Abstract
Waves of Ca2+-induced Ca2+ release occur in various cell types and are involved in the pathology of certain forms of cardiac arrhythmia. These arrhythmias include catecholaminergic polymorphic ventricular tachycardia (CPVT), certain cases of which are associated with mutations in the cardiac calsequestrin gene (CASQ2). To explore the mechanisms of Ca2+ wave generation and unravel the underlying causes of CPVT, we investigated the effects of adenoviral-mediated changes in CASQ2 protein levels on the properties of cytosolic and sarcoplasmic reticulum (SR) Ca2+ waves in permeabilized rat ventricular myocytes. The free [Ca2+] inside the sarcoplasmic reticulum ([Ca2+]SR) was monitored by fluo-5N entrapped into the SR, and cytosolic Ca2+ was imaged using fluo-3. Overexpression of CASQ2 resulted in significant increases in the amplitude of Ca2+ waves and interwave intervals, whereas reduced CASQ2 levels caused drastic reductions in the amplitude and period of Ca2+ waves. CASQ2 abundance had no impact on resting diastolic [Ca2+]SR or on the amplitude of the [Ca2+]SR depletion signal during the Ca2+ wave. However, the recovery dynamics of [Ca2+]SR following Ca2+ release were dramatically altered as the rate of [Ca2+]SR recovery increased ∼3-fold in CASQ2-overexpressing myocytes and decreased to 30% of control in CASQ2-underexpressing myocytes. There was a direct linear relationship between Ca2+ wave period and the half-time of basal [Ca2+]SR recovery following Ca2+ release. Loading the SR with the low affinity exogenous Ca2+ buffer citrate exerted effects quantitatively similar to those observed on overexpressing CASQ2. We conclude that free intra-SR [Ca2+] is a critical determinant of cardiac Ca2+ wave generation. Our data indicate that reduced intra-SR Ca2+ binding activity promotes the generation of Ca2+ waves by accelerating the dynamics of attaining a threshold free [Ca2+]SR required for Ca2+ wave initiation, potentially accounting for arrythmogenesis in CPVT linked to mutations in CASQ2.
In cardiac muscle, the process of excitation–contraction (EC) coupling relies on Ca2+ influx from the extracellular milieu activating the SR Ca2+ release channels (ryanodine receptors, RyRs) in the sarcoplasmic reticulum (SR). This mechanism is known as Ca2+-induced Ca2+ release (CICR) (Fabiato, 1985). In addition to being controlled by cytosolic Ca2+, the RyR channels are modulated by Ca2+ levels inside the SR (Györke et al. 2002). The closure of Ca2+ release channels upon decline in intra-SR Ca2+ levels (luminal Ca2+-dependent deactivation) appears to account for the termination of CICR (Terentyev et al. 2002, 2003). Ca2+ released to the cytosol is taken back by the Sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) to the SR where it is stored on the Ca2+-binding protein CASQ2 before it can be released again during the next activation cycle.
Due to its self-regenerating nature, CICR is an intrinsically unstable process. Under various pathological conditions, generally characterized by an increased SR Ca2+ content (i.e. ‘Ca2+ overload’), CICR can arise spontaneously, leading to self-propagating Ca2+ waves (Orchard et al. 1983; Wier et al. 1987; Lipp & Niggli, 1993; Cheng et al. 1996; Lukyanenko et al. 1996). Although it is clear that Ca2+ wave propagation involves diffusion-coupled CICR (Cheng et al. 1996; Lukyanenko et al. 1999; Izu et al. 2001), the precise roles of cytosolic and luminal Ca2+ in wave generation remain to be defined. The Ca2+ waves are arrythmogenic because they induce inward membrane currents that lead to delayed afterdepolarizations (DADs) and irregular action potentials (APs), accounting for the pathological mechanism of triggered arrhythmia (Kass & Tsien, 1982; Allen et al. 1984; Marban et al. 1986; Lakatta & Guarnieri, 1993).
Catecholaminergic polymorphic ventricular tachycardia is a form of inherited exercise-induced arrhythmia associated with mutations in CASQ2 and RyR (Coumel et al. 1978; Laitinen et al. 2001; Priori et al. 2001; Lahat et al. 2001; Postma et al. 2002). Recently, we showed that reduced CASQ2 levels or expression of a specific CPVT-associated CASQ2 mutant (D307H) leads to the generation of spontaneous Ca2+ transients and arrythmogenic DADs (i.e. cellular arrhythmia) in regularly paced cardiac myocytes (Terentyev et al. 2003; Viatchenko-Karpinski et al. 2004). We provided evidence to suggest that the generation of spontaneous, extrasystolic Ca2+ transients in these myocytes was caused by the premature recovery of the release channels from a luminal Ca2+-dependent refractory state. This refractory state is normally induced by the decline in [Ca2+]SR following SR Ca2+ release (Terentyev et al. 2003). However, the specific molecular mechanisms by which genetic defects in CASQ2 lead to cellular arrhythmia remain to be elucidated.
