Abstract
Ca2+ transfer across the syncytiotrophoblast (ST) of the human placenta is essential for normal fetal development. However, the nature of Ca2+ conductance in the ST and the mechanisms by which it is regulated are poorly understood. With the major signal transduction pathway of endothelin-1 (ET1) acting via phospholipase C (PLC) and Ca2+, we used ET1 to analyse the nature of Ca2+ channels on cultured trophoblastic cells by means of cytofluorimetric analysis using the ratiometric Ca2+ indicator Indo-1. Results indicate that ET1 (10−7 m) stimulates a biphasic (transient and sustained) increase in [Ca2+]i in trophoblastic cells. This response is mediated by the endothelin receptor B (ETB) coupled to PLC, since treatment with BQ788 (10−6 m) or U73122 (2 μm) totally abolished the response. Persistence of the rapid transient rise in [Ca2+]i in Ca2+-free extracellular medium confirms the release of Ca2+ from intracellular stores in response to ET1 stimulation. Furthermore, abolition of the sustained increase in [Ca2+]i in Ca2+-free extracellular medium argues in favour of the entry of Ca2+ during the plateau phase. Abolition of this plateau phase by Ni2+ (1 mm) in the presence of extracellular Ca2+ confirmed the existence of an ET1-induced Ca2+ entry. No evidence for the presence of voltage-operated channels was demonstrated during ET1 action since nifedipine (10−6 m) did not reduce the Ca2+ response and depolarization with a hyper-potassium solution had no effect. Pharmacological studies using the imidazole derivatives SK&F96365 (30 μm) and LOE 908 (10 μm) partially inhibited the ET1-evoked Ca2+ response, thus providing evidence for the presence of both store-operated Ca2+ channels and non-selective cationic channels in the human ST.
In the human placenta, the chorionic villi, which are in direct contact with the maternal blood, consist of trophoblasts surrounding a core of connective tissue that includes the fetal vessels, fibroblasts and Hofbauer cells. The villous trophoblast differentiates from the fusion of cytotrophoblastic (CT) cells into a multinucleated true syncytium, the syncytiotrophoblast (ST). The ST is a transporting epithelium engaged in numerous placental functions required for fetal growth and development (different forms of exchange, metabolism and production of biologically active substances).
Ca2+ ion transfer across the ST is essential for normal fetal development. Its transport takes place in an active manner against a concentration gradient, as the Ca2+ concentration in the fetal circulation is considerably higher than that of the mother. This active transfer is carried out by the ST, for which the first step is that of membrane-gated Ca2+ entry. It has been suggested that the influx of Ca2+ across the ST microvillous membrane is performed by a facilitated diffusion process (Kamath et al. 1992). Recently, the presence of Ca2+ transporter types 1 and 2 was demonstrated in cultured trophoblastic cells (Moreau et al. 2002a). Furthermore, Ca2+ is required in multiple cellular functions that include secretion, ionic conductance, cell-cycle regulation and programmed cell death (Berridge et al. 2000). However, the nature of Ca2+ conductance in the ST, and the mechanisms by which it is regulated, are poorly understood. Previous studies have demonstrated an increase in intracellular Ca2+ in the ST following exposure to various biologically active substances acting in both autocrine and paracrine fashions: GnRH (Currie et al. 1993), ATP and UTP (Petit & Belisle, 1995), ATP and angiotensin II (Karl et al. 1997), endothelin (Cronier et al. 1999), ATP (Clarson et al. 2002). However, the nature of Ca2+ channel activation in the ST remains controversial, with voltage-operated Ca2+ channels (VOCC; Meuris et al. 1994; Petit & Belisle, 1995; Robidoux et al. 2000), non-selective cationic channels (NSCC; Grosman and Reisin, 2000; Llanos et al. 2002; Long & Clarson, 2002), store-operated Ca2+ channels (SOCCs; Clarson et al. 2003) and receptor-operated Ca2+ channels (ROCCs; Bax et al. 1994) all having been described.
We previously demonstrated the presence of endothelin (ET) receptors A and B on the human trophoblastic membrane (Malassiné et al. 1993b). With the major signal transduction pathway of endothelin-1 (ET1) acting via phospholipase C (PLC) and the mobilization of intracellular Ca2+, we have thus used cytofluorimetry and pharmacological analysis to determine the nature of ET1-mediated Ca2+ entry into ST cells in primary culture.
Methods
Materials
ET1, trypsin, DNase I, NiCl2, Indo-1 AM and nifedipine were purchased from Sigma (St Louis, MO, USA), BQ123 and BQ788 were from Neosystem (Strasbourg, France), monoclonal anti-human leucocytic antigen-A, B and C antibody (W6-32HL) was from Sera Laboratory (Crawley Down, UK) and SK&F96365 was from Calbiochem (VWR International, Fontenay-sous-bois, France). LOE 908 was kindly provided by Boehringer Ingelheim (Ingelheim, Germany). All other reagents were from standard suppliers.
Trophoblastic cell culture
Human placentas were obtained after caesarean section from mothers with uncomplicated pregnancies. The use of human placentas for this study was approved by the ethics committee of the Clinique du Fief de Grimoire (Poitiers, France). CT cells were isolated using a previously described method (Malassiné et al. 1993a), which was adapted from that of Kliman et al. (1986). Briefly, after several sequential trypsin/DNase digestions followed by Percoll gradient centrifugation, cells were further purified by means of W6-32HL. CT cells were diluted to a final concentration of 0.5 × 106 ml−1 in minimum essential medium (MEM) containing 10% fetal calf serum (FCS), 25 mm glucose and 50 μg ml−1 gentamicin. Cells were plated onto glass coverslips in 35 mm plastic dishes (Nunclon, Nunc, Roskilde, Denmark) and incubated for 2 days at 37 °C in 5% CO2. The culture medium was renewed daily. Cytokeratin 07 immunocytochemistry (clone OV.TL12/30, Dako, Denmark) was performed to confirm the cytotrophoblastic nature of the attached cells. After the purification procedure, 95% of the cells stained positively for cytokeratin.
