Abstract
Exercise-induced loss of skeletal muscle K+ can seriously impede muscle performance through membrane depolarization. Thus far, it has been assumed that the negative equilibrium potential and large membrane conductance of Cl− attenuate the loss of force during hyperkalaemia. We questioned this idea because there is some evidence that Cl− itself can exert a depolarizing influence on membrane potential (Vm). With this study we tried to identify the possible roles played by Cl− during hyperkalaemia. Isolated rat soleus muscles were kept at 25 °C and twitch contractions were evoked by current pulses. Reducing [Cl−]o to 5 mm, prior to introducing 12.5 mm Ko, prevented the otherwise occurring loss of force. Reversing the order of introducing these two solutions revealed an additional effect, i.e. the ongoing hyperkalaemia-related loss of force was sped up tenfold after reducing [Cl−]o. However, hereafter twitch force recovered completely. The recovery of force was absent at [K+]o exceeding 14 mm. In addition, reducing [Cl−]o increased membrane excitability by 24%, as shown by a shift in the relationship between force and current level. Measurements of Vm indicated that the antagonistic effect of reducing [Cl−]o on hyperkalaemia-induced loss of force was due to low-Cl−-induced membrane hyperpolarization. The involvement of specific Cl− conductance was established with 9-anthracene carboxylic acid (9-AC). At 100 μm, 9-AC reduced the loss of force due to hyperkalaemia, while at 200 μm, 9-AC completely prevented loss of force. To study the role of the Na+−K+−2Cl− cotransporter (NKCC1) in this matter, we added 400 μm of the NKCC inhibitor bumetanide to the incubation medium. This did not affect the hyperkalaemia-induced loss of force. We conclude that Cl− exerts a permanent depolarizing influence on Vm. This influence of Cl− on Vm, in combination with a large membrane conductance, can apparently have two different effects on hyperkalaemia-induced loss of force. It might exert a stabilizing influence on force production during short periods of hyperkalaemia, but it can add to the loss of force during prolonged periods of hyperkalaemia.
The substantial Cl− conductance of mammalian skeletal muscle has a stabilizing influence on membrane potential (Vm). Blocking the ClC-1 channel or replacing extracellular Cl− with foreign anions results in myotonia (Landau, 1952; Bryant & Morales-Aguilera, 1971; Bretag, 1987). As such, Cl− is likely to affect muscular fatigue by influencing the K+-related force depression.
Hyperkalaemia can occur during brief periods of intense exercise. The interstitial [K+] in human muscle can reach values as high as 10 mm during fatiguing exercise (Juel et al. 2000). A K+-induced depolarization of Vm can render the muscle fibre inexcitable and cause loss of force if Vm depolarizes to above −60 mV (Juel, 1986; Clausen & Everts, 1991; Cairns et al. 1995, 1997; Overgaard et al. 1999; Clausen & Overgaard, 2000; Pedersen et al. 2003). In vitro experiments established that stimulating mouse soleus muscle until it fatigues, raises interstitial [K+] from 5 to 10 mm and concomitantly lowers [K+]i from 168 to 136 mm (Juel, 1986). The change in [K+]o/[K+]i depolarized Vm to −58 mV at 37 °C. Therefore, the reported changes in K+ concentration gradients during exercise appear large enough to contribute to the development of muscular fatigue. However, several mechanisms have been identified, which might act concertedly in vivo to diminish both the amount of K+ released during exercise and the effect of a rise in [K+]o on Vm and membrane excitability: increased muscle temperature, circulating catecholamines and intracellular acidification (Clausen et al. 1993; Nielsen et al. 2001; Overgaard & Nielsen, 2001; Yensen et al. 2002; Pedersen et al. 2003). In addition, it has been repeatedly suggested that the large Cl− conductance of mammalian muscle fibres may prevent excessive depolarization during repeated stimulation (Dulhunty, 1979; Coonan & Lamb, 1998; Wallinga et al. 1999; Sejersted & Sjøgaard, 2000).
During hyperkalaemia Cl− ions flow into the muscle fibre because their chemical gradient is no longer balanced by Vm. In turn, the influx of these anions slows down membrane depolarization. Therefore, Cl−o lowers the rate of the K+-induced membrane depolarization (Hodgkin & Horowicz, 1959; Dulhunty, 1978; McCaig & Leader, 1984). In addition, Dulhunty (1978, 1979) demonstrated that Cl− is also capable of reducing the extent of the K+-induced membrane depolarization. This effect of Cl− on Vm is most likely to be related to the transport of Cl− into the muscle fibre by the bumetanide-sensitive Na+−K+−2Cl− (NKCC1) transporter, as shown in rats and mice (Betz et al. 1984; Harris & Betz, 1987; Aickin et al. 1989; Geukes Foppen et al. 2002). As with Na+ and K+, any ion which is actively transported across the sarcolemma has an equilibrium potential that differs from Vm (Dulhunty, 1978; Aickin et al. 1989), i.e. an actively transported ion tends to pull the Vm towards its own equilibrium potential. A strong influence of Cl− on Vm causes the membrane to behave like a weak K+ electrode. In fact, the sensitivity of Vm in extensor digitorum longus (EDL) or soleus muscle to changes in [K+]o ranges from 55 down to 35 mV per decade [K+]o (Dulhunty, 1980; Mølgaard et al. 1980; Chua & Dulhunty, 1988; Siegenbeek van Heukelom, 1991; Cairns et al. 1997; Yensen et al. 2002). So, Cl− is capable of reducing both the rate and the extent of K+-induced membrane depolarization. This implies that a reduction in Cl− conductance should lead to a more rapid and more pronounced loss of force during hyperkalaemia. Recently, Cairns et al. (2004) showed that tetanus depression in mouse soleus muscle, due to raised [K+]o, occurred more rapidly and to a greater extent at low [Cl−]o.
However, one final aspect of the influence of Cl− on Vm has not yet been considered. The intracellular accumulation of Cl−, combined with a large specific conductance (Dulhunty, 1979; Coonan & Lamb, 1998), could lead to substantial membrane depolarization because the equilibrium potential of Cl− has become less negative than Vm (Aickin et al. 1989). Hyperpolarizing responses up to 20 mV have been measured in soleus, EDL, sternomastoid and lumbrical muscle from rat or mouse, after eliminating Cl− conductance or Cl− accumulation (Dulhunty, 1978; Betz et al. 1984; Aickin et al. 1989). In contrast, several other studies found no difference between Vm and the equilibrium potential for Cl− (ECl) under resting conditions (Blum & Westphal, 1981; DeCoursey et al. 1981; Donaldson & Leader, 1984; McCaig & Leader, 1984). If Cl− exerts a substantial depolarizing influence on Vm, and the Vm at which twitch force decreases is fixed at around −60 mV (Cairns et al. 1997), then this could make the fibres more prone to hyperkalaemia-induced loss of force. It has been found that fatigue during tetanic contractions was attenuated after reducing Cl− conductance in EDL muscle of developing rats (De Luca et al. 1990). Most of the results from Cairns et al. (2004) illustrate that low [Cl−]o principally accelerates the fatigue with continuous stimulation, but one graph illustrates that the final loss of force can be less when [Cl−]o is low. Whether an increase in [K+]o caused the tetanus depression was not examined.
The aim of this investigation was to identify the roles played by Cl−o and specific Cl− conductance during hyperkalaemia-induced loss of twitch force in isolated rat soleus muscle. We tested the hypothesis that Cl− has an antagonistic effect on the developmental course of hyperkalaemia-induced loss of force, but adds to the final loss of force during prolonged hyperkalaemia. To this end, twitch force was studied under various conditions: in the presence and near absence of extracellular Cl−, with and without the addition of the ClC-1 blocker 9-anthracene-carboxylic-acid (9-AC), and finally, with and without the addition of the loop-diuretic bumetanide. We expected a more rapid loss of force during hyperkalaemia after reducing [Cl−]o or adding 9-AC, because the stabilizing effect of a large Cl− conductance on Vm is lost. In addition, we expected that less force would be lost after reducing Cl− conductance or blocking the NKCC1 transporter because of the associated hyperpolarization.