In the present study we have directly examined the effects of altered CASQ2 levels on the properties of spontaneous Ca2+ waves monitored in the cytosolic and luminal compartments of myocytes permeabilized with saponin. Our results indicate that CASQ2 influences the frequency of spontaneous Ca2+ waves by determining the rate of recovery of [Ca2+]SR after each release.
Methods
Ca2+ measurements in the cytosolic and SR luminal compartments
Single ventricular myocytes were obtained from adult male Sprague-Dawley rat hearts through enzymatic dissociation (Györke et al. 1997). Rats were anaesthetized with Nembutal and killed by exsanguination. The protocol for animal use was reviewed and approved by the Institutional Animal Care and Use Committee and complied with US Public Health Service policy on humane care and use of laboratory animals. The standard Tyrode solution contained (mm): 140 NaCl, 5.4 KCl, 0.5 MgCl2, 1 CaCl2, 10 Hepes, 0.25 NaH2PO4, 5.6 glucose, pH 7.3). The myocytes were permeabilized with saponin (0.01% for 45–60 s) in an ‘internal’ solution containing (mm): 120 potassium aspartate, 20 KCl, 0.81 MgCl2, 20 Hepes, 3 MgATP, 0.5 EGTA, 0.114 CaCl2 (free [Ca2+]∼100 nm), 10 phosphocreatine, 5 U ml−1 creatine phosphokinase (Lukyanenko & Györke, 1999). Fluo-3 potassium salt (10 μm, TefLabs) was added to the internal solution for measurement of cytosolic [Ca2+] changes. The acetoxymethyl ester form of the low-affinity calcium indicator, fluo-5N (fluo-5N AM; Molecular Probes), entrapped inside the SR (Kabbara & Allen, 2001; Shannon et al. 2003) was used to assess changes in the intraluminal SR Ca2+ concentration. The myocytes were incubated with 10 μm fluo-5N AM and 0.05% pluronic detergent (Molecular Probes, OR, USA) for 6–8 h at 37°C to load the dye into the SR. After fluo-5N AM loading, the myocytes were permeabilized to remove the dye from the cytoplasm. Calcium waves were initiated by lowering the Ca2+ buffering capacity in the internal solution as a result of decreasing the concentration of EGTA from 0.5 mm to 0.1 mm (Lukyanenko & Györke, 1999) at a constant [Ca2+] of 100 nm. Ca2+ levels in the internal solutions were calculated using a computer program (WinMAXC 1.80, Stanford University, CA, USA) and verified by Ca2+ electrode measurements using standards from Calbiochem. In experiments involving the addition of 10 mm caffeine, the fluo-5N traces were corrected for ∼10% fluorescence quenching by this agent (determined at 100 μm Ca2+). In some experiments, potassium salt of citrate was added to the internal solution at a final concentration of 20 mm by replacing osmotically equivalent amounts of potassium aspartate. Myocytes were imaged with a Laser Scanning Confocal System (Bio-Rad MRC-1024ES interfaced to an Olympus IX-70 inverted microscope equipped with an Olympus ×60, 1.4 NA oil objective). Fluo-3 and fluo-5N were excited by light at 488 nm, and fluorescence was recorded at wavelengths > 515 nm in either the x–y or x–t (line scan) mode of the confocal system at a rate of 6 ms per line. All line-scan images were obtained by scanning the myocytes in the longitudinal direction. Values of relative fluorescence from line-scan images were obtained as: (F(x,t) −Fmin)/(Fbaseline−Fmin), where F(x,t) is fluorescence at the linear coordinate x at time t, Fmin is minimal fluorescence during SR depletion, and Fbaseline is baseline fluorescence (Cheng et al. 1999; Lukyanenko et al. 2001). Image processing and analysis was performed using IDL software (Research Systems Inc., Boulder, CO, USA).
Adenoviral gene transfer
Recombinant adenoviruses containing the full-length CASQ2 coding sequence in the sense and antisense orientation (Ad-CASQ2 and Ad-CASQ2as, respectively) and an adenovirus containing a truncated CASQ2 coding region (1–70, Ad-control) were constructed as previously described (Terentyev et al. 2003). Isolated ventricular myocytes were plated on laminin-coated glass coverslips in serum-free medium 199 containing 25 mm NaHCO3, 5 mm creatine, 5 mm taurine, 10 units ml−1 penicillin, 10 μg ml−1 streptomycin, 10 μm gentamicin and incubated at 5% CO2–95% air at 37°C (Terentyev et al. 2003). After approximately 1 h of incubation, the medium was aspirated along with unattached myocytes and replaced with the same medium containing a particular type of adenovirus at a multiplicity of infection of 100. The myocytes were incubated with adenoviruses for 48–56 h and CASQ2 protein levels were determined by immunoblot analysis using antibodies specific for CASQ2 (1: 2500; PA-913, Affinity Bioreagents, Golden, CO, USA) (Terentyev et al. 2003).
Statistics
The results are presented as mean ±s.e.m. Statistical differences were tested using ANOVA (P < 0.05).
