Abstract
Anorexia and fever are important features of the host's response to inflammation that can be triggered by the bacterial endotoxin lipopolysaccharide (LPS) and the appetite suppressant leptin. Previous studies have demonstrated that LPS induces leptin synthesis and secretion in the periphery, and that the action of leptin on appetite suppression and fever are dependent on brain interleukin (IL)-1β. However, the role of leptin as a neuroimmune mediator of LPS-induced inflammation has not been fully elucidated. To address this issue, we neutralized circulating leptin using a leptin antiserum (LAS) and determined how this neutralization affected LPS-induced anorexia, fever and hypothalamic IL-1β. Adult male rats were separated into four treatment groups, namely LPS + normal sheep serum (NSS), LPS + LAS, saline + LAS and saline + NSS. Intraperitoneal injection of LPS (100 μg kg−1) induced a significant reduction in food intake and body weight, which were significantly reversed in the presence of LAS (1 ml kg−1), 8 and 24 h after treatment. In addition, LPS-induced fever was significantly attenuated by LAS over the duration of the fever response (8 h). Lipopolysaccharide induced an increase of circulating IL-6, another potential circulating pyrogen, which was not affected by neutralization of leptin at 2 h. Interleukin-1β mRNA at 1 and 8 h, and IL-1 receptor antagonist (ra) at 2 h were significantly upregulated in the hypothalamus of LPS-treated animals. The induction of these cytokines was attenuated in the presence of LAS. These results are the first to demonstrate that leptin is a circulating mediator of LPS-induced anorexia and fever, probably through a hypothalamic IL-1β-dependent mechanism.
Infection and inflammation result in a number of metabolic changes that are often characterized by negative energy balance, increased thermogenesis and anorexia (Johnson, 1998). In experimental animals, these changes can be induced by exposure to the bacterial cell wall product lipopolysaccharide (LPS), which suppresses appetite and triggers a number of other behavioural responses including sleep, general malaise and fever as part of the brain-coordinated host defence mechanisms. These responses are mediated by cytokines, a family of peripherally generated immune products induced by LPS that can act on brain receptors to activate sickness-type behaviours (Rothwell & Hopkins, 1995; Dantzer, 2001).
Different cytokines have been suggested to act as circulating mediators during LPS-induced inflammation. Interleukin(IL)-1β, the best known member of the cytokine family, acts principally at the local site of infection or inflammation (Luheshi et al. 1996; Miller et al. 1997a,b) or in the brain (Miller et al. 1997a; Cartmell et al. 1999a,b), where it has also been shown to mediate LPS-induced fever and anorexia (Luheshi et al. 1996; Laye et al. 2000). Absence of this cytokine in the circulation of febrile animals (Miller et al. 1997b) precludes it from acting as the probable circulating mediator of LPS-induced sickness responses. In contrast, we and others have demonstrated that circulating levels of another cytokine, IL-6, rise dramatically following LPS injection with a profile that correlates closely with the development of fever (Luheshi et al. 1996; Miller et al. 1997b; Cartmell et al. 2000; Harre et al. 2002), and that the neutralization of endogenous IL-6 (Cartmell et al. 2000) or absence of IL-6 in knock-out mice (Chai et al. 1996) results in an almost total inhibition of the LPS-induced fever. These observations suggest that IL-6 is an essential circulating mediator of the brain-derived fever response (Conti et al. 2004). It would appear, however, that the role of IL-6 is limited to mediating the fever response (Lenczowski et al. 1999; Cartmell et al. 2000), and that this cytokine does not mediate other sickness-type behaviours, such as LPS-induced anorexia, which is still observed in LPS-treated IL-6-deficient mice (Fattori et al. 1994). This suggests that a second factor, possibly working in parallel with IL-6, could be involved.
We hypothesized that the cytokine-like adipocyte product leptin could be a candidate for this role. LPS induced this hormone (Grunfeld et al. 1996; Sarraf et al. 1997; Berkowitz et al. 1998; Faggioni et al. 1998; Finck et al. 1998; Mastronardi et al. 2001) through a cytokine-dependent mechanism (Faggioni et al. 1998; Finck et al. 1998). From the circulation, leptin is then actively transported across the blood–brain barrier (BBB; Banks et al. 1996) to act on hypothalamic receptors (Schwartz et al. 1996; Hakansson et al. 1998), resulting in appetite suppression and increased energy expenditure (Campfield et al. 1995; Halaas et al. 1995; Jequier, 2002). The link with LPS and cytokines prompted investigations that explored the role of leptin as a regulator of immune function and in particular its involvement in mediating the anorexia of infection (Grunfeld et al. 1996; Faggioni et al. 1997). These studies demonstrated that LPS-induced anorexia is inversely proportional to leptin mRNA expression in hamster adipose tissue (Grunfeld et al. 1996), and that mice lacking the leptin receptor are partly resistant to the anorectic effect of LPS (Faggioni et al. 1997). Our own studies (Luheshi et al. 1999) demonstrated that the activity of leptin is not limited to energy balance regulation but can also induce a prostaglandin E2 (PGE2)-dependent fever, equivalent in magnitude to that induced by LPS or cytokines, such as IL-1β. We further demonstrated that the appetite suppressing and pyrogenic effects of leptin are both mediated by hypothalamic IL-1β (Luheshi et al. 1999). These observations suggested that circulating leptin could be acting as a peripheral signal to the brain to induce sickness responses through the induction of hypothalamic IL-1β.
In the present study, we investigated the role of endogenous leptin in LPS-treated animals by utilizing a neutralizing antiserum raised against rat leptin. Specifically, we studied whether LPS-induced appetite suppression, loss of body weight and fever are mediated by circulating leptin through the activation of brain IL-1β.
Methods
Adult male Sprague–Dawley rats (300–350 g; Charles River, Saint Constant, Quebec, Canada) were used in all experiments. Animal care was provided according to protocols and guidelines approved by McGill University and the Canadian Council of Animal Care. The animals were housed individually in a controlled environment at an ambient temperature of 21 ± 2°C. Observations were carried out in a 12 h:12 h light–dark cycle (lights on from 09.00 to 21.00 h) or a 12 h:12 h reverse light–dark cycle (lights on from 21.00 to 09.00 h). All animals had free access to food and water and were handled extensively for at least 2 days before the start of each experiment.
