Abstract
While lowering muscle glycogen availability to an extent that would reduce muscle pyruvate formation during intense exercise, we investigated the importance of muscle pyruvate availability to pyruvate dehydrogenase complex (PDC) activation during intense exercise in human skeletal muscle. The present study demonstrated that regardless of whether pre-exercise muscle glycogen content was at a habitual resting concentration (412 ± 30 mmol (kg dry muscle)−1) or depleted (60 ± 3 mmol (kg dry muscle)−1), the increase in PDC activation from its resting value (5.46 ± 0.96 and 3.67 ± 0.34 nmol acetyl-CoA min−1 (mg protein)−1, respectively) during 10 min of exercise at 75% of the maximum rate of oxygen consumption (V̇O2,max) (Δ12.82 ± 1.72 and Δ13.24 ± 1.42 nmol acetyl-CoA min−1 (mg protein)−1, respectively) was the same, despite pyruvate accumulation during exercise being 3-fold lower in the glycogen depleted state (Δ0.34 ± 0.04 and Δ0.11 ± 0.06 mmol (kg dry muscle)−1, P < 0.001). However, as a result of the reduction in pyruvate availability, calculated flux through the PDC reaction was at least 2-fold lower in the glycogen depleted state compared with normal (21.81 ± 2.62 and 9.41 ± 0.63 nmol acetyl-CoA min−1 (mg protein)−1, respectively; P < 0.001). It is therefore pertinent to conclude that whilst muscle pyruvate availability appears to be important to the rate of flux through the PDC reaction during in vivo contraction, it is not of primary importance to the control of PDC activation under these conditions, which is probably principally regulated by muscle calcium availability. The proposed central role of pyruvate in muscle PDC activation during in vivo contraction may therefore have been over stated.
The magnitude of activation of the mitochondrial pyruvate dehydrogenase complex (PDC) is central to the control of carbohydrate oxidation in contracting skeletal muscle. Accordingly, muscle PDC activation increases, from its relatively inactive or low state at rest, in parallel with exercise intensity up to an intensity of about 75–90%, when the rate of carbohydrate oxidation becomes maximal (Constantin-Teodosiu et al. 1991a; Howlett et al. 1998).
The activity of PDC is regulated principally by a covalent mechanism of competing pyruvate dehydrogenase kinase (PDK) and pyruvate dehydrogenase phosphatase (PDP) reactions. The resulting interconversion cycle determines the amount of pyruvate dehydrogenase component existing in a non-phosphorylated (active) form (PDCa; Wieland, 1983). Calcium and pyruvate would appear to be the principal physiological activators of the PDC in vitro, because they operate by activating the PDP and inhibiting the PDK reactions, respectively.
The relative importance of calcium and pyruvate to PDC activation in vivo is not well established, particularly during contraction. Certainly, at rest, when muscle calcium availability is relatively low, pyruvate formation could be the primary activator of muscle PDC. For example, we have recently shown that intravenous adrenaline administration resulted in a ∼5-fold increase in PDC activity in canine skeletal muscle (Roberts et al. 2002). One possible explanation for this observation is that adrenaline increased muscle glycogenolysis and thereby muscle pyruvate availability, which in turn increased PDC activity. Clearly, muscle contraction increases both muscle calcium and pyruvate availability, and it is generally accepted that both events increase muscle PDC activity and thereby pyruvate oxidation to acetyl-CoA (Constantin-Teodosiu et al. 1991a). However, no in vivo study to date has directly attempted to dissociate any effect of pyruvate on PDC activation during muscle contraction from that of calcium. One way to accomplish this would be to reduce muscle glycogen content to an extent that it would limit pyruvate formation during contraction. In this respect, Putman et al. (1993) reported a blunting of muscle PDC activation during submaximal exercise (75% V˙O2,max) in humans following 3 days of high dietary fat intake, which they suggested could be due to a diet-induced reduction in muscle pyruvate availability. However, when the same research group extended the period of high dietary fat intake to 6 days, they were unable to demonstrate any blunting of PDC activation during prolonged exercise at 65% V˙O2,max (St Amand et al. 2000). On the basis of this, and in contrast to the finding of Putman et al. (1993), the authors concluded that pyruvate accumulation during exercise overrode a dietary fat-mediated inhibition of PDC activation. It is also worthy of comment that studies to date advocating a central role for pyruvate in PDC activation during exercise have been performed under conditions where muscle glycogen availability may not have significantly impaired pyruvate formation, i.e. in the range of 200–300 mmol (kg dry muscle)−1 (Putman et al. 1993; St Amand et al. 2000).
The aim of the present study therefore was to investigate the magnitude of PDC activation during 10 min of exercise at 75% V˙O2,max under conditions in which pre-exercise muscle glycogen stores were either at a habitual resting concentration or depleted to an extent that would reduce muscle pyruvate formation during exercise. If pyruvate formation was limiting to PDC activation in the latter condition, we would expected this to be reflected by a reduction in PDC activation compared to the habitual state. If we observed no difference in PDC activation between experimental conditions during exercise, it could be concluded that pyruvate formation was not central to PDC activation in vivo in human skeletal muscle.
