Abstract
Spinal cord transection produces a marked increase in the response of the isolated rat tail artery to sympathetic nerve stimulation, possibly as a result of a decrease in ongoing sympathetic activity. We have tested the effects of removing ongoing nerve activity on neurovascular transmission by cutting the preganglionic input to postganglionic neurones supplying the tail artery (decentralization). Isometric contractions to nerve stimulation were compared between decentralized arteries and those from age-matched and sham-operated controls. Nerve-evoked responses of decentralized arteries were much larger than those of control arteries at 2 and 7 weeks post operatively. The extent of blockade of nerve-evoked contraction by α-adrenoceptor antagonists prazosin (10 nm) or idazoxan (0.1 μm) was reduced. Decentralized arteries were transiently supersensitive to the α1-adrenoceptor agonist phenylephrine and the α2-adrenoceptor agonist clonidine; the unchanged sensitivity to methoxamine and phenylephrine after 2 weeks indicated no effect on the neuronal noradrenaline uptake transporter. Decentralized arteries were hypersensitive to α,β methylene-ATP, but the P2-purinoceptor antagonist suramin (0.1 mm) did not reduce nerve-evoked contractions. Enlarged responses to 60 mm K+ after both 2 and 7 weeks were correlated with the response of the arteries to nerve stimulation, suggesting that increased postjunctional reactivity contributes to the enhanced contraction. Comparison between data from decentralized arteries and our previous data from spinalized animals showed that the two lesions similarly potentiate nerve-evoked contractions and have similar but not identical postjunctional effects. The enhanced vascular responses following a reduction in tonic nerve activity may contribute to the hypertensive episodes of autonomic dysreflexia in spinally injured patients.
We recently demonstrated that both force and duration of neurally evoked contractions were increased in the rat tail artery for up to 8 weeks following spinal transection at T7–8 (Yeoh et al. 2004). The preganglionic supply to the tail artery arises from T13 to L2 (Rathner & McAllen, 1998; Smith & Gilbey, 1998) so that spinal transection at T7–8 leaves both preganglionic and postganglionic pathways to the tail artery intact, but disconnected from higher centres and from the site of origin of sympathetic vasoconstrictor tone in the rostral ventrolateral medulla (Guyenet et al. 1989). Silencing synaptic connections in other neural pathways leads to enhancement of synaptic efficacy (e.g. Gallego & Geijo, 1987; Murthy et al. 2001). This suggests that enhanced neurovascular transmission in arteries below a spinal lesion (‘spinalized arteries’) might be caused by the decrease in ongoing sympathetic nerve activity (Wallin & Stjernberg, 1984). At least part of the change in spinalized arteries was postjunctional as contractions evoked by exposure to 60 mm K+ were also enhanced. Such increased vascular responses could contribute to the episodes of autonomic dysreflexia (uncontrolled hypertension triggered reflexly by bladder or colon distension or unheeded injuries) in people with cervical or high thoracic spinal injuries.
In sympathetically innervated smooth muscles, silencing ongoing nerve activity by surgically removing the preganglionic input to postganglionic sympathetic neurones (i.e. decentralization) increases the response of the muscle to exogenously applied noradrenaline (NA) as well as a range of other constrictor agents (Fleming & Westfall, 1988). The effect of decentralization on sympathetic nerve-evoked responses of vascular smooth muscle has only been examined in the rabbit ear artery (Tsuru & Bevan, 1980; Tsuru & Uematsu, 1986), where the contractile response to nerve stimulation was increased.
In the present study we have investigated the effects of decentralization on the mechanical responses of the rat tail artery to nerve stimulation. In rats, the postganglionic neurones supplying the tail artery lie in paravertebral ganglia L6–S4 (Sittiracha et al. 1987). This means that bilateral transection of the sympathetic chains below the most caudal white ramus (L2) removes all preganglionic connections to the postganglionic neurones supplying the tail artery.
The perivascular plexus of the tail artery is almost exclusively noradrenergic and it contains very few peptidergic afferent axons (McLachlan, 1996); consequently it lacks vasodilator responses to afferent nerve activation (Li & Duckles, 1993). Neurally evoked contractions of the tail artery are largely blocked by α-adrenoceptor antagonists, with both α1- and α2-adrenoceptors contributing to the postjunctional response (Bao et al. 1993; Brock et al. 1997; Yeoh et al. 2004). A synergistic role for both ATP and NPY coreleased with NA, particularly during short trains of stimuli, has also been suggested (Bradley et al. 2003). Here, the effects of the α1-adrenoceptor antagonist prazosin, the α2-adrenoceptor antagonist idazoxan, and the P2-purinoceptor antagonist suramin on neurally evoked contractions of decentralized tail arteries were assessed. In addition, the effects of decentralization on the sensitivity of the tail artery to the α1-adrenoceptor agonist phenylephrine, the α2-adrenoceptor agonist clonidine, the P2X-purinoceptor agonist α,β-methylene ATP, and 60 mm K+ were examined. To assess the possibility that changes in neuronal uptake affect sensitivity to phenylephrine (Langer & Trendelenburg, 1969), sensitivity to methoxamine (which is not a substrate for the neuronal NA uptake transporter; Trendelenburg et al. 1970) was also determined. The relation between the changes in sensitivity of the vascular smooth muscle to nerve stimulation at various time points following decentralization, and the muscle's responsiveness to the vasoconstrictor agents was evaluated. The data have also been compared directly with those previously reported following spinal transection (Yeoh et al. 2004).
Methods
All experimental procedures conformed to the National Health and Medical Research Council of Australia guidelines and were approved by the University of New South Wales Animal Care and Ethics Committee.
Surgery was performed on female inbred Wistar rats (∼8 weeks of age) under anaesthesia with ketamine (60 mg kg−1) and xylazine (10 mg kg−1) administered i.p. L3 and L4 sympathetic ganglia were exposed, usually via a midline ventral incision, and 1–3 mm of the trunk between them was removed. Oxytetracycline powder was applied before and after the muscle layers were sutured, and the incision was closed with Michel clips. Oxytetracycline (100 mg kg−1) and benzylpenicillin (90 mg kg−1) were administered s.c. The animals recovered uneventfully and were maintained for 2 days (n = 7), 2 weeks (17 ± 1 days, mean ± s.d., n = 13) and 7 weeks (50 ± 2 days, n = 10) postoperatively (p.o.). An additional group of animals was decentralized for 10 ± 1 days (n = 8) and tested for early hypersensitivity to phenylephrine. In sham-operated controls, the trunks were exposed and cleared but not cut; these animals were maintained for 2 days (n = 6) and 2 weeks (14 ± 4 days, n = 7). All operated animals were monitored on a daily basis to ensure their wellbeing. Unoperated animals age-matched to the 10 day (n = 7), 2 week (n = 6) and 7 week (n = 7) decentralized animals were also used as controls.
The animals were killed under deep anaesthesia (pentobarbitone 100 mg kg−1, i.p.) and the proximal part of the tail artery was dissected. Once isolated, the arteries were maintained in physiological saline solution of the following composition (mm): Na+, 150.6; K+, 4.7; Ca2+, 2; Mg2+, 1.2; Cl−, 144.1; H2PO4−, 1.3; HCO3−, 16.3; glucose, 7.8. This solution was gassed with 95% O2/5% CO2 (to pH 7.2) and warmed to 35–36°C.
In all lesioned animals, the site at which the lumbar sympathetic trunk had been divided was checked postmortem, and the absence of detectable regeneration was confirmed.
