Abstract
Oscillatory and negative flows occur normally in the cardiovascular system, which predispose those regions to atherosclerosis. Nitric oxide (NO) production increases in proportion to the magnitude of flow and is known to be athero-protective. What is not known, however, is the effect of flow reversal on NO concentration ([NO]). The hypothesis of the present study is that [NO] is reduced in reverse flow. An additional hypothesis is that the reduction in [NO] is mediated through an increase in superoxide production during flow reversal. These hypotheses were tested in an ex vivo preparation of porcine elastic and muscular arteries. The flow of a physiological solution through the vessels was regulated in the forward and reverse direction and the effluent was assayed for nitrite levels using a combination of a diazo coupling method and high performance liquid chromatography. Our results show that [NO] is significantly reduced during reverse flow. Furthermore, addition of tempol (superoxide dismutase-mimetic) which is a superoxide scavenger returns the [NO] during reverse flow to mirror those of forward flow. These results have important implications since the action of superoxide is implicated in many cardiovascular diseases, and the present finding suggests that flow reversal should be added to the list.
In humans and other mammals, blood flow and wall shear stress (WSS) are related to the nutrient transport, the vascular tone of the blood vessel, the remodelling of the blood vessel wall, and the initiation and development of atherosclerosis (Fry, 1968; Caro et al. 1971; Zarins et al. 1983; Fujii et al. 1991; Schwartz et al. 1995; Corson et al. 1996; Gnasso et al. 1997; Gooch et al. 1997; Marano et al. 1999; Shaaban & Duerinckx, 2000; Tuttle et al. 2001; Lu et al. 2001). Steady-state blood flow stimulates nitric oxide (NO) production by the endothelium in a linear fashion (Mochizuki et al. 1999 and 2003); i.e. NO production increases proportionally with increase in blood flow. In vivo, blood flow is not purely steady state, however, and transient reversals of blood flow occur during diastole with each heart beat (Snow et al. 2001). Thus the pulsatile component of flow is bidirectional, especially at normal resting blood flows in central vessels near bifurcations and in peripheral vessels, even though the mean component is always in the forward direction. These observations have clinical significance since diseased endothelial regions experience slow moving blood flow and changes in flow direction (Caro et al. 1971; Bharadvaj et al. 1982; Zarins et al. 1983; Gnasso et al. 1997). In contrast it is commonly accepted that vessel regions that are exposed to steady blood flow are usually nonatherogenic (Bharadvaj et al. 1982; Gnasso et al. 1997). The hypothesis of the present study is that NO concentration ([NO]) depends not only on the magnitude but also on the direction of blood flow. The former has been recently documented in an ex-vivo vessel preparation (Mochizuki et al. 2003), while the later has not yet been investigated. Our hypothesis is that [NO] is significantly reduced during flow reversal which may attribute to the dysfunction of the blood vessel wall. An additional hypothesis is that the reduction in [NO] is due to an increase in superoxide production during flow reversal.
Direct measurement of NO is difficult since it is a molecule with a short half life and reacts rapidly with free oxygen, oxygen radicals, redox metals, sulphydryls, disulphides and oxygenated haemoglobin (Stamler et al. 1992). However, the measurement of nitrite (NO2−) and nitrate (NO3−) concentrations according to the Griess reaction, supplemented with the reduction of nitrate to nitrite by NADPH-dependent reductase or with cadmium reduction is commonly used for monitoring changes in the concentration of NO released in blood. This technique was adapted in the present study. Accordingly, we used an ex vivo model of porcine carotid and femoral artery perfused with a physiological solution within a wide range of physiological perfusion rates to evaluate the flow-induced [NO] in the effluent of forward and reverse flow. Furthermore, we used tempol, a mimic superoxide dismutase, to elucidate the mechanism of the decrease in NO during reverse flow. The major findings of the present study are that [NO] is significantly reduced during reverse flow and the reduction is mediated through an increase in superoxide production.
Methods
Animal preparation
Eighteen farm pigs weighting 30 ± 5 kg were used in the study. Surgical anaesthesia was induced with ketamine (20 mg kg−1, i.m.) and atropine (0.05 mg kg−1) and maintained with isoflourane (1–2%). Ventilation with 100% O2 was provided with a respirator to maintain values of PO2 and PCO2 (approximately 500 and 35 mmHg, resepctively). A 6–7 cm length of the left carotid artery was dissected and a jugular vein was cannulated for administration of heparin, 100 U kg−1, for anticoagulation. The adjacent tissue around the carotid artery was dissected and the branches were ligated. The proximal portion of the carotid artery was then cannulated for blood pressure measurement with a 6 Fr sheath and the carotid artery was subsequently excised and transferred to Krebs solution. Prior to harvest, two 6-0 sutures were sutured onto the adventitia to determine the change of blood vessel length from in vivo to in vitro state and to identify the proximal and distal segments. Similar lengths of the left and right femoral arteries (5–6 cm) were dissected and the in vivo length and flow direction measured. The animal was killed with an overdose of pentobarbital and the carotid and two femoral arteries were harvested. All animal experiments were performed in accordance with national and local ethical guidelines, including the Institute of Laboratory Animal Research (ILAR) Guide, Public Health Service (PHS) policy, Animal Welfare Act, and UCI policies regarding the use of animals in research.
