Abstract
Prolactin-releasing peptide (PrRP) is a recently discovered neuropeptide implicated in the central control of feeding behaviour and autonomic homeostasis. PrRP-containing neurones and PrRP receptor mRNA are found in abundance in the caudal portion of the nucleus tractus solitarius (NTS), an area which together with the dorsal motor nucleus of the vagus (DMV) comprises an integrated structure, the dorsal vagal complex (DVC) that processes visceral afferent signals from and provides parasympathetic motor innervation to the gastrointestinal tract. In this study, microinjection experiments were conducted in vivo in combination with whole-cell recording from neurones in rat medullary slices to test the hypothesis that PrRP plays a role in the central control of gastric motor function, acting within the DVC to modulate the activity of preganglionic vagal motor neurones that supply the stomach. Microinjection of PrRP (0.2 pmol (20 nl)−1) into the DMV at the level of the area postrema (+0.2 to +0.6 mm from the calamus scriptorius, CS) markedly stimulated gastric contractions and increased intragastric pressure (IGP). Conversely, administration of peptide into the DMV at sites caudal to the obex (0.0 to −0.3 mm from the CS) decreased IGP and reduced phasic contractions. These effects occurred without change in mean arterial pressure and were abolished by ipsilateral vagotomy, indicating mediation via a vagal-dependent mechanism(s). The pattern of gastric motor responses evoked by PrRP mimicked that produced by administration of l-glutamate at the same sites, and both the effects of l-glutamate and PrRP were abolished following local administration of NMDA and non-NMDA-type glutamate receptor antagonists. On the other hand, microinjection of PrRP into the medial or comissural nucleus of the solitary tract (mNTS and comNTS, respectively) resulted in less robust changes in IGP in a smaller percentage of animals, accompanied by marked alterations in arterial pressure. Superfusion of brain slices with PrRP (100–300 nm) produced a small depolarization and increased spontaneous firing in 10 of 30 retrogradely labelled gastric-projecting DMV neurones. The excitatory effects were blocked by administration of TTX (2 μm) or specific glutamate receptor antagonists, indicating that they resulted from interactions of PrRP at a presynaptic site. Congruent with this, PrRP increased the amplitude of excitatory postsynaptic currents (EPSCs, 154 ± 33%, 12 of 25 neurones) evoked by electrical stimulation in mNTS or comNTS. In addition, administration of PrRP decreased the paired-pulse ratio of EPSCs evoked by two identical stimuli delivered 100 ms apart (from 0.95 ± 0.08 to 0.71 ± 0.11, P < 0.05), whereas it did not affect the amplitude of inward currents evoked by exogenous application of l-glutamate to the slice. The frequency, but not amplitude of spontaneous EPSCs and action potential-independent miniature EPSCs was also increased by administration of PrRP, suggesting that the peptide was acting at least in part at receptors on presynaptic nerve terminals to enhance glutamatergic transmission. In recordings obtained from a separate group of slices, we did not observe any direct effects of PrRP on spontaneous discharge or postsynaptic excitability in either mNTS or comNTS neurones (n = 31). These data indicate that PrRP may act within the DVC to regulate gastric motor function by modulating the efficacy of conventional excitatory synaptic inputs from the NTS onto gastric-projecting vagal motor neurones.
Prolactin-releasing peptide (PrRP) is a recently discovered neuropeptide, originally identified as the endogenous ligand for the human orphan G protein-coupled receptor UHR-1 (Hinuma et al. 1998). Although initially named for its ability to stimulate prolactin secretion in cultured anterior pituitary cells, central administration of PrRP has since been shown to cause the secretion of other hypothalamic-pituitary hormones, including oxytocin, adrenocorticotropin, luteinizing and follicle-stimulating hormone (Maruyama et al. 1999a; Seal et al. 2000). However, the absence of immunoreactivity for PrRP in the median eminence in rats (Maruyama et al. 1999b), coupled with the restricted pattern of PrRP mRNA expression found in the brain, argue against its role as a classic hypophysiotropic hormone.
Anatomical studies indicate that PrRP is synthesized by neurones localized exclusively in three regions in the brain, the caudal portion of the hypothalamic dorsomedial nucleus, the nucleus tractus solitarius (NTS) and the ventrolateral medullary reticular formation (Roland et al. 1999; Ibata et al. 2000; Lee et al. 2000), areas implicated in the control of appetite and autonomic regulation. Within the brain stem, PrRP-immunopositive perikarya are restricted to the caudal part of the NTS and to an area corresponding to the ventrolateral reticular nucleus, appearing as dense clusters that account, respectively, for approximately 70% and 25% of all central PrRP-containing neurones. In addition, all of the PrRP-immunoreactive neurones within these brainstem areas were found to coexpress immunoreactivity for tyrosine hydroxylase (TH), identifying them as belonging to the A2 and A1 catecholaminergic cell groups, respectively (Chen et al. 1999; Yano et al. 2001). Results obtained from immunohistochemical mapping studies reveal that PrRP neurones provide a diffuse network of axonal projections to various parts of the brain and spinal cord (Chen et al. 1999; Maruyama et al. 1999b). On the other hand, mRNA encoding for PrRP receptors is localized to specific brain areas and nuclei, being abundantly expressed in the periventricular and dorsomedial hypothalamus, reticular thalamic nucleus, NTS, area postrema (AP) and anterior pituitary (Roland et al. 1999; Lee et al. 2000), suggesting a more limited scope of action for the peptide in the brain.
The highest levels of PrRP protein and the strongest expression of message-encoding PrRP receptors have been reported in the caudal NTS, indicating that the peptide may be involved in modulating activity within this autonomic relay nucleus (Roland et al. 1999). The NTS is the major recipient of visceral afferent information arising from various regions of the gastrointestinal (GI) tract. Neurones located in the caudal two-thirds of the nucleus, including the A2 catecholamine-containing cells (Sumal et al. 1983; Rinaman et al. 1995), relay signals concerning satiety and prandial stimuli conveyed via vagal afferents to more rostral brain sites and provide a major site for their integration with descending commands originating from autonomic centres of the hypothalamus and forebrain. The NTS neurones also provide direct inhibitory and excitatory inputs to preganglionic parasympathetic neurones in the DMV that in turn control gastric motility and secretion via their efferent projections in the vagus nerve (Rogers et al. 1996; Rogers et al. 1999). By way of these interconnections, the NTS and dorsal motor nucleus of the vagus (DMV) comprise a functionally integrated structure, termed the dorsal vagal complex (DVC), providing the synaptic circuitry responsible for the coordination of classic vago-vagal GI reflexes. The vast majority of NTS neurones utilize either GABA or glutamate as their neurotransmitter, and it is generally accepted that their inhibitory and excitatory effects on the excitability of DMV neurones are mediated directly via activation of postsynaptic GABAA receptors and both NMDA- and non-NMDA-type glutamatergic receptors, respectively (Travagli et al. 1991; Willis et al. 1996). However, DMV neurones also receive synaptic input from catecholamine-containing NTS neurones, corresponding to the A2 cell group (Fukuda et al. 1987). The activation of inputs from the latter group of cells has been shown to modulate the activity of vagal efferent neurones, producing both excitation and inhibition via interactions of noradrenaline (norepinephrine) with postsynaptic receptors on DMV neurones, as well as at presynaptic sites (Fukuda et al. 1987; Bertolino et al. 1997). Interestingly, a large percentage (67%) of these catecholaminergic NTS neurones express immunoreactivity for PrRP (Ellacott et al. 2002), although it is not known whether and how this peptide might affect DMV neurone activity.
The aim of the present study was to examine the hypothesis that PrRP functions in the central control of gastric activity, acting within the DMV and/or by way of the NTS to modulate vagal efferent outflow to the upper GI tract. To test this hypothesis, we made use of two complementary experimental approaches. Microinjection experiments were conducted in anaesthetized rats to investigate the effects of administration of PrRP into the DMV or NTS (medial and comissural subnuclei) on gastric tone and motility. In addition, whole-cell patch-clamp recordings were performed in rat brainstem slices to determine the effects of PrRP on the resting membrane properties and synaptically evoked current responses of gastric-projecting vagal motor neurones, and on postsynaptic excitability of NTS neurones. In this study, we demonstrate that PrRP acts within the DVC to regulate gastric motor function and provide electrophysiological evidence that it alters vagal efferent outflow to the stomach by modulating excitatory synaptic input from the NTS onto gastric-projecting DMV neurones.
Methods
Animal preparation and microinjection studies in vivo
Experiments were performed on adult male Sprague-Dawley rats (250–350 g), obtained from Charles River Laboratories (Wilmington, MA, USA), in accordance with NIH guidelines and as approved by the University of Michigan Health Center Institutional Animal Care and Use Committee.
Prior to experimentation, animals were fasted overnight with ad libitum access to water. Anaesthesia was induced in the animals with urethane (1.0–1.25 g kg−1 i.p) and its depth monitored by assessing for the absence of limb withdrawal to a noxious toe pinch. Body temperature was monitored by a rectal probe and maintained at 37 ± 1°C by means of a homeothermic pad placed under the animal. Following the induction of anaesthesia, silicon catheters were inserted into the femoral artery and vein for monitoring arterial blood pressure and drug infusion, respectively. A laparotomy was then performed to expose the stomach. In some experiments, the vagus nerves were carefully exposed along their cervical extent, and ligatures were tied around one or both nerves, after which the area was moistened with mineral oil. An intragastric balloon, fashioned from the little finger of a small latex glove, was tied around polyethylene tubing (PE 160) and inserted into the stomach via a small incision placed in the pylorus to obtain measurements of intragastric pressure (IGP). The balloon was secured with a running suture to prevent movement and the incision made in the pylorus was closed. The tubing was connected to a pressure transducer, interfaced to a bridge amplifier, whose signal was fed into an analog-to-digital converter (MacLab System, A-D Instruments, Grand Junction, CO) and the data stored on a microcomputer for subsequent analysis off-line. The stomach was inflated by introducing 2–3 ml of warm saline into the intragastric balloon to achieve a baseline pressure of ∼5 cm H2O that was used for all microinjection experiments.
