Abstract
An early consequence of brain energy deprivation is an increase in the frequency of spontaneous inhibitory and excitatory postsynaptic currents (sIPSCs and sEPSCs), which may disrupt neural information processing. This increase in spontaneous transmitter release has been reported to occur in calcium-free solution and has been attributed either to calcium release from internal stores or to a direct effect of hypoxia on the transmitter release mechanism. Here we investigate the mechanism of the increase in sIPSC frequency that occurs in area CA1 of rat hippocampus during simulated ischaemia, by making patch-clamp recordings from CA1 pyramidal neurones. When recording in whole-cell mode, exposure to ischaemic solution increased the sIPSC frequency 30-fold (to 49 Hz) after 5 min, and doubled the sIPSC amplitude. Ischaemic sIPSCs were action potential independent, vesicular in origin and, contrary to the results of earlier studies which did not buffer extracellular calcium to a low level, dependent on extracellular calcium. The properties of the ischaemic sIPSCs were not affected by depleting intracellular stores of calcium or by blocking the neuronal GABA transporter GAT-1. Recording from neurones using gramicidin-perforated patch-clamping showed a 10-fold smaller, more transient increase in sIPSC frequency during ischaemia, with no change of sIPSC amplitude, suggesting that whole-cell clamp recording increases the ischaemia-induced sIPSC rate and amplitude by controlling the intracellular milieu.
In hypoxia/ischaemia, when the energy supply to the brain is compromised, there is an increase in the frequency of both spontaneous excitatory postsynaptic currents (sEPSCs) and spontaneous inhibitory postsynaptic currents (sIPSCs; Katchman & Hershkowitz, 1993a; Katchman et al. 1994; Fleidervish et al. 2001; Allen et al. 2004). It is important to understand the cause of these spontaneous postsynaptic currents (sPSCs), since the voltage change they generate will contribute to the alteration of brain information processing that occurs early in ischaemia.
Much interest has focused on the release of glutamate during energy deprivation because of its excitotoxic actions, but GABA release in hypoxia/ischaemia (Hagberg et al. 1985) is also likely to be important, for two reasons. First, the activation of GABAA receptors affects the depolarization of neurones that occurs in ischaemia (Allen et al. 2004) and so will modulate the neurotoxic Ca2+ entry occurring through glutamate-activated NMDA receptors. Second, therapeutic approaches for ischaemia have been proposed, based on modulating the actions of GABA (Green et al. 2000). Here we characterize the increase in frequency of sIPSCs that occurs during simulated hippocampal ischaemia and investigate the mechanisms that lead to this increase.
Previous work on hypoxia suggested that the increase in sEPSC frequency was not affected by removing extracellular calcium, but was dependent on the release of calcium from intracellular stores (Katchman & Hershkowitz, 1993a). Similarly, Fleidervish et al. (2001) showed that sIPSCs recorded in the first 3 min of hypoxia were not sensitive to application of TTX or removal of external calcium, consistent with transmitter release being evoked by calcium released from internal stores or being calcium independent, and they attributed the transmitter release to a direct effect of hypoxia on the release mechanism. However, neither Katchman & Hershkowitz (1993a) nor Fleidervish et al. (2001) buffered free calcium when they removed external calcium, so the exact extracellular calcium concentration ([Ca2+]o) produced is uncertain and may be sufficient to sustain [Ca2+]o-dependent exocytosis. Furthermore, no studies have yet been conducted on spontaneous GABA release in ischaemia as opposed to hypoxia, which probably produces a smaller fall of ATP concentration than ischaemia.
Our results demonstrate that ischaemia-evoked sIPSCs are dependent on external calcium and show intriguingly different properties when studied in whole-cell and perforated patch recording modes.
Methods
Brain slices and extracellular solution
Sprague–Dawley rats, 12 days old, were killed by cervical dislocation in accordance with UK animal experimentation regulations. Thin (225 μm) hippocampal slices were cut using a vibrating slicer, and patch-clamp recordings from visually identified pyramidal cells in area CA1 were performed as described by Allen et al. (2004). Experiments were at 33 ± 1°C, with the slices submerged in flowing (10 ml min−1) extracellular solution containing (mm): 126 NaCl, 24 NaHCO3, 1 NaH2PO4, 2.5 KCl, 2.5 CaCl2, 2 MgCl2 and 10 d-glucose (gassed with 95% O2–5% CO2), pH 7.4. Kynurenic acid (1 mm, Sigma, UK) was included in the dissection and incubation solution (to block glutamate receptors, to reduce potential excitotoxic damage) but was omitted from the superfusion solution. Ischaemia was simulated by replacing 10 mm glucose with 7 mm sucrose, bubbling with 95% N2–5% CO2 and, to get a reproducible fast onset to the ischaemic response, adding 2 mm sodium iodoacetate (Sigma) and 1 mm sodium cyanide (BDH, UK) to block glycolysis and oxidative phosphorylation (Reiner et al. 1990). Drugs were added directly to the external solution from stock solutions in water (GABAzine and SKF 89976A were obtained from Tocris, UK; TTX from Alomone, Israel). For experiments using concanamycin to block vesicle loading with transmitter, slices were presoaked for at least 2 h in 0.5 μm concanamycin (ICN Biomedicine, USA) while control slices were soaked in external solution lacking concanamycin (both solutions contained 1 mm kynurenic acid to block glutamate receptors; concanamycin was made up as a 50 μm stock in DMSO and a corresponding amount of DMSO was added to the control soaking solution). Concanamycin was not present in the subsequent recording solution. Similarly, pretreatment with the irreversible inhibitor of the endoplasmic reticulum calcium pump, thapsigargin (Lytton et al. 1991), involved soaking in 3 μm thapsigargin for 1 h before recording (and thapsigargin was not present in the recording solution).
Electrophysiology
Recording was performed either by whole-cell clamping using BAPTA in the electrode solution to chelate intracellular calcium, since this preserves GABAA receptor function during ischaemia (Allen et al. 2004), or by gramicidin-perforated patch recording (Akaike, 1996) to avoid disturbance of intracellular calcium buffering and chloride concentration by the pipette solution. Electrode junction potentials were compensated for.
Whole-cell clamping
Patch pipettes were pulled from thick-walled borosilicate glass capillaries and filled with an internal solution containing (mm): 130 CsCl (Cs+ was used as the main cation rather than K+ to improve voltage uniformity), 4 NaCl, 10 Hepes, 10 BAPTA, 4 MgATP, 0.5 Na2GTP and 10 QX-314 (to suppress voltage-gated sodium currents), pH adjusted to 7.2 with CsOH. For this internal solution, ECl = 0 mV and GABAA receptor-mediated currents are inward at the holding potential of −33 mV. In some experiments the BAPTA was replaced with 5 mm EGTA and 0.5 mm CaCl2, as described in Results (Perforated patch-clamp recording of sIPSCs in ischaemia). Pipette series resistance was ∼5 MΩ before compensation by ∼60% to reduce it to ∼2 MΩ.
Perforated patch recording
The electrode solution contained (mm): 135 KCl, 10 Hepes, 2 MgCl2, 5 Na2EGTA, 0.5 CaCl2 and 2 MgATP, pH adjusted to 7.2 with KOH, to which gramicidin A (Sigma) was added, at 64 μg ml−1, on the day of use (the tip of the electrode was filled with solution lacking gramicidin to facilitate seal formation). Gramicidin A allows monovalent cations, but not anions or divalent cations, to cross the membrane (Akaike, 1996). The electrode solution also contained Lucifer Yellow (0.8 mg ml−1) to allow visualization of whether the perforated patch had altered to whole-cell mode, allowing Lucifer Yellow into the cell (Allen et al. 2004). Typically, after sealing onto the cell membrane, it took about 30–40 min for sufficient perforant to enter the membrane to lower the series resistance below 25 MΩ. The series resistance was then compensated to about 15 MΩ.