Isolated vessel preparation
The two ends of the segment were cannulated and stretched to the in vivo length in an organ bath with Krebs solution at 36−37°C. The proximal portion of each of the six carotid arterial segments was first connected to an inflow container and the distal segment connected to an outflow container, both of which were filled with Krebs solution with 1% bovine serum albumin at 36−37°C. The distal segment was connected to the outflow container via a short tube with a stopcock that was used to extract the effluent solution. This setup was used for forward flow studies followed by a 180 deg rotation of the vessel where the distal portion was connected to the inflow container and the proximal portion to the outflow container for reverse flow studies. In six additional segments, the order was reversed where the vessel was first exposed to reverse flow followed by forward flow. The flow rate was monitored with an ultrasonic probe (Transonic Systems Inc.) and was varied at 30, 60, 90, 120, and 150 ml min−1 in the forward direction, equivalent to the in vivo direction (i.e. proximal to distal). The flow rate direction was then reversed with identical magnitudes (−30, −60, −90, −120, and −150 ml min−1) but opposite to the in vivo direction (i.e. distal to proximal). The perfusion pressure was maintained at 30 mmHg while the resistance of the outlet tube was varied with a graded clamp to change the flow rate. Once the forward and reverse flow protocols were completed, l-arginine (1 mm) was added to the perfusate and the forward and reverse protocols were repeated. l-Arginine was used to verify the viable response of endothelial cells.
A circulatory system was set up for the two femoral arteries. The left femoral artery of six animals was used for forward flow while the right was used for reverse flow. The system consisted of a hydrostatic column to drive the flow while the effluent was recirculated via a flow pump. The circulating fluid was collected at 10, 30, 60, 120 and 180 min. For both carotid and femoral arteries, control samples were collected from the inlet container to subtract off any nitrite contamination present in the solution.
Measurement of vasodilatation
The degree of vasodilatation was quantified by the ratio D/D0 where D is the external diameter of the vessel under a given flow rate which was measured with a stereo-dissection microscope. D0 is the diameter at zero flow which was measured by ligation of the vessel at the outlet to obtain zero flow and at a distension pressure of 30 mmHg. Measurements of D at various flow rates were also made at perfusion pressure of approximately 30 mmHg. The D for the carotid artery was measured at 30, 90 and 150 ml min−1 (and −30, −90 and −150 ml min−1 for reverse flow) after 3 min of flow, while that for the femoral artery was measured at 90 ml min−1 (and −90 ml min−1 for reverse flow) at various time points as described above.
Effect of tempol on [NO] during flow reversal
The effect of 4-hydroxy-tempol (2,2,6,6-tetramethyl-piperidine 1 oxyl; Sigma), a mimic superoxide dismutase to scavenge superoxide, was determined in six additional animals in the forward and reverse experiments described above. The femoral arterial segments were first perfused with Krebs solution in both forward and reverse direction at flow rates of 60, 90, 120, and 150 ml min−1, and samples collected for each respective flow. These measurements served as controls. The segments were then incubated in Krebs solution containing tempol (1 mm) for 10 min at 37°C. Subsequently, the segments were perfused with Krebs solution containing tempol (1 mm) in forward and reverse direction at identical flow rates to the control. The perfusate was again collected at each flow rate, and the nitrite concentration was measured as described below.
Measurement of NO metabolites: Griess's method
The perfusate was collected after 3 min of circulation and assayed for nitrite and nitrate, the breakdown products of NO in aqueous solution. The nitrite and nitrate ion concentrations in solution were measured by the combination of a diazo coupling method and high performance liquid chromatography (ENO-20 NOx Analyser; EiCom, Kyoto). The method for NOx analysis has been previously described in detail (Zeballos et al. 1995). Briefly, the peak of detected voltage for nitrite was converted into nitrite concentration by use of calibration solution. The signals were corrected for contamination by subtraction of the control nitrite concentration. The endogeneous NO production was then evaluated as the product of perfusion rate and nitrite concentration in the effluent. About 10 µl of the effluent solution was collected after 20, 40, 60 and 180 s at each perfusion rate for measurement of nitrite concentration in the effluent. The minimum detectable concentrations are less than 5 nm in pH-neutral solutions.
