Abstract
We have investigated the acute desensitization of acetylcholine-activated GIRK current (IK(ACh)) in cultured adult rat atrial myocytes. Acute desensitization of IK(ACh) is observed as a partial relaxation of current with a half-time of < 5 s when muscarinic M2 receptors are stimulated by a high concentration (> 2 μmol l−1) of ACh. Under this condition experimental manoeuvres that cause a decrease in the amplitude of IK(ACh), such as partial block of M2 receptors by atropine, intracellular loading with GDP-β-S, or exposure to Ba2+, caused a reduction in desensitization. Acute desensitization was also identified as a decrease in current amplitude and a blunting of the response to saturating [ACh] (20 μmol l−1) when the current had been partially activated by a low concentration of ACh or by stimulation of adenosine A1 receptors. A reduction in current analogous to acute desensitization was observed when ATP-dependent K+ current (IK(ATP)) was activated either by mitochondrial uncoupling using 2,4-dinitrophenole (DNP) or by the channel opener rilmakalim. Adenovirus-driven overexpression of Kir2.1, a subunit of constitutively active inwardly rectifying K+ channels, resulted in a large Ba2+-sensitive background K+ current and a dramatic reduction of ACh-activated current. Adenovirus-driven overexpression of GIRK4 (Kir3.4) subunits resulted in an increased agonist-independent GIRK current paralleled by a reduction in IK(ACh) and removal of the desensitizing component. These data indicate that acute desensitization depends on K+ current flow, independent of the K+ channel species, suggesting that it reflects a reduction in electrochemical driving force rather than a bona fide signalling mechanism. This is supported by the observation that desensitization is paralleled by a significant negative shift in reversal potential of IK(ACh). Since the ACh-induced hyperpolarization shows comparable desensitization properties as IK(ACh), this novel current-dependent desensitization is a physiologically relevant process, shaping the time course of parasympathetic bradycardia.
G protein activated inward-rectifying K+ (GIRK) channels are expressed in the heart, preferentially in the pacemaking and atrial myocytes and in various neurones and endocrine cells (Yamada et al. 1998; Morishige et al. 1999; Dascal, 2001). They represent important mediators of vagally induced bradycardia and synaptic inhibition in the central nervous system (Wickman et al. 1998; Kitamura et al. 2000). These channels are tetrameric complexes formed by assembly of GIRK subunits, of which four mammalian types (GIRK1–4; Kir3.1–4) have been identified. They are activated by direct interaction of their subunits with βγ subunits released from heterotrimeric G proteins upon agonist stimulation of appropriate 7-helix receptors. It is generally believed that these channels are preferentially – but not exclusively – activated by βγ released from pertussis-toxin-sensitive Gi/o (Bender et al. 1998; Leaney et al. 2000; Wellner-Kienitz et al. 2001).
Upon rapid application of a suitable receptor agonist, GIRK current in myocytes and neurones but also in heterologous expression systems is activated on a time scale in the order of 1 s or less, depending on receptor species, receptor density and agonist concentration (Wellner-Kienitz et al. 2000; Bender et al. 2002; Hommers et al. 2003). In the presence of the agonist, activation is followed by a decrease in current, termed desensitization that is also dependent on these parameters. A comparable fade of ACh-induced bradycardia has been observed in the isolated sino-atrial node (Boyett & Roberts, 1987). Desensitization, which is a phenomenological rather than a mechanistical term, occurs in virtually all receptor–effector systems and is important for cellular adaptation to external inputs. In the system under study desensitization, as revealed by the decrease in current and agonist sensitivity during or following agonist exposure, has been shown to consist of several components, depending on the species of the receptor, concentration of agonist(s) and duration of agonist exposure (Bünemann et al. 1996a). Whereas slow components of GIRK current desensitization observed after exposure to agonist on a time scale of minutes to hours are likely to occur on the receptor level, as described for many other G protein coupled receptors (GPCR) (Shui et al. 1997; Bünemann et al. 1999), a fast component (‘fast’ or ‘acute’ desensitization) is unique to the receptor–GIRK channel pathway. This component is characterized by a relaxation of current within seconds immediately following exposure to a high concentration of agonist, provided the expression level of the corresponding GPCR is high enough (Wellner-Kienitz et al. 2000; Bösche et al. 2003a). Moreover, it appears to be heterologous between different receptor species (Bünemann et al. 1996b; Wellner-Kienitz et al. 2001). Although it is commonly accepted that this component of desensitization is localized downstream of the receptor, the underlying mechanism is still a matter of debate (Kurachi et al. 1987; Shui et al. 1998; Chuang et al. 1998; Kim, 1993; Saitoh et al. 2001).
In the present study we show that in atrial myocytes changes in GIRK current analogous to acute desensitization can be induced by opening or induction of other types of K+ channels and are dependent on K+ current flow rather than stimulation of a GPCR or activation of GIRK channels. We provide evidence that acute desensitization is caused by a reduction in electrochemical driving force resulting from K+ current flow, regardless of the K+ current pathway. Comparison of acute desensitization of GIRK current and ACh-induced hyperpolarization demonstrates that acute desensitization determines the time course of the hyperpolarization, i.e. the physiological signal. The present data are in line with an accompanying study demonstrating a negative interference between different inwardly rectifying K+ currents in atrial and ventricular myocytes most likely via a subsarcolemmal K+ gradient (Wellner-Kienitz et al. 2004).
Methods
Isolation and culture of atrial myocytes
The method of enzymatic isolation of adult rat atrial myocytes and serum-free culture conditions have been described in detail elsewhere (e.g. Bünemann et al. 1996a). Atrial and ventricular myocytes were cultured in 35 mm culture dishes under identical conditions. If not stated otherwise, myocytes were used experimentally from about 24 h until 5 days after isolation. Time in culture did not affect the key properties of the membrane currents investigated.
Solutions and chemicals
For whole-cell measurements of membrane currents an extracellular solution of the following composition was used (mmol l−1): NaCl 120; KCl 20; CaCl2 0.5; MgCl2 1.0; Hepes/NaOH 10.0, pH 7.4. The pipette solution contained (mmol l−1): potassium aspartate 110; KCl 20; NaCl 5.0, MgCl2 7.0; Na2ATP 5.0; EGTA 2.0; GTP 0.025; Hepes/KOH 20.0, pH 7.4. The K+ reversal potential under these conditions was calculated as −48 mV. Standard chemicals were from Merck (Darmstadt, Germany). EGTA, Hepes, MgATP, GTP, acetylcholine-chloride, adenosine, pinacidil, glibenclamide and 2,4-dinitrophenole (DNP) were from Sigma (Deisenhofen, Germany). Rilmakalime was obtained from Dr H. Gögelein (Aventis, Frankfurt, Germany).
Current measurement
Membrane currents were measured at ambient temperature (22–24°C) using whole-cell patch clamp as described in detail previously (Wellner-Kienitz et al. 2000). If not otherwise stated, cells were voltage clamped at a holding potential of −90 mV, i.e. negative to EK, resulting in inward K+ currents. Current–voltage relations were routinely determined by means of voltage ramps between −120 and +60 mV within 500 ms. Rapid exposure to agonist-containing solutions was performed by means of a custom-made solenoid-operated flow system.
Adenovirus constructs and gene transfer
The pAd-Easy-1 and shuttle plasmid (pAd-Track-CMV) were kindly provided by Dr B. Vogelstein (Johns Hopkins University, Baltimore, MD, USA). Production and purification of the recombinant virus were performed as described in detail by He et al. (1998). Briefly, the cDNA of rat Kir2.1 subunit (obtained from Dr A. Karschin, Würzburg, Germany), was subcloned into pAd-Track-CMV, using HindIII and EcoRV, to yield pAdTrack-Kir2.1. Recombinant adenovirus (Ad-Kir2.1) was generated by homologous recombination between pAdTrack-Kir2.1 and pAd-Easy-1 in E. coli. An adenoviral vector containing rat GIRK4 (Kir3.4) cDNA, provided by Dr Y. Kurachi, was constructed correspondingly (Ad-Kir3.4) using KpnI and XbaI.