Analysis and display of spontaneous postsynaptic currents (sPSCs)
Data were sampled at 10 kHz and filtered at 2–5 kHz. Spontaneous postsynaptic currents were analysed using Synaptosoft software. When quantifying sPSC occurrence, sPSCs were defined as current deflections which had an amplitude (measured from the mean current) greater than the peak-to-peak amplitude of the current noise, and which had a fast rise and slower decay. The majority of sPSCs had a 10–90% rise time of between 0.5 and 3 ms, and events with a rise time longer than 6 ms were rejected. The decay time of sPSCs was defined as the time taken for the sPSC to decrease to 1/e (37%) of the peak amplitude. The decay time was variable within cells, but in whole-cell mode the majority decayed with a time constant of between 6 and 9 ms, with some events with decay times of up to 25 ms being seen. On average, the decay time was 4-fold longer than the rise time. The values measured for rise and decay times of sIPSCs, and the variability seen amongst the decay times, are consistent with those recorded by other investigators (Otis et al. 1991; Cohen et al. 2000; De Simoni et al. 2003). As detailed in the Results, the kinetics of sIPSCs recorded in perforated patch mode were different.
In ischaemia the frequency of sPSC occurrence increased to greater than 40 Hz (i.e. a new event on average every 25 ms). At this frequency sPSCs were often overlapping (see Fig. 2), with a new sPSC beginning before the previous one had decayed back to the baseline (with a mean decay time of about 7.8 ms). Under these conditions it was possible to measure both the occurrence (i.e. frequency) and amplitude of sPSCs with accuracy. The decay time was also estimated when sPSCs were occurring at high frequency, by subtraction of the pre-event baseline, but should be interpreted with some caution, owing to the incomplete decay of events.
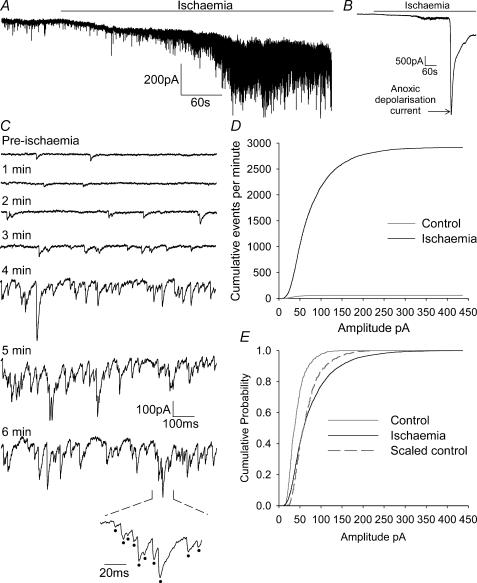
Figure 2. Properties of sPSCs in ischaemia recorded using whole-cell voltage clamping.
A, recording from a CA1 pyramidal neurone at −33 mV showing the increase in spontaneous events (downward current deflections) when ischaemia solution was applied. The anoxic depolarization current is not shown on this record (to allow sPSCs to be seen on a larger scale). B, same record as in A but filtered and on a lower gain and longer time scale, to show the occurrence of the anoxic depolarization current. C, expanded regions of 1 s duration from the trace in A. Pre-ischaemia relates to events before ischaemia, downward deflections are sPSCs. All other traces show events during ischaemia, with the times given representing time elapsed since the ischaemic solution was applied. The bottom trace is an expanded section from the 6 min ischaemia time point, with sPSCs marked with dots. D, cumulative events (per minute) of sPSC amplitudes, in control solution (grey line) and after 5 min ischaemia (black line), averaged over 5 cells. E, the curves in D normalized to the same peak for control (grey line) and ischaemia (black line) conditions. Dashed line is the control curve expanded along the amplitude axis to superimpose on the ischaemia curve.
Statistics
Five cells were analysed for every condition investigated, unless stated otherwise in the main text. Data are presented as means ± s.e.m. Significance of changes was assessed with a one-way ANOVA followed by a comparison of the means. In cases of a non-normal distribution within, or unequal variance between data groups, an ANOVA by rank was performed followed by a comparison of the means. Significance was assessed at the 95 and 99% confidence level. When only two groups were being compared a Student's two-tailed t test was used.
Results
Characteristics of sIPSCs in non-ischaemic conditions
Spontaneous postsynaptic currents recorded in non-ischaemic conditions from a CA1 pyramidal cell whole-cell clamped at −33 mV are shown in Fig. 1A. The intracellular solution was CsCl based (and, unless stated otherwise, used BAPTA as the calcium buffer). Since ECl was 0 mV and GABAA receptor-mediated currents were inward, these currents could include miniature inhibitory and excitatory currents generated by the spontaneous release of single quanta and currents evoked by spontaneous action potentials. Events below 10 pA in amplitude were not detectable above the baseline noise. The sPSCs had an average frequency of 1.57 ± 0.22 Hz (similar to the rate found by De Simoni et al. 2003 at postnatal day 14 (P14)), a mean amplitude of 43.2 ± 2.0 pA, a mean decay time of 7.9 ± 0.4 ms and a mean 10–90% rise time of 2.10 ± 0.11 ms (5848 sPSCs in 33 cells, at 33°C).
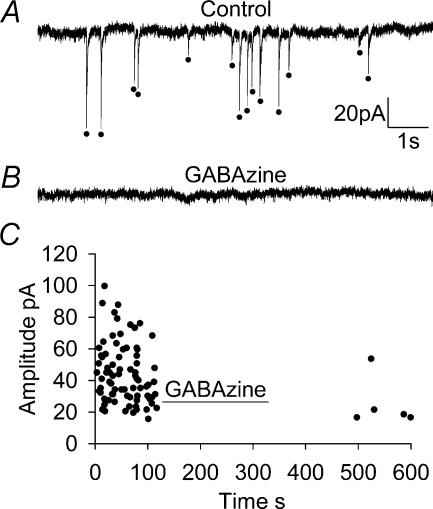
Figure 1. Spontaneous postsynaptic currents in non-ischaemic conditions in a whole-cell clamped CA1 pyramidal neurone.
A, 10 s recording from a CA1 cell at −33 mV showing sPSCs (marked with dots). B, 10 s recording from the same CA1 cell in the presence of 10 μm GABAzine to block GABAA receptors, which abolished almost all spontaneous events. C, time versus amplitude distribution plot (each point is a single sPSC) showing the abolition of sPSCs in the presence of GABAzine to block GABAA receptors (sample data from 1 cell, experiment performed on 3 cells, sPSC rate recovers only slowly from GABAzine application).
When the GABAA receptor blocker GABAzine (10 μm) was added (Fig. 1B and C) the frequency of sPSCs was reduced by 99% (P < 0.05) to 0.01 ± 0.01 Hz in three cells (mean data are shown below in Fig. 4A). There were too few events remaining in the presence of GABAzine to reliably quantify their amplitude, rise time or decay time. This is consistent with several previous studies on the frequency of spontaneous inhibitory and excitatory events in area CA1 pyramidal cells. These have shown most spontaneous currents to be sIPSCs, representing up to 97% of recorded events (Ropert et al. 1990; De Simoni et al. 2003), with smaller sEPSCs being far less numerous. Thus, almost all sPSCs recorded in control conditions are sIPSCs.
