Abstract
Recent studies indicate that the histaminergic system, which is critical for wakefulness, also influences learning and memory by interacting with cholinergic systems in the brain. Histamine-containing neurones of the tuberomammillary nucleus densely innervate the cholinergic and GABAergic nucleus of the medial septum/diagonal band of Broca (MSDB) which projects to the hippocampus and sustains hippocampal theta rhythm and associated learning and memory functions. Here we demonstrate that histamine, acting via H1 and/or H2 receptor subtypes, utilizes direct and indirect mechanisms to excite septohippocampal GABA-type neurones in a reversible, reproducible and concentration-dependent manner. The indirect mechanism involves local ACh release, is potentiated by acetylcholinesterase inhibitors and blocked by atropine methylbromide and 4-DAMP mustard, an M3 muscarinic receptor selective antagonist. This indirect effect, presumably, results from a direct histamine-induced activation of septohippocampal cholinergic neurones and a subsequent indirect activation of the septohippocampal GABAergic neurones. In double-immunolabelling studies, histamine fibres were found in the vicinity of both septohippocampal cholinergic and GABAergic cell types. These findings have significance for Alzheimer's disease and other neurodegenerative disorders involving a loss of septohippocampal cholinergic neurones as such a loss would also obtund histamine effects on septohippocampal cholinergic and GABAergic functions and further compromise hippocampal arousal and associated cognitive functions.
Histamine is an arousal-associated neurotransmitter that is synthesized by a restricted number of neurones located exclusively in the hypothalamic tuberomammillary nucleus (TMN) (Watanabe et al. 1983; Panula et al. 1984; Watanabe et al. 1984). Histamine neurones fire spontaneously (Reiner & McGeer, 1987; Haas & Reiner, 1988) at a rate that is highest during wakefulness and lowest during REM sleep (Vanni-Mercier et al. 1984) leading to a clear circadian rhythmicity in its release pattern (Mochizuki et al. 1992). Inhibition of histamine synthesis increases slow wave sleep, whereas preventing histamine degradation elicits long-lasting arousal (Lin et al. 1989). Lesioning or inactivation of histamine-containing neurones causes hypersomnolence (Lin et al. 1989). Finally, mice lacking histamine decarboxylase, the enzyme responsible for the synthesis of histamine, are unable to remain awake even in conditions requiring vigilance and show increased REM sleep (Parmentier et al. 2002; Ohtsu & Watanabe, 2003).
Exogenously administered histamine elicits arousal and antihistaminergic drugs decrease wakefulness and lead to a drowsy state. The centrally located histamine H1 receptors are largely considered responsible for the sedative actions of antihistamines as H1 receptor antagonists, though H2 and H3 receptors may also be involved. Histamine signalling may also be the downstream mediator of the arousal-promoting effects of the peptide hypocretin (Huang et al. 2001a), absence of which leads to narcolepsy (Peyron et al. 2000; Thannickal et al. 2000).
Histamine-containing neurones have very diverse projections, and form an especially dense fibre network in the cholinergic and GABAergic nucleus of the medial septum/diagonal band of Broca (MSDB) (Panula et al. 1989); the MSDB in turn projects back to the TMN (Wouterlood et al. 1988). Electrical stimulation of the TMN increases histamine release in the MSDB and ACh release in the hippocampus (Mochizuki et al. 1994); the hippocampus derives its ACh almost entirely from the MSDB cholinergic neurones. Through the septohippocampal cholinergic and GABAergic projections, the MSDB controls the hippocampal theta rhythm and associated cognitive functions via both the septohippocampal cholinergic and GABAergic projections.
Intracerebroventricular injections of mast cell degranulating peptide, which releases histamine, produce a quasi-permanent hippocampal theta rhythm in the motionless rat alternating with epileptiform spike waves (Cherubini et al. 1987). Recent findings indicate that histamine directly influences processes underlying learning and memory (Bacciottini et al. 2000; Passani et al. 2000; Blandina et al. 2004). Thus, intraseptal infusions of histamine enhance hippocampal ACh release (Bacciottini et al. 2002), a finding that is consistent with the reported H1-receptor-mediated excitatory effects of histamine on presumed septal cholinergic neurones (Gorelova & Reiner, 1996). Cholinergic nucleus basalis neurones projecting to the cortex are also excited by histamine mostly via H1 but also via H2 receptors (Khateb et al. 1995).
In recent years, the septohippocampal GABAergic neurones have especially been noted for their important role in generating hippocampal theta as theta persists even after selective lesioning of septohippocampal cholinergic neurones (Lee et al. 1994; Bassant et al. 1995). We have therefore investigated the electrophysiological and pharmacological actions of histamine on rat septohippocampal GABAergic neurones and also studied histamine-induced interactions between the septohippocampal cholinergic and GABAergic neurones. In addition, we have performed double immunolabelling studies to investigate the relationship between histamine fibres and the septohippocampal cholinergic and GABAergic neurones.
Methods
All experiments were carried out with the approval of the Yale Animal Care and Use Committee.
Slice preparation for electrophysiological recordings
Brain slices containing the MSDB were prepared from male Sprague-Dawley albino rats (2–4 weeks old) using methods detailed previously (Alreja & Liu, 1996). Briefly, rats were anaesthetized with chloral hydrate (400 mg kg−1 i.p.) and killed by decapitation. The ACSF (pH 7.35–7.38), equilibrated with 95% O2–5% CO2, contained (mm): NaCl, 126; KCl, 3; NaH2PO4, 1.25; d-glucose, 10; NaHCO3, 25; CaCl2, 2; and MgSO4, 2. Following decapitation, the brain was removed and placed in a Petri dish containing ACSF and trimmed to yield a small block containing the MSDB. Coronal slices of 300–600 μm thickness containing the MSDB were cut with a vibrating-knife microtome (Frederick Haer, ME, USA) and transferred to a Plexiglas recording chamber (1.5 ml volume) on the fixed stage of an Olympus BX50WI scope or to an interface-type chamber. The slices were maintained at 33 ± 0.5°C. One to two hours later the slice was used for recording. The chamber was continuously perfused with normal ACSF at a rate of 2–3 ml min−1. Extracellular and intracellular recordings were performed using blind recordings in slices maintained in the interface chamber wherein the recording site was visualized using a dissection microscope (Alreja & Liu, 1996). Visualized recordings on identified septohippocampal projection neurones were performed using the IR-DIC setup (see below).
Labelling of septohippocampal cholinergic neurones using Cy3–192IgG
Septohippocampal cholinergic neurones were identified in the living state using the fluorescent marker, Cy3–192IgG as previously described (Alreja et al. 2000; Wu et al. 2000). This technique exploits the fact that septohippocampal cholinergic neurones, but not the GABAergic neurones of the MSDB, exclusively express the low-affinity nerve growth factor receptor, p75. Thus, Cy3–192IgG, which is a conjugate of the fluorochrome, Cy3, and an antibody against the p75 receptor (192IgG), is taken up only by cholinergic terminals and thus selectively labels only the cholinergic subpopulation. The specificity of this marker and its inert nature have been thoroughly confirmed by us (Alreja et al. 2000; Wu et al. 2000, 2003b) and by others in an earlier study (Hartig et al. 1998).
