Abstract
We have recently reported that inhibition of nitric oxide synthase (NOS) with NG-nitro-l-arginine methyl ester (l-NAME) accelerates the ‘phase II’ pulmonary O2 uptake (V̇O2) kinetics following the onset of moderate and heavy intensity submaximal exercise in humans. These data suggest that the influence of nitric oxide (NO) on mitochondrial function is an important factor in the inertia to aerobic respiration that is evident in the transition from a lower to a higher metabolic rate. The purpose of the present study was to investigate the influence of l-NAME on pulmonary V̇O2 kinetics following the onset of supra-maximal exercise, where it has been suggested that O2 availability represents an additional limitation to V̇O2 kinetics. Seven healthy young men volunteered to participate in this study. Following an incremental cycle ergometer test for the determination of V̇O2max, the subjects returned on two occasions to perform a ‘step’ exercise test from a baseline of unloaded cycling to a work rate calculated to require 105% V̇O2max, preceded either by systemic infusion of l-NAME (4 mg kg−1 in 50 ml saline) or 50 ml saline as a control (Con). Pulmonary gas exchange was measured on a breath-by-breath basis throughout the exercise tests. The duration of ‘phase I’ was greater with l-NAME (Con: 14.0 ± 2.1 versus l-NAME: 16.0 ± 1.6 s; P = 0.03), suggestive of a slower cardiovascular adaptation following the onset of exercise. However, the phase II V̇O2 time constant was reduced by 44% with l-NAME (Con: 36.3 ± 17.3 versus l-NAME: 20.4 ± 8.3 s; P = 0.01). The accumulation of blood lactate during exercise was also reduced with l-NAME (Con: 4.0 ± 1.1 versus l-NAME: 2.7 ± 2.1 mm; P = 0.04). These data indicate that skeletal muscle NO production represents an important limitation to the acceleration of oxidative metabolism following the onset of supra-maximal exercise in humans.
Upon the abrupt transition from rest to exercise, there is an initial lag in the contribution of oxidative phosphorylation to the ATP turnover rate required for muscle force generation, with the deficit being made up by an acceleration of substrate-level phosphorylation (i.e. muscle phosphocreatine hydrolysis and, for higher work rates above the so-called lactate threshold (LT), anaerobic glycolysis) (Krogh & Lindhard, 1920; Whipp & Wasserman, 1972). At submaximal work rates below the LT, muscle O2 consumption, and pulmonary oxygen uptake V̇O2, rise with an exponential time course to reach a steady-state within 2–3 min in young healthy subjects (Whipp & Wasserman, 1972). At higher submaximal work rates (i.e. above the LT), however, a steady state in V̇O2 is either delayed (if the work rate is below the critical power, CP; Monod & Scherrer, 1965) or is not attained at all (if the work rate is above the CP), due to the emergence of a ‘slow component’ of V̇O2 that causes V̇O2 to rise above the expected steady-state value (Whipp & Wasserman, 1972; Linnarsson, 1974). At supra-maximal work rates (i.e. work rates with an energy equivalent above the V̇O2max), it is by definition impossible for a steady-state V̇O2 to be attained. Whether or not a V̇O2 slow component can be reliably discerned at these work rates probably depends on the exercise intensity and the time for which exercise can be sustained (Grassi et al. 2000; Scheuermann & Barstow, 2003). However, at supra-maximal work rates requiring > ∼105–110% V̇O2max, where time to exhaustion is ≤ 2 min, the available evidence indicates that V̇O2 rises exponentially until V̇O2max is achieved or the exercise is terminated by fatigue (Özyener et al. 2001; Hill et al. 2002; Scheuermann & Barstow, 2003; Wilkerson et al. 2004a).
The factor(s) that limit(s) the rise in V̇O2 in the transition from a lower to a higher metabolic rate is/are obscure. For submaximal cycle exercise < LT in young healthy subjects, there is compelling evidence that ‘metabolic inertia’ (e.g. the activation kinetics of key oxidative enzymes) represents the principal limitation to muscle O2 consumption following the onset of exercise (e.g. Whipp & Mahler, 1980; Grassi et al. 1998; Rossiter et al. 1999; Burnley et al. 2000; Koga et al. 2001); the limitations to the rate of rise of muscle oxygen consumption > LT are, however, more controversial (Hughson et al. 2001). Relatively few studies have examined V̇O2 kinetics during supra-maximal exercise. However, at maximal and supra-maximal work rates, there is some evidence that muscle O2 availability provides an additional limitation to V̇O2 dynamics. For example, Grassi et al. (2000) demonstrated that V̇O2 kinetics were ∼25% faster when muscle contractions at an intensity requiring 100% V̇O2max were initiated with blood flow set at the ‘steady-state’ requirement across the rest-to-exercise transition in an isolated in situ canine muscle preparation. However, in contrast, the performance of prior high-intensity exercise, which is known to result in increased muscle blood flow (Krustrup et al. 2001), did not alter phase II pulmonary V̇O2 kinetics during supra-maximal exercise in humans (Wilkerson et al. 2004a). The potential for O2 availability to modulate V̇O2 kinetics during maximal intensity exercise therefore remains unclear.
Nitric oxide (NO) has been implicated in a multitude of physiological functions including the control of skeletal muscle vasodilatation and oxidative metabolism (Moncada et al. 1991; Stamler & Meissner, 2001). Specifically, it has been proposed that NO is one of several vaso-active substances that are released at exercise onset and which interact to regulate vascular conductance and muscle blood flow (e.g. Moncada et al. 1991; Shepherd & Katusic, 1991; Sander et al. 1995). It has also been demonstrated, in vitro, that NO inhibits a number of key oxidative enzymes and competes with O2 for the binding site at cytochrome oxidase (e.g. Shen et al. 1994; Zhang & Snyder, 1995; Cassina & Radi, 1996; Brown, 1999, 2000). It is therefore possible that NO represents at least one source of the metabolic inertia following the onset of exercise. Indeed, inhibition of nitric oxide synthase (NOS, the enzyme responsible for the synthesis of NO) with the l-arginine analogue NG-nitro-l-arginine methyl ester (l-NAME) has been shown to result in a speeding of the phase II pulmonary V̇O2 kinetics during both moderate (< LT) and heavy (> LT) exercise both in the horse (Kindig et al. 2001, 2002) and in humans (Jones et al. 2003, 2004a). These results are important because they demonstrate that V̇O2 kinetics can be speeded by NOS inhibition despite a possible reduction in bulk muscle blood flow during exercise.
The purpose of the present study was to extend our previous investigations (Jones et al. 2003, 2004a) by examining the effect of l-NAME on V̇O2 kinetics during supra-maximal exercise. As mentioned above, there is a stronger possibility that V̇O2 kinetics are limited by muscle O2 availability during supra-maximal compared to submaximal exercise (Grassi et al. 1998, 2000). Infusion of l-NAME therefore potentially permits an exploration of the interaction of intrinsic (metabolic inertia) and extrinsic (muscle O2 delivery) factors on pulmonary V̇O2 kinetics during supra-maximal exercise. It would be expected that sluggish cardiac output (and muscle blood flow) kinetics with l-NAME would be reflected in an increased circulatory transit delay (as evidenced by an increased phase I duration) before the exponential increase in V̇O2 (phase II) towards the steady state (Barstow & Molé, 1987; Barstow et al. 1990). If this is the case, and O2 availability is an important determinant of V̇O2 kinetics during supra-maximal exercise, then the anticipated speeding of the phase II V̇O2 kinetics with l-NAME (Kindig et al. 2001, 2002; Jones et al. 2003, 2004a) should be attenuated or eliminated. Our first hypothesis was that l-NAME would significantly increase the duration of the phase I pulmonary V̇O2 response to exercise, consistent with a reduction in skeletal muscle blood flow in this condition. Our second hypothesis was that the phase II pulmonary V̇O2 kinetics would be faster with l-NAME, despite the possibility of a reduction in skeletal muscle blood flow in this condition.