Methods
Ten healthy male volunteers (age, 20.1 ± 1.0 years; body mass index, 23.2 ± 1.2 kg m−2;, 53.1 (V˙O2,max), ± 1.2 ml (min kg)−1) unaccustomed to strenuous activity participated in the study. All participants were informed about the nature and the risks of the experimental procedures before their written consent was obtained. The study was approved by the University of Nottingham Medical School Ethics Committee and was in accordance with the Declaration of Helsinki.
Prior to participation, each subject underwent routine medical and physiological screening. Upon entry to the study each performed two incremental exercise tests, separated by at least 7 days, on an electrically braked cycle ergometer (Lode NV Intrumenten, Groningen, the Netherlands), to determine the maximum rate of oxygen consumption (V˙O2,max).
Experimental protocol
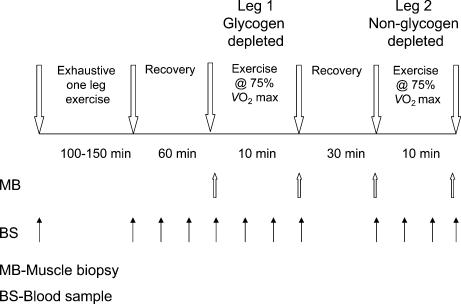
The experimental protocol is summarized in Fig. 1. Having determined V˙O2,max, each subject was familiarized with single-leg cycling exercise. This involved pairs of subjects performing exercise simultaneously with one leg using an electrically braked cycle ergometer whilst being supported in a standing position by the contralateral limb. Subjects were assigned to matched pairs based on their V˙O2,max.
Figure 1. The experimental protocol.
The experimental protocol involved pairs of subjects performing prolonged and exhaustive single-leg exercise simultaneously on an electrically braked cycle ergometer in order to deplete muscle glycogen stores in one limb. After 60 min of recovery, a muscle biopsy sample was obtained from the vastus lateralis of the glycogen-depleted limb of each subject. Each subject then performed 10 min of single-leg exercise at 75% with the glycogen-depleted limb, and a second muscle biopsy sample was obtained at the end of exercise. The subject recovered for 30 min in a supine position after which a third muscle biopsy sample was obtained from the vastus lateralis of the non-glycogen-depleted limb. Subjects then performed a second 10 min bout of single-leg exercise at 75% V̇O2,max, but on this occasion used the non-glycogen-depleted limb, and a muscle biopsy was taken immediately following exercise.
One week later, subjects reported to the laboratory on the morning after an overnight fast, having been instructed to maintain their dietary intake to as close to usual as possible, and having abstained from alcohol and strenuous exercise for the previous 3 days. Subjects rested in a semisupine position for 20 min and a venous cannula was then inserted into an antecubital fossa (under local anaesthesia using 1% lignocaine), which was used subsequently for venous blood sampling. Each pair of subjects then performed single-leg cycling exercise to the point of volitional exhaustion (130 ± 6 min) at a predetermined workload equivalent to 75% V˙O2,max (205 ± 10 W), whilst maintaining a pedalling frequency of 70 r.p.m. Subjects were able to stop exercising at anytime, but after a short rest of up to 5 min, were required to resume exercise. In an attempt to maximize depletion of muscle glycogen stores, this work–rest protocol was repeated until subjects were no longer able to maintain a pedal frequency of 70 r.p.m. for more than 2 min. In the case of the exercise time to exhaustion differing within a pair of subjects, a volunteer from the group of investigators replaced the first subject when he became exhausted; thereby ensuring the remaining subject could continue exercising to the point of exhaustion. Consumption of water was allowed ad libitum throughout exercise. We have previously demonstrated that this protocol of subjects performing one-leg exercise simultaneously on either side of a cycle ergometer results in almost complete muscle glycogen depletion in the exercised leg (Casey et al. 1995).
Immediately following exercise, subjects rested in a supine position for 60 min during which only water was available for consumption. After this, a muscle biopsy sample was obtained under local anaesthesia (lignocaine 1%) from the vastus lateralis of the leg that had been exercised (Bergström, 1962). Subjects then immediately performed 10 min of single-leg exercise at a workload equivalent to 75% V˙O2,max using the same glycogen-depleted leg, and a second muscle biopsy was obtained from this leg at the end of this exercise period. Following this, subjects recovered for 30 min in a supine position, after which a third muscle biopsy sample was obtained, but on this occasion it was taken from the vastus lateralis muscle of the ‘non-exercised’ leg (i.e. the limb used to support the subject during the prolonged single-leg exercise). Subjects then performed a second 10 min bout of one–leg exercise at 75% V˙O2,max using this ‘non-exercised’ leg, after which a final muscle biopsy sample was obtained from the same leg. Upon removal from the muscle, all biopsy samples were immediately frozen in liquid nitrogen. Samples were then stored in liquid nitrogen until analysed at a later date.
Blood samples were taken at regular predetermined intervals throughout the experiment (see Fig. 1) and were used for the determination of whole blood glucose and lactate, and plasma free fatty acid (FFA) concentrations.
Muscle analysis
One portion of muscle was freeze-dried, dissected free of visible connective tissue and powdered. Free carnitine and acetyl carnitine levels were measured in the neutralized extract by radioisotope enzymatic assays (Cederblad et al. 1990). Muscle pyruvate, lactate, malate, fumarate, succinate and citrate concentrations were measured enzymatically with a fluorometer (Hitachi F-2000, Hitachi Ltd, Japan) (Bergmeyer, 1995). ATP, phosphocreatine (PCr), creatine and glycogen concentrations were measured using a modification of the spectrophotometric method of Harris et al. (1974).