Mechanical responses
Arterial ring segments (∼1.5 mm long) from a region of the artery 10–20 mm distal to the base of the tail were mounted isometrically between stainless steel wires (50 μm diameter) in a four-chamber myograph (Multi Myograph Model 610 m, Danish Myo Technology, Denmark). Each chamber of the myograph contained 6 ml of physiological saline that was exchanged at intervals of 8–15 min throughout the recording period. There was some variation in the lengths (median 1.4 mm, range 1–1.8 mm) and lumen diameters (for the range of diameters see Results) of the segments studied. To normalize the basal conditions and the isometric contractions, the measured force was converted to the effective pressure exerted on the luminal surface of the artery using Laplace's equation (see Mulvany & Halpern, 1977). Initially, the effective distending pressure under basal conditions was set at ∼12.7 × 103 N m−2 (∼95 mmHg) and left to equilibrate for 30 min. After this, the basal effective distending pressure stabilized at ∼10.0 × 103 N m−2 (∼75 mmHg). Preliminary measurements had shown that at this pressure both control and decentralized arteries were at the peak of their length–force relations.
Experimental protocols
In the first series of experiments, the experimental protocols were identical to those previously described for tail arteries from spinalized animals (Yeoh et al. 2004) and are only summarized here.
Arterial segments mounted in two chambers of the myograph were used to record responses elicited by electrical stimulation of the perivascular nerves via transmural electrodes. First, a voltage strength–response curve was constructed for contractions evoked by trains of 10 stimuli (1 ms pulse width, 5–30 V) at 10 Hz. Subsequently the responses to trains of supramaximal stimuli (25 V) at 0.1–10 Hz were assessed. In all cases, the peak amplitude of the contractile response recorded during the trains of stimuli was measured. For the contractions evoked by 10 stimuli at 10 Hz, the rise time was the interval between 10 and 90% of the peak contraction and the half-width was the duration at 50% of the peak amplitude.
These segments were also used to determine the sensitivity of contractions evoked by 100 stimuli at 1 Hz to the α1-adrenoceptor antagonist prazosin (10 nm, Sigma), and the α2-adrenoceptor antagonist idazoxan (0.1 μm; Sigma), applied alone and in combination. Following the combined application of prazosin and idazoxan, the effect of the further addition of a relatively high concentration of the nonselective α-adrenoceptor antagonist phentolamine (1 μm; Ciba Geigy) was assessed. The effects of all antagonists were measured 20 min following their application.
Segments of artery in the other two chambers of the myograph were used to construct concentration–response curves for the α1- and α2-adrenoceptor agonists (–)-phenylephrine (0.01–300 μm; Sigma) and clonidine (0.001–10 μm; Sigma). Non-cumulative concentration–response curves were determined by increasing the concentration of each agonist by half-log increments, with exposure to each concentration for 7 min, followed by 9 min washes before the next addition of the agonist. The peak contraction during the period of drug application was measured.
Following both the nerve stimulation and the α-adrenoceptor agonist protocols, responses to 60 mm K+ (equimolar substitution of KCl for NaCl) were recorded from all four tissues in the presence of prazosin (10 nm) and idazoxan (0.1 μm) to prevent the excitatory actions of NA released from the sympathetic nerves by high [K+]. The cotransmitters ATP and neuropeptide Y do not contribute to K+-evoked contractions of the tail artery (Chen & Rembold, 1995). In control tissues, 60 mm K+ produces about 60% of the maximum response to raised [K+] (Chen & Rembold, 1995). The half-decay time of high-[K+]-induced contraction was measured as the time from when the raised K+ solution was removed until the amplitude of the contraction had fallen to 50% of its value at the start of washout.
In another series of experiments, arterial segments from 2 week decentralized or age matched unoperated controls were mounted in two chambers of the myograph. One arterial segment was used to construct noncumulative concentration–response curves to the α1-adrenoceptor agonist methoxamine (0.03–100 μm; Sigma). The concentration of agonist was increased by half-log increments, with washes between each agonist application (as described previously). The peak contraction during the period of drug application was measured.
Following construction of the concentration–response curves to methoxamine, this segment was also used to assess the sensitivity to the P2X-purinoceptor agonist α,β-methylene ATP (0.3–10 μm; Sigma). In this part of the experiment, the concentration of agonist was increased by half-log increments, with exposure to α,β-methylene ATP for 3 min intervals followed by 30 min washes before the next addition of this agent. During each application of α,β-methylene ATP, the contraction produced by this agent peaked and then declined. The peak amplitude of the response to α,β-methylene ATP was measured.
The second arterial segment was used to assess the effects of the P2-purinoceptor antagonist suramin (0.1 mm) on contractions evoked by 25 stimuli at 0.5 and 1 Hz. In these experiments, the segment was stimulated alternately with trains at 0.5 and 1 Hz at 5 min intervals, and the effects of suramin were determined 30–40 min following its addition to the bathing solution. Phentolamine (1 μm) was then added, and the extent of the blockade produced by this agent in combination with suramin was determined 30–40 min following its addition.
At the end of these experiments, the responses of both arterial segments to 60 mm K+ were recorded in the presence of prazosin (10 nm) and idazoxan (0.1 μm).
Statistical analysis
Unless otherwise indicated, Mann–Whitney U tests were used for pairwise comparisons and Kruskal–Wallis tests for multiple comparisons because the groups of raw data had unequal variance. Within-group comparisons between the sequential effects of the antagonists were made with Friedman tests followed by Wilcoxon signed rank tests. When multiple pairwise comparisons were made, the P values were adjusted using the Dunn–Sidak method. P < 0.05 was taken as a significant difference.
All data are presented as median and interquartile range (IQR).
Results
Basal conditions
In the first series of experiments, for all measured parameters there were no differences between arteries from 2 week sham-operated animals and those from age-matched unoperated animals. The effects of decentralization were assessed by comparing data from 2 day decentralized arteries (n = 7) with 2 day sham-operated arteries (n = 6, ‘2 day control arteries’), 2 week decentralized arteries (n = 7) with 2 week sham-operated arteries (n = 7, ‘2 week control arteries’), and 7 week decentralized arteries (n = 10) with unoperated age-matched control arteries (n = 7, ‘7 week control arteries’). For most of the measured parameters there was no difference between any of the groups of control arteries, but where there were differences, these are described.
Comparison between the decentralized arteries and their respective control arteries revealed no differences in the basal effective distending pressures (about 10 × 103 N m−2). There were also no differences between the lumen diameters of control and decentralized arteries after 2 days (control 839 μm, IQR 825–861; decentralized 894 μm, IQR 856–925) or 7 weeks (control 868 μm, IQR 773–878; decentralized 812 μm, IQR 749–845). However, the lumen diameters of 2 week decentralized arteries (730 μm, IQR 688–765) were smaller than those of 2 week control arteries (778 μm, IQR 778–823, P < 0.05).
The lumen diameters of 2 day decentralized arteries were larger than those of 2 week decentralized arteries (P < 0.05). The effective distending pressure of 2 day decentralized arteries (10.9 × 103 N m−2, IQR 10.8–11.1) was also larger than that of 2 week decentralized arteries (10.0 × 103 N m−2, IQR 10.0–10.3, P < 0.05). No other differences in distending pressure or diameter were detected between the groups of decentralized arteries.
In the second series of experiments, no significant differences in lumen diameter or effective basal distending pressure were found between 2 week decentralized arteries (n = 6) and age-matched control arteries (n = 6).