Wall shear stress.
The WSS of the artery was computed by the equation for laminar flow in a circular cylinder as:
| (1) |
where Di is internal diameter of blood vessels, µ is the fluid viscosity and Q is the volumetric blood flow rate. The inner diameter in eqn (1) was calculated from the incompressibility condition which, for a cylindrical vessel, can be expressed as:
| (2) |
where Di and Do are the inner and outer radii at the loaded state, respectively, and Ao is the wall area in the no-load state, which was measured at the midpoint of the vessel length from a transverse section of the blood vessel at the conclusion of the experiment. The stretch ratio in the axial direction, λ = llo−1, was also computed where l and lo are the vessel lengths in the loaded and no-load state, respectively. Hence, measurements of Do, λ and Ao allow the determination of Di using eqn (2) and WSS using eqn (1).
Data analysis
The data are presented as means ± s.d. Statistical significance was determined by the use of Student's t test or analysis of variance (ANOVA). A probability of P < 0.05 was considered statistically significant.
Results
Reproducibility and time of sample
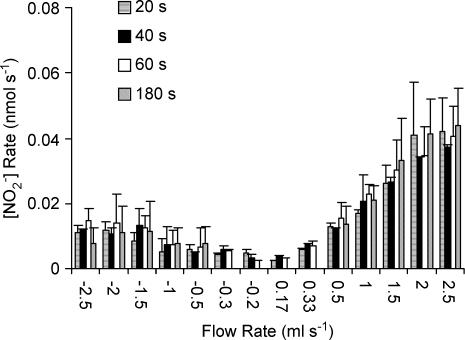
The reproducibility of the NO measurements was assessed by obtaining measurements in quadruplicate. We found the variation to be less than 5%. Subsequently, all measurements were made in duplicate and the mean was reported. To establish the steady-state values of NO production (measured as nitrite, the stable degradation product), we obtained measurements at a given flow rate at multiple time points (20, 40, 60 and 180 s) as shown in Fig. 1. We found no statistically significant differences at various time points for any measured flow (range of P-values: 0.057–0.98). Hence, all subsequent measurements were made at 180 s (3 min).
Figure 1. NO production at different flow rates.
The effluent samples were collected at 20, 40, 60 and 180 s for the carotid artery. The data represent mean ± s.d. for 6 carotid muscles.
Forward versus reverse flow
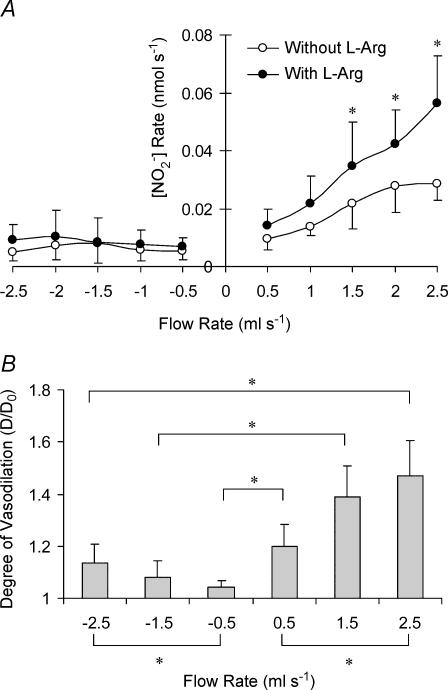
The order of flow direction (first forward and then reverse or vice versa) did not affect the results. Hence, the randomized measurements for the carotid artery were grouped together and presented in Fig. 2A as the relationship between flow rate and rate of NO production. The relationship between NO production and flow rate is linear for positive flow with and without l-arginine (l-Arg; Sigma). A linear least square fit of the data shows a slope of 0.029 ± 0.008 nm(R2 = 0.7669–0.9508) and 0.017 ± 0.005 nm(R2 = 0.5954–0.9577) with and without l-Arg, respectively. The slope is significantly larger in the presence of L-Arg (P < 0.05). The NO production is significantly reduced during reverse flow with and without l-Arg. The degree of vasodilatation (D/D0) for the carotid artery for flow rates of 30, 90 and 150 ml min−1 for forward and reverse flow is shown in Fig. 2A. The degree of vasodilatation was larger for forward flow at all flow rates. Furthermore, the degree of vasodilatation was also greater at 150 as compared to 30 ml min−1 for the respective forward and reverse flows as denoted in Fig. 2B.