In all studies, the animals received a single intraperitoneal (i.p.) injection of LPS at 100 μg kg−1 (extracted from Escherichia coli, 90H4012, Sigma) and/or 1 ml kg−1 i.p. of neutralizing sheep anti-rat leptin antiserum (LAS; NIBSC, Potters Bar, UK). Control animals received normal sheep serum (NSS; Sigma, Quebec, Canada) and/or sterile physiological saline (1 ml kg−1) as vehicles for LAS or LPS, respectively.
Measurements of body weight and food consumption
The effect of LAS on LPS-induced reduction in food intake and change in body weight was tested on animals previously habituated to powdered food (powdered 5012, Ralston Purina, Ottawa, Canada) for 2 days before the start of the experiment. These studies were conducted under reverse light–dark cycle during the active phase of the animals. For accurate measurements of food consumption, the powdered food was placed in special dispensers (PFF16D jar; Allentown Caging, Allentown, NJ, USA) inside the cage and the normal pelleted food removed from the top of the cage. The food dispensers are designed to avoid spillage during feeding and therefore provide an accurate method of assessing food consumed by each animal. On the day of the experiment, the animals were separated into four treatment groups, namely LPS + NSS, LPS + LAS, saline + LAS and saline + NSS (n = 5 per group), and the appropriate drugs or vehicle were administered at the same time using two consecutive i.p. injections of 1 ml kg−1 each. Changes in food consumption were measured by weighing the container (including the food) before treatments and then at 4, 6, 8 and 24 h post-treatment using a top pan balance (Scout, VWR International, Ville Mont-Royale, Quebec, Canada) accurate to 0.1 g. In the same study, changes in body weight were monitored by weighing animals immediately before injections and again at 8 and 24 h post-treatment. Only two time points were chosen to measure changes in body weight in order to minimize the disruption of feeding behaviour.
Monitoring of core body temperature
Changes in core body temperature were measured using remote radio-biotelemetry (Data Sciences, St Paul, MN, USA) as previously described (Cartmell et al. 1999a). Briefly, anaesthetized animals (50 mg kg−1 ketamine, 5 mg kg−1 xylazine, 0.5 mg kg−1 acepromazine, all by i.p. injection) were implanted intraperitoneally with precalibrated temperature-sensitive radio transmitters (TA10TA-F40, Data Sciences) and allowed to recover for 1 week postsurgery. Transmitter output frequency (Hz) was monitored, at 10 min intervals, by an antenna mounted in a receiver board, situated beneath the cage of each animal. The output data from each transmitter were then logged into a peripheral processor (BCM, Data Sciences), which relayed the information to a personal computer for analysis using Dataquest software (Data Sciences). Unlike the food intake study, these experiments were conducted during normal light–dark cycle when the animals were in their resting phase and did not exhibit any changes in basal body temperature as a result of circadian influences. On the day of the experiment, the animals were separated into the same four treatment groups (n = 5 per group) as those used for the food intake and body weight studies described above. The injections were administered between 08.30 and 09.30 h and the changes in core body temperatures monitored for 8 h post-treatment.
Cytokine measurements
To determine plasma IL-6 concentration, blood was collected from animals anaesthetized as described above via cardiac puncture 2 h following treatments (n = 5 per group) using sterile heparinized syringes and placed into sterile tubes containing pyrogen-free heparin (10 IU ml−1). The samples were then centrifuged (5300g, 10 min at 4°C) and stored at −80°C until assays were performed. Plasma IL-6 levels were measured using a rat-specific sandwich enzyme-linked immunosorbent assay (ELISA) for rat IL-6 (NIBSC, Potters Bar, UK) as previously described (Rees et al. 1999). All samples were assayed in duplicate.
Measurement of brain IL-1β and IL-1 receptor antagonist (ra) mRNA
In order to assess changes in hypothalamic IL-1β and IL-1ra mRNA, animals from three separate studies received the same treatment (n = 5 per group) as above and were killed, respectively, 1 or 8 h post-treatment for IL-1β, and 2 h post-treatment for IL-1ra using a lethal i.p. dose of anaesthetic. The animals were then perfused intracardially using diethyl pyrocarbonate (DEPC)-treated saline to remove blood cells and the brains immediately removed and placed on ice. The hypothalami were microdissected and homogenized in 1000 μl TRIzol Reagent (Invitrogen, Burlington, Ontario, Canada). Total RNA was isolated according to the manufacturer's protocol and the air-dried RNA pellet dissolved in 50 μl autoclaved water. RNA (1 μg) was incubated with random primers (5 μm; Applied Biosystems, Streetsville, Ontario, Canada) and deoxyribonucleotide triphosphate (1 mm; Sigma, USA) for 10 min at 65°C. cDNA synthesis was performed from isolated RNA by adding murine myeloleukaemia virus reverse transcriptase (200 units; MMLV, Invitrogen), dithiothreitol (10 μm; Invitrogen) and first strand buffer (Invitrogen), and incubating at 37°C for 1 h, then at 90°C for 5 min to inactivate the enzyme. Polymerase chain reaction amplification of an aliquot (1.5 μl) of cDNA was performed using ReadyMix Taq PCR Reaction Kit (Sigma) and 6 pmol of specific primers for IL-1β (forward: 5′-CCCAAGCACCTTCTTTTCCTTCATCTT-3′; reverse: 5′-CAGGGTGGGTGTGCCGTCTTTC-3′), IL-1ra (forward: 5′-CCTTCTACCTGAGGAACAACC-3′; reverse: 5′-CCTCTAGTGTTGTGCAGAGG-3′) and β-actin (forward: 5′-GCCGTCTTC CCCTCCATCGTG-3′; reverse: 5′-TACGACCAGAGGCATACAGGGACAAC-3′). Using a Gene Amp PCR system 9700 Thermocycler (Applied Biosystems), the following cycling parameters were used for IL-1β and β-actin: (1) 5 min at 94°C; (2) 30 s at 94°C, 30 s at 60°C, followed by 45 s at 72°C for 38 and 26 cycles, respectively; and (3) 72°C for 10 min (Fortier et al. 2004). For IL-1ra, the cycling parameters were: (1) 5 min at 93°C; (2) 1 min at 93°C, 2 min at 55°C and 2.5 min at 72°C for 38 cycles; and (3) 72°C for 10 min (De Simoni et al. 2000). Polymerase chain reaction products were separated by gel electrophoresis (2% agarose), band densities were obtained using Northern Eclipse Software (Empix Imaging Inc., Mississauga, Ontario, Canada) and expressed as a percentage of β-actin density (relative density (gene X/β-actin mRNA × 100)). In initial experiments, control PCR reactions for each pair of primers used total RNA to ensure that PCR products did not result from genomic DNA amplification and the linear phase of PCR amplification was determined by performing RT-PCR on a sample from each treatment group for an increasing number of cycles (20–50). The amount of each amplified product was plotted against the number of cycles. A cycle number within the exponential phase of the reaction was then selected and used for all subsequent PCRs.