Anaesthesia, surgery and postoperative care
Surgical instruments were immersed in chlorine dioxide (Clidox) for 6 h and rinsed in sterile water prior to use. The surgery table was wiped with disinfectant before and after surgery and covered with a benchcoat paper. Rats, 10–14 days old, were anaesthetized with the following cocktail: ketamine 75 mg kg−1, xylazine 4 mg kg−1 and acepromazine 0.075 mg kg−1 administered intraperitoneally. Level of anaesthesia was assessed by doing the toe-pinch test and making sure that there is no withdrawal response. After being anaesthesized, animals were prepped away from the surgical area and the skin cleaned with a disinfectant. Hair was removed from the incision site with clippers. All loose hair and debris was removed using tape. A two-stage scrub, first with a cotton swab soaked in chlorhexadine followed by a cotton swab soaked in isopropyl alcohol, was used to prepare the surgery site, which was cleaned from the centre outwards. The rats were then placed in a stereotaxic apparatus on a warm water circulating pad lined with a towel and draped with cotton gauze. Anatomical landmarks were used to determine the craniotomy site and a 1 cm incision made in the scalp. The topical anaesthetic cetacaine was sprayed and a few minutes later the scalp was wiped clean. Burr holes were made in the skull corresponding to specific coordinates as determined by the rat atlas and Cy3–192IgG (3–5 μl; 0.4 mg ml−1) was stereotaxically injected bilaterally into the lateral ventricle of each rat with a Hamilton syringe (22 gauge needle) at a rate of 0.5 μl min−1. The coordinates used were: 0.8 mm posterior from bregma, 1.2 mm lateral from midline and 3–4 mm below the dura. The incision was closed with skin glue (3M Pronto CA4 instant adhesive).
Animals were monitored from the completion of surgery until they recovered from anaesthesia and were fully awake and ambulatory. Animals were kept warm and dry to prevent hypothermia by placing in a cage lined with a clean towel and placing the cage under a heat lamp to speed recovery. Occurrence of wound infections was prevented by topical use of antibiotics (zinc–neomycin–polymixin-B ointment). Two or more days later, the injected rats were used to prepare brain slices.
Retrograde labelling of septohippocampal neurones with rhodamine beads
Rats were anaesthetized and subjected to the same surgical and postsurgical care as above. Retrograde labelling of septohippocampal neurones was performed by pressure-injecting 50–100 nl of rhodamine-labelled fluorescent latex microspheres (Lumafluor Inc., Naples, FL, USA) at several sites within the hippocampus using a glass micropipette (40–50 μm tip diameter) (Alreja et al. 2000; Wu et al. 2000). The stereotaxic coordinates were (anterior posterior, lateral, vertical): −2.8, −1.4, −2.8; −4, −1.4, −2.8; and −5.8, −4.5, −3.5 to −6 mm track. Two or more days later, the injected rats were used to prepare brain slices and injection sites were confirmed for each experiment.
Unlike Cy3–192IgG, rhodamine beads are not selective for any particular subpopulation of neurones, but label both septohippocampal cholinergic and GABAergic neurones. However, the two neuronal subpopulations can be differentiated on the basis of their electrophysiological characteristics and responsiveness to muscarine. Thus, while septohippocampal cholinergic neurones are never excited by muscarine, > 90% of septohippocampal GABA-type neurones are strongly excited by muscarine in vitro (Alreja et al. 2000; Wu et al. 2000).
Fluorescence and infrared imaging
Infra-red, differential interference contrast imaging (IR-DIC) was performed to visualize neurones for extracellular or patch-clamp recording using an Olympus Optical (Tokyo, Japan) BX-50 microscope using methods described in earlier studies (Alreja et al. 2000; Wu et al. 2000). Rhodamine-labelled and Cy3–192IgG-labelled neurones were visualized using the appropriate fluorescence filter. A neurone viewed with infrared optics was considered to be the same as that viewed with fluorescence optics when the infrared image and the fluorescent image of the neurone had the same position and orientation with the two imaging systems.
Electrophysiology recordings
Extracellular recordings
Extracellular potentials were recorded with glass micropipettes filled with 2 m NaCl (5–10 MΩ) using an Axoclamp-2A amplifier. Single units were discriminated on the basis of their shape, duration and amplitude. A window discriminator counted the frequency of the spikes using an amplitude threshold that was 5–10 times the noise level. Frequency counts were recorded on a chart recorder (Gould 2200 or Gould TA240).
Whole-cell recordings from visualized neurones
The image of the cells in the slice was displayed on a video monitor, and glass pipettes used for electrophysiological recordings were visually advanced through the slice to the surface of the cell from which recordings were made. Whole-cell patch-clamp recordings were performed using previously described methods (Alreja & Liu, 1996). In brief, low resistance (2.5–3.5 MΩ) patch pipettes were filled with a solution containing (mm): potassium methylsulphonate, 120; Hepes, 10; BAPTA-K4, 5; sucrose, 20; CaCl2, 2.38; MgCl2, 1; K2ATP, 1; and GTP, 0.1 (pH 7.32–7.35). Voltage clamp recordings were performed using the continuous single electrode voltage clamp mode.
Immunohistochemical staining and confocal microscopy
Young adult Sprague-Dawley albino rats were anaesthetized with i.p. pentobarbital. Transcardiac perfusion was performed with ice-cold 0.1 m phosphate buffer, pH 7.4 (PB) followed by 4% 1-ethyl-3(3-di-methyl-aminopropyl)-carbodiimide (EDAC; CMS Chemicals, Abingdon, UK) and 0.2% N-hydroxysuccinimide (NHS; Sigma-Aldrich, St Louis, MO, USA) and finally the same fixative including 0.2–0.5% paraformaldehyde (PFA; J. T. Baker, Deventer, the Netherlands). The brains were removed and immersed at +4°C in EDAC and NHS overnight, in PFA for 3 h and in 20% sucrose overnight. All solutions were made in PB. Brains were then frozen and stored at −20°C until use. Sections of 20 μm were cut on a cryostat and incubated at +4°C for 16 h with a rabbit anti-histamine antibody (Panula et al. 1984, 1989) and either a mouse anti-parvalbumin antibody (Swant, Bellinzona, Switzerland) or a mouse anti-choline acetyltransferase antibody (Chemicon, Temecula, CA, USA). Dilutions were 1 : 10 000, 1 : 4000 and 1 : 1000, respectively. One per cent normal goat serum was included in all cases. Secondary, fluorophore-conjugated goat anti-rabbit and goat anti-mouse antibodies (Alexa 488 and 568, respectively, by Molecular Probes, Leiden, the Netherlands) were diluted 1 : 500. Incubation lasted 1–2 h at room temperature.
