Abstract
Reversal of long-term potentiation (LTP) by long trains of low-frequency stimulation is generally referred to as depotentiation. One of the intriguing aspects of depotentiation is that the magnitude of depotentiation is inversely proportional to the time lag of depotentiation stimulation following LTP induction. Although the mechanisms underlying depotentiation have been widely explored, the factors that regulate the susceptibility of LTP to depotentiation stimulation remain largely unclear. We now report that multiple trains of high-frequency stimulation provide immediate synaptic resistance to depotentiation stimulation at the mossy fibre–CA3 synapses. The synaptic resistance to depotentiation stimulation depends on the amount of synaptic stimulation used to induce LTP; it is prevented by protein synthesis inhibitors and is input specific. In contrast, neither the transection of mossy fibre axons near granule cell somata nor the application of RNA synthesis inhibitors influences synaptic resistance to depotentiation stimulation. We also provide evidence that the induction of depotentiation is regulated by GABAB receptors. Application of a GABAB receptor antagonist significantly promoted the synaptic resistance to depotentiation stimulation, whereas inhibition of GABA transport delayed the onset of this synaptic resistance. These results suggest that local protein synthesis is required for the development of synaptic resistance to depotentiation stimulation, whereas the activation of GABAB receptors promotes the susceptibility to depotentiation stimulation. These two factors may crucially regulate the reversal and stability of long-term information storage.
Activity-induced persistent synaptic modification is widely assumed to be the cellular mechanism underlying learning and memory in the brain (Siegelbaum & Kandel, 1991; Martin et al. 2000). Much of our understanding of activity-induced synaptic modifications and their functional relevance comes from studies on the mammalian hippocampus. In the hippocampus, brief trains of high-frequency stimulation (HFS) of afferent pathways can trigger a long-lasting enhancement of synaptic strength, commonly referred to as long-term potentiation (LTP) (Bliss & Collingridge, 1993), whereas prolonged low-frequency stimulation (LFS) results in a long-lasting decrease in synaptic strength, termed as long-term depression (LTD) (Mulkey & Malenka, 1992; Dudek & Bear, 1993). Although both LTP and LTD are remarkable for their stability, evidence accumulated recently suggests that they are initially labile and sensitive to disruption by a variety of interfering events or agents (Huang & Hsu, 2001). The reversal of LTP and LTD has been termed as depotentiation and de-depression (Huang & Hsu, 2001; Meng et al. 2003), respectively, and depotentiation has been studied much more extensively than de-depression (Zhou & Poo, 2004).
Studies in the past decades have revealed several common features of depotentiation. Of interest is the observation that activity can induce depotentiation only when applied within a time window (usually from several to tens of minutes) after the induction of LTP and the extent of depotentiation is inversely related to the interval between LTP induction and delivery of depotentiation stimulation (Fujii et al. 1991; Larson et al. 1993; Huang et al. 1999). This observation has led to a general belief that although the neurochemical processes consolidating LTP are set in motion by synaptic events in the millisecond range, they require many minutes to reach completion (Staübli & Chun, 1996; Staübli et al. 1998; Huang & Hsu, 2001). In this light, the substrates of potentiation resemble those for the encoding of memory (Popik et al. 1994). However, the factors that critically regulate the susceptibility of potentiated synapses to depotentiation stimulation have not been explored formally. Recently, Woo & Nguyen (2003) reported an immediate role for postsynaptic local protein synthesis during hippocampal CA1 LTP, one that is critical for consolidating synaptic potentiation into a stabilized state that is resistant to depotentiation stimulation. Furthermore, we have previously shown that, like the Hebbian form of Schaffer collateral–CA1 LTP, the best characterized non-Hebbian form of hippocampal mossy fibre–CA3 LTP also displays a time-dependent LFS-induced depotentiation (LFS-DEP) in a mouse slice preparation (Chen et al. 2001; Huang et al. 2002). Here, we extended these studies and examined the possibility that the synthesis of new proteins also contributes to the conversion of mossy fibre LTP that is susceptible to depotentiation stimulation to LTP that is resistant to depotentiation stimulation. Our results indicate that the development of mossy fibre synaptic resistance to depotentiation stimulation is induced rapidly, depends critically on the amount of imposed synaptic stimulation and requires presynaptic protein synthesis. Moreover, we demonstrate that GABAB receptor inhibition promotes synaptic resistance to depotentiation stimulation. These results underscore a crucially modulatory role of the protein synthesis process in bidirectional plasticity of hippocampal mossy fibre–CA3 synapses and point to an active role of GABAB receptors in regulating the synaptic plasticity.
Methods
Hippocampal slice preparation
Animal care was consistent with the guidelines set by the Laboratory Animal Center of National Cheng Kung University. All experiments were approved by the National Cheng Kung University Institutional Animal Care and Use Committee governing the participating laboratories. Hippocampal slices, 400 μm thick, were prepared from 4- to 5-week-old B57BL/6 mice according to procedures previously described (Chen et al. 2001; Huang et al. 2002). Briefly, animals were anaesthetized deeply with halothane, decapitated, and hippocampal slices were cut from a tissue block of the brain using a Leica VT1000S tissue slicer (Leica, Nussloch, Germany). After their preparation, slices were placed in a holding chamber of artificial cerebrospinal fluid (aCSF) oxygenated with 95% O2–5% CO2 and kept at room temperature for at least 1 h before recording. The composition of the aCSF solution was (mm): NaCl 117, KCl 4.7, CaCl2 2.5, MgCl2 1.2, NaHCO3 25, NaH2PO4 1.2 and glucose 11 at pH 7.3–7.4 and equilibrated with 95% O2–5% CO2. In some experiments, mossy fibre axons were disconnected from the granule cell somas by surgical transection through the granule cell layer of the dentate gyrus immediately after the slices were prepared. To ensure uniformity of the transection across experiments, the microlesion was made under visual control using a dissecting microscope. The mossy fibre transected slices were allowed to recover in the holding chamber for at least 2 h before recording commenced as described by Calixto et al. (2003).
Field potential and patch clamp recordings
For field potential recording, a single slice was then transferred to a submerge-type recording chamber and held between two nylon nets. The recording chamber consisted of a circular well of a low volume (1–2 ml) and was continuously superfused at 2–3 ml min−1 with the aCSF containing 4 mm CaCl2 and 4 mm MgCl2 at 32.0 ± 0.5°C to minimize polysynaptic activity (Moore et al. 2003). Synaptic responses were evoked by low-intensity stimulation (20 μs duration, < 1 mA intensity) of dentate gyrus granule cells or by placement directly in the stratum lucidum of the CA3 hippocampus to activate mossy fibre afferents at 0.05 Hz via a constant-current isolation unit (SS-104J; Nihon Kohden, Tokyo, Japan) connected to a bipolar stainless steel stimulating electrode. For each experiment, the stimulus intensity was set to the lowest value that reliably evoked a synaptic response without failures. Mossy fibre field excitatory postsynaptic potentials (fEPSPs) were recorded in the stratum lucidum of the CA3 region of the hippocampus using a glass microelectrode filled with 1 m NaCl (resistance 2–3 MΩ). Because the synaptic response to mossy fibre stimulation could be contaminated by responses activated by disynaptic activation of associational collateral fibres and/or association axon reflex inputs (Weisskopf & Nicoll, 1995), the following procedures were done to minimize the contribution of fibres other than mossy fibres to the fEPSPs (Zalutsky & Nicoll, 1990). First, the stimulating electrode was placed at a site in the granule cell layer of the dentate gyrus in which stimulation of the CA3 stratum lucidum produced the maximal antidromic field potential. Second, the reversal of the waveform as the recording microelectrode was moved from the stratum lucidum to the stratum radiatum served to define mossy fibre inputs. Third, positivities of the mossy fibre fEPSP were minimized, as these may reflect contamination by disynaptic excitatory inputs. Mossy fibre synaptic responses were characterized by fast rise times, by discontinuous stimulus–response properties, by their elicitation with low stimulus intensity (< 1 mA), and by the large frequency facilitation that occurred when stimulation frequency was changed from 0.05 to 1 Hz (Salin et al. 1996). Experiments were included for data analysis only if (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV; 0.5 μm), the potent group II metabotropic glutamate receptor agonist that selectively blocks mossy fibre responses, caused a greater than 80% reduction in the synaptic responses (Fig. S1, available online as Supplementary Material) (Castillo et al. 1997). To stimulate independent inputs to the same cell population, two bipolar stimulating electrodes were positioned on both edges of the stratum granulosum of dentate gyrus to activate two different mossy fibre inputs, alternating every 10 s. Their positions were arranged so that the same amount of current evoked two responses that did not differ from each other by > 10%. The absence of cross-pathway paired-pulse facilitation was used to ensure that the two inputs were independent of each other. In all experiments baseline synaptic transmission was monitored for 30 min before drug administration or delivering either high- or low-frequency stimulation. The strength of synaptic transmission was quantified by measuring the amplitude of fEPSPs. The fEPSP amplitudes were calculated after subtracting the mossy fibre volley from the evoked response. The mossy fibre volley was recorded at the end of experiment after blocking synaptic transmission with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μm) (Fig. S1 in Supplementary Material). LTP was induced by applying a single tetanus (25 Hz for 5 s duration at test strength), two trains of HFS (100 Hz, 1 s duration at test strength) or four trains of HFS (100 Hz, 1 s duration at test strength) delivered with an interval of 20 s. d-Aminophosphonovalerate (d-APV, 50 μm) was present for the duration of all experiments to block LTP at the CA3–CA3 collateral synapses. Depotentiation was induced by application of 15 min low-frequency trains of stimuli at 1 Hz at test strength. Responses during the trains were not recorded, and for convenience these periods are not shown on the graph. The values of residual potentiation reported here were calculated as the changes in fEPSP amplitude measured 40 min after the end of LFS. Microelectrodes were pulled from microfibre 1.0 mm capillary tubing on a Brown-Flaming electrode puller (Sutter Instruments, San Rafael, CA, USA). Electrical signals were collected with an Axoclamp-2B amplifier (Axon Instruments, Union City, CA, USA) filtered at 1 kHz, sampled at 10 kHz, and an Intel Pentium-based computer with pCLAMP software (Version 7.0, Axon Instruments) was used for data acquisition and analysis.