The remainder of the frozen muscle was used to determine PDC activity as previously described (Constantin-Teodosiu et al. 1991b) Briefly, the activity of PDC in its dephosphorylated active form (PDCa) was assayed in a buffer containing NaF and dichloroacetate (DCA) and was expressed as rate of acetyl-CoA formation (mmol min−1 (kg muscle wt)−1 or nmol min−1 (mg protein)−1) at 37°C. Protein concentrations were determined using the method of Peterson (1977).
Blood and plasma analysis
Venous blood samples were immediately analysed for blood lactate and glucose concentrations (Yellow Springs Int., Farnborough, Hampshire, UK) and the remainder was centrifuged. Following centrifugation, plasma was stored at −80°C and later analysed for free fatty acid (FFA) concentrations using a commercially available kit (WAKO C kit, Alpha Laboratories, Eastleigh, Hampshire, UK).
Calculations
Estimates of maximal flux through the PDC reaction (nmol acetyl-CoA min−1 (mg protein)−1 at 37°C) during each of the two bouts of exercise lasting 10 min were made based on changes in muscle metabolite concentrations using the following formula: 2ΔGlycogen-Δlactate-Δacetylcarnitine-Δpyruvate-Δtricarboxylic acid cycle intermediates (Σ malate + fumarate + succinate + citrate)/10 and assuming 175 g protein (kg wet wt)−1 (Forsberg et al. 1991) where Δ signifies the increase in concentration during exercise and 10 signifies the duration of the exercise.
Statistical analysis
The data were analysed using two-way analysis of variance for repeated measures (ANOVA) with two within-subject factors (time and treatment). When the ANOVA tests resulted in a significant F ratio (P < 0.05), the location of the difference was identified using Scheffe's test.
Results
Muscle metabolites
Muscle glycogen concentration, determined after 60 min of rest following prolonged glycogen-depleting single-leg exercise, was one seventh of that determined in the non-exercised contralateral limb (‘Pre-10 min exercise’Table 1).
Table 1.
Muscle metabolite concentrations in human skeletal muscle determined immediately before and after a 10 min bout of exercise at 75% V̇O2,max with or without prior muscle glycogen depletion.
| Glycogen-depleted muscle | Non-glycogen-depleted muscle | |||
|---|---|---|---|---|
| Metabolite | Pre-10 min exercise | Post-10 min exercise | Pre-10 min exercise | Post-10 min exercise |
| Glycogen | 60 ± 3* | 21 ± 2*† | 412 ± 30 | 307 ± 78† |
| Glu-6-P | 1.6 ± 0.6 | 1.2 ± 0.3 | 1.7 ± 0.3 | 3.9 ± 0.4† |
| Lactate | 0.8 ± 0.1* | 4.8 ± 1.0*† | 3.1 ± 0.4 | 71.9 ± 8.2† |
| Acetylcarnitine | 15.8 ± 0.6* | 14.0 ± 1.2 | 4.5 ± 0.5 | 15.4 ± 1.0† |
| Free carnitine | 7.8 ± 1.2* | 9.3 ± 1.0† | 16.9 ± 1.0 | 6.7 ± 1.3† |
| TCAI# | 1.29 ± 0.09* | 3.41 ± 0.27*† | 1.56 ± 0.09 | 5.25 ± 0.03† |
| ATP | 24.0 ± 1.2 | 23.3 ± 1.0 | 23.5 ± 0.7 | 23.1 ± 0.6 |
| PCr | 91.1 ± 3.0* | 25.4 ± 1.7† | 77.9 ± 2.3 | 23.4 ± 4.9† |
Values are means ± s.e.m. with n = 10. All concentrations are expressed as mmol (kg dry muscle)−1
Significantly different from the corresponding normal non-glycogen-depleted leg (P < 0.05)
significantly different from rest within the same group (P < 0.05). #Represents the sum of muscle citrate, malate, fumarate and succinate concentrations.
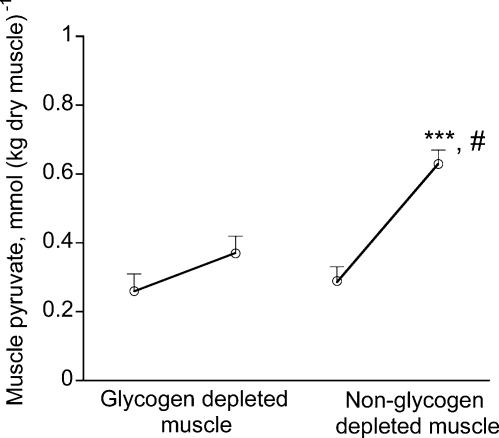
During the two 10 min bouts of exercise that followed, the rate of glycogenolysis in the glycogen-depleted muscle was 2.5-fold lower than that measured in the contralateral limb (P < 0.001).Consequently, muscle glucose 6-phosphate (Glu-6-P) failed to accumulate in the glycogen-depleted muscle during exercise, but increased by more than 2-fold in the other limb (Table 1). Similarly, pyruvate accumulated in the muscle only modestly in the glycogen-depleted leg, whilst its concentration more than doubled in the contralateral limb during exercise (Fig. 2). Correspondingly, the magnitude of lactate accumulation in the glycogen-depleted muscle during exercise was 17-fold lower (P < 0.001) than that of the contralateral limb (Table 1).