For infection, cells were incubated for 3 h, starting about 24 h after plating, with 1 ml culture medium containing approximately 104 transducing particles. Electrophysiological recordings were made on days 3 and 4 after exposure to the virus. Infected cells (≥ 80%) were identified by epifluorescence of green fluorescent protein (GFP). Time-matched GFP-positive cells infected with the empty (GFP-encoding) virus served as controls.
Statistical analysis
Wherever possible, data are presented as mean ± s.e.m. and were analysed using Student's unpaired t test. P < 0.05 was considered significant.
Results
Definition of acute desensitization
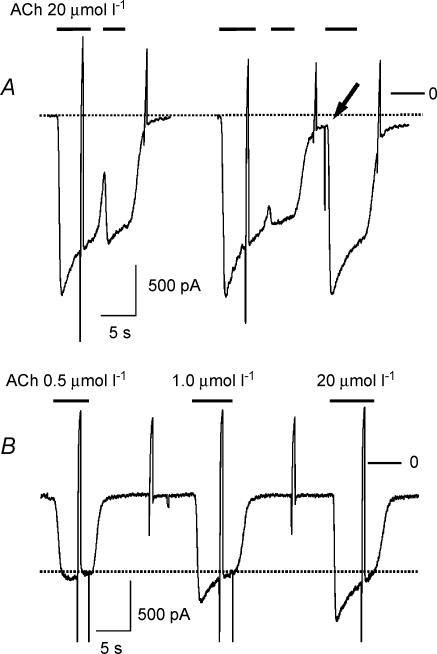
A key property of acute desensitization, as illustrated in Fig. 1A, is represented by its quasi-instantaneous reversibility. As soon as deactivation upon washout of agonist is complete, a full-sized current can be activated by a subsequent challenge by agonist. On average when the current was deactivated to < 10% of its peak following washout of ACh, the response to a second challenge by ACh was 95.3 ± 1.7%(n = 8) Acute desensitization, distinguished from other components of desensitization by this criterion, in terms of a distinct relaxation of current from a peak to a steady-state requires a saturating concentration of agonist and a high expression level of the activating receptor, which in native atrial myocytes is the case only for the M2AChR (Bösche et al. 2003a). The dependence of acute desensitization on ACh concentration is illustrated in Fig. 1B, which shows responses to three different concentrations of ACh (0.5, 1 and 20 μmol l−1). The increase in peak IK(ACh) at concentrations ≥ 0.5 μmol l−1 corresponds to the development of an increasing ‘desensitizing component’. In a series of paired measurements mean desensitization at 0.5 μmol l−1 ACh was 5.1 ± 1.5% as compared with 28.5 ± 2.6% at saturating ACh concentration (20 μmol l−1; n = 12). Traces of currents evoked by different concentrations of agonist do not crossover but relax to a common quasi-steady-state level as indicated by the dotted line. The representative data presented in Fig. 1 are neither in support of nor incompatible with a distinct mechanism of acute desensitization. Phenomenologically they might suggest that acute desensitization is related to the size of the current. The experiments to be described in the following were performed to study validity of this novel hypothesis.
Figure 1. Properties of acute desensitization of atrial GIRK current activated by Ach.
A, instantaneous reversibility of acute desensitization. Superfusion with ACh-containing (20 μmol l−1) solution was performed as indicated. The response marked by the arrow indicates a full-sized response at 94% deactivation. The dotted line was drawn to indicate baseline current. The rapid deflections in this and subsequent figures represent changes in membrane current caused by voltage ramps from −120 to +60 mV (500 ms) that were applied once every 10 s to determine current–voltage relations. B, inward currents activated by rapid superfusion of a cell with solutions containing 0.5, 1.0 and 20 μmol l−1 ACh. The dotted line in this panel indicates the quasi-steady-state current level. Zero current level in this and subsequent figures is indicated by a bar on the right labelled ‘0’.
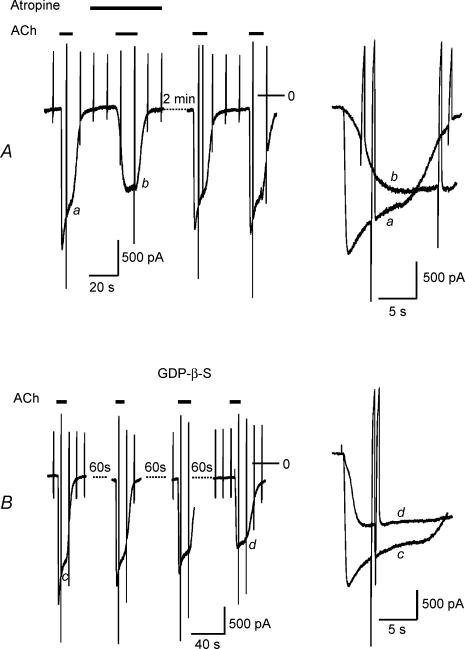
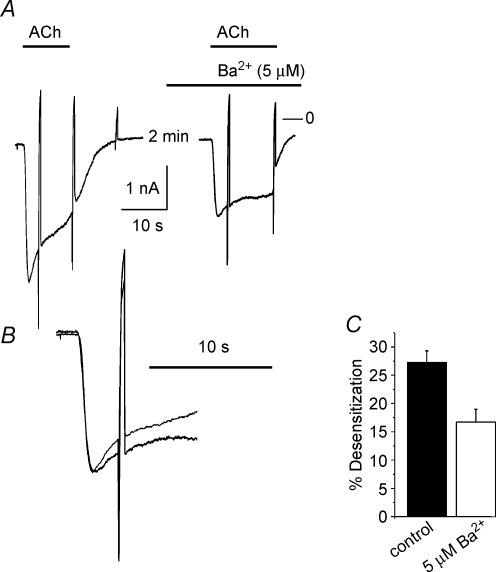
Desensitization as defined in Fig. 1 is reduced by various manoeuvres that cause a slowing in activation rate and a reduction in peak amplitude, such as lowering the concentration of the agonist or reducing the expression level of the activating receptor species (Wellner-Kienitz et al. 2000; Bösche et al. 2003a). One of the key arguments in relating acute desensitization to the G protein cycle is based on the finding that the G protein antagonist GDP-β-S, applied intracellularly, apart from slowing activation of GIRK current, causes a reduction in desensitization (Chuang et al. 1998; Sickmann & Alzheimer, 2003; Leaney et al. 2004). In Fig. 2 we compared the action of a receptor antagonist (atropine) and intracellular GDP-β-S on GIRK current activation amplitude and desensitization. As illustrated in Fig. 2A for a representative experiment, atropine at 10 nmol l−1 caused a reversible inhibition of GIRK current by about 40%. Simultaneously the activation rate was slowed and desensitization was completely abolished. In Fig. 2B a representative experiment is illustrated using the GDP analogue GDP-β-S (500 μmol l−1) included in the pipette-filling solution. Within about 3 min of dialysing the cell this resulted in a gradual reduction in the amplitude of IK(ACh) evoked by repetitive exposures to ACh to 54 ± 7%(n = 5) of peak IK(Ach) recorded immediately after breaking the membrane under the tip of the recording pipette. The decrease in amplitude was paralleled by a slowing of activation kinetics and an almost complete loss in the desensitizing component from 33.3 ± 4.5 to 7.6 ± 3.8% (measured at 5 s). Qualitatively the action of this G protein antagonist resembled the effect of the receptor antagonist. The apparent link between activation rate and desensitization would be compatible with the hypothesis that desensitization reflects a property of the G protein cycle, suggested by Chuang et al. (1998), Sickmann & Alzheimer (2003) and more recently by Leaney et al. (2004). However, these authors did not take into account that intracellular GDP-β-S causes a reduction in current amplitude. In order to test if a reduction in amplitude as such, i.e. without simultaneous manipulation of the signalling pathway on the receptor level or G protein level, respectively, affects desensitization, we used Ba2+ ions. In line with previous publications (e.g. Lancaster et al. 2000), Kir3.0 channels, like other inwardly rectifying K+ channels, are blocked by Ba2+ ions. In the conditions of the present study the apparent Ki was determined as about 20 μmol l−1, significantly higher than for ventricular inward rectifier potassium current (IK1) (data not shown). At a concentration of 5 μmol l−1 Ba2+ caused a reduction of IK(ACh) by 22.7 ± 4.5%(n = 8). As illustrated in Fig. 3, the reduction in amplitude was not paralleled by a slowing in activation rate. Nevertheless, desensitization was significantly reduced, supporting the notion that it is directly related to the amplitude of current rather than activation kinetics. This strongly argues against the G protein cycle or any component of or associated with the signal transduction pathway to determine acute desensitization.