Visualized whole-cell patch clamp recordings of evoked excitatory postsynaptic currents (EPSCs) were conducted at 32.0 ± 0.5°C using standard methods as previously described (Edwards et al. 1989; Huang et al. 2002). CA3 pyramidal neurones were visualized throughout the experiment with an upright microscope (Olympus BX50WI; Olympus, Tokyo, Japan) equipped with a water-immersion ×40 objective using Nomarski-type differential interference contrast optics combined with infrared videomicroscopy. Patch pipettes were pulled from borosilicate capillary tubing and heat polished. The electrode resistance was typically 4–5 MΩ. The composition of the intracellular solution was (mm): potassium gluconate 110, KCl 30, Hepes 10, MgCl2 1, EGTA 0.5, Na2ATP 4, Na3GTP 0.3, phosphocreatine 7, lidocaine (lignocaine) N-ethyl bromide quaternary (QX-314) 5 and sucrose to bring the osmolarity to 290–295 mosmol l−1, pH 7.3 (adjusted with KOH). In part of these experiments, the recording pipette also contained 250 μm 7-methyl-GTP (m7GpppG), a mRNA cap analogue. Tight-seal (> 2 GΩ before breaking into whole-cell mode) whole-cell recordings were made using a patch-clamp amplifier (Axopatch 200B; Axon Instruments). Electrical signals were low-pass filtered at 2 kHz, digitized at 4–10 kHz using a Digidata 1200B interface, and an Intel Pentium-based computer with pCLAMP software (Version 8.0; Axon Instruments) was used for data acquisition and analysis. Postsynaptic series resistance was not compensated but was monitored during the experiment by using the amplitude of the capacitive current in response to a 10 mV pulse; the range was 8–15 MΩ. Experiments in which the series resistance increased by > 20% were discarded. EPSCs were included in the analysis if the rise time and decay time constants were monotonic and possessed no obvious multiple EPSCs or polysynaptic waveforms. The EPSC amplitude was determined from the response during a 1–2 ms window that included the peak of the waveform, and the amplitude of the baseline in a similar time window was subtracted. DCG-IV (0.5 μm) was added routinely at the end of the experiments to verify that the evoked EPSCs were abolished > 80%, confirming that the responses were primarily attributable to the activation of the mossy fibres.
Drug application
All drugs were applied by dissolving them to the desired final concentrations in aCSF and by switching the perfusion from control aCSF to drug-containing aCSF. Appropriate stock solutions of drugs were made and diluted with aCSF just before application. CNQX, cycloheximide, anisomycin, actinomycin-D and SCH50911 were dissolved in dimethylsulfoxide (DMSO) stock solution and stored at −20°C until the day of the experiment. The concentration of DMSO in the perfusing medium was 0.05%, which alone had no effect on the basal mossy fibre synaptic transmission (Chen et al. 2001). CNQX, d-APV (S)-3,5-dihydroxyphenylglycine (DHPG), cycloheximide, anisomycin, actinomycin-D, DCG-IV, SKF89976A and nipecotic acid were obtained from Tocris Cookson Ltd (Bristol, UK) and m7GpppG was purchased from New England Biolabs (Beverly, MA, USA).
Statistical analysis
The data for each experiment were normalized relative to baseline. All data are expressed as mean ± s.e.m. and, unless stated otherwise, the statistical significance was determined using the Mann-Whitney U test. The number of experiments is indicated by n. Probability values of P < 0.05 were considered to represent significant differences.
Results
The amount of synaptic stimulation modulates synaptic resistance to depotentiation stimulation
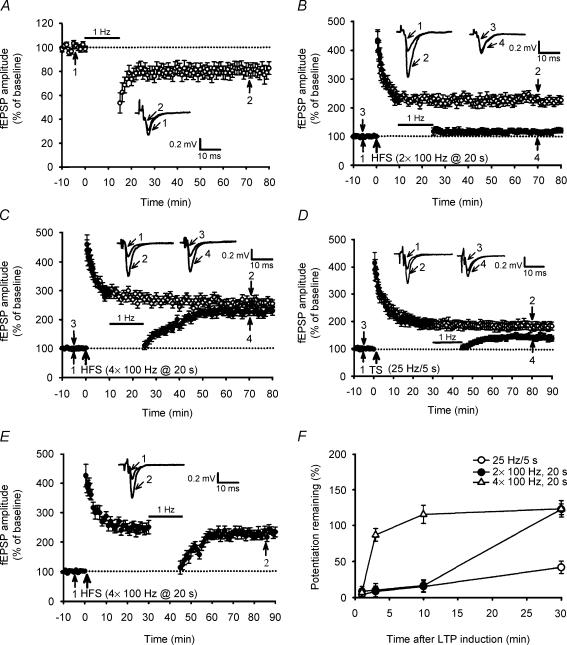
To test the possibility that the increase in the amount of synaptic stimulation may change the susceptibility of potentiated synapses to depotentiation stimulation, we first compared the effects of LFS (1 Hz/900 pulses) on the previously established LTP elicited by diverse patterns of HFS protocol. As reported previously (Kobayashi et al. 1996; Domenici et al. 1998; Tzounopoulos et al. 1998; Chen et al. 2001), at naive synapses 1 Hz/900 pulse stimulation of mossy fibre inputs resulted in a significant LTD of fEPSP (Fig. 1A). On average, the amplitude of fEPSP measured 40 min after the end of 1 Hz stimulation was 75.9 ± 5.7% of baseline (n = 8; P < 0.05; Student's paired t test). When LFS was applied, beginning at 10 min after two trains of 100 Hz LTP induction, LTP was almost completely reversed. The residual potentiation measured 40 min after the end of LFS was 121.5 ± 7.5% (n = 8) of baseline, which was significantly less than the corresponding value of slices receiving HFS only (225 ± 15%; n = 7; P < 0.01) (Fig. 1B). In contrast, adding two additional trains of HFS blocked the development of LFS-induced depotentiation (LFS-DEP). Four trains of 100 Hz stimulation, delivered with an interval of 20 s, induced a robust LTP with mean fEPSP amplitude value of 258 ± 15% of baseline (n = 8; P < 0.05; Student's paired t test), measured 50 min after HFS. However, levels of potentiation in slices receiving four trains of 100 Hz HFS and in slices receiving two trains of 100 Hz HFS were not statistically different (P = 0.14). When 1 Hz LFS was applied, beginning at 10 min after LTP induction, the extent of depotentiation was markedly reduced (Fig. 1C). The mean residual potentiation measured 40 min after the end of LFS was 226 ± 14% (n = 8; P = 0.13 when compared with the corresponding value of slices that received HFS only) of baseline. We next asked whether a reduction in the amount of stimulation can promote depotentiation of the synapses. To test this idea, we used an LTP protocol consisting of a single tetanus (25 Hz for 5 s) and examined the expression of LFS-DEP (Fig. 1D). The mean magnitude of the LTP induced by a 25 Hz protocol, measured 50 min after induction, was 183 ± 12% (n = 7; P = 0.01) of baseline, which was significantly less LTP than for the two trains of 100 Hz and the susceptibility of these two forms of LTP was a little different. Application of LFS, beginning at 30 min after 25 Hz LTP induction, revealed a significant reversal of LTP. The residual potentiation measured 40 min after the end of LFS was 143.5 ± 8.6% of baseline (n = 6; P < 0.01), which was significantly less than the corresponding value of slices that received HFS only. In contrast, the same LFS treatment protocol elicited no significant depotentiation of four trains of 100 Hz LTP (Fig. 1E). The residual potentiation measured 40 min after the end of LFS was 236 ± 15% of baseline (n = 6; P = 0.37), which was not statistically different from the corresponding value of slices receiving HFS only. A summary of all the experiments examining the time-dependent effect of LFS on the magnitude of LTP expression induced by three different LTP induction protocols is shown in Fig. 1F and confirms that the extent of LFS-DEP is inversely related to the interval between LTP induction and delivery of LFS. These results also demonstrate that a close correspondence exists between the amount of stimulation used to induce LTP and the susceptibility of LTP to depotentiation stimulation.
Figure 1. The reversal of long-term potentiation (LTP) by low-frequency stimulation (LFS) is activity and time dependent.