Figure 2. Muscle pyruvate concentrations before and after two 10-min bouts of exercise at 75% V̇O2,max with or without muscle glycogen depletion.
Values are means ± s.e.m. with n = 10. ***Significantly different from the corresponding time point across the treatment (P < 0.001). #Significantly different from rest(P < 0.001)
Acetylcarnitine concentration in the glycogen-depleted leg immediately before the 10 min bout of exercise was significantly greater than that of the contralateral limb (P < 0.001, Table 1). Muscle acetylcarnitine concentration declined by ∼2 mmol in the glycogen-depleted muscle over the course of 10 min exercise, whereas the acetylcarnitine concentration increased 3-fold from its resting concentration during exercise in the contralateral limb (Table 1). The changes in muscle free carnitine concentration during exercise mirrored those of acetylcarnitine, such that the sum of the muscle free carnitine and acetylcarnitine concentrations remained constant throughout the whole experiment.
The rate of expansion of the tricarboxylic acid intermediate (TCAI) pool during exercise in the glycogen-depleted muscle was ∼2-fold lower than that measured in the muscle with a habitual resting glycogen concentration (P < 0.001).
Muscle ATP concentration did not change during 10 min of exercise in both the glycogen-depleted and non-depleted states (Table 1). PCr concentration in the glycogen-depleted muscle immediately prior to the 10 min bout of exercise was significantly greater when compared with the contralateral limb (P < 0.05). During exercise, the rate of PCr hydrolysis was also significantly greater in the glycogen-depleted muscle than in the contralateral limb (P < 0.05, Table 1).
Muscle PDCa and calculated flux through the PDC reaction
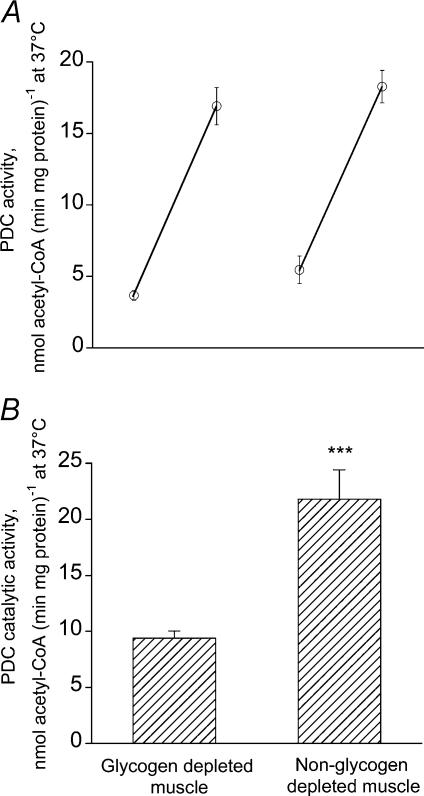
There was no significant difference in muscle PDC activation status when comparing glycogen-depleted and non-depleted muscle immediately prior to 10 min of exercise at 75% V̇O2,max (0.44 ± 0.04 and 0.57 ± 0.09 mmol acetyl-CoA min−1 (kg wet muscle)−1, respectively; P > 0.05 or 3.67 ± 0.34 and 5.46 ± 0.96 nmol acetyl-CoA min−1 (mg protein)−1, respectively). Exercise for 10 min produced a 4- to 5-fold increase of muscle PDC activation in both treatment groups; however, there was no difference when comparing PDC activation between groups (glycogen-depleted muscle, 2.00 ± 0.17 mmol acetyl CoA min−1 (kg wet muscle)−1 or 16.92 ± 1.30 nmol acetyl-CoA min−1 (mg protein)−1; non-depleted muscle, 1.81 ± 0.13 mmol acetyl CoA min−1 (kg wet muscle)−1 or 18.28 ± 1.13 nmol acetyl-CoA min−1 (mg protein)−1). Thus, there was no difference in the magnitude of PDC activation during exercise between glycogen-depleted and non-depleted muscles (Fig. 3A).
Figure 3. Muscle PDC activity and calculated PDC flux during two 10-min bouts of exercise at 75% V̇O2,max with or without muscle glycogen depletion.
A, muscle PDC activity. B, calculated PDC flux. Values are means ± s.e.m. with n =10. ***Significantly different across the treatment (P < 0.001). The PDC activity and flux were expressed as rate of acetyl-CoA formation per mg protein at 37°C, assuming a protein concentration of 175 g (kg wet muscle)−1 for the flux calculation.
The calculated rate of flux through the PDC reaction in the glycogen-depleted muscle was more than 2-fold lower during exercise compared with the same measurement in the contralateral limb (Fig. 3B).