The animal was then positioned in a stereotaxic apparatus, and a partial craniotomy was performed to expose the dorsal surface of the medulla. The cerebellum was retracted slightly and a slit made in the subarachnoid covering to expose the fourth ventricle. The calamus scriptorius (CS), representing the caudal-most pole of the area postrema, was viewed from the dorsal aspect and used as a point of reference for the stereotaxic placement of multibarrel glass micropipette assemblies into the DMV or NTS. For the purposes of this study, the DMV was divided into rostral and caudal regions based on its anatomical relationship with the area postrema (Loewy, 1990). The portion of the DMV extending roughly from the CS to the anterior border of the area postrema was defined as the ‘rostral DMV, and the portion of the nucleus extending caudal from the CS was defined as the ‘caudal DMV’. Microinjection of drug was performed by pneumatic pressure delivered via a pico-pump (model PV-830, World Precision Instruments, Sarasota, FL, USA) to individual barrels containing solutions of PrRP, l-glutamate, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), d-22-amino-5-phosphopentanoic acid (AP5) or saline. An additional saline-filled barrel also contained fluorescein-labelled fluorescent microbeads (Polysciences, Eppelheim, Germany) that were used for marking of the injection site. At the end of an experiment, rats were killed with an overdose of urethane, perfused transcardially with a mixture of 4% paraformaldehyde in phosphate-buffered saline, and the brains removed and placed in fixative for at least 24 h. Coronal sections of 50 μm thickness were cut through the brainstem, counterstained with neutral red, and then examined under a fluorescent microscope equipped with FITC filters to confirm the location of the injection site.
All microinjections were delivered unilaterally, unless indicated otherwise in the text. Injections were given in volumes of 20 nl over a period of 10–15 s. Stereotaxic coordinates were originally chosen based on histological material presented in Paxinos & Watson (1986). Final coordinates for placement of the electrode tip within the rostral DMV (coordinates: 0.2–0.6 mm rostral to the CS, respectively, 0.3–0.6 mm lateral from the midline, and 0.5–0.7 mm ventral from the dorsal surface of the medulla) were selected based on preliminary experiments wherein microinjection of l-glutamate (4 nmol) produced transient elevations in IGP (see below). Conversely, coordinates for placements within the caudal DMV (coordinates: 0.0 mm to 0.3 mm caudal from the CS, 0.3–0.5 mm lateral from the midline, and 0.6–0.8 mm ventral from the dorsal surface of the medulla) were determined from sites where similar injections of l-glutamate evoked gastric relaxation (see below). Coordinates for the medial and comissural nuclei of the solitary tract (mNTS and comNTS) were similarly determined from micropipette placements wherein microinjection of l-glutamate (4 nmol) induced gastric relaxations and ranged from 0.2 to 0.5 mm rostral and 0.0 to 0.3 mm caudal to the CS, respectively, 0.5–0.7 mm lateral from the midline, and from 0.4 to 0.6 mm from the dorsal surface of the medulla. For experiments in which glutamate receptor antagonists were administered, CNQX and AP5 were co-applied at doses (200 pmol and 2 nmol, respectively) that were determined in pilot studies to effectively block gastric motor responses evoked by a single bolus injection of l-glutamate (4 nmol) into the DMV.
Analysis of microinjection data
Data from IGP measurements were analysed using the Chart Software analysis program designed for the MacLab data acquisition system (AD Instruments). Prior to initiation of microinjections, values were calculated over a 5 min control period during which the baseline in the IGP trace remained stable. The lowest points of the IGP trace obtained over that 5 min segment were averaged, and the resultant value used as an index of gastric tone. After microinjections of drug into the DMV or NTS, the maximum or minimum value in the IGP trace (also derived from a 5 min segment) was taken as the largest change in gastric tone. The peak increases and decreases in IGP were compared with the pre-injection baseline levels, and drug-induced changes in IGP reported as means of the absolute value of change in IGP ±s.e.m. Differences between groups were assessed by one-way ANOVA followed by student Newman Keuls multiple-comparisons test. Effects were considered to be statistically different compared to vehicle at P-values < 0.05
Retrograde labelling
Sprague-Dawley rats (10 days old) of either sex were deeply anaesthetized via inhalation of a 4% solution of 2-bromo-2-chloro-1,1,1-trifluorethane (Halothane, Sigma Chemical) in air (500 ml min−1) before abdominal surgery was performed. The depth of anaesthesia was assessed by absence of the limb withdrawal reflex to noxious foot pinch and maintained throughout surgical procedures by placing the head of the rat in a custom-made chamber through which a 2% halothane–air mixture was continuously delivered. The abdominal area was cleaned with 70% ethanol and a laparotomy performed. Crystals of the retrograde tracer 1.1′-dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine perchlorate (DiIC18, Molecular Probes, Eugene, OR, USA) were applied to the serosal surface of the gastric corpus (along the greater curvature) according to procedures developed by Browning et al. (1999). To confine the dye to the site of application, the application site was embedded in a fast-hardening epoxy resin that was allowed to harden (3–5 min) before the surgical area was washed with warm sterile saline solution and blotted dry with cotton swabs. The wound was closed using 5/0 nylon sutures, after which the rats were placed in a chamber warmed to 36°C under a radiant heat lamp, until normal motor activity was restored. Animals were then returned to their home cage and allowed to recover for 7–15 days before being used for electrophysiological study.
Brain slice preparation
Coronal tissue slice preparations of the caudal brainstem were prepared according to procedures previously described (see Grabauskus & Moises, 2003). Briefly, rats were rapidly decapitated and the whole brain carefully removed and placed in ice-cold physiological saline containing (mm) NaCl, 124; KCl, 2.5; CaCl2, 2.5; MgSO4, 1.3; NaHCO3, 26; NaH2PO4, 1.25; and glucose, 10, pregassed with 95% O2–5% CO2 and adjusted to pH 7.3. The cerebellum was removed and the brainstem was transected rostrally at the level of the pons and again at a point several millimetres caudal to the CS. Using a vibratome (Ted Pella, Redding, CA, USA), coronal brain sections containing the DVC were cut at 200 μm thickness and placed in a holding chamber. Following at least 1 h for equilibration, a single slice was then transferred to a custom-designed recording chamber (volume of ∼1 ml) and held in place by a nylon mesh, submerged under continuously flowing (1–2 ml min−1) oxygenated physiological saline at either room temperature (current-clamp recordings) or warmed to 34°C (voltage-clamp recordings).
Electrophysiological recordings
Prior to electrophysiological recording, retrogradely labelled DMV neurones were identified using a Nikon E600FN (Nikon, Melville, NY) microscope equipped with Nomarski optics and epifluorescent filters suitable for visualizing DiI. The DVC was briefly exposed to fluorescent light using TRITC filters to visualize the fluorescent neurone(s), and once the labelled neurone was identified, its location in the slice was confirmed under bright field illumination using DIC (Nomarski) optics. Stimulating and recording electrodes were then placed into position under visual guidance and whole-cell recordings obtained under brightfield illumination.
Whole-cell patch-clamp recordings were performed using patch pipettes (4–5 MΩ), pulled in two stages from 1.5 mm o.d. borosilicate filament glass and filled with a solution containing (mm): K-gluconate, 120; KCl, 10; Hepes, 10; EGTA, 10; MgCl2, 1.0; CaCl2, 1.0; ATP, 2.0; GTP, 0.5. The recording pipette solution was adjusted to pH 7.2–7.3 with KOH and had an osmolarity of 275–292 mosmol. Recordings were obtained from DMV neurones that were unequivocally labelled with DiI and from a smaller number of undefined neurones in the mNTS or comNTS. The data were recorded under voltage- or current-clamp conditions using an Axopach 1D amplifier (Axon instruments, Union City, CA, USA). Data were sampled every 100 μs and filtered at 3 kHz, digitized via an A/D converter (Digidata 1200C, Axon Instruments, Union City, CA, USA) interfaced to a Pentium-based microcomputer, and stored for subsequent analysis off-line. Data acquisition, storage and analysis were performed using pCLAMP software (ver.8 and 9, Axon Instruments, Union City, CA, USA). Records were corrected for a tip potential of approximately 8 mV and were accepted only if series resistance was < 15 MΩ.
Excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs, respectively) were evoked by stimulating the mNTS or comNTS, using rectangular constant current pulses (0.5 ms duration) delivered via a tungsten bipolar stimulating electrode (World Precision Instruments, Sarasota, FL, USA). Single shocks or pairs of stimuli (spaced 100 ms apart) were delivered every 30 s, and the stimulus intensity was adjusted to evoke submaximal (∼65% of maximal) postsynaptic currents (PSCs) that ranged in amplitude from 35 to 350 pA when recording in control medium. The criteria for detecting spontaneous synaptic currents were fast rise times (<1 ms) and exponential decays, with 20 pA (at least twice baseline noise) used as the detection limit for minimum EPSC amplitude. Frequencies of spontaneous synaptic events were obtained over continuous periods of recording lasting from 2 to 6 min, and between 100 and 300 consecutive PSCs were measured for determinations of mean amplitudes.
Data and statistical analysis
Recordings were considered acceptable if the neuronal resting membrane potential was more negative than −50 mV, and the neurone generated overshooting action potentials. In addition, variability in amplitude of NTS-evoked synaptic currents was required to be within 10% between trials during control for neurones to be included in the analysis. Data were expressed as means (s.e.m). Statistical analysis was performed using ANOVA paired tests and the significance was accepted at the level P < 0.05. Analysis of PSC frequency and amplitudes was conducted with Clampfit subroutines available with PClamp version 9 software. This program detects and measures spontaneous synaptic events by application of various user-defined criteria, including the amplitude, waveform, duration and area under the curve (fc). Statistical comparisons of the extracted amplitude and interspike interval distributions for PSCs were obtained using the non-parametric Kolmogorov–Smirnov test, with a significant difference being set at P < 0.01.