A, summary of experiments (n = 8) showing that LFS at 1 Hz for 15 min elicits long-term depression (LTD). B, summary of experiments showing that LFS given 10 min after two trains of 100 Hz high-frequency stimulation (HFS) almost completely reversed LTP (n = 8; •), whereas fEPSPs in slices that received HFS without LFS exhibited persistent potentiation (n = 7; ○). C, summary of experiments showing that four trains of 100 Hz HFS induced a stable LTP (n = 8; ○). Application of LFS at 10 min after HFS initially depressed fEPSPs that subsequently recovered to previously potentiated levels (n = 8; •). D, summary of experiments showing that LFS given 30 min after a single tetanus (25 Hz for 5 s) revealed a significant reversal of LTP (n = 6; •). Field EPSPs in slices that received HFS without LFS exhibited persistent potentiation (n = 7; ○). E, summary of experiments showing that LFS given 30 min after four trains of 100 Hz HFS did not reveal a significant reversal of LTP (n = 6; •). F, summary data comparing the effects of LFS given at 1, 3, 10 and 30 min after LTP induction by three different stimulation protocols. The magnitude of potentiation remaining was calculated at 40 min after the end of LFS. In A–F, each value is the mean ± s.e.m. of independent determinations in six to eight slices. The superimposed fEPSP in the inset of each graph illustrates respective recordings from example experiments taken at the time indicated by number. Upward arrows indicate application of HFS. The horizontal bars denote the period of delivery of 1 Hz LFS. The horizontal dotted lines indicate the average value of the normalized amplitude during the control period.
Presynaptic local protein synthesis confers synaptic resistance to LFS-DEP
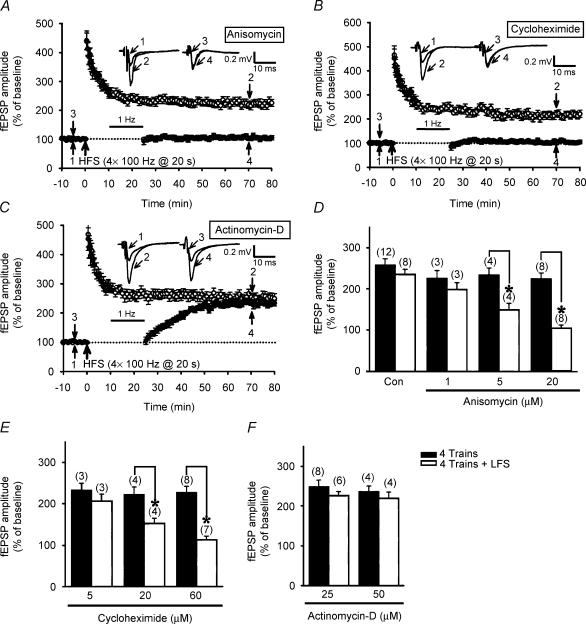
At the hippocampal Schaffer collateral–CA1 synapses, it has recently been shown that local protein synthesis may confer synaptic resistance to depotentiation stimulation at previously potentiated synapses (Woo & Nguyen, 2003). To investigate whether such a modulation is also present at the mossy fibre–CA3 synapses, we tested the effect of two structurally different translational inhibitors, anisomycin and cycloheximide, on the development of LFS-DEP. We found that anisomycin and cycloheximide permitted the induction of LFS-DEP of four trains of 100 Hz LTP in a dose-dependent manner, which was not normally seen in control slices. Preincubation of both 5 μm and 20 μm anisomycin for 60 min promoted the susceptibility of synapses to depotentiation (Fig. 2A and D). The mean residual potentiation measured 40 min after the end of LFS was 149 ± 15% (n = 4; P = 0.014) and 105.3 ± 7.5% (n = 8; P < 0.001) of baseline, respectively, which was statistically less than the corresponding values of anisomycin-treated slices receiving HFS only (5 μm: 235 ± 17% of baseline; n = 4; 20 μm: 226 ± 15% of baseline; n = 8). Similarly, pretreatment of the slices with cycloheximide (20 and 60 μm) for 30–60 min also promoted the induction of LFS-DEP (Fig. 2B and E). In cycloheximide-pretreated slices, the mean residual potentiation measured 40 min after the end of LFS was 151 ± 13% (n = 4; P = 0.007) and 112.5 ± 8.7% (n = 7; P < 0.001) of baseline, respectively, which was statistically less than the corresponding values of cycloheximide-treated slices receiving HFS only (20 μm: 221 ± 18% of baseline; n = 4; 60 μm: 225 ± 14% of baseline; n = 8). In contrast, the induction of LTP by four trains of 100 Hz HFS was not significantly (P > 0.05) affected by either anisomycin or cycloheximide pretreatment (Fig. 2D and E). To examine the possible contribution of mRNA synthesis to the synaptic resistance to LFS-DEP, we tested the effect of the transcriptional inhibitor actinomycin-D on LFS-DEP. In contrast to the translational inhibitors, actinomycin-D (25 or 50 μm) pretreatment for 30–60 min had no effect on the development of either LTP or the synaptic resistance to LFS-DEP (Fig. 2C and F). On average, the residual potentiation measured 40 min after the end of LFS was 229 ± 11% (n = 6; P = 0.28) and 219 ± 16% of baseline (n = 4; P = 0.36), which was not statistically different from the mean value measured from actinomycin-D-treated slices that received HFS only (25 μm: 248 ± 17% of baseline; n = 8; 50 μm: 236 ± 15% of baseline; n = 4). Moreover, treatment with actinomycin-D (25 or 50 μm) alone failed to affect the induction of LTP by four trains of 100 Hz HFS (P > 0.05). These results suggest that local protein synthesis initiated immediately after tetanus, without previous transcription, can confer synaptic resistance to depotentiation.
Figure 2. The development of synaptic resistance to depotentiation stimulation is dependent on protein synthesis but not mRNA synthesis.
A, summary of experiments showing that preincubation of slices with the protein synthesis inhibitor anisomycin (20 μm; 60 min) permitted persistent LFS-induced depotentiation (LFS-DEP) of four trains of 100 Hz LTP (n = 8; •) but had no effect on the induction of LTP (n = 8; ○). B, summary of experiments showing that preincubation of slices with another protein synthesis inhibitor, cycloheximide (60 μm; 30–60 min), also permitted persistent LFS-DEP of four trains of 100 Hz LTP (n = 7; •) but had no effect on the induction of LTP (n = 8; ○). C, summary of experiments showing that preincubation of slices with transcriptional inhibitor, actinomycin-D (25 μm; 30–60 min), did not affect the induction of either the synaptic resistance to LFS-DEP (n = 6; •) or LTP (n = 8; ○). D, summary histogram comparing the effects of different concentrations of anisomycin on the induction of four trains of 100 Hz LTP (filled columns) and LFS-DEP (open columns). E, summary histogram comparing the effects of different concentrations of cycloheximide on the induction of four trains of 100 Hz LTP (filled columns) and LFS-DEP (open columns). F, summary histogram comparing the effects of different concentrations of actinomycin-D on the induction of four trains of 100 Hz LTP (filled columns) and LFS-DEP (open columns). The magnitude of LTP was calculated at 50 min after HFS and the magnitude of LFS-DEP was calculated at 40 min after the end of LFS. In D–F the numbers in parentheses indicate the number of slices tested. Asterisks represent a significant difference compared with slices not receiving LFS.
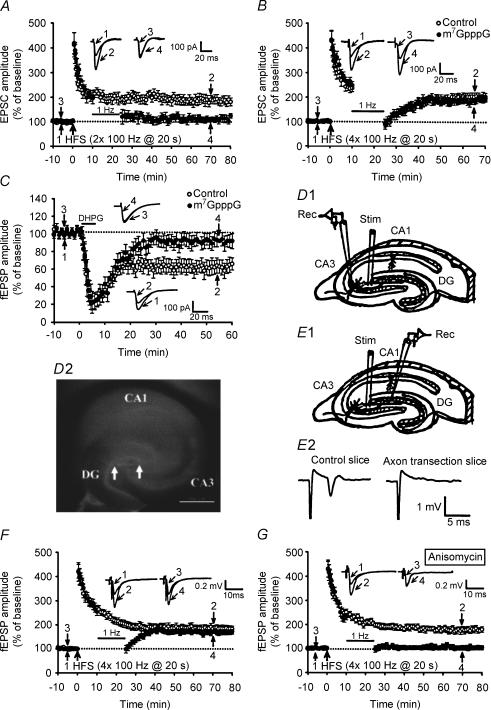
To determine whether a pre- or postsynaptic protein synthesis process is required for the development of synaptic resistance to LFS-DEP, we performed additional whole-cell patch clamp recording experiments. To strengthen the tie between the field potential and whole-cell experiments, we have performed a set of experiments with two trains of 100 Hz HFS, delivered with an interval of 20 s, using whole-cell recordings. Similar to the results described above for fEPSPs, when LFS was applied, beginning 10 min after LTP induction, the LTP of EPSCs was almost completely reversed (Fig. 3A). The residual potentiation measured 40 min after the end of LFS was 112 ± 11% (n = 4; P < 0.01) of baseline, which was significantly less than the corresponding value of slices receiving HFS only (185 ± 16%; n = 6).
Figure 3. Presynaptic local protein synthesis is required for the development of synaptic resistance to depotentiation stimulation.
