Abstract
Infants born to mothers that smoke while pregnant have a relatively high incidence of central respiratory control abnormalities. Recent studies have shown that prenatal nicotine exposure increases GABA release and the frequency of GABAergic currents, leading to an up-regulation of GABAA receptors in central neurones. Activation of GABAA receptors inhibits ventilatory activity, with intense activation causing apnoea. These observations lead us to hypothesize that prenatal nicotine exposure alters GABAergic control of respiratory motor pattern in the early neonatal period. Osmotic minipumps were implanted in pregnant Sprague-Dawley rats on the fifth day of gestation, and filled with nicotine (6 mg kg−1 day−1, 2.5 μl h−1) or physiological saline (2.5 μl h−1). Brainstem–spinal cord preparations from 1- to 3-day-old neonates were studied under in vitro conditions. Electrical activity was recorded from the fourth cervical ventral root (C4 VR), which contains the axons of phrenic motoneurones. Bath application of GABAA receptor agonists muscimol (250 μm) or pentobarbital sodium (60 μm) to the brainstem led to consistent, reversible and significant reductions in C4 VR burst frequency. In saline-exposed animals, frequency (bursts min−1) fell from 6.8 ± 0.4 to a nadir of 2.8 ± 0.5 with muscimol, and from 6.5 ± 0.3 to a nadir of 2.9 ± 0.3 for pentobarbital; in nicotine-exposed animals, frequency fell from 6.3 ± 0.4 to 1.0 ± 0.4 with muscimol and from 6.4 ± 0.2 to 1.7 ± 0.4 with pentobarbital (P < 0.05 in all cases). The decrease in C4 VR frequency was significantly greater in nicotine-exposed compared to saline-exposed preparations with both muscimol and pentobarbital (P < 0.001 for both). There were no changes in the amplitude of C4 VR bursts under any condition. The GABAA receptor antagonist bicuculline methiodide (8 μm) did not change C4 VR frequency or amplitude in either group, although it was effective in reversing the effects of muscimol. These experiments demonstrate that prenatal nicotine exposure alters the GABAergic regulation of respiratory rhythm in a reduced preparation. The results may lead to a better understanding of the perturbed breathing pattern observed in neonates that are exposed to nicotine in utero.
Infants born to mothers that smoke while pregnant have a high incidence of central respiratory control abnormalities (e.g. apnoea, delayed arousal responses, diminished chemoreceptor reflexes) (Gennser et al. 1975; Lewis & Bosque 1995; Ueda et al. 1999; Kahn et al. 2002) and are more likely to die from the sudden infant death syndrome (SIDS) (Kinney et al. 1992; Kahn et al. 2002). Here, we test the hypothesis that prenatal nicotine exposure alters the GABAergic control of the respiratory motor pattern. We developed this hypothesis on the basis of the following observations: first, acute stimulation of nicotinic acetylcholine receptors on presynaptic nerve terminals increases GABA release and the frequency of inhibitory postsynaptic currents in brainstem neurones (Bertolino et al. 1997; Zhu & Chiappinelli, 1999; Neff et al. 2003); second, prenatal nicotine exposure causes a widespread increase in the density of nicotinic acetylcholine receptors on mammalian central neurones (Slotkin et al. 2002); third, prolonged elevations of brain GABA (or its agonists) increases the density of GABAA receptors on postsynaptic neurones in cortex (Sykes et al. 1984), cerebellum (Sykes et al. 1984; Platt et al. 1996) and cultured cells (Pericic et al. 2003). Taken together, these data raise the possibility that prenatal nicotine exposure leads to an up-regulation of GABA receptors on brainstem respiratory neurones, with resultant changes in physiological efficacy. The changes in GABAergic function and morphology reviewed above were generally reversed by the GABAA receptor antagonist bicuculline methiodide. Accordingly, the focus of our initial experiments is the GABAA receptor subtype.
Methods
Animals
Sixty-nine neonatal Sprague-Dawley rats born by spontaneous vaginal delivery were used in the study. Procedures were approved and performed in accordance with guidelines provided by the Institutional Animal Care and Use Committee at the University of Arizona. Adult female rats were housed individually in cages with a continuous supply of food and water, room temperature of 22°C, relative humidity of 20–30%, and a 12 h–12 h light–dark cycle. Neonatal rats were housed together with their mothers and siblings until they were studied.