Drug application
Drugs were applied to the bath in known concentrations via a series of manually operated switching valves. Because of the long duration of effects encountered with PrRP, superfusion of the peptide was terminated once a plateau effect was observed. When experiments were performed to determine concentration–response relationships, the subsequent superfusion of different concentrations of peptide was randomized and a washout period of at least 30 min was allowed between individual trails. Recordings of stimulation-evoked EPSCs were carried out in the presence of bicuculline methiodide (BMI, 30 μm) or picrotoxin (100 μm) to selectively eliminate spontaneous and evoked GABAergic IPSCs (Browning et al. 1999). Administration of these antagonists at the concentrations indicated has been shown previously to eliminate spontaneous and evoked GABA-mediated currents in DMV neurones, without affecting glutamate-mediated excitatory synaptic events (Willis et al. 1996; Bertolino et al. 1997). For all experiments with electrically evoked PSCs, a minimum of 10 control EPSC responses were obtained prior to application of drug, and the effects were assessed using each neurone as its own control, i.e. the results obtained after administration of a receptor antagonist or peptide were compared to those obtained before drug administration.
PrRP (12–31) was purchased from Bachem, DiIC18 was purchased from Molecular Probes (Eugene, OR, USA), and tetrodotoxin (TTX) was purchased from Alamone Laboratories (Jerusalem, Israel). 6-Cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX), dl-2-amino-7-phosphonoheptanoic acid (APV), dl-2-amino-5-phosphonopentanoic acid (AP5) (–)-bicuculline methiodide (BMI) and other chemicals were purchased from Sigma-Aldrich-RBI (St. Louis, MO, USA).
Results
Effects of PrRP on gastric tone and motility
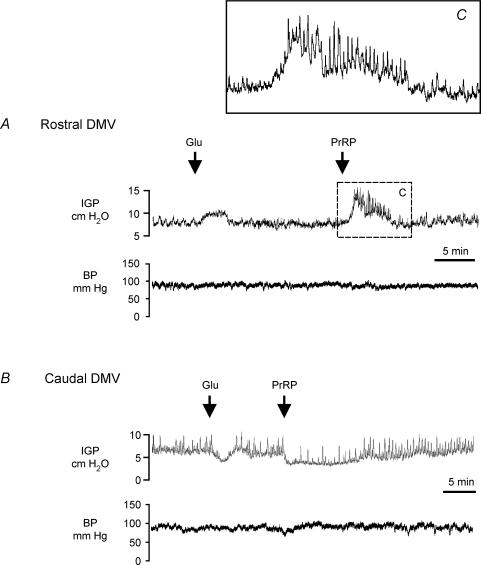
Figure 1 illustrates representative IGP recordings of the gastric motor responses produced by microinjection of l-glutamate (4 nmol in 20 nl) or PrRP (2 pmol in 20 nl) at sites within rostral (upper trace) and caudal portions (lower trace) of the DMV. Microinjection of l-glutamate into the DMV at the level of the area postrema evoked transient increases in IGP (2.1 ± 0.3 cm H2O, 2.8 ± 1.1 min duration, n= 9, P < 0.0.05), accompanied by little or no changes in mean arterial pressure (Fig. 1A, upper trace). Such responses have been attributed to the excitation of vagal neurones that stimulate gastric contraction via activation of cholinergic postganglionic enteric neurones (Rossiter et al. 1990; Krowicki et al. 2002). By contrast, microinjection of l-glutamate into the DMV at sites 0–0.3 mm caudal to the CS decreased baseline IGP (−1.8 ± 0.4 cm H2O, 3.8 ± 1.5 min duration, n = 9, P < 0.05) and inhibited phasic contractions (Fig. 1B, upper trace), independent of changes in mean arterial pressure. The glutamate-induced gastric relaxation is thought to result from the activation of vagal neurones that synapse onto postganglionic inhibitory non-adrenergic, non-cholinergic intragastric motor neurones (Rossiter et al. 1990; Krowicki et al. 2002). Changes in IGP were not observed following microinjection of saline (20 nl) into the DMV at sites in which administration of l-glutamate produced stimulation (n= 8) or reductions (n= 9) in gastric tone or phasic contractions.
Figure 1. Representative chart recordings showing the effect of microinjection of glutamate (4 nmol (20 nl)−1) or PrRP (2 pmol (20 nl)−1) into the rostal (A) and caudal (B) DMV on IGP and arterial blood pressure (BP).
A, microinjection of l-glutamate into the DMV at a level of the area postrema (0.3 mm rostral to the CS) evoked a transient increase in IGP, whereas administration of PrRP at the same site produced marked gastric contraction and evoked phasic increases in IGP (inset shows boxed region at expanded scale). B, administration of l-glutamate or PrRP into the caudal DMV at a level 0.1 mm posterior to the CS induced gastric relaxation, as evidenced by decreases in baseline IGP, and inhibited phasic contractions. Both types of gastric motor response to microinjection of PrRP occurred independently of changes in arterial pressure (A and B, lower traces).
The gastric motor effects produced by microinjection of PrRP (2 pmol in 20 nl) into different regions of the DMV were examined in 32 rats. Microinjection of PrRP into the rostral portion of either the right or left DMV induced strong gastric contraction, characterized by a prolonged (8.9 ± 2.2 min, 4–20 min range) increase in IGP above baseline (4.7 ± 0.8 cm H2O, n= 15, P < 0.01). In all cases, this was associated with an increase in the frequency and amplitude of phasic changes in IGP, indicating that the peptide also stimulated gastric motility (Fig. 1A, upper trace and inset). Although PrRP stimulated gastric contraction when microinjected into the DMV at levels near the anterior border of the area postrema (five of six placements located +0.4 to +0.6 mm rostral to the CS, Fig. 2A), administration of the peptide at more rostral sites corresponding to the anteriormost extent of the DMV was without effect (n= 2, data not included in Fig. 2). By contrast, microinjection of an equivalent amount of PrRP into the caudal portion of the DMV (0.0 to −0.3 mm from the CS) induced marked gastric relaxation. The inhibitory gastric responses to PrRP were characterized by a sustained decrease in IGP (−4.2 ± 0.6 cm H2O, n= 15, P < 0.01) that recovered gradually back to the baseline level, typically within 15 min (9.6 ± 1.5 min) from the point of peak relaxation (Fig. 1B, upper trace, but see Fig. 3B). Additionally, in eight cases the gastric relaxation produced by PrRP was accompanied by attenuation or complete blockade of phasic changes in IGP (Fig. 1B, lower trace and Fig. 3B). Microinjection of the vehicle either before (n= 12) or following administration of the peptide (n= 18) did not produce significant changes in IGP. On the other hand, subsequent injections of an identical amount of PrRP delivered at the same site either in rostral or caudal portions of the nucleus produced progressively smaller changes in IGP, indicating tachyphylaxis to the actions of the peptide. Recovery from tachyphylaxis occurred slowly, with the amplitude of the gastric motor response to a second injection of the peptide attaining roughly 70% of the initial response (79 ± 8% for gastric contraction; 71 ± 6% for gastric relaxation) when 45 min had elapsed between trials.
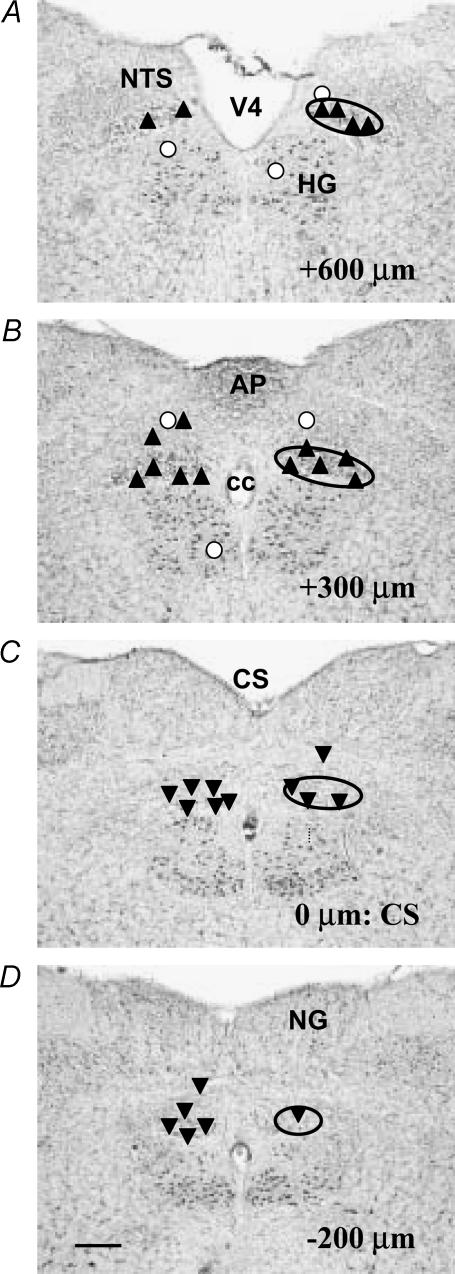
Figure 2. Summary map showing the location of the sites of microinjection of PrRP into the DMV at levels corresponding to the rostral pole of the nucleus (A), the area postrema (B), CS (C) and caudal pole of the DMV (D).
The approximate location of the DMV is outlined by the solid oval. For the purposes of this study, the portion of the DMV extending from the CS to +0.2 mm rostral of the anteriormost border of the area postrema was taken to define the rostral DMV (A and B) and the region extending caudally from the CS (C and D) was defined as the caudal DMV. Numbers in lower right of panels indicate rostral–caudal distance measured relative to the CS, with the caudalmost tip of the area postrema set as 0 μm. Upward triangles indicate sites where microinjection of PrRP increased IGP, whereas downward triangles indicate sites where IGP was decreased in response to PrRP. Open circles indicate sites where administration of the peptide did not evoke changes in IGP. Locations of various NTS subnuclei are not shown for purposes of clarity. Abbreviations: AP, area postrema; cc, central canal; CS, calamus scriptorius; HG, hypoglossal nucleus; NG, nucleus gracilis; NTS, nucleus tractus solitarius; V4, fourth ventricle. Scale bar=300 μm.