Results
Effects of CASQ2 levels on properties of cytosolic Ca2+ waves
We first investigated the effects of increased and decreased CASQ2 protein levels on the properties of cytosolically measured spontaneous Ca2+ waves in permeabilized myocytes. As in our previous studies (Terentyev et al. 2003; Viatchenko-Karpinski et al. 2004), we used adenoviral constructs containing the canine CASQ2 coding region in the sense (Ad-CASQ2) or antisense orientations (Ad-CASQ2as) to either increase or decrease CASQ2 expression in cardiac myocytes, respectively. Myocytes infected with a viral construct (Ad-control) containing a truncated CASQ2 coding region served as a control for the effects of viral infection (Terentyev et al. 2003). CASQ2 levels were assessed by Western blot analysis using a CASQ2-specific antibody. Consistent with our previous report (Terentyev et al. 2003), infection of myocytes with Ad-CASQ2 resulted in ∼3.5-fold increases in CASQ2 abundance, whereas infection with the Ad-CASQ2as viral construct resulted in a decrease in CASQ2 expression to 30% of control at ∼48 h after infection (not shown). As we have shown previously (Terentyev et al. 2003), these acute changes in CASQ2 abundance are not accompanied by the altered expression of other major SR Ca2+ handling proteins, including SERCA and phospholamban.
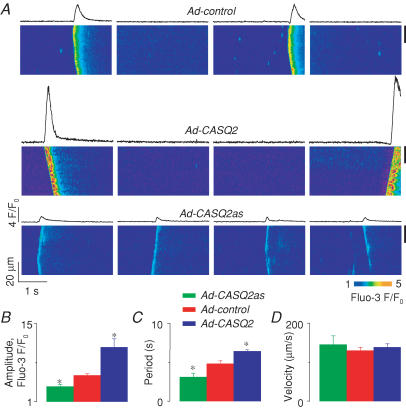
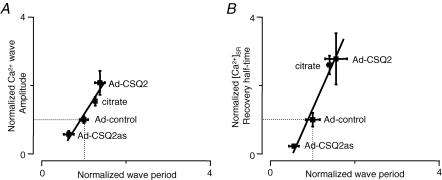
To promote the generation of spontaneous Ca2+ waves, myocytes permeabilized with saponin (Lukyanenko & Györke, 1999) were exposed to an ‘internal’ solution with low buffering strength (0.1 mm EGTA; 10 μm fluo-3, pCa 7). Under these conditions, control myocytes generated Ca2+ waves with a frequency of about 12 per minute. Representative line-scan images of Ca2+ waves along with the corresponding averaged fluo-3 fluorescence profiles measured in control, CASQ2-overexpressing and CASQ2-underexpressing myocytes are shown in Fig. 1A. The amplitude of Ca2+ waves and the length of interwave intervals (period) were greater in myocytes with increased CASQ2 levels (Ad-CASQ2), whereas Ca2+ wave amplitude and period were decreased in myocytes with reduced CASQ2 (Ad-CASQ2as) levels (Fig. 1B and C, respectively). At the same time, alterations in CASQ2 abundance did not affect the propagation velocity of Ca2+ waves (Fig. 1D). The relationship between the amplitude and period of Ca2+ waves is shown in Fig. 7A. The plot indicates that there is a direct linear correlation between wave amplitude and period. Since the amplitude of the spontaneous Ca2+ transients is a rough measure of the SR Ca2+-buffering capacity and the rate of free intra-SR [Ca2+] recovery after each release should be inversely proportional to the concentration of Ca2+-binding sites within the SR, these results suggest that Ca2+ wave generation is influenced by free [Ca2+] inside the SR luminal compartment.
Figure 1. Effects of increased and reduced CASQ2 levels on properties of cytosolic Ca2+ waves in permeabilized myocytes.
A, representative line-scan images of fluo-3 fluorescence and time-dependent profiles of spontaneous cytosolic Ca2+ waves (from the region indicated by the black bar on the right) acquired in permeabilized myocytes infected with Ad-control, Ad-CASQ2 and Ad-CASQ2as vectors (as indicated). B, C and D, pooled data on changes in the amplitude (B), period (C) and velocity (D) of cytosolic Ca2+ waves in myocytes infected with Ad-control, Ad-CASQ2 and Ad-CASQ2as vectors. The data are presented as means ±s.e.m., n = 4–6; * significantly different from control at P < 0.05.
Figure 7. Dependency of Ca2+ wave frequency on [Ca2+]SR recovery dynamics.
A, Ca2+ wave period as a function of wave amplitude for different levels of CASQ2 and citrate present in the SR compartment. B, Ca2+ wave period as a function of basal [Ca2+]SR recovery half-time following release for different levels of CASQ2 and citrate present in the SR compartment. The data were normalized with respect to control.