Responses to perivascular nerve stimulation
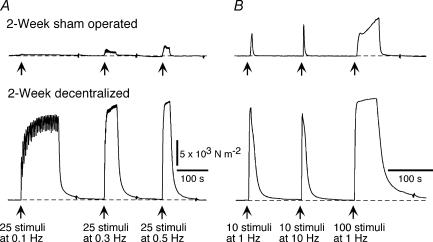
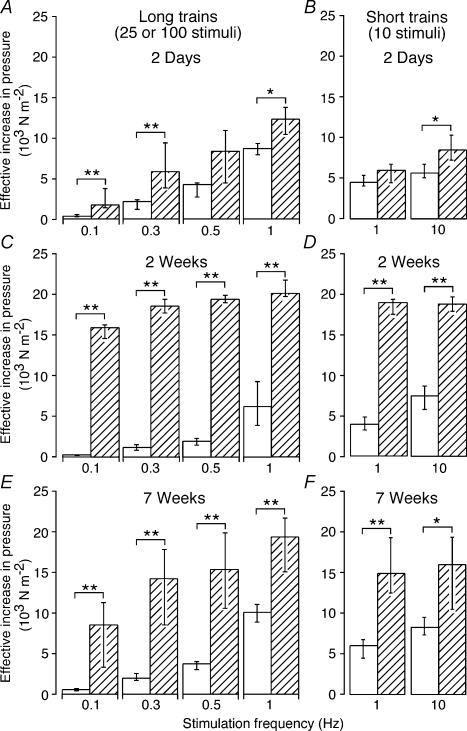
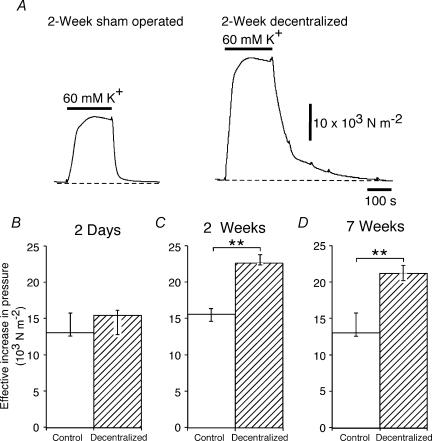
Figure 1 shows contractile responses of a 2 week sham operated artery and a 2 week decentralized artery to trains of stimuli at 0.1–10 Hz. In comparison with their respective control arteries, both 2 week and 7 week decentralized arteries generated greater increases in effective pressure to all the trains of stimuli (Fig. 2C–F). The responses of 2 day decentralized arteries to 25 pulses at 0.5 Hz and to 10 pulses at 1 Hz were not different from those of 2 day control arteries, but the responses of these arteries to all the other trains of stimuli were enhanced (Fig. 2A and B).
Figure 1. Decentralization enhances contractions to perivascular nerve stimulation.
A, contractions of 2 week sham-operated (upper trace) and 2 week decentralized arteries (lower trace) to stimulation of the perivascular nerves with 25 pulses at 0.1, 0.3 and 0.5 Hz. B, contractions in the same tissues evoked by 10 pulses at 1 and 10 Hz, and 100 pulses at 1 Hz. The pressure calibration in A applies also in B. In comparison with the sham-operated artery, the decentralized artery generated greater increases in effective pressure during all trains of stimuli. The contractions of the decentralized artery were also prolonged in time course.
Figure 2. Enhancement of contraction in decentralized arteries is greatest at low frequencies of nerve stimulation.
A–F, peak increases in effective pressure evoked by different frequencies of perivascular stimulation in (A, B) 2 day control (n = 6) and decentralized (n = 7) arteries, (C, D) 2 week control (n = 7) and decentralized (n = 7) arteries, and (E, F) 7 week control (n = 7) and decentralized (n = 10) arteries. Open and hatched columns represent data for control and decentralized arteries, respectively. A, C and E, responses to 25 pulses at 0.1, 0.3 and 0.5 Hz, and 100 pulses at 1 Hz. B, D and F, responses to 10 pulses at 1 and 10 Hz. Data are presented as median and interquartile range, and statistical differences are indicated (*P < 0.05 and **P < 0.01). Arteries from decentralized animals had enlarged responses to neural activation at all postoperative (p.o.) times, and the effects were greater at lower frequencies of stimulation.
For all trains of stimuli, the peak amplitude of the responses of 2 day decentralized arteries was smaller than that of 2 week decentralized arteries (P < 0.01). However, no differences were detected between the responses of 2 week and 7 week decentralized arteries although the responses of 7 week decentralized arteries were generally more variable. Only the responses to 10 and 100 stimuli at 1 Hz were smaller in 2 day decentralized arteries than in 7 week decentralized arteries (P < 0.05).
At all time points, the enhancement of neurally evoked contractions was most marked at 0.1 Hz and decreased as the frequency of stimulation was increased (Fig. 2). For example, the response of 2 week decentralized arteries to long trains of stimuli (Fig. 2C) was increased 55-fold at 0.1 Hz, 16-fold at 0.3 Hz, 10-fold at 0.5 Hz and 3-fold at 1 Hz. It was notable that the responses to trains at low frequencies were relatively less enhanced in 7 week decentralized arteries.
Time course of contractions to short trains of stimuli
The mechanical responses of decentralized arteries to electrical stimulation were prolonged (Fig. 1A and B). To exemplify this change, contractions to trains of 10 stimuli at 10 Hz in control and decentralized arteries were compared. Table 1 shows the rise time and the half-width of contractions produced by these trains of stimuli. The 2 day decentralized arteries contracted with time courses similar to their controls. In the 2 week decentralized arteries, the half-width of the contractions was increased about threefold whereas the rise time was increased by ∼60% (Table 1). Thus the change in time course was due primarily to an increase in relaxation time. Although the half-width of the contractions of 7 week decentralized arteries was not significantly different from that of their control arteries, their rise time was prolonged.
Table 1.
Characteristics of nerve-evoked contractions evoked by 10 stimuli at 10 Hz in decentralized arteries
| 2 days | 2 weeks | 7 weeks | |
|---|---|---|---|
| Rise time | |||
| Control | 1.7a | 1.3a,b | 1.4b |
| (1.5–1.9) | (1.2–1.3) | (1.4–1.5) | |
| n = 6 | n = 7 | n = 7 | |
| Decentralized | 1.6c | 2.1**,c | 1.9* |
| (1.5–1.6) | (2.0–2.1) | (1.6–2.1) | |
| n = 6 | n = 7 | n = 10 | |
| Half-width | |||
| Control | 6.0d | 3.7d | 4.8 |
| (5.5–6.5) | (3.4–4.2) | (4.7–5.2) | |
| n = 6 | n = 7 | n = 7 | |
| Decentralized | 4.5e | 16.8**,e,f | 7.7f |
| (4.1–8.9) | (14.2–18.0) | (6.0–9.1) | |
| n = 6 | n = 7 | n = 10 | |
The rise time was the period between 10 and 90% of the peak contraction, and the half-width was the duration of the contraction at 50% of its peak. Interquartile range (IQR) values are shown in parentheses below the median. Significant differences between decentralized and control arteries are indicated by
P < 0.5 and
P < 0.01.
Significant differences between groups of arteries at different time points indicated by superscript letters a–f
P < 0.05
P < 0.01.
The rise time of the contractions was longer in 2 week decentralized arteries than in 2 day decentralized arteries (Table 1). In addition, the half-width of the contractions of 2 week decentralized arteries was much longer than that of both 2 day and 7 week decentralized arteries (Table 1).
The rise time and half-width for the contractions of 2 day control arteries were significantly longer than those of 2 week control arteries (Table 1), possibly reflecting some effect of surgery. The rise time for the contractions of 7 week control arteries was longer than that of 2 week control arteries, but the half-width did not differ significantly between these two groups (Table 1).