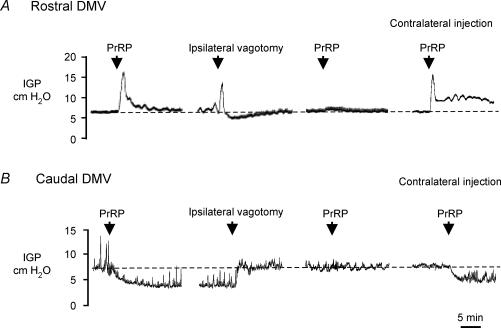
Figure 3. Representative chart recordings showing gastric contraction (A) and relaxation (B) produced by microinjection of PrRP into the rostral DMV and caudal portion of the DMV, respectively, and elimination of the gastric motor response to peptide administration following ipsilateral vagotomy.
A, microinjection of PrRP (2 pmol/20 nL) into the rostral portion of the right DMV evoked a marked increase in IGP. Following ipsilateral vagotomy, administration of PrRP in the same site had no effect on IGP (third trace from left). Microinjection of the same dose of PrRP into the corresponding site in the DMV on the contralateral (left) side produced a large increase in IGP despite prior sectioning of the right vagus nerve. B, in another rat, microinjection of PrRP (2 pmol (20 nl)−1) into the left DMV at the level of the CS induced a sustained decrease in IGP and inhibited phasic contractions. IGP returned to the baseline level abruptly upon sectioning of the ipsilateral vagus nerve (second trace from left), whereas a subsequent injection of the peptide at the same site made 45 min after vagotomy did not affect IGP. In contrast, microinjection of PrRP into a similar location within the caudal DMV on the contralateral (right) side induced gastric relaxation (trace at far right).
The prolonged delay required for complete recovery of sensitivity to the gastric motor effects of PrRP precluded accurate determination of dose–response relationships. In addition, the magnitude of the changes in IGP produced by microinjection of 2 pmol PrRP into rostral or cuadal portions of the DMV varied markedly between placements. Nevertheless, in several of our pilot experiments (three of five) we were able to evoke an increase in IGP following microinjection of 0.5 pmol PrRP (the lowest dose tested) into the rostral DMV at sites where administration of l-glutamate also evoked gastric contraction. Furthermore, in a separate group of animals, administration of 6 pmol (20 nl)−1 PrRP into the DMV at the level of or caudal to the area postrema evoked changes in IGP (4.5 ± 1.4 cm H2O, n = 3; and −3.7 ± 1.1, P < 0.05, n = 2, respectively) comparable in magnitude to the gastric responses observed following microinjection of 2 pmol of peptide into corresponding regions of the nucleus. Thus, 2 pmol of PrRP probably evoked a maximal response.
Mapping of the microinjection sites in terms of the response to PrRP indicated that the increases and decreases in gastric contractility resulted, with rare exception, from administering the peptide within the confines of the rostral and caudal DMV, respectively (Fig. 2). These results appeared to conflict with findings from in situ hybridization analyses of mRNA for PrRP receptors in brain, which indicate that these receptors are found in moderate to high density throughout the NTS and in the area postrema, but are not expressed in DMV neurones (Roland et al. 1999; Ibata et al. 2000). Therefore, additional kinds of experiments were performed to identify the neuronal substrate(s) and site of action through which exogenous application of PrRP effected changes in vagal motor output to the stomach. To determine whether the PrRP-induced increases in IGP were due to activation of preganglionic motor neurones in the DMV projecting to the stomach, ipsilateral vagotomy was performed in six rats. In five of the experiments, vagotomy was performed after IGP had returned to the baseline level and remained stable following the response to an initial microinjection of 2 pmol of PrRP into the DMV. In addition, to avoid confounding effects owing to drug tachyphylaxis, a minimum of 45 min was allowed following termination of the initial response to PrRP before administration of the second dose of peptide (see above). In all three cases in which peptide was administered into the rostal DMV, the contractile response to a second dose of PrRP was prevented by ipsilateral vagotomy (peak increase in IGP was 3.5 ± 1.2 cm H2O before vagotomy and 0.3 ± 0.2 cm H2O after vagotomy, P < 0.05). However, unilateral vagotomy did not prevent an increase in IGP (3.9 ± 0.8 cm H2O, compared to vehicle, P < 0.05) following microinjection of an identical dose of peptide into corresponding sites in the contralateral DMV (Fig. 3A). Similarly, in a separate group of rats (n= 3), reductions in IGP produced by administration of PrRP into the caudal DMV were eliminated after ipsilateral vagotomy (3.3 ± 0.9 cm H2O before versus 0.3 ± 0.2 cm H2O after, P < 0.05), whereas this manipulation did not interfere with the ability of the peptide to induce gastric relaxation when administered on the contralateral side. The results from such an experiment are depicted in Fig. 3B. In this animal, microinjection of PrRP at a site 0.1 mm caudal to the CS evoked a sustained decrease in IGP lasting beyond 20 min. Despite partial recovery in phasic contractions, gastric tone remained depressed 25 min after microinjection of peptide, at which time ipsilateral vagotomy was performed, resulting in an abrupt return of IGP to the baseline level. After waiting 1 h from the time of initial injection of PrRP, administration of a second dose of PrRP at the same site in the DMV did not alter baseline IGP. Taken together, these data indicate that the PrRP-induced changes in IGP were dependent on vagal motor pathways and probably resulted from actions of the peptide within the DMV, rather than in adjacent structures (Ferreira et al. 2000). Furthermore, the fact that microinjection of PrRP into the DMV had little or no effect on mean arterial pressure, as noted above (Fig. 1), suggests that diffusional spread of the drug from the site of injection into the adjacent NTS was limited.
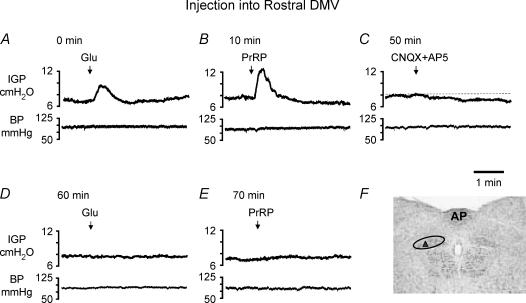
The differential gastric motor effects obtained following microinjection of PrRP into rostral versus caudal regions of the DMV mirrored the regionally dependent pattern of gastric stimulation and relaxation produced by glutamatergic excitation of preganglionic vagal motor neurones within the corresponding areas (see also Krowicki et al. 2002). This finding, coupled with failure to detect mRNA encoding for PrRP receptors in DMV neurones (Roland et al. 1999; Ibata et al. 2000), led us to hypothesize that PrRP might affect the activity of gastric-projecting vagal motor neurones, at least in part, through modulation of their glutamatergic input. To examine this hypothesis, we compared the gastric motor responses evoked by microinjection of the peptide into the rostral (n = 3) or caudal DMV (n = 3) before and after local blockade of NMDA-type and non-NMDA-type glutamate receptors by combined administration of 20 nl of AP5 (200 pmol) and CNQX (200 pmol), respectively. To avoid confounding effects associated with tachyphylaxis to the peptide (see above), at least one hour elapsed between termination of the initial response to PrRP and trials in which the effects of the peptide were examined following microinjection of the glutamate receptor antagonists. In addition, gastric responses evoked by microinjection of l-glutamate (4 nmol in 20 nl) were concurrently determined to assess the effectiveness of antagonist administration in preventing excitation of DMV neurones through postsynaptic glutamate receptors. In all three experiments in which microinjections were made into the DMV rostral to the CS, the stimulation of gastric motor activity produced by microinjection of PrRP was blocked by prior administration of CNQX and AP5 (increase in IGP of 2.8 ± 0.15 cm H2O before versus 0.1 ± 0.01 cm H2O after CNQX + AP5, P < 0.01). Concomitant with the blockade of responses to PrRP, administration of CNQX + AP5 prevented increases in IGP evoked by microinjection of l-glutamate at the same site (1.5 ± 0.1 cm H2O before versus 0.1 ± 0.02 cm H2O after, P < 0.01). The paired traces depicted in Fig. 4, from a representative experiment, illustrate the effects on IGP (upper) and mean arterial pressure (lower) produced by microinjection of PrRP or l-glutamate before and after administration of the antagonists. Analagous results were obtained with CNQX and AP5 in a related series of experiments (n = 3), wherein local blockade of NMDA- and non-NMDA-type glutamate receptors prevented the inhibition in gastric motor activity produced by microinjection of either PrRP or l-glutamate into the caudal DMV (−2.2 ± 0.85 cm H2O before versus 0.2 ± 0.25 cm H2O after CNQX + AP5, P < 0.05, Fig. 5). Interestingly, in three of the six animals examined, IGP was transiently decreased and increased following microinjection of CNQX + AP5 into the rostral (n = 2) and caudal DMV (n = 1), respectively, indicating that the excitability of gastric-projecting DMV neurones was governed in part via a tonically active glutatmatergic input under our experimental conditions (Figs 4C and 5C).
Figure 4. Representative chart recordings showing the effects of microinjection of l-glutamate (4 nmol) and PrRP (2 pmol) into the rostral DMV on IGP (upper traces in paired recordings) and mean arterial pressure (lower traces) before and when performed following local application of the glutamate receptor antagonists CNQX (200 pmol) and AP5 (2 nmol).
A and B, microinjection of l-glutamate or PrRP increased IGP without change in blood pressure, and D and E, the gastric responses evoked by either drug were abolished by prior administration of the glutamate receptor antagonists. C, microinjection of CNQX + AP5 on their own transiently reduced baseline IGP, after which sufficient time was allowed for its recovery back to the control level before repeating the administration of l-glutamate or PrRP. The times shown with each pair of recordings indicate the continuous time course of the experiment and are referenced to the initial delivery of l-glutamate, denoted as 0 min. F, coronal section of brainstem at the level of the AP illustrates the injection site (upward triangle) within the DMV (outlined by oval) from which the IGP responses shown in traces (A–E) were obtained.