Data analysis
All data are presented as mean values ± s.e.m. and were analysed using Datasim software (Desktop Press, Lewiston, ME, USA). Food intake and body weight data were analysed by parametric two-way analysis of variance (ANOVA) with treatment as the between-factor and time as the within-subject factor. Tukey–Kramer multiple comparisons post hoc test was used to compare the effect of each treatment. For the fever study, the area under the curve (AUC) was calculated on the core body temperature of individual animals in each group from 2 to 8 h post-treatment and a one-way ANOVA using treatment as a one between factor, performed. One-way ANOVA was also used to analyse the data from the ELISA and PCR studies. In cases where the comparisons using ANOVA were shown to be significant, a further test using Newman–Keuls post hoc analyses was performed to compare the effect of each treatment. In all cases, values less than 5% were deemed statistically significant.
Results
LAS reverses LPS effects on food intake and body weight
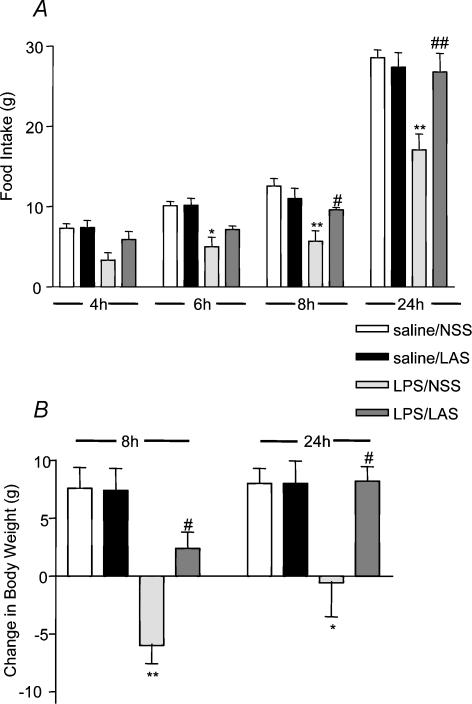
In order to assess the contribution of endogenous circulating leptin to the LPS-induced changes in food intake, animals were injected with LPS in the presence and absence of LAS. As expected, LPS (100 μg kg−1, i.p.) alone resulted in a reduction in the amount of food consumed by the animals, which was significantly different at 6, 8 and 24 h after the injection when compared to the counterpart saline-treated controls (see Fig. 1A). In the presence of LAS (1 ml kg−1, i.p.), the effects of LPS on food intake were significantly attenuated at 8 and 24 h post-treatment. At these time points, the LPS-treated animals consumed, respectively, 5.72 ± 1.30 g and 17.08 ± 1.95 g compared to 12.56 ± 0.95 g at 8 h and 28.56 ± 0.98 g at 24 h in the corresponding saline-treated controls (ANOVA F3,16 = 4.38; P < 0.001, LPS + NSS versus saline + NSS, 8 h post hoc q 4,32.43 = 5.69, P < 0.01; 24 h post hoc q4,32.43 = 9.55, P < 0.01). In the presence of the LAS, the food consumed by the LPS-treated group was 9.62 ± 0.28 g at 8 h and 26.78 ± 2.31 g at 24 h (LPS + LAS versus LPS + NSS, 8 h post hoc q4,32.43 = 3.25, P < 0.05, 24 h post hoc q4,32.43 = 8.07, P < 0.01). LAS alone (11.02 ± 1.27 g at 8 h and 27.38 ± 1.81 g at 24 h) did not affect food intake in saline-treated rats, which consumed 12.56 ± 0.95 g at 8 h and 28.56 ± 0.98 g at 24 h.
Figure 1. Effects of LAS on LPS-induced anorexia and body weight.
A, food intake was reduced significantly over 6, 8 and 24 h in rats by LPS (100 μg kg−1, i.p.). These effects were abolished completely over 8 and 24 h, by administration of LAS (1 ml kg−1, i.p.). B, similarly, administration of LAS reversed LPS-induced loss of body weight over 8 and 24 h. LPS + NSS versus saline + NSS: *P < 0.05, **P < 0.01; LPS + LAS versus LPS + NSS #P < 0.05, ##P < 0.01.