Recording of [Ca2+]i transients
Intracellular free Ca2+ concentrations ([Ca2+]i) were measured by means of the ratiometric method with an inverted epifluorescence microscope (Olympus IX 70). Briefly, the Ca2+ indicator Indo-1 was used, for which fluorescence emissions of the Ca2+-free (485 nm) and Ca2+-bound (405 nm) forms of the indicator were collected using a dichroic filter and two photomultiplier tubes (excitation wavelength 355 nm). The Ca2+ activity was estimated as the ratio of the 405/485 nm fluorescence emission intensities. For loading of the probe, trophoblastic cells were incubated for 45 min in the dark in Tyrode solution (mm: 144 NaCl, 5.4 KCl, 2.5 CaCl2, 1 MgCl2, 0.3 NaH2PO4, 5 Hepes, 5.6 glucose, pH 7.4) containing the lipophilic form of the dye (Indo-1 AM dissolved in DMSO 0.3%) at a concentration of 3 μm. After carefully washing off the unincorporated fluorogenic dye, cells were incubated in Tyrode solution for a further 15 min in the dark to obtain complete de-esterification of the dye. STs with between six and eight nuclei accumulated in a central nuclei mount were identified with the aid of the inverted epifluorescence microscope. Variations of [Ca2+]i with time were measured in a defined area located approximately in the centre of the trophoblastic cells. By means of a home-made gravity-based microperfusion system, test solutions were applied rapidly onto the ST under investigation by using a streamline flow directed from the opening of a stainless steel capillary tube (internal diameter 50 μm) positioned in the bath. Switching between different solutions was performed with electrovalves controlling different juxtaposed capillaries. Treatments were performed by perfusion of Tyrode solution containing ET1 or pharmacological agents. All experiments were conducted at room temperature (20 ± 1 °C).
Ratio analyses and statistics
Indo-1 signals were not calibrated in terms of absolute values since this was not necessary for the monitoring of variations of Ca2+ levels. Intracellular Ca2+ concentration changes were expressed as changes in the ratio of the 405/485 nm fluorescence emissions of the dye. Reported data represent the mean ± s.e.m. of the percentage difference in ratio between the basal level and peak or plateau levels, with n being the number of STs tested. One-way analysis of variance followed by a Dunnett's post hoc test was used to compare peak ratio values, while paired Student's t tests were used for statistical comparisons of baseline and plateau Ca2+ levels for different treatments.
Results
ST formation
When purified CT cells are cultured in the presence of 10% FCS, after adhesion and flattening, cells make initial contacts by pseudopodia with neighbouring cells, transform into cellular aggregates and fuse to form STs. This differentiation process was monitored by the immunostaining of cells for desmoplakin and βhCG secretion as previously described (Cronier et al. 1994, 2003; Frendo et al. 2003). Under the experimental conditions used here, a large proportion of mononuclear cells had differentiated into ST after 48 h of culture. Only STs with between six and eight nuclei amassed in a central mount were selected for further study.
Effect of ET1 on [Ca2+]i in STs
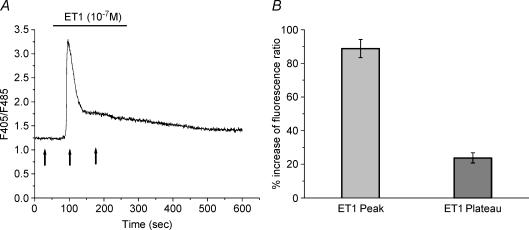
Previous studies have demonstrated that, in our experimental conditions, ET1 (100 nm) is effective in inducing a Ca2+ response in around 75% of investigated cells (Cronier et al. 1999). As shown in Fig. 1, a stable resting [Ca2+]i level is followed by a rapid increase in fluorescence ratio upon the addition of 100 nm ET1. This was followed by a sustained prolongation of [Ca2+]i in 41% of ET1-responding cells. The effect of ET3 on Ca2+ activity presented an identical profile (data not shown).
Figure 1. Effect of endothelin-1 (ET1) on [Ca2+]i in cultured syncytiotrophoblastic cells.
A, Ca2+ change in response to exposure of a syncytiotrophoblast to ET1 (10−7 m). After a rapid rise to peak of the fluorescence ratio, a decrease in [Ca2+] to a level higher than that of the basal level was observed (plateau). Arrows indicate the time of measurement for calculation of the percentage change in ratio expressed in B. B, averaged data for the percentage increase in fluorescence ratio (F405/F485). Data are means ± s.e.m.; peak n = 49; plateau n = 20.
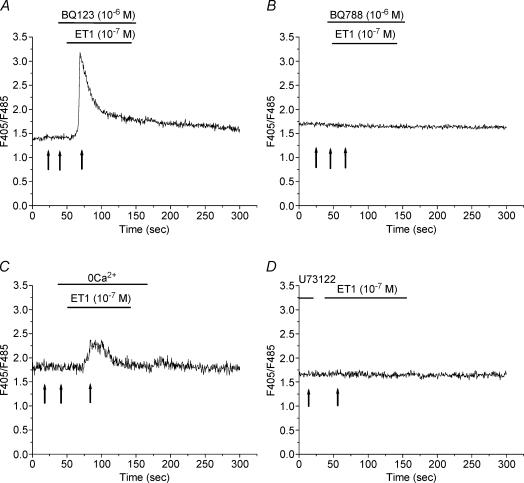
The nature of ET receptor(s) implicated in the ET1-induced change in [Ca2+]i was subsequently investigated using specific antagonists. As shown in Fig. 2A and also Fig. 4, prior and concomitant perfusion of cells with BQ123 (10−6 m), a specific ETA receptor antagonist, did not change the Ca2+ response to ET1 (n = 24), while the presence of BQ788 (10−6 m; Figs 2B and 4), a specific ETB receptor antagonist, completely inhibited the ET1-stimulated increase in Ca2+ (n = 13).