A, summary of experiments showing that LFS given 10 min after two trains of 100 Hz HFS almost completely reversed LTP (n = 4; •), whereas EPSCs in slices that received HFS without LFS exhibited persistent potentiation (n = 6; ○). Whole-cell patch clamp recordings were made of CA3 pyramidal cells at a holding potential of −70 mV. B, summary of experiments showing that the synaptic resistance to LFS-DEP was not affected by postsynaptic injection of the cap analogue m7 GpppG. Whole-cell patch clamp recordings were made of CA3 pyramidal cells at a holding potential of −70 mV. When LFS was applied 10 min after four trains of 100 Hz HFS in CA3 pyramidal cells loaded with m7 GpppG (250 μm) (n = 6; •), transient synaptic depression was seen, but EPSCs recovered to potentiated levels not significantly different those seen in control cells without m7 GpppG (n = 6; ○). C, summary of experiments showing that CA1 LTD induced by DHPG (50 μm, 5 min) was prevented by postsynaptic injection of m7 GpppG (250 μm) (n = 6; •). Whole-cell patch clamp recordings were made of CA1 pyramidal cells at a holding potential of −70 mV. D1, schematic diagram showing the incision (thick black line) made to transect mossy fibre axons from the granule cell somata and the positions of recording (Rec) and stimulation (Stim) electrodes. D2, bright-field image of a hippocampal slice after the isolation of mossy fibre axons from the granule cell somata. Arrows indicate the site of lesion. Scale bar, 500 μm. E1, schematic diagram showing a mossy fibre axon transection slice, where an electrical stimulus was delivered to the stratum lucidum of the CA3 region and the evoked antidromic population spikes were recorded from the granulosum of the dentate gyrus (DG). E2, absence of antidromic population spikes in stratum granulosum of the dentate gyrus when stimulation was applied at the stratum lucidum of the CA3 region in the mossy fibre axon transection slice, whereas an antidromic population spike was successfully evoked in control slices. F, summary of experiments showing that mossy fibre axon transection had no effect on either the synaptic resistance to LFS-DEP (n = 6; •) or the induction of LTP (n = 8; ○). G, summary of experiments showing that preincubation of mossy fibre axon transection slices with protein synthesis inhibitor anisomycin (20 μm; 60 min) permitted persistent LFS-DEP of four trains of 100 Hz LTP (n = 6; •) but had no effect on the induction of LTP (n = 6; ○).
Is a postsynaptic protein synthesis process required for the development of synaptic resistance to LFS-DEP? To answer this question, CA3 pyramidal cells were loaded with the mRNA cap analogue m7GpppG to inhibit protein synthesis by competing with endogenous capped mRNA for the cap-binding brain protein eIF-4E (Gingras et al. 1999). Cells were dialysed for at least 30 min before HFS application to ensure complete dialysis of m7GpppG into the cell. In m7GpppG (250 μm)-loaded cells, application of LFS at 10 min after four trains of 100 Hz HFS only elicited an initial synaptic depression followed by a recovery of EPSC to potentiated levels (Fig. 3B). The mean EPSC amplitude measured 40 min after the end of LFS was 196 ± 12% of baseline (n = 6; P = 0.43), which was not significantly different from those of control cells without m7GpppG (203 ± 14% of baseline; n = 6). It may be argued that the m7GpppG treatment protocol used in the present study may not be enough to abolish the postsynaptic protein synthesis process. At the Schaffer collateral–CA1 synapses, it has been shown that the induction of metabotropic glutamate receptor-dependent LTD by DHPG can be blocked by the loading of m7GpppG into CA1 pyramidal cells (Huber et al. 2000). If the m7GpppG-pretreatment protocol used in the present study can effectively block postsynaptic protein synthesis, it should also be expected to block the induction of DHPG-induced LTD in the hippocampal CA1 region. As shown in Fig. 3C, although the acute depression of synaptic transmission was still observed, stable LTD failed to occur in response to DHPG in cells loaded with m7GpppG (250 μm). In contrast, control cells without m7GpppG elicited a stable LTD following DHPG application. Thus, presynaptic protein synthesis appears to be required for the development of synaptic resistance to depotentiation stimulation at the mossy fibre–CA3 synapses.
Our findings that presynaptic protein synthesis may be critical in regulating the susceptibility of potentiated synapses to LFS-DEP raised an immediate question of whether granule cell somata contribute to this process, because the major protein synthesis sites are cell bodies. To address this question, we transected the mossy fibre axons near the granule cell bodies at least 2 h before transferring to the chamber for recordings (Fig. 3D1 and D2). We confirmed the complete dissociation of mossy fibre axons from granule cell bodies by placing a recording electrode in the stratum granulosum of the dentate gyrus to record antidromic population spikes evoked by stimulating the stratum lucidum of the CA3 region (Fig. 3E1). In no such instances did we detect a population spike (Fig. 3E2). These cut slices exhibited normal synaptic transmission, paired-pulse facilitation and frequency facilitation when compared with the control slices. For example, the ratio of fEPSPs in cut slices versus control slices was ∼1 for any given presynaptic strength (n = 6). The paired-pulse ratio calculated from the response to paired-pulse stimulation with an interval of 40 ms in cut and control slices was 2.2 ± 0.4 (n = 6; P = 0.45) and 2.0 ± 0.3 (n = 6), respectively. In addition, when the stimulation frequency was increased from 0.05 to 1 Hz for 1 min, there was a 238 ± 32% (n = 6; P = 0.36) increase in the fEPSP amplitude in cut slices, which was not significantly different from those of control slices (251 ± 28%; n = 6). When four trains of 100 Hz HFS was applied to the cut slices, a robust LTP was elicited. On average, the amplitude of fEPSP measured 50 min after LTP induction was 192 ± 14% of baseline (n = 6; P < 0.05; Student's paired t test) (Fig. 3F). When subsequent LFS was applied to these slices, there was an initial depression followed by a recovery of fEPSP to the potentiated state with a mean value of 178 ± 10% of baseline, measured 40 min after the end of LFS (n = 6; P = 0.32, when compared with cut slices that received HFS only) (Fig. 3F). Pretreatment of cut slices with anisomycin (20 μm) for 60 min permitted subsequent induction of LFS-DEP after four trains of 100 Hz HFS (Fig. 3G). The mean residual potentiation measured 40 min after the end of LFS was 105.7 ± 8.9% of baseline (n = 6; P < 0.01 when compared with control LFS-DEP cut slices). Moreover, the fEPSP amplitude measured 50 min after LTP induction was 185.6 ± 12% of baseline (n = 6) in anisomycin-pretreatment cut slices receiving four trains of 100 Hz HFS only. These results suggest that the integrity of communication between soma and terminals of the presynaptic granule cell may be not involved in regulating the susceptibility of potentiated synapses to LFS-DEP.
Synaptic resistance to LFS-DEP is input specific
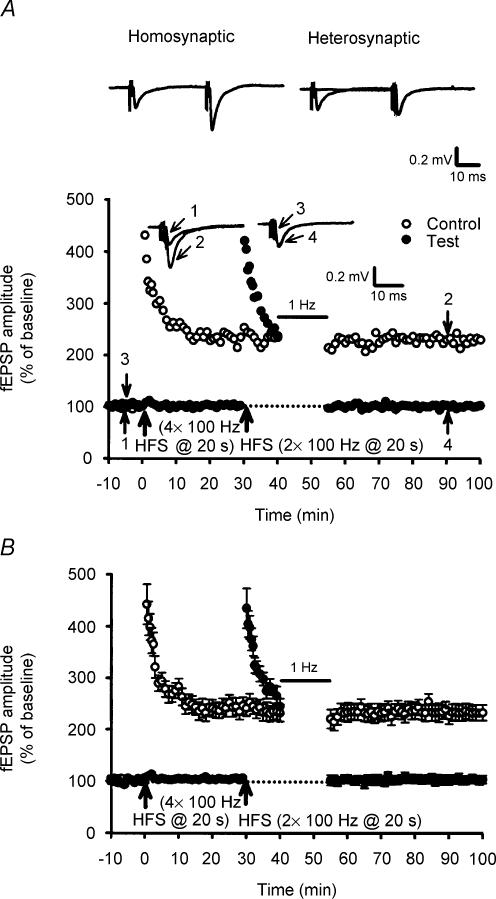
We next asked whether the synaptic resistance to depotentiation stimulation can be transferred between two independent pathways by examining whether stimulation of one pathway can block LFS-DEP in an adjacent pathway. To address this issue, two stimulatory electrodes were placed on both edges of the stratum granulosum of dentate gyrus to activate two group inputs to the same cell population. The independence of inputs activated by two stimulatory electrodes was assessed by verifying the absence of heterosynaptic facilitation between the two inputs using paired stimuli applied at interval of 30 ms (Fig. 4A). Having confirmed the independence of inputs activated, one (control) pathway received only four trains of 100 Hz HSF to induced synaptic resistance to LFS-DEP and the second (test) pathway received two trains of 100 Hz HFS followed 10 min later by LFS to test the inducibility of homosynaptic LFS-DEP. As a typical example illustrated in Fig. 4A, application of LFS to the test pathway almost completely reversed the established two trains of 100 Hz LTP. This phenomenon was observed in all six slices tested in this study. At the test pathway, the mean residual potentiation measured 40 min after the end of LFS was 108.5 ± 8.2% of baseline (n = 6) (Fig. 4B). These data indicate that synaptic resistance to LFS-DEP is input specific.
Figure 4. Synaptic resistance to depotentiation stimulation is input specific.
A, a typical example shows that two stimulating electrodes were used to activate two independent groups of afferent inputs. Field EPSPs were evoked by paired stimulations applied at 30 ms intervals to the first and/or second afferents. Paired-pulse facilitation was present when stimuli were applied twice to the same afferent (homosynaptic facilitation) but not when the stimuli were applied to different afferents (no heterosynaptic facilitation). Having confirmed the independence of afferent inputs activated, four trains of 100 Hz HFS were applied to one pathway (Control) to induce synaptic resistance to LFS-DEP. This treatment had no significant effect on the induction of depotentiation at a second adjacent pathway (Test) when LFS was applied at 10 min after two trains of 100 Hz HFS. B, summary of data from six experiments performed as in A.
