Abstract
The awakening of the gonadotrophic axis at puberty is the end-point of a complex cascade of sex developmental events that leads to the attainment of reproductive capacity. Recently, loss-of-function mutations of the gene encoding GPR54, the putative receptor for the KiSS-1-derived peptide metastin, have been linked to hypogonadotrophic hypogonadism, both in rodents and humans. However, the actual role of the KiSS-1/GPR54 system in the timing of puberty onset remains unexplored. We report herein that chronic central administration of KiSS-1 peptide to immature female rats induced the precocious activation of the gonadotrophic axis, as estimated by advanced vaginal opening, elevated uterus weight, and increased serum levels of luteinizing hormone (LH) and oestrogen. The central effect of KiSS-1 upon LH release appeared to be mediated via the hypothalamic LH-releasing hormone. In contrast, despite the well-documented permissive role of body fat stores and the adipocyte-derived hormone leptin in puberty maturation, acute activation of the gonadotrophic axis by KiSS-1 was persistently observed in pubertal animals under food deprivation, after central immunoneutralization of leptin, and in a model of leptin resistance. Overall, the present results, together with our recent data on maximum expression of KiSS-1 and GPR54 genes in the hypothalamus at puberty, provide novel evidence for a role of the KiSS-1 system as a downstream element in the hypothalamic network triggering the onset of puberty.
Puberty is the culmination of a complex sequence of maturational events that leads to the activation of the gonadotrophic axis, with increased serum levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and the attainment of reproductive capacity (Ojeda & Urbanski, 1994; Parent et al. 2003). Normal puberty development requires the precise interplay of a plethora of central and peripheral signals that are integrated at the neuroendocrine hypothalamus, where the decapeptide LH-releasing hormone (LHRH) plays an essential role as the end-point regulatory signal which ultimately dictates the pulsatile release of LH and FSH from pituitary gonadotrophs (Ojeda & Urbanski, 1994; Parent et al. 2003). Thus, it is assumed that puberty onset in mammals is triggered by the concerted enhancement of excitatory signals and the lowering of inhibitory inputs upon hypothalamic LHRH neurones, under the permissive action of a number of peripheral metabolic signals, such as leptin (Ojeda & Urbanski, 1994; Cheung et al. 1997; Parent et al. 2003). Yet, the molecular determinants for the timing of normal puberty and its alterations (i.e. precocious, delayed or absent puberty onset) remain largely unknown (Parent et al. 2003).
Among the signals potentially involved in the control of the gonadotrophic axis, an unexpected reproductive role for the KiSS-1/GPR54 system has recently emerged (de Roux et al. 2003; Seminara et al. 2003). GPR54 was initially cloned in the rat as an orphan G protein-coupled receptor with 45% sequence similarity to galanin receptors, its human orthologue being termed AXOR12 or hOT7T175 (Kotani et al. 2001; Muir et al. 2001; Ohtaki et al. 2001). The endogenous ligand of this receptor was identified as a 54-amino-acid secreted peptide, derived from the proteolytic processing of the product of the metastasis suppressor gene KiSS-1, and termed metastin (Kotani et al. 2001; Muir et al. 2001). A number of additional KiSS-1 derived peptides, structurally related to metastin and globally termed kisspeptins, were identified thereafter (Kotani et al. 2001). Expression of KiSS-1 and GPR54 has been described in a variety of tissues, including placenta, different brain areas and pituitary (Ohtaki et al. 2001; Muir et al. 2001). Yet, the physiological role of this system remains to be fully established. Nevertheless, it has been demonstrated that metastin has potent antimetastasis activity in several tumours (Ohtaki et al. 2001), it is involved in the control of trophoblast invasion (Bilban et al. 2004), and it may participate in the regulation of specific neuroendocrine systems (e.g. oxytocin) (Kotani et al. 2001).