Effects of CASQ2 levels on SR luminal Ca2+ waves
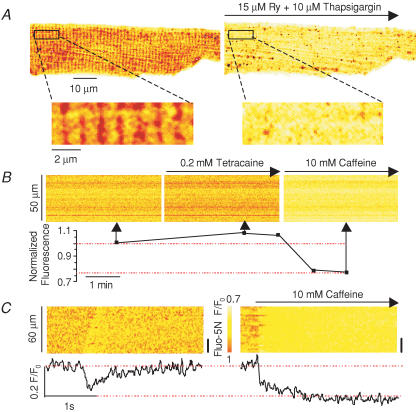
The free intra-SR [Ca2+] was monitored in the same three groups of myocytes (i.e. Ad-control, Ad-CASQ2 and Ad-CASQ2as) with the low affinity Ca2+ indicator fluo-5N entrapped in the SR (Kabbara & Allen, 2001; Shannon et al. 2003). Loading the myocytes with fluo-5N resulted in a characteristic cross-striated staining pattern at about 2 μm that was largely eliminated by emptying the SR Ca2+ stores with either ryanodine (15 μm) and thapsigargin (10 μm) (Fig. 2A) or caffeine (10 mm) (not shown). In addition, line-scan image recordings from fluo-5N-stained myocytes revealed that the inhibition of Ca2+ leakage from the SR by tetracaine (0.2 mm), an inhibitor of RyR2, led to an increase in intra-SR fluo-5N fluorescence (8 ± 1%, n = 3). Conversely, discharging the SR Ca2+ stores by caffeine (10 mm) application reduced the fluorescence signal (20 ± 1%; n = 4). Representative examples of these experiments are shown in Fig. 2B. The line-scan images at the top were obtained at different times before and after exposing the cell to 0.2 mm tetracaine and 10 mm caffeine; the graph below shows the corresponding changes of average fluorescence in the course of the experiment. These results indicate that fluo-5N can be loaded into the SR to monitor luminal Ca2+ in permeabilized myocytes. The increase of the intra-SR fluorescence signal in response to tetracaine suggests that fluo-5N was not saturated in our experiments. These data also confirm that Ca2+ leakage through RyRs plays an important role in establishing the Ca2+ gradient across the SR (Lukyanenko et al. 2001).
Figure 2. Fluorescence signals from intra-SR fluo-5N in permeabilized myocytes.
A, images of a fluo-5N-loaded myocyte before and 10 min after application of 15 μm ryanodine and 10 μm thapsigargin (upper panels). Enlargement of the area indicated by the rectangular boxes (lower panels). B, line-scan images of fluo-5N fluorescence from a myocyte before (left) and after sequential additions of 0.2 mm tetracaine (middle) and 10 mm caffeine (right). The lower panel plots averaged fluo-5N fluorescence in the course of the experiment. C, representative line-scan images of fluo-5N fluorescence and time-dependent profiles of an intra-SR Ca2+ wave (left) and caffeine-induced intra-SR Ca2+ transient (right). The time-dependent profiles were taken from the region indicated by black bars on the right.
Spontaneous Ca2+ waves were marked by a transient decrease in the spatially resolved fluo-5N fluorescence along the line scan and of the averaged fluorescence signal (Fig. 2C, left). To assess the portion of total SR Ca2+ released by the wave, the SR Ca2+ stores were emptied by caffeine application. The amplitude of the luminal Ca2+ depletion signal was about 80% (n = 20) of that induced by caffeine (Fig. 2C), consistent with the notion that regenerative Ca2+ waves result in the liberation of nearly all of the Ca2+ available for release from the SR.
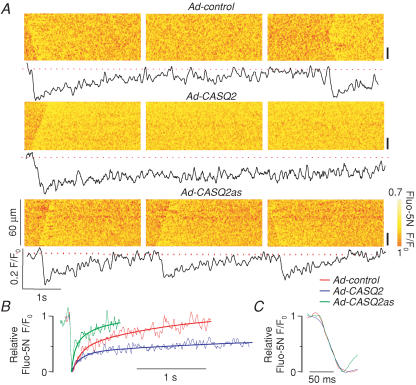
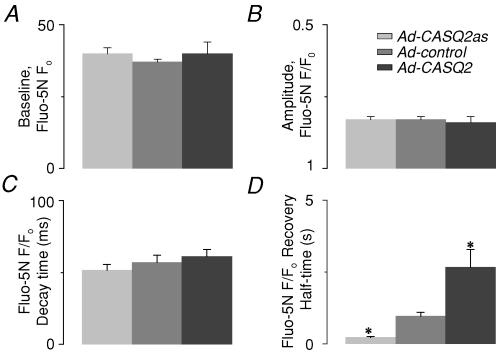
The effects of CASQ2 protein abundance on the properties of SR luminal Ca2+ waves are illustrated in Fig. 3A by a series of line-scan image recordings and corresponding averaged fluo-5N fluorescence signals from a representative control, CASQ2-overexpressing and CASQ2-underexpressing myocytes. CASQ2 abundance had no impact on basal (‘diastolic’) [Ca2+]SR or on the amplitude of the maximum [Ca2+]SR depletion signal during the wave (Fig. 4A and B, respectively). It is important to consider that the steady-state free [Ca2+] in a closed compartment such as the SR is determined by the transport mechanisms in the enclosing membrane. Therefore, Ca2+ binding to interluminal binding sites may affect the kinetics of luminal [Ca2+] changes but not the final steady-state [Ca2+] which is set by a balance between the Ca2+ uptake by SERCA and Ca2+ leak through the RyR channels. Indeed, the dynamics of [Ca2+]SR recovery following release were dramatically altered by changes in CASQ2 protein levels. The SR Ca2+ wave signals at each pixel along the line-scan were aligned at the peak and averaged to assess the quantitative effects of alterations in CASQ2 protein levels on Ca2+ dynamics inside the SR during Ca2+ release and the subsequent refilling of the SR. Whilst the rate of [Ca2+]SR decline was similar for all conditions (Figs 3C and 4C), the half-time of recovery of basal [Ca2+]SR increased ∼3-fold in CASQ2-overexpressing cells and decreased to ∼25% of control levels in CASQ2-underexpressing cells (Figs 3B and 4D). These changes in intra-SR [Ca2+] recovery kinetics were in agreement with the observed changes in the period of cytosolically measured Ca2+ waves (Fig. 1A and C). Our SR luminal Ca2+ measurements suggest that initiation of spontaneous Ca2+ waves always occurs at the same free intra-SR [Ca2+] regardless of the amount of total Ca2+ in the SR, and thus provide evidence for the role of RyR luminal Ca2+ modulation in the initiation of Ca2+ waves.