In addition to monitoring changes of food consumption, the body weight of the same animals was recorded at 0 h (immediately before treatment), then at 8 and 24 h following the injections (see Fig. 1B). As with food intake, treatment with LPS resulted in significant weight loss of 6.00 ± 1.58 g over 8 h and 0.60 ± 2.93 g over 24 h compared to the saline-treated controls, which gained 7.60 ± 1.78 g at 8 h and 8.00 ± 1.31 g at 24 h (ANOVA F3,16 = 3.55, P < 0.05, LPS + NSS versus saline + NSS, 8 h post hoc q4,22.8 = 7.42, P < 0.01, 24 h post hoc q4,22.8 = 4.69, P < 0.05). The effects of LPS on body weight were partly reversed at 8 h and totally abolished at 24 h in the presence of LAS (LPS + LAS versus LPS + NSS, 8 h post hoc q4,22.8 = 4.58, P < 0.05, 24 h post hoc q4,22.8 = 4.8, P < 0.05). Control animals injected with saline and LAS gained 7.40 ± 1.91 g at 8 h and 8.00 ± 1.95 g at 24 h, i.e. almost exactly the same gain as their counterpart NSS-injected controls, which gained 7.60 ± 1.78 g at 8 h and 8.00 ± 1.31 g at 24 h.
Neutralization of leptin inhibits LPS-induced fever
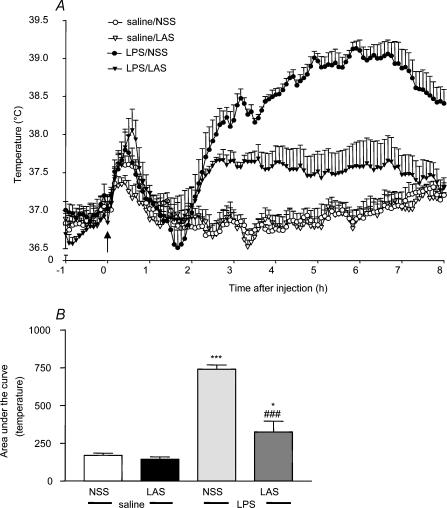
All animals exhibited a similar basal body temperature that ranged between 36.8 and 37.0°C at the time of injection. The temperature in the saline-treated control group remained stable throughout the study apart from a transient increase during the time of injection due to handling stress. Injection with LPS (100 μg kg−1, i.p.) resulted in a significant increase in core body temperature starting between 1.5 and 2 h and peaking at around 6 h (LPS + NSS, 39.1 ± 0.1°C, see Fig. 2A). The temperature of this group of animals remained elevated up to 8 h following the injection and was significantly higher than that of the control group throughout (ANOVA F3,16 = 47.72, P < 0.0001, LPS + NSS versus saline + NSS post hoc q3,16 = 14.31, P < 0.001, see Fig. 2B). In the presence of LAS (1 ml kg−1, i.p.), the increase in body temperature induced by LPS was significantly attenuated over 8 h (LAS + LPS versus LPS + NSS, post hoc q (2, 16) = 10.41, P < 0.001; LAS + LPS versus saline + NSS, post hoc q2,16 = 3.897, P < 0.05, see Fig. 2B), reaching a peak of 37.37 ± 0.2°C at 6 h (see Fig. 2A). LAS alone had no effect on the body temperature of animals in this treatment group, with no significant deviation from the saline-treated control group observed.
Figure 2. LAS attenuates LPS-induced fever.
A, injection of LPS (100 μg kg−1, i.p. injection at 09.00 h) induced a significant rise in core body temperature. This rise was significantly inhibited by administration of LAS (1 ml kg−1, i.p.). The arrow indicates the time of injection. B, statistical analysis was performed on area under the curve data. LPS + NSS versus saline + NSS: ***P < 0.001; LPS + LAS versus LPS + NSS: ###P < 0.001; LPS + LAS versus saline + NSS: *P < 0.05.
LAS had no effect on LPS-induced circulating IL-6
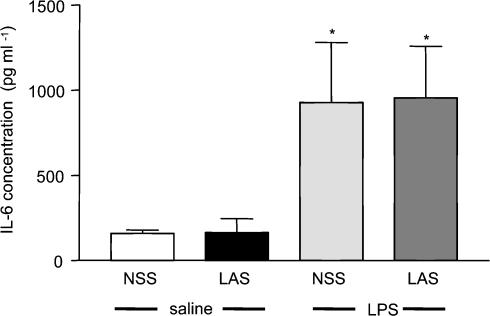
It was possible that the observed effects of LAS on LPS-induced changes were not specific to the neutralization of circulating leptin and were instead caused by another circulating mediator. To assess this hypothesis, we measured changes in the levels of circulating IL-6, which we have previously shown to be a major circulating signal that mediates LPS-induced fever in rats (Cartmell et al. 2000). Peripheral administration of LPS (100 μg kg−1, i.p.) induced a rise in plasma IL-6 (928 ± 352 pg ml−1) at 2 h after injection (a time point that was previously shown to represent the peak plasma concentration of this cytokine following LPS injection; Luheshi et al. 1996), which was significantly higher than circulating IL-6 levels in control animals (159 ± 20 pg ml−1) (ANOVA; LPS + NSS versus saline + NSS Newman–Keuls, P < 0.05; see Fig. 3). This increase was not affected by administration of LAS (1 ml kg−1, i.p.) to the LPS-treated group, in which the circulating levels of IL-6 (955 ± 303 pg ml−1) were similar to the values obtained from the animals treated with LPS alone.
Figure 3. No effect of LAS on LPS-induced IL-6 in the circulation.
Injection of LPS (100 μg kg−1, i.p.) induced a significant increase in the concentration of circulating IL-6 2 h after the injection. This increase was not affected by administration of LAS (1 ml kg−1, i.p.). LPS + NSS and LPS + LAS versus saline + NSS: *P < 0.05.