Figure 5. Representative chart recordings showing the effects of microinjection of l-glutamate (4 nmol) and PrRP (2 pmol) into the caudal DMV on IGP (upper traces in paired recordings) and mean arterial pressure (lower traces) before and following application of the glutamate receptor antagonists CNQX (200 pmol) and AP5 (2 nmol).
A and B, microinjection of l-glutamate or PrRP decreased baseline IGP and phasic contractions without change in blood pressure. D and E, the inhibitory effects of both drugs on gastric motor activity were abolished following administration of the glutamate receptor antagonists, with F, reponses to PrRP showing partial recovery after 40 min. Microinjection of CNQX + AP5 on their own transiently increased baseline IGP, which recovered back to the control level prior to repeating the administration of l-glutamate or PrRP. The times shown with each pair of recordings indicate the continuous time course of the experiment and are referenced to the initial delivery of l-glutamate, denoted as 0 min.
To further establish that the gastric motor responses evoked by administration of PrRP into the DMV arose locally from actions of the peptide within the nucleus, we examined the gastric motor effects produced by microinjection of PrRP into the NTS in a separate group of animals (n= 7). Administration of the peptide into the mNTS at sites 0.3–0.6 mm rostral to the CS produced transient increases in IGP in two rats (0.8 and 3.3 cm H2O of 1.5 and 5 min duration, respectively), whereas it had little or no effect on gastric motor function in three additional animals (Fig. 2B). On the other hand, microinjection of peptide into the comNTS (0.1 rostral to 0.2 mm caudal to the CS) decreased IGP and reduced phasic contractions in two rats. Here too, the changes in IGP were of short duration (lasting 2–3 min) and much smaller in amplitude (−0.8 and −1.2 cm H2O) than the gastric responses produced following administration of PrRP into corresponding portions of the DMV. Furthermore, in nearly all cases administration of the peptide into the mNTS or comNTS produced an appreciable increase in mean arterial pressure. By contrast, neither increases nor decreases in IGP, or changes in mean arterial pressure were observed when PrRP was microinjected into the hypoglossal nucleus (n= 3) (Fig. 2).
PrRP indirectly affects excitability of gastric-projecting DMV neurones
Whole-cell recordings were obtained from identified vagal motor neurones in rat medullary slices to investigate the mechanisms by which PrRP acts in the DMV to modulate gastric motor activity. The effects of bath administration of PrRP were examined on the resting membrane properties (n= 30) and synaptically evoked currents (n= 35) in DMV neurones retrogradely labelled following placement of DiI to the gastric corpus. For these experiments, the peptide was administered at a concentration of 100 or 300 nm, which was determined in pilot studies to be suprathreshold for evoking changes in spontaneous firing and modulating the amplitude of synaptically evoked currents.
Superfusion of medullary slices with PrRP for 1–3 min increased spontaneous firing in 10 of 30 (33%) corpus-projecting DMV neurones recorded under current clamp at −54 mV holding potential. In eight of these neurones, the PrRP-induced increase in spontaneous firing was accompanied by a small depolarization (4.6 ± 1.3 mV, range 2–6 mV, P < 0.05 compared to control) (Fig. 6). The excitatory effects of PrRP developed slowly, reaching a peak after 1–3 min, and were of prolonged duration with recovery to the baseline occurring 15–45 min after switching the bath perfusate back to control medium.
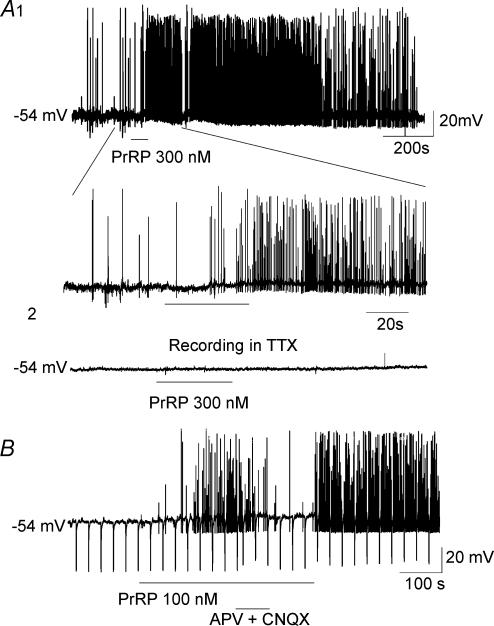
Figure 6. Representative current-clamp recordings showing PrRP-induced depolarization and increase in spontaneous firing in gastric-projecting DMV neurones.
A1 (upper trace), superfusion of PrRP (300 nm) produced a small membrane depolarization, accompanied by a marked increase in spontaneous discharge that persisted for many minutes following washout of the peptide. (lower trace). A segment of same recording displayed at faster time sweep illustrates slowly developing depolarization of 4 mV in response to PrRP. A2, administration of TTX (2 μm) blocked spontaneous action potential firing in the same neurone, and in its presence PrRP had no effect on the membrane potential or spike discharge. Note different time scales for upper and lower traces. B, current-clamp recording from another gastric-projecting DMV neurone showing attenuation of the excitatory effects of PrRP on membrane potential and spike firing during coadministration of the glutamate receptor antagonists APV (50 μm) and CNQX (20 μm). The excitatory effects of PrRP were re-established and the elevation in spike firing increased further following washout of APV and CNQX from the bath. Downward deflections in the trace represent electrotonic potentials evoked in response to the passage of constant hyperpolarizing current pulses through the electorde that were used for measurement of membrane conductance. Holding potential for both neurones was −54 mV. Slow sampling rate of digitization resulted in truncation in the amplitudes of some action potentials.
Repeated applications of the peptide produced similar, but often less robust effects in the same neurone, suggesting desensitization of PrRP receptors. On the other hand, changes in resting membrane potential were not observed in any of five responsive neurones when testing of the peptide was performed during blockade of impulse-dependent synaptic transmission with TTX (2 μm) (Fig. 6A2). In addition, administration of PrRP either in the absence or presence of TTX resulted in little or no change in membrane input resistance (699 ± 79 MΩ, control versus 721 ± 89 MΩ, n= 13), as determined by measurement of electrotonic potentials elicited by injection of constant, hyperpolarizing current pulses through the recording electrode (Fig. 6B). These results indicated that the peptide acted indirectly to modulate activity in DMV neurones via interactions at presynaptic sites. Interestingly, responsiveness to PrRP did not appear to vary among neurones sampled from different regions of the DMV. Thus, the excitatory effects of PrRP on spontaneous firing were encountered with equal probability among DMV neurones found at the level of the area postrema (5 of 16 tested) and those located in more caudal portions of the DMV (5 among 14 tested).
To determine if the excitation of DMV neurones by PrRP was dependent on activation of postsynaptic glutamate receptors, we next examined for sensitivity of the peptide-induced firing to blockade by selective antagonists for NMDA- (APV) and non-NMDA-type (CNQX) glutamatergic receptors. Co-administration of these glutamate receptor antagonists attenuated the membrane depolarization and eliminated the increase in spontaneous activity produced by application of PrRP (n= 6, Fig. 6B). Moreover, the elevation in firing caused by the peptide resumed after washout of the antagonists, indicating their actions were likely to be specific and not the result of tachyphylaxis to PrRP. The results obtained with these pharmacological antagonists strongly suggest that the stimulatory effects of PrRP on DMV neurone activity were mediated indirectly and resulted, at least in part, from an enhancement of glutamatergic input to the neurones.
Effects of PrRP on EPSCs evoked by NTS stimulation
In additional experiments conducted under voltage-clamp, we examined the effects of PrRP on postsynaptic currents evoked by electrical stimulation in the NTS to determine if the peptide modulated glutamatergic excitatory synaptic inputs to gastric-projecting DMV neurones. EPSCs were tested in isolation for modulation by PrRP by recording in the presence of the GABAA receptor antagonist BMI (30 μm) or picrotoxin (100 μm). Bath application of PrRP (100 or 300 nm) produced little change in resting membrane currents recorded at the holding potential (−60 mV), but increased the amplitude of evoked EPSCs by 162 ± 29% (P < 0.05, range 111% to 215%) in 17 of 35 neurones examined, including 10 of 19 and 7 of 16 cells sampled from rostral and caudal portions of the DMV, respectively (Fig. 7A). Among 12 neurones examined in which PrRP increased the amplitude of the evoked EPSC, the ratio (S2/S1) of the EPSCs evoked by two identical pulses delivered 100 ms apart was decreased from 0.95 ± 0.08 to 0.71 ± 0.11 (P < 0.05) in the presence of the peptide (Fig. 7C). Such an alteration in the paired-pulse ratio is consistent with a presynaptic action of PrRP (Harris & Cotman, 1983; Trombley & Westbrook, 1992). In addition, we found that PrRP did not affect inward currents produced by direct applications of l-glutamate (1 mm) to the slice via an auxillary pipette (n= 3, Fig. 8). Thus, it was unlikely that the increase in amplitude of evoked EPSCs observed during administration of PrRP resulted from changes in postsynaptic sensitivity to the excitatory transmitter.
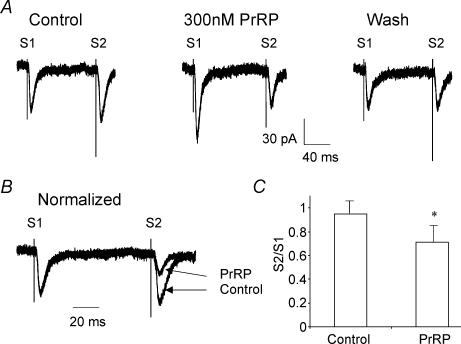
Figure 7. PrRP enhanced synaptically evoked excitation in DMV neurones.