Fluorescence was detected with a Leica TCS-SP confocal laser scanning microscope system (Leica, Heidelberg, Germany) equipped with an Ar–Kr laser (Melles Griot, Carlsbad, CA, USA). Excitation wavelengths were 488 and 568 nm and fluorescence detection windows approximately 495–550 and 590–690 nm, respectively. Image stacks were acquired and maximum projection images constructed with Leica TCS NT/SP Scanware software.
Materials
Histamine dihydrochloride, dimaprit dihydrochloride (R)(–)-α-methylhistamine dihydrochloride, pyrilamine maleate salt, cimetidine, imetit dihydrobromide, ranitidine hydrochloride, triprolidine hydrochloride, muscarine chloride, atropine methyl bromide, neostigmine bromide, 4-diphenylacetoxy-N-(2-chloroethyl) piperidine hydrochloride were obtained from Sigma-RBI (St Louis, MO). Tetrodotoxin (TTX) was obtained from Alomone labs (Jerusalem, Israel). All drugs were diluted in ACSF from previously prepared stock solutions that were prepared in water and stored at −20°C. Rhodamine microspheres were obtained from Lumafluor Inc. (Naples, FL, USA). Cy3–192IgG was obtained from Advanced Targeting Systems (San Diego, CA, USA).
Results
Histamine excites retrogradely labelled septohippocampal GABA-type neurones
Similar to our previous studies, retrogradely labelled septohippocampal neurones were classified as GABA-type based on their electrophysiological characteristics and/or their excitatory response to muscarine (see Methods). Previously published studies on histamine effects in brain slices have used histamine in concentrations ranging from 0.3 to 1000 μm with most studies using 10 or 30 μm (Li et al. 1999; Bell et al. 2000; Tian et al. 2000). Histamine, was therefore used at concentrations of 10–30 μm in this study. In cell-attached recordings, bath-applied histamine (10–30 μm) produced a profound excitatory effect in 93.4% of the septohippocampal GABA-type neurones tested (113/121) and had no effect in the remaining neurones (Fig. 1).
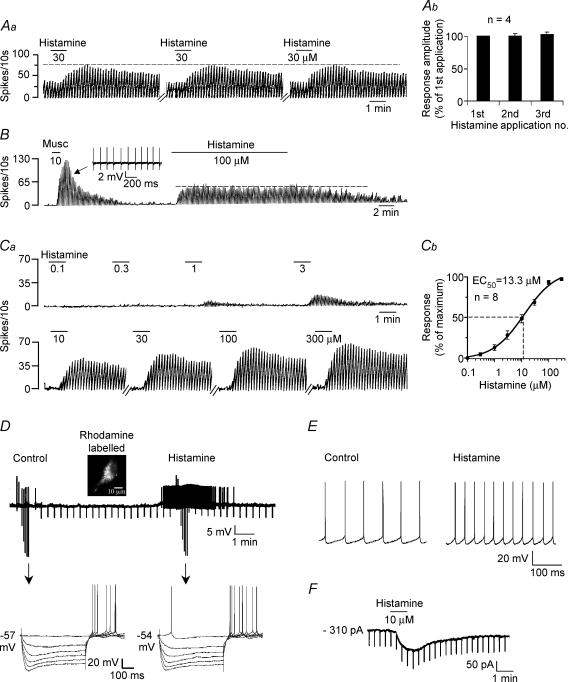
Figure 1. Septohippocampal GABA-type neurones are excited by histamine in a reversible, reproducible, concentration-dependent manner.
Aa, rate-meter record of action potentials recorded during a cell-attached recording showing the response of a GABA-type neurone to repeated applications of histamine. Note the lack of desensitization. This cell was also excited by muscarine (not shown). b, bar chart summarizing the reproducibility of the histamine response. B, another rate-meter record showing the response of a muscarine-excited MSDB GABA-type neurone to a 15 min application of a high concentration of histamine. Note the lack of desensitization. Inset shows extracellular spikes. Ca, rate-meter record of a cell-attached recording showing the response of a septohippocampal GABA-type neurone to 8 different concentrations of histamine. b, concentration–response curve showing that histamine excites septohippocampal GABA-type neurones with an EC50 value of 13.3 μm. D, whole-cell current-clamp recording showing a rhodamine-labelled septohippocampal GABA-type neurone that was quiescent and responded to 10 μm histamine with a 3 mV depolarization and spontaneous firing activity. Arrows refer to the points at which a step protocol of depolarizing and hyperpolarizing currents was applied to the cell. E, another whole-cell current clamp recording from a cell showing action potentials. Note that histamine increased the firing rate of this cell. F, whole-cell voltage-clamp recording showing that histamine produces an inward current at a holding potential of −65 mV.
The histamine-induced excitation was reproducible, reversible and non-desensitizing as repeated applications (3–6 times at intervals of 3–40 min) produced responses of comparable magnitude with the mean magnitude of the repeated responses being 96.5 ± 5% of the first response (range: 83–98%; Fig. 1Aa and b). Prolonged applications (15 min) of high concentrations of histamine (30–100 μm) also did not result in any kind of reduction in the response to histamine (Fig. 1B).
The histamine excitation was concentration dependent, with an EC50 value of 13.3 μm as determined by plotting 8-point concentration curves (100 nm to 300 μm; n = 8; Fig. 1Ca and b). This EC50 value is comparable to the EC50/KD values of 6.3 μm (Bell et al. 2000) and 8 ± 2 μm (Soria-Jasso et al. 1997) that have previously been reported in brain slices.
The excitatory effects of histamine could also be observed in a vast majority of neurones during whole-cell recordings (95.3%; n = 129). In current-clamp recordings, histamine produced a 3.6 ± 0.6 mV depolarization and in voltage-clamp recordings at a holding potential of −65 mV, histamine produced a 109 ± 8.5 pA inward current with no significant change in input conductance (Figs 1D–F). Little or no change in input conductance has previously been noted to accompany histamine-induced activation of supraoptic vasopressin neurones (Smith & Armstrong, 1996). This may reflect involvement of a dual ionic mechanism involving opening of a cationic conductance and closing of a K+ channel, such that there is no change in net conductance or activation of a sodium–calcium exchanger (Keele et al. 1997; Eriksson et al. 2001; Wu et al. 2004). Thus, histamine excites a vast majority of septohippocampal GABA-type MSDB neurones in a reversible, reproducible and concentration-dependent manner.