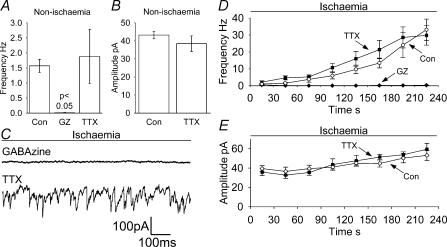
Figure 4. Effects on sPSCs of GABAzine (GZ, 10 μm) and TTX (1 μm).
A and B, effect of GABAzine (3 cells) and TTX (5 cells) on the sPSC frequency (A) and amplitude (B) in non-ischaemic solution (no amplitude data are plotted for GABAzine because of the very low number of events). P value is compared with control conditions. C–E, data in ischaemia solution. C, sample traces at −33 mV showing sPSCs, taken 4 min after the start of the ischaemic episode in GABAzine- or TTX-containing solution. Average data from 5 cells for frequency (D) and amplitude (E) of sPSCs during ischaemia; points represent 30 s bins of events. Control data from Fig. 3 (Con) are superimposed for comparison. Amplitude data are not shown for GABAzine due to the small number of events measured.
Characteristics of sPSCs in ischaemic conditions
During ischaemia there was an increase in the frequency of sPSCs (we will show below that these are essentially all GABAergic sIPSCs). Figure 2A is a sample trace showing the increase in sPSCs occurring in the build up to the anoxic depolarization (AD), which happens after 6–7 min (Rossi et al. 2000; Allen et al. 2004; the current associated with the AD is shown in the longer time scale, lower gain trace in Fig. 2B). Figure 2C shows 1 s long expansions of the full trace, taken at 1 min intervals as the ischaemic episode progressed, showing the increase in sPSC frequency.
The cumulative distribution of sPSC number as a function of amplitude shows a large increase in the sPSC rate after 5 min ischaemia (Fig. 2D). In addition, when this graph is normalized to produce a cumulative probability distribution (Fig. 2E), it shows that ischaemia not only increased the frequency of events, but also increased their amplitude. Scaling up the amplitude axis of the control curve in Fig. 2E by a constant factor for all amplitudes gave the dashed curve in Fig. 2E, which suggests that in ischaemia there was not a uniform scaling of all sPSCs by the same factor, but that larger sPSCs were selectively increased. This would rule out the idea that the amplitude increase results purely from a change of reversal potential for the GABAergic currents (see Discussion), which would scale all currents by the same factor.
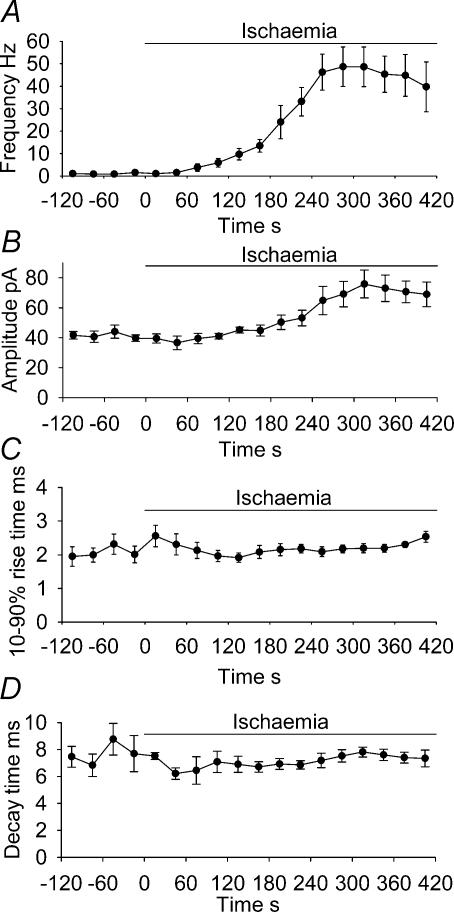
Figure 3 shows the time course of the changes in the properties of the ischaemia-evoked sPSCs, averaged over five cells. The frequency of sPSCs increased with each subsequent minute (Fig. 3A) until about 2 min before the AD, when it peaked at 48.6 ± 8.8 Hz, before decreasing to 39.7 ± 11.1 Hz just before the AD, although this decrease was not significant (P = 0.6, comparing the values 2 min before the AD with the values just before the AD). The peak sPSC frequency of 48.6 Hz was 47-fold higher than the mean pre-ischaemic sPSC frequency measured in these five cells, and 31-fold higher than the mean pre-ischaemic frequency measured in all 33 cells studied. The amplitude of sPSCs also increased as the AD was approached, peaking at 75.9 ± 9.3 pA 2 min before the AD, significantly larger than the 41.9 ± 3.2 pA measured in non-ischaemic solution (P = 0.02; Fig. 3B). The sPSCs then remained around 70 pA in amplitude until the AD occurred. Their decay times and 10–90% rise times remained fairly constant and near their control values throughout ischaemia (Fig. 3C and D).
Figure 3. Summary of sPSC properties (at −33 mV) in ischaemia solution up to the time of the anoxic depolarization.
Plots show frequency (A), amplitude (B), 10–90% rise time (C) and decay time of sPSCs during ischaemia (D); points represent 30 s bins of events, averaged over 5 cells.
To test the effect of drug treatments on the sPSCs produced in ischaemia, the sPSC frequency in different conditions was measured for the period between 3.5 and 4 min after the application of ischaemia solution (referred to as 4 min for brevity in the rest of the paper), just before the peak increase of sPSC frequency that occurred at 4–5 min (Fig. 3A). Measurement at 3.5–4 min was a compromise between choosing a time at which a dramatic increase of sPSC frequency had occurred and avoiding the period of peak sPSC activity, during which measurement of sPSC decay times is less accurate (see Methods).
Spontaneous postsynaptic curents in ischaemia are predominantly mediated via GABAA receptors
When 10 μm GABAzine was present throughout the ischaemic episode the frequency of sPSCs reached only 0.3 ± 0.1 Hz after 4 min (Fig. 4C and D), which is only 0.9% of the frequency reached after 4 min in control ischaemia solution, i.e. 33.1 ± 6.3 Hz (Fig. 3A; P < 0.01). This 99% reduction is the same as the 99% reduction of sPSC frequency produced by GABAzine in non-ischaemic solution (Figs 1 and 4A), and suggests that the majority of spontaneous events measured during ischaemia are GABAergic in origin. There were too few events remaining in the presence of GABAzine to reliably quantify their amplitude, rise time or decay time. From now on, since 99% of sPSCs were abolished when GABAA receptors were blocked during ischaemia, all sPSCs detected in the absence of GABAA blockers will be assumed to be sIPSCs.
Spontaeous inhibitory postsynaptic currents in ischaemia are not action potential evoked
Tetrodotoxin blocks voltage-gated Na+ channels, so preventing action potentials and removing one of the triggers for vesicular release. Tetrodotoxin (1 μm) had no significant effect on the frequency (Fig. 4A), size (Fig. 4B), rise time or decay kinetics (not shown) of sIPSCs in non-ischaemic conditions, implying that most events are not triggered by action potentials.