The nicotine-exposed neonates were derived from eight different litters, and the saline-exposed from six litters; 4–10 animals were used from each litter. The influence of muscimol was studied in 12 saline-exposed and 12 nicotine-exposed neonates. The influence of pentobarbital sodium was also studied in 12 saline-exposed and 12 nicotine-exposed neonates. The influence of bicuculline methiodide was studied in seven saline-exposed and seven nicotine-exposed neonates. Finally, we also studied the effects of muscimol, followed by a mixture of muscimol and bicuculline, in seven saline-exposed neonates.
Prenatal exposure to nicotine or saline
A 28-day osmotic mini pump (2ML4, Alzet, Cupertino, CA, USA) was implanted into 14 pregnant dams on day five of gestation. Pumps were set to deliver nicotine (6 mg kg−1 day−1 at a rate of 2.5 μl h−1) in eight rats, and physiological saline (also at a rate of 2.5 μl h−1) in six rats. The rats were anaesthetized with a subcutaneous injection of a mixture of xylazine (8.0 mg kg−1), ketamine (25 mg kg−1) and acepromazine (1.0 mg kg−1); buprenorphine (0.5 mg kg−1) was given for control of postoperative pain. A small incision was made over the scapulae under aseptic conditions, and the pump was inserted under the skin longitudinally. The incision was sutured and the rats were given a subcutaneous injection of penicillin and returned to their cages after the effects of the anaesthetic had subsided.
Brainstem–spinal cord preparation
One- to three-day-old pups were used for the en bloc preparation, as previously described (Fregosi et al. 2004). After the pups were anaesthetized with ether using a small nose cone, the brainstem and spinal cord were removed en bloc. The en bloc preparation was chosen because it allows us to examine pharmacological influences on respiratory neurones in the brainstem and spinal cord without the confounding influences from higher central nervous system elements, or sensory feedback from the periphery. This preparation also allows us to precisely control the extracellular concentration of the drugs of interest.
The preparation was fixed ventral side up in either a single-bath (24 animals) or a split-bath Plexiglasrecording chamber (45 animals), both described in detail previously (Fregosi et al. 2004). The single-bath chamber was used for all experiments where pentobarbital sodium was administered (n = 24 animals, 12 nicotine-exposed and 12 saline-exposed), because previous studies demonstrated that changes in central respiratory output were identical if the drug was delivered to the entire preparation or to the brainstem alone (Fregosi et al. 2004). The chamber was continuously superfused with modified Krebs solution (2.5–3.0 ml min−1, at 25 ± 1°C) via a tube placed adjacent to the ventral medullary surface. The Krebs solution consisted of (mm): 124 NaCl, 5 KCl, 2.4 CaCl2, 1.3 MgSO4, 26 NaHCO3, 1.2 KH2PO4 and 30 d-glucose, bubbled with 95% O2–5% CO2, pH 7.4.
We applied muscimol and bicuculline methiodide to the brainstem using the split-bath chamber, because there are some data suggesting that these drugs influence phrenic motoneurones directly (Su & Chai, 1998) and we wished to examine drug effects on brainstem neurones. The chamber was separated into brainstem and spinal cord compartments at the level of the C1–C2 segments with 1 mm aluminium plates (Fregosi et al. 2004). Each compartment was continuously superfused with modified Krebs solution. The adequacy of the seal between the two chambers was documented at the end of each experiment by injecting 0.5 ml of black ink into the brainstem chamber while viewing the preparation through the microscope; the absence of ink flow into the spinal cord chamber confirmed a tight, leak free partition.
Measurements
The electroneurogram (ENG) of the C4 VR was recorded with a glass suction electrode. Because bicuculline methiodide often causes seizure-like activity that is conveyed to all spinal motoneurones (Tribble et al. 1983), we also recorded motor activity from the fourth or fifth lumbar (L4 or L5) VR, since the L4/L5 VR contains few motor axons to the respiratory muscles. Segments of experiments that included seizure-like activity occurring concurrently in the C4 and L4/L5 VR recordings were not used for data analysis; by using the lowest effective dose of bicuculline methiodide we were able to minimize seizure-like activity, and only had to discard data from three preparations.