In this context, two independent reports recently provided conclusive evidence demonstrating that a number of point mutations and deletions of the GPR54 gene, which are transmitted with an autosomal recessive trait, are found in patients suffering familiar forms of idiopathic hypogonadotrophic hypogonadism (de Roux et al. 2003; Seminara et al. 2003). Such a clinical syndrome was reproduced in mouse models carrying null mutations of the GPR54 gene (Seminara et al. 2003; Funes et al. 2003). On this basis, the KiSS-1/GPR54 system was proposed to play a previously unexpected role in the regulation of the development and/or function of the hypothalamic–pituitary–gonadal axis. Accordingly, three simultaneous studies have recently reported the ability of KiSS-1 peptide to markedly elicit LH secretion in vivo (Gottsch et al. 2004; Matsui et al. 2004; Navarro et al. 2004). However, the mechanism(s) whereby KiSS-1 carries out its modulatory action upon the reproductive axis and whether it is actually involved in normal pubertal development remain to be fully characterized. This limited availability of functional data has so far hampered our complete understanding of the physiological function of the KiSS-1/GPR54 system in reproduction. In this context, the present experimental work aimed at characterizing the putative role of this system in the central activation of the gonadotrophic axis at puberty. To this end, functional in vivo studies, involving chronic administration of KiSS-1 peptide and analysis of the acute interaction between KiSS-1, LHRH and leptin, were conducted in immature female rats. Overall, our results are the first to provide conclusive evidence for a role of the KiSS-1 system as a relevant downstream element in the central network triggering puberty onset.
Methods
Animals and drugs
Wistar female rats bred in the vivarium of the University of Córdoba were used, unless otherwise stated. The day the litters were born was considered as day 1 of age. The animals were maintained under constant conditions of light (14 h of light, from 07.00 h) and temperature (22°C), and were weaned at day 21 of age in groups of five rats per cage with free access to pelleted food and tap water. Experimental procedures, including surgical manipulations for cannulae implantation, were approved by the Córdoba University Ethical Committee for animal experimentation and were conducted in accordance with the European Union guidelines for care and use of experimental animals. The animals were humanely killed by decapitation at the end of the experiments. Mouse KiSS-1 (110–119)-NH2, the rodent analogue of the C-terminal KiSS-1 decapeptide KiSS-1 (112–121)-NH2, was obtained from Phoenix Pharmaceuticals Ltd (Belmont, CA, USA), and the potent LHRH antagonist Org 30276 (Ac-d-pClPhe-d-pClPhe-d-Trp-Ser-Tyr-d-Arg-Leu-Arg-Pro-d-Ala-NH2CH3COOH) was generously supplied by Organon (Oss, Netherlands).
Experimental design
In Experiment 1, the effects of chronic central administration of KiSS-1 peptide on puberty onset in immature female rats were monitored. Daily intracerebroventricular (i.c.v.) administration of KiSS-1 peptide in the lateral cerebral ventricle was conducted between days 26 and 31 postpartum in females (n = 20), as described in detail elsewhere (Pinilla et al. 2000). Briefly, animals were implanted on day 25 postpartum with i.c.v. cannulae under light ether anaesthesia. To allow delivery of KiSS-1 peptide into the lateral cerebral ventricle, the cannulae were lowered to a depth of 3 mm beneath the surface of the skull; the insert point was 1 mm posterior and 1.2 mm lateral to bregma. The treatment regimen was set at 1 nmol KiSS-1 per animal in 10 μl, every 12 h. This dose was selected on the basis of our initial data on the ability of 1 nmol KiSS-1 to potently elicit LH secretion (Navarro et al. 2004), and previous references testing the neuroendocrine actions of different centrally administered peptides (Furuta et al. 2001; Tena-Sempere et al. 2004). Pair-aged females (n = 15) injected with vehicle (NaCl 0.9%) served as controls. The animals were i.c.v. injected under conscious conditions after careful handling to avoid any stressful influence, in keeping with our previous references (Pinilla et al. 2000). Daily inspection of the cannulae was conducted in each animal in order to exclude those showing obvious displacement or de-attachment. In addition, correct positioning of the cannulae was confirmed at autopsy. In all experimental animals, body weight and vaginal opening were monitored daily. On the latter, detailed inspection was conducted in each animal to determine the date of complete canalization of the vagina. At the end of treatment (31 days postpartum), all the animals within each group were killed by decapitation, 60 min after the last injection of KiSS-1. Upon decapitation, trunk blood was collected, and the uterus was dissected out of the surrounding fat and its weight recorded after brief drying of the periphery with cellulose paper. For determination of the normal date of vaginal opening in our colony, an additional group of females injected with vehicle (n = 15) were maintained on daily inspection of canalization of vagina up to day 39 postpartum.