Methods
Subjects
Seven healthy males (mean ± s.d.: age 25 ± 4 years, body mass 78.5 ± 9.2 kg) volunteered to participate in this study. All subjects were informed of the experimental procedures, the potential risks and discomfort, and that they could withdraw from the study at any time without prejudice. All subjects gave their written informed consent. The experiments were approved by the Manchester Metropolitan University and South Cheshire Local Research Ethics Committees.
Procedures
The subjects were required to visit the laboratory on three occasions. On the first visit, they completed an incremental exercise test to exhaustion on an electronically braked cycle ergometer that maintained work rate independent of pedal rate within the range 30–120 rev min−1 (Jaeger Ergoline E800, Mindjhaart, The Netherlands). Following 3 min of ‘unloaded’ cycling, work rate was increased by 5 W every 10 s (i.e. 30 W min−1) until the subject was unable to continue. The subjects cycled at a self-selected pedal rate (60–90 rev min−1) and this pedal rate, and the saddle and handlebar heights and configurations were recorded and reproduced in subsequent tests. Pulmonary gas exchange was measured on a breath-by-breath basis (see below). The gas exchange threshold (GET) was determined from a cluster of measurements including: (1) the first disproportionate increase in V̇CO2 from visual inspection of individual plots of V̇CO2 versus V̇O2 (V-slope method; Beaver et al. 1986); (2) the first increase in the ventilatory equivalent for O2 with no increase in the ventilatory equivalent for CO2; and (3) an increase in end-tidal O2 tension with no fall in end-tidal CO2 tension. The V̇O2max was determined as the highest value recorded in any 30 s period before the subject's volitional termination of the test. The work rate that would require 105% of the V̇O2max was calculated as 1.05 times the maximal work rate attained in the incremental test minus 20 W (to account for the ‘lag’ in the V̇O2 response during the incremental test; Whipp et al. 1981).
On the second and third visits to the laboratory, the subjects performed a ‘step’ bout of exercise at the work rate corresponding to 105% V̇O2max following infusion of either l-NAME or saline. The conditions were presented in a counter-balanced design with the subjects blinded to the nature of the infusate. For each subject, the conditions were separated by 7 days. Following arrival at the laboratory, the subjects lay supine and a cannula was placed in a hand vein. The subjects then rested for 20 min before either l-NAME (Merck Biosciences AG, Nottingham, UK; 4 mg (kg body mass)−1 in 50 ml saline), or an equivalent volume of saline (as a placebo), was infused over 60 min. Our protocol for the l-NAME infusion was based on that described by Frandsen et al. (2001) who demonstrated a 67% reduction in NOS activity in skeletal muscle with this procedure. Throughout the infusions, blood pressure and heart rate (HR) were monitored with an automated sphygmomanometer and a HR monitor (Polar Electro, Finland), respectively. The mean arterial blood pressure (MAP) was calculated as the diastolic pressure plus one-third of the pulse pressure.
Following 60 min rest, the subjects performed a supra-maximal exercise bout for as long as possible. The exercise protocol began with 3 min of baseline pedalling at 20 W (the lowest available work rate on the cycle ergometer), followed by an abrupt transition to the 105% V̇O2max work rate. The subjects received strong verbal encouragement to continue to exhaustion in each condition. Pulmonary gas exchange was measured breath-by-breath and HR was recorded at 5 s intervals throughout all exercise tests. The subjects wore a nose-clip and breathed through a low dead space, low resistance mouthpiece and volume sensor assembly. Pulmonary gas exchange was measured with a mass spectrometer and volume turbine system (Morgan EX670, Morgan Medical Limited, Gillingham, Kent). The system was calibrated prior to each test using gases of known concentration and a precision 3 l calibration syringe. A fingertip blood sample was collected into a capillary tube immediately before and after exercise in each condition and subsequently analysed for blood [lactate] (YSI 1500 Sport Lactate Analyzer, Yellow Springs Instruments, OH, USA). Blood pressure was measured within the first 30 s of recovery from exercise.
Analysis of V̇O2 kinetics
The breath-by-breath V̇O2 data for each transition were initially scrutinized to identify the phase I–phase II transition point (i.e. respiratory exchange ratio (RER) and end-tidal O2 partial pressure (PET,O2) starting to fall and end-tidal CO2 partial pressure (PET,CO2) starting to increase; Whipp et al. 1982). The breath-by-breath data were subsequently interpolated to give second-by-second values and the time course of after the onset of exercise was initially described for each subject using a mathematical model which featured two exponential terms:
where V̇O2B is the baseline V̇O2, and Ap and As are the response amplitudes; TDp and TDs are the time delays, and τp and τs are the time constants for the primary (p) and slow (s) components of the response, respectively. The first 20 s of data (containing the phase I response, see above) were not included in the model fit. Model parameters were determined by using a least-squares non-linear regression in which minimizing the sum of squared errors was the criterion for convergence. The amplitude of the V̇O2 slow component was described as the increase in V̇O2 from TDs to the end of exercise (As′).
Owing to concerns over the accuracy with which a slow component phase can be discerned in a single transition to supra-maximal exercise, we also described the overall V̇O2 response to exercise (i.e. with no attempt to separate the response into its constituent primary and slow component phases), with a single exponential function following the removal of the first 20 s of data after the onset of exercise:
where A is the amplitude of the V̇O2 response above baseline, TD represents a time delay and τ is the ‘effective’ time constant describing the kinetics of the change in V̇O2 over the exercise bout.
Statistics
Paired t tests were used to test for significant differences in the V̇O2 kinetic parameters between the control and l-NAME conditions. Pearson product moment correlation coefficients were used to examine the relationship between variables. Statistical significance was accepted when P < 0.05. Results are reported as mean ± s.d.
Results
The subjects' mean (± s.d.) was 52.3 ± 7.5 ml kg−1 min−1 with GET occurring at 50 ± 8% V̇O2max. The increase in work rate above baseline cycling (20 W) to 105% V̇O2max was 360 ± 44 W. The time to exhaustion was not significantly different between the conditions (Con: 169 ± 35 versus l-NAME: 161 ± 34 s; P = 0.08).
As was expected (Sander et al. 1999), l-NAME infusion resulted in an elevation of MAP and a reduction in HR. At the end of the unloaded cycling period, MAP was significantly higher (Con: 96 ± 12 versus l-NAME: 116 ± 7 mmHg; P = 0.04) and heart rate was significantly lower (Con: 86 ± 14 versus l-NAME: 75 ± 8 beats min−1; P = 0.04) following l-NAME infusion. In the immediate recovery period following exercise, MAP remained elevated (Con: 107 ± 18 versus l-NAME: 123 ± 22 mmHg; P = 0.07). HR was significantly lower in the l-NAME condition for the first 50 s of exercise, and at exhaustion (Fig. 1).
Figure 1.
Mean (± s.d.) heart rate response to supra-maximal exercise in the placebo condition (•) and following the infusion of l-NAME (○). *P < 0.05; **P < 0.01. Time ○ represents onset of supra-maximal exercise.