Application of TTX throughout the ischaemic episode had no effect on the increase in frequency of sIPSCs (Fig. 4C and D). The rate of sIPSCs increased to 29.8 ± 5.8 Hz after 4 min, not significantly different from the 33.1 ± 6.3 Hz seen in control ischaemia solution lacking TTX in Fig. 3. This suggests that the ischaemia-evoked neurotransmitter release is spontaneous and does not rely on action potentials to depolarize the presynaptic terminal. The amplitudes (Fig. 4E), rise and decay times (not shown) of the events were also not significantly different from those seen in control ischaemia solution, with an increase of amplitude and no change of rise or decay time as the ischaemic episode progressed.
Spontaneous inhibitory postsynaptic currents in ischaemia are vesicular in origin
Although most neurotransmitter release is assumed to be vesicular, various non-vesicular transmitter release mechanisms exist (Schwartz, 1987; Szatkowski et al. 1990; Demarque et al. 2002). We tested whether the sIPSCs were generated by vesicular release by pretreating slices with concanamycin, which depletes vesicles of neurotransmitter by blocking the vacuolar H+-ATPase, thus abolishing the proton gradient required for vesicular filling (Drose & Altendorf, 1997; Zhou et al. 2000). In non-ischaemic conditions, pretreatment with concanamycin (0.5 μm) reduced the frequency (by 84%, P < 0.05) and the average amplitude (by 37%, P < 0.01) of sIPSCs, compared to those recorded in non-concanamycin-treated slices (Fig. 5A and B), but did not affect the decay time or 10–90% rise time (data not shown). The effect of concanamycin on the event frequency is as expected, since it effectively blocks vesicular release. The incomplete abolition of sIPSCs and the reduced event amplitude after concanamycin pretreatment may reflect the presence of a remaining population of vesicles with a low GABA level inside, which would release smaller packets of GABA into the synaptic cleft. This is likely because concanamycin was present in the presoaking solution but not in the recording solution (for cost reasons), so some vesicle refilling may occur if concanamycin partly washes out while locating and recording from the cell.
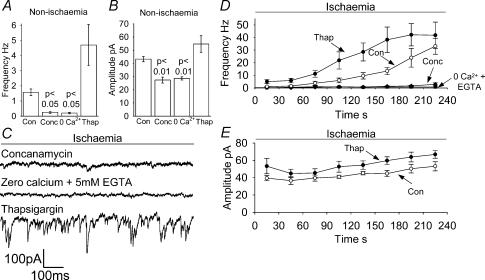
Figure 5. Effects on sIPSCs of pretreatment with concanamaycin, Ca2+-free solution containing 5 mm EGTA, or pretreatment with thapsigargin.
A and B, effect of concanamycin pretreatment (Conc, 0.5 μm for at least 2 h, 5 cells), Ca2+-free solution containing 5 mm EGTA (5 cells) and thapsigargin pretreatment (Thap, 3 μm for 1 h, 5 cells) on the sIPSC frequency (A) and amplitude (B) in non-ischaemic solution. P values are compared with control conditions. C–E, data in ischaemia solution. C, sample traces at −33 mV showing sIPSCs, taken 4 min after the start of the ischaemic episode after concanamycin pretreatment, in Ca2+-free/5 mm EGTA solution, and after thapsigargin pretreatment. Average data from 5 cells for frequency (D) and amplitude (E) of sIPSCs during ischaemia; points represent 30 s bins of events. Control data (Con) from Fig. 3 are superimposed for comparison. Amplitude data are not shown for Ca2+-free conditions or concanamycin because of the small number of events measured.
When concanamycin pretreated slices were exposed to ischaemic conditions, the frequency of sPSCs reached 2.9 ± 2.6 Hz after 4 min (Fig. 5C and D), 92% (P < 0.01) lower than the 33.1 ± 6.3 Hz seen after 4 min in control ischaemic conditions (Fig. 3A), and not a significant increase over the 0.25 ± 0.06 Hz recorded before ischaemia was applied (P = 0.08). Thus, as expected, the majority of sIPSCs seen in ischaemia are caused by the release of neurotransmitter from vesicles. The amplitude of the events seen after 4 min ischaemia was significantly smaller than the amplitude of those seen without concanamycin pretreatment, i.e. 33.9 ± 4.1 compared to 53.1 ± 5.2pA (P < 0.05). This is very similar to the reduction described above for non-ischaemic solution which was attributed to the fact that concanamycin was not present in the solution used for recording, so some vesicles may have partly refilled in the time between removal of the slice from the concanamycin-containing presoaking solution and recording from the cell, leading to smaller sIPSCs. The events remaining are unlikely to be sEPSCs, since in ischaemia solution containing GABAzine these occurred at a much lower rate (0.3 Hz, P < 0.01, see Fig. 4D) than the 2.9 Hz seen here.
Spontaneous inhibitory postsynaptic currents in ischaemia are inhibited by removal of extracellular calcium
Removal of external calcium prevents vesicular release by removing the major trigger for that release, external calcium entry (Katz & Miledi, 1970), although some spontaneous vesicular release has been shown to occur in the absence of external calcium (reviewed by Edwards, 1995). Cells recorded from in non-ischaemic calcium-free external solution (with 5 mm EGTA added to chelate residual calcium) displayed sPSCs at a lower frequency (reduced by 87%, P < 0.05) than those recorded in calcium-containing solution (Fig. 5A). The decrease of sPSC frequency in calcium-free solution is consistent with spontaneous transmitter release being activated by Ca2+ entry across the cell membrane. The sPSC amplitude (Fig. 5B) was also reduced in calcium-free external solution, by 34% (P < 0.01); the mechanism of this change was not investigated. The sPSC decay time and 10–90% rise time were not affected by removal of extracellular calcium (data not shown).
With external calcium absent throughout the ischaemic episode, the frequency of sIPSCs reached only 0.75 ± 0.09 Hz 4 min into the ischaemic episode (Fig. 5C and D), a reduction of 98% (P < 0.01) from the 33.1 ± 6.3 Hz seen after 4 min in control conditions (Fig. 3A), but a small increase (P = 0.004) on the frequency (0.20 ± 0.11 Hz) recorded in calcium-free solution in the 2 min before the ischaemia solution was applied (Fig. 5A). This is consistent with external calcium entry being required in order to trigger the majority of the ischaemia-evoked vesicular release of neurotransmitter, and contrasts with the results of Fleidervish et al. (2001), who reported that the hypoxic increase in sIPSCs was insensitive to external calcium removal, but who did not chelate trace calcium with EGTA.
Spontaneous inhibitory postsynaptic currents in ischaemia are not affected by depletion of calcium from intracellular stores
In order to exclude the possibility that application of calcium-free solution to the slices for 2 min had depleted intracellular calcium stores, slices in normal calcium solution were pretreated with thapsigargin (3 μm for 1 h) to irreversibly block the endoplasmic reticulum calcium pump, and thereby deplete internal stores of calcium (Thastrup et al. 1990; Lytton et al. 1991; Irving et al. 1992; Garaschuk et al. 1997). In non-ischaemic solution, the sIPSC frequency was increased (but not significantly) following thapsigargin treatment to 4.7 ± 1.4 Hz (Fig. 5A; P > 0.05 compared to 1.6 ± 0.2 Hz in control conditions). Although not a significant increase, this rise could reflect a higher basal [Ca2+] in the presynaptic terminal causing more spontaneous release, resulting from the inhibition of sequestration into internal stores. The amplitude of events was not affected (55 ± 6.6 pA; Fig. 5B), nor were the rise and decay times (data not shown).