Figure 3. Effects of increased and reduced CASQ2 levels on properties of intra-SR Ca2+ waves in permeabilized myocytes.
A, representative line-scan images of fluo-5N fluorescence and time-dependent profiles of intra-SR Ca2+ waves acquired in permeabilized myocytes infected with Ad-control, Ad-CASQ2 and Ad-CASQ2as vectors (as indicated). The time-dependent fluorescence profiles were taken from the region indicated by the black bars on the right. B, superimposed relative fluo-5N fluorescence signals acquired during and following SR Ca2+ release in myocytes infected with Ad-control, Ad-CASQ2 and A-CASQ2as vectors (as indicated). The recovery phases of the relative fluo-5N fluorescence signals were fitted with single exponential functions C, expansion of the descending phase of the relative fluorescence signals.
Figure 4. Spatio-temporal properties of intra-SR Ca2+ waves for different levels of CASQ2 in the SR compartment.
Pooled data on changes in the baseline (‘diastolic’) [Ca2+]SR (A), amplitude of [Ca2+]SR reduction (B), time to peak [Ca2+]SR reduction (C) and half-time of [Ca2+]SR recovery (D) in myocytes infected with Ad-control, Ad-CASQ2 and Ad-CASQ2as vectors. The data are presented as means ±s.e.m., n = 4–6; * significantly different from control at P < 0.05.
Effects of SR luminal citrate on properties of cytosolic-and luminal Ca2+ waves
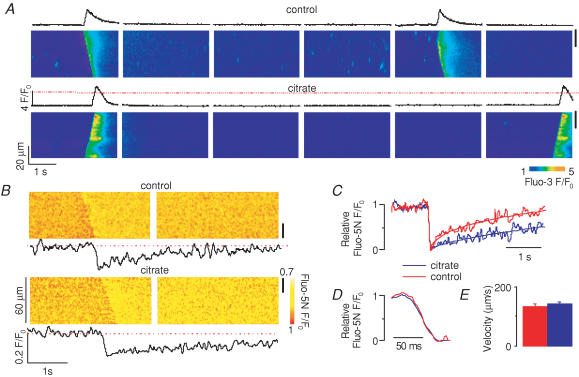
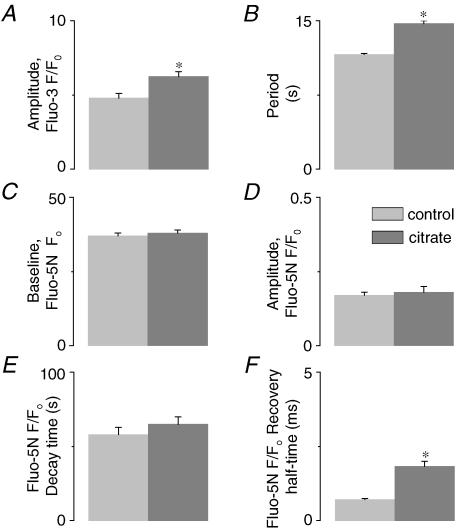
In addition to influencing the SR Ca2+ release channels by modulating the free [Ca2+]SR near their luminal side, CASQ2 may also affect the Ca2+ release mechanism more directly through protein–protein interactions with RyR, triadin and junctin (Zhang et al. 1997; Györke et al. 2004). To distinguish between these possibilities, we explored the effects of loading the SR with citrate, an exogenous low-affinity Ca2+ buffer (Terentyev et al. 2002). We reasoned that if the effects of CASQ2 were entirely due to its action as a buffer, loading the SR with citrate would have the same effect as increasing CASQ2 protein levels. Permeabilized myocytes were incubated in a citrate-containing internal solution for 20 min as previously described (Terentyev et al. 2002), after which citrate was removed from the cytosol, effectively leaving this Ca2+ chelator entrapped in the SR. Representative line-scan images of cytosolic and intra-SR Ca2+ waves are shown in Fig. 5A and B, respectively. Loading the SR with citrate resulted in increased amplitude and period of cytosolic Ca2+ waves (Fig. 6A and B, respectively) in a manner similar to that observed in CASQ2-overexpressing myocytes. Additionally, the recovery dynamics of [Ca2+]SR were slowed in the presence of luminal citrate (Figs 5C and 6F), an effect similar to that seen in myocytes overexpressing CASQ2. Furthermore, in agreement with the effects of increased CASQ2 expression, luminal citrate did not alter the propagation velocity of Ca2+ waves (Fig. 5E). Citrate loading also did not change the rate of [Ca2+]SR decline (Figs 5D and 6E) or the resting [Ca2+]SR (Fig. 6C) or maximum [Ca2+]SR depletion signal (Fig. 6D). Examining the relationship between the amplitude and period of cytosolic Ca2+ waves for control, citrate-loaded, as well as CASQ2 overexpressing and underexpressing myocytes shows that all data points fall on a single straight line (Fig. 7A). Similarly, there was a direct linear relationship between the half-time of basal [Ca2+]SR recovery and Ca2+ wave period measured in the same four groups of cells (Fig. 7B). These results suggest that the effects of CASQ2 levels on Ca2+ wave properties could be attributed to its action as a Ca2+ buffer.
Figure 5. Effects of SR intraluminal citrate loading on properties of cytosolic and luminal Ca2+ waves.