Figure 2. Analysis of ET1-induced Ca2+ response.
A and B, the effects of BQ123 (A; 10−6 m) and BQ788 (B; 10−6 m) on the [Ca2+]i increase induced by ET1 (10−7 m). C, recording of the Ca2+ response to ET1 (10−7 m) for a syncytiotrophoblast (ST) bathed in Ca2+-free extracellular medium. D, Ca2+ response to ET1 (10−7 m) after pre-incubation of the ST for 15 min in the presence of the phospholipase C (PLC) inhibitor, U73122 (2 μm). Arrows in A–D indicate the time of measurement used for the calculation of the percentage changes in ratios expressed in Fig. 4.
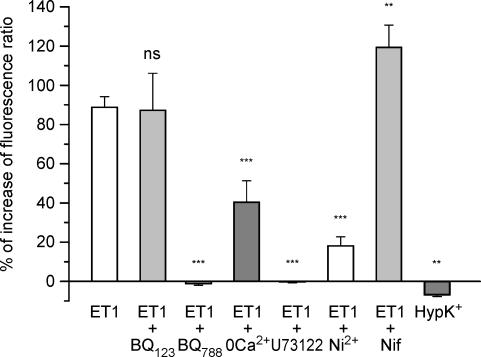
Figure 4. Average of percentage variation of fluorescence ratio during the spike obtained after various pharmacological treatments.
Data represent means ± s.e.m.; ET1 alone (n = 49); ET1 + BQ123 (n = 24); ET1 + BQ788 (n = 13); ET1 +0 Ca2+ (n = 10); ET1 + U73122 (n = 12); ET1 + Ni2+ (n = 13); ET1 + nifedipine (n = 20); 100 mm K+ (n = 15). One-way analysis of variance revealed significant differences between treatments (F = 39.1; P < 0.0001; d.f. = 7). Dunnett's post hoc test showed a non-significant difference between ET1 alone and ET1 + BQ123, and that BQ788, 0 Ca2+, U73122 and Ni2+ significantly inhibited ET1-mediated effects on Ca2+ activity (***P < 0.01). On the other hand, nifedipine (Nif) slightly increased the ET1 response (**P < 0.05). Furthermore, hyper-potassium (HypK+) perfusion induced a slight decrease of the baseline Ca2+ level (two-tailed paired Student's t test; **P < 0.05).
The signal transduction pathway involved in the Ca2+ response in human STs could be produced by the mobilization of intracellular Ca2+ following the ET-induced activation of PLC and/or by an influx of extracellular Ca2+. Therefore, the relative contribution of extracellular Ca2+ was evaluated. In Ca2+-free medium (Figs 2C and 4), the resting [Ca2+]i was not affected and ET1 was able to induce a spike in [Ca2+]i in 67% of STs tested. However, under these conditions, the Ca2+ spike was less than that observed in control STs, and the plateau phase was not obtained (n = 10). Therefore an influx of extracellular Ca2+ represents a large portion of the ET1-induced elevation of cytosolic Ca2+ under control conditions.
Coupling of the ETB receptor to PLC was examined pharmacologically using U73122, a membrane-permeable inhibitor which inhibits PLC by disrupting its coupling to G protein. As shown in Figs 2D and 4, pre-incubation of cells with 2 μm U73122 completely abolished the ET1-induced [Ca2+]i increase (spike and plateau; n = 12) indicating that the mobilization of Ca2+ from inositol 1,4,5-trisphosphate (IP3)-sensitive intracellular Ca2+ stores is a pre-requisite for the ET1-induced Ca2+ response.
Characterization of channels involved in Ca2+ entry
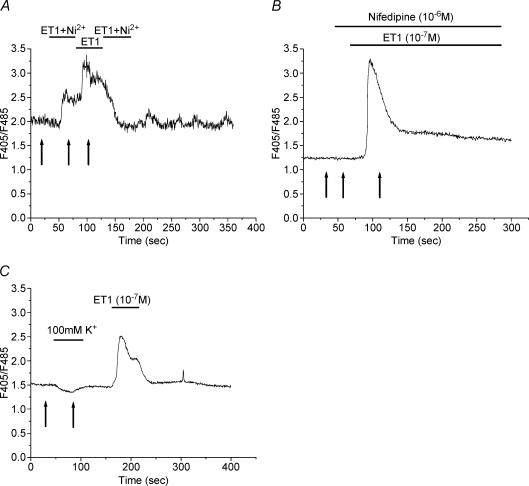
To characterize the nature of Ca2+ channels involved in ET1-evoked Ca2+ entry in STs, a panel of Ca2+ channel inhibitors was tested. Ni2+ is an inorganic, non-specific inhibitor of Ca2+ channels, and it has been demonstrated to block Ca2+ entry in other cell types. As illustrated in Fig. 3A and Fig. 4, 1 mm Ni2+ significantly reduced the ET1-evoked initial Ca2+ entry by 70% and inhibited the plateau phase (n = 13). The inhibitory effects of Ni2+ were reversible.
Figure 3. Effects of Ni2+, nifedipine and hyper-potassium solution on the ET1-induced Ca2+ response in a ST.
Ca2+ response to ET1 (10−7 m) in the presence of 1 mm Ni2+ (A) or nifedipine (10−6 m; B). C, representative recording of the Ca2+ response to 100 mm K+ perfusion and subsequent response to ET1 perfusion (10−7 m). Arrows in A–C indicate the time of measurement used for the calculation of the percentage changes in ratios expressed in Fig. 4.