Blood and plasma metabolite
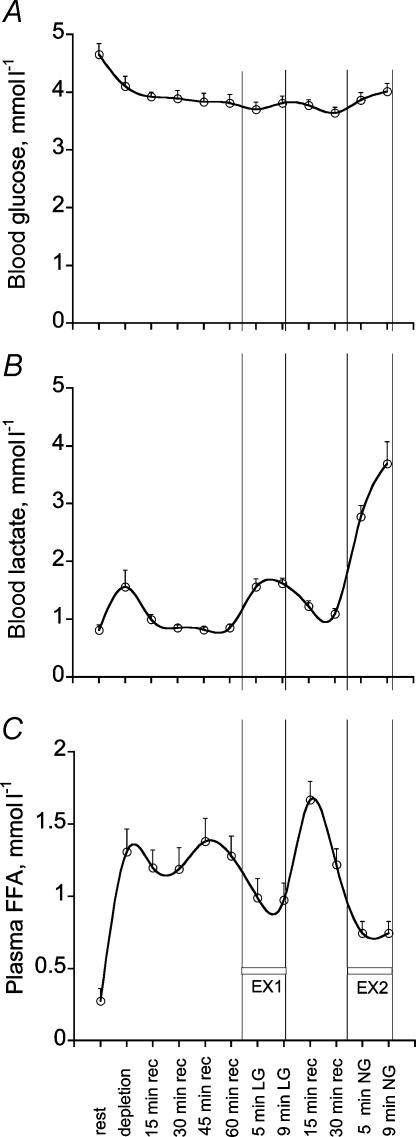
Blood and plasma metabolite concentration data are presented in Fig. 4. Given the nature of the protocol (single-leg exercise) and the relatively insensitive nature of blood and plasma markers in reflecting changes in muscle metabolism, no direct treatment effect on blood metabolites could be identified. However, some time effects were identified. Blood glucose concentration declined gradually during the single-leg exercise performed to deplete muscle glycogen, but thereafter remained relatively constant at ∼4 mmol l−1 (Fig. 4A). Blood lactate concentration was elevated above basal at three points during the protocol. This corresponded to the end of glycogen-depleting exercise, the 10 min bout of exercise with the glycogen-depleted limb and the second 10 min bout of exercise with the contralateral limb (Fig. 4B). Plasma free fatty acid concentration was elevated considerably above basal at the end of glycogen-depleting exercise, but was increased further during recovery following the 10 min of exercise with the glycogen-depleted limb (Fig. 4C).
Figure 4. Blood glucose, lactate and plasma free fatty acid concentrations over the course of the experimental protocol.
LG, glycogen depleted; NG, non-glycogen depleted; rec, recovery. Values are means ± s.e.m. with n = 10.
Discussion
The results of the present study demonstrate that muscle glycogen depletion, achieved by prolonged exercise, measurably diminished muscle glycogenolysis and pyruvate accumulation during subsequent intense exercise. However, this clearly has no effect on the transformation of pyruvate dehydrogenase complex (PDC) to its active form (PDCa) during exercise. Moreover, this latter response was observed under conditions when flux through the PDC reaction during exercise was reduced by more than 2-fold by glycogen depletion. It is therefore pertinent to conclude that, whilst pyruvate production is important to the rate of flux through the PDC reaction during muscle contraction in vivo, it is not of primary importance to the control of PDC activation during exercise, which is probably primarily regulated by muscle calcium availability under these conditions. The reported central role of pyruvate in PDC activation during in vivo muscle contraction may therefore have been over stated.
It could be contended that the exercise performed by subjects to deplete muscle glycogen in the present study may have negatively impacted upon motor recruitment, calcium release, excitation–contraction coupling and/or force production during the subsequent 10 min of exercise (Vøllestad et al. 1988; Stephenson et al. 1999; Leppik et al. 2004) and in such a way that influenced PDC activation. However, work from our own laboratory (Casey et al. 1996), and by others (Symons & Jacobs, 1989; Ren et al. 1990) has shown that initial power output during maximal intensity isokinetic and concentric cycling exercise, and isometric force generation during electrically evoked contraction, is not influenced by glycogen depleting exercise in humans, implying therefore that motor recruitment and calcium handling are unchanged. Furthermore, assuming these confounding variables produced as a result of glycogen-depleting exercise could have indeed negatively impacted upon motor recruitment and calcium handling, one would reasonably predict this would have resulted in lower activation of PDC in the glycogen-depleted leg. However, as we clearly demonstrate PDC activation was very similar across experimental treatments. Nevertheless, given that subjects were performing submaximal exercise in the present study, it can not be ruled out that an impairment in Ca2+ handling as a result of glycogen-depleted exercise (Leppik et al. 2004) might have been partially offset by increased fibre recruitment, and therefore Ca2+ release, during the second bout of exercise. It is worthy of note, however, that such a scenario, if anything, underlines the importance of Ca2+ to PDC activation during exercise, which is the central premise of this work.
The activity of PDC is regulated by a covalent mechanism involving a competing PDK and PDP pair. The outcome of this antagonism determines the amount of PDC existing in a non-phosphorylated (active) form, i.e. PDCa. The flux through PDCa is also acutely regulated by an allosteric mechanism; that is, the products of pyruvate oxidation (NADH and acetyl-CoA) inhibit the overall reaction by reversing the partial reactions catalysed by the components of the complex (Wieland, 1983).