Figure 2. Production of nitrite and vasodilation of the porcine carotid artery for forward and reverse flow.
A, production of NO metabolite (nitrite) in the porcine carotid artery for forward and reverse flow with (○) and without l-Arg (•). The data represent mean ± s.d. The asterisk denotes statistical significance between groups at the respective flows (P < 0.05). B, the degree of vasodilatation was expressed as outer diameter ratio (D/D0) of carotid arterial segment where D is the diameter at a given flow and D0 is the diameter at zero flow with same pressure. The ratio was significantly larger during forward flow as denoted by the asterisk.
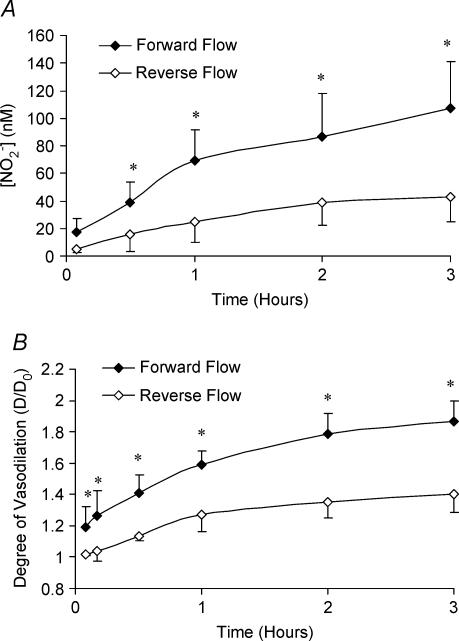
In order to establish the same phenomena observed in an elastic vessel (carotid artery) in a muscular vessel, we made similar measurements in the femoral artery. The two femoral arteries of the same animal were examined, one in forward flow and the other in reverse. We considered a circulating system to assess the accumulation of [NO] over time at a constant flow rate of 90 ml min−1. Figure 3A shows that NO accumulation is significant for both forward and reverse flow in the right and left femoral arteries, respectively. The increase in NO production, however, is significantly larger for forward compared to reverse flow for time periods equal to or greater than 30 min, as shown in Fig. 3A. The curve corresponding to degree of vasodilatation for the right and left femoral arteries (forward and reverse flow, respectively) of the same animal are shown in Fig. 3B. Again, it is clear that the vessels with larger NO production have greater vasodilatation. The increase in vessel diameter over time is also statistically significant for both forward and reverse flow.
Figure 3. Accumulation of nitrite and vasodilation of the porcine femoral artery for forward and reverse flow.
A, accumulation of NO (nitrite) concentration over time for a continuous flow set-up in the right and left femoral arteries (forward • and reverse flow ◊, respectively). The asterisk denotes the statistical significance between forward flow and reverse flow (P < 0.05) at various time points. The increase of NO concentration over time of both forward and reverse flow was statistically significant (P < 0.001). B, the degree of vasodilatation expressed as the diameter ratio (D/D0) increased with time in continuous flow in femoral arteries in both forward and reverse flow (P < 0.001). D is the diameter at a given flow and D0 is the diameter at zero flow with same pressure. The asterisk indicates statistical significance between forward flow and reverse flow at the respective time points (P < 0.05).
The inner diameter of the vessel at the midpoint of the segment was computed from eqn (2). We found that the inner diameter and wall thickness of the carotid artery at perfusion pressure of 30 mmHg and flow rate of 2.5 ml s−1 were 3.1 ± 0.27 mm and 0.29 ± 0.06 mm for forward flow and 2.5 ± 0.24 mm and 0.33 ± 0.07 mm for reverse flow, respectively. The inner diameter and wall thickness of the femoral artery at perfusion pressure of 30 mmHg and flow rate of 1.7 ml s−1 were 2.8 ± 0.23 mm and 0.28 ± 0.05 mm for forward flow and 2.3 ± 0.22 mm and 0.3 ± 0.06 mm for reverse flow, respectively. The WSS was computed based on the imposed flow rates, measured diameters and viscosity of Krebs solution (8.5 × 10−4 Pa s) as given by eqn (1). We found the range of WSS to vary between approximately −0.8 and 0.8 Pa. The value of 0.8 Pa is within the physiological range reported in the literature.