The ET-induced Ca2+ entry could occur via voltage-operated or voltage-insensitive Ca2+ channels. The dihydropyridine compound nifedipine is an L-type Ca2+ channel blocker frequently used to demonstrate the presence of voltage-operated channels. As illustrated in Figs 3B and 4, perfusion with 1 mm nifedipine did not block ET1-induced Ca2+ entry (n = 20). Moreover, trophoblastic membrane depolarization induced by the perfusion of cells with a hyper-potassium solution did not induce an influx of Ca2+ into cells (Figs 3C and 4), thereby confirming the absence of voltage-operated Ca2+ channels. It should be noted that the antagonists and blockers used here (BQ123, BQ788, U73122 and nifedipine) had no significant effect on baseline Ca2+ levels (two-tailed paired Student's t test).
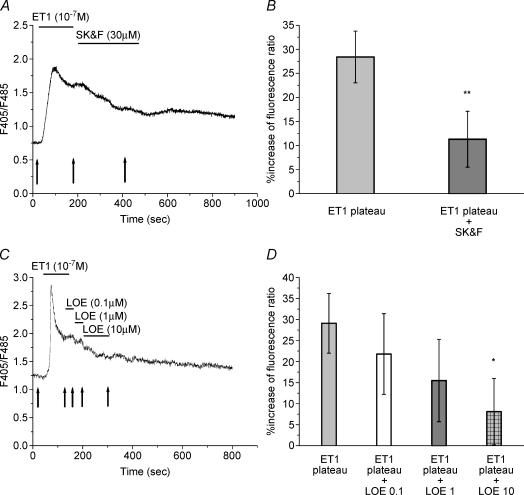
It is possible, therefore, that the ET-induced Ca2+ entry occurs via voltage-insensitive Ca2+ channels. Recently, two types of organic compounds have been used as pharmacological tools to block L-type Ca2+ channel blocker-insensitive Ca2+ influx mechanisms, they are the imidazole derivatives SK&F96365 and LOE 908. Importantly, NSCCs and SOCCs can be distinguished in terms of their sensitivities to these compounds (Kawanabe et al. 2001, 2002). NSCCs are sensitive to LOE 908, whereas SOCCs are resistant to LOE 908 and sensitive to SK&F96365. As shown in Fig. 5A and B, perfusion of STs with 30 μm SK&F96365 during the plateau phase decreased the ET1-evoked Ca2+ entry by 80% (n = 10). However, SK&F96365 did not totally abolished the plateau phase. Perfusion of STs with LOE 908 during the plateau phase induced a dose-dependent decrease of the ET1-evoked Ca2+ entry without totally abolishing the ET1 response (Fig. 5C and D).
Figure 5. Effects of SK&F96365 and LOE 908 on the ET1-induced calcium plateau phase.
A and C, recordings of Ca2+ changes induced in response to ET1 (10−7 m) in the presence of 30 μm SK&F96365 (A) and 0.1, 1 and 10 μm LOE 908 (C) during the plateau phase. Arrows indicate the time of measurement used for the calculation of the percentage changes in ratios expressed in B and D. B, average percentage change in fluorescence ratio (F405/F485) for results of SK&F96365 treatment presented in A. Data represent means ± s.e.m.; ET1 plateau (n = 10); ET1 plateau + SK&F96365 (n = 10); (**P < 0.01; one-tailed paired Student's t test). D, average of percentage increases in fluorescence ratio (F405/F485) for results of LOE 908 treatment presented in A. Data represent means ± s.e.m.; ET1 plateau (n = 10); ET1 plateau + LOE 908 0.1 μm (n = 6); ET1 plateau + LOE 908 1 μm (n = 6); ET1 plateau + LOE 908 10 μm (n = 6); (*P < 0.05; one-tailed paired Student's t test).
These ET1-evoked Ca2+ responses in the presence of SK&F96365 or LOE 908 suggest the participation of two types of voltage-insensitive Ca2+ channels, i.e. NSCCs and SOCCs.
Discussion
In the present study, an attempt has been made to pharmacologically characterize the nature of channels involved in Ca2+ entry in human trophoblasts in culture. Owing to its position in the human chorionic villi, bathed with maternal blood in the intervillous space, the ST is the site of numerous placental functions including various forms of exchange, as well as metabolism and the synthesis of hormones required for fetal growth and development (Benirschke & Kaufmann, 2000). It is now well established that the villous trophoblast differentiates from the fusion of CT cells into a ST, and that culture of trophoblastic cells provides a useful way to study trophoblast physiology (Kliman et al. 1986; Cronier et al. 1994; Malassiné & Cronier, 2002).
The results presented here indicate that ET1 stimulated a biphasic (transient and sustained) increase in [Ca2+]i in trophoblastic cells. This Ca2+ response is mediated by the ETB receptor, since BQ788 totally abolished the response. ETB receptors were previously demonstrated in the term trophoblastic microvillous membrane (Malassiné et al. 1993b), in first trimester trophoblastic cells (Cervar et al. 1997) and in a human extravillous trophoblast cell line (Chakraborty et al. 2003). In various cell types, ETB receptors are protein Gq-coupled receptors activating PLC to produce IP3 as well as 1,2-diacylglycerol (DAG). IP3 stimulates the release of Ca2+ from IP3-sensitive stores and subsequently the activation of SOCCs. Furthermore, it was recently demonstrated that a non-metabolizable analogue of DAG (OAG) could directly activate members of the transient receptor potential (TRP) superfamily, thereby inducing an increase of intracellular Ca2+ in placental explants (Clarson et al. 2003). Abrogation of the ET1-mediated Ca2+ response after pre-treatment with U73122, a PLC inhibitor, indicates firstly that in human trophoblastic cells ETB receptors are coupled to PLC via Gq protein. The abrogation of both the spike and plateau phases indicates that the inhibition of PLC could also induce inhibition of other functional Ca2+ channels such as SOCC, NSCC or other members of the TRP superfamily. The persistence of the rapid transient rise in [Ca2+]i in Ca2+-free extracellular medium confirms the release of Ca2+ from intracellular Ca2+ stores in response to ET1 stimulation. Furthermore, abolition of the sustained increase in [Ca2+]i in Ca2+-free extracellular medium argues for the entry of extracellular Ca2+ during the plateau phase. Inhibition of the plateau phase by Ni2+ confirms the existence of an ET1-induced Ca2+ entry.