Methods
Animal handling and muscle preparation
Male Wistar rats were obtained from laboratory stock (GDL, Utrecht University, the Netherlands). All twitch force experiments were performed using rats weighing 190–200 g. For measurements of Vm we used rats weighing 140 g. These smaller animals were chosen because of the size and limitations of the set-up. Animals were fed ad libitum and kept under a 12 h light (during the daytime)/12 h dark regime, at 21 °C. Rats were killed by cervical dislocation. Next, intact soleus muscles weighing between 95 and 115 mg (wet weight) were dissected out in approximately 15 min. During dissection muscles were kept moist with a modified Krebs–Ringer bicarbonate buffer solution. All procedures were approved by the Utrecht University Committee for experiments on animals and were in accordance with the Dutch law on experimental animals.
After dissection, soleus muscles were mounted vertically in thermostatically controlled Perspex measuring chambers (40 ml). A small part of the proximal fibula was used to fix the muscle at the bottom of the chamber. At the other end of the muscle, a size 10 fishing hook was pierced trough the tendon and connected to the force tranducer using Ethicon EH781P 5-0 thread (Johnson & Johnson Medical, Amersfoort, the Netherlands). Next, a tension of 50 mN was applied to the soleus by adjusting the position of the force transducer. In preliminary experiments we had established that this pre-tension resulted in optimal twitch force.
All muscles were allowed to acclimatize for 45 min at 25 °C in modified Krebs–Ringer bicarbonate buffer solution. A temperature of 25 °C was chosen for the following two reasons. First, to avoid myotonia; reducing Cl− conductance triggered myotonia at 35 °C, but not at 25 °C, although an occasional myotonic contraction could still be observed. Thus, we were compelled to perform our experiments at 25 °C. Second, a bath temperature of 25 °C reduces metabolic requirements and thus ensures sufficient oxygenation of the central muscle fibres. It has been shown (Segal & Faulkner, 1985) that as incubation temperature exceeds 25 °C, the critical radius for oxygen diffusion becomes less than the average radius of 70–90 mg muscles. Although the weight of our soleus muscles was around 100 mg (without tendons), we did not find any indication of a progressively larger anoxic core with time. Stability of the muscle preparation was established during preliminary experiments lasting 250 min. During these experiments, both peak isometric twitch force and membrane excitability, as determined by the isometric twitch-force–current-strength relationship, were measured repeatedly and remained stable.
Signal conditioning and stimulation protocol
Muscles were stimulated directly through two platinum/iridium (90/10%) wire electrodes of 0.2 mm diameter (Drijfhout, Amsterdam, the Netherlands). These were inserted horizontally through the muscle, 1 cm apart. Electrodes had been mechanically sharpened.
Single 0.2 ms TTL voltage pulses were obtained once each minute from a programmable pulse-generator (Master-8, A.M.P.I., Israel) and converted into constant unipolar current pulses with a two-channel stimulus isolator (BSI-2, BAK Electronics Inc., Germany). The performance and accuracy of the stimulus isolator at different current levels was routinely checked at 1 and 10 kΩ with a Tektronix TDS 3012 digital oscilloscope (Beaverton, OR, USA). During stimulation, the muscles were no longer submerged in conductive fluid. The fluid was pumped out of the chamber 5 s in advance of the stimulus, only to be pumped back in 1 s after stimulation. Timing was controlled by the programmable pulse generator. The advantages of stimulating muscle suspended in air are that much less current is needed to evoke the contractions and, more importantly, the results become much more reproducible (Van der Heijden et al. 1998).
Membrane excitability of each muscle was repeatedly established during an experiment. This was done by increasing the current level from 0 to 0.5 mA in 0.05 mA steps, and from 0.5 to 1.5 mA in 0.1 mA steps, and recording twitch force at each stimulus level. These twitch-force–stimulus-level relationships are referred to as twitch curves. All further stimulation was performed at a stimulus level of 1.5 mA, which was at least 1.5 times the current needed to evoke maximum twitch force.
Isometric force development was measured using FT03 force displacement transducers (GRASS FT03, W. Warwick, RI, USA). The force transducers were connected with a signal conditioner (Cyber Amp 380, Axon Instruments). The output signal from the transducer was amplified 2000 times and low-pass filtered at 100 Hz before A/D conversion took place. The signal was sampled (250 Hz) using LabVIEW 3.1 (National Instruments, Austin, TX, USA).
Before each experiment transducers were calibrated using weights of 5, 10, 20 and 50 g. The relation between weight and voltage output was highly linear. The inverse slope of this relation was used for off-line analyses of muscle twitch force (1 g = 9.8 mN).
Solutions and chemicals
In the first set of experiments, four different solutions were used: modified Krebs–Ringer solution, high K+o, low Cl−o, and high K+o/low Cl−o. Modified Krebs–Ringer solution contained 5 mm KHCO3− instead of 5 mm KCl. The low Cl−o solutions were made by replacing NaCl with sodium isethionate (isethionic acid, sodium salt; Aldrich). We chose isethionate (sodium salt) to replace Cl−o because it is known as one of the monovalent anions that can be used to substitute for Cl− without affecting divalent cation activity, osmolality or Na+ activity. Moreover, it does not seem to interact with Cl− channels (Bretag, 1987). In an additional control experiment we used methanesulphonate (CH3SO3−, methanesulphonic acid, sodium salt; Aldrich) to replace Cl−o. The salt and ionic composition of the solutions is given in Table 1.
Table 1.
Composition of the extracellular solutions
| Modified Krebs–Ringer solution | High K+o | Low Cl−o | High K+o/ Low Cl−o | |
|---|---|---|---|---|
| NaCl | 125 | 125 | 0 | 0 |
| Sodium isethionate | 0 | 0 | 125 | 125 |
| KHCO3 | 5 | 12.5 | 5 | 12.5 |
| NaHCO3 | 20 | 12.5 | 20 | 12.5 |
| NaH2PO4·H2O | 1.2 | 1.2 | 1.2 | 1.2 |
| MgSO4 | 1.2 | 1.2 | 1.2 | 1.2 |
| CaCl2·2H2O | 2.6 | 2.6 | 2.6 | 2.6 |
| d-Glucose | 5 | 5 | 5 | 5 |
| K+ | 5 | 12.5 | 5 | 12.5 |
| Na+ | 146.2 | 138.7 | 146.2 | 138.7 |
| Cl− | 130.2 | 130.2 | 5.2 | 5.2 |
Concentrations are given in millimoles per litre. The [K+]o was increased by equimolar replacement of NaHCO3 with KHCO3. The [Cl−]o was reduced by replacement of NaCl by sodium isethionate (isethionic acid, sodium salt). The three rows at the bottom provide the concentrations of the three ions being involved in setting membrane excitability. All fluids were continuously aerated with a mixture of 95% O2 and 5% CO2 (pH 7.4) through glass filter plates (16–40 μm pores, duran, Schott Glas, Mainz, Germany).
In the second and third set of experiments, the modified Krebs–Ringer solution and high K+o solutions, as mentioned previously, were used. To these two control solutions we added 9-AC to a final concentration of 100 μm (20 mm stock, solvent ethanol) or bumetanide to a final concentration of 100 or 400 μm (100 mm stock, solvent methanol). In the experiments with 9-AC and bumetanide, the control solutions contained an equimolar amount of the solvent, i.e. 0.5% v/v ethanol and 0.1–0.4% v/v methanol, respectively. Because (m)ethanol is a permeant solute it should only have a transient effect on cell volume. In an additional set of experiments 9-AC was tested at a concentration of 200 μm. In this case the stock solution contained 9-AC (400 mm) dissolved in DMSO.
Prior to each experiment, the Na+, K+ and Cl− concentrations of the solutions were measured with ion-sensitive electrodes (AVL 983-S). The osmotic value of the solutions was verified with a freezing-point osmometer (Osmomat 030/Gonotec, Berlin, Germany). The osmolality of the solutions in Table 1 varied between 302 and 309 mosmol kg−1. The osmolality of the solutions containing 0.1 or 0.4% v/v methanol ranged from 315 to 325 and from 389 to 399 mosmol kg−1, respectively. The osmolality of the solutions containing 0.5% v/v ethanol ranged from 376 to 390 mosmol kg−1.
All chemicals were of analytical grade. Bumetanide and 9-AC were from Sigma-Aldrich (Steinheim, Germany), TTX was from Alomone Laboratories (Jerusalem, Israel), methanol was from Sigma Diagnostics (St Louis, MO, USA) and ethanol was from Riedel-de Haën (Sigma-Aldrich, Seelze, Germany).