As stated earlier, the results from in vitro experiments show that pyruvate and calcium seem to be the most important physiological activators of PDC, and act by inhibiting PDK and activating PDP reactions, respectively. Low concentrations of pyruvate (25–100 µmoll−1) have been shown to increase the activity of PDK (but not when thiamine pyrophosphate was absent), whereas higher concentrations (approaching 500 µmoll−1) were inhibitory (Cooper et al. 1975). In skeletal muscle, pyruvate inhibits PDK at a concentration of ∼3 mmoll−1 in the presence of Ca2+ ions (Fuller & Randle, 1984), but this concentration is not physiological. Nevertheless, Cate & Roche (1978) showed that phosphate ions augment the inhibitory effect of pyruvate on PDK. Taken together, these in vitro data suggest that the inhibitory effect of pyruvate on PDK is rather complex.
It is well established that muscle contraction increases pyruvate generation by enhancing glycogenolysis/glycolysis and leg glucose uptake. Furthermore, exercise increases pyruvate oxidation to acetyl-CoA by increasing the activity of PDC. It has been proposed that the primary mechanism regulating the activity of PDC during in vivo contraction is the calcium-mediated activation of PDP (Constantin-Teodosiu et al. 1991a). However, others have put equal, if not more, emphasis on pyruvate formation, and therefore PDK inhibition, being the principal regulator of PDC activation during contraction (Putman et al. 1993). For example, Putman et al. (1993) reported significantly less activation of muscle PDC during contraction both at rest and during exercise at 75% V˙O2,max following 3 days of high dietary fat intake, when compared with a high carbohydrate diet. The authors suggested that the high fat diet produced a reduction in muscle pyruvate accumulation which diminished PDK inhibition and thereby blunted PDC activation. However, the same research group were unable to reproduce this response in a subsequent study involving 6 days of high dietary fat intake and a lower exercise workload (65% V˙O2, max; St Amand et al. 2000). On the basis of this, and in contrast to their previous suggestion, the authors concluded that ‘pyruvate overrode a dietary fat mediated inhibition on PDC activation’. It is also worthy of comment that studies to date advocating a central role for pyruvate in PDC activation during exercise have been performed under conditions where muscle glycogen availability may not have significantly impaired pyruvate formation, i.e. in the range of 200–300 mmol (kg dry muscle)−1 (Putman et al. 1993; St Amand et al. 2000).
The protocol employed in the present study assured, for the first time, that muscle glycogen concentration was indeed very low (i.e. 60 mmol (kg dry muscle)−1), before exercise commenced. Accordingly, the rates of glycogen degradation and Glu-6-P, pyruvate and lactate accumulation were ∼3, 2, 3 and 17-fold lower, respectively, in glycogen-depleted muscle during 10 min of exercise at 75% V˙O2,max when compared with the corresponding rates determined in the contralateral, non-glycogen-depleted muscle (Fig. 2, Table 1). Nevertheless, despite these striking reductions in glycogenolysis and glycolysis during exercise in the glycogen-depleted muscle, the rates of PDC activation during exercise were almost identical when comparing muscles (Fig. 3A). This therefore clearly demonstrates that it is highly unlikely that pyruvate availability can limit PDC activation in human skeletal muscle in vivo. This is particularly the case when one considers that the in vitro Michaelis-Menten constant (Km) of human PDC for pyruvate is reported to be 25 µmol l−1 (Kiselevsky et al. 1990), which is likely to be exceeded during muscle contraction in almost all physiological conditions.
In support of this contention, we have previously demonstrated that intravenous infusion of pyruvate (36 mmol over 30 min) in healthy human volunteers had no effect on PDC activation in resting skeletal muscle (Constantin-Teodosiu et al. 1999). From our calculations, the amount of pyruvate that theoretically entered the tissue space in this study (∼0.67 mmol (l intracellular water)−1) should have favoured the activation of PDC to PDCa by inhibiting PDK. However it could be argued that the lack of PDC activation might have been attributable to a large fraction of the administrated pyruvate being sequestered by the liver, which would have been better perfused during infusion when compared with muscle. Against this view, is the observation that the infusion of DCA, whose structure is very similar to that of pyruvate, has been shown repeatedly to maximally activate muscle PDC at rest in humans (Timmons et al. 1996; Gibala & Saltin, 1999), and at a similar concentration to that employed in the study involving pyruvate infusion described above (Constantin-Teodosiu et al. 1999). However it is perhaps worthy of note in the context of this discussion, that DCA might also activate PDC by facilitating calcium entry into mitochondria (Browning et al. 1981). One potential mechanism being that a DCA-mediated reduction in pyruvate availability would diminish the inhibitory effect of pyruvate on sarcoplasmic reticulum Ca2+ release channels (Zima et al. 2003).