As results from Experiment 1 evidenced a potent stimulatory action of KiSS-1 upon the gonadotrophic axis at puberty, the next experiments aimed at addressing the potential interplay between KiSS-1, the hypothalamic LHRH and the adipocyte-derive hormone leptin in the control of LH secretion. In Experiment 2, immature (31-day-old) female rats (n = 10–12/group) were twice subcutaneously injected with a potent LHRH antagonist (5 mg kg−1·24 h) in order to completely block endogenous LHRH actions, as previously reported (Tena-Sempere et al. 1995). Vehicle-injected groups served as controls. Twenty-four hours after the last dose of the antagonist, the animals were i.c.v. injected with a single dose of 1 nmol KiSS-1 or vehicle into the lateral cerebral ventricle, and trunk blood samples were collected 15 min later. Procedures for implantation of i.c.v. cannulae and peptide administration were similar to those described above.
In addition, in Experiment 3, the ability of KiSS-1 to elicit LH secretion after severe food restriction was monitored. Immature (31-day-old) female rats (n = 10–12 per group) were subjected to food deprivation for 48 h, and central i.c.v. administration of a single dose of 1 nmol KiSS-1 or vehicle was conducted as described above. Trunk blood samples were collected upon decapitation at 15 min and 60 min after KiSS-1 injection.
In Experiment 4, the effects of immunoneutralization of endogenous leptin on the ability of KiSS-1 peptide to stimulate LH secretion were assessed. To this end, immature (28-day-old) female rats (n = 10–15/group) were daily i.c.v. injected for 6 days either with a specific leptin antiserum (Considine et al. 1996) or normal rabbit serum. Twenty-four hours after the last injection of the antibody, the animals were i.c.v. injected with a single dose of 1 nmol KiSS-1 or vehicle into the lateral cerebral ventricle, and trunk blood samples were collected 15 min later.
Finally, in Experiment 5, the potential interaction between KiSS-1 and leptin in the control of LH secretion at puberty was further monitored using a model of leptin resistance, i.e. the obese Zucker rat. Five-week-old Zucker (fa/fa) female rats and their corresponding (fa?/+) lean controls were purchased from Charles River (Barcelona, Spain). Upon acclimatization of the animals, central i.c.v. injection of a single dose of 1 nmol KiSS-1 or vehicle, and collection of trunk blood samples were conducted as described above in control and obese, leptin-resistant animals (n = 10 per group), at 15 min after administration of KiSS-1.
Hormone measurement by specific RIAs
Serum LH levels were measured in a volume of 25 μl using a double-antibody method and radioimmunoassay (RIA) kits kindly supplied by the NIH (Dr A. F. Parlow, NIDDK National Hormone and Peptide Program, Bethesda, MD, USA). Rat LH-I-9 was labelled with 125I by the chloramine-T method and the hormone concentrations were expressed using the reference preparation LH-RP-3 as standards. Intra- and interassay coefficients of variation were below 8 and 10%, respectively. The sensitivity of the assay was 5 pg per tube. In addition, in selected serum samples (Experiment 1), serum oestradiol levels were determined using a commercial kit from MP Biomedicals (Costa Mesa, CA, USA), following the instructions of the manufacturer. The sensitivity of the assay was 0.5 pg per tube and the intra-assay coefficient of variation was below 5%.
Presentation of data and statistics
Serum LH and oestradiol determinations were conducted in duplicate, with a minimum total number of 10 samples per group. Hormonal data are presented as means ± s.e.m. Results were analysed for statistically significant differences using Student's t test or ANOVA followed by a Student-Newman-Keuls multiple range test (SigmaStat 2.0, Jandel Corp., San Rafael, CA, USA). P ≤ 0.05 was considered significant.