Nerve recordings were amplified (gain: 1000–10 000), band pass filtered (30–3000 Hz), digitized at 6410 Hz (Spike II, Cambridge Electronic Design, Cambridge, UK) and stored on a hard disc for offline data analysis. As previously described, we quantified the amplitude of each nerve burst by first rectifying the activity and then computing the total area of the rectified burst. We next computed the ‘mean activity’ of each burst by dividing the total activity by the duration of the burst.
Experimental protocol
In all experiments, the effects of drugs (pentobarbital sodium, muscimol, bicuculline methiodide) on C4 VR nerve discharges were assessed with the following protocol: 30 min of control recording, a 30 min bath application of a drug, and a 30 min washout period to insure recovery of C4 VR nerve discharge. Pentobarbital sodium (Nembutal, Abbott Laboratories, North Chicago, USA, at a dose of 60 μm, or 15 mg l−1), muscimol (Sigma, USA, at a dose of 250 μm) and bicuculline methiodide (Sigma, at a dose of 8 μm) were prepared 30 min before the beginning of each experiment, and added to separate perfusion flasks that were filled with Krebs solution and bubbled with the 95% O2–5% CO2 gas mixture. The dose of pentobarbital sodium was chosen based on our previous study showing that this dose resulted in consistent and reversible changes in respiratory rhythm, and because the effects were completely blocked by bicuculline methiodide (Fregosi et al. 2004). The dose of muscimol was also chosen on the basis of our previous work showing that this dose evoked changes that were very similar to those evoked by 60 μm pentobarbital. As indicated above, 8 μm bicuculline effectively reverses the effects of muscimol or pentobarbital sodium without evoking seizure activity on ventral nerve roots (Fregosi et al. 2004).
Data analysis
The average C4 VR nerve burst frequency and amplitude within each 5-min epoch of the 90-min experiment was obtained using Spike II software, as previously described (Fregosi et al. 2004). The data were analysed with two-way repeated measures ANOVA, with time and drug treatment (nicotine or saline) the independent variables, and C4 VR burst frequency and mean amplitude the dependent variables. To determine if surgery or nicotine exposure had adverse effects on growth, we also used repeated measures ANOVA to compare body weight changes in saline-exposed pups, nicotine-exposed pups, and pups born to dams that had not undergone surgery (from earlier studies). In all cases where the ANOVA was significant (P < 0.05), pair-wise comparisons were tested with the Student-Neuman-Keuls post hoc analysis.
Results
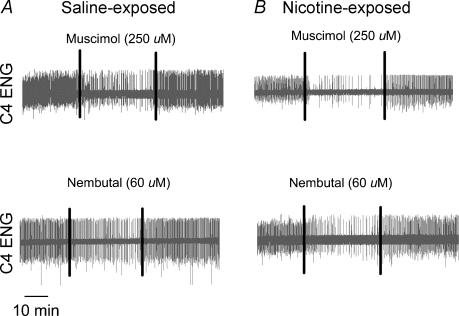
Neither the surgical interventions performed on the pregnant dams nor prenatal nicotine exposure influenced neonatal growth (Table 1). Prenatal nicotine exposure had no effect on baseline C4 VR ENG burst frequency or mean activity. Baseline frequency remained constant within a group for the 30 min measurement period in all experiments (Figs 1–3). Application of the GABAA receptor agonists muscimol (250 μm) or pentobarbital sodium (60 μm) slowed the C4 VR burst frequency reversibly in all preparations; representative recordings are shown in Fig. 1. Note that the slowing evoked by either drug was significantly greater in the nicotine-exposed compared to the saline-exposed animals (Fig. 1). Average data are shown in Fig. 2A and B. Consistent with the recordings shown in Fig. 1, both muscimol and pentobarbital slowed C4 VR burst frequency significantly (Fig. 2). Drug washout was rapid with pentobarbital, as the frequency returned to baseline levels within 5 min of washout in saline-exposed animals and within 15 min in nicotine-exposed animals. In contrast, muscimol was more difficult to washout, and after 25–30 min the frequency was slightly but significantly below predrug baseline levels in both groups of animals. Importantly, the extent of the slowing was significantly greater in the nicotine-exposed than in the saline-exposed animals with either muscimol or pentobarbital, although the difference appeared more quickly upon muscimol application (see asterisks in Fig. 2). There were no significant changes in C4 VR ENG mean activity with either drug (data not shown).