Treatment of slices with thapsigargin did not reduce the frequency (41.9 ± 10.4 Hz) of sIPSCs after 4 min ischaemia (Fig. 5C and D); indeed, this elevated frequency was reached more quickly after thapsigargin treatment (Fig. 5D). Thus, the release of calcium from intracellular stores is apparently not involved in the ischaemic increase in sIPSC frequency, contrary to the suggestion of Katchman & Hershkowitz (1993a) for sEPSCs. The amplitude of ischaemic sIPSCs was not altered by thapsigargin (66.8 ± 4.4 pA; P > 0.05 compared with control ischaemia; Fig. 5E). The 10–90% rise time and decay time were not different from those in control ischaemia (data not shown).
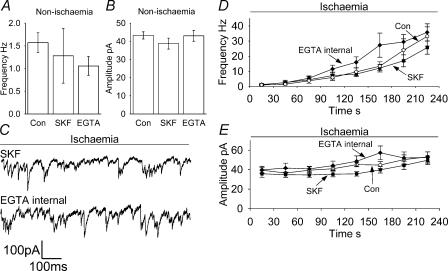
Spontaneous inhibitory postsynaptic currents in ischaemia are not affected by blocking GAT-1
The run-down of ion gradients occurring during ischaemia eventually leads to a reversal of the neuronal GABA transporter GAT-1 and release of large amounts of GABA (Allen et al. 2004). To test whether any rise of [GABA]o produced by a slowing or reversal of GAT-1 affects ischaemia-evoked sIPSCs, we blocked GAT-1 using 100 μm SKF 89976A (Borden et al. 1994). In non-ischaemic solution, SKF 89976A had no effect on the frequency (Fig. 6A), amplitude (Fig. 6B), 10–90% decay time or rise time (data not shown) of sIPSCs. Blocking GAT-1 throughout the ischaemic episode had no effect on the frequency (25.7 ± 4.3 Hz) or amplitude of sIPSCs (49.7 ± 4.4 pA) after 4 min of ischaemia (Fig. 6C, D and E), compared to control ischaemia after 4 min (Fig. 3). The kinetics of the rise and decay times were no different from those seen in control ischaemia (data not shown). The lack of a prolongation of sIPSCs by SKF 89976A is consistent with previous studies where blocking uptake had no effect on the decay of small events such as sIPSCs, despite prolongation of the decay time of larger evoked events (Thompson & Gähwiler, 1992; Isaacson et al. 1993; Overstreet et al. 2000).
Figure 6. Effects on sIPSCs of the GAT-1 blocker SKF 89976A and of using EGTA rather than BAPTA in the whole-cell pipette to buffer internal calcium concentration.
A and B, effect of SKF 89976A (SKF, 5 cells) and EGTA internal (EGTA, 5 cells) on the sIPSC frequency (A) and amplitude (B) in non-ischaemic solution. C–E, data in ischaemia solution. C, sample traces at −33 mV showing sIPSCs, taken 4 min after the start of the ischaemic episode, in SKF 89976A-containing solution, or in normal ischaemic solution when recording with the EGTA internal solution. Average data from 5 cells for frequency (D) and amplitude (E) of sIPSCs during ischaemia; points represent 30 s bins of events. Control data from Fig. 3 are superimposed for comparison (Con).
Characteristics of sIPSCs recorded using perforated patch-clamping
The experiments described so far have used whole-cell clamping, with BAPTA buffering of intracellular calcium concentration, because under these conditions GABAA receptors stay functional and can be used to monitor the release of GABA onto the pyramidal cell recorded from (Allen et al. 2004). In vivo, the intracellular calcium concentration rises during ischaemia, which decreases the response of GABAA receptors (Allen et al. 2004), and ischaemia may induce changes of the chloride concentration across the cell membrane (Jiang et al. 1992), which will alter the driving force on Cl− movements through GABAA receptor channels.
To determine how sIPSCs alter in ischaemia in a non-dialysed cell, we used perforated patch-clamping with gramicidin as the perforant. This allows us to record sPSCs with minimal disruption of the intracellular environment, by preventing the wash-out of intracellular substances and allowing the chloride and calcium concentrations within the cell to behave in a natural manner. At a holding potential of −33 mV sIPSCs now appeared as outward currents (Fig. 7A and B). Under these conditions sIPSCs had average amplitudes of 31.4 ± 1.2 pA in five cells. The average frequency of events in control (non-ischaemic) solution was lower than that recorded in whole-cell mode; sIPSCs occurred at 0.8 ± 0.2 Hz (480 events detected in 5 cells; P = 0.03 compared with the sIPSC frequency of 1.6 ± 0.2 Hz in whole-cell mode). Possible reasons for this are considered in the Discussion. The 10–90% rise time of sIPSCs was 1.55 ± 0.08 ms, significantly shorter than in whole-cell mode (2.10 ± 0.11 ms; P = 0.0007). The decay time of sIPSCs was 3.5 ± 0.6 ms, significantly shorter than that recorded in whole-cell mode (7.9 ± 0.4 ms; P = 10−6), which may be explained by the intracellular [Ca2+] being higher in perforated patch mode than in whole-cell mode, since elevated ]Ca2+]i shortens the open time of GABAA receptor channels in some cells (Behrends et al. 1988; but see De Koninck & Mody, 1996).
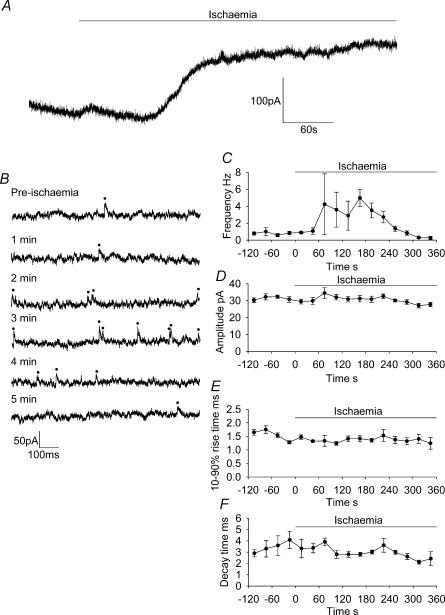
Figure 7. Properties of sIPSCs in ischaemia recorded using gramicidin perforated patch-clamping.
A, recording from a CA1 pyramidal neurone at −33 mV showing the increase in spontaneous IPSCs, and the development of an outward current, when ischaemia solution was applied; an AD occurred a few seconds after the end of the trace, but is not shown in order to display the pre-AD current on a larger scale. B, expanded regions of 1 s duration from the trace in A. Pre-ischaemia represents events before ischaemia, upward deflections are sIPSCs (marked with dots). All other traces show events during ischaemia, with the times stated representing time elapsed since the ischaemic solution was applied. C–F, plots of frequency (C), amplitude (D), 10–90% rise time (E) and decay time of sIPSCs during ischaemia (F); points represent 30 s bins of events, averaged over 5 cells.
Perforated patch-clamp recording of sIPSCs in ischaemia
Figure 7A shows the response of a CA1 pyramidal neurone to ischaemia in perforated patch conditions, with expanded sections of the trace shown in Fig. 7B. An outward current developed during the ischaemic episode before the anoxic depolarization (Allen et al. 2004; the AD is not shown on this trace in order to display the pre-AD trace on a larger scale). After 1.5 min there was an increase in the frequency of sIPSCs, peaking at 5.0 ± 1.0 Hz after 3 min, but this decreased again to 0.3 ± 0.2 Hz just before the AD (Fig. 7C; note that this decrease occurred earlier than the smaller decrease seen after 5–6 min in Fig. 3A). The amplitude, rise and decay time of sIPSCs were not significantly changed during ischaemia (Fig. 7D, E and F). As expected, recording in perforated patch mode in ischaemia in the presence of GABAzine prevented the majority of sIPSCs from occurring (reduced by 99%; data not shown).