The role of superoxide
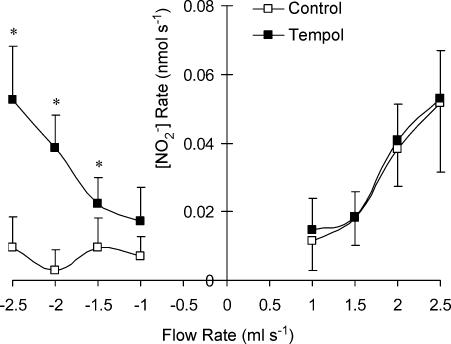
To assess the role of superoxide production during flow reversal, we added tempol (a cell-permeable superoxide scavenger) to the perfusate and measured the [NO] as shown in Fig. 4. The increase in NO production was not statistically significant during forward flow in the presence of tempol. The neutralization of superoxide by tempol, however, did significantly increase NO production during reverse flow. A two-way ANOVA shows a significant increase of NO production with flow rate in the reverse direction.
Figure 4. Production of NO metabolite (nitrite) in the porcine femoral artery for forward and reverse flow with and without tempol.
The data represent mean ± s.d. The asterisk denotes statistical significance between groups at the respective flows (P < 0.05).
Discussion
The occurrence of flow reversal in normal and diseased cardiovascular system
Numerous methods have been used to document flow reversal in the cardiovascular system. In vivo, angiocardiography, echocardiography and especially magnetic resonance imaging (MRI) have enabled the construction of the stream lines and flow velocities throughout the cardiac cycle (Moore & Ku, 1994; Oshinski et al. 1995; Bogren et al. 1997; Krams et al. 1997). In in vitro studies, using transparent models, digital particle image velocity and MRI have been utilized to examine the complex flow patterns in the vicinity of bifurcations (Moore & Ku, 1994). Finally, in silico computational fluid dynamics simulations have been used to solve the Navier–Stokes equation for the temporal and spatial velocity profile (Marques et al. 2003). The conclusion of the various methods has been that flow reversal does occur in the normal cardiovascular system near bifurcations, along curved vessels and in the abdominal aorta, infrarenal, below the renal artery (Shaaban & Duerinckx, 2000). These regions have been of particular interest because of their predilection to atherosclerosis. Indeed, the connection between flow or WSS and atherogenesis has been investigated for the past four decades. Abnormally high shear stresses can cause endothelial damage (Fry, 1968), whereas the location of atherosclerotic plaques is related to regions of low and oscillatory shear stress (Caro et al. 1969; Gidden et al. 1993; Barakat et al. 1999).
Numerous studies have reported an increase in arterial blood flow reversal in cardiovascular dysfunction (Milnor, 1989; Oyre et al. 1997; Pedersen et al. 1999; Bonnefous et al. 2000;). Gharib & Benzai have recently shown, in an in vitro system, that reduced blood flow combined with increased heartbeat can lead to near-wall retrograde flow adjacent to the wall and thereby high negative flow during the cardiac cycle (Gharib & Beizaie, 2003). In heart failure, for example, the cardiac output is reduced, usually accompanying low ejection fraction. In these patients the heart tries to compensate by beating faster, but reduced blood flow combined with increased heart rate can lead to retrograde flow and negative WSS along the vessel walls during each cardiac cycle (Gharib & Beizaie, 2003).
Flow reversal and NO
When oscillatory flow (with reversing component) is imposed on cultured endothelial cells, there is a lack of endothelial NO synthase upregulation (Silacci et al. 2000), chronic increase in superoxide anion production (De Keulenaer et al. 1998), increased expression of adhesion molecules including VCAM-1, ICAM-1 and E-selectin (Chappell et al. 1998) and lack of cell alignment (Zhao et al. 1995). Our novel finding that reverse flow significantly reduces the NO production by the endothelium in ex vivo elastic and muscular arteries (Figs 1, 2A and 3A, respectively) complements these observations and has important clinical implications. Since regions of the cardiovascular system that are susceptible to atherogenesis experience flow reversal, NO production in those regions is likely to be significantly reduced. It is well known that reduced NO can lead to vascular dysfunction including intimal hyperplasia, cell activation and platelet adhesion (Traub & Berk, 1998; Li & Forstermann, 2000). Hence, our finding makes a direct connection between negative flow and the aetiology of vascular dysfunction; i.e. reduction in [NO].
Flow reversal and vasoreacitivity
The reduction in degree of vasodilatation found for reverse flow in elastic and muscular arteries (Figs 2B and 3B, respectively) is consistent with the observed decrease in [NO]. This finding is consistent with the results of Markos et al. (2002) who recently examined the effect of reverse flow on the dilatation of the iliac artery in an anaesthetized dog (Markos et al. 2002). It was found that an increase in forward flow caused a significantly greater change in arterial diameter than an equivalent increase in the reverse direction for the same increase in flow. They verified that the increase in arterial diameter in response to an increase in forward or reverse shear stress was attenuated by l-NAME, indicating that arterial dilatation was mediated by NO. They inferred that NO release occurs irrespective of the direction in which the flow occurs but is attenuated in the reverse direction. In a related study, Sorop et al. recently suggested that superoxides, which inhibit NO, may play an important role during pulsatile flow (with negative component) in subendocardial and subepicardial coronary arteriolar vessels (Sorop et al. 2003). They showed that pulsatile flow can only induce vasodilatation if the superoxide production is inhibited with superoxide dismutase (SOD).