Effects of blocking α-adrenoceptors on nerve-evoked contractions
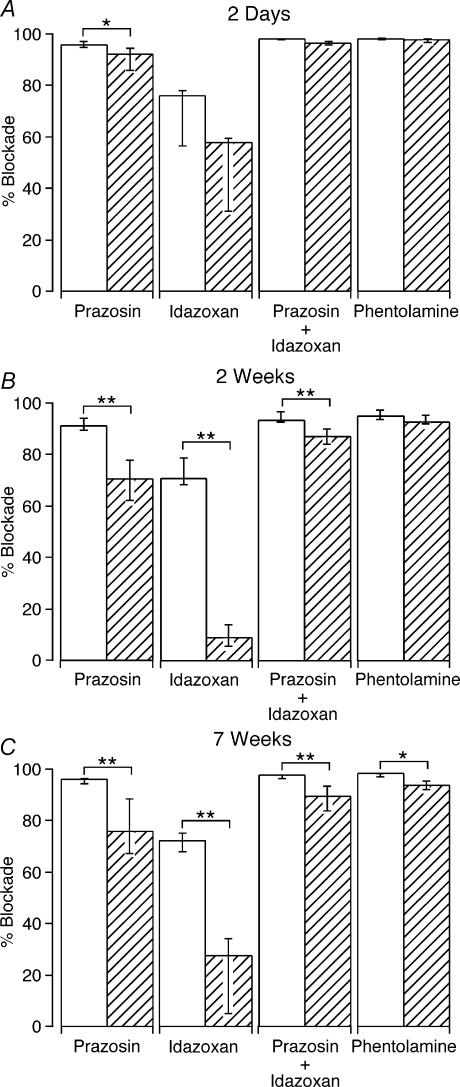
The effects of α-adrenoceptor antagonists on contractions evoked by 100 stimuli at 1 Hz were assessed. This pattern of stimulation produces a prolonged steady-state contraction. In control arteries, prazosin (10 nm) and idazoxan (0.1 μm) reduced the amplitude of the contractions by 95 and 69%, respectively (Fig. 3). The blockade produced by the combined application of prazosin and idazoxan was slightly greater than that of prazosin alone (approximately 97%, Wilcoxon signed rank test, P < 0.01) and the subsequent addition of phentolamine (1 μm) produced a further small increase in the blockade of contraction (to 98% blockade of the pre-drug-treatment values, Wilcoxon signed rank test, P < 0.01).
Figure 3. Blockade of nerve-evoked transmission by α-adrenoceptor antagonists is reduced in decentralized arteries.
A–C, blockade of contractions evoked by 100 pulses at 1 Hz produced by the α-adrenoceptor antagonists prazosin (10 nm), idazoxan (0.1 μm) and phentolamine (1 μm) in (A) 2 day control (n = 6) and decentralized (n = 7) arteries, (B) 2 week control (n = 7) and decentralized (n = 7) arteries, and (C) 7 week control (n = 7) and decentralized (n = 10) arteries. Open and hatched columns represent data for control and decentralized arteries, respectively. Prazosin and idazoxan were applied alone and together as indicated. The column labelled ‘Phentolamine’ indicates the percentage blockade after adding this agent in the presence of both prazosin and idazoxan. Data are presented as median and interquartile range, and statistical differences are indicated (*P < 0.05 and **P < 0.01). In comparison with control, the blockade of electrically evoked contractions produced by prazosin and idazoxan was reduced in 2 week and 7 week decentralized arteries. For 2 day decentralized arteries, only the blockade produced by prazosin was reduced.
In comparison with control arteries, the blockade produced by prazosin was reduced in 2 day decentralized arteries (Fig. 3A), whereas in 2 week and 7 week decentralized arteries, the blockades produced by prazosin and by idazoxan were both reduced (Fig. 3B and C). The effect of the combined application of prazosin and idazoxan was slightly (∼10%) less in 2 week and 7 week decentralized arteries than in control arteries. For both 2 and 7 week decentralized arteries, the subsequent addition of phentolamine reduced the contractions by a further ∼5% of the pre-drug-treatment values (Wilcoxon signed rank test, P < 0.05). The combined application of all three antagonists reduced the contraction of 2 and 7 week decentralized arteries by about 94%, but the combined effect of these agents was significantly less than control only for 7 week decentralized arteries (Fig. 3C).
In all tissues, the small component of contraction resistant to α-adrenoceptor antagonists was fully blocked by tetrodotoxin (0.5 μm), confirming that the electrically evoked contractions were due entirely to activation of the perivascular nerves.
Responses to α-adrenoceptor agonists
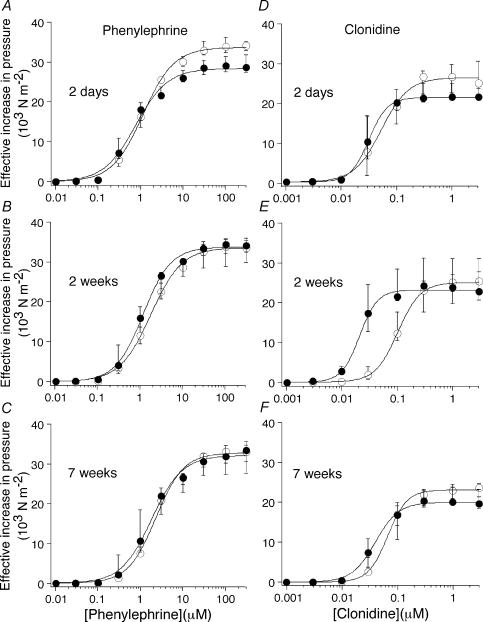
Figure 4 shows the concentration–response curves for the α1-adrenoceptor agonist phenylephrine, and the α2-adrenoceptor agonist clonidine, in control and decentralized groups of arteries.
Figure 4. Decentralized arteries are transiently hypersensitive to clonidine.
Concentration–response curves for (A–C) phenylephrine, and (D–F) clonidine, in control arteries (○) and at 2 days (A and D), 2 weeks (B and E), and 7 weeks (C and F) following decentralization (•). Data are presented as median and interquartile range. The curves are the best fits to the Hill equation. The sensitivity to α-adrenoceptor agonists was similar to control at all time points except for a transient hypersensitivity to clonidine at 2 weeks p.o. Note, however, that arteries decentralized for only 10 days were hypersensitive to both clonidine and phenylephrine (see text).
The maximum increases in effective pressure produced both by phenylephrine and by clonidine did not differ significantly between decentralized and control arteries (Fig. 4), although the maximum contraction to clonidine was significantly smaller than that to phenylephrine. This difference may not reflect differences in the maximum response to α1- and α2-adrenoceptor activation, as clonidine is a partial agonist at α2-adrenoceptors.
The EC50 for phenylephrine in the decentralized arteries did not differ significantly from that of the control arteries (Fig. 4A–C, Table 2). In contrast, the EC50 for clonidine was significantly reduced in the 2 week decentralized arteries (Fig. 4E, Table 2). This hypersensitivity to clonidine was transient, as it had disappeared after 7 weeks (Fig. 4F, Table 2). To test for an early transient phase of hypersensitivity to phenylephrine, one group of animals was decentralized for only 10 days; in this group, the EC50 for phenylephrine was reduced (Table 2), but there was no change in the maximum response to this agent (control 33.9 × 103 N m−2, IQR 33.0–36.1; decentralized 36.4 × 103 N m−2, IQR 34.2–39.4). Arteries from this group of animals also showed hypersensitivity to clonidine (Table 2).