Histamine excites septohippocampal GABA-type neurones via both H1 and H2 receptors
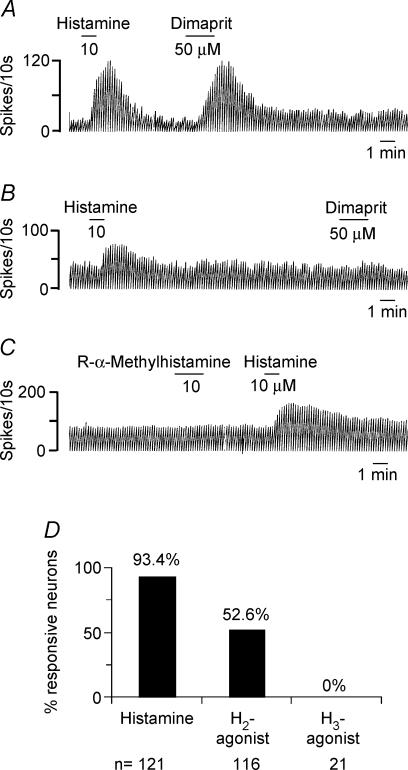
In order to determine the pharmacology of the histamine response, we tested the effect of histamine receptor subtype-selective agonists and antagonists. One hundred and sixteen histamine-responsive neurones were tested with dimaprit, an H2-receptor selective agonist that has a KD value of 1100 nm for the H2 receptor and more than > 10 000 for the H1 receptor (Hough, 2001); 61/116 neurones responded to dimaprit (20–50 μm) with an excitation, suggesting presence of an H2 receptor in at least 52.6% of the neurones tested (Fig. 2A, B and D). In voltage-clamp recordings at −65 mV, dimaprit induced an inward current of 58.2 ± 8.5 pA (n = 28; not shown). The H3 receptor agonists, R-α-methylhistamine (n = 11) or imetit (n = 10), had no effect in any of the 21 neurones tested (Figs 2C and D).
Figure 2. Histamine receptor agonists suggest the presence of H1 and H2 but not H3 receptor-mediated responses in septohippocampal GABA-type neurones.
A–C, rate-meter record showing the effect of histamine receptor agonists on 3 MSDB neurones recording using the cell-attached mode. The cell in A was excited by histamine as well as by dimaprit, a selective H2 receptor agonist. The cell in B responded to histamine but not to dimaprit. The cell in C responded to histamine but not to the H3 receptor agonist R-α-methylhistamine. D, bar chart summarizing the agonist data and showing that while histamine activated 93.3% of neurones, the H2 agonist activated only 52.6% neurones and R-α-methylhistamine did not have any effect on the 21 neurones tested.
H1 receptor blockade was achieved using pyrilamine, also known as mepyramine, a first choice competitive antagonist with a KD of 0.8 nm and good receptor selectivity. Triprolidine, another high affinity H1 receptor antagonist (KD value 0.1 nm), was also used in some experiments. Cimetidine and ranitidine with KD values of 800 nm and 200 nm, respectively, were used for H2 receptor antagonism (Hough, 2001).
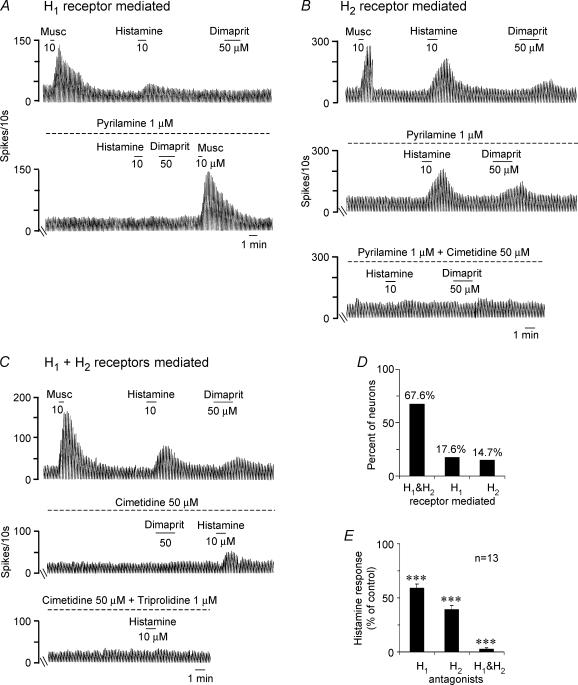
The effect of the H1 receptor antagonists pyrilamine and triprolidine (1 μm, 15−20 min), and/or the H2 receptor antagonists cimetidine (100–200 μm) or ranitidine (2 μm), was tested in a total of 34 neurones. In 18 neurones, the H1 receptor antagonists pyrilamine (n = 15) and triprolidine (n = 3) were applied first and in 16 neurones, the H2 receptor antagonists were applied first; 20 neurones received both antagonists. The histamine receptor antagonists had no effect on baseline firing of any of the neurones tested (Fig. 3A–C), suggesting lack of an endogenous H1 or H2 receptor-mediated tone in the brain slice preparation which is devoid of histaminergic cell bodies. In 17.6% of septohippocampal GABA-type neurones the histamine response was completely blocked by the H1 receptor antagonist (Fig. 3A–D), in 14.7% of neurones, the histamine effect was completely blocked by the H2 receptor antagonist (Fig. 3B) and in 67.6% of neurones both H1 and H2 receptor antagonists were required to block the histamine effect (Figs 3C and D). Of the cells expressing both H1 and H2 receptor-mediated responses, the H1 receptor antagonists reduced the histamine response to 59 ± 3.9% of control values and the H2 receptor antagonist reduced the response to 39 ± 3.6% of control values. The two antagonists together reduced the histamine response to 2.7 ± 1.3% of control values (Fig. 3E).
Figure 3. Subtype selective H1 and/or H2 receptor antagonists block the histamine-induced excitation in septohippocampal GABA-type neurones.
A, rate-meter record of action potentials recorded during a cell-attached recording showing an example of a cell in which the histamine response was completely blocked by the H1 receptor antagonist pyrilamine. Note that dimaprit had no effect in this cell. Also note that the muscarine response was not affected by pyrilamine, indicating specificity of blockade. B, example of another cell-attached recording in which both histamine and dimaprit activated the cell. The H1 receptor antagonist pyrilamine (1 μm, 15 min) had no effect on the histamine or the dimaprit response, suggesting lack of an H1 receptor-mediated effect. Addition of the H2 receptor antagonist cimetidine, however, completely blocked the response to the two agonists, indicating exclusive involvement of the H2 receptor. C, rate-meter record of a cell-attached recording from a septohippocampal GABA-type neurone that is excited both by histamine and by the H2 receptor agonist dimaprit. Note that cimetidine (50 μm, 20 min) completely blocked the dimaprit and reduced the histamine response in this cell. Addition of the H1 receptor antagonist triprolidine (1 μm, 20 min) blocked the remaining histamine response, indicating the presence of both an H1 and an H2 receptor-mediated excitation in this cell. D, bar chart summarizing the percentage of neurones expressing H1, H2 or H1 and H2 receptor-mediated responses. E, bar chart summarizing the relative contribution of the 2 receptor subtypes in cells exhibiting a mixed H1 and H2 receptor-mediated response.
The effect of dimaprit (20–50 μm) was completely blocked by the H2 receptor antagonists (Fig. 3B and C) but not by the H1 receptor antagonists (Fig. 3B), confirming the selectivity of dimaprit for the H2 receptor at the concentrations used.