A, representative line-scan images of fluo-3 fluorescence and time-dependent profiles of cytosolic Ca2+ waves acquired in permeabilized myocytes before and after loading their SR with 20 mm citrate (as indicated). B, representative line-scan images of fluo-5N fluorescence and time-dependent profiles of intra-SR Ca2+ waves acquired in permeabilized myocytes before and after SR loading with 20 mm citrate (as indicated). The time-dependent profiles were taken from the regions indicated by black bars on the right. C, superimposed relative fluo-5N fluorescence signals acquired during and following SR Ca2+ release before (red) and after (blue) SR loading with 20 mm citrate in myocytes infected with Ad-control, Ad-CASQ2 and Ad-CASQ2as vectors (as indicated). The recovery phases of the relative fluo-5N fluorescence signals were fitted with single exponential functions. D, expansion of the descending phase of the relative fluorescence signals. E, pooled data on changes in the velocity of Ca2+ waves.
Figure 6. Effects of luminal citrate on spatio-temporal properties of cytosolic and luminal Ca2+ waves.
A and B, pooled data on changes in the amplitude (A) and period (B) of cytosolic Ca2+ waves measured with fluo-3. C and D, pooled data on changes in the baseline [Ca2+]SR (C) and amplitude (D) of the [Ca2+]SR depletion signals measured with SR-entrapped fluo-5N. E and F, pooled data on changes in time to peak reduction (F) and half-time of recovery of [Ca2+]SR (E) during and following Ca2+ wave-induced Ca2+ release in myocytes before and after SR loading with 20 mm citrate. The data are presented as means ±s.e.m., n = 3–4; * significantly different from control at P < 0.05.
Discussion
Intracellular Ca2+ waves produced by the release of Ca2+ from the SR have been implicated in various cardiac arrhythmias, including effort-induced tachycardia linked to mutations in CASQ2 (Priori & Corr, 1990; Lakatta & Guarnieri, 1993; Leenhardt et al. 1995; Terentyev et al. 2003; Viatchenko-Karpinski et al. 2004). Ca2+ waves are generally associated with an increased SR Ca2+ content. Therefore, defining the role of free luminal Ca2+ in the generation of Ca2+ waves is of considerable interest. In the present study, we directly measured free intra-SR Ca2+ in rat ventricular myocytes expressing different levels of CASQ2 protein to explore the underlying mechanisms of Ca2+ wave generation.
Role of luminal Ca2+ in Ca2+ wave generation
In principle, increased SR Ca2+ content (Ca2+ overload) could result in the generation of spontaneous Ca2+ waves through two different mechanisms. One possibility is that increased amounts of releasable Ca2+ stored in the SR stimulate the process of CICR, which is also involved in normal EC coupling, by increasing the positive feedback of released Ca2+ on the Ca2+-activated RyR channels (Stern et al. 1988). The other possibility is that elevated free [Ca2+] in the SR compartment enhances the functional activity of the Ca2+ release channels by acting at RyR-associated luminal Ca2+-sensing sites (Lukyanenko et al. 1999). Our direct measurements of [Ca2+]SR indicate that spontaneous Ca2+ wave generation in cardiac myocytes consistently occurred at the same threshold free intra-SR [Ca2+] regardless of the amount of total Ca2+ available for release inside the SR and released to the cytosol. Enhancing the intra-SR Ca2+ buffering capacity through either overexpressing CASQ2 or loading the SR with citrate delayed the functional restitution of release sites after each release (as assessed from changes in Ca2+ wave period) by slowing the recovery of free intra-SR [Ca2+]. In contrast, reducing the intra-SR Ca2+-buffering capacity by decreasing CASQ2 protein levels accelerated the functional restitution or readiness of release sites by accelerating the recovery of [Ca2+]SR. Thus, our results suggest that the modulation of RyR channel activity by luminal Ca2+ is a critical factor in the initiation of spontaneous cardiac Ca2+ waves.