Comparison of the frequency of events after 3 min (when events peaked in perforated patch mode) in perforated patch and whole-cell modes showed that less events were occurring in perforated patch mode at this time (P = 0.04; frequency in whole-cell mode was 13.5 ± 2.9 Hz), suggesting that the overall increase in sIPSC frequency was reduced, rather than following the same initial pattern as in whole-cell mode. The 5 Hz peak frequency of sIPSCs in perforated patch mode was much lower than was seen in whole-cell mode (48.6 ± 8.8 Hz, 5 min after the application of ischaemia solution; P = 0.007).
We considered the possibility that the higher series resistance inherent in perforated patch-clamp recordings (∼15 versus ∼2 MΩ in whole-cell recordings) may have caused greater filtering of the sIPSCs to occur, so making them less detectable so that they appeared to be less frequent. To investigate this, we filtered a whole-cell recording to mimic the level of filtering occurring in perforated patch recordings (using a one-pole RC filter with a half power frequency of 1/2πτ, where τ (0.55 ms) was the decay time constant of a typical capacity transient during perforated patch-clamp recording). In practice, this led to a 6% increase in the number of detected sIPSCs, since the greater filtering improved resolution of the fact that events had occurred (but slightly distorted their waveform). Thus the higher series resistance present in perforated patch-clamp recordings is not responsible for the decrease in the frequency of sIPSCs detected.
Next, we considered whether the lower sIPSC frequency seen in perforated patch mode could be because the perforated patch mode allowed the [Ca2+]i to rise higher and desensitize GABAA receptors (Allen et al. 2004), thus reducing sIPSC amplitudes and making sIPSCs harder to detect (although there was no obvious change in sIPSC amplitude as the frequency of events decreased late in ischaemia; Fig. 7D). To test this we recorded from pyramidal cells in whole-cell mode using EGTA as the calcium buffer rather than BAPTA (we have previously shown that using EGTA allows desensitization of GABAA receptors to occur after the AD, whereas BAPTA prevents it; Allen et al. 2004). Recording from cells in non-ischaemic solution using EGTA rather than BAPTA as the whole-cell solution calcium buffer had no significant effect on the frequency (Fig. 6A), amplitude (Fig. 6B), decay time or 10–90% rise time of sIPSCs (data not shown), showing that the different calcium-buffering solutions did not affect the properties of the GABAA receptors in non-ischaemic conditions. In ischaemia, use of EGTA as the whole-cell calcium buffer still allowed an increase in sIPSC frequency to occur, to 35.8 ± 5.6 Hz after 4 min in ischaemia solution (Fig. 6D), which is not significantly different from when BAPTA was used as the calcium buffer in whole-cell mode (33.1 ± 6.3 Hz; Fig. 3A), suggesting that calcium-induced desensitization of GABAA receptors is not responsible for the lower sIPSC frequency during ischaemia in perforated patch mode.
Discussion
We have shown that: (i) ischaemia greatly increases the sIPSC frequency; (ii) the sIPSCs are generated by vesicular release dependent on external calcium, but are independent of Na+ action potentials; and (iii) in perforated patch mode, the properties of ischaemia-evoked sIPSCs are intriguingly different, most notably in that they occur at a 10-fold lower frequency.
Characteristics of sIPSCs in non-ischaemic conditions
In control conditions the frequency of spontaneous postsynaptic currents was around 1.6 Hz. Blocking GABAA receptors abolished over 99% of sPSCs, showing that these were largely sIPSCs (Fig. 1 of the present paper; Ropert et al. 1990; De Simoni et al. 2003).
Blocking vesicular release of transmitter, by depleting vesicles of transmitter using concanamycin, or removing external Ca2+, greatly reduced the frequency of sIPSCs in control conditions, while irreversibly blocking the endoplasmic reticulum Ca2+ pump with thapsigargin and thus depleting internal Ca2+ stores had little effect on the sIPSCs (in fact their frequency was slightly increased; Fig. 5). Similarly, a great reduction of spontaneous IPSCs in Ca2+-free solution was reported by Gaspary et al. (1998) in cultured hippocampal neurones and by Kojima & Takahashi (1985) in spinal neurones. In contrast, Ca2+ removal did not affect the sIPSC rate (in TTX) in cerebellar neurones (Llano & Gerschenfeld, 1993), nor did it alter sIPSC rate in CA3 pyramidal neurones (Savic & Sciancalepore, 1998). Tetrodotoxin had no effect on the frequency of sIPSCs (Fig. 4), showing them to be spontaneous miniature events and not driven by action potentials. By contrast, De Simoni et al. (2003) found that TTX halved the IPSC frequency (under similar recording conditions except their cells were at −70 mV and 24°C, while ours were at −33 mV and 33°C); the cause of this difference is unclear.
Characteristics of sIPSCs in ischaemia
Ischaemia solution increased the frequency and amplitude of sPSCs (Fig. 3). These were mainly sIPSCs, since GABAzine abolished 99% of the sPSCs. Hershkowitz et al. (1993) showed that sEPSCs in area CA1 also increase in frequency during hypoxia, but both the frequency and the amplitude of these events are much smaller than for sIPSCs, being on average ∼1 Hz and 12 pA at a holding potential of −60 mV. In our experiments, with neurones voltage clamped at −33 mV, sEPSCs are even smaller (∼6 pA) owing to the reduction in the driving force for Na+ entry. The peak-to-peak noise in our recordings is around 10 pA, hindering the detection of sEPSCs. Because of this, and the 50-fold higher frequency of sIPSCs, almost all the ischaemic sPSCs detected were GABAergic.
GABA release during early ischaemia is vesicular and [Ca2+]o dependent
Tetrodotoxin did not alter the frequency or amplitude of sIPSCs during ischaemia, implying that action potential-induced depolarization of presynaptic terminals did not drive spontaneous GABA release (Fig. 4). Blocking vesicular release with concanamycin or removal of Ca2+ greatly reduced the ischaemia-evoked sIPSC frequency, while depleting internal Ca2+ stores with thapsigargin did not reduce the sIPSC frequency (Fig. 5). Thus, the majority of GABA release in early ischaemia is from vesicles, and this vesicular release is dependent on external Ca2+. The external Ca2+ dependence of release is in contrast to the reports of Fleidervish et al. (2001) and Katchman & Hershkowitz (1993a), who found that sIPSCs and sEPSCs in hypoxia occurred at normal rates in Ca2+-free external solution (and that sEPSCs were greatly reduced if Ca2+ release from internal stores was blocked). In both these studies, however, extracellular Ca2+ was simply removed, and no EGTA was added to chelate trace Ca2+. Katchman & Hershkowitz (1993a) also applied cadmium to block voltage-dependent Ca2+ channels, which had no effect on the frequency of sEPSCs in hypoxia, again suggesting that they were independent of external Ca2+ entry. Fleidervish et al. (2001) recorded from mouse layer 5 pyramidal neurones in the somatosensory cortex, so the differential calcium sensitivity may be attributable to cell type or species differences. The fact that sIPSCs are [Ca2+]o dependent in our ischaemia experiments, and sEPSCs are [Ca2+]o independent in the hypoxia experiments (in area CA1) using Cd2+ of Katchman & Hershkowitz (1993a), may reflect a different mechanism of energy deprivation-induced release of transmitter from glutamatergic and GABAergic terminals, or it could reflect a difference between the effects and severity of hypoxia and ischaemia.