Intracellular measurement of resting membrane potential
The steady-state membrane potential of soleus muscle fibres was measured during incubations with media containing a range of K+o concentrations (5, 8, 11, 12.5, 14 and 17 mm), at either a low (5 mm) or standard (130 mm) Cl−o concentration. All media contained 1 μm TTX to prevent action potentials and contractions. Soleus muscles were dissected and pinned down on the silicone-rubber-lined bottom of a small plastic Petri dish (∼3 ml bath volume), containing either the modified Krebs–Ringer solution (5 mm K+ and 130.2 mm Cl−) or the low-Cl− medium (5 mm K+ and 5.2 mm Cl−). So, each rat contributed one muscle to the high Cl−o group and one to the low Cl−o group. The bath temperature was kept at 25 °C using a Peltier device. Muscles were allowed to acclimatize for 45 min. Intracellular recordings were made using a glass capillary microelectrode filled with 3 m KCl (∼15 MΩ resistance) connected to standard microelectrode equipment. During each measuring session of about 5 min, different muscle fibres were impaled and the steady-state membrane potential recorded. Thereafter, the muscle was washed at least three times with 3 ml of the medium containing 8 mm K+o. This took place during a 5 min equilibration period, which is long enough to allow the superficial fibres to reach a new steady state. Subsequently, a new measurement session was performed. Completing the measurements, from 5 to 17 mm K+o, took about 1 h.
Calculations and statistics
Results are presented in the figures and text as means ±s.e.m.; n is the number of measurements from different preparations. Statistical analysis was performed on the twitch-curve results as follows. Each individual twitch curve was characterized by curve fitting and the obtained sigmoidal function was used to calculate the current level at which half the maximal twitch force was developed (I½). These I½ values were used to compare groups of measurements with one another by two-tailed Student's paired t test. The I½ values given in the Results represent these averaged group results.
Vm values were measured at a range of [K+]o. Each muscle was exposed to the full range of [K+]o, at either low or high Cl−o. At each condition, the Vm was obtained from a number of fibres (7–8). Next, these Vm values were averaged (n = 1). These averaged values were obtained from a number of muscles (n = 4) and used to run the statistical analysis. The analysis of the data was performed with a linear mixed-effect (LME) model with fixed effects: K+o (covariate), Cl−o (factor), and their interaction; and rat as a random effect. This approach was chosen to account for the interdependence of observations within and between the two muscles from the same rat.
Results
Effect of hyperkalaemia on twitch force in rat soleus muscle
The first experiment served to establish the relationship between the concentration of K+o and muscle twitch force, at two time points (Table 2). After 45 min at 12.5 mm K+o, the muscle preparations had lost around 50% of their twitch force. Steady state had not yet been obtained, as indicated by the total loss of force after 90 min of 12.5 mm K+o. The loss of force at 10, 12, 12.5 or 14 mm K+o was completely reversed by reintroducing 5 mm K+o (not shown). Based on these results, we decided to investigate the effect of a reduction in [Cl−]o on hyperkalaemic force development after 45 min exposure to 12.5 mm K+o. These conditions should allow us to ascertain a dynamic effect of Cl−o concentration on force development in either direction.
Table 2.
Hyperkalaemia-induced loss of twitch force
| [K+]o (mm) | % Twitch force after 45 min high K+o | % Twitch force after 90 min high K+o |
|---|---|---|
| 5→10 | 97.6 ± 4.0 | 70.6 ± 9.2 |
| 5→12 | 88.7 ± 4.4 | 34.5 ± 7.5 |
| 5→12.5 | 51.2 ± 3.1 | 3.1 ± 0.1 |
| 5→14 | 18.5 ± 4.3 | 0 |
Twitch force was recorded using supramaximal stimuli (1.5 mA). After 45 min of incubation at a [K+]o of 5 mm, twitch force was measured and [K+]o was increased to 10, 12, 12.5 or 14 mm. Twitch force was again measured after 45 min and 90 min of hyperkalaemia. Force was expressed as a percentage of the original force measured at 5 mm K+o. Each value represents the mean of five muscle preparations (±s.e.m.), resulting in a total of 20 muscle preparations. K+o concentrations were increased by equimolar replacement of NaHCO3 with KHCO3.
Effect of low [Cl−]o on hyperkalaemia-induced loss of twitch force
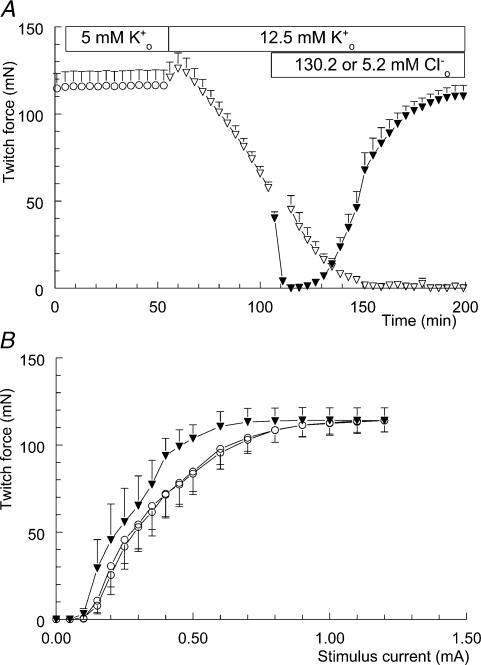
Figure 1A shows the influence of lowering [Cl−]o on twitch-force development during hyperkalaemia. For 55 min twitch force was recorded in modified Krebs–Ringer solution to demonstrate the stability of the preparation. During this period the twitch curve was reconstructed twice, once at the beginning of the experiment at t = 0 min and once at t = 50 min. As shown in Fig. 1B the excitability of the muscles remained stable during this period. The I½ values from the control situation at t = 0 and t = 50 min were both 0.38 ± 0.07 mA (n = 6). Next, the [K+]o was increased to 12.5 mm. After an initial increase, twitch force started to decline. At t = 160 min, force was zero and no sign of recovery was present during the final 40 min of the experiment.
Figure 1. Effect of lowering [Cl−]o on twitch-force development during hyperkalaemia.
A, twitch force recorded from soleus muscle is shown once every 4 min, although measurements were actually made once every minute. After 55 min, the modified KR solution (○; n = 10, +s.e.m.) was replaced by the high-K+o solution (▽; n = 10). This 12.5 mm K+o solution was maintained in two preparations (▽; n = 2, +s.d.), while the other eight preparations were introduced to the high-K+o/low-Cl−o solution at t = 105 min (▾; n = 8, +s.e.m.). B, isometric twitch-force–stimulation-strength relationships. These three twitch curves were recorded during the experiment depicted in A. Twice during the presence of the control solution, at time points t = 0 and t = 50 min (○; n = 6, −s.e.m.), and once during the presence of the high-K+o/low-Cl−o solution at t = 200 min (▾; n = 6, +s.e.m.).
This picture changed noticeably when [Cl−]o was reduced from 130.2 to 5.2 mm at t = 105 min. The ongoing rundown of twitch force was accelerated, leading to a total loss of force in just 5 min. However, hereafter the soleus muscle started to recover gradually, almost reaching control values at t = 200 min. At the end of this experiment, twitch force was stable enough to record another twitch curve. As can be seen from Fig. 1B, this twitch curve had shifted to the left. The I½ value from the high-K+o/low-Cl−o situation was 0.27 ± 0.05 mA (n = 6), and differed significantly from the control value of 0.38 ± 0.07 mA (P < 0.05). This shift indicates increased membrane excitability with low Cl−o levels.
In order to verify that low [Cl−]o was responsible for the recovery of twitch force during hyperkalaemia, and not the presence of its substitute isethionate, we performed an additional experiment (n = 2) with methanesulphonate as the major anion. The result was identical, i.e. a rapid loss of twitch force followed by complete recovery (not shown).
In an additional set of experiments (n = 4) the [K+]o was increased to 14 mm. Again, loss of force was accelerated after reducing [Cl−]o. However, at this slightly higher [K+]o, no sign of recovery was seen (not shown).