Table 2.
Effects of decentralization on EC50 values for phenylephrine and clonidine
| 2 days | 10 days | 2 weeks | 7 weeks | |
|---|---|---|---|---|
| Phenyleprine (μm) | ||||
| Control | 1.25 | 1.69 | 1.69 | 2.02 |
| (0.90–1.47) | (1.36–2.54) | (1.55–2.04) | (1.92–2.18) | |
| n = 6 | n = 7 | n = 7 | n = 7 | |
| Decentralized | 0.72 | 0.74** | 1.05 | 1.59 |
| (0.61–0.75) | (0.33–0.93) | (0.79–1.44) | (0.89–2.22) | |
| n = 7 | n = 8 | n = 7 | n = 10 | |
| Clonidine (nm) | ||||
| Control | 62 | 67 | 100 | 69 |
| (32–79) | (59–120) | (67–112) | (52–76) | |
| n = 6 | n = 7 | n = 7 | n = 7 | |
| Decentralized | 32 | 32** | 20* | 49 |
| (22–53) | (27–41) | (19–27) | (33–79) | |
| n = 7 | n = 8 | n = 7 | n = 10 | |
IQR values are shown in parentheses below the median. Significant differences between decentralized and control arteries are indicated by
P < 0.05
P < 0.01.
In order to test whether the absence of hypersensitivity to phenylephrine at 2 weeks could be explained by a change in neuronal uptake, the sensitivity of 2 week decentralized arteries and age-matched control arteries to the α1-adrenoceptor agonist methoxamine, was also assessed. As with phenlyephrine at this stage, no change in the EC50 (control 1.20 μm, IQR 0.95–1.50; decentralized 1.01 μm, IQR 0.91–1.05) or the maximum response (control 33.9 × 103 N m−2, IQR 33.1–36.6; decentralized 35.1 × 103 N m−2, IQR 33.0–36.4) to methoxamine was detected between control and decentralized arteries.
Effects of suramin on nerve-evoked contractions
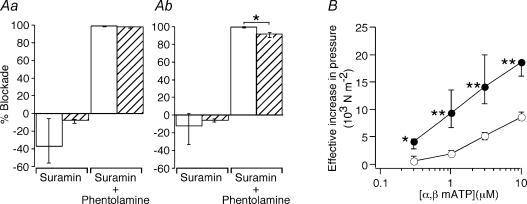
In the second series of experiments, the effects of the P2-purinoceptor antagonist suramin (0.1 mm) on responses to 25 pulses at 0.5 and 1 Hz were assessed in control and 2 week decentralized arteries (Fig. 5Aa and Ab). While the contractions of decentralized arteries to stimulation at lower frequencies were the most markedly increased, the responses of control arteries were too small to accurately assess the effects of suramin. As in the first series of experiments, the contractile responses of 2 week decentralized arteries (0.5 Hz, 21.3 × 103 N m−2, IQR 16.4–24.4; 1 Hz, 23.8 × 103 N m−2, IQR 19.6–26.3) were markedly increased in comparison with control arteries (0.5 Hz, 3.9 × 103 N m−2, IQR 3.3–6.4, P < 0.05; 1 Hz, 8.6 × 103 N m−2, IQR 6.0–12.7, P < 0.05). In control arteries, the effects of suramin were variable (four segments showed increased responses and two showed decreased responses to nerve stimulation) and no significant change in the response to electrical stimulation at either 0.5 Hz (Fig. 5Aa) or 1 Hz (Fig. 5Ab) was detected. In contrast, suramin slightly increased the response of all decentralized arteries to stimulation at 0.5 and 1 Hz (Fig. 5Aa and Ab; Wilcoxon signed rank test P < 0.05). The subsequent addition of phentolamine (1 μm) abolished contractions of both control and decentralized arteries to stimulation at 0.5 Hz, and reduced those to stimulation at 1 Hz by 98 and 92% of the pre-drug-treatment values, respectively (Fig. 5Aa and Ab). The residual component of the contraction to stimulation at 1 Hz in the presence of both antagonists was proportionately larger in decentralized arteries (Fig. 5Ab, P < 0.05). In all tissues, the residual response was fully blocked by tetrodotoxin (0.5 μm).
Figure 5. Lack of purinergic nerve-evoked responses in either control or decentralized tail arteries despite hypersensitivity to α,β-methylene ATP.
A, effects of suramin (0.1 mm) applied alone or together with phentolamine (1 μm) on contractions of control arteries (open columns, n = 6) and 2 week decentralized arteries (hatched columns, n = 6) to trains of 25 stimuli at 0.5 (a) and 1 (b) Hz. B, the peak amplitude of the responses of control arteries (○) and 2 week decentralized arteries (•) to 0.3, 1, 3 and 10 μmα,β-methylene ATP (α,β-mATP). Data are presented as median and interquartile range, and statistical differences are indicated (*P < 0.05 and **P < 0.01). While suramin did not inhibit nerve-evoked contractions, responses of decentralized arteries to α,β-methylene ATP were increased.
Responses to α,β-methylene ATP
The sensitivity of control and 2 week decentralized arteries to α,β-methylene ATP (0.3–10 μm) was determined. Over this range of concentrations, although the peak amplitudes of the responses of 2 week decentralized arteries to α,β-methylene ATP were quite variable, they were significantly greater than those of control arteries (Fig. 5B). The response to α,β-methylene ATP (3 μm) was fully blocked by suramin (0.1 mm, n = 3).
Responses to 60 mm K+ solution
Figure 6A shows the contractile response of a 2 week sham-operated artery and a 2 week decentralized artery to 60 mm K+. At 2 days p.o., the response of decentralized arteries did not differ from that of control arteries (Fig. 6B). However, the contractile responses of 2 week and 7 week decentralized arteries were greater in amplitude than those of their respective control arteries (Fig. 6C and D).
Figure 6. Enhanced responses to 60 mm K+ suggest a nonspecific increase in reactivity in decentralized arteries.
A, contractions of 2 week sham-operated and 2 week decentralized arteries to 60 mm K+ in the presence of α-adrenoceptor antagonists (10 nm prazosin + 0.1 μm idazoxan). B–D, comparison of responses to 60 mm K+ in (B) 2 day control (n = 6) and decentralized (n = 7) arteries, (C) 2 week control (n = 7) and decentralized (n = 7) arteries, and (D) 7 week control (n = 7) and decentralized (n = 10) arteries. Data are presented as median and interquartile range, and statistical differences are indicated (**P < 0.01). In comparison with the arteries from control animals, the 2 week and 7 week decentralized arteries had larger responses to 60 mm K+. In addition, on washout, the decay of the K+-evoked contraction was much slower in 2 week decentralized arteries (see A).
The contraction of 2 day decentralized arteries to 60 mm K+ was smaller than that of 2 week and 7 week decentralized arteries (P < 0.01). However, there was no difference between the contractile responses of 2 week and 7 week decentralized arteries to 60 mm K+.
On returning to the normal bathing solution, the half-decay time of the K+-evoked contraction was about twice as long in 2 week decentralized arteries (15.9 s, IQR 10.4–19.3) as in 2 week control arteries (7.8 s, IQR 6.4–8.4) (Fig. 6A). However, there were no differences in the half-decay time at the other time points (2 day, control 6.1 s, IQR 5.9–6.6, decentralized 6.5 s, IQR 5.7–9.4; 7 week, control 11.3 s, IQR 8.2–11.9, decentralized 10.6 s, IQR 9.2–13.5).