Results
Central KiSS-1 administration and puberty onset in immature female rats
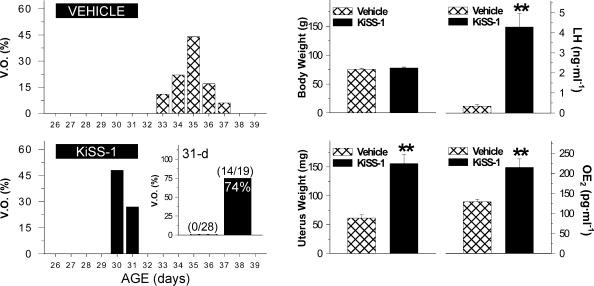
The effects of KiSS-1 upon the precocious activation of the reproductive axis were monitored in immature female rats, after chronic i.c.v. administration of the peptide between 26 and 31 days postpartum. Note, a model of repeated intracerebral (lateral ventricle) injection of KiSS-1 was selected in order to target specific central effects of the peptide. This setting did not allow us to extend the treatment protocol beyond 31 days postpartum (i.e. 11 injections of KiSS-1 over 6 days), in order to avoid misadministration of KiSS-1 peptide due to displacement of the cannulae which takes place (approximately) after 7 days following their surgical implantation. As an external index of puberty onset, vaginal opening (defined as complete canalization of the vagina) was monitored daily. Such a marker was selected as it has been conventionally considered a reliable external sign of puberty (for example see Chehab et al. 1997; Pinilla et al. 2000), which allows recapitulation of proper functional activation of all the levels of the reproductive axis. Control reference females showed complete vaginal opening at a mean age of 34.8 ± 0.35 days postpartum, with a mean body weight of 100.3 ± 2.3 g. By 31 days postpartum, none of the vehicle-injected females (mean body weight: 75.1 ± 1.8 g) presented vaginal opening. In contrast, chronic i.c.v. administration of KiSS-1 (1 nmol/12 h) between 26 and 31 days postpartum induced complete vaginal opening in 14 out of 19 treated females (74%), with a mean body weight of 77.8 ± 1.4 g (Fig. 1). As additional indices of pubertal maturation and activation of the reproductive axis, uterus weight, and serum LH and oestradiol levels were monitored in vehicle- and KiSS-1-treated groups at 31 days postpartum, 60 min after the last injection. As shown in Fig. 1, central i.c.v. administration of KiSS-1 elicited significant increases in uterus weight (∼3-fold), serum LH levels (∼10-fold), and serum oestradiol levels (∼2-fold) over control values.
Figure 1. Compilation of indices of pubertal maturation recorded in immature female rats chronically i.c.v. injected with 1 nmol KiSS-1 peptide or vehicle.
Dates of vaginal opening (expressed as a percentage of total number of animals per experimental group) are shown in the left panels. Administration of 1 nmol KiSS-1 every 12 h between 26 and 31 days postpartum induced vaginal opening in ∼75% of females at the age of 31 days, an age-point when none of the animals injected with vehicle presented canalization of the vagina (inset). Data on body and uterus weights, as well as serum LH and oestradiol (OE2) levels, in vehicle- and KiSS-1-injected animals are shown in the right panels. Values are the mean ±s.e.m. of at least 15 independent determinations per group. **P < 0.01 versus vehicle-injected group (Student's unpaired t test).
Interaction between KiSS-1, LHRH and leptin in the central control of LH secretion at puberty
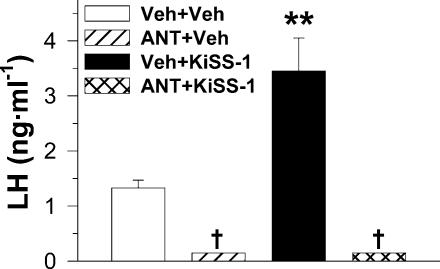
As results from the initial experiments involving its repeated administration provided evidence that KiSS-1 is a potent activator of the gonadotrophic axis at puberty, in the next series of experiments we aimed at evaluating the potential interaction of this novel factor with two key elements in the pubertal activation of the reproductive system: LHRH and leptin. In order to specifically target central (hypothalamic) events, a protocol of acute i.c.v. administration of KiSS-1 was selected, and serum LH levels were used as an index to monitor activation of the gonadotrophic axis. LHRH dependency for the stimulatory actions of KiSS-1 was first assessed in a model of blockade of the actions of endogenous LHRH, by means of administration of the potent synthetic antagonist, ORG 30276. Treatment with the LHRH antagonist resulted in a significant drop in serum LH levels to nearly undetectable values, in line with previous reports (Tena-Sempere et al. 1995). In this setting, central acute administration of 1 nmol KiSS-1 failed to induce a significant increase in serum LH levels in LHRH antagonist-treated animals, despite a potent LH releasing activity of KiSS-1 being confirmed in paired vehicle-injected animals (Fig. 2).