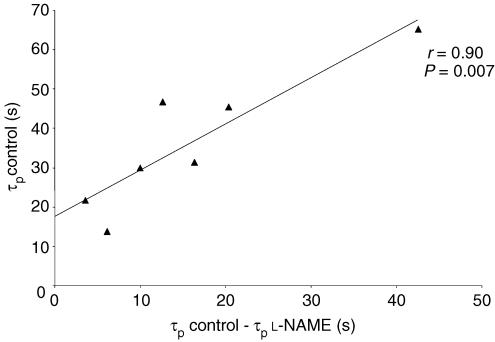
The V̇O2 kinetic response data are presented in Table 1, and example responses are shown in Figs 2 and 3. l-NAME infusion resulted in a significant lengthening of the duration of phase I (Con: 14.0 ± 2.1 versus l-NAME: 16.0 ± 1.6 s; P = 0.03; Fig. 2). The O2 pulse (i.e. V̇O2/HR) was not significantly different between the conditions at 5 s of exercise (Con: 11.1 ± 2.7 versus l-NAME: 12.4 ± 2.2 ml beat−1; P = 0.23) but was significantly greater with l-NAME at 10 s (Con: 11.5 ± 2.8 versus l-NAME: 14.2 ± 2.3 ml beat−1; P < 0.01) and 15 s (Con: 12.2 ± 1.9 versus l-NAME: 13.8 ± 2.3 ml beat−1; P < 0.01) of exercise. L-NAME infusion resulted in a significant reduction in the time constant describing the phase II V̇O2 response (Con: 36.3 ± 17.3 versus l-NAME: 20.4 ± 8.3 s; P = 0.01; Fig. 3). The 95% confidence interval for the estimation of the phase II time constant was 6–7 s, on average. The increased duration of phase I and the reduction of the phase II time constant with l-NAME were not significantly correlated (r = 0.08; P = 0.87). The individual changes in the duration of phase I and in the phase II V̇O2 time constant with l-NAME are illustrated in Fig. 4. The speeding of the phase II time constant with l-NAME was significantly correlated with the control value for the phase II time constant (r = 0.90; P = 0.006; Fig. 5), indicating that those individuals with the slowest phase II V̇O2 kinetics in the control condition evidenced a greater speeding of these kinetics following the infusion of l-NAME. In addition to effects on the dynamic adjustment of V̇O2 following the onset of exercise, the infusion of l-NAME was also associated with a reduction in the amplitude of the phase II V̇O2 response (Table 1).
Table 1.
Pulmonary oxygen uptake kinetics during supramaximal exercise in the control (C) and l-NAME (L) conditions
| V̇O2B (ml min−1) | Ap′ (ml min−1) | TDp (s) | τp (s) | As′ (ml min−1) | TDs (s) | V̇2EE (ml min−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subj. | C | L | C | L | C | L | C | L | C | L | C | L | C | L |
| 1 | 654 | 606 | 2521 | 2438 | 21.0 | 29.8 | 31.3 | 15.0 | 275 | 368 | 114 | 65 | 2796 | 2806 |
| 2 | 831 | 708 | 3308 | 3193 | 16.9 | 19.7 | 46.7 | 34.1 | 474 | 475 | 133 | 122 | 3782 | 3668 |
| 3 | 640 | 664 | 2708 | 2585 | 12.1 | 13.3 | 21.8 | 18.2 | — | — | — | — | 2708 | 2585 |
| 4 | 738 | 783 | 2050 | 1853 | 14.8 | 23.6 | 45.5 | 25.2 | — | 344 | — | 120 | 2050 | 2197 |
| 5 | 886 | 913 | 3763 | 3325 | 15.6 | 20.7 | 65.2 | 22.7 | — | — | — | — | 3763 | 3325 |
| 6 | 782 | 650 | 2276 | 2126 | 13.2 | 17.6 | 30.0 | 20.0 | 1154 | 703 | 121 | 86 | 3430 | 2829 |
| 7 | 748 | 687 | 2755 | 2395 | 20.8 | 25.1 | 13.9 | 7.7 | 306 | 878 | 80 | 78 | 3061 | 3273 |
| Mean | 754 | 716 | 2769 | 2559 | 16.3 | 21.4 | 36.3 | 20.4 | 552 | 606 | 112 | 88 | 3084 | 2955 |
| s.d. | 89 | 103 | 592 | 535 | 3.5 | 5.4 | 17.3 | 8.3 | 411 | 229 | 25 | 24 | 627 | 499 |
| P | 0.21 | 0.007 | 0.003 | 0.01 | 0.81 | 0.11 | 0.29 | |||||||
V̇O2B, baseline V̇O2; Ap′, amplitude of phase II V̇O2 response; TDp, time delay before phase II V̇O2 response; τp, time constant of phase II V̇O2 response; As′, amplitude of slow component at the end of exercise; TDs, time delay before onset of slow component; V̇O2EE, end-exercise V̇O2; Subj., subject.
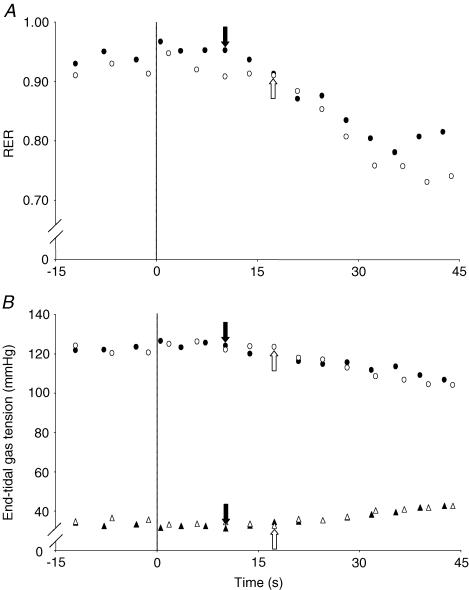
Figure 2.
Respiratory exchange ratio (RER; A) and end-tidal gas profiles (B) following the transition from unloaded cycling to a supra-maximal work rate in the placebo (•) and l-NAME (○) conditions in a typical subject. Notice the delay in the l-NAME condition before RER (A) and PET,O2 (B, top) begin to fall and PET,CO2 begins to rise. These data suggest a more sluggish cardiac output response in the l-NAME condition.
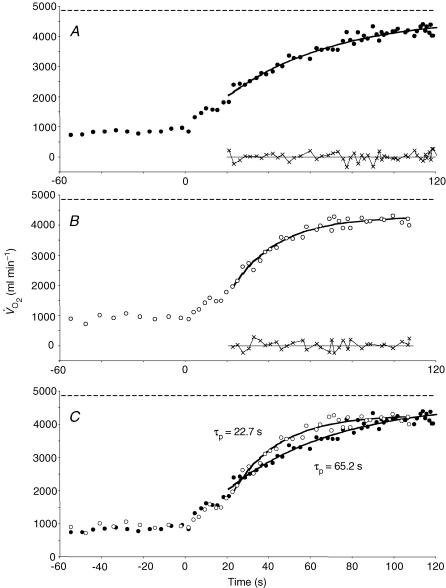
Figure 3.
Pulmonary O2 uptake response to supra-maximal exercise in the placebo condition (A) and following the infusion of l-NAME in one subject (B). The residuals at the bottom of each plot indicate the goodness of the model fit. In C, the O2 uptake responses to exercise in the placebo (•) and l-NAME (○) conditions are overlaid. Note the dramatic speeding of the phase II V̇O2 kinetics in this subject. The horizontal dashed line in all three plots represents the subject's V̇O2max value as determined from the preliminary incremental exercise test.
Figure 4.
Individual changes in the duration of phase I and the time constant (τp) for the phase II V̇O2max response in the placebo condition (•) and following the infusion of l-NAME (○). The bold lines and square symbols represent the group mean responses.
Figure 5.
Relationship between the time constant of the phase II V̇O2max response (τp) in the placebo condition and the speeding of the phase II V̇O2max kinetics resulting from the infusion of l-NAME.
A V̇O2 slow component was evident in 4/7 subjects in the control condition and in 5/7 subjects in the l-NAME condition. The existence or otherwise of the slow component was discerned from the goodness of fit of the single and double exponential models to each data set. On average, the mean squared error was reduced by 20% in those data sets in which a double exponential model provided a better description of the data than a single exponential model. There was no significant difference in the amplitude of the V̇O2 slow component between conditions, but there was a tendency for the slow component to emerge earlier with l-NAME (Table 1). There was no significant difference in the end-exercise V̇O2 between the conditions.
When the V̇O2 data were described with a mono-exponential model with time delay following the removal of the phase I response, the principal findings were essentially unchanged. Specifically, the effective time constant was significantly shorter (Con: 50.5 ± 22.8 versus 34.1 ± 12.6 s; P = 0.04) following the infusion of l-NAME.