Relation between postjunctional reactivity and nerve-evoked responses
The degree of association between the responses of arteries to electrical activation (100 pulses at 1 Hz) and their sensitivity to phenylephrine, clonidine and 60 mm K+ was assessed using Spearman's coefficient of rank correlation. While there was no correlation between the EC50 for phenylephrine and the response to electrical activation (P = 0.10), a significant negative correlation was found between the EC50 value for clonidine and the response to electrical activation (ρ = −0.45, P < 0.01). In addition, a positive correlation was found between the increase in effective pressure produced by 60 mm K+ and that produced by 100 stimuli at 1 Hz (ρ = 0.84, P < 0.0001).
Comparison between arteries from decentralized and spinalized animals
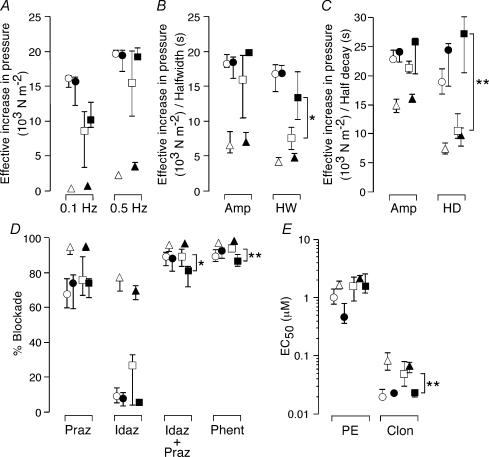
Figure 7 shows the data from decentralized arteries together with data from spinalized arteries (data from Yeoh et al. 2004). At 2 weeks and 7–8 weeks p.o., there were no significant differences between the contractile responses of decentralized and spinalized arteries to nerve stimulation (Fig. 7A and B) or to 60 mm K+ (Fig. 7C) except that, at 7–8 weeks, the half-width of the contraction to 10 stimuli at 10 Hz (Fig. 7B) and the 50% decay of the contraction elicited by 60 mm K+ (Fig. 7C) were longer in spinalized arteries than in decentralized arteries.
Figure 7. Decentralization and spinal transection produce similar changes in the reactivity of tail arteries.
A–E, comparison of data obtained from decentralized arteries (2 weeks ○; 7 weeks □) with those from spinalized arteries (2 weeks • 8 weeks ▪) (Yeoh et al. 2004). Combined data for age-matched and sham-operated control arteries are also shown (2 weeks Δ 7–8 weeks ▴). A, peak amplitudes of contractions to 25 pulses at 0.1 and 0.5 Hz. B, peak amplitude (Amp) and half-width (HW) of contractions to 10 pulses at 10 Hz. C, peak amplitude (Amp) and half-decay time (HD) of contractions to 60 mm K+. D, percentage blockade of contractions evoked by 100 pulses at 1 Hz produced by prazosin (10 nm, Praz) and idazoxan (0.1 μm, Idaz) applied alone and together as indicated. The data points labelled ‘Phent’ indicate the percentage blockade after adding phentolamine (1 μm) in the presence of both prazosin and idazoxan. E, EC50 values for phenylephrine (PE) and clonidine (Clon). Data are presented as median and interquartile range and, statistical differences between decentralized and spinalized arteries are indicated (*P < 0.05 and **P < 0.01). Decentralized and spinalized arteries had indistinguishable responses at 2 weeks. After 7–8 weeks, contractions to both nerve stimulation and raised K+ were no longer prolonged in decentralized arteries and these were less sensitive to clonidine. In contrast, the combinations of α-adrenoceptor antagonists blocked the contractions of spinalized arteries to a lesser extent.
The blockades produced by prazosin and idazoxan did not differ between decentralized and spinalized arteries at 2 weeks and 7–8 weeks, but the blockades produced by the combination of these agents and following the addition of phentolamine were smaller in spinalized arteries after 8 weeks (Fig. 7D). In addition, there was no difference in the sensitivity to phenylephrine between decentralized and spinalized arteries at either time point, but, at 7–8 weeks, spinalized arteries were slightly more sensitive to clonidine than decentralized arteries.
Relative to control arteries, the changes produced in decentralized and spinalized arteries were remarkably similar (Fig. 7). There was a slight difference in the duration of the transient hypersensitivity to phenylephrine (see Yeoh et al. 2004). In addition, the spinalized arteries had reduced sensitivity to the combination of α-adrenoceptor antagonists at 2 weeks p.o. (see Yeoh et al. 2004), but this was not seen in decentralized arteries until 7 weeks. Spinalized arteries, unlike decentralized arteries, showed a maintained supersensitivity to clonidine. Similarly, the prolongation of the contractions to both 10 stimuli at 10 Hz and 60 mm K+ was maintained after spinalization but transient in decentralized arteries (see Yeoh et al. 2004).
Discussion
After decentralization, responses of the isolated rat tail artery to electrical activation of sympathetic perivascular nerve terminals were greatly enhanced in amplitude. This increased responsiveness was clearly evident 2 days after decentralization, had increased further after 2 weeks, and was maintained for 7 weeks. In addition, the responses to nerve stimulation were prolonged. These changes were remarkably similar in kind and extent to those observed in tail arteries after mid-thoracic spinal transection (Fig. 7A and B; see Yeoh et al. for details). After both interventions, tail arteries also showed enhanced sensitivity to the α1-adrenoceptor agonist phenylephrine, to the α2-adrenoceptor agonist clonidine, and to raised extracellular [K+]. In both groups of arteries, the increased sensitivity to phenylephrine was transient but, unlike the spinalized arteries, neither the increased sensitivity to clonidine nor the prolongation of the nerve-evoked contractions was maintained in the decentralized arteries. These findings show that, despite the marked parallels between the changes in vascular reactivity, the effects of decentralization and spinalization are not identical.
In the rabbit ear artery, decentralization also produced an increase in the contractile response to nerve stimulation (Tsuru & Bevan, 1980; Tsuru & Uematsu, 1986) and the facilitatory effect of decentralization was most marked at low frequencies of stimulation (0.1 Hz), as in the rat tail artery. In decentralized rabbit ear arteries, the effect on nerve-evoked contractions developed relatively slowly, reaching a plateau 4 weeks after the intervention (Tsuru & Uematsu, 1986). However, in contrast to the rat tail artery, supersensitivity to NA was fully developed after 1 week and did not change significantly over 8 weeks. The difference between postjunctional sensitivity and responsiveness to nerve stimulation might be explained by the increase in stimulus-evoked release of NA from chronically inactive nerve terminals (Tsuru et al. 1993), as has been shown for other decentralized tissues (Brown et al. 1967; Farnebo & Hamberger, 1973). Direct measurement of NA overflow would clarify whether decentralization increased transmitter release in rat tail artery. However, NA overflow experiments were not performed because the amounts of NA in the perfusion fluid were below the level of detection of the available techniques (combined gas chromatography/mass spectrometry).
The sensitivity of neurally evoked contraction to blockade both by prazosin and by idazoxan was reduced in decentralized tail arteries after both 2 and 7 weeks. At 7 weeks, reduced blockade by these agents was not associated with supersensitivity to phenylephrine or clonidine, so it is unlikely that an increase in postjunctional receptors is the main cause of the reduced effectiveness of the antagonists. Furthermore, as complete blockade of α-adrenoceptors reduced neurally evoked contraction by about 95% in both control and decentralized arteries, the decreased effectiveness of prazosin and idazoxan cannot be explained by an increased role for cotransmitters in neural activation. Our data are therefore consistent with increased release of NA from perivascular sympathetic nerves after decentralization.