Figure 2. Effects of the blockade of endogenous LHRH actions upon the ability of KiSS-1 to acutely stimulate LH secretion.
Immature female rats were subcutaneously treated with a potent LHRH antagonist, ORG 30276 (ANT; 5 mg kg−1 24 h, 2 doses) or vehicle (Veh), and were subsequently i.c.v. injected with vehicle or 1 nmol KiSS-1. Hormonal values are the mean ±s.e.m. of at least 10 independent determinations per group. Groups with different symbols (* and †) are significantly different (P < 0.01, ANOVA followed by Student-Newman-Keuls multiple range test).
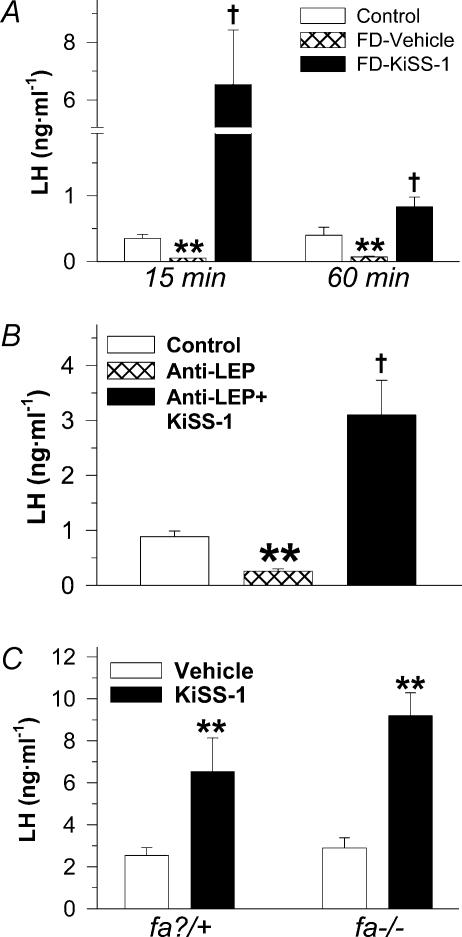
In addition, the acute interaction between KiSS-1 and leptin in the control of LH secretion at puberty was monitored in three different experimental settings. Firstly, the ability of central KiSS-1 to elicit LH secretion was evaluated in immature females subjected to 48 h fasting, a regimen that was able to reduce body weight by 12.5% (78.1 ± 2.4 g in fasting animals versus 88.5 ± 2.5 g in control animals fed ad libitum), and to significantly lower serum LH levels. In such fasting conditions, acute i.c.v. administration of 1 nmol KiSS-1 potently stimulated LH secretion, with serum levels significantly elevated from controls at 15 and 60 min after central KiSS-1 injection (Fig. 3A). In addition, the acute effect of central administration of 1 nmol KiSS-1 on serum LH levels was evaluated after immunoneutralization of endogenous leptin in immature rats, by means of i.c.v. injection of a specific antileptin antibody in a regimen able to suppress LH secretion and to block oestrous cyclicity in adult female rats (Carro et al. 1997). In this setting, serum LH levels were significantly lowered by chronic central administration of antileptin antibody. However, acute central KiSS-1 injection elicited a significant increase in serum LH levels in animals treated with the blocking antileptin antibody (Fig. 3B). Finally, the ability of KiSS-1 to acutely elevate serum LH levels was studied in a rat model of leptin resistance, i.e. the obese Zucker rat, and its corresponding lean controls. Immature (5-week-old) Zucker rats were used (body weight: 132.5 ± 1.73 g versus 100.5 ± 1.5 g in lean controls). In keeping with observations in immature Wistar rats (see Figs 1 and 2), KiSS-1 administration to lean (fa?/+) rats elicited a significant rise in serum LH levels. Similarly, KiSS-1 carried out a potent acute LH releasing effect in leptin-resistant Zucker rats (Fig. 3C).