Flow reversal and superoxide production
Superoxide anion radical (O2−) is implicated in ageing, hypertension, diabetes, hyperlipidaemia, atherosclerosis, heart failure and smoking (Freeman et al. 1998). Superoxide production will scavenge NO to form peroxynitrite anion and therefore lower the [NO] in these regions. Hence, controlling the amount of O2− is critically important for preserving NO bioactivity in the vessel wall and this balance is of major importance in vascular function (Fukai et al. 2002). Our present results show that flow reversal affects this balance. Our experiments suggest that little O2− is produced in the forward direction since tempol does not significantly increase [NO] as shown in Fig. 4. Conversely, significant O2− is produced in the reverse flow such that administration of tempol returns the level of [NO] to mirror that in the forward direction. The mechanism of increased O2− when the endothelial cell is sheared in the reverse direction has not been contemplated.
Critique of method
In several experiments, we used a distension pressure of 80 mmHg and found evidence of endothelial injury in vessels exposed to forward and reverse flow. Hence, the transmural pressure was set at 30 mmHg to avoid large circumferential stress and strain in the arterial wall during the ex vivo perfusion. In vivo, the femoral and carotid arteries are surrounded by connective tissue and muscle. Our group has previously shown that the circumferential stress and strain are smaller in vivo compared to ex vivo, at the same distension pressure, because of the mechanical support of the surrounding tissue (Hamza et al. 2003). We chose a transmural pressure of 30 mmHg to match the circumferential stretch ratio to the in vivo condition. Both forward and reverse vessels were tested at the same pressure of 30 mmHg.
It is well know that WSS is the stimulus for the release of NO from the endothelium (Kuchan & Frangos, 1994) and is known to acutely influence smooth muscle tone (Smiesko & Johnson, 1993) and chronically influence the remodelling of vascular wall structure (Lu et al. 2001). We expressed the present results in terms of flow rate, which is proportional to WSS, rather than directly in terms of WSS because of the tapered structure of the relatively long blood vessel. In order to maximize the surface area of endothelium and hence NO production, we dissected the longest possible segment of carotid (6–7 cm) and femoral arteries (5–6 cm). As such, the distal segment of the artery was smaller than the proximal. For the carotid artery, the difference was small (about 10%) while for the femoral artery it was much more significant (30–40% difference). Hence, the WSS varies along the length of the vessel significantly since the WSS is inversely proportional to the diameter cubed (eqn (1)). The values of WSS reported above correspond to the midpoint of the vessel length with smaller proximal and larger distal values. Since, NO production occurs along the entire length of the vessel, we opted to report NOx concentrations in terms of flow rate which is constant along the length of the vessel.
A possible mechanism for increased superoxide production
There is theoretical evidence that tension and hence deformation can be very large in endothelial cells depending on the cell–cell junction (Fung & Liu, 1993). Fung and Liu considered the shear stress imposed by the flowing blood as the loading and examined the resulting tensile stresses in the membrane of the cell (Fung & Liu, 1993). The analysis showed that stress in one cell depends on the stress in other cells and on the geometric shape of the cell junction. This led to the feature of stress transmission between neighbouring cells and possible stress accumulation. Shearing the endothelial cell in the reverse direction can change the geometric shape of the cell junction and hence increase the tension development in the cell. High tension and deformation of endothelial cells may lead to increased ‘leakage’ of electrons and consequent creation of superoxide. This may occur in analogy to an electric circuit when there is a break in the insulation. The exploration of this hypothesis remains a laudable goal of a future investigation.
Acknowledgments
This research was supported by the American Heart Association 0140036N. Dr Kassab is the recipient of the American Heart Association Established Investigator Award.