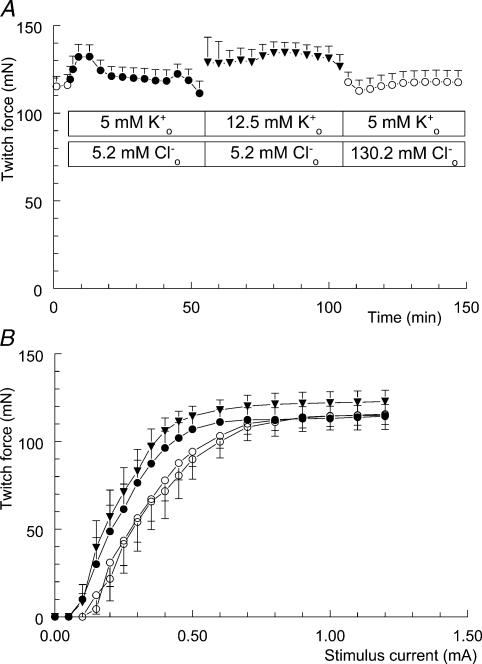
If reduction of [Cl−]o can antagonize hyperkalaemia-induced loss of twitch force, then reducing [Cl−]o before introducing the hyperkalaemia should prevent loss of force. To test this hypothesis we also performed the high K+o/low Cl−o experiment in the reverse order, as shown in Fig. 2A.
Figure 2. Lowering [Cl−]o prevents hyperkalaemia-induced loss of twitch force and affects muscle excitability.
A, twitch force recorded from soleus muscle is shown once every 4 min. After 5 min, the modified Krebs–Ringer solution (○; n = 11, +s.e.m.) was replaced by the low-Cl−o solution (•); at t = 55 min the low-Cl−o solution was replaced by the high-K+o/low-Cl−o solution (▾); the modified Krebs–Ringer solution was reintroduced at t = 105 min (○). B, isometric twitch-force–stimulation-strength relationships. These four twitch curves were recorded during the experiment depicted in A. Twice during the presence of the control solution, at time points t = 0 (○) and t = 150 min (○), once during the presence of the low-Cl−o solution at t = 50 min (•), and once during the presence of the high-K+o/low-Cl−o solution at t = 100 min (▾).
Five minutes after the start of the experiment, [Cl−]o was reduced to 5.2 mm by replacing it with isethionate. After a temporary increase, twitch force stabilized at the same level as before the reduction of [Cl−]o. At t = 50 min, twitch force appears to be going down. This was due to the fact that 6 out of the 11 preparations started to show an occasional myotonic contraction after stimulation, i.e. twitch prolongation directly followed by a nonevoked tetanic contraction lasting about 1 min. Consequently, the next evoked twitch response was smaller.
Introducing the high-K+o/low-Cl−o solution, at t = 55 min, resulted in variable twitch force for about 5 min, which explains the increase in s.e.m. During the next 45 min, twitch force remained stable, in contrast to the 50% rundown of twitch force seen with high K+o only (cf. Table 2 and Fig. 1). Next, the modified Krebs–Ringer solution was reintroduced and twitch force stabilized at exactly the same level as 2 h previously.
Replacement of Cl−o with isethionate not only prevented hyperkalaemia-induced loss of twitch force, but also induced a leftward shift of the twitch curve (Fig. 2B). The calculated I½ value from the low-Cl−o twitch curve (•) is 0.26 ± 0.03 mA (n = 11), which lies 0.08 mA below the calculated I½ value from the control twitch curve (○), being 0.34 ± 0.04 mA. This shift was highly significant (P < 0.0001) and can be regarded as a 24% increase in membrane excitability. Adding extra K+ to the low Cl− medium (▾) did not cause an additional shift of the twitch curve (I½ = 0.24 ± 0.03 mA). The leftward shift in the twitch curve, due to reducing [Cl−]o to 5.2 mm, was completely reversed after returning to 130 mm Cl−o (I½ = 0.35 ± 0.05 mA).
Effect of low [Cl−]o on hyperkalaemia-induced membrane depolarization
The data presented in the previous sections show that a reduction of [Cl−]o modifies the response of rat soleus muscle to hyperkalaemia. Since hyperkalaemia-induced loss of force is due to membrane depolarization (Cairns et al. 1997), it is reasonable to assume that lowering [Cl−]o causes membrane hyperpolarization. We measured Vm to substantiate our idea that the interaction between the effects of low [Cl−]o and high [K+]o on twitch force occurs at the level of Vm.
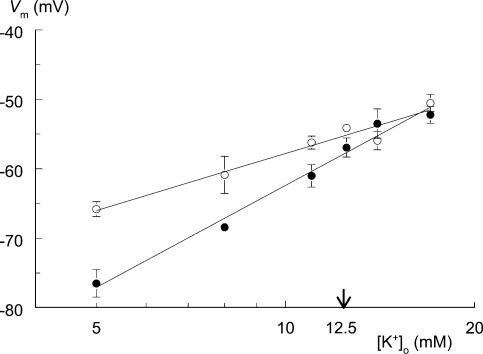
Figure 3 shows the depolarizing response of Vm to hyperkalaemia under two different conditions, 130.2 and 5.2 mm Cl−o. The LME model (see Calculations and statistics) showed that there was a significant interaction (P < 0.001) between the effects of [Cl−]o and [K+]o on Vm, i.e. the effect of a change in [K+]o on Vm is dependent upon [Cl−]o, and vice versa. The regression lines at 130 and 5 mm Cl−o are Vm = −85.1 + 27.3 log[K+]o and Vm = −111.2 + 48.8 log[K+]o, respectively. Therefore, lowering [Cl−]o from 130 to 5 mm increased the sensitivity of Vm to changes in [K+]o from 27.3 mV per decade [K+]o to 48.8 mV per decade [K+]o. The estimated difference in Vm between high and low Cl−, at 12.5 mm K+o, is 2.5 mV, with Vm being more negative at low Cl−o.
Figure 3. Lowering [Cl−]o affects the response of membrane potential (Vm) to changes in [K+]o.
Resting membrane potential was recorded from rat soleus muscle for a range of K+o concentrations (5, 8, 11, 12.5, 14 and 17 mm). The solutions contained either 130.2 mm Cl−o (○, control) or 5.2 mm Cl−o (•). Extracellular Cl− was replaced by equimolar amounts of isethionate. Muscles incubated at either 5 or 130 mm Cl− were subjected to the full range of K+o concentrations, starting at 5 mm. Each point is the mean Vm value of four muscles under steady-state conditions. Error bars represent s.e.m. There is a significant interaction between the effects of [Cl−]o and [K+]o on Vm (P < 0.001, linear mixed-effect model); the regression lines at 130 and 5 mm Cl−o are Vm=−85.1 + 27.3 log [K+]o and Vm=−111.2 + 48.8 log[K+]o, respectively. These lines intersect at a [K+]o of 16.4 mm.
Effect of 9-AC on hyperkalaemia-induced loss of twitch force
The results presented above show the influence of Cl− on hyperkalaemia-induced membrane depolarization and loss of twitch force. In these experiments we lowered [Cl−]o to a level comparable with the low intracellular concentration. This should have diminished the influence of Cl− on Vm. Another way to reduce the influence of Cl− on Vm is by blocking specific Cl− conductance. We used 9-AC to block the ClC-1 Cl− channel.
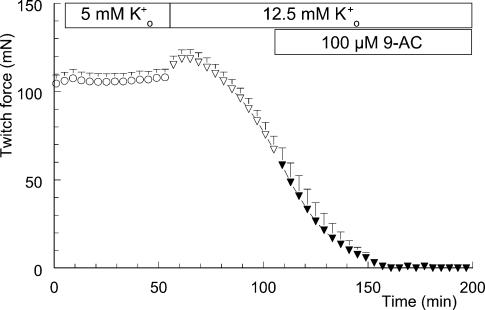
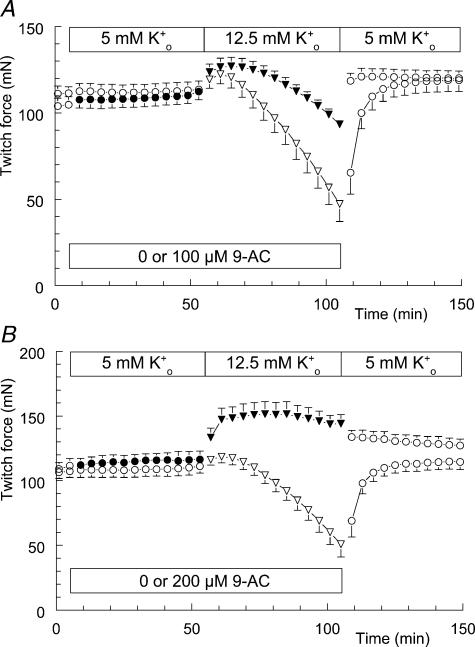
Figure 4 shows the absence of an effect of 100 μm 9-AC on force decline during hyperkalaemia. Introducing the high-K+o solution at t = 55 min resulted in a gradual loss of twitch force. Addition of 9-AC to the high-K+o solution at t = 105 min had no effect on this ongoing loss of force, as stated above. The time course was similar to the hyperkalaemia-induced loss of twitch force shown in Fig. 1A.