Based on the findings presented here, several candidate Ca2+ channel types could mediate Ca2+ entry. These include voltage-operated channels, store-operated Ca2+ channels, receptor-operated channels and non-selective cationic channels. Abrogation of the Ca2+ response in the presence of U73122 argues for the absence of a receptor-operated channel stimulation induced by ET1. No evidence for the presence of voltage-operated channels was demonstrated during ET1 stimulation, since nifedipine did not reduce the ET1-induced Ca2+ response, and depolarization with a hyper-potassium solution had no effect. These results confirm findings from a previous electrophysiological study by this group (Cronier et al. 1999). Other studies have suggested the presence of nifedipine-sensitive channels in the ST of term placenta (Polliotti et al. 1994; Meuris et al. 1994; Cemerikic et al. 1998; Robidoux et al. 2000). However, Bax et al. (1994) using Ca2+ measurements could not demonstrate the presence of these voltage-operated channels in cultured trophoblastic cells. Furthermore, it was reported that in a trophoblastic cell line (BeWo cells), Ca2+ uptake was not influenced by L-type Ca2+ channel modulators (Moreau et al. 2001). This insensitivity towards blockers of voltage-operated Ca2+ channels was also observed with other experimental models including placental perfusion (Stulc et al. 1994), ST membrane vesicles (Kamath et al. 1992) and placental explants (Long & Clarson, 2002; Clarson et al. 2003). It should be pointed out that physiological studies require a clear identification of the investigated cells. Under the experimental conditions employed here, the procedure for trophoblast isolation prevents contamination by other placental cells such as macrophages, fibroblasts, endothelial or smooth muscle cells.
Recently, SK&F96365 (Merrit et al. 1990) and LOE 908 (Encabo et al. 1996) have been used as pharmacological tools to analyse L-type Ca2+ channel blocker-insensitive Ca2+ influx mechanisms. These compounds have permitted characterization of Ca2+ entry routes in excitable and non-excitable cells. Indeed, NSCCs are sensitive to LOE 908, whereas SOCCs are resistant to LOE 908 and sensitive to SK&F96365 (Kawanabe et al. 2001, 2002). From the pharmacological experiments reported here, the presence of both SOCC and NSCC activation during the ET1-induced Ca2+ response in cultured ST cells has been demonstrated.
SOCCs serve as an important class of Ca2+ entry channel which are activated by depletion of intracellular Ca2+ stores upon stimulation of G-protein-coupled receptors (Berridge et al. 2000; Peng et al. 2003). In electrically non-excitable cells, SOCCs serve as one of the main routes for the entry of extracellular Ca2+. The presence of SOCCs in cultured cytotrophoblastic cells was previously suggested in response to stimulation by various ligands (Petit & Belisle, 1995; Karl et al. 1997; Cronier et al. 1999; Clarson et al. 2002). Recently, using placental explants, Clarson et al. (2003) demonstrated pharmacologically the presence of SOCC in term placenta, the expression of transient receptor potential canonical (TRPC) mRNA in first trimester and term placentas, and the immunolocalization of TRPC3, 4 and 6 in term ST cells. They concluded that store-operated Ca2+ entry occurs in human term placenta and that it may be gestationally regulated. Here we have demonstrated the activation of SOCCs by ET1 in cultured human term trophoblastic cells. The molecular identity of this SOCC needs to be identified, but based on previous studies, CaT1 (Moreau et al. 2002a), TRPC (Clarson et al. 2003) and polycystin-2 (Ong et al. 1999) could be candidates for this function. In situ hybridization studies have demonstrated the presence of CaT1 in STs (Peng et al. 2001; Wissenbach et al. 2001). Its expression seems to be correlated to Ca2+ uptake activity and hCG secretion (Moreau et al. 2002a). Moreover, another member of the TRP superfamily, polycystin-2, is present in term human STs (Gonzalez-Perret et al. 2001).
NSCC form a mixed group of ion channels that include ligand-gated, mechanosensitive and hyperpolarization- or stress-activated channels. NSCCs are widely distributed in numerous tissue types and could be permeable to Ca2+ ions. Many of their biophysical and regulatory properties have been described (for review, see Nilius, 2003; Clapham, 2003), but channel functions remain unknown in many cases. The presence of NSCCs in the human placenta has been previously considered by means of electrophysiological studies on placental CT cells (Clarson et al. 1999), after fusion of microvillous membranes with planar lipid bilayers (Grosman & Reisin, 2000) or after reconstitution of brush border membranes into giant liposomes (Riquelme et al. 1995; Llanos et al. 2002). Moreover, in excitable (Van Renterghem et al. 1988; Enoki et al. 1995; Minowa et al. 1997) and non-excitable cells (Enoki et al. 1995; Lee et al. 1999), ET1 was able to activate NSCCs.
SOCCs and NSCCs appear to be essential in replenishing ST Ca2+ stores (Putney, 1999, 2001) and serve as an important means of Ca2+ entry in trophoblastic cells (Shennan & Boyd, 1987; Illsley & Sellers, 1992). Moreover, in non-excitable cell types, activation of NSCCs and SOCCs leading to Ca2+ influx seems to play a role in processes that include secretion, cell proliferation, gene transcription and cell death (Berridge et al. 2000). In the human trophoblast, transient intracellular Ca2+ variations could affect these processes. It has been demonstrated that in purified human trophoblastic cells, raising [Ca2+]i induces an increase of K+ and Cl− efflux (Kibble et al. 1996; Turner et al. 1999; Clarson et al. 2002). As the placental transfer of maternal calcium is carried out in vivo by the ST, SOCCs and Ca2+-permeable NSCCs could also represent regulated modes of Ca2+ entry. Furthermore, Ca2+ ions could serve as mediators implicated in gap junctional intercellular communication during trophoblastic fusion (Cronier et al. 1994, 1999, 2003).