Figure 3. Effects of acute central administration of KiSS-1 peptide upon LH secretion in different models of leptin insufficiency.
In A, immature female rats were subjected to fasting (food-restriction: FD) for 48 h. The animals were subsequently i.c.v. injected with vehicle or 1 nmol KiSS-1 and serum LH levels determined at 15 and 60 min after injection. For comparative purposes, serum LH levels in control pair-aged female rats fed ad libitum are also shown. Hormonal values are the mean ±s.e.m. of at least 10 independent determinations. **P < 0.01 versus corresponding control fed group; †P < 0.01 versus fasting animals i.c.v. injected with vehicle. In B, immunoneutralization of endogenous leptin was conducted in immature female rats by means of central administration of a specific antileptin antibody (Anti-LEP). Thereafter, anti-LEP-treated female rats were i.c.v. injected with vehicle or 1 nmol KiSS-1 and serum LH levels determined at 15 min after injection. For comparative purposes, serum LH levels in control pair-aged female rats i.c.v. injected with normal rabbit serum are also shown. Hormonal values are the mean ±s.e.m. of at least 10 independent determinations. **P < 0.01 versus corresponding control group; †P < 0.01 versus anti-LEP treated animals. In C, i.c.v. injection of vehicle or 1 nmol KiSS-1 was carried out in leptin-resistant Zucker (fa–/–) rats and their corresponding lean (fa?/+) controls, and serum LH levels were determined at 15-min after injection. Hormonal values are the mean ±s.e.m. of at least 10 independent determinations. **P < 0.01 versus corresponding vehicle-injected group (ANOVA followed by Student-Newman-Keuls multiple range test).
Discussion
Maturation of the gonadotrophic axis at puberty is driven by a complex cascade of regulatory circuits that ultimately leads to maximal activation of the pulsatile release of LHRH at the hypothalamus. The transition from the quiescent prepubertal stage is believed to derive from the concerted decrease in the activity of inhibitory networks and the increased activity of excitatory pathways (Ojeda & Urbanski, 1994; Terasawa & Fernandez, 2001). Over the last years, the nature of these signals has been partially elucidated, for both excitatory (e.g. glutamate, neuropeptide Y (NPY), noradrenaline) and inhibitory (e.g. gamma amino butyric acid (GABA), endogenous opioids) factors (Ojeda & Urbanski, 1994; Terasawa & Fernandez, 2001). However, it is likely that additional, as yet unknown, central neurotransmitters may participate in the pubertal activation of the LHRH neuronal system. Our current data provide conclusive, functional evidence for the ability of KiSS-1 peptide to potently activate the reproductive axis in immature females. Thus, chronic central administration to females at the juvenile period elicited a clear-cut advancement of the age of vaginal opening (as an external index of pubertal maturation), together with a significant increase in uterus weight and serum levels of LH and oestrogen. Although the ultimate occurrence of ovulation as definitive proof of complete puberty was not monitored in our experimental setting, the different nature of the end-points assayed (pituitary LH secretion, ovarian-derived serum oestrogen, and its biomarkers of action: uterus weight and vaginal opening) strongly support the hypothesis that central KiSS-1 is sufficient to trigger puberty onset. Interestingly, recent expression analyses in the hypothalamus conducted at our laboratory provided evidence that maximum expression of KiSS-1 and GPR54 genes is observed at puberty, both in male and female rats (Navarro et al. 2004). Thus, in the context of the recent evidence of hypogonadotrophic hypogonadism associated with null mutations of the GPR54 gene in rodents and humans (Funes et al. 2003; de Roux et al. 2003; Seminara et al. 2003), our data on the developmental profile of expression of KiSS-1 and its putative receptor at the hypothalamus, and the effects of central administration of KiSS-1 upon several parameters of pubertal maturation, strongly indicate that the hypothalamic KiSS-1/GPR54 system is likely to play a major role in the activation of the gonadotrophic axis at puberty.