Figure 4. No influence of 9-anthracene carboxylic acid (9-AC) on hyperkalaemia-induced loss of twitch force.
Twitch force recorded from soleus muscle is shown once every 4 min. At t = 5 min, the modified Krebs–Ringer (KR) solution (○; n = 6, +s.e.m.) was replaced by modified KR containing 0.5% v/v ethanol (○). At t = 55 min this solution was replaced by the high-K+o solution, again containing 0.5% v/v ethanol (•). This latter fluid was replaced by the high-K+o solution containing 100 μm 9-AC and 0.5% v/v ethanol at t = 105 min (▾).
Reversing the order of changing the solutions did reveal an effect of 9-AC on hyperkalaemia-induced loss of twitch force (Fig. 5A). In this series of experiments 9-AC was added to half of the preparations at t = 5 min (filled symbols), while the other half received the solvent ethanol (0.5% v/v). Introducing the high-K+o solution at t = 55 min revealed a difference between the preparations with and without 100 μm 9-AC. The loss of force due to 50 min of hyperkalaemia was reduced in the presence of 100 μm 9-AC. Changing the solutions back to modified Krebs–Ringer solution, including 0.5% v/v ethanol, quickly restored full muscle force. This experiment was repeated with a concentration of 200 μm 9-AC (Fig. 5B). To this end DMSO was used to dissolve 9-AC. The experimental solutions contained 0.05% v/v DMSO. Figure 5B shows that 9-AC has reversed the hyperkalaemia-induced loss of twitch force into a situation with increased twitch force. At t = 100 min the difference in force has grown to 100 mN. Changing the solutions back to modified Krebs–Ringer solution, including 0.05% v/v DMSO, quickly reduced the difference in force between the two groups.
Figure 5. Effect of pre-incubating soleus muscle with 9-AC on hyperkalaemia-induced loss of twitch force.
The effect of hyperkalaemia on twitch force in the absence (open symbols) and in the presence of 100 or 200 μm 9-AC (filled symbols, A and B, respectively). Four animals were used in the experiment depicted in A, and each animal contributed to both groups (with and without 9-AC). Muscles from the right and left legs were evenly distributed between the two groups. The same holds for the experiment depicted in B. The 9-AC groups were exposed to 100 or 200 μm 9-AC at t = 5 min (•; n = 4, ±s.e.m.), while the control groups received only the solvent (○; n = 4, ±s.e.m.). In the experiment with 100 μm 9-AC, the solutions contained 0.5% v/v ethanol. In the experiment with 200 μm 9-AC, the solutions contained 0.05% v/v DMSO. In addition, all groups were exposed to 12.5 mm K+o at t = 55 min (▾,▽). After the measurement of t = 105 min fluids were replaced by modified Krebs–Ringer solution, still containing the solvent (○).
Effect of bumetanide on hyperkalaemia-induced loss of twitch force
The results presented above show that a hyperkalaemia-induced loss of twitch force can be prevented or antagonized by a reduction of either [Cl−]o or specific Cl− conductance. It has been reported that both these manipulations can cause hyperpolarization, and that this hyperpolarization depends on intracellular accumulation of Cl− via the bumetanide-sensitive NKCC1 (Betz et al. 1984; Harris & Betz, 1987; Aickin et al. 1989; van Mil et al. 1997). In order to see whether we could trace back our results to this transporter-mediated influx of Cl−, the experiments were repeated in the presence of bumetanide.
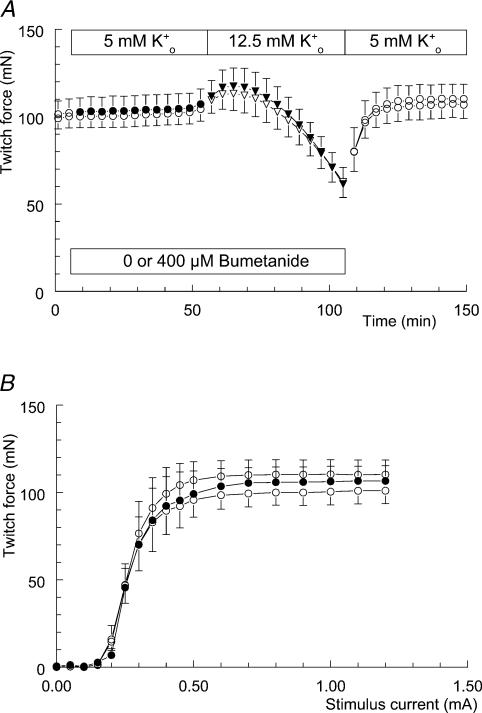
No effect of bumetanide on hyperkalaemia-induced loss of twitch force was found, either at a concentration of 100 μm or at a concentration of 400 μm. The result of one series of experiments is shown in Fig. 6A. During these experiments half of the preparations received 400 μm bumetanide before [K+]o was raised to 12.5 mm (filled symbols). Irrespective of the presence of bumetanide, both groups responded to the hyperkalaemia with a 40% drop in twitch force. Twitch force was completely restored after changing the solutions back to modified Krebs–Ringer solution. In addition, 400 μm bumetanide had no effect on the left–right position of the twitch curves (Fig. 6B). These three twitch curves belong to the bumetanide group and were recorded in the absence (t = 0 min), presence (t = 50 min) and again in the absence (t = 150 min) of bumetanide. At the end of these experiments twitch force had increased by about 10 mN, most likely because of the presence of 0.4% v/v methanol.
Figure 6. No effect of pre-incubating soleus muscle with bumetanide on hyperkalaemia-induced loss of twitch force.
A, the effect of hyperkalaemia on twitch force in the absence (open symbols) and in the presence of 400 μm bumetanide (filled symbols). The bumetanide group was exposed to 400 μm bumetanide at t = 5 min (•; n = 4, +s.e.m.), while the control group only received the solvent methanol (0.4% v/v) (○; n = 4, −s.e.m.). In addition, both groups were exposed to 12.5 mm K+o at t = 55 min (▾,▽). After the measurement of t = 105 min, fluids were replaced by modified Krebs–Ringer solution, still containing 0.4% v/v methanol (○). B, isometric twitch-force–stimulation-strength relationships from the bumetanide group. These three twitch curves were recorded during the experiment depicted in A at time points t = 0 (○, lower curve; modified Krebs–Ringer solution), t = 50 (•; modified Krebs–Ringer solution containing 400 μm bumetanide and 0.4% v/v methanol) and t = 150 min (○, upper curve; modified Krebs–Ringer solution containing 0.4% v/v methanol).