Interestingly, CASQ2 abundance and SR luminal citrate had no discernable impact on the propagation velocity of Ca2+ waves despite the drastic changes in Ca2+ wave amplitude. Increased amounts of Ca2+ released to the cytosol would be expected to enhance Ca2+ wave propagation via classical CICR. Why was this not observed? One possibility is that Ca2+ wave propagation involves mechanisms other than CICR. One such model postulates that Ca2+ release occurs when a heavily Ca2+-loaded SR region reaches its limit, then extra Ca2+ uptake in neighbouring SR regions contributes to activation in the adjacent regions by raising [Ca2+]SR. If Ca2+ wave propagation was due to this kind of local SR Ca2+ overload spreading ahead of the wave front, then increased luminal Ca2+ buffering should slow Ca2+ wave propagation by delaying the attainment of a threshold [Ca2+]SR required for release activation. However, our direct measurements of [Ca2+]SR did not reveal a local elevation of [Ca2+]SR ahead of the wave front predicted by this model. Additionally, we have shown previously that Ca2+ wave propagation is not reduced but rather is enhanced when SERCA is inhibited by thapsigargin in myocytes whose SR was preloaded with Ca2+ (Lukyanenko et al. 1999). Thus this explanation does not seem likely. Another possibility relates to the observation that CASQ2 modulates both focal and global Ca2+ signals by changing the duration of their rising phases without affecting the rate of raise of these signals (Terentyev et al. 2003). These results were attributed to the ability of CASQ2 to influence Ca2+ release termination by buffering local free [Ca2+] in the vicinity of the RyR luminal Ca2+-sensing sites (Terentyev et al. 2003). Because local cytosolic [Ca2+] gradients tend to dissipate rapidly due to diffusion, CICR should be more sensitive to the rate of SR Ca2+ release (Ca2+ flux intensity) than to the overall amounts of Ca2+ released. This could account for the lack of effects of CASQ2 levels on Ca2+ wave propagation velocity. More studies, both experimental and theoretical, are needed to clearly elucidate the effects of SR luminal Ca2+ buffers on Ca2+ waves.
Molecular mechanisms of arrhythmia linked to CASQ2 mutations
Recently, we showed that reduced CASQ2 levels (or expression of a specific CPVT-related CASQ2 mutant) led to the generation of spontaneous Ca2+ transients and arrhythmogenic DADs in paced cardiac myocytes (Terentyev et al. 2003; Viatchenko-Karpinski et al. 2004). We hypothesized that the generation of spontaneous Ca2+ waves in these myocytes is due to a premature recovery of the release channels from a luminal Ca2+-dependent refractory state. In normal myocytes, the release channels become refractory when the Ca2+ level inside the SR declines as a consequence of release and stay refractory until the SR is refilled by the SERCA pump (Terentyev et al. 2002). The recovery of [Ca2+]SR will be faster when the concentration of luminal Ca2+ binding sites is reduced, potentially resulting in premature functional recovery of the RyR channels from refractoriness in myocytes underexpressing CASQ2. In the present study we directly demonstrate that reducing CASQ2 both increased spontaneous Ca2+ wave frequency and accelerated the rate of attaining diastolic [Ca2+]SR. Therefore these results provide compelling evidence for the proposed role of abnormal luminal [Ca2+] dynamics in the aetiology of CPVT.
Molecular mechanisms of CASQ2 effects
Although the precise nature of luminal Ca2+-sensing sites remains to be determined, it has been shown that the luminal proteins CASQ2, triadin and junctin physically associate with RyR2 in cardiac myocytes and modulate its functional activity (Zhang et al. 1997; Györke et al. 2004). Based on these studies, we proposed that CASQ2 confers on RyR the ability to sense luminal [Ca2+] by either dissociating from the RyR complex or by directly regulating the activity of RyRs in response to changes in [Ca2+] (Györke et al. 2004). In the present study, we demonstrated that the effects of increasing CASQ2 protein levels were similar to those observed in myocytes in which the SR was loaded with the exogenous low affinity Ca2+ chelator, citrate. These results therefore suggest that the role of CASQ2 in Ca2+ handling in cardiac myocytes can be attributed to its action as a Ca2+ buffer in the SR lumen. To reconcile this apparent discrepancy it is important to consider that CASQ2 is expressed in the SR at much higher levels than RyR, triadin or junctin (Zhang et al. 1997). Consequently, lowering CASQ2 abundance to 30% of control levels may be sufficient to observe significant differences in its buffering function, but may leave sufficient CASQ2 in the SR to preserve functions based on interactions between CASQ2 with RyR (and possibly triadin and junctin). Therefore, these results do not rule out the possibility that CASQ2 regulates RyR activity by functioning as its luminal Ca2+ sensor. Experiments that achieve more complete depletion of CASQ2 (e.g. by using RNA interference approaches) could clarify this issue.