Figure 2. Effects of the muscarinic receptor antagonist atropine (A) and the G protein antagonist GDP-β-S (B) on IK(ACh).
ACh and atropine were applied as indicated in the left panels. GDP-β-S (500 μmol l−1) was included in the pipette-filling solution. Right panels show expanded current recordings from sections labelled in the slow recordings (left).
Figure 3. Inhibition of IK(ATP) and reduction in desensitization by Ba2+ (5 μmol l−1).
A, recordings of ACh-induced inward currents with and without Ba2+. B, superimposed and expanded traces from A, vertically scaled to match the peaks. C, summarized data from a total of 8 analogous experiments. Bars represent percentage desensitization determined 5 s after switching to ACh-containing solution (P < 0.002).
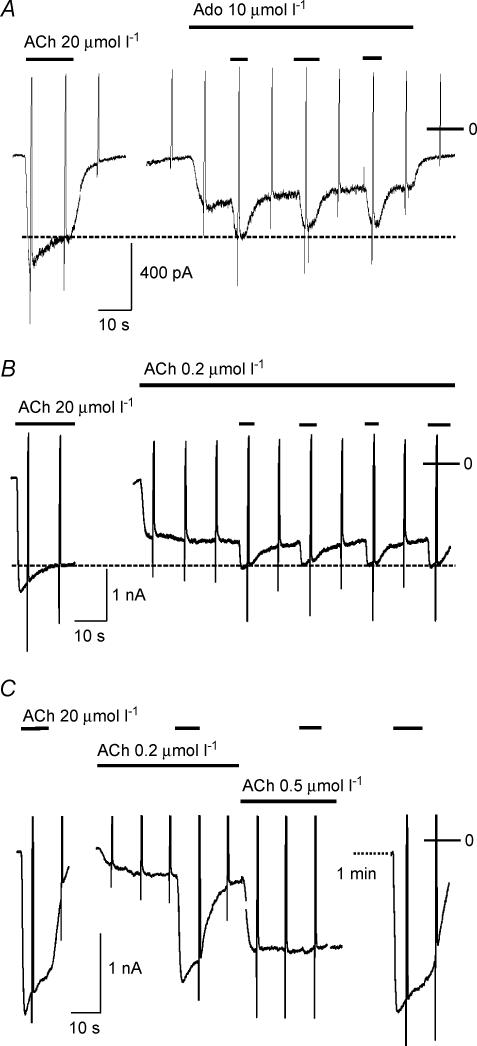
The data presented in Figs 1 and 2 might suggest that acute desensitization only occurs when a cell is challenged by a high concentration of ACh. However, as shown previously, conditions causing slow activation of a fraction of total available current, e.g. by stimulation of A1AdoR or by exposure to a subsaturating concentration of ACh, also cause acute desensitization, which is not discernible as a distinct relaxation, but develops during the activation phase. Three representative examples are illustrated in Fig. 4. In the cell represented by panel A, exposure to Ado (10 μmol l−1) resulted in a current of ∼45% of peak IK(ACh), devoid of a desensitizing component. The effects of ACh and Ado, applied simultaneously, are subadditive. The total level of current in this cell amounted to about 80% of peak IK(ACh) and approximately corresponded to the steady-state level of IK(ACh) in the presence of 20 μmol l−1 ACh. An analogous behaviour was observed when a low concentration of ACh (e.g. 0.2 μmol l−1) was used for fractional activation of IK(ACh). As shown in Fig. 4B the effects of 20 μmol l−1 ACh applied following pre-stimulation of the system with 2 μmol l−1 ACh yields a subadditive response. Due to slight variations in the concentration–response curves for activation of IK(ACh) between individual cells, the inhibition by low concentrations of ACh of the response to a saturating concentration showed a high degree of variability, as shown in Fig. 4C. In that cell 0.2 μmol l−1 ACh induced a current of only 18% of peak IK(ACh); concomitantly the response to 20 μmol l−1 of the agonist was reduced by less than 10%. Raising [ACh] to 0.5 μmol l−1 resulted in a current of 75% of Imax and no additional activation by 20 μmol l−1 ACh.
Figure 4. Desensitization of IK(ACh) by low-level activation.
A, nonadditivity of current evoked by Ado (10 μmol l−1) plus ACh (20 μmol l−1) and removal of desensitizing component. B and C, two examples of nonadditivity and removal of the desensitizing component of IK(ACh) evoked by 20 μmol l−1 ACh by low-level activation of current using 0.2 μmol l−1 (B) or 0.2 and 0.5 μmol l−1 ACh (B). Agonists were applied as indicated by horizontal bars.
The apparent heterologous nature of acute desensitization as demonstrated in Fig. 4A between the A1AdoR and the M2 AChR provided the key argument against the activating receptor(s) as molecular site(s) of this phenomenon (Kurachi et al. 1987). The observation that low level activation of GIRK current via A1AdoR or via any other endogenous or heterologously expressed GPCR species (Sodickson & Bean, 1998; Wellner-Kienitz et al. 2001; Bösche et al. 2003a) results in a preferential reduction of the desensitizing component of IK(ACh), has been interpreted in terms of acute desensitization taking place in parallel to activation of the current.
In a recent study we have provided evidence that in atrial and ventricular myocytes the total macroscopic inward current through inwardly rectifying K+ channels (IK1, IK(ATP), IK(ACh)) is limited due to a reduction in driving force caused by subsarcolemmal K+ accumulation and or depletion in an extracellular membrane-adjacent compartment (Wellner-Kienitz et al. 2004). Based on the data of that study and the data presented in Figs 2–4 of the present study, we hypothesized that acute desensitization of IK(ACh) might be related to K+ current flow rather than to a bona fide cellular signal. In that case one would expect activation of a different endogenous inwardly rectifying K+ current to exert an inhibitory effect on GIRK current comparable with the apparent desensitization caused by low level GIRK activation.