Histamine excites MSDB neurones via a direct postsynaptic mechanism and an indirect mechanism
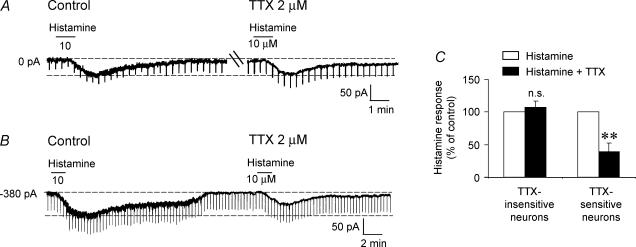
In the presence of TTX the histamine-induced inward current was significantly reduced, being 70.2 ± 13.4% of control values (P= 0.05; Student's paired t test; n = 11). However, within the population of 11 cells tested with TTX, the response to TTX was not uniform. In 54.5% of cells tested (5/11), the histamine response was reduced by no more than 10% of control values (mean response in TTX: 107 ± 9.5% of control; P= 0.47; Fig. 4A and C), suggesting a possible direct effect. However, in the remaining 54.5% cells, the histamine response was reduced by 0–76% (mean: 39 ± 13.3% of control; P = 0.006; Fig. 4B and C). This is in contrast to the less than 17% reduction seen after repeated applications of histamine in control ACSF at time intervals similar to those used in the TTX experiments (see above). These experiments therefore suggested that the actions of histamine on MSDB neurones could be mediated via direct as well as indirect effects.
Figure 4. Histamine activates septohippocampal GABA-type neurones via direct and indirect mechanisms.
A, whole-cell, voltage-clamp recording showing an inward current in response to histamine. Note that the magnitude of the histamine response was similar in the absence and presence of TTX. B, in contrast, in another neurone the amplitude of the histamine response was significantly attenuated in the presence of TTX. C, bar chart showing that in 45.5% of cells, the histamine response remained unchanged in the presence of TTX, whereas in the remaining 54.5% cells the histamine response was reduced to 39 ± 13.3% of control values.
Histamine has previously been reported to excite septal cholinergic neurones located within the MSDB via an H1 receptor (Gorelova & Reiner, 1996). Since septohippocampal cholinergic neurones synaptically innervate septohippocampal GABAergic neurones and activate them via released ACh acting on muscarinic M3 receptors (Brauer et al. 1998; Alreja et al. 2000; Wu et al. 2003b), we hypothesized that the indirect effect of histamine on septohippocampal GABA-type neurones may be mediated via an increase in local ACh release. We speculated that if locally released ACh contributes to the histamine response, then preventing its degradation by using an acetylcholinesterase (AChE) inhibitor should enhance the response of the septohippocampal GABAergic neurones to histamine. Conversely, blocking the effect of locally released ACh using atropine methylbromide or 4-diphenylacetoxy-N-(2-chloroethyl) piperidine hydrochloride (4-DAMP mustard), a selective M3 receptor antagonist, should reduce the histamine response.
Before testing this hypothesis, we confirmed the findings of Gorelova & Reiner (1996) by testing the effects of histamine on septohippocampal cholinergic neurones in our own laboratory.
Septohippocampal cholinergic neurones are excited by histamine via H1 but not H2 receptors
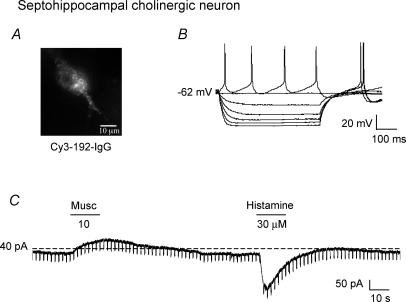
Septohippocampal cholinergic neurones were identified in living brain slices using the selective marker, Cy3–192IgG (Alreja et al. 2000; Wu et al. 2000, 2003a,b; Xu et al. 2004; Hajszan et al. 2004). Ninety-six per cent of Cy3–192IgG-labelled septohippocampal cholinergic neurones were excited by histamine (n = 47; Fig. 5A), but not affected by the H2 (n = 10) or H3 receptor agonists (n = 6). The histamine-induced inward current was TTX insensitive (n = 7) and was blocked by the H1 receptor antagonist pyrilamine (n = 5; not shown), thus confirming the Gorelova & Reiner study.
Figure 5. Excitatory effect of histamine on identified septohippocampal cholinergic neurones.
A and B, whole-cell current-clamp recording showing electrophysiological characteristics of a Cy3–192IgG-labelled septohippocampal cholinergic neurone. Note the lack of a depolarizing sag in response to hyperpolarizing pulses. C, voltage-clamp recording from the same cholinergic neurone showing that histamine induced an inward current whereas muscarine had the opposite effect.
Next, we tested the hypothesis that histamine-activation of septohippocampal cholinergic neurones contributes to the activation of septohippocampal GABA-type neurones via activation of muscarinic, M3 receptors.
Acetycholinesterase inhibitors potentiate the histamine response in septohippocampal GABA-type neurones
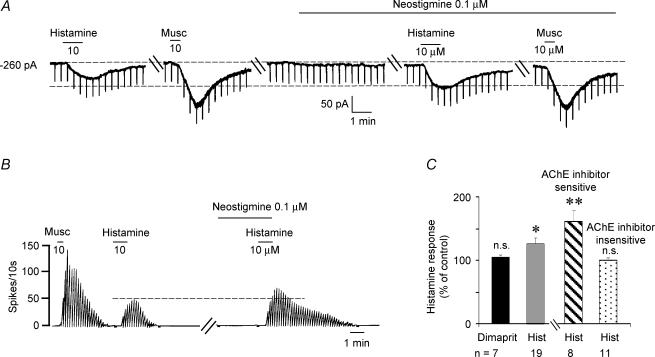
The effect of AChE inhibitors, neostigmine or ambenonium, were tested using a low concentration of 100 nm. At this concentration AChE inhibitors produced a minimal change in baseline but produced a significant increase in the histamine response (126 ± 10.3%; n = 19; P= 0.02), although effects were not uniform across cells. Thus 11/19 cells tested showed no change or less than 20% increase in the histamine response (mean increase 100.4 ± 4.1%; P= 0.9, not significant) and the remaining eight neurones showed a 161 ± 17.3% increase over control (P = 0.009; Fig. 6A–C). Thus, an indirect component of the histamine response that was potentiated by AChE inhibitors was observed in 57.8% of neurones tested. Interestingly, in the same cells the muscarine response was not altered by AChE inhibitor treatment (Fig. 6A), presumably, due to the fact that septohippocampal cholinergic neurones are not excited by muscarine (Wu et al. 2000).
Figure 6. AChE inhibitors potentiate the response to histamine in septohippocampal GABA-type neurones.