As indicated above, the hypothalamic decapeptide LHRH plays an undisputed central role in the onset of puberty (Ojeda & Urbanski, 1994; Terasawa & Fernandez, 2001). To gain knowledge on the mechanism whereby KiSS-1 is able to potently activate the reproductive axis at puberty, an indirect approach, based in the transient blockade of LHRH actions by means of a potent synthetic antagonist, was used. Our results demonstrate that, in the absence of endogenous LHRH actions, the ability of KiSS-1 peptide to centrally elicit LH secretion is totally abrogated; data which are in agreement with recent reports in the adult male rat (Gottsch et al. 2004; Matsui et al. 2004). Although i.c.v. injection into the lateral ventricle does not exclude the possibility of additional actions at brain areas outside the hypothalamus, as a whole these results strongly indicate that the effects of KiSS-1 on LH secretion are mainly conducted through modulation of the hypothalamic LHRH system. In this sense, it is noticeable that GPR54 gene expression has been very recently demonstrated in LHRH neurones from the non-mammalian species tilapia (Oreochromis niloticus; cichlid fish) (Parhar et al. 2004). Similarly, our preliminary data evidence that the GPR54 gene is also expressed in the murine LHRH neuronal cell line GT1-7 (P. Magni & M. Tena-Sempere, unpublished observations). Taken together, the available information makes it tempting to propose that KiSS-1 peptide, acting through its receptor GPR54, is able to directly elicit LHRH secretion by hypothalamic LHRH neurones. It is noteworthy that, despite low LHRH release before puberty, LHRH neurones of immature (prepubertal) animals are intrinsically able to increase their secretory activity upon certain experimental manipulations, such as electrical stimulation or administration of NMDA, an agonist of ionotropic excitatory amino acid receptors (Ojeda & Urbanski, 1994). Thus, it is possible that the precocious activation of the reproductive axis by chronic central administration of KiSS-1 reported herein may derive from a direct stimulatory action upon LHRH neurones. The precise mechanisms whereby this effect is eventually conducted are at present under investigation in our laboratory.
It is well known that pubertal development is critically dependent on sufficient energy stores (Kennedy & Mitra, 1963). This phenomenon seems to be mediated by the adipocyte hormone leptin, which signals the amount of adiposity to the brain centres governing reproductive function, thus playing a permissive role in pubertal development in mammals (Cheung et al. 1997; Casanueva & Dieguez, 1999). The mechanism(s) by which such a role is carried out is still matter of debate, and direct and indirect effects of leptin upon LHRH neurones have been indicated (Casanueva & Dieguez, 1999; Tena-Sempere & Barreiro, 2002). Considering the proposed function of KiSS-1 in puberty onset (de Roux et al. 2003; Seminara et al. 2003; and the present results), we aimed at evaluating the potential interaction between leptin (as peripheral permissive signal) and KiSS-1 (as central triggering signal) in the acute regulation of the gonadotrophic axis at puberty. To this end, three different animal settings were used: (a) a model of food deprivation, which is known to lower circulating leptin levels (Casanueva & Dieguez, 1999), and was able to significantly reduce body weight and serum LH levels; (b) a model of immunoneutralization of endogenous leptin, which was previously reported to suppress LH secretion and to block oestrous cyclicity in adult female rats (Carro et al. 1997); and (c) a model of genetically induced leptin resistance. On the latter, fatty Zucker (fa/fa) rats were used, as they bear a mutation in the leptin receptor that renders them insensitive to the actions of endogenous leptin. This results in a clear-cut metabolic phenotype together with multiple reproductive abnormalities, including delayed pubertal maturation and defective pulsatile LH release (Todd et al. 2003). In all models of leptin insufficiency, acute KiSS-1 administration was able to potently induce LH secretory responses in immature female rats. This observation suggests that KiSS-1 is placed at a step distal to (or independent of) leptin actions within the central circuitry governing LHRH release, and that the stimulatory effect of KiSS-1 does not require the permissive presence of peripheral leptin. These observations reinforce the contention that KiSS-1 acts as a trigger, rather than a permissive factor, for puberty onset. An intriguing possibility, at present under evaluation in our laboratory, is that leptin might regulate the secretory activity of LHRH neurones by modulating the central expression of KiSS-1. An analogous model has been recently proposed for the interaction among leptin, galanin-like peptide and LHRH (Seth et al. 2004). In addition, considering that leptin insufficiency is associated with delayed or absent puberty onset (Casanueva & Dieguez, 1999), it will be relevant to evaluate whether repeated administration of KiSS-1 is able to prevent such a phenotype in the above experimental settings.