What triggers the vesicular release of GABA during ischaemia? Presumably [ATP] falls, inhibiting the sodium pump, depolarizing the presynaptic terminal and opening voltage-sensitive Ca2+ channels, and the subsequent Ca2+ influx causes vesicular release of GABA. At the neuromuscular junction the spontaneous vesicular release rate increases with presynaptic depolarization (Del Castillo & Katz, 1954), consistent with the increase of sIPSC frequency seen in whole-cell recording as the ischaemic episode progresses; the longer energy deprivation lasts, the more depolarized cells become and the more vesicles are released.
There is a second possible explanation for the increase in frequency of sIPSCs in the build up to the anoxic depolarization (AD). Engel et al. (2001) showed that blocking GABA breakdown by GABA transaminase, using γ-vinyl-GABA, led to an increased GABA level in presynaptic terminals and an increase in the frequency and amplitude of sIPSCs. They attributed this to increased vesicular filling and turnover causing more (and more easily detectable) spontaneous release. In ischaemia GABA levels also rise in presynaptic terminals due to increased synthesis and decreased degradation (Madl & Royer, 2000), so there may be increased filling of vesicles and increased spontaneous release. This could also explain the increase in amplitude of the sIPSCs seen in whole-cell recording (Fig. 3B). However, this increased vesicular filling will not continue indefinitely because it is an ATP-dependent process and ATP levels fall in ischaemia. Another possible contributor to the increased sIPSC amplitude (when [Cl−1]i is high, as in our whole-cell clamp experiments) is the fall of [Cl−]o which occurs as a result of Cl− entry into depolarized cells in energy deprivation (Jiang et al. 1992), which will increase the driving force for Cl− to leave the cell through GABAA receptor channels.
Spontaneous inhibitory postsynaptic currents occur at a 10-fold lower rate in ischaemia using perforated patch-clamping
Using perforated patch-clamping, to allow the cells' [Ca2+]i and [Cl−]i to behave in a normal manner, gave a response to ischaemia different from that in whole-cell recordings. The most dramatic difference was in the sIPSC frequency, which increased from 0.8 to 5 Hz in the first 3 min of ischaemia, but started to decrease in frequency after 4 min, reducing to around 0.3 Hz just before the AD (Fig. 7). This is in contrast to whole-cell conditions, where there was a steady increase of sIPSC frequency up to 49 Hz 2 min before the AD, and then a decrease (statistically insignificant) to 40 Hz just before the AD.
We considered whether the apparent reduction in sIPSC frequency seen in perforated patch mode was due to a shift in the chloride reversal potential. A redistribution of Cl− can occur during ischaemia, with movement of Cl− into the cell lowering [Cl−]o and raising [Cl−]i (Jiang et al. 1992; Katchman et al. 1994), moving ECl from its normal value of approx. −65 mV closer to the holding potential of −33 mV. This would reduce the amplitude of sIPSCs (or might even reverse them), possibly making them harder to detect and decreasing the apparent sIPSC frequency. However, both the Jiang et al. (1992) and Katchman et al. (1994) studies were carried out on thick slices perfused at a low rate (0.5–2 ml min−1) in interface chambers, i.e. in conditions more likely to produce a large ischaemia-evoked shift of ECl than in the conditions of the thin submerged slices superfused at a high rate (10 ml min−1) that we used here. Indeed, in our study no decrease in sIPSC amplitude (and no reversal of polarity) was detected during the ischaemic episode during perforated patch recording (Fig. 7D), suggesting that a reduction in sIPSC amplitude caused by a shift of ECl is not the explanation of the lower sIPSC frequency.
Alternative explanations for the difference in sIPSC frequency between perforated patch and whole-cell recording would include: (i) differences in the sensitivity of GABAA receptors when recording in perforated patch and whole-cell modes, altering the size and detectability of sIPSCs; or (ii) the change of the internal environment that occurs in whole-cell mode preventing the production of retrograde messengers that feed back on presynaptic terminals and decrease GABA release.
Desensitization of the GABA response in perforated patch mode might occur due to calcium influx causing dephosphorylation of GABAA receptors (Allen et al. 2004), but whole-cell clamping using the weak calcium buffer EGTA did not reduce the increase in sIPSC frequency seen in ischaemia (Fig. 6). Another possibility is that, if GABAA receptors need to be phosphorylated to remain functional (Chen et al. 1990), then although in whole-cell mode the internal solution contains ATP to keep the receptors in a functional state, in perforated patch mode the cell will experience an ischaemic run-down of ATP levels. Harata et al. (1997) demonstrated that, in isolated CA1 pyramidal cells in perforated patch mode, the response to exogenous GABA decreased with time, with this decrease being enhanced in oxygen- and glucose-free solution, and that the same occurred in cells whole-cell clamped with an ATP-free solution. Thus, desensitization of GABAA receptors, allowed by the lower ATP in perforated patch mode, could reduce the size and detectability of sIPSCs, but the lack of a decrease in sIPSC amplitude (Fig. 7D) argues against this as an explanation for the decreased sIPSC frequency.
Alternatively, adenosine and cannabinoids, which inhibit GABA release from interneurones (Hoffman & Lupica, 2000; Jeong et al. 2003) might be released at a higher rate from cells studied with perforated patch clamping. Adenosine is released from metabolically compromised cells due to enhanced ATP breakdown, and in striatal ischaemia it inhibits evoked IPSCs (Centonze et al. 2001), but it may be less important in hippocampal ischaemia (Katchman & Hershkowitz, 1993b). Cannabinoid release depends on postsynaptic [Ca2+]i (Wilson & Nicoll, 2001), which during ischaemia presumably rises higher in perforated patch mode.
The 10-fold lower sIPSC frequency, with no change of sIPSC amplitude, when perforated patch-clamping during ischaemia, suggests that enhanced release of one of these retrograde messengers when perforated patch-clamping may underlie the lower GABA release observed (assuming that the local concentration of retrograde messenger at presynaptic release sites is determined mainly by release from the postsynaptic (recorded) cell and not from other surrounding cells). Lack of control of the intracellular medium could also explain the roughly constant sIPSC amplitude when perforated patch-clamping, whereas an increase is seen in whole-cell mode. Movement of Cl− into the cell will lower [Cl−]o and raise [Cl−]i (Jiang et al. 1992), moving ECl from its normal value of around −65 mV closer to the holding potential of −33 mV and reducing the size of the sIPSCs. Combined with the tendency for sIPSC amplitude to increase as a result of the ischaemia-evoked increase in presynaptic [GABA]i (see above), this may result in the relatively constant sIPSC amplitude which is observed.
Although the cause of the difference in sIPSC properties seen in whole-cell and perforated patch recording remains to be explained, our data imply that perforated patch recording may give better information on the rate and effects of spontaneous GABA release in ischaemia than is provided by whole-cell clamping.
Acknowledgments
This work was supported by the Wellcome Trust, the European Union and a Wolfson-Royal Society Award to D.A.. Nicola Allen was in the 4 year PhD Programme in Neuroscience at University College London.