GABAB receptors regulate synaptic resistance to LFS-DEP
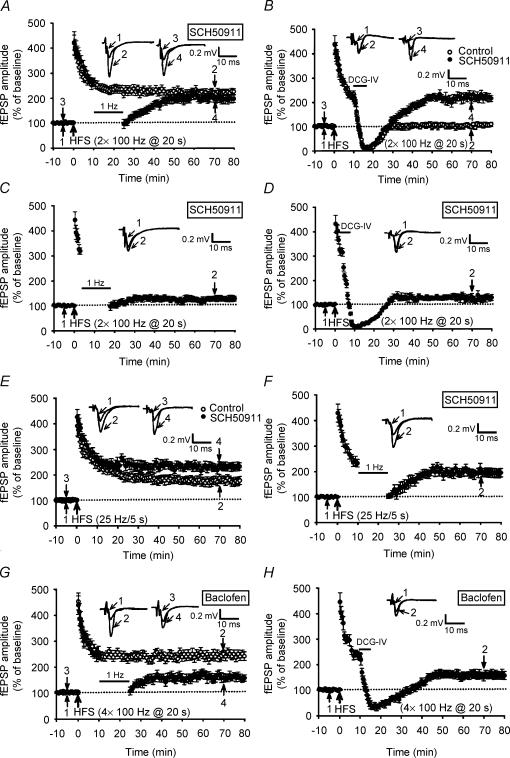
GABAergic inhibition is an important regulatory factor during the induction of LTP in the mossy fibre system (Vogt & Nicoll, 1999) and elsewhere (Davies et al. 1991; Paulsen & Moser, 1998) in the hippocampus. Regardless of the source of GABA release, a brief train of stimulation to mossy fibres and axons of GABAergic interneurones can evoke enough GABA release to activate presynaptic GABAB receptors and results in an increase in the threshold for mossy fibre LTP (Vogt & Nicoll, 1999). We therefore conducted a series of experiments to assess the influence of GABAB receptor inhibition in regulating the susceptibility of synapses to LFS-DEP. We found that the amount of LTP elicited by two trains of 100 Hz HFS was unaffected by the GABAB receptor antagonist SCH50911 (20 μm). The mean fEPSP amplitude measured 50 min after HFS was 226 ± 15% of baseline (n = 6; P = 0.36 when compared with control LTP slices). In contrast, SCH50911 treatment blocked the development of persistent LFS-DEP of two trains of 100 Hz LTP (Fig. 5A). The application of LFS, beginning at 10 min after LTP induction, initially depressed fEPSP amplitude that subsequently recovered to the potentiated level; the mean fEPSP amplitude measured 40 min after LFS was 198 ± 15% of baseline (n = 8; P > 0.28 when compared with the corresponding value of slices receiving HFS only). To further establish that GABAB receptor inhibition promotes synaptic resistance to LFS-DEP, attempts were made to see whether the induction of depotentiation by direct application of the selective group II metabotropic glutamate receptor agonist DCG-IV (Chen et al. 2001; Huang et al. 2002) was also blocked by SCH50911. As shown in Fig. 5B in control slices, similar to LFS application, a 5 min application of DCG-IV (3 μm), beginning at 10 min after LTP induction, almost completely reversed previously established synaptic potentiation (DCG-IV-DEP); i.e. the amplitude of fEPSP measured 40 min after washout of DCG-IV was 102.3 ± 9.2% of baseline (n = 6) (Fig. 5B). In contrast, SCH50911 treatment inhibited the DCG-IV-DEP in all seven slices tested. The mean fEPSP amplitude measured 40 min after washout of DCG-IV was 215 ± 18% of baseline (n = 7; P = 0.32 when compared with the corresponding value of slices that received HFS only). However, SCH50911 treatment failed to affect the induction of LFS-DEP and DCG-IV-DEP when LFS or DCG-IV was applied at 3 min after LTP induction (Fig. 5C and D). The mean fEPSP amplitude measured 40 min after the end of LFS and washout of DCG-IV was 125.3 ± 8.9% (n = 6; P < 0.01 when compared with the corresponding value of slices that received HFS only) and 139 ± 11% (n = 6; P < 0.01 when compared with the corresponding value of slices that received HFS only) of baseline, respectively. We also tested for the possible effects of GABAB receptor inhibition on the ability to induce LTP by a single tetanus of 25 Hz for 5 s. After tetanization of mossy fibre afferent pathways, the fEPSP amplitude showed a higher potentiation in SCH50911 treatment slices than those of control slices (Fig. 5E). The mean fEPSP amplitude measured 50 min after 25 Hz stimuli was 231 ± 16% (n = 7; P = 0.03) and 183 ± 15% of baseline (n = 6), respectively. In marked contrast to control slices, the application of LFS, 10 min after 25 Hz LTP induction, failed to reverse 25 Hz LTP (Fig. 5F); the mean fEPSP amplitude measured 40 min after the end of LFS was 189 ± 17% of baseline (n = 6; P = 0.24 when compared with the corresponding value of slices that received HFS only). These results suggest that the relief of GABAB receptor inhibition promotes the synaptic resistance to depotentiation stimulation at the mossy fibre–CA3 synapses.
Figure 5. GABAB receptor inhibition promotes the development of synaptic resistance to depotentiation stimulation.
A, summary of experiments showing that in slices treated with GABAB receptor antagonist SCH50911 (20 μm) the application of LFS at 10 min after two trains of 100 Hz HFS initially depressed fEPSPs that subsequently recovered to previously potentiated level (n = 8; •). B, summary of experiments showing that in contrast to control slices (n = 6; ○), a 5 min application of DCG-IV (3 μm) at 10 min after two trains of 100 Hz HFS failed to elicit a persistent depotentiation in SCH50911-treated slices (n = 7; •). C, summary of experiments showing that SCH50911 treatment did not significantly affect the induction of LFS-DEP of two trains of 100 Hz LTP when LFS was applied at 3 min after LTP induction (n = 6). D, summary of experiments showing that SCH50911 treatment had no effect on the induction of DCG-IV-DEP of two trains of 100 Hz LTP when DCG-IV was applied at 3 min after LTP induction (n = 6). E, summary of experiments showing that with 25 Hz/125 pulse stimulation of mossy fibre pathways, the fEPSP amplitude showed a higher potentiation in SCH50911-treated slices (n = 7; •) than in control slices (n = 6; ○). F, summary of experiments showing that in slices treated with the GABAB receptor antagonist SCH50911 (20 μm), application of LFS at 10 min after 25 Hz/125 pulse stimulation did not produce a persistent depotentiation (n = 6; •). G, summary of experiments showing that treatment of slices with baclofen (0.2 μm) permitted persistent LFS-DEP of four trains of 100 Hz LTP (n = 5; •) but had no effect on the induction of LTP (n = 5; ○). H, summary of experiments showing that treatment of slices with baclofen also permitted persistent DCG-IV-DEP of four trains of 100 Hz LTP (n = 5).
To test whether the direct activation of GABAB receptors was able to promote the susceptibility of synapses to depotentiation stimulation, we bath-applied the GABAB receptor agonist baclofen (0.2 μm) to hippocampal slices. Addition of baclofen, which depressed synaptic transmission by ∼60%, had no effect on the induction of four trains of 100 Hz LTP. The mean fEPSP amplitude measured 50 min after LTP induction in the presence of baclofen was 235 ± 16% (n = 5; P = 0.34), which was not statistically different from the corresponding values of control slices. In contrast to control slices, application of LFS, 10 min after LTP induction, successfully reversed the four trains of 100 Hz LTP in baclofen-treated slices (Fig. 5G). The mean fEPSP amplitude measured 40 min after the end of LFS was 154 ± 14% (n = 5; P = 0.012 when compared with the corresponding value of baclofen-treated slices that received HFS only). Furthermore, baclofen treatment also promoted the induction of DCG-IV-DEP (Fig. 5H). The mean fEPSP amplitude measured 40 min after washout of DCG-IV (3 μm) was 163 ± 18% (n = 5; P = 0.07 when compared with the corresponding value of baclofen-treated slices that received HFS only).