A, whole-cell voltage-clamp recording showing that the acetylcholinesterase inhibitor neostigmine increased the histamine-induced inward current from 50 to 80 pA. Neostigmine alone applied for the same duration produced only a 10 pA inward current. Also note that as expected, the muscarine effect was not enhanced by neostigmine (see text for details). B, rate-meter record of action potentials recorded during a cell-attached recording from another cell showing that neostigmine increased the amplitude and duration of the histamine response. Note that at the 0.1 μm concentration used, neostigmine had no effect on basal firing. C, bar chart showing that AChE inhibitors potentiated the histamine response but not the response to the H2 receptor agonist dimaprit. The histamine-tested cells varied in their sensitivity to neostigmine, with 42% of the cells responding with a 161 ± 17.3% increase in the histamine response. The remaining cells were not affected by neostigmine.
In contrast to histamine, the response to the H2 receptor agonist dimaprit was not enhanced by neostigmine (104.9 ± 3.8% of control; n = 7; P= 0.24, not significant; Fig. 6C), suggesting that the H2 component of the histamine response is not mediated by ACh release. This finding is consistent with the lack of involvement of H2 receptors in septohippocampal cholinergic neurones.
Next, we tested the effects of muscarinic receptor antagonists on histamine-induced excitation of septohippocampal GABA-type neurones.
Muscarinic receptor antagonists reduce histamine effect
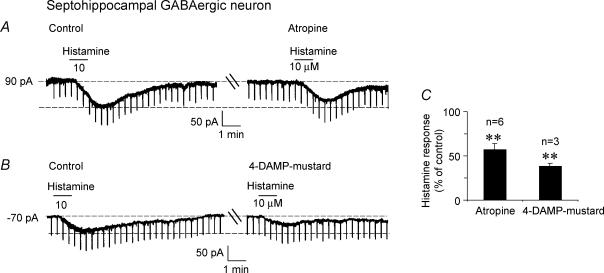
In the five neurones tested, bath-applied atropine methylbromide (0.1–1 μm, 20 min) reduced the histamine response by 56.7 ± 7.3% (range: 37.5–84%; P = 0.002; Fig. 7A and C), suggesting the presence of a muscarinic component to the histamine response. In the three cells tested, the M3 receptor antagonist 4-DAMP mustard (200 nm, 20 min) reduced the histamine response to 37.9 ± 3.6% of control values (P= 0.003; Fig. 7B and C). The H2 receptor antagonist, dimaprit had no effect in these neurones. Thus, locally released ACh contributes to H1 receptor-mediated histamine-induced excitation via M3 muscarinic receptors.
Figure 7. Muscarinic receptor antagonists reduce histamine-induced excitation in septohippocampal GABA-type neurones.
A, voltage-clamp recording showing that the muscarinic receptor antagonist atropine methyl bromide (100 nm, 20 min) reduces histamine-induced inward current in a septohippocampal GABA-type neurone. B, the M3 receptor antagonist, 4-DAMP mustard also reduces the response to histamine, consistent with previous findings that suggest that locally released ACh in septal slices excites septohippocampal GABA-type neurones via M3 receptors. The H2 receptor agonist, dimaprit had no effect on these neurones. C, bar chart summarizing the effect of the 2 muscarinic receptor antagonists on the histamine response.
Next we used double immunolabelling studies to determine the relationship of histamine-immunoreactive (-ir) fibres with septohippocampal cholinergic and GABAergic neurones.
Histamine-immunoreactive fibres innervate both septohippocampal cholinergic and GABAergic neurones
Histamine-ir fibres were seen throughout the MSDB and were most dense along the midline, especially in the MS. In coronal sections, fibres of the ventrolateral DB were running along the ventrolateral–dorsomedial axis, towards the MS, where they were orientated along the dorsoventral axis.
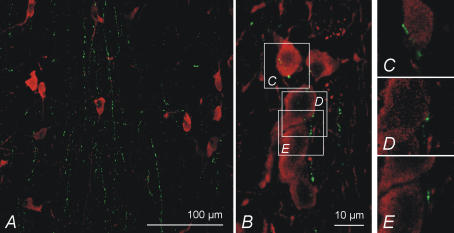
ChAT-ir neurones were abundant in both the MS and DB. In MS, most neurones were located in the lateral parts of the nucleus and in DB they were distributed throughout the nucleus. Immunohistochemical double-stainings revealed that histamine-ir fibres innervated the ChAT-ir neurone containing areas (Fig. 8A) and made close contacts with approximately 1/3 of the neurones in both MS and DB, as evaluated by 3D confocal microscopy on a resolution level of approximately 500 nm (n = 30 for each nucleus). High-resolution confocal microscopy (theoretical resolution less than 150 nm) of some such contacts suggested that they may be putative axosomatic contacts (Fig. 8B–E).
Figure 8. The relationship of histamine fibres to septohippocampal cholinergic neurones.
A, coronal section showing that histamine-ir fibres running in a dorsoventral direction alongside the midplane innervate ChAT-positive neurones in the medial septum. B, a close-up on a few neurones revealing putative axosomatic connections, marked by arrows. C–E, images of single optical planes confirming that histamine-ir and ChAT-ir are indeed located very close to each other: a water immersion objective with a theoretical maximum resolution of less than 150 nm was used. A and B, are maximum projection images of 15 μm thick image stacks.
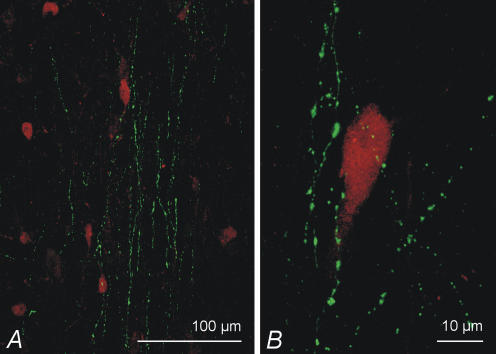
PV-ir neurones were also abundant in both the MS and DB and were distributed throughout the nuclei. Thus, the histamine-ir fibre innervation of the PV-ir neurones was similar to that of the ChAT-ir neurones (Fig. 9). The number of close contacts between histamine-ir fibres and PV-ir neurones was also similar, amounting to approximately 1/3 in the MS and slightly fewer in the DB (n = 30 for each nucleus).
Figure 9. Relationship of histamine fibres to septohippocampal GABAergic neurones.
A, histamine-ir fibres (green) intermingling with PV-ir (red) neurones in the medial septum. A close look at a single PV-positive neurone shows histamine-ir fibres running very close to the soma. B, maximum projection images of 15 μm and 17 μm thick image stacks, respectively.
Discussion
The main finding of this study is that histamine excites septohippocampal GABA-type neurones via H1 and H2 receptors by utilizing both direct and indirect mechanisms. The indirect mechanism involves activation of muscarinic M3 receptors by locally released ACh subsequent to an H1 receptor-mediated activation of septohippocampal cholinergic neurones. In double immunolabelling studies, histamine fibres were within close proximity of both the septohippocampal neuronal populations and formed putative synaptic contacts with the septohippocampal cholinergic neurones.