Activation of IK(ATP) causes inhibition of IK(ACh)
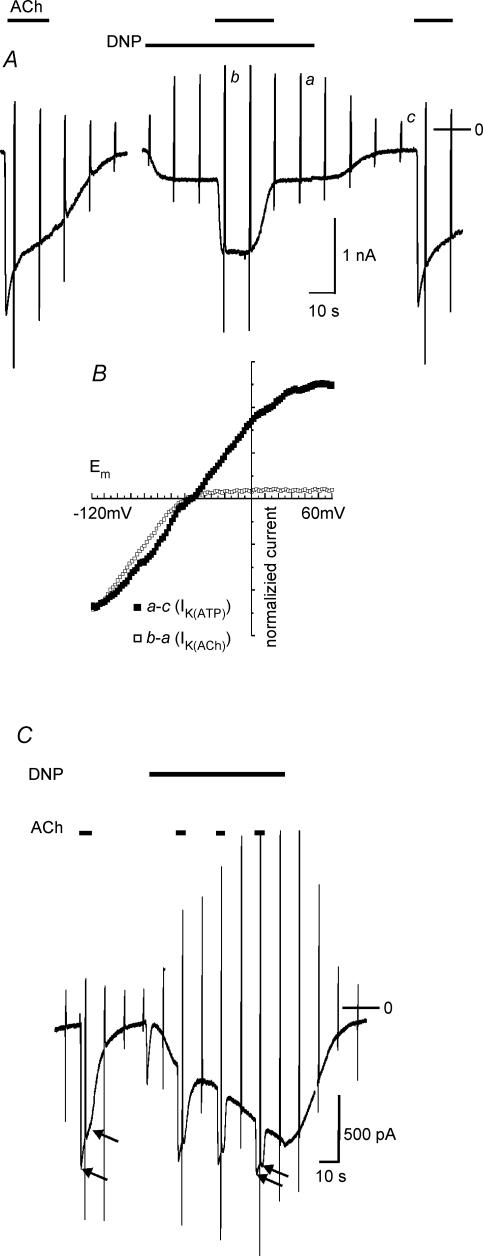
Atrial and ventricular myocytes express functional ATP-dependent K+ (K(ATP)) channels that can be opened by ATP depletion via metabolic inhibition or by channel opening drugs (e.g. Terzic et al. 1995). In Fig. 5 representative recordings of membrane current from myocytes, in which IK(ATP) was activated by mitochondrial uncoupling using DNP are illustrated. As a rule, exposure to the uncoupling agent resulted in activation of IK(ATP) within seconds. In this series of experiments there was a large range of variability regarding the amplitude of IK(ATP) in individual cells. In the cell represented by Fig. 5A and B stimulation by ACh (20 μmol l−1) caused activation of a current exhibiting a distinct desensitizing component. Exposure to DNP (50 μmol l−1) resulted in activation of a small inward current, reminiscent of the current evoked by Ado or a low concentration of ACh (compare Fig. 4). However, comparison of the current–voltage relations of IK(ACh) and DNP-induced current (Fig. 5B) clearly identifies the latter as IK(ATP) by its weak inward rectification (e.g. Brandts et al. 1998; Wellner-Kienitz et al. 2004). Analogous to an experiment using application of ACh in the presence of Ado (Fig. 4A), IK(ACh) was substantially reduced in amplitude and was devoid of a desensitizing component in the presence of DNP. Analogous results, i.e. a subadditivity of IK(ACh) and IK(ATP) and a reduction in the desensitizing component, were obtained in all 22 myocytes studied using a protocol as in Fig. 5, though amplitudes or densities, respectively, of IK(ATP) showed a large variability. In the cell represented by Fig. 5C, IK(ATP) was activated with a slower time course. Superimposed pulses of ACh reveal a gradual inhibition of IK(ACh) in parallel with activation of IK(ATP) and a reduction of the desensitizing component.
Figure 5. Activation of IK(ATP) by DNP negatively interferes with IK(ACh).
A, representative recording of membrane current from a myocyte with small DNP-activated current. ACh and DNP were applied as indicated. B, background-subtracted current–voltage relations of ACh- and DNP-activated current, normalized to current at −120 mV. C, sample recording of membrane current from a cell with large DNP-activated current and inhibition of IK(ACh) in parallel to activation of IK(ATP). The arrows indicate peak IK(ACh) and the current level 3.5 s after the peak.
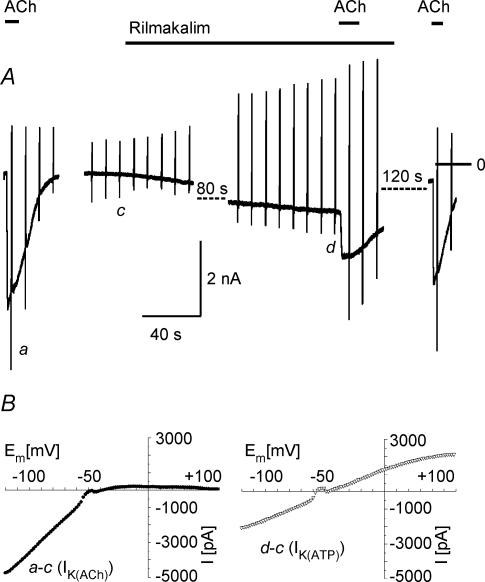
Nucleoside phosphates in a cell are, in equilibrium, catalysed by various enzymes such as adenylate kinase, creatine kinase and nucleoside diphosphate kinase (e.g. Dzeja & Terzic, 1998; Heidbüchel et al. 1992). Metabolic inhibition or uncoupling, therefore, is likely to also affect the concentration of GTP in the cell and thus potentially interferes with agonist-induced G protein cycling. Moreover, depletion of ATP by DNP via F1Fo-ATPase (Sasaki et al. 2001) might result in depletion of phosphatidylinositol bisphosphate (PIP2), an important co-factor for activation of GIRK channels (Hilgemann et al. 2001; Meyer et al. 2001). Thus it cannot be excluded that inhibition of IK(ACh) by DNP is due to or at least contaminated by impairment of G protein signalling or PIP2 depletion, respectively. We therefore used the channel opener rilmakalim (Ril, Englert et al. 2001) to activate IK(ATP) via a different mechanism. In the present conditions, Ril (50 μmol l−1) caused acute activation of IK(ATP) in only about 20% of myocytes tested. In these cells, as a rule, activation was slower than using DNP. A result qualitatively representative of eight Ril-responsive myocytes is illustrated in Fig. 6A. In principle the effect of activating IK(ATP) by Ril on ACh-induced current is analogous to the effect of DNP. On the background of IK(ATP), identified by its I–V curve (Fig. 6B), IK(ACh) was reversibly reduced in amplitude and apparent desensitization was almost completely removed. Thus, current flowing through K(ATP) channels negatively interferes with IK(ACh), independent of how IK(ATP) is activated.
Figure 6. Activation of IK(ATP) and inhibition of IK(ACh) by rilmakalim.
A, representative current recording. B, difference I–V curves of ACh- and rilmakalim-activated current.