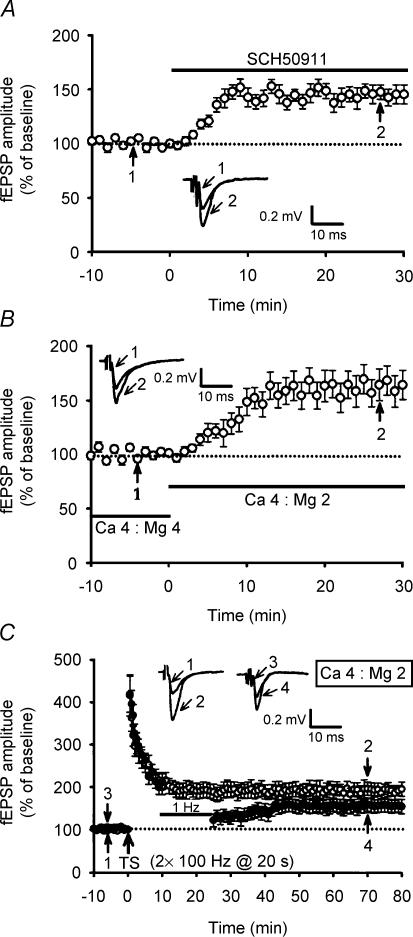
Because the application of SCH50911 caused a dramatic increase in the baseline response to 147.6 ± 8.7% (n = 5; P < 0.01; Student paired t test) of baseline (Fig. 6A), the enhancement resistance to depotentiation with SCH50911 probably arises as a consequence of the increase in glutamate release probability. To examine this possibility directly, we increased the Ca2+/Mg2+ ratio in aCSF from 4 mm/4 mm to 4 mm/2 mm. This increased the amplitude of fEPSP to 159 ± 14% (n = 10; P = 0.36) (Fig. 6B), comparable in magnitude to the enhancement by SCH50911. Under these conditions, two trains of 100 Hz HFS elicited a stable potentiation with a mean fEPSP amplitude of 189 ± 12% (n = 5; P < 0.01; Student's paired t test), measured 50 min after LTP induction. It should be noted that two trains of 100 Hz HFS elicited significantly less LTP in slices in a 4 mm/2 mm Ca2+/Mg2+ ratio aCSF than in slices in control 4 mm/4 mm Ca2+/Mg2+ ratio aCSF (P < 0.01). Similar to the results described above for SCH50911, after the application of 1 Hz LFS, beginning at 10 min after LTP induction, the extent of depotentiation was markedly reduced (Fig. 6C). The mean residual potentiation measured 40 min after the end of LFS was 158 ± 16% (n = 5; P = 0.15 when compared with the corresponding value of slices that received HFS only in 4 mm/4 mm Ca2+/Mg2+ ratio aCSF) of baseline. These data imply that an increase in transmitter release probability by blocking GABAB receptors may account, at least in part, for the enhancement effect of SCH50911 on the synaptic resistance to depotentiation stimulation.
Figure 6. An increase of the ratio of extracellular Ca2+ and Mg2+ concentration promotes the development of synaptic resistance to depotentiation stimulation.
A, summary of experiments showing that bath application of SCH50911 (20 μm) caused a significant increase in the amplitude of mossy fibre fEPSPs (n = 5). B, summary of experiments showing that an increase in the extracellular Ca2+/Mg2+ concentration ratio from 4 mm/4 mm to 4 mm/2 mm mimicked the effect of SCH50911 on baseline mossy fibre transmission (n = 10). C, summary of experiments showing that in 4 mm Ca2+ and 2 mm Mg2+ aCSF, application of LFS at 10 min after two trains of 100 Hz HFS initially depressed fEPSPs that subsequently recovered to previously potentiated level (n = 5; •). Field EPSPs in slices that received HFS without LFS exhibited persistent potentiation (n = 5; ○).
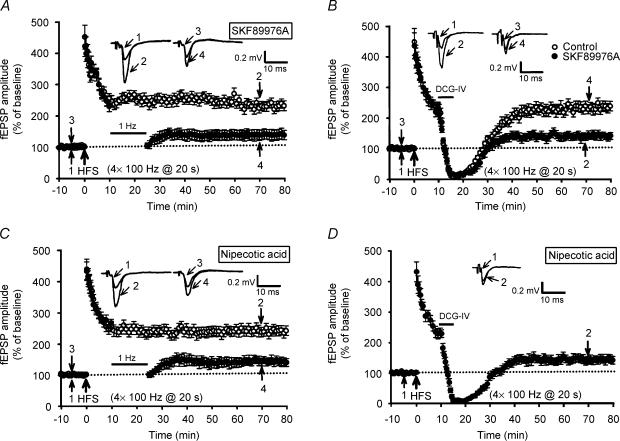
To further establish the role of GABA in regulating synaptic resistance to LFS-DEP, it is essential to demonstrate that LFS-DEP should be enhanced by the blockade of GABA uptake. For this purpose, the effects of specific GABA transport inhibitors SKF89976A and nipecotic acid on the induction of LFS-DEP were investigated (Borden et al. 1994; Stokes et al. 2001). Neither SKF89976A (50 μm) nor nipecotic acid (500 μm) treatment alone affected the basal synaptic transmission or the induction of four trains of 100 Hz LTP. The mean fEPSP amplitude measured 50 min after LTP induction in the presence of either SKF89976A or nipecotic acid was 251 ± 15% (n = 6; P = 0.63) and 238 ± 15% of baseline (n = 6; P = 0.37), respectively, which was not statistically different from the corresponding values of control slices. In marked contrast to control slices, application of LFS, 10 min after LTP induction, successfully reversed the four trains of 100 Hz LTP in both SKF89976A- and nipecotic acid-treated slices (Fig. 7A and C). The mean fEPSP amplitude measured 40 min after the end of LFS was 138 ± 16% (n = 6; P < 0.001 when compared with the corresponding value of SKF89976A-treated slices that received HFS only) and 129 ± 12% (n = 6; P < 0.001 when compared with the corresponding value of nipecotic acid-treated slices that received HFS only) of baseline, respectively. Furthermore, both SKF89976A and nipecotic acid treatments also promoted the induction of DCG-IV-DEP (Fig. 7B and D). The mean fEPSP amplitude measured 40 min after washout of DCG-IV (3 μm) was 137 ± 12% (n = 6; P < 0.001 when compared with the corresponding value of SKF89976A-treated slices that received HFS only) and 134 ± 14% (n = 6; P < 0.001 when compared with the corresponding value of nipecotic acid-treated slices that received HFS only) of baseline, respectively. These results suggest that GABA plays a role in mediating the delayed onset of synaptic resistance to LFS- and DCG-IV-DEP at mossy fibre–CA3 synapses.
Figure 7. GABA transport inhibitors delayed the development of synaptic resistance to depotentiation stimulation.
A, summary of experiments showing that treatment of slices with the GABA transport inhibitor SKF89976A (50 μm) permitted persistent LFS-DEP of four trains of 100 Hz LTP (n = 6; •) but had no effect on the induction of LTP (n = 6; ○). B, summary of experiments showing that a 5 min application of DCG-IV (3 μm) at 10 min after four trains of 100 Hz HFS failed to elicit a persistent depotentiation in control slices (n = 6; ○). In contrast, treatment of slices with SKF89976A (50 μm) permitted persistent DCG-IV-DEP of four trains of 100 Hz LTP (n = 6; •). C, summary of experiments showing that treatment of slices with another GABA transport inhibitor, nipecotic acid (500 μm), permitted persistent LFS-DEP of four trains of 100 Hz LTP (n = 6; •). Field EPSPs in slices that received HFS without LFS exhibited persistent potentiation (n = 6; ○). D, summary of experiments showing that treatment of slices with nipecotic acid (500 μm) also permitted persistent DCG-IV-DEP of four trains of 100 Hz LTP (n = 6).
Effect of a silent period on the synaptic resistance to depotentiation stimulation
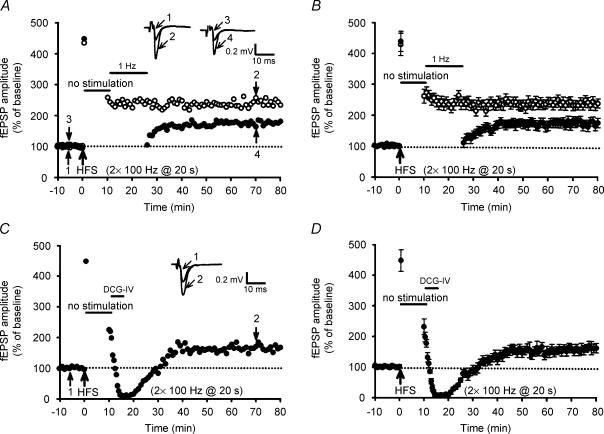
Mossy fibres themselves have been shown to contain GABA (Sloviter et al. 1996) and may contribute to the GABAergic inhibition in the hippocampal CA3 region. Indeed, application of GABAB receptor antagonists can cause a significant increase in mossy fibre baseline response (Vogt & Nicoll, 1999; Moore et al. 2003), suggesting that under basal low-frequency stimulation conditions, the released GABA potentially inhibits mossy fibre synaptic transmission by activating GABAB receptors. To determine whether the basal afferent stimulation immediately after the induction of LTP could evoke a sufficient amount of GABA release to influence the development of synaptic resistance to LFS- and DCG-IV-DEP, the basal afferent stimulation was interrupted for 10 min commencing 20 s after HFS. As in the typical example shown in Fig. 8A, the magnitude of LTP induced by two trains of 100 Hz HFS was not significantly affected by the interrupted basal afferent stimulation after HFS. The mean fEPSP amplitude measured 50 min after LTP induction was 236 ± 16% (n = 6; P = 0.27 when compared with control LTP slices) (Fig. 8B). In contrast, application of LFS or DCG-IV (3 μm), beginning at 10 min after LTP induction, initially depressed fEPSP amplitude that subsequently recovered to the potentiated level (Fig. 8A and C); the mean fEPSP amplitude measured 40 min after the end of LFS or washout of DCG-IV was 178 ± 13% (n = 6; P < 0.01 when compared with the corresponding value of control LFS-DEP slices) and 162 ± 15% (Fig. 8B and D) (n = 6; P < 0.01 when compared with the corresponding value of control LFS-DEP slices). These data are consistent with the GABAB receptor inhibition observations (Fig. 5) and imply that basal afferent stimulation may delay the onset of synaptic resistance to depotentiation stimulation through the release of GABA to activate GABAB receptors. However, the present data cannot exclude the possibility that other neurotransmitters released during basal synaptic stimulation may also contribute to the regulation of the susceptibility of synapses to depotentiation.