LPS-induced IL-1β and IL-1ra mRNA in the hypothalamus are significantly inhibited in the presence of LAS
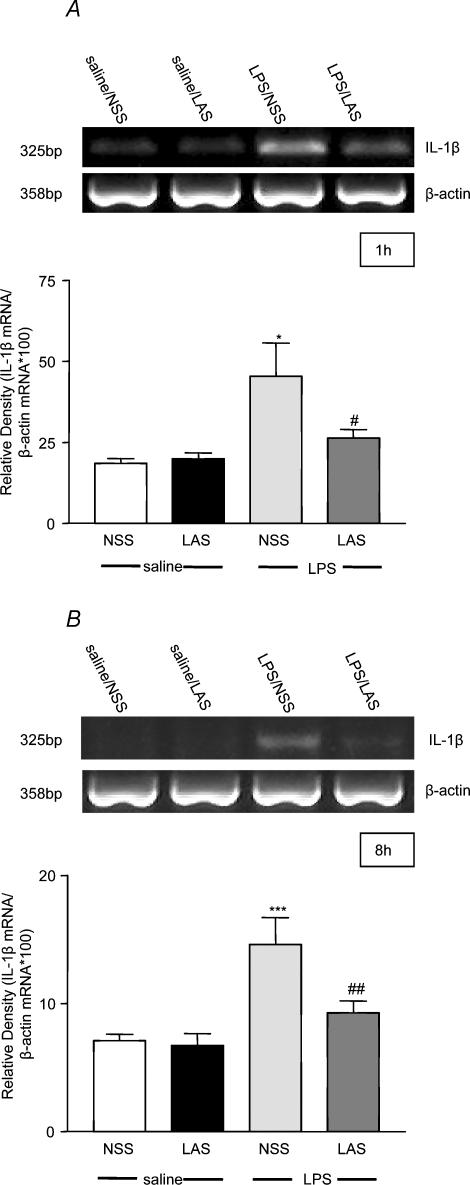
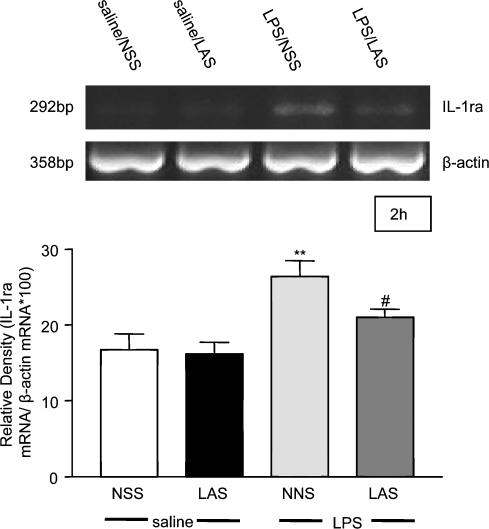
Changes in hypothalamic IL-1β mRNA were measured at two time points post-treatment. The first, 1 h, corresponding to a time when leptin is known to induce hypothalamic IL-1β (Hosoi et al. 2002b), was used as a representative time point that preceded the rising phase of the fever response while the second, 8 h, was used as a representative point at which LAS significantly inhibited the effects of LPS on both food intake and body weight. LPS (100 μg kg−1, i.p.) induced a significant (2-fold) increase in hypothalamic IL-1β mRNA expression 1 h (ANOVA F3,16 = 5.31, P < 0.01; LPS + NSS versus saline + NSS post hoc q4,16 = 4.99, P < 0.05, see Fig. 4A) and 8 h (ANOVA F3,28 = 8.99, P < 0.001; LPS + NSS versus saline + NSS post hoc q3,28 = 6.2, P < 0.001, see Fig. 4B) after the injection compared to the saline-treated group. This response was significantly attenuated at both time points tested (LAS + LPS versus LPS + NSS 1 h post hoc q2,16 = 3.55 P < 0.05; 8 h post hoc q2,28 = 4.38 P < 0.01) in the presence of the systemically administered LAS (1 ml kg−1, i.p.). Interleukin-1β mRNA expression in the hypothalamus did not differ significantly between the LPS + LAS and saline + NSS groups. No change in IL-1β mRNA expression was detected in either of the control groups tested. In addition to monitoring changes in IL-1β mRNA, we further tested whether similar changes would be observed in the level of IL-1ra mRNA. These studies were based on our earlier in vivo observations demonstrating that LPS-induced IL-1β release is closely followed after a short period by upregulation of IL-1ra, which acts to ‘dampen’ the effects of the pro-inflammatory cytokine (Cartmell et al. 2001). Since leptin is known to induce hypothalamic IL-1ra mRNA at 2 h (Hosoi et al. 2002a), we tested hypothalamic IL-1ra levels at this time, as opposed to the 1 h time point used for IL-1β. At 2 h, LPS induced a significant increase in hypothalamic IL-1ra mRNA (ANOVA F3,16 = 7.95, P < 0.01; LPS + NSS versus saline + NSS post hoc q3,16 = 5.76, P < 0.01, see Fig. 5), which was significantly attenuated by neutralization of circulating leptin using LAS (LAS + LPS versus LPS + NSS post hoc q2,16 = 3.20, P < 0.05). Interleukin-1ra mRNA levels were similar in the LAS and saline control groups.
Figure 4. LAS reverses LPS-induced hypothalamic IL-1β mRNA induction.
Injection of LPS (100 μg kg−1, i.p.) induced a significant increase of IL-1β mRNA expression in the hypothalamus of rats 1 (A) and 8 h (B) after the injection. These responses were significantly attenuated by administration of LAS (1 ml kg−1, i.p.). LPS + NSS versus saline + NSS: *P < 0.05, ***P < 0.001; LPS + LAS versus LPS + NSS: #P < 0.05, ##P < 0.01. Each band on the gel represents one animal per group.
Figure 5. LAS reverses LPS-induced IL-1ra mRNA in the hypothalamus.
Injection of LPS (100 μg kg−1, i.p.) induced a significant increase of IL-1ra mRNA expression in the hypothalamus of rats 2 h after the injection. This response was significantly attenuated by administration of LAS (1 ml kg−1, i.p.). LPS + NSS versus saline + NSS: **P < 0.01; LPS + LAS versus LPS + NSS: #P < 0.05. Each band on the gel represents one animal per group.