Various bioactive substances have been demonstrated to induce an increase in intracellular Ca2+ in isolated trophoblastic cells. These include GnRH (Currie et al. 1993), ATP and UTP (Petit & Belisle, 1995), ATP and angiotensin II (Karl et al. 1997), ET (Cronier et al. 1999) and ATP (Clarson et al. 2002). The human placenta appears to be an important source of ETs; cultured trophoblastic cells have been shown to release ET1 and to express pre-pro-ET1 and pre-pro-ET3 mRNA (Malassiné et al. 1993a; Robert et al. 1996). Since the first description of ETs, it is evident that these peptides display a multitude of biological functions controlling cellular ion fluxes, cell-to-cell communication, hormone release, cell chemokinesis, cell proliferation, cell differentiation, and the growth and progression of various tumours (Nelson et al. 2003). We have previously demonstrated that ET1 impairs trophoblast differentiation (Cronier et al. 1999). Furthermore, ET1 stimulates the invasion of first trimester trophoblastic cells (Cervar et al. 1997) and the migration of a human extravillous trophoblast cell line (Chakraborty et al. 2003). Further studies are required to determine the implications of ET1-induced Ca2+ increases in the human trophoblast.
Acknowledgments
We are grateful to the Clinique du Fief de Grimoire for providing us with placental tissue. We would like to thank Boehringer Ingelheim for the generous gift of LOE 908 and Dr F. Gaillard (UMR 6187 CNRS) for his expertise in the statistical analysis. This research was supported in part by grants from the Ligue nationale contre le cancer to C. Niger.
References
- Bax C, Bax B, Bain M, Zaidi M. Ca2+ channels in human term trophoblast cells in vitro. A study using the Ca2+-sensitive dye FURA 2. Trophoblast Res. 1994;8:573–580. [Google Scholar]
- Benirschke K, Kaufmann P. Pathology of the Human Placenta. 4. New York: Springer-Verlag; 2000. [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Cemerikic B, Zamah R, Ahmed MS. Idendification of L-type calcium channels associated with kappa opioide receptors in human placenta. J Mol Neurosci. 1998;10:261–272. doi: 10.1007/BF02761779. [DOI] [PubMed] [Google Scholar]
- Cervar M, Kainer F, Desoye G. Endothelin-1 stimulates the invasion of first trimester trophoblast specifically via the B-type receptor, whereas its action on proliferation is mediated by both receptor subtypes. Placenta. 1997;18:A15. 10.1016/S0143-4004(97)90059-X. [Google Scholar]
- Chakraborty C, Barbin YP, Chakrabarti S, Chidiac P, Dixon SJ, Lala PK. Endothelin-1 promotes migration and induces elevation of [Ca2+]i and phosphorylation of MAP kinase of a human extravillous trophoblast cell line. Mol Cell Endocrinol. 2003;201:63–73. doi: 10.1016/s0303-7207(02)00431-8. [DOI] [PubMed] [Google Scholar]
- Clapham E. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clarson LH, Greenwood SL, Sibley CP. The effect of a non-selective cation (NSCC) channel blocker on ATP-induced 86Rb efflux from cytotrophoblast cells. Placenta. 1999;20:A18. [Google Scholar]
- Clarson LH, Roberts VH, Greenwood SL, Elliott AC. ATP-stimulated Ca2+-activated (K+) efflux pathway and differentiation of human placental cytotrophoblast cells. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1077–1085. doi: 10.1152/ajpregu.00564.2001. [DOI] [PubMed] [Google Scholar]
- Clarson LH, Roberts VH, Hamark B, Elliott AC, Powell T. Store-operated Ca2+ entry in first trimester and term human placenta. J Physiol. 2003:515–528. doi: 10.1113/jphysiol.2003.044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronier L, Bastide B, Hervé JC, Délèze J, Malassiné A. Gap junctional communication during human trophoblast differentiation: Influence of human chorionic gonadotropin. Endocrinology. 1994;135:402–408. doi: 10.1210/endo.135.1.8013377. [DOI] [PubMed] [Google Scholar]
- Cronier L, Dubut A, Guibourdenche J, Malassiné A. Effects of endothelin on villous trophoblast differentiation and free intracellular calcium. Trophoblast Res. 1999;13:69–86. [Google Scholar]
- Cronier L, Frendo JL, Defamie N, Pidoux G, Bertin G, Guibourdenche J, Pointis G, Malassiné A. Requirement of gap junctional intercellular communication for human villous trophoblast differentiation. Biol Reprod. 2003;69:1472–1480. doi: 10.1095/biolreprod.103.016360. [DOI] [PubMed] [Google Scholar]
- Currie D, Setoyama T, Lee TS, Baimbridge KL, Church J, Ho Yuen B, Leung CK. Cytosolic free Ca2+ in human syncytiotrophoblast cells increased by gonadotropin-releasing hormone. Endocrinology. 1993;133:2220–2226. doi: 10.1210/endo.133.5.8404673. [DOI] [PubMed] [Google Scholar]