A constitutive inward rectifier current due to overexpression of Kir2.1 causes a reduction of IK(ACh)
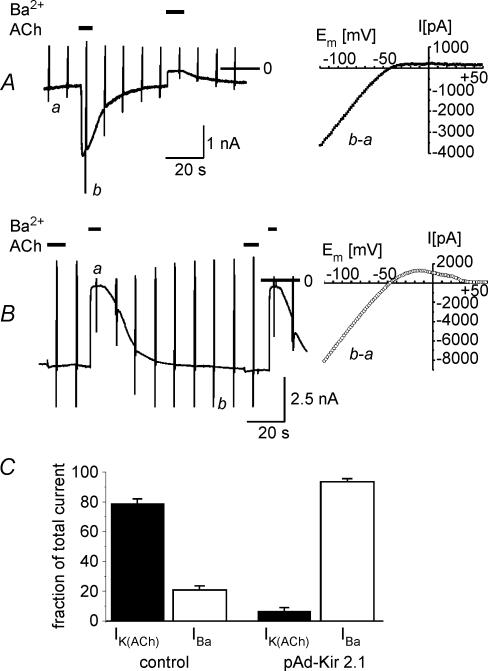
Taken together, the data presented in Figs 5 and 6 demonstrate a negative interference of inward current carried by different inward-rectifying K+ channels and suggest that acute desensitization might be related to K+ current rather than to a genuine cellular signal. To further challenge this hypothesis we overexpressed Kir2.1 driven by adenoviral gene transfer. Kir2.1 subunits form constitutively active inwardly rectifying K+ channels and are likely to assemble with other Kir2.x subunits to form cardiac IK1 channels (Liu et al. 2001; Zaritsky et al. 2001; Zobel et al. 2003). Representative current recordings from this series of experiments are shown in Fig. 7. Panel A displays a current recording from a GFP-positive myocyte infected with the empty viral vector. Ba2+ (2 mmol l−1) was used to quantify the background K+ current, which on average was about 25% of peak IK(ACh). In Fig. 7B an analogous experiment on a representative time-matched cell infected with pAd-Kir2.1 is illustrated. In line with a recent study, atrial cells overexpressing the Kir2.1 subunit are characterized by a large inward Ba2+-sensitive holding current, which shows strong inwardly rectifying properties but displays an I–V relation which differed from that of IK(ACh) by its negative slope at positive membrane potentials (Wellner-Kienitz et al. 2004). Two exposures to ACh (20 μmol l−1) caused hardly discernable currents. This behaviour is reminiscent of ventricular myocytes, whose background current–voltage relation is governed by IK1, and which show little receptor-activated GIRK current (e.g. Ito et al. 1995). The differences in background inwardly rectifying current probed by 2 mmol l−1 Ba2+, and ACh-induced current between mock-infected and Ad-Kir2.1-infected cells were highly significant, as demonstrated by the summarized data in Fig. 7C.
Figure 7. Reduction of IK(ACh) in myocytes overexpressing Kir2.1.
A, recording of IK(ACh) and Ba2+-sensitive background current from a GFP-positive myocyte infected with the empty virus (left) and background-subtracted I–V relation of IK(ACh) (right). B, recording of IK(ACh) and Ba2+-sensitive background current from a GFP-positive myocyte infected with Ad-Kir2.1 (left) and I–V relation of Ba2+-sensitive background current (right). C, summarized data from 10 time-matched cells in each group comparing fractional Ba2+-sensitive background current (IBa) and ACh-activated current (n = 8 for each group).
A constitutive inward rectifier current due to overexpression of Kir3.4 causes a reduction of ACh-activated current
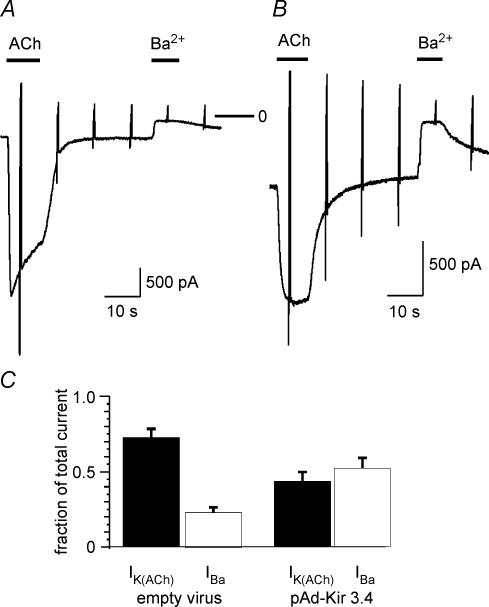
In a previous study we have shown that GIRK4 subunits or concatemeric GIRK4 constructs expressed in CHO cells form functional homomeric channels that can be activated by stimulation of co-expressed A1AdoR. Overexpression of monomeric GIRK4 subunits in atrial myocytes resulted in ACh-induced currents that were completely devoid of a desensitizing component (Bender et al. 2001). Based on these data it was proposed that acute desensitization represents a property related to the heterotetrameric GIRK1/GIRK4 channel. This is contradictory to the hypothesis that acute desensitization results from K+ current flow. We therefore re-investigated the properties of ACh-induced current in myocytes overexpressing GIRK4 by adenoviral gene transfer. Figure 8 compares ACh-induced current and Ba2+-sensitive background current in GFP-positive myocytes infected with the empty vector (A) and Ad-Kir3.4, respectively (B). The representative traces and summarized data (C) reveal a significantly larger Ba2+-sensitive constitutive current in the Ad-Kir3.4-infected cell as compared with the control myocyte. The larger constitutive K+ current was paralleled by a significant reduction in amplitude of receptor-stimulated GIRK current. In line with our previous publication, ACh-induced currents in GIRK4 (Kir3.4) overexpressing myocytes are completely devoid of acute desensitization. In accord with our current hypothesis, the lack of desensitization of receptor-activated current carried by homomeric GIRK4 channels probably results from an increased constitutive K+ current carried by GIRK4 homomers.
Figure 8. Increased background current and reduced ACh-activated current in myocytes overexpressing GIRK4 (Kir3.4).
A, representative current recordings from two myocytes infected with the empty virus (A) and Ad-Kir3.4 (B). C, summarized data showing IK(ACh) and Ba2+-sensitive background current (IBa) as fraction of total current (n = 8 for each group).
Acute desensitization is paralleled by a shift of the reversal potential of IK(ACh) in the negative direction
If acute desensitization reflects a reduction in driving force, the reduction in current should be paralleled by a corresponding shift in the reversal potential of IK(ACh).
Using the standard ramp protocol, i.e. a linear change in membrane potential from −130 to +60 mV within 500 ms, corresponding to a dV/dt of 360 mV s−1, the reversal potential of the agonist-activated current was constant throughout, regardless of if it was determined near the peak of IK(ACh) or in the desensitized state (not shown). We hypothesized that because of the slow rate of the ramp, a hypothetical submembrane [K+] gradient dissipates, and the system is in equilibrium throughout the ramp.
We therefore generated more rapid voltage ramps and compared the resulting I–V curves at peak IK(ACh) and in the desensitized state. Following a step from −90 mV to −60 mV a ramp was generated to −30 mV within 7.5 ms (4 V s−1). To eliminate contamination by current through voltage-activated Na+ and Ca2+ channels the solution contained TTX (10 μmol l−1) and CdCl (1 mmol l−1). All curves were background subtracted as described above to eliminate an offset caused by capacitive current.
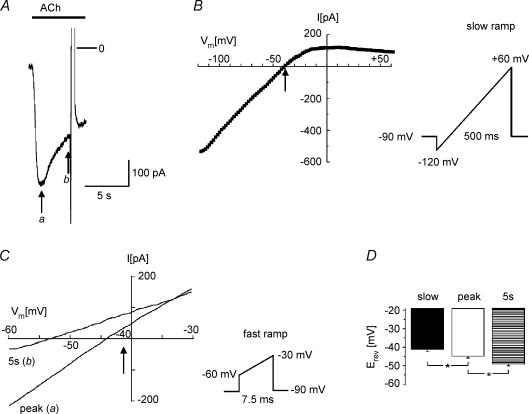
Figure 9A shows a representative current trace of IK(ACh) elicited by 20 μmol l−1 ACh. The current desensitized by > 50% within 5 s. The I–V curve determined by the standard slow ramp intersected the voltage axis at −40.5 mV as illustrated in Fig. 9B. Figure 9C shows I–V curves determined by application of fast ramps at the times labelled a and b in panel A (for further details see legend). In line with our hypothesis, in the desensitized state the reversal potential was consistently more negative than at peak IK(ACh) (10 mV in the experiment shown). Moreover, the I–V curve at peak had a reversal potential that was consistently a few millivolts more negative than the Erev obtained by the slow ramp protocol, which is in line with the hypothesis that desensitization already develops in parallel with activation. The summarized data from 10 myocytes analysed in the same way (Fig. 9C) demonstrate significant differences in reversal potentials between slow (‘equilibrium’) voltage ramps and fast ramps applied at peak IK(ACh) and in the desensitized state.