Discussion
The data from the present study suggest that leptin is acting as a classical inflammatory signal, in a manner similar to other pro-inflammatory cytokines, to mediate responses to acute infection/inflammation. Like other cytokines, leptin is now clearly implicated in the immune response (La Cava & Matarese, 2004), but its exact role remains to be determined because a number of contradictions relating to its function in infection and inflammation have arisen from different studies. Some data, for example, suggest that absence of leptin in genetically modified mice or starvation-induced decrease of leptin in mice increases susceptibility to infection (Faggioni et al. 1999, 2000b), whereas in other studies leptin deficiency was found to be protective against concanavalin-A-induced hepatotoxicity (Faggioni et al. 2000a; Siegmund et al. 2002). From these observations, it would appear that the role of leptin as an immune mediator may depend on the degree of inflammation and/or the physiological state of the animal at the time of infection.
In the present study, we tested the involvement of circulating leptin in triggering brain-mediated responses, such as LPS-induced anorexia. Support for this notion was provided by observations that this hormone was significantly upregulated in the circulation of rodents following LPS treatment (Grunfeld et al. 1996; Sarraf et al. 1997; Berkowitz et al. 1998; Faggioni et al. 1998; Finck et al. 1998; Mastronardi et al. 2001) and that its induction inversely correlated with reduction in food intake (Grunfeld et al. 1996). However, none of these studies provided direct evidence linking leptin with the appetite-reducing effects of LPS. In our experiments, we addressed this question by neutralizing endogenous leptin using a specific antiserum. The results from these experiments demonstrate for the first time that inhibition of leptin activity using this approach significantly reversed the effect of LPS on food intake at 8 and 24 h, culminating in 54% reduction in food consumption, and completely reversed the LPS-induced reduction in body weight at 24 h postadministration. Interestingly, administration of LAS alone did not appear to have an effect on basal levels of food intake or body weight, which indicates that the dose of LAS we used to neutralize circulating leptin does not affect its normal regulatory action and confirms that the anti-anorectic effect of LAS in the present study is specific to the anorexia associated with inflammation. These results suggest that leptin constitutes a major factor by which the inflammation-triggered peripheral satiety signal is relayed to hypothalamic appetite-regulating centres in the brain. This is supported by studies in leptin receptor-deficient (db/db mice) (Faggioni et al. 1997), which present a partial resistance to LPS-induced anorexia. However, the same study (Faggioni et al. 1997) demonstrated that the anorexic effects of LPS remained intact in leptin-deficient mice (ob/ob), suggesting that leptin is not involved in mediating this response. The multiplicity of the appetite-regulating system could have been a significant factor determining the outcome of that particular study. This would have most likely involved compensatory mechanisms, with other cytokines or hormones being activated to act as substitutes for leptin. Indeed, previous studies have shown that the anorectic effects of LPS were partly mediated via cytokines such as tumour necrosis factor (TNF)-α (Plata-Salaman, 2001). Inhibition of TNF-α production by pentoxifylline, for example, eliminated LPS-induced hypophagia (Porter et al. 2000) and peripheral administration of TNF-α can induce a decrease in food intake (McCarthy, 2000). Interestingly, TNF-α has been shown to interact with leptin with several studies (Grunfeld et al. 1996; Sarraf et al. 1997; Finck et al. 1998), demonstrating that it stimulates leptin synthesis from adipocytes and leptin release in the circulation. Conversely, other studies (Loffreda et al. 1998b; Santos-Alvarez et al. 1999; Zarkesh-Esfahani et al. 2004) reported that leptin can in fact upregulate LPS-induced TNF-α production by murine macrophages, induce TNF-α in human monocytes and activate human neutrophils via TNF-α in vitro. Collectively, these data suggest that leptin may interact with TNF-α to mediate LPS-induced sickness behaviours; however, the specifics of this interaction require further study.
In addition to testing the role of leptin in the anorexia of inflammation, we also investigated the possibility that it could be acting as a mediator of LPS-induced fever. These studies stem from our earlier observations (Luheshi et al. 1999) that leptin, when injected systemically or directly into the brain of rats, is pyrogenic. The present study confirmed the role of leptin in fever by demonstrating that LPS-induced increase in core body temperature was significantly attenuated in the presence of LAS over the duration of the experiment (8 h). These data suggest strongly that leptin is involved in mediating the fever response to exogenous inflammatory stimuli such as LPS. This is in contrast with observations made in a recent study, which reported failure to induce fever with intravenously injected leptin (Kelly et al. 2004). It is difficult to reconcile these data with our previous results (Luheshi et al. 1999), but the difference in the source of leptin and the routes of injection (i.p. in our study versus i.v. in the study of Kelly et al. 2004) may be responsible for this discrepancy. Nevertheless, the result from the present study where endogenous leptin was targeted as opposed to using exogenous recombinant protein strongly suggests a pyrogenic role for leptin in LPS-induced fever.
The failure of LAS to completely abolish LPS-induced increases in core body temperature, as observed in our study, would suggest that leptin may be acting in conjunction with another circulating pyrogen such as IL-6. We have previously demonstrated the importance of IL-6 as a circulating mediator of fever (Cartmell et al. 2000) by abolishing the febrile response to LPS in the presence of an IL-6 antiserum. In the same study, however, we reported that systemic injection of this cytokine in rodents, at concentrations severalfold higher than those found in the circulation during fever, fails to induce a febrile response (Cartmell et al. 2000). This is in contrast to leptin, which is pyrogenic when injected systemically (Luheshi et al. 1999). It is therefore possible that the two mediators could be acting in tandem, although the precise mechanism underlying this relationship remains to be elucidated. There is evidence to suggest a significant interaction between the two proteins, which also share some structural homology at the level of the receptor (Baumann et al. 1996; Nakashima et al. 1997; Tartaglia, 1997). For example, leptin has been shown to induce IL-6 in human monocytes (Santos-Alvarez et al. 1999) and to upregulate LPS-induced IL-6 production by murine macrophages (Loffreda et al. 1998). In the present in vivo study, however, LAS failed to affect the LPS-induced rise in circulating IL-6 at 2 h (Fig. 3). A recent study (Hubschle et al. 2001) suggested a specific role for each mediator during inflammation by showing that leptin and IL-6 activate different brain nuclei, the first being associated primarily with appetite regulation (arcuate nucleus, dorsomedial nucleus and ventromedial nucleus) and the second with regulation of the fever response (medial preoptic area and ventromedial preoptic area). Although these data were obtained following direct injection of both leptin and IL-6 into the brain, which may not reflect accurately the contribution of the circulating cytokines in vivo, they suggest that the primary function of leptin is to mediate the anorexia of inflammation, while IL-6 is more implicated in the fever response. Moreover, and to the best of our knowledge, there are no data available to suggest a role for IL-6 in mediating LPS-induced anorexia, even though IL-6 itself has been demonstrated to influence appetite and body weight in a number of studies (McCarthy, 2000; Sakic et al. 2001; Wallenius et al. 2002a,b). Additional studies are required to determine the relationship between IL-6 and leptin and their respective roles as mediators of LPS-induced inflammation.