Figure 8. Stimulus interruption for 10 min immediately after the induction of LTP promotes the development of synaptic resistance to depotentiation stimulation.
A, a typical example showing that stimulus interruption for 10 min, commencing 20 s after HFS, prevented the induction of LFS-DEP when LFS was applied 10 min after two trains of 100 Hz HFS. B, summary of data from six experiments performed as in A. C, a typical example showing that stimulus interruption for 10 min commencing 20 s after HFS prevented the induction of DCG-IV-DEP (3 μm for 5 min) when LFS was applied 10 min after two trains of 100 Hz HFS. D, summary of data from six experiments performed as in C.
Discussion
Mechanisms underlying depotentiation are presumably the reversal of cellular changes associated with LTP induction (Huang & Hsu, 2001; Zhou & Poo, 2004). Although the molecular mechanisms responsible for mossy fibre LTP have not been well characterized, it is widely believed that mossy fibre LTP is triggered by a tetanus-induced rise in presynaptic Ca2+ that results in the activation of a Ca2+–calmodulin-dependent adenylyl cyclase, which in turn causes an increase in presynaptic cAMP levels and activation of protein kinase A that, via mechanisms perhaps involving Rab3A and its interacting protein, RIM1α, causes a long-lasting enhancement of transmitter release (Huang et al. 1994; Weisskopf et al. 1994; Castillo et al. 2002). Our previous work has revealed several common features of mossy fibre depotentiation and has provided evidence that it is expressed presynaptically through the activation of both group II mGluRs and protein phosphatase-coupled signalling cascades (Chen et al. 2001; Huang & Hsu, 2001). However, the factors that critically regulate the susceptibility of mossy fibre LTP to depotentiation stimulation have not been identified. Besides the confirmation of our previous work demonstrating that the extent of depotentiation is inversely related to the interval between LTP induction and the delivery of depotentiation stimulation, the present study provides four new insights into the molecular mechanisms that critically regulate the susceptibility of potentiated synapses to depotentiation. First, multiple trains of HFS can provide an immediate synaptic resistance to depotentiation stimulation. Second, the development of synaptic resistance to depotentiation stimulation is dependent on the amount of synaptic stimulation used to induce LTP and is input specific. Third, the synaptic resistance to depotentiation stimulation is strongly dependent on presynaptic protein synthesis but not on RNA synthesis. Finally, GABAB receptor inhibition or stimulus interruption immediately after LTP induction stimulation promotes the onset of synaptic resistance to depotentiation stimulation. Together, our data provide strong support for the idea that the synaptic resistance to depotentiation stimulation is achieved by local protein synthesis and can be regulated by GABAB receptor activation.
The activity commonly found to effectively induce mossy fibre LTP is brief or long trains of HFS (Henze et al. 2000; Kakegawa et al. 2002). The results of the present study demonstrate that the amount of imposed synaptic stimulation is an important factor in determining the time window of the susceptibility of potentiated synapses to depotentiation stimulation. The synaptic resistance to depotentiation stimulation is clearly evident when stronger stimulation protocols, such as multiple trains of 100 Hz, are used to induce LTP. In contrast, a weaker (25 Hz for 5 s) stimulation protocol is less effective at eliciting the synaptic resistance to depotentiation stimulation (Fig. 1D). An intriguing observation made in the present study is that the addition of two trains of 100 Hz HFS (in changing from a two trains to a four trains of tetanus protocol) was sufficient to convert LTP from a form that was vulnerable to depotentiation stimulation to one that was resistant to depotentiation stimulation (Fig. 1C). Although we prefer to interpret this finding as a result of accelerating the expression of new protein necessary for the consolidation processes of mossy fibre LTP, it is possible that additional synaptic activity imposed during four trains of 100 Hz HFS may recruit other signalling pathways leading to synaptic resistance to depotentiation stimulation. However, this possibility is unlikely, because application of anisomycin and cycloheximide, both inhibitors of protein synthesis, enabled depotentiation of four trains of 100 Hz LTP without affecting the magnitude of LTP (Fig. 2A and B) and because interrupting basal afferent stimulation for a certain time period after LTP induction was sufficient to convert two trains of 100 Hz LTP from a susceptible state into a resilient state that is resistant to depotentiation stimulation (Fig. 8). Our results clearly demonstrate an early role of local protein synthesis in promoting synaptic resistance to depotentiation stimulation at the mossy fibre–CA3 synapses. However, our results cannot exclude the possibility that the protein required for the initial development of potentiated synapse resistance to depotentiation stimulation is constitutively present and maintained critically at a level required for LTP consolidation by an unusually high turnover rate against fast degradation. When translational inhibitors block protein synthesis, the level of protein would decrease, resulting in blockade of synaptic resistance to depotentiation stimulation. It remains to be elucidated how conditioning tetanization interacts with the protein synthesis process in consolidating synaptic modifications.
An important question that arises from this study is whether pre- or postsynaptic protein synthesis is required for the development of synaptic resistance to depotentiation stimulation. The present result that loading postsynaptic CA3 cells with mRNA cap analogue had no effect on synaptic resistance to LFS-DEP (Fig. 3B) strongly supports the view that presynaptic protein synthesis contributes to the conversion of the initial synaptic potentiation into a persistent and not disrupted state. Traditionally, it has been thought that new proteins are synthesized in the cell body of the neurones and transported to the synapses or nerve terminals. In the present study, the synaptic resistance to LFS-DEP in slices with transected granule cell somas was comparable with that measured in slices with intact mossy fibre axons (Fig. 3F). It is therefore possible that the synthesis of proteins necessary for the development of synaptic resistance to depotentiation stimulation takes place solely in the presynaptic nerve terminal or axonal compartment.
Recently, Calixto et al. (2003) have reported, in contrast to our results, that the application of protein synthesis inhibitors before HFS blocked mossy fibre LTP. The reason for this discrepancy is unclear but could be attributable partly to the use of different animal species (4- to 5-week-old B57BL/6 mice versus 90–120 g Sprague-Dawley rats) and the methods used to induce LTP (two or four trains of 100 Hz HFS at 20 s intervals versus three trains of 100 Hz HFS at 10 s intervals), resulting in the activation of different cellular processes that may vary in their mode of action. However, our results are generally in agreement with the previous findings of Huang & Kandel (1994) and Nguyen & Kandel (1996), who demonstrated that the early maintenance phase of mossy LTP is independent of protein synthesis.
As in the case for mossy fibre LTP (Higashima & Yamamoto, 1985; Zalutsky & Nicoll, 1992), the synaptic resistance to depotentiation stimulation exhibits strong input specificity, because the expression of this phenomenon is restricted to the activated inputs and not non-activated inputs, although they terminated onto the same postsynaptic neurones (Fig. 4). Although the identities of the presynaptic proteins required for synaptic resistance to depotentiation stimulation remain to be elucidated, our present study clearly demonstrates that the candidate proteins should be rapidly upregulated within a few minutes of the cessation of LTP-inducing stimulation and synthesized from local constitutive mRNA, and that their synthesis does not require basal afferent stimulation. There is increasing evidence that the localization of translation machinery and mRNAs in axonal and presynaptic compartments endows individual axons or nerve endings with the capability to independently control synaptic strength through the local synthesis of proteins (Giuditta et al. 2002). To date, HFS has been shown to effectively induce an increase in expression of multiple proteins involved in vesicular neurotransmitter release, such as synapsin I, synaptotagmin, and synaptophysin (Lynch et al. 1994; Hicks et al. 1997; Sato et al. 2000). It is therefore possible that the rapid synthesis of these or other unidentified presynaptic proteins is involved in the development of synaptic resistance to depotentiation stimulation at the mossy fibre–CA3 synapses. Further work, involving the use of function knockouts of candidate proteins, is required to assess the potential roles of specific candidate proteins required for conferring the synaptic resistance to depotentiation stimulation.
The importance of GABAB receptors in homosynaptic and heterosynaptic depression of glutamate release has been extensively investigated at the mossy fibre–CA3 synapses, where it may provide an additional mechanism that further increases the sparseness of the input signal and thereby enhance the storage capacity of the CA3 network (Vogt & Nicoll, 1999). Recent evidence has further indicated that the loss of GABAB receptor-mediated synaptic modulation at the mossy fibres may contribute to the development of spontaneous seizures after status epilepticus (Chandler et al. 2003). In the present study, we extend these earlier findings and demonstrate that synaptically released GABA by afferent stimulation may activate GABAB receptors, thereby delaying the onset of stabilization processes set in motion during LTP induction. GABAB receptors are present at both presynaptic and postsynaptic sites within the hippocampal CA3 area (Fritschy et al. 1999). Blockade of GABAB receptors may produce different effects on glutamatergic transmission depending on the receptor location in the circuit. For example, if the antagonist primarily blocks presynaptic GABAB receptors, synaptic transmission would be enhanced (Vogt & Nicoll, 1999). In the present study, we observed that SCH50911 application caused an enhancement of basal synaptic transmission and promoted the synaptic resistance to depotentiation, which is consistent with the presynaptic site of action. However, the present data cannot exclude other sources where GABAB receptors may also contribute to the regulation of synaptic susceptibility to depotentiation, such as possible postsynaptic and network effects. What could be the underlying mechanisms for the influence of GABAB receptor activation on the development of synaptic resistance to depotentiation stimulation? One potential possibility is that the released GABA acting on GABAB receptors may suppress neurotransmitter release probability, engaging the potentiated synapses to a state more responsible to depotentiation stimulation. A prediction of this hypothesis, which we have confirmed, is that the increase of glutamate release by increasing the Ca2+/Mg2+ ratio of aCSF mimicked the GABAB receptor antagonist SCH50911 effect of promoting the synaptic resistance to depotentiation (Fig. 7C). However, we could not exclude the possibility that activation of GABAB receptors may directly or indirectly interfere with the protein synthesis process that is critical for establishing the synaptic resistance to depotentiation stimulation. Another straightforward prediction is that the GABAB receptor antagonist SCH50911 inhibits the reversal process itself. However, this prediction is unlikely, because the development of LFS- and DCG-IV-DEP was unchanged when they were delivered at 3 min after LTP induction (Fig. 6C and D). In addition, we have shown that stronger LTP induction protocols, such as four trains of 100 Hz HFS, presumably overcome the effects of GABAB receptors and stabilize synaptic modifications. Although our observations strongly suggest that GABAB receptor activation plays an important role in the regulation of synaptic susceptibility to depotentiation at mossy fibre–CA3 synapses, it should also be mentioned that Wagner & Alger (1995) reported a lack of GABAB receptor effect on depotentiation at Schaffer collateral–CA1 synapses.