Figure 9. Desensitization affects the reversal potential of IK(ACh).
A, inward current induced by exposure to ACh (20 μmol l−1). B, current–voltage relation obtained by slow voltage-ramp protocol as outlined in the scheme. The arrow indicates the reversal potential (−41.2 mV). C, current–voltage relations obtained by fast voltage ramps, as outlined in the scheme, at peak IK(ACh) and 5 s later. These curves were obtained during a second exposure to ACh, with the slow ramp protocol turned off, which resulted in an inward current with identical properties as shown in A. The corresponding times have been labelled a and b in panel A. The arrow corresponds to the reversal potential obtained by the slow ramp (B). D, summarized data from 10 myocytes. The column labelled ‘slow’ represents the mean reversal potential obtained by a slow voltage ramp. Peak and 5 s have the same meaning as in C.
Physiological relevance of current-dependent desensitization
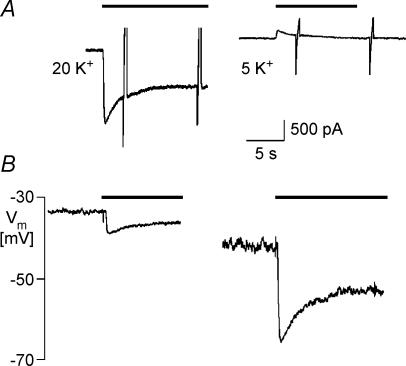
The data of the present study were obtained using an unphysiological K+ gradient and holding potential. These conditions were chosen to measure K+ channel currents in the inward direction, which is standard in numerous studies related to GIRK current because of its strong inward rectification. It has been shown previously that outward IK(ACh) in atrial myocytes shows comparable desensitization properties, though with faster kinetics (Brandts et al. 1997). Nevertheless, the question remains, if acute desensitization is of physiological relevance in terms of shaping the ACh-induced hyperpolarization in myocytes or IPSPs in neuronal synapses. Therefore in a series of experiments we applied ACh to the same cell under both voltage-clamp and current-clamp conditions at two different [K+]o. A representative result from a total of eight cells is shown in Fig. 10. The left trace in panel A shows the inward IK(ACh) induced by ACh at 20 mmol l−1[K+]o and −90 mV holding potential. The unclamped cell had a resting potential of about −35 mV (panel B). The corresponding hyperpolarization (panel B) was about 5 mV in amplitude. When [K+]o was reduced to 5 mmol l−1, resting potential stabilized at −42 mV. Rapid exposure to 20 μmol l−1 ACh resulted in a hyperpolarization of 24 mV. The corresponding outward current, recorded at −40 mV holding potential, was small, as compared with inward IK(ACh) due to the strong inwardly rectifying properties of the charge-carrying channel species, but showed desensitization, which was similar but consistently faster and stronger than for inward IK(ACh) (Brandts et al. 1997). In both conditions also the ACh-induced hyperpolarization desensitized with comparable time courses. Although this might be coincidental, it might suggest that the rapid fade (Boyett & Roberts, 1987) of the bradycardic action of ACh in the sinoatrial node at least partially reflects current-dependent desensitization.
Figure 10. Comparison of desensitization of IK(ACh) and ACh-induced hyperpolarization in a representative myocyte.
A, IK(ACh) recorded at 20 mmol l−1 (left) and 5 mmol l−1 [K+]o (right). Holding potentials were −90 mV (20 mmol l−1) and −40 mV (5 mmol l−1). B, ACh-induced hyperpolarizations recorded in current-clamp mode.
Discussion
In this study we demonstrated that inward current through different types of K+ channels affects the relaxation of IK(ACh) termed rapid or acute desensitization. Receptor-activated GIRK current was reduced when a K+ inward current was simultaneously active. This negative interference was observed with: (i) receptor-activated endogenous GIRK (Kir3.1/Kir3.4), independent of the receptor species; (ii) IK(ATP) (Kir6.2/SUR2A); (iii) constitutively active IK1 (Kir2.1); (iv) constitutively active GIRK4 (Kir3.4). Moreover, as shown in an accompanying paper this negative interference was mutual between IK(ACh) and IK(ATP), and it was also observed between IK1 and IK(ATP) (Wellner-Kienitz et al. 2004).
Rapid desensitization of atrial GIRK current was first demonstrated and discussed by Kurachi et al. (1987) as possibly related to the coupling G protein. Due to its apparent heterologous nature it is generally believed to be localized downstream of the receptor(s). A kinase phosphatase mechanism was suggested by Kim (1993). In that study, however, desensitization had a half-time of approximately 20 s as opposed to ≤ 5 s in the present and previous studies and was not instantaneously reversible, and thus, conceivably reflected or was contaminated by slower receptor desensitization. A different mechanism, related to the G protein cycle, had been proposed by Chuang et al. (1998). These authors studied responses of GIRK channels in inside-out macro patches to rapid application of guanosine nucleotides (GDP/GTP). The key observations were: (i) that priming the channels with GDP followed by exposure to GTP resulted in a current of slower activation kinetics, smaller amplitude and less desensitization than upon activation with GTP from a GDP-unbound state; and (ii) that desensitization was enhanced by RGS4. Using a simple model, desensitization could be accounted for by the nucleotide exchange and hydrolysis cycle. The experimental data as such are not contradictory to the present hypothesis, since a distinct ‘desensitizing component’ requires fast activation of IK(ACh). As discussed in that publication, however, the model cannot satisfactorily explain desensitization in an intact cell, where an empty (guanosine nucleotide-free) state of the Gα subunit is unlikely to exist. Moreover, RGS4 has been shown to increase the rate of activation of heterologously expressed GIRK channels (Doupnik et al. 1997; Saitoh et al. 1997), which, in line with the present study, would also result in an augmentation of the ‘desensitizing component’. More recently a similar hypothesis was put forward for fast desensitization of receptor-activated GIRK currents in neurones (Sickmann & Alzheimer, 2003) and for heterologously expressed GIRK channels (Leaney et al. 2004). These authors found that intracellular loading with GDP or GDP-β-S reduced fast desensitization. These experimental manoeuvers without doubt interfere with the G protein cycle. This, however, affects the activation rate and also, as shown in the present study, the amplitude of the receptor-evoked current, and therefore those data are not contradictory to the hypothesis of the present study. Thus, the kinetics of the G protein cycle is likely to have an effect on the ‘desensitizing component’ of the current by virtue of its slowing effect on the activation kinetics and, more importantly, the reduction in current amplitude. Our data using Ba2+ to partially block IK(ACh), clearly show that reducing only the amplitude of the current per se causes a significant reduction in desensitization.
Kobrinsky et al. (2000) proposed that fast desensitization of IK(ACh) is brought about by depletion of PIP2 due to co-stimulation of muscarinic M3 receptors linked to phospholipase C (PLC). This hypothesis, however, is not in line with the observation that in atrial myocytes stimulation of any receptor, endogenous or heterologously expressed, causes acute desensitization (Wellner-Kienitz et al. 2001; Bösche et al. 2003a). Moreover, inhibition of GIRK current upon activation of receptors that cause depletion of PIP2 is too slow to account for this phenomenon (Meyer et al. 2001; Bender et al. 2002; Cho et al. 2002).