The increased sensitivity of decentralized arteries to phenylephrine lasted less than 2 weeks, and that to clonidine less than 7 weeks. Increased neuronal uptake of phenylephrine cannot explain the loss of hypersensitivity by 2 weeks as, at this time, there was no change in sensitivity to methoxamine, which is not a substrate for the neuronal NA transporter. The responses of decentralized arteries to nerve stimulation were negatively correlated with their EC50 for clonidine but not with that for phenylephrine. The spinalized arteries also showed only a transient supersensitivity to phenylephrine although it was still detectable at 2 weeks p.o. (Yeoh et al. 2004). As for decentralized arteries, the responses to nerve stimulation and sensitivity to clonidine (but not phenylephrine) were correlated in the spinalized arteries. Therefore, it is likely that changes in the sensitivity of the vascular smooth muscle to α2-adrenoceptor activation contribute, in part, to the increased response of both decentralized and spinalized arteries to nerve stimulation.
The blockade produced by each of prazosin and idazoxan was similar in decentralized and spinalized arteries (Fig. 7D). However, by 8 weeks, the blockade produced by the combination of prazosin and idazoxan and by the subsequent addition of phentolamine was less in the spinalized arteries (Fig. 7D). The α-adrenoceptor-antagonist-resistant component of the nerve-evoked contraction increased to about 15% of pre-drug-treatment values. This finding may indicate an increased role for cotransmitters in spinalized arteries that was not evident after decentralization.
Previous studies have reported that the P2-purinoceptor antagonist suramin inhibits the α-adrenoceptor-antagonist-resistant component of contraction in rat tail artery evoked by trains of stimuli at 20 Hz (Bao & Stjärne, 1993; Fukumitsu et al. 1999; Bradley et al. 2003). However, contractions of both control and 2 week decentralized arteries evoked by stimulation at 1 Hz (100 stimuli or 25 stimuli) were blocked to a similar extent by the combination of prazosin, idazoxan and phentolamine. and by suramin together with phentolamine. When applied alone, suramin potentiated contractions evoked by stimulation at 0.5 and 1 Hz in 2 week decentralized arteries and in some of the control arteries, as previously reported (Bao & Stjärne, 1993; Bao et al. 1993).
The concentration of suramin used in this study markedly reduces the amplitude of purinergic excitatory junction potentials (McLaren et al. 1995) and abolishes contractions to α,β-methylene ATP, consistent with blockade of P2X1-purinoceptors. It is unlikely that the augmentation of the nerve-evoked contraction produced by suramin is due to a nonselective action of this agent, as similar increases in the amplitude of electrically evoked responses are observed with the P2-purinoceptor antagonist, PPADS (unpublished observations), and when the P2X-purinoceptors are desensitized with α,β-methylene ATP (Bao et al. 1993). A presynaptic site of action of suramin is also unlikely, as this agent does not increase the release of endogenous NA evoked by 10 or 100 stimuli at 20 Hz (Bao & Stjärne, 1993; Bao et al. 1993). To explain the facilitatory action of suramin, it has been proposed that neuronally released ATP has a postjunctional inhibitory action on the NA-mediated component of contraction (Bao & Stjärne, 1993).
The amplitudes of the responses to 60 mm K+ in decentralized and spinalized arteries were increased to a similar extent after 2 weeks and after 7–8 weeks (Fig. 7C). In contrast, the prolonged relaxation following washout of 60 mm K+ was transient in decentralized arteries (Fig. 7C), but sustained in spinalized ones. Nevertheless, as the amplitude of responses of both decentralized and spinalized arteries to electrical stimulation was positively correlated with the amplitude of their responses to 60 mm K+, it seems likely that hyperreactivity of these vessels to neural activation can, in part, be attributed to a postjunctional change. While it was not possible to demonstrate a purinergic component of the nerve-evoked contractions, the 2 week decentralized arteries had augmented responses to α,β-methylene ATP, indicating that the smooth muscle had increased sensitivity to a range of contractile agents. As contractions of the tail artery to clonidine, α,β-methylene ATP and high [K+] are all dependent on the influx of extracellular Ca2+ (Abe et al. 1987; Chen & Rembold, 1995; McLaren et al. 1998), a possible explanation for the increased response to these agents is that the contractile mechanism is selectively sensitized to Ca2+ entering from outside the cell. Alternatively, as these agents depolarize the muscle, depolarization-induced Ca2+ entry may be enhanced.
The differences between some decentralization-induced changes and those after spinal transection may reflect their exposure to different conditions in vivo. (a) Arterial blood pressure in rats spinalized at T7–8 is reduced initially but recovers to normal by 4 weeks (Krassioukov et al. 2002; Rodenbaugh et al. 2003), whereas decentralization of the vasculature of the tail and lower hindlimbs is unlikely to modify blood pressure. (b) Circulating catecholamines are reduced after spinalization, as the major preganglionic input to the adrenal gland arises below T7 (Strack et al. 1988), but should be normal in decentralized animals. (c) Decentralized arteries receive no centrally derived input and denervated postganglionic neurones do not develop spontaneous activity (McLachlan, 1974; Jänig, 1995; Ireland, 1999). In contrast, spinalized vessels are exposed to low levels of sympathetic activity with intermittent reflex bursts, e.g. during the periodic manual expression of urine in our experiments (Yeoh et al. 2004). Reflexes caudal to an injury may be enhanced by novel intraspinal connections (Weaver et al. 1997) and/or inflammation (Popovich & Jones, 2003).
In conclusion, decentralization produced a marked increase in the response of the tail artery to nerve stimulation. As the responses of control and decentralized arteries to nerve stimulation were correlated with their responses to 60 mm K+ and with their sensitivity to clonidine, postjunctional changes probably contribute to the hyperreactivity to neural activation. The findings also suggest that silencing sympathetic postganglionic axons increases neurotransmitter release from their nerve terminals, as it does at neuronal synapses (Gallego & Geijo, 1987; Murthy et al. 2001). Responses of decentralized and spinalized arteries to nerve stimulation, 60 mm K+ and clonidine were all similarly augmented, suggesting that the major factor responsible for initiating the vascular hyperreactivity to sympathetic reflexes following spinal transection is decreased ongoing nerve activity.
Acknowledgments
This work was supported by the Christopher Reeve Paralysis Foundation (Contract Nos BAC1-0101-1 and BAC1-0101-2) and National Health and Medical Research Council of Australia (Project Grant I.D. 209632). J.A.B. is a National Health and Medical Research Council of Australia Senior Research Fellow.