Immediately before the onset of exercise, blood [lactate] was significantly higher in the l-NAME condition compared to control (Con: 1.1 ± 0.2 versus l-NAME: 1.6 ± 0.3 mm; P = 0.01). There was no significant difference between the conditions at the end of exercise (Con: 5.1 ± 1.2 versus l-NAME: 4.3 ± 2.0 mm; P = 0.10) but blood lactate accumulation (i.e. Δ blood [lactate]) was significantly reduced with l-NAME (Con: 4.0 ± 1.1 versus l-NAME: 2.7 ± 2.1 mm; P = 0.04).
Discussion
In support of our experimental hypotheses, l-NAME resulted in a small but significant increase in the duration of phase I and a significant reduction in the time constant describing the phase II increase in V̇O2. The significant increase in the duration of phase I suggests that NOS inhibition reduced cardiac output early in the transition from light to supra-maximal cycle exercise. However, the phase II time constant was reduced by 44% with l-NAME suggesting that the inhibition of the NO production reduced some of the metabolic inertia to the increase in V̇O2 following the onset of exercise. The present study therefore suggests that any reduction in muscle blood flow, and therefore O2 availability, was insufficient to fully cancel out the speeding of V̇O2 kinetics brought about by the reduction in metabolic inertia with NOS inhibition.
Phase I
We found that the duration of phase I was slightly but significantly longer in the l-NAME condition compared to the control condition (i.e. ∼14 versus 16 s; Fig. 2). These data imply that muscle blood flow was at least transiently reduced following NOS inhibition. This interpretation is supported by the significant increase in the O2 pulse with l-NAME at 10 and 15 s of exercise. Other situations in which the duration of phase I is increased include subjects with chronic heart failure and in heart transplant recipients where the cardiovascular response to exercise is sluggish (Sietsema et al. 1986, 1994; Mettauer et al. 2000; Borrelli et al. 2003). In our study, the precise cause of the increased duration of phase I with l-NAME is somewhat unclear, but it was associated with a reduced HR both at rest and throughout exercise with the differences being significant over the first 50 s of exercise and at exhaustion. It has been proposed that the reduced vascular conductance with NOS inhibition causes a reflex reduction in HR as the result of a baroreceptor-mediated withdrawal of sympathetic outflow (Sheriff et al. 2000; Joyner & Tschakovsky, 2003). It is possible therefore that l-NAME reduces cardiac output and bulk muscle blood flow during exercise. However, it is also possible that the higher MAP with l-NAME arises from vasoconstriction in the vascular beds of other organs including non-active skeletal muscle, with blood flow to contracting skeletal muscle being well preserved (Frandsen et al. 2001). It has also been suggested that the reduced vascular conductance at rest with NOS inhibition could reduce the effectiveness of the muscle pump at exercise onset (Shoemaker & Hughson, 1999) which would also impact upon the duration of phase I. Interestingly, there was no appreciable difference in the duration of phase I with l-NAME in our two previous studies which were conducted at lower intensities (Jones et al. 2003, 2004a), suggesting that NO might be relatively more important in the regulation of muscle blood flow at the onset of high compared to low intensity cycle exercise.
In humans, NO is important in the regulation of vascular tone at rest and during recovery from exercise, but its role in the regulation of skeletal muscle blood flow during exercise is more controversial. For example, it has been recently reported that (partial) NOS inhibition does not significantly influence muscle blood flow during submaximal or maximal exercise with small muscle groups (Shoemaker et al. 1997; Radegran & Saltin, 1999; Frandsen et al. 2001). However, simultaneous inhibition of NOS and either prostaglandins (Boushel et al. 2002) or endothelial-derived hyperpolarizing factors (Hillig et al. 2003) does result in a reduction of skeletal muscle blood flow. It should be borne in mind that in the study of Frandsen et al. (2001) in which no effect of l-NAME on muscle hyperaemia was reported, discrete measurements of muscle blood flow were made at 10 and 20 min of submaximal exercise and at an unspecified time point during maximal exercise. The present study suggests, however, that NO might be important in the regulation of muscle blood flow early in the transition to exercise, i.e. over (at least) the first ∼15 s following the onset of supra-maximal exercise.
Phase II
Despite the possibility that muscle blood flow was reduced, at least transiently, the phase II V̇O2 kinetics were substantially faster (τ reduced from ∼36 to ∼20 s) in the l-NAME condition. These results corroborate our earlier work which demonstrated faster phase II V̇O2 kinetics with l-NAME during moderate and heavy submaximal exercise (Jones et al. 2003, 2004a) and extend them to show that NO represents an important component of the metabolic inertia to the V̇O2 dynamics during supra-maximal exercise also. The precise mechanism by which NO contributes to the metabolic inertia at exercise onset is unclear but, in vitro, it has been demonstrated that NO inhibits a number of key tricarboxylic acid and electron transport chain enzymes and also competitively inhibits mitochondrial respiration by competing with O2 for the binding site at cytochrome oxidase (e.g. Shen et al. 1994; Zhang & Snyder, 1995; Cassina & Radi, 1996; Brown, 1999, 2000).
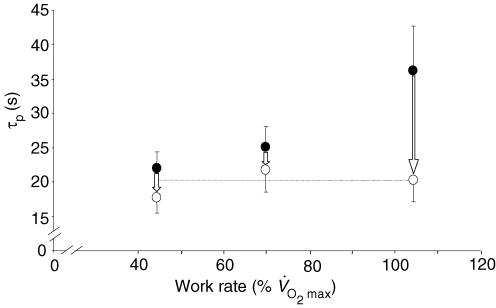
Interestingly, the magnitude of the speeding of the phase II V̇O2 kinetics in the present study (44%) was greater than we have previously observed during exercise at ∼45% V̇O2max (19% speeding; Jones et al. 2003) and ∼70% V̇O2max (13% speeding; Jones et al. 2004a). The possible limitations to V̇O2 kinetics are complex and depend, in part, upon the intensity domain in which the exercise is performed (Whipp & Ward, 1990; Tschakovsky & Hughson, 1999; Özyener et al. 2001). It is generally reported that the phase II time constant is longer at higher work rates and this has been attributed to an increasing O2 supply limitation (Hughson & Morrissey, 1982; Hughson et al. 2001) as well as to differences in the characteristics of the population of muscle fibres contributing to force production (Brittain et al. 2001; Jones et al. 2002; Koppo et al. 2004). In our three studies to date which have investigated the influence of l-NAME on V̇O2 kinetics during constant work rate exercise (Jones et al. 2003, 2004a; present study) in essentially the same population of young, healthy, moderately trained males, the phase II time constant in the control condition was ∼22 s (moderate exercise), ∼25 s (heavy exercise) and ∼36 s (supra-maximal exercise). Following the infusion of l-NAME, the phase II time constant was reduced to ∼18 s, ∼22 s and ∼20 s, respectively, in these three studies (Fig. 6). These data might be interpreted to suggest that the longer phase II time constant at higher work rates is related to a greater pernicious influence of NO on oxidative metabolism at higher work rates, and not to an O2 delivery limitation. That the speeding of the V̇O2 kinetics following NOS inhibition was significantly correlated with the absolute value of the phase II time constant in the control condition (Fig. 5) is consistent with this notion. Certainly, the faster phase II V̇O2 kinetics during supra-maximal exercise following l-NAME administration cannot be ascribed to an enhanced muscle O2 availability; on the contrary, as discussed above, the longer duration of phase I suggests that cardiac output (and muscle blood flow) might be at least transiently reduced with NOS inhibition. Although it is generally accepted that muscle O2 delivery ultimately limits the highest attainable V̇O2 amplitude (i.e. the V̇O2max), it does not necessarily follow that the rate at which V̇O2 adjusts following the onset of supra-maximal exercise is similarly limited (Whipp, 1994). Consistent with this interpretation, we have recently reported that the performance of prior high intensity exercise, which has been shown to result in an increased muscle blood flow (Krustrup et al. 2001), does not significantly alter the phase II time constant during subsequent supra-maximal exercise, although it does increase the V̇O2 attained at end-exercise (Wilkerson et al. 2004a).