Discussion
This study shows that reducing Cl− conductance, either by lowering [Cl−]o or blocking specific Cl− conductance with 9-AC, can have two distinct effects on hyperkalaemia-induced loss of twitch force. On one hand it was found that lowering [Cl−]o during hyperkalaemia accelerates ongoing rundown of twitch force. Unexpectedly, 9-AC did not produce this effect, probably because it diffuses too slowly into the core of the muscle to affect the ongoing rundown of twitch force. The rapid decline of force after lowering [Cl−]o most likely reflects both the loss of a stabilizing influence of Cl− on Vm and a temporary change in equilibrium potential of Cl− (Hodgkin & Horowicz, 1959; Dulhunty, 1978). The increased membrane excitability with low [Cl−]o clearly shows the stabilizing influence of Cl− on Vm. As such, this result is consistent with the general notion that the negative ECl, in combination with a large specific membrane conductance, stabilizes Vm during depolarization (Hodgkin & Horowicz, 1959; Dulhunty, 1978, 1979; McCaig & Leader, 1984; Wallinga et al. 1999). On the other hand we found that blocking Cl− channels or lowering [Cl−]o could completely prevent hyperkalaemia-induced loss of force. The range of [K+]o associated with loss of force was shifted to higher values after lowering [Cl−]o. This was due to a low-Cl−o-induced hyperpolarization of the membrane. This result is in line with studies showing that ECl is less negative than Vm (Dulhunty, 1978; Aickin et al. 1989). Since the NKCC1 transporter is held responsible for the difference between ECl and Vm, we expected that blocking this transporter would also prevent hyperkalaemia-induced loss of force. This was not the case. The most significant conclusion to be drawn from this study is that the normal high Cl− conductance of rat soleus muscle can add to the loss of force during hyperkalaemia.
Methodology and limitations
We studied twitch contractions instead of tetanic contractions because it has been demonstrated that hyperkalaemia-induced fatigue can be overcome by high-frequency stimulation, probably by activation of the electrogenic Na+,K+-ATPase (Overgaard & Nielsen, 2001). Another aspect of high-frequency stimulation is that the amount of K+ released from the muscle fibres can cause a significant rise in [K+]o (Juel, 1986). To prevent these confounding influences on our hyperkalaemia experiments we used single twitches.
Hyperkalaemia-induced loss of twitch force in the near absence of extracellular Cl−
In order to investigate the influence of Cl− on the loss of twitch force during hyperkalaemia, [Cl−]o was reduced to 5 mm. This value was chosen because it lies at the bottom of the range of values reported for intracellular Cl− activity and, as such, is nearly identical to the intracellular Cl− activity associated with passive Cl− distribution (Donaldson & Leader, 1984; McCaig & Leader, 1984; Aickin et al. 1989; Cairns et al. 1997). As a consequence, Cl− should rapidly lose its influence on Vm. Lowering [Cl−]o from 130 to 5 mm accelerated the ongoing loss of twitch force around 10 times (Fig. 1A). Our result is consistent with results from mouse EDL and soleus muscle (Cairns et al. 2004). It was shown that low [Cl−]o mainly increased the rate of fatigue with tetanic stimulation. Whether an increase in [K+]o caused the tetanus depression was not examined.
The rapid loss of force shown in Fig. 1 indicates that normal [Cl−]o exerts a stabilizing influence on Vm during hyperkalaemia. It has been shown that membrane depolarization during hyperkalaemia is sped up in the absence of extracellular Cl−, most likely due to the loss of an influx of Cl−o (see also Introduction) (Hodgkin & Horowicz, 1959; Dulhunty, 1978, 1979; McCaig & Leader, 1984). Dulhunty (1978) showed that the depolarization proceeded around 10 times faster in the absence of Cl− conductance. So, it appears that the unusual rapid loss of force during hyperkalaemia reflects the changes occurring in Vm without the stabilizing influence of Cl− conductance.
However, lowering [Cl−]o is impossible without inducing a concomitant change in the ECl. It has been shown that the Vm of muscle fibres is transiently depolarized when [Cl−]o is reduced and Cl− loses its influence on Vm (Hodgkin & Horowicz, 1959; Dulhunty, 1978; Aickin et al. 1989). Aickin et al. (1989) reported 20 mV depolarizations, lasting about 5 min, when [Cl−]o was reduced to zero. Certainly, the fibres will experience a change in ECl when we quickly reduce [Cl−]o from 130 to 5 mm. ECl will temporarily become less negative due to the reduction in the chemical gradient. This change in ECl will depolarize the muscle fibre and add to the rapidly developing hyperkalaemia-induced depolarization. So, the rapid decline of twitch force during high [K+]o and low [Cl−]o most likely reflects both the loss of a stabilizing influence of Cl− on Vm and the temporary change in ECl.
The rapid loss of twitch force was followed by a remarkable recovery, despite the high concentration of 12.5 mm K+o (Fig. 1). The experiment shown in Fig. 2 also shows that low [Cl−]o protects muscle twitch force against hyperkalaemia. This is most likely to be due to the depolarizing influence of Cl− on Vm, under normal resting conditions. This became clear when we recorded Vm. The regression lines in Fig. 3 show that low [Cl−]o produced steady-state hyperpolarizations at [K+]o below 16.4 mm (the intersect of the regression lines). As such, our results are in line with studies showing that ECl is less negative than Vm and, thereby, exerts a depolarizing influence on Vm under normal resting conditions (Dulhunty, 1978; Aickin et al. 1989). Lowering [Cl−]o at 5 mm K+o produced a hyperpolarizing response of 11.1 mV. This falls within the range of values reported in literature. Hyperpolarizations ranging from a few to 20 mV have been measured after reducing [Cl−]o or blocking Cl− channels (Dulhunty, 1978; Aickin et al. 1989; van Mil et al. 1997). However, our Vm data seem in conflict with data presented by Cairns et al. (2004) who showed no change in the resting Vm of mouse soleus muscle following equilibration at 10 mm[Cl−]o. Perhaps the absence of an effect in their study was due to the less pronounced reduction in [Cl−]o at 25°C.
Although the estimated difference in Vm between high and low Cl−o, at 12.5 mm K+o, is only 2.5 mV, we still consider it feasible that this small change in Vm is responsible for the recovery of force. Cairns et al. (1995, 1997) showed that the dependence of twitch force on Vm is very steep. While depolarizations up to around −60 mV induce twitch potentiation, all force is lost when fibres are depolarized above −55 mV. Thus, a substantial recovery of twitch force is possible with a hyperpolarization of just a few millivolts. In addition, the small hyperpolarization occurs within the narrow range of Vm values associated with loss of twitch force (Fig. 3).
Figure 2A shows a 15% increase in peak twitch force when the soleus muscle is exposed to a combination of low Cl−o and high K+o (middle segment). Therefore, lowering [Cl−]o has reversed the hyperkalaemia-induced loss of twitch force into a situation with increased twitch force (cf. Figs 1 and 2). Twitch potentiation is known to occur at values of Vm, which are slightly more negative than the values needed to depress twitch force (Cairns et al. 1997; Yensen et al. 2002). Therefore, this result is in line with our data on Vm.
Since no sign of force recovery was seen at 14 mm K+o, we conclude that low [Cl−]o shifts the range of [K+]o associated with loss of twitch force to higher values.
Finally, we point out that the interactive effect of [Cl−]o and [K+]o on Vm, as shown in Fig. 3, has been previously reported by Geukes Foppen et al. (2002). Those authors showed that decreasing the accumulation of Cl− in lumbrical muscle of mice, by decreasing medium osmolality, decreases ΔVm/Δ[K+]o from 50 to 31 mV per decade between 5.7 and 22.8 mm K+o. Concomitantly, the membrane was hyperpolarized by 18 mV at 5.7 mm K+o. Consequently, the relationships between Vm and [K+]o appear to intersect at an estimated value of 20 mm K+o.
Hyperkalaemia-induced loss of twitch force in the presence of 9-AC
Next, we wanted to test the effect of 9-AC on hyperkalaemia-induced loss of twitch force. Since 9-AC is a potent blocker of Cl− channels (Palade & Barchi, 1977), it should eliminate the influence of Cl− on Vm. The results obtained using 9-AC are in line with those we obtained with low [Cl−]o.
The ongoing loss of twitch force was unaffected when 9-AC was applied 45 min after the onset of hyperkalaemia (Fig. 4). However, when 9-AC was already in the bath for 45 min when hyperkalaemia was induced, we did find an effect of 9-AC on the development of hyperkalaemia-induced loss of twitch force (Fig. 5). This indicates that 9-AC diffuses slowly into the core of the soleus muscle.
At a concentration of 100 μm, 9-AC caused a delay in the development of hyperkalaemia-induced loss of twitch force, while 200 μm 9-AC completely prevented the loss of force. This latter result is identical to the effect of low [Cl−]o on hyperkalaemia-induced loss of twitch force (cf. Figs 2 and 5). Moreover, the combination of 200 μm 9-AC and high [K+]o produced twitch potentiation, similar to the effect of low [Cl−]o and high [K+]o on twitch force (cf. Figs 2 and 5).