Table 1.
Body mass (g) over the first three postnatal days in animals that were born to dams that were surgically implanted with osmotic mini-pumps, loaded to deliver either saline or nicotine
| Postnatal age (days) | Saline exposed | Nicotine exposed | Control |
|---|---|---|---|
| 1 | 6.7 ± 0.6 | 6.9 ± 0.3 | 7.1 ± 0.2 |
| 2 | 8.0 ± 0.5* | 8.0 ± 0.3* | 7.8 ± 0.2* |
| 3 | 9.1 ± 0.7* | 8.8 ± 0.4* | 8.6 ± 0.3* |
The control animals were born to dams that did not undergo any surgical interventions. Data are the mean ± 1 s.e.m.
P < 0.05 compared to postnatal day 1. There were no significant differences in growth between the three groups.
Figure 1. Influence of GABAA receptor agonists on respiratory rhythm in saline or nicotine-exposed neonatal rat brainstem-spinal cord preparations.
Segments of experimental records demonstrating the influence of 250 μm muscimol and 60 μm pentobarbital sodium (Nembutal) in four representative animals. A, the effects of muscimol (top) and Nembutal (bottom) on C4 ENG activity in preparations that were saline-exposed in the neonatal period. B, the effects of the two drugs in preparations that were exposed to nicotine in the prenatal period. The vertical lines on each record indicate the period of drug application, which is bracketed by the control period and the washout period. Drugs were applied to the brainstem compartment using the split bath (see Methods). Note that both drugs slowed the frequency in all cases, but more so following prenatal nicotine exposure.
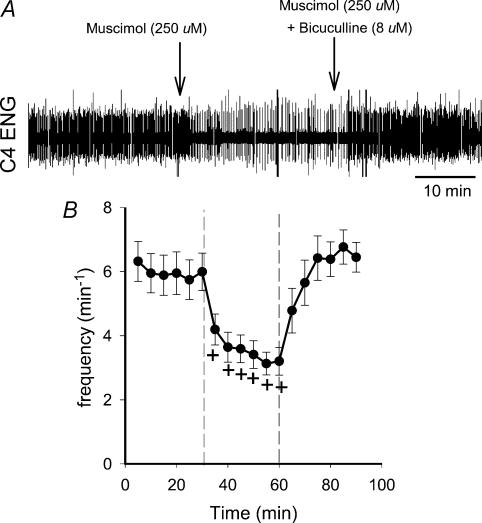
Figure 3. Reversal of the muscimol-induced frequfency slowing by bicuculline methiodide.
A, recording showing changes in C4 ENG burst frequency when 30 min of muscimol application is followed by application of a mixture of muscimol and bicuculline methiodide. Bicuculline methiodide completely reversed the frequency slowing evoked by muscimol. Drugs were applied to the brainstem compartment of the split-bath preparation (see Methods). B, meandata from seven such experiments. +P < 0.05 versus baseline activity.
Figure 2. Influence of GABA receptor agonists and antagonists on C4 nerve burst frequency in nicotine and saline-exposed preparations.
Average changes in C4 nerve burst frequency as a function of time in response to 250 μm muscimol (A), 60 μm pentobarbital sodium (Nembutal, B) and 8 μm bicuculline methiodide (C) in saline-exposed (•) and nicotine-exposed preparations (○). Drug application occurred between minutes 30 and 60 (vertical dashed lines in the top panel), and this period was bracketed by a 30 min baseline period and a 30 min period of drug washout. *Difference between nicotine-exposed and saline-exposed preparations at a given time point (P < 0.05). +Frequency significantly different from baseline frequency within a group (P < 0.05). Baseline frequency was taken as the average of the 6 baseline data points, and statistical contrasts between this average baseline value and each subsequent data point were made for all three drugs. Bicuculline did not result in any significant differences, either within or between groups, although there is trend towards an increase in frequency in both groups.