Figure 6.
Mean (± s.d.) values for the time constant of the phase II V̇O2 response (τp) in the placebo condition (•) and following the infusion of l-NAME (○) for moderate exercise (Jones et al. 2003), heavy exercise (Jones et al. 2004a), and supra-maximal exercise (present study). The dotted line represents the mean τp (∼20 s) following the infusion of l-NAME in the three studies. These data suggest that the lengthening of τp at higher work rates in the control condition might be related to a nitric oxide-mediated metabolic inertia. See text for further discussion.
Despite the dramatic effect of NOS inhibition on the phase II time constant, it should be emphasized that most of the metabolic (or other) limitation to V̇O2 kinetics following the onset of exercise remains unexplained. For example, even when the phase II V̇O2 kinetics become faster as the result of an intervention, the time constant has rarely been reported to be less than ∼17–21 s either in isolated muscle preparations (Grassi et al. 1998, 2000) or in the exercising human (Phillips et al. 1995; Scheuermann et al. 2002; Jones et al. 2003, 2004a; Tordi et al. 2003), although it can be as fast as 9–12 s in equine (Langsetmo et al. 1997; Kindig et al. 2002) and human (Koppo et al. 2004) athletes. Similar minimum values for the time constant for the fall in Po2 or the rise in V̇O2 of ∼10–20 s have been reported in single isolated fibre studies (Hogan, 2001; Kindig et al. 2003), suggesting that this is an inherent (metabolic) limitation to oxidative respiration that is essentially independent of O2 availability. There is compelling evidence that the phase II pulmonary V̇O2 response following the onset of exercise is functionally linked to the rate of intramuscular phosphocreatine hydrolysis (Whipp & Mahler, 1980; Rossiter et al. 1999; Roman et al. 2002). It is likely therefore that the ‘remaining’ limitation to the phase II V̇O2 kinetics following the infusion of l-NAME is linked to a feedback mechanism involving one or more of the products of high-energy phosphate hydrolysis, to the kinetics of creatine kinase activation, or possibly to mitochondrial Ca2+ dynamics.
An additional novel feature of the present study was the significant 8% reduction in the amplitude of the phase II V̇O2 response in the l-NAME condition compared to control, although there was no significant difference in the V̇O2 attained at the end of exercise in the two conditions. The ‘gain’ of the phase II V̇O2 response (i.e. Δ V̇O2/ Δ work rate) was reduced from ∼7.7 ml min−1 W−1 in the control condition to ∼7.1 ml min−1 W−1 in the l-NAME condition. This lower-than-expected phase II V̇O2 gain during peri-maximal exercise in the control condition (Fig. 3) has been previously described (Jones et al. 2002; Pringle et al. 2003a; Scheuermann & Barstow, 2003; Wilkerson et al. 2004a, b) and might be attributed to a number of factors including the influence of pH on mitochondrial function (Conley et al. 2001) and the recruitment of type II muscle fibres in which blood flow and microvascular Po2 might be sufficiently low during high intensity exercise to limit the increase in V̇O2 (Behnke et al. 2003). We have recently observed that prior high-intensity exercise increases the phase II V̇O2 gain during subsequent peri-maximal exercise (Wilkerson et al. 2004a), suggesting that muscle blood flow and/or its distribution might limit the amplitude to which V̇O2 can rise following the onset of peri-maximal exercise in the ‘control’ condition. The cause of the reduction in the phase II V̇O2 gain with l-NAME is unclear, although it is known that this parameter is sensitive to a number of factors including the performance of prior exercise, muscle fibre type and fibre recruitment patterns, other pharmacological and nutritional interventions, and O2 availability (Koga et al. 1999; Burnley et al. 2000; Jones et al. 2002; Pringle et al. 2003a, b; Rossiter et al. 2003). For example, a lower phase II V̇O2 gain during heavy cycle exercise has been reported in subjects with a high percentage of type II fibre distribution in the working muscles (Barstow et al. 1996; Pringle et al. 2003a) and at high compared to low pedal rates, where type II fibre recruitment is likely to be enhanced (Pringle et al. 2003b). However, it is difficult to see how l-NAME administration could alter muscle fibre recruitment patterns during exercise, although this should not, of course, be ruled out.
A lower phase II V̇O2 gain has also been reported during supine compared to upright cycle exercise, where there is a reduced pressure head for muscle blood flow (Koga et al. 1999). One possibility for the lower phase II V̇O2 gain with l-NAME, therefore, is that NOS inhibition partially reduced muscle blood flow during supra-maximal exercise (see earlier discussion). This postulate is strengthened when one considers that the phase II V̇O2 gain is not altered by l-NAME during moderate or heavy submaximal exercise (Jones et al. 2003, 2004a) and that V̇O2max is reduced during maximal intensity treadmill running in the Thoroughbred horse (Kindig et al. 2000). In a recent study (Jones et al. 2004b), we observed that l-NAME infusion resulted in a small (∼6%) but significant reduction in V̇O2max during incremental cycle exercise in humans, which was associated with a significant reduction in maximal HR (and presumably maximal cardiac output). Assuming that there was indeed a similar reduction in V̇O2max with l-NAME in the present study, then the same absolute work rate imposed in both conditions would have represented a slightly higher relative exercise intensity in the l-NAME condition. However, because phase II V̇O2 kinetics tend to become slower at higher relative exercise intensities, the reduction of the phase II time constant with l-NAME might be considered to be even more impressive. It should also be pointed out that the time to fatigue was not significantly different in the two conditions implying either that the relative exercise intensity was not appreciably different with and without l-NAME, or that l-NAME infusion preserved exercise tolerance during constant work rate supra-maximal exercise despite a reduction in V̇O2max resulting from a reduced muscle blood flow.
It has also been suggested that NO might extend the zone of effective tissue respiration by increasing the O2 gradient away from the blood vessel (Thomas et al. 2001). One other consideration therefore is that NOS inhibition might have reduced the gradient for O2 diffusion away from the capillaries thereby altering the distribution of perfusion to metabolic demand (Q̇/V̇O2, where Q̇ is blood flow; Richardson et al. 2002). However, it is important to reiterate here that if muscle blood flow or O2 distribution were indeed reduced with l-NAME, then this did not significantly impact on the acceleration of V̇O2 kinetics caused by removal of the inhibitory effect of NO on mitochondrial function.
Slow component
A V̇O2 slow component was observed in 4 of the 7 subjects in the control condition and in 5 of the 7 subjects in the l-NAME condition (Table 1). The existence of a V̇O2 slow component during supra-maximal exercise is somewhat controversial since in some (Özyener et al. 2001; Wilkerson et al. 2004a), though not all (Hughson et al. 2000), studies it has been reported that the V̇O2 response is adequately described with a mono-exponential function during such exercise. Whether or not a V̇O2 slow component can be discerned during supra-maximal exercise possibly depends on the sustainable exercise duration since the slow component typically emerges at ∼120 s into high-intensity exercise (e.g. Burnley et al. 2000; Pringle et al. 2003a; Koppo et al. 2004). For example, in a recent study in which the time to exhaustion at a supra-maximal work rate was ∼110–150 s, we reported that the V̇O2 data were adequately described with a mono-exponential function (Wilkerson et al. 2004a). However, in the present study (time to exhaustion ∼160–170 s, on average), the V̇O2 response of some subjects was better described with a bi-exponential model. The subjects in whom a V̇O2 slow component could not be discerned had the shortest times to exhaustion (∼120 s).