References
- Barakat AI, Leaver EV, Pappone PA, Davies PF. A flow-activated chloride-selective membrane current in vascular endothelial cells. Circ Res. 1999;85:820–828. doi: 10.1161/01.res.85.9.820. [DOI] [PubMed] [Google Scholar]
- Bharadvaj BK, Mabon RF, Giddens DP. Steady flow in a model of the human carotid bifurcation, flow visualization. J Biomech. 1982;15:349–362. doi: 10.1016/0021-9290(82)90057-4. [DOI] [PubMed] [Google Scholar]
- Bogren HG, Mohiaddin RH, Kilner PJ, Jimenez-Borreguero LJ, Yang GZ, Firmin DN. Blood flow patterns in the thoracic aorta studied with three-directional MR velocity mapping: The effects of age and coronary artery disease. J Magn Reson Imaging. 1997;7:784–793. doi: 10.1002/jmri.1880070504. [DOI] [PubMed] [Google Scholar]
- Bonnefous O, Luizy F, Kownator S. Arterial wall motion imaging – a new ultrasound approach to vascular characterization. Medicamundi. 2000;44:37–43. [Google Scholar]
- Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear stress and distribution of early atheroma in man. Nature. 1969;223:1159–1161. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- Caro CG, Fitz-Gerald JM, Schroter RC. Atheroma and arterial wall shear – observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci. 1971;177:109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res. 1996;79:984–991. doi: 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- De Keulenaer G, Chappel DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of superoxide-producting NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- Freeman BA, Jackson RM, Matalon S, Harding SM. Endothelial Cells. Boca Raton FL USA: CRC; 1998. pp. 13–32. [Google Scholar]
- Fry DL. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res. 1968;22:165–197. doi: 10.1161/01.res.22.2.165. [DOI] [PubMed] [Google Scholar]
- Fujii K, Heistad DD, Faraci FM. Flow-mediated dilatation of the basilar artery in vivo. Circ Res. 1991;69:697–705. doi: 10.1161/01.res.69.3.697. [DOI] [PubMed] [Google Scholar]
- Fukai T, Folz RJ, Landmesser U, Harrison DG. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res. 2002;55:239–249. doi: 10.1016/s0008-6363(02)00328-0. [DOI] [PubMed] [Google Scholar]
- Fung YC, Liu SQ. Elementary mechanics of endothelium of blood vessels. J Biomech Eng. 1993;115:1–12. doi: 10.1115/1.2895465. [DOI] [PubMed] [Google Scholar]
- Gharib M, Beizaie M. On the correlation between negative near-wall shear stress in human aorta and various stages of congestive heart failure. Ann Biomed Eng. 2003;31:678–685. doi: 10.1114/1.1574025. [DOI] [PubMed] [Google Scholar]
- Giddens DP, Zarins CK, Glagov S. The role of fluid mechanics in the localization and detection of atherosclerosis. J Biomech Eng. 1993;115:588–594. doi: 10.1115/1.2895545. [DOI] [PubMed] [Google Scholar]
- Gnasso A, Irace C, Carallo C, De Franceschi MS, Motti C, Mattioli PL, Pujia A. vivo association between low wall shear stress and plaque in subjects with asymmetrical carotid atherosclerosis. Stroke. 1997;28:993–998. doi: 10.1161/01.str.28.5.993. [DOI] [PubMed] [Google Scholar]
- Gooch KJ, Dangler CA, Frangos JA. Exogenous, basal, and flow-induced nitric oxide production and endothelial cell proliferation. J Cell Physiol. 1997;171:252–258. doi: 10.1002/(SICI)1097-4652(199706)171:3<252::AID-JCP3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hamza LH, Dang Q, Lu X, Mian A, Molloi S, Kassab GS. Effect of passive myocardium on the compliance of porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2003;285:H653–H660. doi: 10.1152/ajpheart.00090.2003. [DOI] [PubMed] [Google Scholar]
- Krams R, Wentzel JJ, Oomen JAF, Vinke R, Schuurbiers JCH, de Feyter PJ, Serruys PW, Slager CJ. Evaluation of endothelial shear stress and 3D reconstruction from angiography and IVUs (ANGUS) with computational fluid dynamics. Arteriosc Thromb Vasc Bio. 1997;17:2061–2065. doi: 10.1161/01.atv.17.10.2061. [DOI] [PubMed] [Google Scholar]
- Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol Cell Physiol. 1994;266:C628–C636. doi: 10.1152/ajpcell.1994.266.3.C628. [DOI] [PubMed] [Google Scholar]
- Li H, Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Lu X, Zhao JB, Wang GR, Gregersen H, Kassab GS. Remodeling of the zero-stress state of femoral arteries in response to flow overload. Am J Physiol Heart Circ Physiol. 2001;280:H1547–H1559. doi: 10.1152/ajpheart.2001.280.4.H1547. [DOI] [PubMed] [Google Scholar]