Bicuculline methiodide, a GABAA receptor antagonist, did not significantly change C4 VR frequency or mean activity in either saline-exposed or nicotine-exposed animals (Fig. 2C), although there was a trend for a rise in frequency in both groups. Because bicuculline did not influence C4 VR frequency or mean activity, we conducted additional experiments in seven saline-exposed animals to demonstrate that bicuculline could reverse the effects of GABAA receptor agonists in this preparation. A representative recording, as well as the average data from these experiments, is shown in Fig. 3. Note that the frequency slowing evoked by 250 μm muscimol was reversed when muscimol application was followed by application of a mixture of 250 μm muscimol and 8 μm bicuculline. The average data are entirely consistent with this example, as frequency was back to baseline levels within 5 min after the mixture of muscimol plus bicuculline was applied.
Discussion
We found that bath application of the GABAA receptor agonists muscimol or pentobarbital sodium caused a significantly greater reduction in the frequency of the respiratory rhythm in brainstem–spinal cord preparations derived from nicotine-exposed compared to saline-exposed neonatal rats. The drugs had no effect on mean C4 ENG activity in either nicotine-exposed or saline-exposed preparations, indicating that prenatal nicotine exposure does not alter GABAergic control of respiratory drive.
Methodological issues
A critique of the continuous infusion model that we used is provided in our recent paper (Fregosi et al. 2004), and in a recent review (Slotkin, 1998). The salient points are as follows: first, a fixed dose close to levels experienced by human smokers or humans wearing transdermal nicotine patches can be delivered to the animal (Slotkin, 1998); second, unlike episodic nicotine injections, continuous infusion is not confounded by the effects of transient ischaemia, which include significant increases in brain GABA release (Nunez et al. 2003); third, the dose of nicotine delivered to the pregnant rats (plasma levels of about 6 mg kg−1) is associated with physiological effects that are similar to those observed in moderately smoking humans (Slotkin, 1998). Indeed, our nicotine-exposed neonates grew normally, providing strong support for the notion that the dose was in the moderate range, inasmuch as high levels of prenatal nicotine exposure retards growth in the rat (Slotkin, 1998).
The utility of the reduced neonatal rat brainstem–spinal cord preparation as a model for mammalian respiratory control has been debated at length (Smith & Feldman, 1987; St John, 1990; Ramirez et al. 2002), and it is generally accepted that this is a useful model for studying the mechanisms underlying respiratory motor pattern and rhythm generation. Its main advantage in the context of the present experiments is that the extracellular levels of neurotransmitter agonists and antagonists could be precisely controlled. In addition, it allowed us to reproduce the dose from animal to animal without concern for differences in blood flow, drug metabolism, depth of anaesthesia, and other variables that are difficult to control in anaesthetized animal preparations. Thus, for the specific issue that we addressed the in vitro brainstem–spinal cord preparation proved useful.
The use of muscimol and pentobarbital sodium to assay the change in GABAergic control of respiratory rhythm and pattern was also carefully considered because these drugs can activate other neurotransmitter receptors. Nevertheless, in this and in our earlier study (Fregosi et al. 2004) we show that bicuculline methiodide fully reverses the decline in frequency evoked by muscimol or pentobarbital sodium, indicating that the GABAA receptor subtype mediated the effects that we observed (Fig. 3).
Prenatal nicotine exposure and GABAergic control of respiratory motor output
The major goal of the experiments was to test the hypothesis that prenatal nicotine exposure would alter the GABAergic control of the respiratory motor pattern. We found that agonists of the GABAA receptor subtype slowed the rhythm in all preparations, but to a greater extent in the preparations that had been exposed to nicotine in the prenatal period. These results are consistent with other data showing a decline in frequency with muscimol in the neonatal rat brainstem–spinal cord preparation (Brockhaus & Ballanyi, 1998), and in the in situ juvenile rat preparation (Hayashi & Lipski, 1992; St-John & Paton, 2002). Similar reductions in ventilation and/or breathing frequency have been obtained with muscimol in neonatal animals studied in vivo (Hedner et al. 1980; Curran et al. 2001).