We have previously reported that l-NAME resulted in a significantly greater amplitude of the V̇O2 slow component during heavy exercise at 40% ‘Δ’ (∼70% V̇O2max) with no difference in the time at which the slow component emerged (Jones et al. 2004a). In the present study, there was no significant difference in the slow component amplitude in the four subjects who demonstrated a slow component response in both conditions but there was a tendency for the slow component to emerge earlier in the l-NAME condition (Table 1). Kindig et al. (2001) have previously reported that the V̇O2 slow component emerged earlier (125 versus 65 s, on average) during high-intensity treadmill running in the Thoroughbred horse following the administration of l-NAME. The mechanism by which NOS inhibition might cause an earlier onset of the slow component during supra-LT exercise is not immediately apparent, although it has been suggested that alterations in muscle O2 availability and/or muscle fibre recruitment might be responsible (Kindig et al. 2001).
Blood [lactate] and exercise tolerance
The resting blood [lactate] was significantly greater in the l-NAME compared to the control condition. We have noted a non-significant tendency for this same effect in our previous studies with l-NAME (Jones et al. 2003, 2004a). It is possible that this effect is related to the reduced vascular conductance at rest that occurs with NOS inhibition (Radegran & Saltin, 1999; Frandsen et al. 2001). However, the accumulation of blood lactate from the start to the end of exercise (i.e. Δ blood [lactate]) was significantly reduced with l-NAME compared to control. This is consistent with a reduction in the muscle O2 deficit resulting from a speeding of the V̇O2 kinetics. However, this reduced blood lactate accumulation with exercise in the l-NAME condition did not facilitate exercise tolerance because the time to exhaustion was not significantly different between the two conditions. It is possible that the enhanced mitochondrial respiration resulting from an alleviation of some of the metabolic inertia following NOS inhibition was counterbalanced by a reduced blood flow and maximal attainable V̇O2 during maximal intensity exercise (Kindig et al. 2000; Jones et al. 2004b).
Modelling
The most appropriate procedure for describing the pulmonary V̇O2 response to supra-maximal exercise has been debated (Hughson et al. 2000; Scheuermann & Barstow, 2003). In the present study, we chose to use standard exponential curve-fitting procedures since this approach makes no assumption with regard to the amplitude to which V̇O2 should theoretically project (Hughson et al. 2000; Scheuermann & Barstow, 2003). One possible limitation was that our subjects only completed one transition to exercise in each condition. Breath-by-breath respiratory gas exchange data is inherently variable but the signal-to-noise ratio can be enhanced by averaging the response to several repeat transitions and/or maximizing the V̇O2 response amplitude (Lamarra et al. 1987). Although our subjects only completed one transition, the fact that they were healthy physically active males performing supra-maximal exercise meant that the response amplitude was appreciable (i.e. ∼3 l min−1 above baseline). That time to exhaustion was ∼3 min also meant that sufficient data were collected for adequate curve fitting (see Fig. 3 for an example of the fidelity of the data collected). The 95% confidence interval surrounding the estimation of the phase II time constant was 6–7 s, on average, which, although larger than the ideal, was much smaller than the difference between the two conditions. In several data sets, the goodness of fit was significantly improved by the inclusion of a second exponential term to describe the slow component phase of the response (in these cases, the mean squared error was reduced by 20%, on average). It should be pointed out, however, that the inclusion of additional exponential terms in the modelling procedure has the potential to reduce statistical confidence in any of the parameters so derived including the primary variable of interest, the phase II time constant. To be certain that this did not inadvertently influence our results or interpretation, we also performed additional analysis in which the first 20 s of data following the onset of exercise was omitted and a mono-exponential model was applied to the remaining data. However, this did not appreciably affect our principal results or conclusions (i.e. the ‘effective’ time constant was significantly reduced from 50.5 ± 22.8 to 34.1 ± 12.6 s with l-NAME).
The increased duration of phase I that we have reported here suggests that muscle blood flow was at least transiently reduced following the onset of supra-maximal cycle exercise with NOS inhibition. As we have noted previously (Jones et al. 2003, 2004a), changes in cardiovascular dynamics can confound simple interpretation of the pulmonary V̇O2 dynamics during exercise (Barstow & Molé, 1987; Barstow et al. 1990). Specifically, in a mathematical modelling study, Barstow et al. (1990) estimated that a slowing of cardiac output kinetics (with constant muscle O2 consumption kinetics) would result in an increased duration of phase I and a reduced phase II time constant, as indeed we found in the present study. This might suggest that muscle V̇O2 kinetics were unaltered in this and previous studies which have reported faster phase II pulmonary V̇O2 kinetics with l-NAME (Kindig et al. 2001, 2002; Jones et al. 2003, 2004a). However, in our two previous studies (Jones et al. 2003, 2004a), we were unable to determine any significant effect of l-NAME on the duration of phase I but we still found a significant speeding of the phase II V̇O2 kinetics. Furthermore, in the present study, there was no significant correlation between the increase in the duration of phase I and the speeding of the phase II V̇O2 kinetics with l-NAME, suggesting that the reduced phase II time constant was not dependent on the altered cardiovascular response to exercise. Finally, the significant 33% reduction in Δ blood [lactate] that we observed with l-NAME suggests that the muscle O2 deficit was reduced in this condition. For these reasons, we believe that the speeding of the phase II V̇O2 kinetics that we report here resulted primarily from a removal of the inhibitory effect of NO on mitochondrial respiration rather than from an ‘artefactual’ effect caused by substantially altered cardiovascular dynamics. However, further studies involving ‘direct’ measurements of muscle V̇O2 kinetics are necessary to confirm this.
In summary, we have shown that the inhibition of NOS with l-NAME causes a small but significant increase in the duration of phase I (of ∼2 s), and a significant reduction in the phase II V̇O2 time constant (of ∼16 s) following the onset of exhaustive constant-load cycle exercise at a work rate calculated to require 105% V̇O2max. These results demonstrate that phase II V̇O2 kinetics can be speeded with NOS inhibition even during supra-maximal exercise, despite the possibility that cardiac output is simultaneously reduced. That NOS inhibition can significantly reduce the phase II V̇O2 time constant during supra-maximal exercise to values that are typically reported for moderate intensity exercise (i.e. ∼20 s), presumably by alleviating the pernicious effects of NO on mitochondrial respiration, suggests that an NO-dependent metabolic inertia represents an important limitation to V̇O2 kinetics following the onset of high-intensity cycle exercise.
Acknowledgments
We would like to dedicate this article to the memory of the late Casey A. Kindig, whose pioneering research with David C. Poole on the influence of NOS inhibition on respiratory control in the horse was the inspiration for our own work in this area.