References
- Akaike N. Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol. 1996;65:251–264. doi: 10.1016/s0079-6107(96)00013-2. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Rossi DJ, Attwell D. Sequential release of GABA by exocytosis and reversed uptake leads to neuronal swelling in simulated ischemia of hippocampal slices. J Neurosci. 2004;24:3837–3849. doi: 10.1523/JNEUROSCI.5539-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends JC, Maruyama T, Tokutomi N, Akaike N. Ca2+-mediated suppression of the GABA-response through modulation of chloride channel gating in frog sensory neurones. Neurosci Lett. 1988;86:311–316. doi: 10.1016/0304-3940(88)90502-2. [DOI] [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966 and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Centonze D, Saulle E, Pisani A, Bernardi G, Calabresi P. Adenosine-mediated inhibition of striatal GABAergic synaptic transmission during in vitro ischaemia. Brain. 2001;124:1855–1865. doi: 10.1093/brain/124.9.1855. [DOI] [PubMed] [Google Scholar]
- Chen QX, Stelzer A, Kay AR, Wong RK. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Coulter DA. Protracted postnatal development of inhibitory synaptic transmission in rat hippocampal area CA1 neurons. J Neurophysiol. 2000;84:2465–2476. doi: 10.1152/jn.2000.84.5.2465. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. The effects of raising intracellular calcium on synaptic GABAA receptor channels. Neuropharmacol. 1996;35:1365–1374. doi: 10.1016/s0028-3908(96)00063-9. [DOI] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954;124:586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol. 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drose S, Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J Exp Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- Edwards FA. Anatomy and electrophysiology of fast central synapses lead to a structural model for long-term potentiation. Physiol Rev. 1995;75:759–787. doi: 10.1152/physrev.1995.75.4.759. [DOI] [PubMed] [Google Scholar]
- Engel D, Pahner I, Schulze K, Frahm C, Jarry H, Ahnert-Hilger G, Draguhn A. Plasticity of rat central inhibitory synapses through GABA metabolism. J Physiol. 2001;535:473–482. doi: 10.1111/j.1469-7793.2001.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish IA, Gebhardt C, Astman N, Gutnick MJ, Heinemann U. Enhanced spontaneous transmitter release is the earliest consequence of neocortical hypoxia that can explain the disruption of normal circuit function. J Neurosci. 2001;21:4600–4608. doi: 10.1523/JNEUROSCI.21-13-04600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Yaari Y, Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J Physiol. 1997;502:13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspary HL, Wang W, Richerson GB. Carrier-mediated GABA release activates GABA receptors on hippocampal neurons. J Neurophysiol. 1998;80:270–281. doi: 10.1152/jn.1998.80.1.270. [DOI] [PubMed] [Google Scholar]
- Green AR, Hainsworth AH, Jackson DM. GABA potentiation: a logical pharmacological approach for the treatment of acute ischaemic stroke. Neuropharmacol. 2000;39:1483–1494. doi: 10.1016/s0028-3908(99)00233-6. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Lehmann A, Sandberg M, Nystrom B, Jacobson I, Hamberger A. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J Cereb Blood Flow Metab. 1985;5:413–419. doi: 10.1038/jcbfm.1985.56. [DOI] [PubMed] [Google Scholar]
- Harata N, Wu J, Ishibashi H, Ono K, Akaike N. Run-down of the GABAA response under experimental ischaemia in acutely dissociated CA1 pyramidal neurones of the rat. J Physiol. 1997;500:673–688. doi: 10.1113/jphysiol.1997.sp022052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkowitz N, Katchman AN, Veregge S. Site of synaptic depression during hypoxia: a patch-clamp analysis. J Neurophysiol. 1993;69:432–441. doi: 10.1152/jn.1993.69.2.432. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABAA synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving AJ, Collingridge GL, Schofield JG. Interactions between Ca2+ mobilizing mechanisms in cultured rat cerebellar granule cells. J Physiol. 1992;456:667–680. doi: 10.1113/jphysiol.1992.sp019360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Jang IS, Nabekura J, Akaike N. Adenosine A1 receptor-mediated presynaptic inhibition of GABAergic transmission in immature rat hippocampal CA1 neurons. J Neurophysiol. 2003;89:1214–1222. doi: 10.1152/jn.00516.2002. [DOI] [PubMed] [Google Scholar]
- Jiang C, Agulian S, Haddad GG. Cl− and Na+ homeostasis during anoxia in rat hypoglossal neurons: intracellular and extracellular in vitro studies. J Physiol. 1992;448:697–708. doi: 10.1113/jphysiol.1992.sp019065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchman AN, Hershkowitz N. Early anoxia-induced vesicular glutamate release results from mobilization of calcium from intracellular stores. J Neurophysiol. 1993a;70:1–7. doi: 10.1152/jn.1993.70.1.1. [DOI] [PubMed] [Google Scholar]
- Katchman AN, Hershkowitz N. Adenosine antagonists prevent hypoxia-induced depression of excitatory but not inhibitory synaptic currents. Neurosci Lett. 1993b;159:123–126. doi: 10.1016/0304-3940(93)90814-2. [DOI] [PubMed] [Google Scholar]
- Katchman AN, Vicini S, Hershkowitz N. Mechanism of early anoxia-induced suppression of the GABAA-mediated inhibitory postsynaptic current. J Neurophysiol. 1994;71:1128–1138. doi: 10.1152/jn.1994.71.3.1128. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970;207:789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Takahashi T. Characterization of miniature inhibitory post-synaptic potentials in rat spinal motoneurones. J Physiol. 1985;368:627–640. doi: 10.1113/jphysiol.1985.sp015880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Gerschenfeld HM. Inhibitory synaptic currents in stellate cells of rat cerebellar slices. J Physiol. 1993;468:177–200. doi: 10.1113/jphysiol.1993.sp019766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- Madl JE, Royer SM. Glutamate dependence of GABA levels in neurons of hypoxic and hypoglycemic rat hippocampal slices. Neuroscience. 2000;96:657–664. doi: 10.1016/s0306-4522(99)00548-5. [DOI] [PubMed] [Google Scholar]
- Otis TS, Staley KJ, Mody I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991;545:142–150. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Jones MV, Westbrook GL. Slow desensitization regulates the availability of synaptic GABAA receptors. J Neurosci. 2000;20:7914–7921. doi: 10.1523/JNEUROSCI.20-21-07914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner PB, Laycock AG, Doll CJ. A pharmacological model of ischemia in the hippocampal slice. Neurosci Lett. 1990;119:175–178. doi: 10.1016/0304-3940(90)90827-v. [DOI] [PubMed] [Google Scholar]
- Ropert N, Miles R, Korn H. Characteristics of miniature inhibitory postsynaptic currents in CA1 pyramidal neurones of rat hippocampus. J Physiol. 1990;428:707–722. doi: 10.1113/jphysiol.1990.sp018236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Savic N, Sciancalepore M. Intracellular calcium stores modulate miniature GABA-mediated synaptic currents in neonatal rat hippocampal neurons. Eur J Neurosci. 1998;10:3379–3386. doi: 10.1046/j.1460-9568.1998.00342.x. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release γ-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Gähwiler BH. Effects of the GABA uptake inhibitor tiagabine on inhibitory synaptic potentials in rat hippocampal slice cultures. J Neurophysiol. 1992;67:1698–1701. doi: 10.1152/jn.1992.67.6.1698. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Petersen CC, Nicoll RA. Effects of reduced vesicular filling on synaptic transmission in rat hippocampal neurones. J Physiol. 2000;525:195–206. doi: 10.1111/j.1469-7793.2000.t01-1-00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]