- Encabo A, Romanin C, Birke FW, Kukovetz WR, Groschner K. Inhibition of a store-operated Ca2+ entry pathway in human endothelial cells by the isoquinoline derivative LOE 908. Br J Pharmacol. 1996;119:702–706. doi: 10.1111/j.1476-5381.1996.tb15729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki T, Miwa S, Sakamoto S, Minowa T, Komuro T, Kobayashi T, Ninomiya H, Masaki T. Long-lasting activation of cation current by low concentration of endothelin-1 in mouse fibroblast and smooth muscle cells of rabbit aorta. Br J Pharmacol. 1995;115:479–485. doi: 10.1111/j.1476-5381.1995.tb16358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frendo JL, Cronier L, Bertin G, Guibourdenche J, Vidaud M, Evain-Brion D, Malassiné A. Involvement of connexin 43 in human trophoblast cell fusion and differentiation. J Cell Sci. 2003;116:3413–3421. doi: 10.1242/jcs.00648. 10.1242/jcs.00648. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli L, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci U S A. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. 10.1073/pnas.021456598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman C, Reisin IL. Single-channel characterization of a nonselective cation channel from human placental microvillous membranes. Large conductance, multiplicity of conductance states, and inhibition by lanthanides. J Membr Biol. 2000;174:59–70. doi: 10.1007/s002320001032. 10.1007/s002320001032. [DOI] [PubMed] [Google Scholar]
- Illsley NP, Sellers MM. Ion conductances in the microvillous and basal membrane vesicles isolated from human placental syncytiotrophoblast. Placenta. 1992;13:25–34. doi: 10.1016/0143-4004(92)90004-d. [DOI] [PubMed] [Google Scholar]
- Kamath SG, Kelley LK, Friedman CH, Smith CH. Transport and binding in calcium uptake by microvillous membrane of human placenta. Am J Physiol Cell Physiol. 1992;262:C789–794. doi: 10.1152/ajpcell.1992.262.3.C789. [DOI] [PubMed] [Google Scholar]
- Karl PI, Chusid J, Tagoe C, Fisher SE. Ca2+ flux in human placental trophoblast. Am J Physio Cell Physioll. 1997;272:C1776–1780. doi: 10.1152/ajpcell.1997.272.6.C1776. [DOI] [PubMed] [Google Scholar]
- Kawanabe Y, Okamoto Y, Enoki T, Hashimoto N, Masaki T. Ca2+ channels activated by endothelin-1 in CHO cells expressing endothelin-A or endothelin-B receptors. Am J Physiol Cell Physiol. 2001;281:C1676–1685. doi: 10.1152/ajpcell.2001.281.5.C1676. [DOI] [PubMed] [Google Scholar]
- Kawanabe Y, Okamoto Y, Miwa S, Hashimoto N, Masaki T. Molecular mechanism for the activation of voltage-independent Ca2+ channels by endothelin-1 in chinese hamster ovary cells stably expressing human endothelin A receptors. Mol Pharmacol. 2002;62:62–75. doi: 10.1124/mol.62.1.75. [DOI] [PubMed] [Google Scholar]
- Kibble JD, Greenwood SL, Clarson LH, Sibley CP. A Ca2+-activated whole-cell Cl− conductance in human placental cytotrophoblast cells activated via G protein. J Membr Biol. 1996;151:131–138. doi: 10.1007/s002329900064. 10.1007/s002329900064. [DOI] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger J, Strauss J., III Purification, characterization and in vitro differentiation of cytotrophoblasts from human term placenta. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Lee K, Morita H, Iwamuro Y, Zhang XF, Okamoto Y, Nakagawa T, Hasegawa H, Futurani H, Miwa S, Masaki T. Pharmacological characterization of receptor-mediated Ca2+ entry in endothelin-1 induced catecholamine release from cultured bovine adrenal chromaffin cells. Nauyn Schmiedebergs Arch Pharmacol. 1999;360:616–622. doi: 10.1007/s002109900132. 10.1007/s002109900132. [DOI] [PubMed] [Google Scholar]
- Llanos P, Henriquez M, Riquelme G. A low conductance, non-selective cation channel from human placenta. Placenta. 2002;23:184–191. doi: 10.1053/plac.2001.0766. 10.1053/plac.2001.0766. [DOI] [PubMed] [Google Scholar]
- Long O, Clarson LH. The effect of Ca2+-permeable channel blockers on human chorionic gonadotrophin (hCG) secretion by villous fragments from term placentas. J Physiol. 2002;539.P:126. P. [Google Scholar]
- Malassiné A, Cronier L. Hormones and human trophoblast differentiation. Endocrine. 2002;19:3–11. doi: 10.1385/ENDO:19:1:3. 10.1385/ENDO:19:1:3. [DOI] [PubMed] [Google Scholar]
- Malassiné A, Cronier L, Mondon F, Mignot TM, Ferré F. Localization and production of immunoreactive endothelin-1 in the trophoblast of human placenta. Cell Tissue Res. 1993a;271:491–497. doi: 10.1007/BF02913732. [DOI] [PubMed] [Google Scholar]
- Malassiné A, Mondon F, Robaut C, Vial M, Bandet J, Tanguy G, Rostène W, Cavero I, Ferré F. Trophoblastic localisation of [125I]endothelin-1 binding sites in human placenta. Trophoblast Res. 1993b;7:271–285. doi: 10.1210/jcem.76.1.8421091. [DOI] [PubMed] [Google Scholar]
- Merrit JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarty SA, Moores KE, Rink TJ. SK & F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuris S, Polliotti B, Robyn C, Lebrun P. Ca2+ entry through L-type voltage-sensitive Ca2+ channels stimulates the release of human chorionic gonadotrophin and placental lactogen by placental explants. Biochim Biophys Acta. 1994;1220:101–106. doi: 10.1016/0167-4889(94)90124-4. 10.1016/0167-4889(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Minowa T, Miwa S, Kobayashi S, Enoki T, Zhang XF, Komuro T, Iwamuro Y, Masaki T. Inhibitory effect of nitrovasodilators and cyclic 6MP on ET-1-activated Ca2+-permeable nonselective cation channel in rat aortic smooth muscle cells. Br J Pharmacol. 1997;120:1536–1544. doi: 10.1038/sj.bjp.0701059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau R, Daoud G, Bernachez R, Simoneau L, Masse A, Lafond J. Calcium uptake and calcium transporter expression by torphoblast cells from human term placenta. Biochim Biophys Acta. 2002a;1564:325–332. doi: 10.1016/s0005-2736(02)00466-2. [DOI] [PubMed] [Google Scholar]