References
- Barstow TJ, Jones AM, Nguyen P, Casaburi R. Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol. 1996;81:1642–1650. doi: 10.1152/jappl.1996.81.4.1642. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Lamarra N, Whipp BJ. Modulation of muscle and pulmonary O2 uptakes by circulatory dynamics during exercise. J Appl Physiol. 1990;68:979–989. doi: 10.1152/jappl.1990.68.3.979. 10.1063/1.346664. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Molé PA. Simulation of pulmonary O2 uptake during exercise transients in humans. J Appl Physiol. 1987;63:2253–2261. doi: 10.1152/jappl.1987.63.6.2253. [DOI] [PubMed] [Google Scholar]
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol. 2003;549:597–605. doi: 10.1113/jphysiol.2002.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E, Pogliaghi S, Molinello A, Diciolla F, Maccherini M, Grassi B. Serial assessment of peak VO2 and VO2 kinetics early after heart transplantation. Med Sci Sports Exerc. 2003;35:1798–1804. doi: 10.1249/01.MSS.0000093610.71730.02. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain CJ, Rossiter HB, Kowalchuk JM, Whipp BJ. Effect of prior metabolic rate on the kinetics of oxygen uptake during moderate-intensity exercise. Eur J Appl Physiol. 2001;86:125–134. doi: 10.1007/s004210100514. 10.1007/s004210100514. [DOI] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta. 1999;1411:351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide as a competitive inhibitor of oxygen consumption in the mitochondrial respiratory chain. Acta Physiol Scand. 2000;168:667–674. doi: 10.1046/j.1365-201x.2000.00718.x. [DOI] [PubMed] [Google Scholar]
- Burnley M, Jones AM, Carter H, Doust JH. Effects of prior heavy exercise on phase II pulmonary oxygen uptake kinetics during heavy exercise. J Appl Physiol. 2000;89:1387–1396. doi: 10.1152/jappl.2000.89.4.1387. [DOI] [PubMed] [Google Scholar]
- Cassina A, Radi R. Different inhibitory actions of NO and peroxynitrite on mitochondrial electron transport. Arch Biophys Biochem. 1996;328:309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- Conley KE, Kemper WF, Crowther GJ. Limits to sustainable muscle performance: interaction between glycolysis and oxidative phosphorylation. J Exp Biol. 2001;204:3189–3194. doi: 10.1242/jeb.204.18.3189. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with NG-nitro-L-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Grassi B, Hogan MC, Kelley KM, Aschenbach WG, Hamann JJ, Evans RK, Patillo RE, Gladden LB. Role of convective O2 delivery in determining VO2 on-kinetics in canine muscle contracting at peak VO2. J Appl Physiol. 2000;89:1293–1301. doi: 10.1152/jappl.2000.89.4.1293. [DOI] [PubMed] [Google Scholar]
- Hill DW, Poole DC, Smith JC. The relationship between power and the time to achieve VO2 max. Med Sci Sports Exerc. 2002;34:709–714. doi: 10.1097/00005768-200204000-00023. 10.1097/00005768-200204000-00023. [DOI] [PubMed] [Google Scholar]
- Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. Cytochrome P450 2C9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol. 2003;546:307–314. doi: 10.1113/jphysiol.2002.030833. 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC. Fall in intracellular PO2 at the onset of contractions in Xenopus single skeletal muscle fibers. J Appl Physiol. 2001;90:1871–1876. doi: 10.1152/jappl.2001.90.5.1871. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Morrissey M. Delayed kinetics of VO2 in the transition from prior exercise. Evidence for O2 transport limitation of VO2 kinetics: a review. Int J Sports Med. 1982;4:31–39. doi: 10.1055/s-2008-1026013. [DOI] [PubMed] [Google Scholar]
- Hughson RL, O'Leary DD, Betik AC, Hebestreit H. kinetics of oxygen uptake at the onset of exercise near or above peak oxygen uptake. J Appl Physiol. 2000;88:1812–1819. doi: 10.1152/jappl.2000.88.5.1812. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Tschakovsky ME, Houston ME. Regulation of oxygen consumption at the onset of exercise. Exerc Sport Sci Rev. 2001;29:129–133. doi: 10.1097/00003677-200107000-00008. 10.1097/00003677-200107000-00008. [DOI] [PubMed] [Google Scholar]
- Jones AM, Carter H, Pringle JSM, Campbell IT. Effect of creatine supplementation on oxygen uptake kinetics during sub-maximal cycle exercise. J Appl Physiol. 2002;92:2571–2577. doi: 10.1152/japplphysiol.01065.2001. [DOI] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, Koppo K, Wilmshurst S, Campbell IT. Inhibition of nitric oxide synthase by L-NAME speeds VO2 kinetics in the transition to moderate intensity exercise in man. J Physiol. 2003;552:265–272. doi: 10.1113/jphysiol.2003.045799. 10.1113/jphysiol.2003.045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, Wilmshurst S, Campbell IT. Influence of L-NAME on pulmonary O2 uptake kinetics during heavy-intensity cycle exercise. J Appl Physiol. 2004a;96:1033–1038. doi: 10.1152/japplphysiol.00381.2003. 10.1152/japplphysiol.00381.2003. [DOI] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, Campbell IT. Nitric Oxide synthase inhibition with L-NAME reduces maximal oxygen uptake but not gas exchange threshold during incremental cycle exercise in man. J Physiol. 2004b;560:329–338. doi: 10.1113/jphysiol.2004.065664. 10.1113/jphysiol.2004.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Tschakovsky ME. Nitric oxide and physiologic vasodilation in human limbs: where do we go from here? Can J Appl Physiol. 2003;28:475–490. doi: 10.1139/h03-035. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Gallatin LL, Erickson HH, Fedde MR, Poole DC. Cardiorespiratory impact of the nitric oxide synthase inhibitor L-NAME in the exercising horse. Respir Physiol Neurobiol. 2000;120:151–166. doi: 10.1016/s0034-5687(00)00096-7. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Kelley KM, Howlett RA, Stary CM, Hogan MC. Assessment of O2 uptake dynamics in isolated single skeletal myocytes. J Appl Physiol. 2003;94:353–357. doi: 10.1152/japplphysiol.00559.2002. [DOI] [PubMed] [Google Scholar]
- Kindig CA, McDonough P, Erickson HH, Poole DC. Effect of L-NAME on oxygen uptake kinetics during heavy-intensity exercise in the horse. J Appl Physiol. 2001;91:891–896. doi: 10.1152/jappl.2001.91.2.891. [DOI] [PubMed] [Google Scholar]
- Kindig CA, McDonough P, Erickson HH, Poole DC. Nitric oxide synthase inhibition speeds oxygen uptake kinetics in horses during moderate domain running. Respir Physiol Neurobiol. 2002;132:169–178. doi: 10.1016/s1569-9048(02)00068-x. 10.1016/S1569-9048(02)00068-X. [DOI] [PubMed] [Google Scholar]
- Koga S, Barstow TJ, Shiojiri T, Takaishi T, Fukuba Y, Kondo N, Shibasaki M, Poole DC. Effect of muscle mass on VO2 kinetics at the onset of work. J Appl Physiol. 2001;90:461–468. doi: 10.1152/jappl.2001.90.2.461. [DOI] [PubMed] [Google Scholar]
- Koga S, Shiojiri T, Shibasaki M, Kondo N, Fukuba Y, Barstow TJ. kinetics of oxygen uptake during supine and upright heavy exercise. J Appl Physiol. 1999;87:253–260. doi: 10.1152/jappl.1999.87.1.253. [DOI] [PubMed] [Google Scholar]
- Koppo K, Jones AM, Bouckaert J. Effects of training status and exercise intensity on phase II VO2 kinetics. Med Sci Sports Exerc. 2004;36:225–232. doi: 10.1249/01.MSS.0000113473.48220.20. [DOI] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The changes in respiration at the transition from work to rest. J Physiol. 1920;53:431–439. doi: 10.1113/jphysiol.1920.sp001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Gonzalez-Alonso J, Quistorff B, Bangsbo J. Muscle heat production and anaerobic energy turnover during repeated intense dynamic exercise in humans. J Physiol. 2001;536:947–956. doi: 10.1111/j.1469-7793.2001.00947.x. 10.1111/j.1469-7793.2001.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarra N, Whipp BJ, Ward SA, Wasserman K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol. 1987;62:2003–2012. doi: 10.1152/jappl.1987.62.5.2003. 10.1063/1.339541. [DOI] [PubMed] [Google Scholar]