Flux through the PDC reaction, or its catalytic activity, is dependent on the amount of PDCa and the availability of pyruvate, coenzyme A (CoASH) and NAD+. As a result of the PDC reaction, pyruvate and CoASH are oxidized to acetyl-CoA, while NAD+ is reduced to NADH. The acetyl group formed is either further oxidized in the tricarboxylic acid (TCA) cycle or, when the availability of acetyl-CoA becomes greater than its rate of entry into TCA cycle, is retained mainly as acetylcarnitine. Muscle acetylcarnitine concentration declined by ∼2 mmol (kg dry muscle)−1 during exercise in the glycogen-depleted state, but was seen to increase by ∼11 mmol (kg dry muscle)−1 in the non-glycogen-depleted state. It is reasonable to conclude therefore that the rate of flux through the PDC reaction was markedly lower in glycogen-depleted muscle during contraction. Indeed, the calculated flux through the PDC reaction (estimated from the measured rates of glycogen degradation from which the rates of non-oxidative pyruvate disposal were subtracted, see Methods) was 2-fold lower in the glycogen-depleted state compared with the non-depleted state (Fig. 3B). The most likely explanation for this observation is that the rate of muscle pyruvate formation was markedly lower during exercise in the glycogen-depleted state, which is supported by the data presented in Table 1 and Fig. 2.
It is worth mentioning the significantly lower expansion of muscle TCAIs during exercise in the glycogen-depleted state compared with the non-glycogen-depleted state in the present study. This observation of a blunting of TCAI expansion in glycogen-depleted muscle is at odds with our previous observation of greater TCAI expansion during two-leg exercise in the presence of reduced muscle glycogen levels (Gibala et al. 2002). Closer inspection of these two studies offers some insight as to why this disparity may have occurred. Firstly, the study of Gibala et al. (2002) involved two-leg exercise and it is reasonable to suggest that the intensity of the exercise employed in the present study, that is one leg-exercise at 75% of two-leg V˙O2,max, might in reality have been greater than 75% V˙O2,max at the single leg level. Indeed, the magnitude of muscle lactate and acetylcarnitine accumulation observed in the non-glycogen-depleted leg after 10 min of exercise was 2- to 3-fold greater than we observed previously during two-leg cycling exercise (Gibala et al. 2002), which may also explain the difference in the degree of PDC activation between these two studies. Secondly, and probably more importantly, the magnitude of muscle glycogen depletion achieved in the present study was greater than that achieved in the study of Gibala et al. (2002), i.e. 60 versus 200 mmol (kg dry muscle)−1. Therefore it is not unreasonable to suggest that muscle pyruvate accumulation, and thus TCAI expansion, would have been lower in the present study. Furthermore, because PDC activation during exercise in the glycogen-depleted state in the study of Gibala et al. (2002), was lower than in the present study it is likely that more pyruvate would have been diverted towards TCAI expansion, even under conditions of identical pyruvate availability.
The present study showed that the near-complete depletion of glycogen in human skeletal muscle markedly reduced pyruvate production and flux through the PDC reaction during 10 min of exercise at 75% V̇O2, max, but did not affect the activation of PDC during exercise. It is therefore pertinent to conclude that whilst pyruvate production is important to the magnitude of flux through the PDC reaction, it is of little importance to PDC activation during contraction, which we believe is due to the dominant role of calcium-mediated activation of PDC under these conditions. The proposed central role of pyruvate in muscle PDC activation during in vivo contraction may therefore have been over stated.
References
- Bergrneyer HU, editor. Methods of Enzymatic Analysis. 3. Verlag Chemie: Weinheim; 1995. [Google Scholar]
- Bergström J. Muscle electrolytes in man. Determined by neutron activation analysis on needle biopsy specimens. A study on normal subjects, kidney patients, and patients with chronic diarrhoea. Scand J Clin Lab Invest. 1962;14:1–110. [Google Scholar]
- Browning M, Baudry M, Bennett WF, Lynch G. Phosphorylation-mediated changes in pyruvate dehydrogenase activity influence pyruvate-supported calcium accumulation by brain mitochondria. J Neurochem. 1981;36:1932–1940. doi: 10.1111/j.1471-4159.1981.tb10817.x. [DOI] [PubMed] [Google Scholar]
- Casey A, Short AH, Curtis S, Greenhaff PL. The effect of glycogen availability on power output and the metabolic response to repeated bouts of maximal, isokinetic exercise in man. Eur J Appl Physiol Occup Physiol. 1996;72:249–255. doi: 10.1007/BF00838647. [DOI] [PubMed] [Google Scholar]
- Casey A, Short AH, Hultman E, Greenhaff PL. Glycogen resynthesis in human muscle fibre types following exercise-induced glycogen depletion. J Physiol. 1995;483:265–271. doi: 10.1113/jphysiol.1995.sp020583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate RL, Roche TE. A unifying mechanism for stimulation of mammalian pyruvate dehydrogenase(a) kinase by reduced nicotinamide adenine dinucleotide, dihydrolipoamide, acetyl coenzyme A, or pyruvate. J Biol Chem. 1978;253:496–503. [PubMed] [Google Scholar]