- Moreau R, Hamel A, Daoud G, Simoneau L, Lafond J. Expression of calcium channels along the differentiation of cultured trophoblast cells from human term placenta. Biol Reprod. 2002b;67:1473–1479. doi: 10.1095/biolreprod.102.005397. 10.1095/biolreprod.102.005397. [DOI] [PubMed] [Google Scholar]
- Moreau R, Simoneau L, Lafond J. Characteristic of calcium uptake by BeWo cells, a human trophoblast cell line. Placenta. 2001;22:768–775. doi: 10.1053/plac.2001.0719. 10.1053/plac.2001.0719. [DOI] [PubMed] [Google Scholar]
- Nelson J, Bagnato A, Basttistini B, Nisen P. The endothelin axis: emerging role in cancer. Nature Rev Cancer. 2003;3:110–116. doi: 10.1038/nrc990. 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- Nilius B. Calcium-impermeable monovalent cation channels: a TRP connection? Br J Pharmacol. 2003;138:5–7. doi: 10.1038/sj.bjp.0705073. 10.1038/sj.bjp.0705073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong ACM, Ward CJ, Butler RJ, Biddolph S, Bowker C, Torra R, Pei Y, Harris PC. Coordinate expression of the autosomal dominant polycystic kidney disease proteins, polycystin-2 and polycystin-1 in normal and cystic tissue. Am J Pathol. 1999;154:1721–1729. doi: 10.1016/S0002-9440(10)65428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JB, Brown EM, Hediger MA. Structural conversion of the genes encoding CaT1, CaT2, and related cation channels. Genomics. 2001;76:99–109. doi: 10.1006/geno.2001.6606. 10.1006/geno.2001.6606. [DOI] [PubMed] [Google Scholar]
- Peng JB, Brown EM, Hediger M. Epithelial Ca2+ entry channels: transcellular Ca2+ transport and beyond. J Physiol. 2003;551:729–740. doi: 10.1113/jphysiol.2003.043349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A, Belisle S. Stimulation of intracellular calcium concentration by adenosine triphosphate and uridine 5′-triphosphate in human term placental cells: evidence for purinergic receptors. J Clin Endocrinol Metab. 1995;80:1809–1815. doi: 10.1210/jcem.80.6.7775628. 10.1210/jc.80.6.1809. [DOI] [PubMed] [Google Scholar]
- Polliotti B, Lebrun P, Robyn C, Meuris S. The release of human chorionic gonadotrophin and placental lactogen by placental explants can be stimulated by Ca2+ entry through a Na+–Ca2+ exchange process. Placenta. 1994;15:477–485. doi: 10.1016/s0143-4004(05)80417-5. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr TRP, inositol 1,4,5-trisphosphate receptors, and capacitive calcium entry. Proc Natl Acad Sci U S A. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr Channelling calcium. Nature. 2001;410:648–649. doi: 10.1038/35070704. 10.1038/35070704. [DOI] [PubMed] [Google Scholar]
- Riquelme G, Stutzin A, Barros LF, Liberona JL. A chloride channel from human placenta reconstituted into giant liposomes. Am J Obstet Gynecol. 1995;173:733–738. doi: 10.1016/0002-9378(95)90332-1. 10.1016/0002-9378(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Robert B, Malassiné A, Bourgeois C, Mignot MT, Cronier L, Ferré F, Duc-Doiran P. Expression of endothelin precursor genes in human trophoblast in culture. Eur J Endocrinol. 1996;134:490–496. doi: 10.1530/eje.0.1340490. [DOI] [PubMed] [Google Scholar]
- Robidoux J, Simoneau L, St-Pierre S, Masse A, Lafond J. Characterization of neuropeptide Y-mediated corticotropin-releasing factor synthesis and release from human placental trophoblasts. Endocrinology. 2000;141:2795–2804. doi: 10.1210/endo.141.8.7601. 10.1210/en.141.8.2795. [DOI] [PubMed] [Google Scholar]
- Shennan D, Boyd C. Ion transport by the placenta: a of membrane transport system. Biochem Biophys Acta. 1987;906:437–457. doi: 10.1016/0304-4157(87)90019-0. [DOI] [PubMed] [Google Scholar]
- Stulc J, Stulcova B, Smid M, Sach I. Parallel mechanisms of Ca++ transfer across the perfused human placental cotyledon. Am J Obstet Gynecol. 1994;170:162–167. doi: 10.1016/s0002-9378(94)70403-1. [DOI] [PubMed] [Google Scholar]
- Turner MA, Sides MK, Sibley CP, Greenwood SL. Anion efflux from cytotrophoblast cells derived from normal term human placenta is stimulated by hyposmotic challenge and extracellular A23187 but not by membrane-soluble cAMP. Exp Physiol. 1999;84:27–40. doi: 10.1111/j.1469-445x.1999.tb00069.x. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C, Vigne P, Barhanin J, Schmid Alliana A, Frelin C, Lazdunski M. Molecular mechanism of action of the vasoconstrictor peptide endothelin. Biochem Biophys Res Commun. 1988;157:977–985. doi: 10.1016/s0006-291x(88)80970-7. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Niemeyer BAZ, Fixemer T, Schneidewind A, Trost C, Cavalie A, Reus K, Meese E, Bonkhoff H, Flockerzi V. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem. 2001;276:19461–19468. doi: 10.1074/jbc.M009895200. 10.1074/jbc.M009895200. [DOI] [PubMed] [Google Scholar]