A, pairs of EPSCs were evoked 100 ms apart by delivering two identical electrical stimuli (S1, S2) to the mNTS before, during administration and after washout of PrRP (300 nm). Superfusion of PrRP increased the amplitude of the evoked EPSCs (compare S1 response of control and PrRP traces), with recovery of the response back to control amplitude following 25 min of superfusion with normal Krebs solution (trace labelled wash). In addition, comparison of the amplitude of the second EPSC with that of the first synaptic current response of the pair revealed that administration of PrRP altered the paired pulse ratio (S2/S1). B, the alteration of the paired pulse ratio of evoked EPSCs by PrRP can be observed more readily following normalization of the responses recorded in PrRP to control amplitude. C, summary histogram of results from 12 experiments illustrating the decrease in ratio of the paired EPSCs (S2/S1) following administration of PrRP, indicating a presynaptic effect of the peptide. *P < 0.05. Traces in A and B are the average of responses to 10 stimulus trials. Holding potential was −60 mV. Error bars show s.e.m.
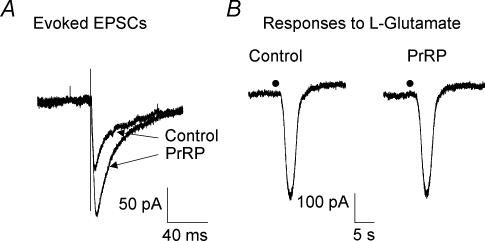
Figure 8. Enhancement of synaptically evoked excitation by PrRP does not reflect changes in postsynaptic sensitivity to glutamate.
A, voltage-clamp recordings from a representative experiment illustrate enhancement by PrRP of mNTS-evoked EPSCs in a gastric-projecting DMV neurone. Traces are the average of responses to 10 stimulus trials. B, PrRP did not affect inward currents evoked in the same neurone in response to application of the putative excitatory transmitter l-glutamate (1 mm) to the surface of the slice from an extracellular glass micropipette. Filled circles indicate the time of delivery of l-glutamate to the slice by pneumatic pressure ejection. Holding potential was −60 mV.
We next examined for an effect of PrRP on spontaneous EPSCs (sEPSCs) to determine whether the facilitation of excitatory input onto DMV neurones arose indirectly from increased firing in glutamatergic NTS neurones or from presynaptic actions of the peptide on their nerve terminals. For these experiments, sEPSCs were recorded at a holding potential of −60 mV in the presence of BMI (30 μm) from corpus-projecting DMV neurones in which PrRP enhanced excitation evoked by electrical stimulation in NTS. Administration of PrRP (300 nm) reversibly increased sEPSC frequency by 24–191% in six of eight neurones, with mean frequency being increased by 86 ± 24% (P < 0.01) (Fig 9A and B). The increase in frequency of sEPSCs was not accompanied by a significant change in mean amplitude of these postsynaptic currents (22.9 ± 2.6 pA, control versus 24.8 ± 3.5 pA, PrRP; P > 0.5) (Fig. 9C). Additionally, application of PrRP in the presence of TTX (2 μm) enhanced the frequency of action potential-independent miniature EPSCs (mEPSCs, increased by 24–50%) in two of three neurones in which sEPSC frequency was increased previously by PrRP in normal Krebs solution (Fig. 9D). The finding of increased frequency of sEPSCs (and mEPSCs) without change in amplitude strongly suggests that PrRP acted, at least in part, at receptors located on the terminals of glutamatergic neurones.
Figure 9. Presynaptic mechanism for PrRP to facilitate glutamatergic transmission between NTS and DMV neurones.
A, representative current traces from a corpus-projecting DMV neurone show that administration of PrRP (300 nm) reversibly increased the frequency of spontaneous EPSCs (sEPSCs). Voltage-clamp recordings were obtained at a holding potential of −60 mV and in the presence of bicuculline methiodide (30 μm) to permit the measurement of sEPSC activity uncomtaminated by sIPSCs. B, cumulative distributions of interevent intervals (left) and C, histogram plots of sEPSC amplitude (right) for the same neurone before and during administration of PrRP reveal that the peptide increased the frequency of sEPSCs, without appreciable change in their amplitude. D, traces from another corpus-projecting DMV neurone recorded in the presence of TTX (2 μm) and BMI (30 μm) show that the administration of PrRP (100 nm) increased the frequency of spontaneous mEPSCs. Recordings were made at a holding potential of −70 mV to facilitate the measurment of these currents by increasing unitary amplitude.
Effects of PrRP on excitability of NTS neurones
Results from in situ hybridization studies reveal that mRNA encoding for PrRP receptors is abundantly expressed within the caudal NTS (Roland et al. 1999; Lee et al. 2000). Thus, it seems plausible that receptors for PrRP might be expressed postsynaptically in somatodendritic regions of mNTS or comNTS neurones and play a role in modulating their excitability. If so, peptide-induced changes in the excitability and/or impulse activity of NTS neurones might contribute as well to the ability of PrRP to modulate spontaneous firing in vagal motor neurones and facilitate synaptic transmission between the NTS and DMV. This possibility was examined in a final series of experiments by determining the effects of PrRP on the spontaneous firing and intrinsic membrane properties of a random sample (n= 31) of mNTS and comNTS neurones. Contrary to expectations, administration of the peptide (100–300 nm) did not affect spontaneous firing or alter the membrane potential in any NTS neurones recorded under current clamp at −60 mV holding potential (Fig. 10). In addition, PrRP did not alter membrane conductance in these neurones, as determined from examination of the current–voltage relationships generated in the absence and presence of peptide in response to passage of an incremental series of hyperpolarizing current pulses through the recording electrode (Fig. 10). On the basis of these data, we suggest that PrRP regulates excitability in gastric-projecting vagal motor neurones primarily by acting presynaptically on the axonal terminals of glutamatergic neurones to facilitate excitatory synaptic input to the DMV.
Figure 10. PrRP does not affect postsynaptic excitability of NTS neurones.
A, family of electrotonic potentials recorded in an mNTS neurone in response to passage of an incremental series of 200 ms hyperpolarizing and depolarizing current pulses (shown below) through the recording electrode in the absence (control) and presence of PrRP (300 nm). Administration of PrRP had no effect on the membrane potential and did not alter spontaneous or current evoked action potential firing in this cell, or in any of 30 additional NTS neurones examined. B, current–voltage relationship generated from the responses shown in the traces above indicates a lack of effect of PrRP on membrane conductance over a range of membrane potentials. Neurone was current clamped at −55 mV holding potential.
Discussion
Stimulation of gastric contraction by PrRP
To the best of our knowledge this report provides the first evidence that suggests a role for PrRP in the central control of gastric motor function. The results of our in vivo experiments in anaesthetized rats indicate that PrRP evokes marked and prolonged increases in IGP and stimulates phasic contractions when microinjected into the rostral DMV at the level of the area postrema. Conversely, microinjection of the peptide into the caudal DMV at or slightly posterior to the CS induced pronounced gastric relaxation and inhibited phasic contractions. Several lines of evidence indicate that the gastric motor responses produced following microinjection of PrRP into the DMV can be attributed to actions of the peptide within the nucleus, resulting in corresponding changes in vagally mediated parasympathetic efferent outflow to the stomach. First, mapping studies demonstrated that the most effective sites for producing either gastric contraction or relaxation were located directly in the DMV, albeit in distinct regions of the nucleus. By contrast, placements of PrRP slightly outside the boundaries of the DMV either in the mNTS or comNTS produced weak responses, whereas those made in the hypoglossal nucleus were without effect. Second, both the stimulatory and depressant effects on gastric contractility produced by microinjection of PrRP into the DMV occurred independently of changes in mean arterial pressure. In addition, they were not mimicked by administration of an identical volume of saline, indicating the actions were specific and unlikely to result from diffusion of peptide outside the DMV. Third, the stimulatory effects of PrRP on gastric motor function were abolished following ipsilateral vagotomy, confirming their mediation via vagal efferent pathways. Earlier results from anatomic tracing studies indicate that each DMV provides ipsilateral projections to the stomach (Blessing et al. 1991), whereas neurones of the mNTS send projections to both the right and left DMVs (Blessing et al. 1991). Hence, the finding that ipsilateral vagotomy was sufficient to block the gastric stimulation produced by microinjection of PrRP in the DMV provides further evidence that the effects of the peptide were mediated locally and unlikely to involve changes in the activity of NTS neurones, per se. Similarly, the fact that the reductions in IGP produced by microinjection of PrRP into the caudal DMV were abolished following ipsilateral vagotomy argues that the inhibitory effects of the peptide on gastric motor function also derived from actions within the DMV.