Histamine excites septohippocampal GABAergic neurones via both direct and indirect mechanisms
Histamine produced a reproducible, reversible and concentration-dependent excitation of septohippocampal GABAergic neurones that was partially mimicked by the selective H2 receptor agonist dimaprit in some but not all neurones. The selective H3 receptor agonist (R)-α-methyl-histamine had no effect on any of the neurones tested. The effects of histamine were blocked by H1 and/or H2 receptor antagonists and indicated the exclusive presence of H1 and H2 receptors in 17.6% and 14.7% of neurones, respectively, while the remaining neurones expressed mixed H1 and H2 responses. The presence of H1 and H2 receptor-mediated effects is consistent with the high levels of H1 (Bouthenet et al. 1988) and lower levels of H2 receptors reported in this area (Vizuete et al. 1997).
Locally released ACh via activation of muscarinic receptors contributes to an indirect H1-receptor mediated activation of septohippocampal GABAergic neurones
The indirect TTX-sensitive component of the histamine response was concluded to be mediated via an H1 receptor mediated increase in ACh release, because (1) the non-selective muscarinic receptor antagonist atropine methyl bromide and the M3-selective antagonist 4-DAMP mustard reduced the response to histamine but not to the H2 agonist dimaprit; and (2) AChE inhibitors, which increase synaptic levels of available ACh, increased the magnitude and duration of the histamine but not of the H2 agonist response. These effects are consistent with the previously reported H1 receptor-mediated activation of septal cholinergic neurones (Gorelova & Reiner, 1996), a finding that was also confirmed in the present study by testing the effects of histamine, and histamine receptor agonists and antagonists on selectively labelled septohippocampal cholinergic neurones. More importantly, these conclusions are consistent with the demonstrated physiological and anatomical connectivity between the septohippocampal cholinergic and GABAergic neurones (Brauer et al. 1998; Alreja et al. 2000; Wu et al. 2003b).
The presence of a cholinergic component in the histamine response in septohippocampal GABAergic neurones is significant as in the face of a loss of septohippocampal cholinergic neurones, there will be a reduced activation of the septohippocampal cholinergic pathway by the released histamine and the excitation of septohippocampal GABAergic neurones will also be obtunded. These obtunded effects of histamine may exaggerate the deficits in arousal in patients with Alzheimer's disease, which by itself is also associated with reduced brain histamine levels and turnover (Panula et al. 1998). In this regard, cholinesterase inhibitors by rescuing the available ACh from degradation could also restore some of the effects of the released histamine and thus contribute to improved cognition and arousal. The hippocampus itself is also directly innervated by histamine fibres; however, the MSDB is much more densely innervated than the hippocampus as electrical stimulation of the histaminergic cell bodies in the tuberomammillary nucleus produces a 6-fold higher histamine release in the MSDB as compared to the hippocampus (Mochizuki et al. 1994). Stimulation of the tuberomammilary nucleus also produces a 170% increase in basal ACh release in the hippocampus. Considering that the hippocampus obtains its ACh entirely from the septum, loss of cholinergic neurones would significantly reduce histamine effects on the hippocampus via the septohippocampal cholinergic as well as GABAergic pathway. Thus, histamine actions on the septum may be critical for hippocampal arousal.
Histamine, acting via H1 receptors, may also be the down-stream mediator for the effects of hypocretin, as exogenous hypocretin effects on wakefulness occur in normal but not H1 receptor knockout mice (Huang et al. 2001b). Both the septohippocampal cholinergic and GABAergic neurones express Hcrt-R2 (Wu et al. 2002, 2004) as well as the histamine H1 and/or H2 receptors.
The physiological significance of the observed effects of histamine is further complemented by the findings that histaminergic fibres innervate septohippocampal cholinergic and GABAergic neurones. Histamine-ir fibres were seen very close to the neurones and close contacts were interpreted as putative axosomatic contacts. The true nature of these contacts needs to be elucidated using electron microscopy. However, no specific high-resolution methods for immunohistochemical demonstration of histamine in electron microscopic samples have been reported. In general, histaminergic fibres in the brain have low density in most areas (Panula et al. 1989), and they make few if any direct synaptic contacts with target cells which are affected by histamine released from fibres (Wada et al. 1991; Haas & Panula, 2003). Thus, synaptic contacts may not be necessary for histaminergic modulation of GABAergic and cholinergic neurones of the MSDB.
Interestingly, the septum and the hippocampus have also been suggested to be key elements of the network involved in hibernation, which is controlled by the hypothalamus and brainstem (Heller, 1979). Histamine levels and turnover are significantly increased in hibernation (Sallmen et al. 1999), supporting the concept that this amine may be one of the key regulators of general activity in the brain.
In conclusion, histamine innervation and H1 and H2 receptor-mediated activation of septohippocampal GABAergic and cholinergic neurones are likely to be important in hibernation as well as in normal arousal and cognition.
Acknowledgments
This work was supported by NIH grants MH61465, and a NARSAD to Meenakshi Alreja, and by the Academy of Finland to Pertti Panula. We thank Ms N. Margiotta for technical help and Ms Leslie Rosello for secretarial help.
References
- Alreja M, Liu W. Noradrenaline induces IPSCs in rat medial septal/diagonal band neurons: involvement of septohippocampal GABAergic neurons. J Physiol. 1996;494:201–215. doi: 10.1113/jphysiol.1996.sp021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alreja M, Wu M, Liu W, Atkins JB, Leranth C, Shanabrough M. Muscarinic tone sustains impulse flow in the septohippocampal GABA but not cholinergic pathway: implications for learning and memory. J Neurosci. 2000;20:8103–8110. doi: 10.1523/JNEUROSCI.20-21-08103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacciottini L, Giovannelli L, Passani MB, Schunack W, Mannaioni PF, Blandina P. Ciproxifan and cimetidine modulate c-fos expression in septal neurons, and acetylcholine release from hippocampus of freely moving rats. Inflamm Res. 2000;49(suppl. 1):S41–S42. doi: 10.1007/PL00000174. [DOI] [PubMed] [Google Scholar]
- Bacciottini L, Passani MB, Giovannelli L, Cangioli I, Mannaioni PF, Schunack W, Blandina P. Endogenous histamine in the medial septum-diagonal band complex increases the release of acetylcholine from the hippocampus: a dual-probe microdialysis study in the freely moving rat. Eur J Neurosci. 2002;15:1669–1680. doi: 10.1046/j.1460-9568.2002.02005.x. [DOI] [PubMed] [Google Scholar]