The present experiments came about by linking three independent observations pertaining to GABA and nicotine exposure. First, stimulation of presynaptic nicotinic receptors on GABAergic interneurones increases the frequency of GABAergic inhibitory postsynaptic currents in central neurones (Zhu & Chiappinelli, 1999). Second, prenatal nicotine exposure exaggerates the frequency and amplitude of spontaneous GABAergic currents in neonatal cardiac parasympathetic neurones, suggesting augmented cholinergic control of GABAergic interneurones (Neff et al. 2003). Third, prolonged elevations of brain GABA (or its agonists) increases the density of GABAA receptors on postsynaptic neurones in cortex (Sykes et al. 1984), cerebellum (Sykes et al. 1984; Platt et al. 1996) and cultured cells (Pericic et al. 2003), and these effects are blocked by bicuculline (Platt et al. 1996; Pericic et al. 2003). That prolonged elevations in brain GABA can lead to up-regulation of GABAA receptors has been overlooked because short-term exposure to GABA leads to receptor down-regulation; this is consistent with the biphasic response reported by others (Platt et al. 1996; Pericic et al. 2003). Taken together, these observations suggest that prenatal nicotine exposure results in an increased release of GABA from GABAergic interneurones, and an increased expression and/or functional efficacy of GABAA receptors on the postsynaptic cell. Our data are consistent with this thesis as both muscimol and pentobarbital act on the GABAA receptor subtype via postsynaptic mechanisms, albeit by slightly different actions. Because the drugs were bath-applied to the entire medulla, additional experiments are needed to determine which respiratory neurones are involved.
A final point is that prenatal nicotine exposure had no significant effect on baseline breathing frequency. This is consistent with findings in awake rats (St-John & Leiter, 1999; Fewell et al. 2000) and mice (Robinson et al. 2002), although hypoxic responses are clearly perturbed (St-John & Leiter, 1999; Fewell et al. 2001; Robinson et al. 2002). On the other hand, a recent study in awake neonatal rats from our own laboratory (Huang et al. 2004) showed that prenatal nicotine exposure increases apnoea incidence in the early neonatal period (postnatal days 1 and 2), and alters frequency and tidal volume later in the neonatal period (i.e. between days 10 and 14). Taken together, these observations suggest that prenatal nicotine exposure may cause subtle changes in baseline ventilatory activity, but more dramatic effects on the azbility of neonates to tolerate hypoxic, asphyxic or pharmacological challenge.
References
- Bertolino M, Kellar KJ, Vicini S, Gillis RA. Nicotinic receptor mediates spontaneous GABA release in the rat dorsal motor nucleus of the vagus. Neuroscience. 1997;79:671–681. doi: 10.1016/s0306-4522(97)00026-2. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur JNeurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Curran AK, Darnall RA, Filiano JJ, Li A, Nattie EE. Muscimol dialysis in the rostral ventral medulla reduced the CO2 response in awake and sleeping piglets. J Appl Physiol. 2001;90:971–980. doi: 10.1152/jappl.2001.90.3.971. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, Ng VK. Prenatal exposure to nicotine impairs protective responses of rat pups to hypoxia in an age-dependent manner. Respir Physiol. 2001;127:61–73. doi: 10.1016/s0034-5687(01)00232-8. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, Ng VK, Wong VH, Wang Y. Postnatal age influences the ability of rats to autoresuscitate from hypoxic-induced apnea. Am J Physiol Regul Integr Comp Physiol. 2000;279:R39–R46. doi: 10.1152/ajpregu.2000.279.1.R39. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Luo Z, Iizuka M. GABAA receptors mediate postnatal depression of respiratory frequency by barbiturates. Respir Physiol Neurobiol. 2004;140:219–230. doi: 10.1016/j.resp.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Gennser G, Marsal K, Brantmark B. Maternal smoking and fetal breathing movements. Am J Obstet Gynecol. 1975;123:861–867. doi: 10.1016/0002-9378(75)90863-7. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Lipski J. The role of inhibitory amino acids in control of respiratory motor output in an arterially perfused rat. Respir Physiol. 1992;89:47–63. doi: 10.1016/0034-5687(92)90070-d. [DOI] [PubMed] [Google Scholar]