Preganglionic neurones in the DMV that supply the upper gastrointestinal tract can be functionally partitioned into a ‘gastro-stimulatory group’ located rostral to the obex and an ‘inhibitory group’ found in caudal portions of the nucleus posterior to the obex, where stimulation results in relaxation of the lower oesophageal sphincter and gastric smooth muscle (Rossiter et al. 1990; Sivarao et al. 1999; Krowicki et al. 2002). In keeping with this, we found that glutamatergic stimulation of neurones in the rostral DMV at the level of the AP produced gastric contraction, reflected by an increase in IGP, whereas decreases in IGP occurred following microinjection of l-glutamate into more caudal portions of the DMV. Others have shown that the gastric motor responses produced by stimulation of neurones in the rostral and caudal DMV involve activation of separate groups of preganglionic vagal neurones that synapse, respectively, onto postganglionic cholinergic excitatory elements and non-adrenergic, non-cholinergic (NANC) inhibitory motor neurones in the gastric wall (Rossiter et al. 1990; Rogers et al. 1996; Takahashi & Owyang, 1997). In the present study, microinjection of PrRP into rostral or caudal portions of the DMV evoked the same pattern of gastric excitation or relaxation produced by administration of l-glutamate at that site. Thus, PrRP may exert its opposing effects on gastric motor function in the rostral and caudal DMV through activation of functionally distinct populations of preganglionic vagal motor neurones involved in cholinergic excitatory and NANC inhibitory projections to the stomach. Nonetheless, the results from our electrophysiological experiments indicate that PrRP does not act directly to regulate the excitability of gastric-projecting vagal motor neurones, in contrast to l-glutamate which produces depolarization and increases firing in gastric-related DMV neurones via activation of postsynaptic AMPA- and NMDA-type glutamate receptors (Travagli et al. 1991; Willis et al. 1996; Bertolino et al. 1997). Furthermore, we showed that local blockade of AMPA- and NMDA-type glutamate receptors with CNQX and AP5 prevented the gastric contractions and relaxations produced by microinjection of PrRP into rostral and caudal DMV, respectively. The blockade by CNQX and AP5 of responses to PrRP was probably due to specific antagonism of glutamate receptors, as evidenced by the concomitant blockade of gastric motor responses evoked by microinjection of l-glutamate. Morever, the fact that administration of these antagonists produced transient reductions (injections into the rostral DMV) or elevations in baseline IGP (caudal DMV) is consistent with results from earlier studies which suggest that gastric-projecting DMV neurones receive an underlying tonic excitation. (Sivarao et al. 1998). A reasonable conclusion to be drawn from these data is that PrRP acts within the DMV to regulate gastrostimulatory and gastroinhibitory vagal motor outflow by way of an indirect, glutamatergic-dependent mechanism.
Neuronal target and site of PrRP actions in the DVC
High levels of PrRP, PrRP mRNA and mRNA encoding for PrRP receptors have been reported in the caudal NTS, AP and the A1 catecholaminergic cell group in the ventrolateral medulla (Roland et al. 1999: Ibata et al. 2000). Each of these areas sends projections into the DMV (Rogers et al. 1980; Fukuda et al. 1987), providing an anatomical substrate whereby endogenous release of PrRP could modulate the excitability of vagal efferent neurones that supply the stomach. However, the DMV shows sparse specific binding sites for PrRP (Roland et al. 1999; Ibata et al. 2000), and vagal neurones do not express mRNA encoding for PrRP receptors (Roland et al. 1999; Lee et al. 2000; Ibata et al. 2000). These considerations suggest that PrRP may be acting indirectly to alter the activity of vagal neurones that regulate gastric motor function, presumably by modulating the level and/or effectiveness of excitatory afferent input converging on these cells. In keeping with this, results from brain slice recordings showed that PrRP produced excitation in a subset of corpus-projecting DMV neurones, but that these effects were abolished during blockade of impulse-dependent synaptic transmission with TTX. In addition, elevations in spontaneous neuronal firing produced by PrRP were sensitive to blockade by administration of APV and CNQX, indicating mediation of these excitatory effects via release of glutamate and activation of postsynaptic NMDA- and non-NMDA-type receptors. NTS neurones located in the subnuclei medialis and commisuralis provide a major source of excitatory input to the DMV, and electrical stimulation within these areas produces glutamatergic synaptic currents and potentials in a large percentage of gastric-projecting vagal neurones (Willis et al. 1996; Browning & Travagli, 2001; Browning et al. 2002; and this study). Furthermore, Lawrence et al. (2002) have recently demonstrated using c-fos functional mapping that neurones in the caudal NTS, which includes these subnuclei, show marked activation in response to intracerebroventricular administration of PrRP. Thus, NTS neurones and/or their axonal endings may provide the neurological substrate whereby the primary actions of endogenous PrRP in the DVC are transformed into changes in vagal efferent activity and gastric-directed outflow.
PrRP modulates NTS-DMV synaptic transmission
Although central administration of PrRP has been shown to produce marked activation of neurones in caudal NTS, no significant changes were found in the number of neurones expressing c-fos in the DMV following these treatments (Lawrence et al. 2002). Similarly, only a limited percentage of the DMV neurones (∼33%) recorded under current-clamp conditions showed increased spontaneous firing or a depolarizing shift in holding current during superfusion of brainstem slices with PrRP. On the other hand, administration of PrRP had marked effects on postsynaptic responses evoked by activation of excitatory afferent inputs from the NTS, producing enhancement of glutamatergic EPSCs in 48% of the corpus-projecting vagal motor neurones examined. In addition, PrRP altered the paired pulse ratio for EPSCs evoked by presentation of two identical NTS stimuli, spaced closely apart, but it did not affect the responses of DMV neurones to postsynaptic applications of l-glutamate, the putative excitatory neurotransmitter. Such data are consistent with the notion that PrRP acts via a presynaptic mechanism (see Trombley & Westbrook, 1992; Travagli & Williams, 1996) within the DVC to modulate the efficacy of excitatory synaptic connections between NTS neurones and vagal efferent neurones that innervate the stomach. This conclusion is supported further by the fact that in most neurones in which PrRP altered the paired pulse ratio, administration of the peptide also increased the frequency without change in amplitude of spontaneous EPSCs. Furthermore, the finding in several of these neurones that PrRP increased the frequency of action potential-independent mEPSCs strongly suggests that the facilitating effects of the peptide resulted, at least in part, from actions exerted at receptors located on glutamatergic nerve terminals.
Lastly, we should add that our inability to demonstrate direct postsynaptic effects of PrRP in NTS neurones does not preclude the possibility that PrRP might affect membrane excitability or spike firig indirectly, for example, by modulating responsiveness to or efficacy in the release of afferent neurotransmitters. Accordingly, it remains to be determined whether the PrRP-induced enhancement of excitatory input into the DMV reflects actions confined to the nerve terminals of glutamatergic NTS neurones or also includes more remote effects of the peptide that influence spike activity in these neurones. Still, the fact that ipsilateral vagotomy abolished the gastric contraction and relaxation produced by microinjection of PrRP into the DMV indicates that the responses themselves are not dependent on changes in NTS neurone activity. In Fig. 11 we present a schematic model that depicts the anatomical relationships within rostral and caudal portions of the DMV whereby PrRP is hypothesized to activate vagal efferent pathways to evoke gastric contraction and relaxation, respectively. In both situations, specific receptors for PrRP are hypothesized to reside presynaptically on the nerve endings of glutamatergic NTS neurones, rather than on the cell bodies of DMV or NTS neurones.
Figure 11. Schematic model depicting the anatomical relationships within rostral and caudal portions of the DMV whereby PrRP is hypothesized to activate vagal efferent pathways to evoke gastric contraction and relaxation, respectively.
In both cases, specific receptors for PrRP are hypothesized to reside presynaptically on the nerve endings of glutamatergic NTS neurones, but not on the cell bodies of DMV or NTS neurones. Dotted line denotes approximate boundary between the NTS and DMV, without reference to anatomical exactness. Note that PrRP is shown to be colocalized with tyrosine hydroxylase (TH) in NTS neurones of noradrenergic phenotype, comprising the A2 cell group. Abbreviations: Chol PPN, cholinergic postganglionic parasympathetic neurone; NANC PPN, non-adrenergic, non-cholinergic postganglionic parasympathetic neurone. Unshaded terminals represent presynaptic elements of excitatory synapses, whereas darkened terminals represent presynaptic inhibitory elements.
Physiological implications of PrRP-induced modulation of gastric motor function
A remaining issue to consider is how the gastric motor effects of PrRP demonstrated here might fit within a wider perspective of the peptide's postulated involvement in feeding behaviour and energy balance. Recent reports have provided strong evidence for a physiological role for PrRP in the regulation of feeding, particularly with respect to the signalling of satiety in response to gastrointestinal stimuli. In this regard, central administration of PrRP has been shown to reduce food intake and body weight in both free-feeding and fasted rats (Lawrence et al. 2000). In addition, PrRP mRNA is downregulated in the dorsomedial hypothalamus, NTS and ventrolateral medulla (VLM) in several rat models of negative energy balance (Lawrence et al. 2000). It is well established that signals from the gastrointestinal tract concerned with satiety are detected by vagal nerve afferents that project to neurones in the NTS (McCann et al. 1989), and it is likely that many of these neurones contain PrRP. Thus, Lawrence et al. (2002) found that a large number of PrRP immunoreactive neurones in the caudal NTS are activated by intraperitoneal injection of the satiety factor cholecystokinin (CCK). These investigators also showed that central administration of PrRP elicits a pattern of c-fos activation in brainstem and forebrain structures closely resembling that produced by peripheral administration of CCK (also see Rinaman et al. 1993, 1995). Furthermore, all PrRP-containing neurones in the NTS coexpress tyrosine hydroxylase (Chen et al. 1999; Ellacott et al. 2002), suggesting that at least some of them correspond to noradrenergic neurones of the A2 cell groups that convey the satiating actions of CCK to the hypothalamus (Luckman, 1992; Rinaman et al. 1995) and stimulate release of oxytocin, leading to reduced food intake and delayed gastric emptying (Verbalis et al. 1987; Lawrence et al. 2000). The present data provide evidence that PrRP-containing neurones in the NTS may also be components of a short-feedback loop, acting in a regionally specific manner within the DVC to modulate gastric motility. Conceivably, PrRP might be released from axonal collaterals of A2 neurones into the DMV (Rea et al. 1982; Fukuda et al. 1987) after feeding, and act locally either to constrain (rostral of the obex) or augment (caudal to the obex) the gastro-inhibitory effects produced by activation of oxytocin-containing input from the hypothalamus. Interestingly, nearly all of the NTS neurones that contain PrRP have also been shown to express receptors for leptin (Ellacott et al. 2002). Moreover, their anatomical location in close proximity to the AP may enable them to monitor leptin concentrations in the blood plasma, owing to the lack of a blood–brain barrier in this area. These considerations lead us to suggest an alternative possibility that the effects produced by actions of PrRP within the DMV may have little to do with events surrounding feeding episodes per se, but instead provide a mechanism for adjusting the level of parasympathetic autonomic outflow in accordance with nutrient intake to maintain energy homeostasis.
Acknowledgments
This work was supported by The University of Michigan GI Peptide Center Grant No.2 P30-DK34933 (S-YZ) and NIH grants DK061423 (HCM) and DK39199 (CO).