In conclusion, we have provided evidence that presynaptic local protein synthesis mediates input-specific synaptic resistance to depotentiation stimulation at the mossy fibre–CA3 synapses and that the onset of this synaptic resistance was delayed if the GABAB receptors were activated by basal afferent stimulation. To the extent that LTP represents the cellular correlate of memory encoding and storage, the processes involved in the regulation of synaptic susceptibility to depotentiation stimulation may be important for controlling the neural threshold for the consolidation of long-term memory (Huang & Hsu, 2001; Woo & Nguyen, 2003).
Supplementary Material
Acknowledgments
This work was financially supported by a research grant from the National Health Research Institute (NHRI-EX92-9215NI) of Taipei, Taiwan.
Supplementary material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2004.072546
http://jp.physoc.org/cgi/content/full/jphysiol.2004.072546/DC1
and contains a supplementary figure and legend entitled:
An example of pharmacological characterization of mossy fibre synaptic responses.
References
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Calixto E, Thiels E, Klann E, Barrionuevo G. Early maintenance of hippocampal mossy fiber – long-term potentiation depends on protein and RNA synthesis and presynaptic granule cell integrity. J Neurosci. 2003;23:4842–4849. doi: 10.1523/JNEUROSCI.23-12-04842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1α is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Chandler KE, Princivalle AP, Fabian-Fine R, Bowery NG, Kullmann DM, Walker MC. Plasticity of GABAB receptor-mediated heterosynaptic interactions at mossy fibers after status epilepticus. J Neurosci. 2003;23:11382–11391. doi: 10.1523/JNEUROSCI.23-36-11382.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Huang CC, Hsu KS. Time-dependent reversal of long-term potentiation by low-frequency stimulation at the hippocampal mossy fiber-CA3 synapses. J Neurosci. 2001;21:3705–3714. doi: 10.1523/JNEUROSCI.21-11-03705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Domenici MR, Berretta N, Cherubini E. Two distinct forms of long-term depression coexist at the mossy fiber-CA3 synapse in the hippocampus during development. Proc Natl Acad Sci U S A. 1998;95:8310–8315. doi: 10.1073/pnas.95.14.8310. 10.1073/pnas.95.14.8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Fujii S, Saito K, Miyakawa H, Ito K, Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 1991;555:112–122. doi: 10.1016/0006-8993(91)90867-u. 10.1016/0006-8993(91)90867-U. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Giuditta A, Kaplan BB, van Minnen J, Alvarez J, Koenig E. Axonal and presynaptic protein synthesis: new insights into the biology of the neuron. Trends Neurosci. 2002;25:400–404. doi: 10.1016/s0166-2236(02)02188-4. 10.1016/S0166-2236(02)02188-4. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. 10.1016/S0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Hicks A, Davis S, Rodger J, Helme-Guizon A, Laroche S, Mallet J. Synapsin I and syntaxin 1B: key elements in the control of neurotransmitter release are regulated by neuronal activation and long-term potentiation in vivo. Neuroscience. 1997;79:329–340. doi: 10.1016/s0306-4522(96)00700-2. 10.1016/S0306-4522(96)00700-2. [DOI] [PubMed] [Google Scholar]
- Higashima M, Yamamoto C. Two components of long-term potentiation in mossy fiber-induced excitation in hippocampus. Exp Neurol. 1985;90:529–539. doi: 10.1016/0014-4886(85)90150-5. 10.1016/0014-4886(85)90150-5. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chen YL, Liang YC, Hsu KS. Role for cAMP and protein phosphatase in the presynaptic expression of mouse hippocampal mossy fibre depotentiation. J Physiol. 2002;543:767–778. doi: 10.1113/jphysiol.2002.025668. 10.1113/jphysiol.2002.025668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Progress in understanding the factors regulating reversibility of long-term potentiation. Rev Neurosci. 2001;12:51–68. doi: 10.1515/revneuro.2001.12.1.51. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Hsu KS. A role for extracellular adenosine in time-dependent reversal of long-term potentiation by low-frequency stimulation at hippocampal CA1 synapses. J Neurosci. 1999;19:9728–9738. doi: 10.1523/JNEUROSCI.19-22-09728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Kakegawa W, Yamada N, Iino M, Kameyama K, Umeda T, Tsuzuki K, Ozawa S. Postsynaptic expression of a new calcium pathway in hippocampal CA3 neurons and its influence on mossy fiber long-term potentiation. J Neurosci. 2002;22:4312–4320. doi: 10.1523/JNEUROSCI.22-11-04312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Manabe T, Takahashi T. Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- Larson J, Xiao P, Lynch G. Reversal of LTP by theta frequency stimulation. Brain Res. 1993;600:97–102. doi: 10.1016/0006-8993(93)90406-d. 10.1016/0006-8993(93)90406-D. [DOI] [PubMed] [Google Scholar]
- Lynch MA, Voss KL, Rodriguez J, Bliss TV. Increase in synaptic vesicle proteins accompanies long-term potentiation in the dentate gyrus. Neuroscience. 1994;60:1–5. doi: 10.1016/0306-4522(94)90197-x. 10.1016/0306-4522(94)90197-X. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–176. doi: 10.1016/s0896-6273(03)00368-4. 10.1016/S0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2003;100:14397–14402. doi: 10.1073/pnas.1835831100. 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. 10.1016/0896-6273(92)90248-C. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen O, Moser EI. A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci. 1998;21:273–278. doi: 10.1016/s0166-2236(97)01205-8. 10.1016/S0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- Popik P, Nalepa I, Mamczarz J, Vetulani J. Retrieval associated cholinergic activity and its inhibition by memory updating. Life Sci. 1994;54:1251–1257. doi: 10.1016/0024-3205(94)00852-3. 10.1016/0024-3205(94)00852-3. [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Morimoto K, Suemaru S, Sato T, Yamada N. Increased synapsin I immunoreactivity during long-term potentiation in rat hippocampus. Brain Res. 2000;872:219–222. doi: 10.1016/s0006-8993(00)02460-4. 10.1016/S0006-8993(00)02460-4. [DOI] [PubMed] [Google Scholar]
- Siegelbaum SA, Kandel ER. Learning-related synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1991;1:113–120. doi: 10.1016/0959-4388(91)90018-3. 10.1016/0959-4388(91)90018-3. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.3.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Staübli U, Chun D. Factors regulating the reversibility of long-term potentiation. J Neurosci. 1996;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staübli U, Chun D, Lynch G. Time-dependent reversal of long-term potentiation by an integrin antagonist. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes AH, Bernard LP, Nicklas WJ, Zeevalk GD. Attenuation of malonate toxicity in primary mesencephalic cultures using the GABA transport blocker, NO-711. J Neurosci Res. 2001;64:43–52. doi: 10.1002/jnr.1052. 10.1002/jnr.1052. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Janz R, Sudhof TC, Nicoll RA, Malenka RC. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron. 1998;21:837–845. doi: 10.1016/s0896-6273(00)80599-1. 10.1016/S0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- Vogt KE, Nicoll RA. Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci U S A. 1999;96:1118–1122. doi: 10.1073/pnas.96.3.1118. 10.1073/pnas.96.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JJ, Alger BE. GABAergic and developmental influences on homosynaptic LTD and depotentiation in rat hippocampus. J Neurosci. 1995;15:1577–1586. doi: 10.1523/JNEUROSCI.15-02-01577.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Nicoll RA. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature. 1995;376:256–259. doi: 10.1038/376256a0. 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- Woo NH, Nguyen PV. Protein synthesis is required for synaptic immunity to depotentiation. J Neurosci. 2003;23:1125–1132. doi: 10.1523/JNEUROSCI.23-04-01125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Mossy fiber long-term potentiation shows specificity but no apparent cooperativity. Neurosci Lett. 1992;138:193–197. doi: 10.1016/0304-3940(92)90503-y. 10.1016/0304-3940(92)90503-Y. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Poo MM. Reversal and consolidation of activity-induced synaptic modifications. Trends Neurosci. 2004;27:378–383. doi: 10.1016/j.tins.2004.05.006. 10.1016/j.tins.2004.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.