 2001;34:213–228. doi: 10.1002/glia.1055. 10.1002/glia.1055. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. J Neurophysiol. 1998;80:3047–3061. doi: 10.1152/jn.1998.80.6.3047. [DOI] [PubMed] [Google Scholar]
- Gipson K, Bordey A. Analysis of the K+ current profile of mature rat oligodendrocytes in situ. J Membrane Biol. 2002;189:201–212. doi: 10.1007/s00232-002-1014-8. 10.1007/s00232-002-1014-8. [DOI] [PubMed] [Google Scholar]
- Goldman S. Glia as neural progenitor cells. Trends Neurosci. 2004;26:590–596. doi: 10.1016/j.tins.2003.09.011. 10.1016/j.tins.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- He W, Ingraham C, Rising L, Goderie S, Temple S. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocyte to the cerebral cortex during embryogenesis. J Neurosci. 2001;21:8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Thallmair M, Gage FH. Defining the NG2-expressing cell of the adult CNS. J Neurocytol. 2002;31:469–480. doi: 10.1023/a:1025739630398. 10.1023/A:1025739630398. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Blankenfeld GV, Trotter J. Physiological properties of oligodendrocytes during development. Ann N Y Acad Sci. 1991;633:64–77. doi: 10.1111/j.1749-6632.1991.tb15596.x. [DOI] [PubMed] [Google Scholar]
- Knutson P, Ghiani CA, Zhou JM, Gallo V, McBain CJ. K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells. J Neurosci. 1997;17:2669–2682. doi: 10.1523/JNEUROSCI.17-08-02669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Schlichter LC. A Kv1.5 to Kv1.3 switch in endogenous hippocampal microglia and a role in proliferation. J Neurosci. 1999;19:10680–10693. doi: 10.1523/JNEUROSCI.19-24-10680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. 10.1016/S0166-2236(00)01691-X. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2003;7:24–32. doi: 10.1038/nn1162. 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Mangoni M, Lazzari A, DiFrancesco D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J Neurophysiol. 1993;69:2129–2136. doi: 10.1152/jn.1993.69.6.2129. [DOI] [PubMed] [Google Scholar]
- MacFarlane SN, Sontheimer H. Modulation of Kv1.5 currents by Src tyrosine phosphorylation: potential role in the differentiation of astrocytes. J Neurosci. 2000;20:5245–5253. doi: 10.1523/JNEUROSCI.20-14-05245.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mienville JM, Maric I, Maric D, Clay JR. Loss of IA expression and increased excitability in postnatal rat Cajal-Retzius cells. J Neurophysiol. 1999;82:1303–1310. doi: 10.1152/jn.1999.82.3.1303. [DOI] [PubMed] [Google Scholar]
- Morales MJ, Wee JO, Wang S, Strauss HC, Rasmusson RL. The N-terminal domain of a K+ channel beta subunit increases the rate of C-type inactivation from the cytoplasmic side of the channel. Proc Natl Acad Sci U S A. 1996;93:15119–15123. doi: 10.1073/pnas.93.26.15119. 10.1073/pnas.93.26.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci. 2001;21:5429–5438. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Dahlin KJ, Prince JT, Johnstone SR, Stallcup WB. The primary structure of NG2, a novel membrane-spanning proteoglycan. J Cell Biol. 1991;114:359–371. doi: 10.1083/jcb.114.2.359. 10.1083/jcb.114.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Picken Bahrey HL, Moody WJ. Early development of voltage-gated ion currents and firing properties in neurons of the mouse cerebral cortex. J Neurophysiol. 2003;89:1761–1773. doi: 10.1152/jn.00972.2002. [DOI] [PubMed] [Google Scholar]
- Pongracz F, Firestein S, Shepherd GM. Electrotonic structure of olfactory sensory neurons analyzed by intracellular and whole cell patch techniques. J Neurophysiol. 1991;65:747–758. doi: 10.1152/jn.1991.65.3.747. [DOI] [PubMed] [Google Scholar]
- Rakic P. Adult neurogenesis in mammals: an identity crisis. J Neurosci. 2002;22:614–618. doi: 10.1523/JNEUROSCI.22-03-00614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, et al. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. 10.1002/(SICI)1097-4547(19970301)47:5<455::AID-JNR1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Trotter J, Schachner M, Kettenmann H. Channel expression correlates with differentiation stage during the development of oligodendrocytes from their precursor cells in culture. Neuron. 1989;2:1135–1145. doi: 10.1016/0896-6273(89)90180-3. 10.1016/0896-6273(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Stallcup WB. The NG2 proteoglycan: Past insights and future prospects. J Neurocytol. 2002;31:423–435. doi: 10.1023/a:1025731428581. 10.1023/A:1025731428581. [DOI] [PubMed] [Google Scholar]
- Verheugen JAH, Fricker D, Miles R. Noninvasive measurements of the membrane potential and GABAergic action in hippocampal interneurons. J Neurosci. 1999;19:2546–2555. doi: 10.1523/JNEUROSCI.19-07-02546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheugen JAS, Vijverberg HPM, Oortgiesen M, Cahalan MD. Voltage-gated and Ca2+-activated K+ channels in intact human T-lymphocytes. Noninvasive measurements of membrane currents, membrane potential and intracellular calcium. J General Physiol. 1995;105:765–794. doi: 10.1085/jgp.105.6.765. 10.1085/jgp.105.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol. 2003;550:785–800. doi: 10.1113/jphysiol.2003.042572. 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V. Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse for developmental and physiological studies. J Neurosci Res. 2002;70:529–545. doi: 10.1002/jnr.10368. 10.1002/jnr.10368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.