- Langsetmo I, Weigle GE, Fedde MR, Erickson HH, Barstow TJ, Poole DC. VO2 kinetics in the horse during moderate and heavy exercise. J Appl Physiol. 1997;83:1235–1241. doi: 10.1152/jappl.1997.83.4.1235. [DOI] [PubMed] [Google Scholar]
- Linnarsson D. Dynamics of pulmonary gas exchange and heart rate changes at start and end of exercise. Acta Physiol Scand. 1974;415:1–68. [PubMed] [Google Scholar]
- Mettauer B, Zhao QM, Epailly E, Charloux A, Lampert E, Heitz-Naegelen B, Piquard F, di Prampero PE, Lonsdorfer J. VO2 kinetics reveal a central limitation at the onset of subthreshold exercise in heart transplant recipients. J Appl Physiol. 2000;88:1228–1238. doi: 10.1152/jappl.2000.88.4.1228. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Monod H, Scherrer J. The work capacity of a synergic muscle group. Ergonomics. 1965;8:329–338. [Google Scholar]
- Özyener F, Rossiter HB, Ward SA, Whipp BJ. Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol. 2001;533:891–902. doi: 10.1111/j.1469-7793.2001.t01-1-00891.x. 10.1111/j.1469-7793.2001.t01-1-00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, MacDonald MJ, Hughson RL. Progressive effect of endurance training on VO2 kinetics at the onset of submaximal exercise. J Appl Physiol. 1995;79:1914–1920. doi: 10.1152/jappl.1995.79.6.1914. [DOI] [PubMed] [Google Scholar]
- Pringle JSM, Doust JH, Carter H, Tolfrey K, Campbell IT, Sakkas GK, Jones AM. Oxygen uptake kinetics during moderate, heavy and severe intensity ‘submaximal’ exercise in humans: the influence of muscle fibre type and capillarisation. Eur J Appl Physiol. 2003a;89:289–300. doi: 10.1007/s00421-003-0799-1. [DOI] [PubMed] [Google Scholar]
- Pringle JSM, Doust JH, Carter H, Tolfrey K, Jones AM. Effect of pedal rate on primary and slow-component oxygen uptake responses during heavy cycle exercise. J Appl Physiol. 2003b;94:1501–1507. doi: 10.1152/japplphysiol.00456.2002. 10.1063/1.1586961. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Haseler LJ, Bluml S, Frank LR. Evolving techniques for the investigation of muscle bioenergetics and oxygenation. Biochem Soc Trans. 2002;30:232–237. doi: 10.1042/. 10.1042/0300-5127:0300232. [DOI] [PubMed] [Google Scholar]
- Roman BB, Meyer RA, Wiseman RW. Phosphocreatine kinetics at the onset of contractions in skeletal muscle of MM creatine kinase knockout mice. Am J Physiol Cell Physiol. 2002;283:C1776–C1783. doi: 10.1152/ajpcell.00210.2002. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol. 1999;518:921–932. doi: 10.1111/j.1469-7793.1999.0921p.x. 10.1111/j.1469-7793.1999.0921p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Howe FA, Wood DM, Kowalchuk JM, Griffiths JR, Whipp BJ. Effects of dichloroacetate on VO2 and intramuscular 31P metabolite kinetics during high-intensity exercise in humans. J Appl Physiol. 2003;95:1105–1115. doi: 10.1152/japplphysiol.00964.2002. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Victor RG. A large blood pressure raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33:937–942. doi: 10.1161/01.hyp.33.4.937. [DOI] [PubMed] [Google Scholar]
- Sander M, Hansen PG, Victor RG. Sympathetically mediated hypertension caused by chronic inhibition of nitric oxide. Hypertension. 1995;26:691–695. doi: 10.1161/01.hyp.26.4.691. [DOI] [PubMed] [Google Scholar]
- Scheuermann BW, Barstow TJ. O2 uptake kinetics during exercise at peak O2 uptake. J Appl Physiol. 2003;95:2014–2022. doi: 10.1152/japplphysiol.00590.2002. [DOI] [PubMed] [Google Scholar]
- Scheuermann BW, Bell C, Paterson DH, Barstow TJ, Kowalchuk JM. Oxygen uptake kinetics for moderate exercise are speeded in older humans by prior heavy exercise. J Appl Physiol. 2002;92:609–616. doi: 10.1152/japplphysiol.00186.2001. [DOI] [PubMed] [Google Scholar]
- Shen W, Xu X, Ochoa M, Zhao G, Wolin MS, Hintze TH. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ Res. 1994;75:1086–1095. doi: 10.1161/01.res.75.6.1086. [DOI] [PubMed] [Google Scholar]
- Shepherd JT, Katusic ZS. Endothelium-derived vasoactive factors. I. Endothelium-dependent relaxation. Hypertension. 1991;18:76–85. doi: 10.1161/01.hyp.18.5_suppl.iii76. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Nelson CD, Sundermann RK. Does autonomic blockade reveal a potent contribution of nitric oxide to locomotion-induced vasodilation? Am J Physiol. 2000;279 doi: 10.1152/ajpheart.2000.279.2.H726. , H726–H732. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Halliwell JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol. 1997;273:H2388–H2395. doi: 10.1152/ajpheart.1997.273.5.H2388. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Hughson RL. Adaptation of blood flow during the rest to work transition in humans. Med Sci Sports Exerc. 1999;31:1019–1026. doi: 10.1097/00005768-199907000-00015. 10.1097/00005768-199907000-00015. [DOI] [PubMed] [Google Scholar]
- Sietsema KE, Ben-Dov I, Zhang YY, Sullivan C, Wasserman K. Dynamics of oxygen uptake for submaximal exercise and recovery in patients with chronic heart failure. Chest. 1994;105:1693–1700. doi: 10.1378/chest.105.6.1693. [DOI] [PubMed] [Google Scholar]
- Sietsema KE, Cooper DM, Perloff JK, Rosove MH, Child JS, Canobbio MM, Whipp BJ, Wasserman K. Dynamics of oxygen uptake during exercise in adults with cyanotic congenital heart disease. Circulation. 1986;73:1137–1144. doi: 10.1161/01.cir.73.6.1137. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Liu X, Kantrow SP, Lancaster JR. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001;98:355–360. doi: 10.1073/pnas.011379598. 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordi N, Perrey S, Harvey A, Hughson RL. Oxygen uptake kinetics during two bouts of heavy cycling separated by fatiguing sprint exercise in humans. J Appl Physiol. 2003;94:533–541. doi: 10.1152/japplphysiol.00532.2002. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Hughson RL. Interaction of factors determining oxygen uptake at the onset of exercise. J Appl Physiol. 1999;86:1101–1113. doi: 10.1152/jappl.1999.86.4.1101. [DOI] [PubMed] [Google Scholar]
- Whipp BJ. The slow component of O2 uptake kinetics during heavy exercise. Med Sci Sports Exerc. 1994;26:1319–1326. [PubMed] [Google Scholar]
- Whipp BJ, Davis JA, Torres F, Wasserman K. A test to determine parameters of aerobic function during exercise. J Appl Physiol. 1981;50:217–221. doi: 10.1152/jappl.1981.50.1.217. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Mahler M. Dynamics of pulmonary gas exchange during exercise. In: West JB, editor. Pulmonary Gas Exchange, Organism and Environment. II. London: Academic Press; 1980. pp. 33–96. [Google Scholar]
- Whipp BJ, Ward SA. Physiological determinants of pulmonary gas exchange kinetics during exercise. Med Sci Sports Exerc. 1990;22:62–71. [PubMed] [Google Scholar]
- Whipp BJ, Ward SA, Lamarra N, Davis JA, Wasserman K. Parameters of ventilatory and gas exchange dynamics during exercise. J Appl Physiol. 1982;52:1506–1513. doi: 10.1152/jappl.1982.52.6.1506. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Wasserman K. Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol. 1972;33:351–356. doi: 10.1152/jappl.1972.33.3.351. [DOI] [PubMed] [Google Scholar]
- Wilkerson DP, Koppo K, Barstow TJ, Jones AM. Effect of prior multiple sprint exercise on pulmonary O2 uptake kinetics following the onset of peri-maximal exercise. J Appl Physiol. 2004a;97:1227–1236. doi: 10.1152/japplphysiol.01325.2003. 10.1152/japplphysiol.01325.2003. [DOI] [PubMed] [Google Scholar]
- Wilkerson DP, Koppo K, Barstow TJ, Jones AM. Effect of work rate on the functional gain of phase II pulmonary O2 uptake response to exercise. Physiol Neurobiol. 2004b;142:211–223. doi: 10.1016/j.resp.2004.06.001. 10.1016/j.resp.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Zhang J, Snyder SH. Nitric oxide and the nervous system. Annu Rev Pharmacol Toxicol. 1995;35:213–233. doi: 10.1146/annurev.pa.35.040195.001241. 10.1146/annurev.pa.35.040195.001241. [DOI] [PubMed] [Google Scholar]