- Hedner J, Hedner T, Bergman B, Lundberg D. Respiratory depression by GABA-ergic drugs in the preterm rabbit. J Dev Physiol. 1980;2:401–407. [PubMed] [Google Scholar]
- Huang YH, Brown AR, Costy-Bennett S, Luo Z, Fregosi RF. Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respir Physiol Neurobiol. 2004;143:1–8. doi: 10.1016/j.resp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kahn A, Groswasser J, Franco P, Scaillet S, Sawaguchi T, Kelmanson I, Bernanrd D. Sudden infant deaths: arousal as a survival mechanism. Sleep Med. 2002;3(Suppl. 2):S11–S14. doi: 10.1016/s1389-9457(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, Harper RM. The neuropathology of the sudden infant death syndrome. A review. J Neuropathol Exp Neurol. 1992;51:115–126. doi: 10.1097/00005072-199203000-00001. [DOI] [PubMed] [Google Scholar]
- Lewis KW, Bosque EM. Deficient hypoxia awakening response in infants of smoking mothers: possible relationship to sudden infant death syndrome. J Pediatr. 1995;127:691–699. doi: 10.1016/s0022-3476(95)70155-9. [DOI] [PubMed] [Google Scholar]
- Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res. 2003;93:565–572. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Alt JJ, McCarthy MM. A new model for prenatal brain damage. I. GABAA receptor activation induces cell death in developing rat hippocampus. Exp Neurol. 2003;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericic D, Strac DS, Jembrek MJ, Rajcan I. Prolonged exposure to gamma-aminobutyric acid up-regulates stably expressed recombinant alpha 1 beta 2 gamma 2s GABAA receptors. Eur J Pharmacol. 2003;482:117–125. doi: 10.1016/j.ejphar.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Platt KP, Zwartjes RE, Bristow DR. The effect of GABA stimulation on GABAA receptor subunit protein and mRNA expression in rat cultured cerebellar granule cells. Br J Pharmacol. 1996;119:1393–1400. doi: 10.1111/j.1476-5381.1996.tb16051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Zuperku EJ, Alheid GF, Lieske SP, Ptak K, McCrimmon DR. Respiratory rhythm generation: converging concepts from in vitro and in vivo approaches? Respir Physiol Neurobiol. 2002;131:43–56. doi: 10.1016/s1569-9048(02)00036-8. [DOI] [PubMed] [Google Scholar]
- Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, Funk GD. Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J Physiol. 2002;538:957–973. doi: 10.1113/jphysiol.2001.012705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Auman JT, Qiao D, Seidler FJ. Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain. Brain Res Dev Brain Res. 2002;133:175–179. doi: 10.1016/s0165-3806(02)00281-x. [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Meth. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- St John WM. Neurogenesis, control, and functional significance of gasping. J Appl Physiol. 1990;68:1305–1315. doi: 10.1152/jappl.1990.68.4.1305. [DOI] [PubMed] [Google Scholar]
- St-John WM, Leiter JC. Maternal nicotine depresses eupneic ventilation of neonatal rats. Neurosci Lett. 1999;267:206–208. doi: 10.1016/s0304-3940(99)00364-x. [DOI] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Neurogenesis of gasping does not require inhibitory transmission using GABAA or glycine receptors. Respir Physiol Neurobiol. 2002;132:265–277. doi: 10.1016/s1569-9048(02)00079-4. [DOI] [PubMed] [Google Scholar]
- Su CK, Chai CY. GABAergic inhibition of neonatal rat phrenic motoneurons. Neurosci Lett. 1998;248:191–194. doi: 10.1016/s0304-3940(98)00361-9. [DOI] [PubMed] [Google Scholar]
- Sykes C, Prestwich S, Horton R. Chronic administration of the GABA-transaminase inhibitor ethanolamine O-sulphate leads to up-regulation of GABA binding sites. Biochem Pharmacol. 1984;33:387–393. doi: 10.1016/0006-2952(84)90230-2. [DOI] [PubMed] [Google Scholar]
- Tribble GL, Schwindt PC, Crill WE. Reduction of postsynaptic inhibition tolerated before seizure initiation: brain stem. Exp Neurol. 1983;80:304–320. doi: 10.1016/0014-4886(83)90284-4. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Stick SM, Hall G, Sly PD. Control of breathing in infants born to smoking mothers. J Pediatr. 1999;135:226–232. doi: 10.1016/s0022-3476(99)70026-0. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Chiappinelli VA. Nicotine modulates evoked GABAergic transmission in the brain. J Neurophysiol. 1999;82:3041–3045. doi: 10.1152/jn.1999.82.6.3041. [DOI] [PubMed] [Google Scholar]