References
- Bertolino M, Vicini S, Gillis RA, Travagli RA. Presynaptic α2-adrenoreceptors inhibit excitatory synaptic transmission in rat brain stem. Am J Physiol Gastrointest Liver Physiol. 1997;272:G654–G661. doi: 10.1152/ajpgi.1997.272.3.G654. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Li YW, Wesselingh SL. Transneuronal transport of herpes simplex virus from the cervical vagus to brain neurons with axonal inputs to central vagal sensory nuclei in the rat. Neuroscience. 1991;4:261–274. doi: 10.1016/0306-4522(91)90163-i. [DOI] [PubMed] [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci. 2002;22:2998–3004. doi: 10.1523/JNEUROSCI.22-08-02998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999;517:521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol. 2001;531:45–435. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-T, Dun SL, Dun NJ, Chang J-K. Prolactin-releasing peptide-immunoreactivity in A1 and A2 noradrenergic neurons of the rat medulla. Brain Res. 1999;822:276–279. doi: 10.1016/s0006-8993(99)01153-1. [DOI] [PubMed] [Google Scholar]
- Ellacott KLJ, Lawrence CB, Rothwell NJ, Luckman SM. PRL-releasing peptide interacts with leptin to reduce food intake and body weight. Endocrinology. 2002;143:368–374. doi: 10.1210/endo.143.2.8608. 10.1210/en.143.2.368. [DOI] [PubMed] [Google Scholar]
- Ferreira M, Singh A, Dretchen KL, Kellar KJ, Gillis RA. Brainstem nicotinic receptor subtypes that influence intragastric and arterial blood pressure. J Pharmacol Exp Therap. 2000;294:230–238. [PubMed] [Google Scholar]
- Fukuda A, Minami T, Nabekura J, Oomura Y. The effects of noradrenaline on neurons in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol. 1987;393:213–231. doi: 10.1113/jphysiol.1987.sp016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabauskus G, Moises HC. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol. 2003;549:37–56. doi: 10.1113/jphysiol.2002.029546. 10.1113/jphysiol.2002.029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EW, Cotman CW. Effects of acidic amino acid antagonists on paired pulse potentiation at the lateral perforant path. Exp Brain Res. 1983;52:455–460. doi: 10.1007/BF00238039. [DOI] [PubMed] [Google Scholar]
- Hinuma S, Habata Y, Fujii R, Kawamata Y, Hosoya M, Fukusumi S, Kitada C, Masuo Y, Asano T, Matsumoto H, Sekiguchi M, Kurokawa T, Nishimura O, Onda H, Fujino M. A prolactin-releasing peptide in the brain. Nature. 1998;393:272–276. doi: 10.1038/30515. 10.1038/30515. [DOI] [PubMed] [Google Scholar]
- Ibata Y, Iijima N, Kataoka Y, Kakihara K, Tanaka M, Hosoya M, Hinuma S. Morphological survey of Prolactin-releasing peptide and its receptor with special reference to their functional roles in the brain. Neurosci Res. 2000;38:223–230. doi: 10.1016/s0168-0102(00)00182-6. 10.1016/S0168-0102(00)00182-6. [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Burmeister MA, Bethoud H-R, Scullion RT, Fuchs K, Hornby PJ. Orexins in rat dorsal motor nucleus of the vagus potently stimulate gastric motor function. Am J Physiol Gastrointest Liver Physiol. 2002;283:G465–G472. doi: 10.1152/ajpgi.00264.2001. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Celsi F, Brennand J, Luckman SM. Alternative role for prolactin-releasing peptide in the regulation of food intake. Nature Neurosci. 2000;3:645–646. doi: 10.1038/76597. 10.1038/76597. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Ellacott KLJ, Luckman SM. PRL-releasing peptide reduces food intake and may mediate satiety signaling. Endocrinology. 2002;143:360–367. doi: 10.1210/endo.143.2.8609. 10.1210/en.143.2.360. [DOI] [PubMed] [Google Scholar]
- Lee Y, Yang S, Soares MJ, Voogt JL. Distribution of prolactin-releasing peptide mRNA in the rat brain. Brain Res Bull. 2000;51:171–176. doi: 10.1016/s0361-9230(99)00212-9. 10.1016/S0361-9230(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Loewy AD. Central autonomic pathways. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 88–103. [Google Scholar]
- Luckman SM. Fos-like immunoreactivity in the brainstem of the rat following peripheral administration of cholecystokinin. J Neuroendocrinol. 1992;4:149–152. doi: 10.1111/j.1365-2826.1992.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Matsumoto H, Fujiwara K, Kitada C, Hinuma S, Onda H, Fujino M, Inoue K. Immunocytochemical localization of prolactin-releasing peptide in the rat brain. Endocrinology. 1999b;140:2326–2333. doi: 10.1210/endo.140.5.6685. 10.1210/en.140.5.2326. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Matsumoto H, Fujiwara K, Noguchi J, Kitada C, Hinuma S, Onda H, Nishimura O, Fujino M, Higuchi T, Inoue K. Central administration of prolactin-releasing peptide stimulates oxytocin release in rats. Neurosci Lett. 1999a;276:193–196. doi: 10.1016/s0304-3940(99)00831-9. 10.1016/S0304-3940(99)00831-9. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Verbalis JG, Stricker EM. LiCl and CCK inhibit gastric emptying and feeding and stimulate OT secretion in rats. Am J Physiol Regul Integr Physiol. 1989;256:R463–R468. doi: 10.1152/ajpregu.1989.256.2.R463. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Rea MA, Aprison MH, Felten DL. Catecholamines and serotonin in the caudal medulla of the rat: combined neurochemical-histofluorescence study. Brain Res Bull. 1982;9:227–236. doi: 10.1016/0361-9230(82)90135-6. 10.1016/0361-9230(82)90135-6. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Hoffman GE, Dohanics J, Le WW, Stricker EM, Verbalis JG. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1995;360:246–256. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Verbalis JG, Stricker EM, Hoffman GE. Distribution and neurochemical phenotypes of caudal medullary neurons activated to express c-Fos following peripheral administration of cholecystokinin. J Comp Neurol. 1993;338:475–490. doi: 10.1002/cne.903380402. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Herman GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, Kita H, Butcher LL, Novin D. Afferent projections to the dorsal motor nucleus of the vagus. Brain Res Bull. 1980;5:365–373. doi: 10.1016/s0361-9230(80)80006-2. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McTigue DM, Herman GE. Vagal control of digestion: modulation by central neuronal and peripheral endocrine factors. Neurosci Biobehav Rev. 1996;20:57–66. doi: 10.1016/0149-7634(95)00040-l. 10.1016/0149-7634(95)00040-L. [DOI] [PubMed] [Google Scholar]
- Roland BL, Sutton SW, Wilson SJ, Luo L, Pyati J, Huvar R, Erlander MG, Lovenberg TW. Anatomical distribution of prolactin-releasing peptide and its receptor suggests additional functions in the central nervous system and periphery. Endocrinology. 1999;140:5736–5745. doi: 10.1210/endo.140.12.7211. 10.1210/en.140.12.5736. [DOI] [PubMed] [Google Scholar]
- Rossiter CD, Norman PW, Jain M, Hornby PJ, Benjamin S, Gillis RA. Control of lower esophageal sphincter pressure by two sites in the dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 1990;259:G899–G906. doi: 10.1152/ajpgi.1990.259.6.G899. [DOI] [PubMed] [Google Scholar]
- Seal LJ, Small CJ, Kim MS, Stanley SA, Taheri S, Ghatei MA, Boom SR. Prolactin releasing peptide (PrRP) stimulates luteinizing hormone (LH) and follicle stimulating hormone (FSH) via a hypothalamic mechanism in male rats. Endocrinology. 2000;141:1909–1912. doi: 10.1210/endo.141.5.7528. 10.1210/en.141.5.1909. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK, Abrahams TP, Hornby PJ. Vagally-mediated gastric motor activity: evidence for kainite and NMDA receptor mediation. Eur J Pharmacol. 1999;368:173–182. doi: 10.1016/s0014-2999(99)00015-1. 10.1016/S0014-2999(99)00015-1. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998;10:305–313. doi: 10.1046/j.1365-2982.1998.00110.x. 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- Sumal KK, Blessing WW, Joh TH, Reis DJ, Pickel VM. Synaptic interaction of vagal afferents and catecholaminergic neurons in the rat nucleus tractus solitarius. Brain Res. 1983;277:31–40. doi: 10.1016/0006-8993(83)90904-6. 10.1016/0006-8993(83)90904-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504:479–488. doi: 10.1111/j.1469-7793.1997.479be.x. 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 1991;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Williams JT. Endogenous monoamines inhibit glutamate transmission in the spinal trigeminal nucleus of the guinea-pig. J Physiol. 1996;491:177–185. doi: 10.1113/jphysiol.1996.sp021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ, Westbrook GL. Excitatory synaptic transmission in the cultures of rat olfactory bulb. J Neurophysiol. 1992;64:598–606. doi: 10.1152/jn.1990.64.2.598. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1987;232:1417–1419. doi: 10.1126/science.3715453. [DOI] [PubMed] [Google Scholar]
- Willis A, Mihalevich M, Neff RA, Mendelowitz D. Three types of postsynaptic glutamate receptors are activated in DMNX neurons upon stimulation of NTS. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1614–R1619. doi: 10.1152/ajpregu.1996.271.6.R1614. [DOI] [PubMed] [Google Scholar]
- Yano T, Iijima N, Kataoka Y, Hinuma S, Tanaka M, Ibata Y. Developmental expression of prolactin releasing peptide in the rat brain: Localization of messenger ribonucleic acid and immunoreactive neurons. Brain Res Dev Brain Res. 2001;128:101–111. doi: 10.1016/s0165-3806(01)00148-1. 10.1016/S0165-3806(01)00148-1. [DOI] [PubMed] [Google Scholar]