References
- Abe K, Matsuki N, Kasuya Y. Pharmacological and electrophysiological discrimination of contractile responses to selective α1- and α2-adrenoceptor agonists in rat tail artery. Jpn J Pharmacol. 1987;45:249–261. doi: 10.1254/jjp.45.249. [DOI] [PubMed] [Google Scholar]
- Bao JX, Gonon F, Stjärne L. Frequency- and train length-dependent variation in the roles of postjunctional α1- and α2-adrenoceptors for the field stimulation-induced neurogenic contraction of rat tail artery. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:601–616. doi: 10.1007/BF00166943. [DOI] [PubMed] [Google Scholar]
- Bao JX, Stjärne L. Dual contractile effects of ATP released by field stimulation revealed by effects of α,β-methylene ATP and suramin in rat tail artery. Br J Pharmacol. 1993;110:1421–1428. doi: 10.1111/j.1476-5381.1993.tb13979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E, Law A, Bell D, Johnson CD. EfVects of varying impulse number on cotramsmitter contributions to sympatketic vasoconstriction in rat tail artery. Am J Physiol Heart Circ Physiol. 2003;284:H2007–2014. doi: 10.1152/ajpheart.01061.2002. [DOI] [PubMed] [Google Scholar]
- Brock JA, McLachlan EM, Rayner SE. Contribution of alpha-adrenoceptors to depolarization and contraction evoked by continuous asynchronous sympathetic nerve activity in rat tail artery. Br J Pharmacol. 1997;120:1513–1521. doi: 10.1038/sj.bjp.0701055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Dearnaley DP, Geffen LB. Noradrenaline storage and release in the decentralized spleen. Proc R Soc Lond B Biol Sci. 1967;168:48–56. doi: 10.1098/rspb.1967.0050. [DOI] [PubMed] [Google Scholar]
- Chen XL, Rembold CM. Phenylephrine contracts rat tail artery by one electromechanical and three pharmacomechanical mechanisms. Am J Physiol Heart Circ Physiol. 1995;268:H74–81. doi: 10.1152/ajpheart.1995.268.1.H74. [DOI] [PubMed] [Google Scholar]
- Farnebo LO, Hamberger B. Chronic decentralization prevents alpha-receptor mediated regulation of noradrenaline release in the field stimulated rat iris. Brain Res. 1973;62:477–482. doi: 10.1016/0006-8993(73)90711-7. [DOI] [PubMed] [Google Scholar]
- Fleming WW, Westfall DP. Adaptive supersensitivity. In: Trendelenburg U, editor. Handbook of Experimental Pharmacology: Catecholamines. Vol. 90. Berlin: Springer-Verlag; 1988. pp. 509–559. [Google Scholar]
- Fukumitsu A, Takano Y, Iki A, Honda K, Saito R, Katsuragi T, Kamiya H. Endogenous ATP released by electrical field stimulation causes contraction via P2x- and P2y-purinoceptors in the isolated tail artery of rats. Jpn J Pharmacol. 1999;81:375–380. doi: 10.1254/jjp.81.375. [DOI] [PubMed] [Google Scholar]
- Gallego R, Geijo E. Chronic block of the cervical trunk increases synaptic efficacy in the superior and stellate ganglia of the guinea-pig. J Physiol. 1987;382:449–462. doi: 10.1113/jphysiol.1987.sp016377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Haselton JR, Sun MK. Sympathoexcitatory neurons of the rostroventrolateral medulla and the origin of the sympathetic vasomotor tone. Prog Brain Res. 1989;81:105–116. doi: 10.1016/s0079-6123(08)62002-6. [DOI] [PubMed] [Google Scholar]
- Ireland DR. Preferential formation of strong synapses during re-innervation of guinea-pig sympathetic ganglia. J Physiol. 1999;520:827–837. doi: 10.1111/j.1469-7793.1999.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig WM. Ganglionic transmission in vivo. In: McLachlan EM, editor. Autonomic Ganglia. Luxembourg: Harwood Academic Publishers; 1995. pp. 349–395. [Google Scholar]
- Krassioukov AV, Johns DG, Schramm LP. Sensitivity of sympathetically correlated spinal interneurons, renal sympathetic nerve activity, and arterial pressure to somatic and visceral stimuli after chronic spinal injury. J Neurotrauma. 2002;19:1521–1529. doi: 10.1089/089771502762300193. [DOI] [PubMed] [Google Scholar]
- Langer SZ, Trendelenburg U. The effect of a saturable uptake mechanism on the slopes of dose–response curves for sympathomimetic amines and on the shifts of dose–response curves produced by a competitive antagonist. J Pharmacol Exp Ther. 1969;167:117–142. [PubMed] [Google Scholar]
- Li Z, Duckles SP. Acute effects of nicotine on rat mesenteric vasculature and tail artery. J Pharmacol Exp Ther. 1993;264:1305–1310. [PubMed] [Google Scholar]
- McLachlan EM. The formation of synapses in mammalian sympathetic ganglia reinnervated with preganglionic or somatic nerves. J Physiol. 1974;237:217–242. doi: 10.1113/jphysiol.1974.sp010479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM. Inceased number of SP/CGRP axons associated with the rat tail artery after reinnervation. Proc Aust Neuroscience Soc. 1996;7:128. [Google Scholar]
- McLaren GJ, Burke KS, Buchanan KJ, Sneddon P, Kennedy C. Evidence that ATP acts at two sites to evoke contraction in the rat isolated tail artery. Br J Pharmacol. 1998;124:5–12. doi: 10.1038/sj.bjp.0701772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren GJ, Kennedy C, Sneddon P. The effects of suramin on purinergic and noradrenergic neurotransmission in the rat isolated tail artery. Eur J Pharmacol. 1995;277:57–61. doi: 10.1016/0014-2999(95)00065-s. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Jones TB. Manipulating neuroinflam matory reactins in the injured spinal cord: back to basics. Trends Pharmacol Sci. 2003;24:13–17. doi: 10.1016/s0165-6147(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Rathner JA, McAllen RM. The lumbar preganglionic sympathetic supply to rat tail and hindpaw. J Auton Nerv Syst. 1998;69:127–131. doi: 10.1016/s0165-1838(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Rodenbaugh DW, Collins HL, Nowacek DG, DiCarlo SE. Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am J Physiol Heart Circ Physiol. 2003;285:H2605–2613. doi: 10.1152/ajpheart.00319.2003. [DOI] [PubMed] [Google Scholar]
- Sittiracha T, McLachlan EM, Bell C. The innervation of the caudal artery of the rat. Neuroscience. 1987;21:647–659. doi: 10.1016/0306-4522(87)90150-3. [DOI] [PubMed] [Google Scholar]
- Smith JE, Gilbey MP. Segmental origin of sympathetic preganglionic neurones regulating the tail circulation in the rat. J Auton Nerv Syst. 1998;68:109–114. doi: 10.1016/s0165-1838(97)00124-0. [DOI] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res. 1988;455:187–191. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U, Maxwell RA, Pluchino S. Methoxamine as a tool to assess the importance of intraneuronal uptake of 1-norepinephrine in the cat's nictitating membrane. J Pharmacol Exp Ther. 1970;172:91–99. [PubMed] [Google Scholar]
- Tsuru H, Bevan RD. Presynaptic sympathetic supersensitivity following long-term preganglionic denervation. Experientia. 1980;36:968–969. doi: 10.1007/BF01953824. [DOI] [PubMed] [Google Scholar]
- Tsuru H, Negita S, Teranishi Y, Sasa M. Release of sympathetic neurotransmitter evoked by electrical stimulation is increased in the chronically decentralized artery. Jpn J Pharmacol. 1993;63:285–294. doi: 10.1254/jjp.63.285. [DOI] [PubMed] [Google Scholar]
- Tsuru H, Uematsu T. Time course of the development of pre- and postjunctional supersensitivity in the rabbit ear artery after decentralization. Jpn J Pharmacol. 1986;40:273–282. doi: 10.1254/jjp.40.273. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Stjernberg L. Sympathetic activity in man after spinal cord injury. Outflow to skin below the lesion. Brain. 1984;107:183–198. doi: 10.1093/brain/107.1.183. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Cassam AK, Krassioukov AV, Llewellyn-Smith IJ. Changes in immunoreactivity for growth associated protein-43 suggest reorganization of synapses on spinal sympathetic neurons after cord transection. Neuroscience. 1997;81:535–551. doi: 10.1016/s0306-4522(97)00151-6. [DOI] [PubMed] [Google Scholar]
- Yeoh M, McLachlan EM, Brock JA. Tail arteries from chronically spinalized rats have potentiated responses to nerve stimulation in vitro. J Physiol. 2004;556:545–555. doi: 10.1113/jphysiol.2003.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]