- Marano G, Palazzesi S, Vergari A, Ferrari AU. Protection by shear stress from collar-induced intimal thickening: role of nitric oxide. Arterioscler Thromb Vasc Biol. 1999;19:2609–2614. doi: 10.1161/01.atv.19.11.2609. [DOI] [PubMed] [Google Scholar]
- Markos F, Hennessy BA, Fitzpatrick M, O'ullivan J, Snow HM. Reverse arterial wall shear stress causes nitric oxide-dependent vasodilatation in the anaesthetised dog. Pflugers Arch. 2002;445:51–54. doi: 10.1007/s00424-002-0915-9. [DOI] [PubMed] [Google Scholar]
- Marques PF, Oliveira MEC, Franca AS, Pinotti M. Modeling and simulation of pulsatile blood flow with a physiologic wave pattern. Artificial Organs. 2003;27:478–485. doi: 10.1046/j.1525-1594.2003.07239.x. [DOI] [PubMed] [Google Scholar]
- Milnor RM. Hemodynamics. Baltimore: Williams & Wilkins; 1989. [Google Scholar]
- Mochizuki S, Goto M, Chiba Y, Ogasawara Y, Kajiya F. Flow dependence and time constant of the change in nitric oxide concentration measured in the vascular media. Med Biol Eng Comput. 1999;37:497–503. doi: 10.1007/BF02513336. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol. 2003;285:H722–H726. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- Moore JE, Ku DN. Pulsatile velocity measurements in a model of the human abdominal aorta under resting conditions. J Biomech Eng. 1994;116:337–346. doi: 10.1115/1.2895740. [DOI] [PubMed] [Google Scholar]
- Oshinski JN, Ku DN, Mukundan S, Loth F, Pettigrew RI. Determination of wall shear stress in the aorta with the use of MR phase velocity mapping. J Magn Reson Imaging. 1995;5:640–647. doi: 10.1002/jmri.1880050605. [DOI] [PubMed] [Google Scholar]
- Oyre S, Pedersen EM, Ringgaard S, Boesiger P, Paaske WP. vivo wall shear stress measured by magnetic resonance velocity mapping in the normal human abdominal aorta. Eur J Vasc Endovasc Surg. 1997;13:263–271. doi: 10.1016/s1078-5884(97)80097-4. [DOI] [PubMed] [Google Scholar]
- Pedersen EM, Kim WY, Staalsen NH, Hasenkam JM, Nygaard H, Paulsen PK. Development of velocity profiles and retrograde flow in the porcine abdominal aorta under different haemodynamic conditions. Scand Cardiovasc J. 1999;33:206–214. doi: 10.1080/14017439950141632. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, deBlois D, O'rien ERM. The intima: soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- Shaaban AM, Duerinckx AJ. Wall shear stress and early atherosclerosis: a review. AJR Am J Roentgenol. 2000;174:1657–1665. doi: 10.2214/ajr.174.6.1741657. [DOI] [PubMed] [Google Scholar]
- Silacci P, Formentin K, Bouzourene K, Daniel F, Brunner HR, Hayoz D. Unidirectional and oscillatory shear stress differentially modulate NOS III gene expression. Nitric Oxide. 2000;4:47–56. doi: 10.1006/niox.2000.0271. [DOI] [PubMed] [Google Scholar]
- Smiesko V, Johnson PC. The arterial lumen is controlled by flow-related shear stress. News Physiol Sci. 1993;8:34–38. [Google Scholar]
- Snow HM, Markos F, O'Regan D, Pollock K. Characteristics of arterial wall shear stress which cause endothelium-dependent vasodilatation in the anaesthetized dog. J Physiol. 2001;531:843–848. doi: 10.1111/j.1469-7793.2001.0843h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorop O, Spaan JAE, Sweeney TE, VanBavel E. Effect of steady versus oscillating flow on porcine coronary arterioles: involvement of NO and superoxide anion. Circ Res. 2003;92:1344–1351. doi: 10.1161/01.RES.0000078604.47063.2B. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transducer an atheroprotective force. Arterioscler Thromb Vasc Bio. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281:H1380–H1389. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis-quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- Zeballos GA, Bernstein RD, Thompson CI, Forfia PR, Seyedi N, Shen W, Kaminiski PM, Wolin MS, Hintze TH. Pharmacodynamics of plasma nitrate/nitrite as an indication of nitric oxide formation in conscious dogs. Circulation. 1995;91:2982–2988. doi: 10.1161/01.cir.91.12.2982. [DOI] [PubMed] [Google Scholar]
- Zhao S, Suciu A, Ziegler T, Moore JEJ, Burki E, Meister JJ, Brunner HR. Synergisitic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler Thromb Vasc Biol. 1995;15:1781–1786. doi: 10.1161/01.atv.15.10.1781. [DOI] [PubMed] [Google Scholar]