In a recent study acute desensitization has been discussed in relation to a novel G protein-independent inhibition of atrial GIRK current (Bösche et al. 2003b) that is particularly pronounced when GIRK current is activated via overexpressed A1AdoR (see also Leaney et al. 2004). The present data suggest that both phenomena are unlikely to share a common mechanism.
The results of the present study clearly demonstrate that acute desensitization is due to flux of K+ ions. This in turn suggests that the decrease in current reflects a reduction in driving force caused by current-dependent changes in subsarcolemmal K+ concentration and/or extracellular depletion. Both alternatives are difficult to distinguish experimentally, particularly in the case of a current pathway that conducts very little outward current. A similar hypothesis has been suggested previously to account for an inhibitory interference between delayed rectifier K+ current and IK(ATP) in smooth muscle cells (McHugh & Beech, 1995).
Integrating IK(ACh) from the start of activation to peak, and relating this charge to the volume of a sphere estimated from the approximated surface (capacitance) measurement yields a global rise in [K+]i in the order of magnitude of 1–5 mmol l−1, a change that would result in a negligible change in Nernst potential. However, the assumption that a significant gradient of [K+]i from the membrane to the centre of the cell during K+ current flow is realistic and supported by experimental data. In a recent study it has been demonstrated that the subsarcolemmal rise in [Na+]i caused by Na+ inward current via voltage-gated Na+ channels can exceed the increment in bulk [Na+]i by a factor of 60 (Weber et al. 2003). The resulting concentration gradient has been shown to dissipate with a time constant of 15 ms, i.e. slower than one would expect assuming a normal diffusion coefficient. Using fast ramp protocols we could detect a shift in the reversal potential of ACh-activated current related to desensitization, which can only be accounted for by a shift in driving force unless one assumes a change in ion selectivity of GIRK channels associated with desensitization. As stated above, current-related changes in driving force could also be accounted for by changes in K+ concentration in an extracellular membrane-adjacent compartment. Accumulation of K+ in such a structurally not yet defined compartment has been suggested to affect delayed (outward) rectifier current by virtue of the driving force in vascular smooth muscle cells (Smirnov & Aaronson, 1994).
Acknowledgments
We thank Anke Galhoff, Bing Liu and Gabriele Reimus for unfailing technical assistance. A.R. is holder of a scholarship of the International Graduate School of Neurosciences, NRW. This work was supported by the DFG (Po 212-9).
References
- Bender K, Wellner-Kienitz M-C, Inanobe A, Meyer T, Kurachi Y, Pott L. Overexpression of monomeric and multimeric GIRK4 subunits in rat atrial myocytes removes fast desensitization and reduces inward rectification of muscarinic K+ current (IK(ACh)) J Biol Chem. 2001;276:28873–28880. doi: 10.1074/jbc.M102328200. [DOI] [PubMed] [Google Scholar]
- Bender K, Wellner-Kienitz M-C, Meyer T, Pott L. Activation of muscarinic K+ current by β-adrenergic receptors in cultured atrial myocytes transfected with β1 subunit of heterotrimeric G proteins. FEBS Lett. 1998;439:115–120. doi: 10.1016/s0014-5793(98)01350-7. [DOI] [PubMed] [Google Scholar]
- Bender K, Wellner-Kienitz M-C, Pott L. Transfection of a phosphatidyl-4-phosphate 5-kinase gene into rat atrial myocytes removes inhibition of GIRK current by endothelin and α-adrenergic agonists. FEBS Lett. 2002;529:356–360. doi: 10.1016/s0014-5793(02)03426-9. [DOI] [PubMed] [Google Scholar]
- Bösche LI, Bender K, Wellner-Kienitz M-C, Pott L. Transfection with 5-HT1A receptor gene and antisense directed against muscarinic M2 receptors reveal a mutual influence between Gi/o-coupled receptors in rat arial myoctes. J Mol Cell Cardiol. 2003a;35:99–107. doi: 10.1016/s0022-2828(02)00283-3. [DOI] [PubMed] [Google Scholar]
- Bösche LI, Wellner-Kienitz M-C, Bender K, Pott L. G Protein-independent inhibition of GIRK current by adenosine in rat atrial myocytes overexpressing A1-adenosine receptors after adenovirus-mediated gene transfer. J Physiol. 2003b;550:707–717. doi: 10.1113/jphysiol.2003.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett MR, Roberts A. The fade of the response to acetylcholine at the rabbit isolated sino-atrial node. J Physiol. 1987;393:171–194. doi: 10.1113/jphysiol.1987.sp016818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandts B, Brandts A, Wellner-Kienitz M-C, Zidek W, Schlüter H, Pott L. Non-receptor-mediated activation of IK(ATP) and inhibition of IK(ACh) by diadenosine polyphosphates in guinea-pig atrial myocytes. J Physiol. 1998;512:407–420. doi: 10.1111/j.1469-7793.1998.407be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandts B, Bünemann M, Hluchy J, Sabin GV, Pott L. Inhibition of muscarinic K+ current in guinea-pig atrial myocytes by PD 81,723, an allosteric enhancer of adenosine binding to A1 receptors. Br J Pharmacol. 1997;121:1217–1223. doi: 10.1038/sj.bjp.0701254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M, Brandts B, Pott L. Downregulation of muscarinic M2 receptors linked to K+ current in cultured guinea-pig atrial myocytes. J Physiol. 1996a;492:351–362. doi: 10.1113/jphysiol.1996.sp021497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M, Lee KB, Pals-Rylaarsdam R, Roseberry AG, Hosey MM. Desensitization of G-protein-coupled receptors in the cardiovascular system. Annu Rev Physiol. 1999;61:169–192. doi: 10.1146/annurev.physiol.61.1.169. [DOI] [PubMed] [Google Scholar]
- Bünemann M, Liliom K, Brandts B, Pott L, Tseng J-L, Desiderio GM, Sun G, Miller D, Tigyi G. A novel receptor with high affinity for lysosphingomyelin and sphingosine 1-phosphate in atrial myocytes. EMBO J. 1996b;15:5527–5534. [PMC free article] [PubMed] [Google Scholar]
- Cho H, Hwang JY, Kim D, Shin HS, Kim Y, Earm YE, Ho WK. Acetylcholine-induced phophatidylinositol 4,5-bisphosphate depletion does not cause short-term desensitization of G-protein gated inwardly rectifying K+ current in mouse atrial myocytes. J Biol Chem. 2002;277:27742–27747. doi: 10.1074/jbc.M203660200. [DOI] [PubMed] [Google Scholar]
- Chuang H, Yu M, Jan YN, Jan LY. Evidence that the nucleotide exchange and hydrolysis cycle of G proteins causes acute desensitization of G-protein gated inward rectifier K+ channels. Proc Natl Acad Sci U S A. 1998;95:11727–11732. doi: 10.1073/pnas.95.20.11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. Ion-channel regulation by G proteins. Trends Endocrinol Metab. 2001;12:391–398. doi: 10.1016/s1043-2760(01)00475-1. [DOI] [PubMed] [Google Scholar]
- Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proc Natl Acad Sci U S A. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- Englert HC, Gerlach U, Goegelein H, Hartung J, Heitsch H, Mania D, Scheidler S. Cardioselective KATP channel blockers derived from a new series of m-anisamidoethylbenzenesulfonylthioureas. J Med Chem. 2001;44:1085–1098. doi: 10.1021/jm000985v. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou SB, Da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbüchel H, Callewaert G, Vereecke J, Carmeliet E. Membrane-bound nucleoside diphosphate kinase activity in atrial cells of frog, guinea pig, and human. Circ Res. 1992;71:808–820. doi: 10.1161/01.res.71.4.808. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;111:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- Hommers LG, Lohse MJ, Bünemann M. Regulation of the inward rectifying properties of G-protein-activated inwardly rectifying K+ (GIRK) channels by Gβγ subunits. J Biol Chem. 2003;278:1037–1043. doi: 10.1074/jbc.M205325200. [DOI] [PubMed] [Google Scholar]