Conclusions
In summary, our results show that the level of free Ca2+ inside the SR is a critical factor in the generation of spontaneous Ca2+ waves in cardiac myocytes. Ca2+ waves arise only when [Ca2+]SR reaches a certain threshold level that sensitizes the RyR channel to CICR. This model would therefore predict that mutations in CASQ2 that act either by reducing CASQ2 expression or by impairing the Ca2+-binding activity of CASQ2 promote the generation of Ca2+ waves by accelerating the dynamics of attaining this threshold free [Ca2+]SR, thereby providing a mechanism for arrhythmia in CPVT.
Acknowledgments
This work was supported by the American Heart Association Grant 0245088N (S.C.W.) and NIH Grants HL-74045 and HL-63043 (S.G.).
References
- Allen DG, Eisner DA, Orchard CH. Characterization of oscillations of intracellular calcium concentration in ferret ventricular muscle. J Physiol. 1984;352:113–128. doi: 10.1113/jphysiol.1984.sp015281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J. 1999;76:606–617. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumel P, Fidelle J, Lucet V, Attuel P, Bouvrain Y. Catecholaminergic-induced severe ventricular arrhythmias with Adam–Stokes syndrome in children: report of four cases. Br Heart J. 1978;40:28–37. [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85:247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S, Györke I, Lukyanenko V, Terentyev D, Viatchenko-Karpinski S, Wiesner TF. Regulation of sarcoplasmic reticulum calcium release by luminal calcium in cardiac muscle. Front Biosci. 2002;7:d1454–1463. doi: 10.2741/A852. [DOI] [PubMed] [Google Scholar]
- Györke I, Hester NA, Jones LR, Györke S. Role of calsequestrin, triadin and junctin, in conferring the responsiveness of cardiac RyR to luminal Ca. Biophys J. 2004;84:428a. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S, Lukyanenko V, Györke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes. J Physiol. 1997;500:297–309. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izu LT, Wier WG, Balke CW. Evolution of cardiac calcium waves from stochastic calcium sparks. Biophys J. 2001;80:103–120. doi: 10.1016/S0006-3495(01)75998-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbara AA, Allen DG. The use of the indicator fluo-5N to measure sarcoplasmic reticulum calcium in single muscle fibres of the cane toad. J Physiol. 2001;534:87–97. doi: 10.1111/j.1469-7793.2001.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RS, Tsien RW. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys J. 1982;38:259–269. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, Donarum EA, Marino M, Tiso N, Viitasalo M, Toivonen L, Stephan DA, Kontula K. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Guarnieri T. Spontaneous myocardial calcium oscillations: are they linked to ventricular fibrillation. J Cardiovasc Electrophysiol. 1993;4:473–489. doi: 10.1111/j.1540-8167.1993.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- Lipp P, Niggli E. Microscopic spiral waves reveal positive feedback in subcellular calcium signaling. Biophys J. 1993;65:2272–2276. doi: 10.1016/S0006-3495(93)81316-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Györke S. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J Physiol. 1999;521:575–585. doi: 10.1111/j.1469-7793.1999.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Györke I, Györke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 1996;432:1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Subramanian S, Györke I, Wiesner TF, Györke S. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricular myocytes. J Physiol. 1999;518:173–186. doi: 10.1111/j.1469-7793.1999.0173r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Györke S. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+-sensitive leak in rat ventricular myocytes. Biophys J. 2001;81:785–798. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest. 1986;78:1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard CH, Eisner DA, Allen DG. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983;304:735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, Mannens MM, Wilde AA, Guicheney P. Absence of calsequestrin2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- Priori SG, Corr PB. Mechanisms underlying early and delayed afterdepolarizations induced by catecholamines. Am J Physiol. 1990;258:H1796–H1805. doi: 10.1152/ajpheart.1990.258.6.H1796. [DOI] [PubMed] [Google Scholar]
- Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino VV, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res. 2003;93:40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- Stern MD, Capogrossi MC, Lakatta EG. Spontaneous calcium release from the sarcoplasmic reticulum in myocardial cells: mechanisms and consequences. Cell Calcium. 1988;9:247–256. doi: 10.1016/0143-4160(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Györke I, Volpe P, Williams SC, Györke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci U S A. 2003;100:11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Györke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S, Terentyev D, Györke I, Terentyeva R, Volpe P, Priori SG, Napolitano C, Nori A, Williams SC, Gyorke S. Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ Res. 2004;94:471–477. doi: 10.1161/01.RES.0000115944.10681.EB. [DOI] [PubMed] [Google Scholar]
- Wier WG, Cannell MB, Berlin JR, Marban E, Lederer WJ. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987;235:325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]