Since leptin, in a similar manner to LPS, induces hypothalamic IL-1β (Luheshi et al. 1999; Hosoi et al. 2002b), a key cytokine in the CNS control of sickness behaviours (Rothwell & Luheshi, 2000), we assessed its activity in the brain by measuring hypothalamic IL-1β expression following systemic injection of LPS in the absence and presence of LAS. The results from these series of experiments demonstrate quite clearly that the LPS-induced hypothalamic IL 1β is significantly inhibited in animals that were cotreated with LAS, suggesting that this particular effect of LPS is leptin mediated. Since hypothalamic IL-1β is known to mediate the anorectic and febrile effects of LPS (Miller et al. 1997a; Cartmell et al. 1999a; Laye et al. 2000) and leptin (Luheshi et al. 1999), we further assessed the possible association between the changes in hypothalamic IL-1β and the physiological parameters measured in our study at 1 h (representing the rising phase of the fever response) and 8 h (representing the peak reduction in food intake post-treatment). In both cases, the LPS-induced upregulation of IL-1β mRNA was significantly attenuated, suggesting an association between changes in hypothalamic IL-1β and the physiological modifications observed. In addition to measuring changes in IL-1β expression, we also analysed changes in the expression of its endogenously occurring receptor antagonist (IL-1ra). Lipopolysaccharide-induced IL-1β induction and release is normally followed by upregulation of IL-1ra (Laye et al. 1994; Gabellec et al. 1995), which mainly acts as a regulator of IL-1β activity by ‘dampening down’ the effects of the endogenous pro-inflammatory cytokine (Cartmell et al. 2001). We demonstrate here that, similar to IL-1β, neutralization of circulating leptin significantly inhibited the LPS-induced hypothalamic IL-1ra mRNA upregulation 2 h following treatment.
In conclusion, our data strongly indicate that leptin is a circulating mediator of LPS-induced sickness-type behaviours, which acts as a classical inflammatory signal to activate responses to acute inflammation, probably through the activation of brain cytokines.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research. G.N.L. is a receipient of a senior chercheurs-boursiers from the Fonds de la recherché en santé du Québec.
References
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz DE, Brown D, Lee KM, Emala C, Palmer D, et al. Endotoxin-induced alteration in the expression of leptin and β3-adrenergic receptor in adipose tissue. Am J Physiol. 1998;274:E992–E997. doi: 10.1152/ajpendo.1998.274.6.E992. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Luheshi GN, Hopkins SJ, Rothwell NJ, Poole S. Role of endogenous interleukin-1 receptor antagonist in regulating fever induced by localised inflammation in the rat. J Physiol. 2001;531:171–180. doi: 10.1111/j.1469-7793.2001.0171j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell T, Luheshi GN, Rothwell NJ. Brain sites of action of endogenous interleukin-1 in the febrile response to localized inflammation in the rat. J Physiol. 1999a;518:585–594. doi: 10.1111/j.1469-7793.1999.0585p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell T, Poole S, Turnbull AV, Rothwell NJ, Luheshi GN. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J Physiol. 2000;526:653–661. doi: 10.1111/j.1469-7793.2000.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell T, Southgate T, Rees GS, Castro MG, Lowenstein PR, Luheshi GN. Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain. J Neurosci. 1999b;19:1517–1523. doi: 10.1523/JNEUROSCI.19-04-01517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Z, Gatti S, Toniatti C, Poli V, Bartfai T. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1 β: a study on IL-6-deficient mice. J Exp Med. 1996;183:311–316. doi: 10.1084/jem.183.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–1449. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 β mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–R208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, et al. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–R142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Fuller J, Moser A, Feingold KR, Grunfeld C. LPS-induced anorexia in leptin-deficient (ob/ob) and leptin receptor- deficient (db/db) mice. Am J Physiol. 1997;273:R181–R186. doi: 10.1152/ajpregu.1997.273.1.R181. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor α and IL-18. Proc Natl Acad Sci U S A. 2000a;97:2367–2372. doi: 10.1073/pnas.040561297. 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol. 2000b;156:1781–1787. doi: 10.1016/S0002-9440(10)65049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, et al. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Kelley KW, Dantzer R, Johnson RW. In vivo and in vitro evidence for the involvement of tumor necrosis factor-α in the induction of leptin by lipopolysaccharide. Endocrinology. 1998;139:2278–2283. doi: 10.1210/endo.139.5.6012. 10.1210/en.139.5.2278. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic: polycytidylic acid (poly I: C), induces fever in rats via an interleukin-1 dependant mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Gabellec MM, Griffais R, Fillion G, Haour F. Expression of interleukin 1 α, interleukin 1 β and interleukin 1 receptor antagonist mRNA in mouse brain: regulation by bacterial lipopolysaccharide (LPS) treatment. Brain Res Mol Brain Res. 1995;31:122–130. doi: 10.1016/0169-328x(95)00042-q. 10.1016/0169-328X(95)00042-Q. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Zhao C, Fuller J, Pollock A, Moser A, et al. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. A role for leptin in the anorexia of infection. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Harre EM, Roth J, Pehl U, Kueth M, Gerstberger R, Hubschle T. Selected contribution: role of IL-6 in LPS-induced nuclear STAT3 translocation in sensory circumventricular organs during fever in rats. J Appl Physiol. 2002;92:2657–2666. doi: 10.1152/japplphysiol.00822.2001. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Okuma Y, Nomura Y. Leptin induces IL-1 receptor antagonist expression in the brain. Biochem Biophys Res Commun. 2002a;294:215–219. doi: 10.1016/S0006-291X(02)00486-2. 10.1016/S0006-291X(02)00486-2. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Okuma Y, Nomura Y. Leptin regulates interleukin-1β expression in the brain via the STAT3-independent mechanisms. Brain Res. 2002b;949:139–146. doi: 10.1016/s0006-8993(02)02974-8. 10.1016/S0006-8993(02)02974-8. [DOI] [PubMed] [Google Scholar]
- Hubschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W. Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci. 2001;21:2413–2424. doi: 10.1523/JNEUROSCI.21-07-02413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- Johnson RW. Immune and endocrine regulation of food intake in sick animals. Domest Anim Endocrinol. 1998;15:309–319. doi: 10.1016/s0739-7240(98)00031-9. 10.1016/S0739-7240(98)00031-9. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Elias CF, Lee CE, Ahima RS, Seeley RJ, Bjorbaek C, et al. Ciliary neurotrophic factor and leptin induce distinct patterns of immediate early gene expression in the brain. Diabetes. 2004;53:911–920. doi: 10.2337/diabetes.53.4.911. [DOI] [PubMed] [Google Scholar]
- La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Laye S, Gheusi G, Cremona S, Combe C, Kelley K, et al. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–R98. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. 10.1016/0169-328X(94)90197-X. [DOI] [PubMed] [Google Scholar]
- Lenczowski MJ, Bluthe RM, Roth J, Rees GS, Rushforth DA, van Dam AM, et al. Central administration of rat IL-6 induces HPA activation and fever but not sickness behavior in rats. Am J Physiol. 1999;276:R652–R658. doi: 10.1152/ajpregu.1999.276.3.R652. [DOI] [PubMed] [Google Scholar]
- Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ. Leptin actions on food intake and body temperature are mediated by IL-1. Proc Natl Acad Sci U S A. 1999;96:7047–7052. doi: 10.1073/pnas.96.12.7047. 10.1073/pnas.96.12.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luheshi G, Miller AJ, Brouwer S, Dascombe MJ, Rothwell NJ, Hopkins SJ. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in the rat. Am J Physiol. 1996;270:E91–E95. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- McCarthy DO. Tumor necrosis factor α and interleukin-6 have differential effects on food intake and gastric emptying in fasted rats. Res Nurs Health. 2000;23:222–228. doi: 10.1002/1098-240x(200006)23:3<222::aid-nur6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mastronardi CA, Yu WH, Srivastava VK, Dees WL, McCann SM. Lipopolysaccharide-induced leptin release is neurally controlled. Proc Natl Acad Sci U S A. 2001;98:14720–14725. doi: 10.1073/pnas.251543598. 10.1073/pnas.251543598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Hopkins SJ, Luheshi GN. Sites of action of IL-1 in the development of fever and cytokine responses to tissue inflammation in the rat. Br J Pharmacol. 1997a;120:1274–1279. doi: 10.1038/sj.bjp.0701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Luheshi GN, Rothwell NJ, Hopkins SJ. Local cytokine induction by LPS in the rat air pouch and its relationship to the febrile response. Am J hysiol. 1997b;272:R857–R861. doi: 10.1152/ajpregu.1997.272.3.R857. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Narazaki M, Taga T. Overlapping and distinct signals through leptin receptor (OB-R) and a closely related cytokine signal transducer, gp130. FEBS Lett. 1997;401:49–52. doi: 10.1016/s0014-5793(96)01430-5. 10.1016/S0014-5793(96)01430-5. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR. Cytokines and feeding. Int J Obes Relat Metab Disord. 2001;25(Suppl. 5):S48–S52. doi: 10.1038/sj.ijo.0801911. 10.1038/sj/ijo/0801911. [DOI] [PubMed] [Google Scholar]
- Porter MH, Hrupka BJ, Altreuther G, Arnold M, Langhans W. Inhibition of TNF-α production contributes to the attenuation of LPS-induced hypophagia by pentoxifylline. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2113–R2120. doi: 10.1152/ajpregu.2000.279.6.R2113. [DOI] [PubMed] [Google Scholar]
- Rees GS, Ball C, Ward HL, Gee CK, Tarrant G, Mistry Y, et al. Rat interleukin 6: expression in recombinant Escherichia coli, purification and development of a novel ELISA. Cytokine. 1999;11:95–103. doi: 10.1006/cyto.1998.0408. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: actions and mechanisms of action. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. 10.1016/0166-2236(95)93890-A. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. 10.1016/S0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- Sakic B, Gauldie J, Denburg JA, Szechtman H. Behavioral effects of infection with IL-6 adenovector. Brain Behav Immun. 2001;15:25–42. doi: 10.1006/brbi.1999.0576. 10.1006/brbi.1999.0576. [DOI] [PubMed] [Google Scholar]
- Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- Sarraf P, Frederich RC, Turner EM, Ma G, Jaslowiak NT, Rivet DJ, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund B, Lear-Kaul KC, Faggioni R, Fantuzzi G. Leptin deficiency, not obesity, protects mice from Con A-induced hepatitis. Eur J Immunol. 2002;32:552–560. doi: 10.1002/1521-4141(200202)32:2<552::AID-IMMU552>3.0.CO;2-H. 10.1002/1521-4141(200202)32:2<552::AID-IMMU552>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- Wallenius K, Wallenius V, Sunter D, Dickson SL, Jansson JO. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun. 2002;293:560–565. doi: 10.1016/S0006-291X(02)00230-9. 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Zarkesh-Esfahani H, Pockley AG, Wu Z, Hellewell PG, Weetman AP, Ross RJ. Leptin indirectly activates human neutrophils via induction of TNF-α. J Immunol. 2004;172:1809–1814. doi: 10.4049/jimmunol.172.3.1809. [DOI] [PubMed] [Google Scholar]