- Cederblad G, Carlin JI, Constantin-Teodosiu D, Harper P, Hultman E. Radioisotopic assays of CoASH and carnitine and their acetylated forms in human skeletal muscle. Anal Biochem. 1990;185:274–278. doi: 10.1016/0003-2697(90)90292-h. [DOI] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Carlin JI, Cederblad G, Harris RC, Hultman E. Acetyl group accumulation and pyruvate dehydrogenase activity in human muscle during incremental exercise. Acta Physiol Scand. 1991a;143:367–372. doi: 10.1111/j.1748-1716.1991.tb09247.x. [DOI] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Cederblad G, Hultman E. A sensitive radioisotopic assay of pyruvate dehydrogenase complex in human muscle tissue. Anal Biochem. 1991b;198:347–351. doi: 10.1016/0003-2697(91)90437-x. [DOI] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Simpson EJ, Greenhaff PL. The importance of pyruvate availability to PDC activation and anaplerosis in human skeletal muscle. Am J Physiol. 1999;276:E472–E478. doi: 10.1152/ajpendo.1999.276.3.E472. [DOI] [PubMed] [Google Scholar]
- Cooper RH, Randle PJ, Denton RM. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975;257:808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- Forsberg AM, Nilsson E, Werneman J, Bergstrom J, Hultman E. Muscle composition in relation to age and sex. Clin Sci (Lond) 1991;81:249–256. doi: 10.1042/cs0810249. [DOI] [PubMed] [Google Scholar]
- Fuller SJ, Randle PJ. Reversible phosphorylation of pyruvate dehydrogenase in rat skeletal-muscle mitochondria. Effects of starvation and diabetes. Biochem J. 1984;219:635–646. doi: 10.1042/bj2190635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, Peirce N, Constantin-Teodosiu D, Greenhaff PL. Exercise with low muscle glycogen augments TCA cycle anaplerosis but impairs oxidative energy provision in humans. J Physiol. 2002;540:1079–1086. doi: 10.1113/jphysiol.2001.012983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, Saltin B. PDH activation by dichloroacetate reduces TCA cycle intermediates at rest but not during exercise in humans. Am J Physiol. 1999;277:E33–E38. doi: 10.1152/ajpendo.1999.277.1.E33. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Laboratory Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Howlett RA, Parolin ML, Dyck DJ, Hultman E, Jones NL, Heigenhauser GJ, Spriet LL. Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs. Am J Physiol. 1998;275:R418–R425. doi: 10.1152/ajpregu.1998.275.2.R418. [DOI] [PubMed] [Google Scholar]
- Kiselevsky YV, Ostrovtsova SA, Strumilo SA. Kinetic characterization of the pyruvate and oxoglutarate dehydrogenase complexes from human heart. Acta Biochim Pol. 1990;37:135–139. [PubMed] [Google Scholar]
- Leppik JA, Aughey RJ, Medved I, Fairweather I, Carey MF, McKenna MJ. Prolonged exercise to fatigue in humans impairs skeletal muscle Na+-K+-ATPase activity, sarcoplasmic reticulum Ca2+ release, and Ca2+ uptake. J Appl Physiol. 2004;97:1414–1423. doi: 10.1152/japplphysiol.00964.2003. [DOI] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Putman CT, Spriet LL, Hultman E, Lindinger MI, Lands LC, McKelvie RS, Cederblad G, Jones NL, Heigenhauser GJ. Pyruvate dehydrogenase activity and acetyl group accumulation during exercise after different diets. Am J Physiol. 1993;265:E752–E760. doi: 10.1152/ajpendo.1993.265.5.E752. [DOI] [PubMed] [Google Scholar]
- Ren JM, Broberg S, Sahlin K, Hultman E. Influence of reduced glycogen level on glycogenolysis during short-term stimulation in man. Acta Physiol Scand. 1990;139:467–474. doi: 10.1111/j.1748-1716.1990.tb08948.x. [DOI] [PubMed] [Google Scholar]
- Roberts PA, Loxham SJ, Poucher SM, Constantin-Teodosiu D, Greenhaff PL. The temporal relationship between glycogen phosphorylase and activation of the pyruvate dehydrogenase complex during adrenaline infusion in resting canine skeletal muscle. J Physiol. 2002;545:297–304. doi: 10.1113/jphysiol.2002.021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Amand TA, Spriet LL, Jones NL, Heigenhauser GJ. Pyruvate overrides inhibition of PDH during exercise after a low-carbohydrate diet. Am J Physiol Endocrinol Metab. 2000;279:E275–E283. doi: 10.1152/ajpendo.2000.279.2.E275. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Nguyen LT, Stephenson GM. Glycogen content and excitation-contraction coupling in mechanically skinned muscle fibres of the cane toad. J Physiol. 1999;519:177–187. doi: 10.1111/j.1469-7793.1999.0177o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons JD, Jacobs I. High-intensity exercise performance is not impaired by low intramuscular glycogen. Med Sci Sports Exerc. 1989;21:550–557. [PubMed] [Google Scholar]
- Timmons JA, Poucher SM, Constantin-Teodosiu D, Worrall V, Macdonald IA, Greenhaff PL. Increased acetyl group availability enhances contractile function of canine skeletal muscle during ischemia. J Clin Invest. 1996;97:879–883. doi: 10.1172/JCI118490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vøllestad NK, Sejersted OM, Bahr R, Woods JJ, Bigland-Ritchie B. Motor drive and metabolic responses during repeated submaximal contractions in humans. J Appl Physiol. 1988;64:1421–1427. doi: 10.1152/jappl.1988.64.4.1421. [DOI] [PubMed] [Google Scholar]
- Wieland OH. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]
- Zima AV, Kockskamper J, Mejia-Alvarez R, Blatter LA. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J Physiol. 2003;550:765–783. doi: 10.1113/jphysiol.2003.040345. [DOI] [PMC free article] [PubMed] [Google Scholar]