The concentration of 200 μm 9-AC is twice as high as the concentration that is commonly used (Fahlke & Rudel, 1995; Coonan & Lamb, 1998). The need for this high concentration could be related to the presence of two different Cl− channels in mammalian muscle fibres (Gurnett et al. 1995). ClC-1 is blocked by 9-AC from the extracellular side (Steinmeyer et al. 1991), but the T-tubular Cl− channel is probably blocked by 9-AC from the myoplasmic side (Ide et al. 1995; Coonan & Lamb, 1998). The fact that several authors have reported a partial effect of 9-AC on Vm might be due to the use of 9-AC at concentrations of 75–100 μm (Aickin et al. 1989; Geukes Foppen et al. 2002).
The protective effect of 9-AC on twitch force during high [K+]o is most likely to be due to membrane hyperpolarization because: (1) low [Cl−]o, which also eliminates the influence of Cl− on Vm, produced membrane hyperpolarization and protected twitch force against hyperkalaemia; (2) 9-AC is known to produce membrane hyperpolarization in rat and mouse lumbrical muscle (Betz et al. 1984; Aickin et al. 1989; Geukes Foppen et al. 2002); (3) the combination of 9-AC and high [K+]o produced substantial twitch potentiation. Twitch potentiation is known to occur at values of Vm that are slightly more negative than the values needed to depress twitch force (Cairns et al. 1997; Yensen et al. 2002).
Hyperkalaemia-induced loss of twitch force in the presence of bumetanide
Thus far, we showed that our results give evidence of a depolarizing influence of Cl− on Vm. To exert such an influence the [Cl−]i needs to be above that predicted by a passive Donnan type of equilibrium. The transporter held responsible for the uphill transport of Cl− into the muscle fibre is the NKCC1. This conclusion comes from experiments with rat lumbrical muscle, in which it was shown that the internal Cl− activity can be reduced by addition of furosemide or lowering either the [Na+]o or the [K+]o (Harris & Betz, 1987; Aickin et al. 1989) even at room temperature. Blocking the Cl−/HCO3− antiporter was of no consequence to the accumulation of Cl−. Moreover, both the reduction of Cl− conductance and the presence of loop diuretics can lead to membrane hyperpolarization in lumbrical muscle of rat or mouse (Betz et al. 1984; Aickin et al. 1989; van Mil et al. 1997; Geukes Foppen et al. 2002). Therefore, we investigated whether the effect of lowering Cl− conductance on hyperkalaemia-induced loss of force could be mimicked by blocking the NKCC1.
We found that this was not the case. Addition of bumetanide at a concentration of 100 or 400 μm had no effect on either the developmental course or the extent of the hyperkalaemia-induced loss of twitch force (Fig. 6). This result is not easily explained, but several possibilities will be discussed.
Whether or not there is a bumetanide-sensitive Cl− influx in rat soleus muscle has not yet been verified by radioisotope measurements, but a few studies indicate that it is present. These studies demonstrated the presence of either a Cl−-dependent, Na+-dependent, bumetanide-sensitive 86Rb+ influx (Wong et al. 1999) or a Cl−-dependent, K+-independent, bumetanide-sensitive 22Na+ influx (Dørup & Clausen, 1996). In order to obtain complete inhibition of the NKCC1, it was necessary to use concentrations of at least 100 μm (Dørup & Clausen, 1996).
Thus, the absence of an effect of bumetanide on hyperkalaemia-induced loss of twitch force in rat soleus muscle indicates that the NKCC1 is not involved in setting Vm through accumulation of Cl−. But, blocking the NKCC1 is likely to disturb the influx of Na+ and K+. Since the NKCC1 is held responsible for 15% of the influx of K+ at rest, at either 30 or 37 °C (Wong et al. 1999, 2001; Lindinger et al. 2001, 2002), there might be a small increase in [K+]o/[K+]i, leading to a depolarization of a few millivolts. Likewise, a bumetanide-related change in [Na+]i might depolarize Vm and, thereby, oppose a bumetanide-induced Cl−-related hyperpolarization. More specifically, since the activity of the electrogenic Na+,K+-ATPase is dependent upon [Na+]i, although in a complex way, a reduction in total Na+ influx is likely to reduce pump activity (Nielsen & Clausen, 1997; Sejersted & Sjøgaard, 2000; Buchanan et al. 2002). A reduced activity of the Na+,K+-ATPase causes membrane depolarization, since blocking the Na+,K+-ATPase with ouabain in rat soleus muscle causes 8 mV depolarization, at 30 °C (Clausen & Flatman, 1977).
In conclusion, the present study suggests that the [Cl−]i in rat soleus muscle is above that predicted by a passive Donnan type of equilibrium and, as a consequence, exerts a depolarizing influence on Vm. The accumulation of Cl− is most likely to be related to the regulation of the volume of the muscle fibres (Chinet, 1993; van Mil et al. 1997; Russell, 2000; Lindinger et al. 2002; Gosmanov et al. 2003). A side effect of the accumulation of Cl− is that muscle fibres can become more prone to losing their excitability during periods of hyperkalaemia. It was shown that the range of [K+]o associated with loss of twitch force is shifted to higher values during periods of reduced Cl− conductance.
References
- Aickin CC, Betz WJ, Harris GL. Intracellular chloride and the mechanism for its accumulation in rat lumbrical muscle. J Physiol. 1989;411:437–455. doi: 10.1113/jphysiol.1989.sp017582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz WJ, Caldwell JH, Kinnamon SC. Physiological basis of a steady endogenous current in rat lumbrical muscle. J Gen Physiol. 1984;83:175–192. doi: 10.1085/jgp.83.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum R, Westphal W. On the actions of a chloride conductance blocking agent (9-AC) on the resting membrane potential of single mammalian skeletal muscle fibres. Pflugers Arch. 1981;389:45–45. [Google Scholar]
- Bretag AH. Muscle chloride channels. Phys Rev. 1987;67:618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- Bryant SH, Morales-Aguilera A. Chloride conductance in normal and myotonic muscle fibres and the action of monocarboxylic aromatic acids. J Physiol. 1971;219:367–383. doi: 10.1113/jphysiol.1971.sp009667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan R, Nielsen OB, Clausen T. Excitation- and β2-agonist-induced activation of the Na+–K+pump in rat soleus muscle. J Physiol. 2002;545:229–240. doi: 10.1113/jphysiol.2002.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns SP, Flatman JA, Clausen T. Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+–K+ pump. Eur J Physiol. 1995;430:909–915. doi: 10.1007/BF01837404. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Hing WA, Slack JR, Mills RG, Loiselle DS. Different effects of raised [K+]o on membrane potential and contraction in mouse fast- and slow-twitch muscle. Am J Physiol Cell Physiol. 1997;273:C598–611. doi: 10.1152/ajpcell.1997.273.2.C598. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Ruzhynsky V, Renaud JM. Protective role of extracellular chloride in fatigue of isolated mammalian skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C762–770. doi: 10.1152/ajpcell.00589.2003. [DOI] [PubMed] [Google Scholar]
- Chinet A. Ca2+-dependent heat production by rat skeletal muscle in hypertonic media depends on Na+–Cl− co-transport stimulation. J Physiol. 1993;461:689–703. doi: 10.1113/jphysiol.1993.sp019536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua M, Dulhunty AF. Inactivation of excitation-contraction coupling in rat extensor digitorum longus and soleus muscles. J Gen Physiol. 1988;91:737–757. doi: 10.1085/jgp.91.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Andersen SL, Flatman JA. Na+–K+ pump stimulation elicits force recovery of contractility in K+-paralysed rat muscle. J Physiol. 1993;472:521–536. doi: 10.1113/jphysiol.1993.sp019960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Everts ME. K+-induced inhibition of contractile force in rat skeletal muscle: role of active Na+–K+ transport. Am J Physiol Cell Physiol. 1991;261:C799–807. doi: 10.1152/ajpcell.1991.261.5.C799. [DOI] [PubMed] [Google Scholar]