- Ito H, Hosoya Y, Inanobe A, Tomoike H, Endoh M. Acetylcholine and adenosine activate the G protein-gated muscarinic K+ channel in ferret ventricular myocytes. Naunyn-Schmiedebergs Arch Pharmacol. 1995;351:610–617. doi: 10.1007/BF00170160. [DOI] [PubMed] [Google Scholar]
- Kim D. Mechanism of rapid desensitization of muscarinic K+ current in adult rat and guinea pig atrial cells. Circ Res. 1993;73:89–97. doi: 10.1161/01.res.73.1.89. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Yokoyama M, Akita H, Matsushita K, Kurachi Y, Yamada M. Tertiapin potently and selectively blocks muscarinic K+ channels in rabbit cardiac myocytes. J Pharmacol Exp Ther. 2000;293:196–205. [PubMed] [Google Scholar]
- Kobrinsky E, Mirshani T, Zhang H, Logothetis DE. Receptor mediated hydrolysis of the plasma messenger PIP2 provides a novel mechanism for K+ current desensitization. Nature Cell Biol. 2000;2:507–514. doi: 10.1038/35019544. [DOI] [PubMed] [Google Scholar]
- Kurachi Y, Nakajima T, Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987;410:227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Lancaster MK, Dibb KM, Quinn CC, Leach R, Lee JK, Findlay JB, Boyett MR. Residues and mechanisms for slow activation and Ba2+ block of the cardiac muscarinic K+ channel, Kir3.1/Kir3.4. J Biol Chem. 2000;275:35831–35839. doi: 10.1074/jbc.M006565200. [DOI] [PubMed] [Google Scholar]
- Leaney JL, Benians A, Brown S, Nobles M, Kelly D, Tinker A. Rapid desensitization of G protein-gated inwardly rectifying K+ currents is determined by G protein cycle. Am J Physiol Cell Physiol. 2004;287:C182–191. doi: 10.1152/ajpcell.00540.2003. [DOI] [PubMed] [Google Scholar]
- Leaney JL, Milligan G, Tinker A. The G protein α subunit has a key role in determining the specificity of coupling to, but not the activation of, G protein-gated inwardly rectifying K+ channels. J Biol Chem. 2000;275:921–929. doi: 10.1074/jbc.275.2.921. [DOI] [PubMed] [Google Scholar]
- Liu GX, Derst C, Schlichthörl G, Heinen S, Seebohm G, Brüggemann A, Kummer W, Veh RW, Daut J, Preisig-Müller R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Beech DJ. Inhibition of delayed rectifier K+-current by levcromakalim in single intestinal smooth muscle cells: effects of cations and dependence on K+-flux. Br J Pharmacol. 1995;114:391–399. doi: 10.1111/j.1476-5381.1995.tb13239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Wellner-Kienitz M-C, Biewald A, Bender K, Eickel A, Pott L. Depletion of phosphatidylinositol 2,4 bisphosphate by activaton of PLC-coupled receptors causes slow inhibition but not desensitisation of GIRK current in atrial myocytes. J Biol Chem. 2001;276:5650–5658. doi: 10.1074/jbc.M009179200. [DOI] [PubMed] [Google Scholar]
- Morishige K-I, Inanobe A, Yoshimoto Y, Kurachi H, Murata Y, Tokunaga Y, Maeda T, Maruyama Y, Kurachi Y. Secretagogue-induced exocytosis recruits G protein-gated K+ channels to plasma membrane in endocrine cells. J Biol Chem. 1999;274:7969–7974. doi: 10.1074/jbc.274.12.7969. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature. 1997;390:525–529. doi: 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Masuho I, Terakawa I, Nomoto S, Asano T, Kubo Y. Regulator of G protein signaling 8 (RGS8) requires its NH2 terminus for subcellular localization and acute desensitization of G protein-gated K+ channels. J Biol Chem. 2001;276:5052–5058. doi: 10.1074/jbc.M006917200. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Sato T, Marban E, O’Rourke B. ATP consumption by uncoupled mitochondria activates sarcolemmal K(ATP) channels in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2001;280:H1882–1888. doi: 10.1152/ajpheart.2001.280.4.H1882. [DOI] [PubMed] [Google Scholar]
- Shui Z, Boyett MR, Zang WJ. ATP-dependent desensitization of the muscarinic K+ channel in rat atrial cells. J Physiol. 1997;505:77–93. doi: 10.1111/j.1469-7793.1997.077bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui Z, Khan IA, Tsuga H, Haga T, Boyett MR. Role of receptor kinase in short-term desensitization of cardiac muscarinic K+ channels expressed in chinese hamster ovary cells. J Physiol. 1998;507:325–334. doi: 10.1111/j.1469-7793.1998.325bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmann T, Alzheimer C. Short-term desensitization of G-protein-activated, inwardly rectifying K+ (GIRK) currents in Pyramidal neurons of rat neocortex. J Neurophysiol. 2003;90:2494–2503. doi: 10.1152/jn.00112.2003. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Aaronson PI. Alteration of the transmembrane K+ gradient during development of delayed rectifier in isolated rat pulmonary arterial cells. J General Physiol. 1994;104:241–264. doi: 10.1085/jgp.104.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. Neurotransmitter activation of inwardly rectifying potassium current in dissociated hippocampal CA3 neurons: Interactions among multiple receptors. J Neurosci. 1998;18:8153–8162. doi: 10.1523/JNEUROSCI.18-20-08153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic A, Jahangir A, Kurachi Y. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. Am J Physiol. 1995;269:C525–545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- Weber CR, Ginsburg KS, Bers DM. Cardiac submembrane [Na+] transients sensed by Na+-Ca2+ exchange current. Circ Res. 2003;92:950–952. doi: 10.1161/01.RES.0000071747.61468.7F. [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz M-C, Bender K, Meyer T, Bünemann M, Pott L. Overexpressed A1 adenosine receptors reduce activation of IK(ACh) by native muscarinic M2 receptors in rat atrial myocytes. Circ Res. 2000;86:643–648. doi: 10.1161/01.res.86.6.643. [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz MC, Bender K, Pott L. Overexpression of β1 and β2 adrenergic receptors in rat atrial myocytes. Differential coupling to G protein-gated inward rectifier K+ channels via Gs and Gi/o. J Biol Chem. 2001;276:37347–37354. doi: 10.1074/jbc.M106234200. [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz M-C, Bender K, Rinne A, Pott L. Voltage dependence of ATP-dependent K+ current in rat cardiac myocytes is affected by IK1 and IK(ACh) J Physiol. 2004;561:459–469. doi: 10.1113/jphysiol.2004.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman K, Nemec J, Gendler SJ, Clapham DE. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 1998;20:103–114. doi: 10.1016/s0896-6273(00)80438-9. [DOI] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacol Rev. 1998;50:723–757. [PubMed] [Google Scholar]
- Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K+ current (IK1) as revealed by the targeted deletion of the murine Kir 2.1 and Kir 2.2 genes. J Physiol. 2001;533:697–710. doi: 10.1111/j.1469-7793.2001.t01-1-00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel C, Cho HC, Nguyen T-T, Pekhletski R, Diaz RJ, Wilson GJ, Backx PH. Molecular dissection of the inward rectifier potassium current (IK1) in rabbit cardiomyocytes: evidence for heteromeric co-assembly of Kir2.1 and Kir2.2. J Physiol. 2003;550:365–372. doi: 10.1113/jphysiol.2002.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]