In summary, we have shown here that chronic central administration of KiSS-1 peptide significantly advances the activation of the reproductive axis at puberty in immature female rats, as estimated by the date of vaginal opening and conventional indices of maturation of the gonadotrophic system (elevated uterus weight and increased serum levels of LH and oestrogen). The central effect of KiSS-1 upon LH release appeared to be mediated via the hypothalamic LHRH, and did not require the permissive presence of leptin. Overall, it is proposed that the KiSS-1/GPR54 system is a novel, essential downstream element in the central network triggering the onset of puberty.
Acknowledgments
Radioimmunoassay kits for hormone determinations were kindly supplied by Dr A. F. Parlow, NIDDK National Hormone and Peptide Program, Bethesda, MD, USA. The authors are indebted with R. Nogueiras and S. Tovar at the University of Santiago de Compostela (Spain) for their outstanding assistance during conduction of some of the experimental studies. This work was supported by grants BFI 2000-0419-CO3-03 and BFI 2002-00176 from DGESIC (Ministerio de Ciencia y Tecnología, Spain), and EU research contract EDEN QLK4-CT-2002-00603.
References
- Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–1328. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- Carro E, Pinilla L, Seoane LM, Considine RV, Aguilar E, Casanueva FF, et al. Influence of endogenous leptin tone on the estrous cycle and luteinzing hormone pulsatility in female rats. Neuroendocrinology. 1997;66:375–377. doi: 10.1159/000127262. [DOI] [PubMed] [Google Scholar]
- Casanueva FF, Dieguez C. Neuroendocrine regulation and actions of leptin. Front Neuroendocrinol. 1999;20:317–363. doi: 10.1006/frne.1999.0187. [DOI] [PubMed] [Google Scholar]
- Chehab FF, Mounzih K, Lu R, Lim ME. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes S, Hendrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun. 2001;288:780–785. doi: 10.1006/bbrc.2001.5854. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol. 1963;166:408–418. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor GPR54 in rat hypothalamus and potent LH releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. New York: Raven Press; 1994. pp. 363–410. [Google Scholar]
- Parent A-S, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Parhar IS, Ogawa S, Sakuma Y. Laser captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (GPR54) during maturation in cichlid fish. Endocrinology. 2004;145:3615–3618. doi: 10.1210/en.2004-0395. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Gonzalez L, Tena-Sempere M, Aguilar E. Activation of AMPA receptors inhibits prolactin and estradiol secretion and delays the onset of puberty in female rats. J Steroid Biochem Mol Biol. 2000;75:277–281. doi: 10.1016/s0960-0760(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. New Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Seth A, Stanley S, Jethwa P, Gardiner J, Ghatei M, Bloom S. Galanin-like peptide stimulates the release of gonadotropin-releasing hormone in vitro and may mediate the effects of leptin on the hypothalamo-pituitary-gonadal axis. Endocrinology. 2004;145:743–750. doi: 10.1210/en.2003-0873. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Aguilar E, Fernandez-Fernandez R, Pinilla L. Ghrelin inhibits prolactin secretion in prepubertal rats. Neuroendocrinology. 2004;79:133–141. doi: 10.1159/000077271. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Barreiro ML. Leptin in male reproduction: the testis paradigm. Mol Cell Endocrinol. 2002;188:9–13. doi: 10.1016/s0303-7207(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Pinilla L, Aguilar E. Orchidectomy selectively increases follicle-stimulating hormone secretion in gonadotropin-releasing hormone antagonist-treated male rats. Eur J Endocrinol. 1995;132:357–362. doi: 10.1530/eje.0.1320357. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Ladyman SR, Grattan DR. Suppression of pulsatile luteinizing hormone secretion but not luteinizing hormone surge in leptin resistant obese Zucker rats. J Neuroendocrinol. 2003;15:61–68. doi: 10.1046/j.1365-2826.2003.00871.x. [DOI] [PubMed] [Google Scholar]