- Clausen T, Flatman JA. The effect of catecholamines on Na–K transport and membrane potential in rat soleus muscle. J Physiol. 1977;270:383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Overgaard K. The role of K+ channels in the force recovery elicited by Na+–K+ pump stimulation in Ba2+-paralysed rat skeletal muscle. J Physiol. 2000;527:325–332. doi: 10.1111/j.1469-7793.2000.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonan JR, Lamb GD. Effect of transverse-tubular chloride conductance on excitability in skinned skeletal muscle fibres of rat and toad. J Physiol. 1998;509:551–564. doi: 10.1111/j.1469-7793.1998.551bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Bryant SH, Owenburg KM. Dependence of membrane potential on extracellular ionic concentrations in myotonic goats and rats. Am J Physiol Cell Physiol. 1981;240:C56–63. doi: 10.1152/ajpcell.1981.240.1.C56. [DOI] [PubMed] [Google Scholar]
- De Luca A, Conte Camerino D, Connold A, Vrbova G. Pharmacological block of chloride channels of developing rat skeletal muscle affects the differentiation of specific contractile properties. Pflugers Arch. 1990;416:17–21. doi: 10.1007/BF00370216. 10.1007/BF00370216. [DOI] [PubMed] [Google Scholar]
- Donaldson PJ, Leader JP. Intracellular ionic activities in the EDL muscle of the mouse. Pflugers Arch. 1984;400:166–170. doi: 10.1007/BF00585034. [DOI] [PubMed] [Google Scholar]
- Dørup I, Clausen T. Characterization of bumetanide-sensitive Na+ and K+ transport in rat skeletal muscle. Acta Physiol Scand. 1996;158:119–127. doi: 10.1046/j.1365-201X.1996.542296000.x. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF. The dependence of membrane potential on extracellular chloride concentration in mammalian skeletal muscle. J Physiol. 1978;276:67–82. doi: 10.1113/jphysiol.1978.sp012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty AF. Distribution of potassium and chloride permeability over the surface and T-tubule membranes of mammalian skeletal muscle. J Membr Biol. 1979;45:293–310. doi: 10.1007/BF01869290. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF. Potassium contractures and mechanical activation in mammalian skeletal muscles. J Membr Biol. 1980;57:223–233. doi: 10.1007/BF01869590. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Rudel R. Chloride currents across the membrane of mammalian skeletal muscle fibres. J Physiol. 1995;484:355–368. doi: 10.1113/jphysiol.1995.sp020670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geukes Foppen RJ, van Mil HG, Siegenbeek van Heukelom J. Effects of chloride transport on bistable behaviour of the membrane potential in mouse skeletal muscle. J Physiol. 2002;542:181–191. doi: 10.1113/jphysiol.2001.013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosmanov AR, Schneider EG, Thomason DB. NKCC activity restores muscle water during hyperosmotic challenge independent of insulin, ERK, and p38 MAPK. Am J Physiol Regul Integr Comp Physiol. 2003;284:R655–665. doi: 10.1152/ajpregu.00576.2002. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, Kahl SD, Anderson RD, Campbell KP. Absence of the skeletal muscle sarcolemma chloride channel ClC-1 in myotonic mice. J Biol Chem. 1995;270:9035–9038. doi: 10.1074/jbc.270.16.9035. [DOI] [PubMed] [Google Scholar]
- Harris GL, Betz WJ. Evidence for active chloride accumulation in normal and denervated rat lumbrical muscle. J Gen Physiol. 1987;90:127–144. doi: 10.1085/jgp.90.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Horowicz P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T, Hidaka J, Kasai M. An anion channel from transverse tubular membranes incorporated into planar bilayers. Biochim Biophys Acta. 1995;1237:115–120. doi: 10.1016/0005-2736(95)00091-g. [DOI] [PubMed] [Google Scholar]
- Juel C. Potassium and sodium shifts during in vitro isometric muscle contraction, and the time course of the ion-gradient recovery. Pflugers Arch. 1986;406:458–463. doi: 10.1007/BF00583367. [DOI] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R400–406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Landau WM. The essential mechanism in myotonia. An electromyographic study. Neurology. 1952;2:369–388. doi: 10.1212/wnl.2.9-10.369. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, Hawke TJ, Lipskie SL, Schaefer HD, Vickery L. K+ transport and volume regulatory reponse by NKCC in resting rat hindlimb skeletal muscle. Cell Physiol Biochem. 2002;12:279–292. doi: 10.1159/000067898. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, Hawke TJ, Vickery L, Bradford L, Lipskie SL. An integrative, in situ approach to examining K+ flux in resting skeletal muscle. Can J Physiol Pharmacol. 2001;79:996–1006. [PubMed] [Google Scholar]
- McCaig D, Leader JP. Intracellular chloride activity in the extensor digitorum longus (EDL) muscle of rat. J Membr Biol. 1984;81:9–17. doi: 10.1007/BF01868805. [DOI] [PubMed] [Google Scholar]
- Mølgaard H, Stürup-Johansen M, Flatman JA. A dichotomy of the membrane potential response of rat soleus muscle fibers to low extracellular potassium concentrations. Pflugers Arch. 1980;383:181–184. doi: 10.1007/BF00581880. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, Clausen T. Regulation of Na+,K+ pump activity in contracting rat muscle. J Physiol. 1997;503:571–581. doi: 10.1111/j.1469-7793.1997.571bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, de Paoli F, Overgaard K. Protective effect of lactic acid on force production in rat skeletal muscle. J Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard K, Nielsen OB. Activity-induced recovery of excitability in K+-depressed rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2001;280:R48–55. doi: 10.1152/ajpregu.2001.280.1.R48. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Nielsen OB, Flatman JA, Clausen T. Relations between excitability and contractility in rat soleus muscle: role of the Na+–K+ pump and Na+/K+ gradients. J Physiol. 1999;518:215–225. doi: 10.1111/j.1469-7793.1999.0215r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade PT, Barchi RL. On the inhibition of muscle membrane chloride conductance by aromatic carboxylic acids. J Gen Physiol. 1977;69:879–896. doi: 10.1085/jgp.69.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Clausen T, Nielsen OB. Loss of force induced by high extracellular [K+] in rat muscle: effect of temperature, lactic acid and β2-agonist. J Physiol. 2003;551:277–286. doi: 10.1113/jphysiol.2003.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JM. Sodium–potassium–chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol Cell Physiol. 1985;248:C265–270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1412–1465. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Siegenbeek van Heukelom J. Role of the anomalous rectifier in determining membrane potentials of mouse muscle fibres at low extracellular K+ J Physiol. 1991;434:549–560. doi: 10.1113/jphysiol.1991.sp018485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991;354:301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Van der Heijden EPA, Kroese ABA, Werker PMN, Grabietz PD, de Jong MB, Kon M. The function of rat skeletal muscles following storage at 10 °C in various preservation solutions. Clin Sci (Lond) 1998;94:271–278. doi: 10.1042/cs0940271. [DOI] [PubMed] [Google Scholar]
- van Mil HGJ, Geukes Foppen RJ, Siegenbeek van Heukelom J. The influence of bumetanide on the membrane potential of mouse skeletal muscle cells in isotonic and hypertonic media. Br J Pharmacol. 1997;120:39–44. doi: 10.1038/sj.bjp.0700887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallinga W, Meijer SL, Alberink MJ, Vliek M, Wienk ED, Ypey DL. Modelling action potentials and membrane currents of mammalian skeletal muscle fibres in coherence with potassium concentration changes in the T-tubular system. Eur Biophys J. 1999;28:317–329. doi: 10.1007/s002490050214. [DOI] [PubMed] [Google Scholar]
- Wong JA, Fu L, Schneider EG, Thomason DB. Molecular and functional evidence for Na+–K+–2Cl− cotransporter expression in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 1999;277:R154–161. doi: 10.1152/ajpregu.1999.277.1.R154. [DOI] [PubMed] [Google Scholar]
- Wong JA, Thomason DB, Gosmanov AR, Schneider EG. Insulin-independent, MAPK-dependent stimulation of NKCC activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2001;281:R561–571. doi: 10.1152/ajpregu.2001.281.2.R561. [DOI] [PubMed] [Google Scholar]
- Yensen C, Matar W, Renaud JM. K+-induced twitch potentiation is not due to longer action potential. Am J Physiol Cell Physiol. 2002;283:C169–177. doi: 10.1152/ajpcell.00549.2001. [DOI] [PubMed] [Google Scholar]