- Bassant MH, Apartis E, Jazat-Poindessous FR, Wiley RG, Lamour YA. Selective immunolesion of the basal forebrain cholinergic neurons: effects on hippocampal activity during sleep and wakefulness in the rat. Neurodegeneration. 1995;4:61–70. doi: 10.1006/neur.1995.0007. [DOI] [PubMed] [Google Scholar]
- Bell MI, Richardson PJ, Lee K. Histamine depolarizes cholinergic interneurones in the rat striatum via a H1-receptor mediated action. Br J Pharmacol. 2000;131:1135–1142. doi: 10.1038/sj.bjp.0703692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandina P, Efoudebe M, Cenni G, Mannaioni P, Passani MB. Acetylcholine, histamine, and cognition: two sides of the same coin. Learn Mem. 2004;11:1–8. doi: 10.1101/lm.68004. [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Ruat M, Sales N, Garbarg M, Schwartz JC. A detailed mapping of histamine H1-receptors in guinea-pig central nervous system established by autoradiography with [125I]iodobolpyramine. Neuroscience. 1988;26:553–600. doi: 10.1016/0306-4522(88)90167-4. [DOI] [PubMed] [Google Scholar]
- Brauer K, Seeger G, Hartig W, Rossner S, Poethke R, Kacza J, Schliebs R, Bruckner G, Bigl V. Electron microscopic evidence for a cholinergic innervation of GABAergic parvalbumin-immunoreactive neurons in the rat medial septum. J Neurosci Res. 1998;54:248–253. doi: 10.1002/(SICI)1097-4547(19981015)54:2<248::AID-JNR12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Ben Ari Y, Gho M, Bidard JN, Lazdunski M. Long-term potentiation of synaptic transmission in the hippocampus induced by a bee venom peptide. Nature. 1987;328:70–73. doi: 10.1038/328070a0. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Stevens DR, Haas HL. Serotonin excites tuberomammillary neurons by activation of Na+/Ca2+-exchange. Neuropharmacology. 2001;40:345–351. doi: 10.1016/s0028-3908(00)00175-1. 10.1016/S0028-3908(00)00175-1. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Reiner PB. Histamine depolarizes cholinergic septal neurons. J Neurophysiol. 1996;75:707–714. doi: 10.1152/jn.1996.75.2.707. [DOI] [PubMed] [Google Scholar]
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- Haas HL, Reiner PB. Membrane properties of histaminergic tuberomammillary neurones of the rat hypothalamus in vitro. J Physiol. 1988;399:633–646. doi: 10.1113/jphysiol.1988.sp017100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Alreja M, Leranth C. Intrinsic vesicular glutamate transporter 2-immunoreactive input to septohippocampal parvalbumin-containing neurons: novel glutamatergic local circuit cells. Hippocampus. 2004;14:499–509. doi: 10.1002/hipo.10195. 10.1002/hipo.10195. [DOI] [PubMed] [Google Scholar]
- Hartig W, Seeger J, Naumann T, Brauer K, Bruckner G. Selective in vivo fluorescence labelling of cholinergic neurons containing p75 (NTR) in the rat basal forebrain. Brain Res. 1998;808:155–165. doi: 10.1016/s0006-8993(98)00792-6. 10.1016/S0006-8993(98)00792-6. [DOI] [PubMed] [Google Scholar]
- Heller HC. Hibernation: neural aspects. Annu Rev Physiol. 1979;41:305–321. doi: 10.1146/annurev.ph.41.030179.001513. 10.1146/annurev.ph.41.030179.001513. [DOI] [PubMed] [Google Scholar]
- Hough LB. Genomics meets histamine receptors: new subtypes, new receptors. Mol Pharmacol. 2001;59:415–419. [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, Urade Y, Hayaishi O. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A. 2001a;98:9965–9970. doi: 10.1073/pnas.181330998. 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, Urade Y, Hayaishi Op. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A. 2001b;98:9965–9970. doi: 10.1073/pnas.181330998. 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele NB, Arvanov VL, Shinnick-Gallagher P. Quisqualate-preferring metabotropic glutamate receptor activates Na+-Ca2+ exchange in rat basolateral amygdala neurones. J Physiol. 1997;499:87–104. doi: 10.1113/jphysiol.1997.sp021913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khateb A, Fort P, Pegna A, Jones BE, Muhlethaler M. Cholinergic nucleus basalis neurons are excited by histamine in vitro. Neuroscience. 1995;69:495–506. doi: 10.1016/0306-4522(95)00264-j. 10.1016/0306-4522(95)00264-J. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Li WC, Tang XH, Li HZ, Wang JJ. Histamine excites rat cerebellar granule cells in vitro through H1 and H2 receptors. J Physiol Paris. 1999;93:239–244. doi: 10.1016/s0928-4257(99)80157-0. 10.1016/S0928-4257(99)80157-0. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Okakura-Mochizuki K, Horii A, Yamamoto Y, Yamatodani A. Histaminergic modulation of hippocampal acetylcholine release in vivo. J Neurochem. 1994;62:2275–2282. doi: 10.1046/j.1471-4159.1994.62062275.x. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Yamatodani A, Okakura K, Horii A, Inagaki N, Wada H. Circadian rhythm of histamine release from the hypothalamus of freely moving rats. Physiol Behav. 1992;51:391–394. doi: 10.1016/0031-9384(92)90157-w. 10.1016/0031-9384(92)90157-W. [DOI] [PubMed] [Google Scholar]
- Ohtsu H, Watanabe T. New functions of histamine found in histidine decarboxylase gene knockout mice. Biochem Biophys Res Commun. 2003;305:443–447. doi: 10.1016/s0006-291x(03)00696-x. 10.1016/S0006-291X(03)00696-X. [DOI] [PubMed] [Google Scholar]
- Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A. 1984;81:2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passani MB, Bacciottini L, Mannaioni PF, Blandina P. Central histaminergic system and cognition. Neurosci Biobehav Rev. 2000;24:107–113. doi: 10.1016/s0149-7634(99)00053-6. 10.1016/S0149-7634(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Panula P, Rinne J, Kuokkanen K, Eriksson KS, Sallmen T, Kalimo H, Relja M. Neuronal histamine deficit in Alzheimer's disease. Neuroscience. 1998;82:993–997. doi: 10.1016/s0306-4522(97)00353-9. 10.1016/S0306-4522(97)00353-9. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Reiner PB, McGeer EG. Electrophysiological properties of cortically projecting histamine neurons of the rat hypothalamus. Neurosci Lett. 1987;73:43–47. doi: 10.1016/0304-3940(87)90028-0. 10.1016/0304-3940(87)90028-0. [DOI] [PubMed] [Google Scholar]
- Sallmen T, Beckman AL, Stanton TL, Eriksson KS, Tarhanen J, Tuomisto L, Panula P. Major changes in the brain histamine system of the ground squirrel Citellus lateralis during hibernation. J Neurosci. 1999;19:1824–1835. doi: 10.1523/JNEUROSCI.19-05-01824.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BN, Armstrong WE. The ionic dependence of the histamine-induced depolarization of vasopressin neurones in the rat supraoptic nucleus. J Physiol. 1996;495:465–478. doi: 10.1113/jphysiol.1996.sp021607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Jasso LE, Bahena-Trujillo R, Arias-Montano JA. Histamine H1 receptors and inositol phosphate formation in rat thalamus. Neurosci Lett. 1997;225:117–120. doi: 10.1016/s0304-3940(97)00209-7. 10.1016/S0304-3940(97)00209-7. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. 10.1016/S0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wen YQ, Li HZ, Zuo CC, Wang JJ. Histamine excites rat cerebellar Purkinje cells via H2 receptors in vitro. Neurosci Res. 2000;36:61–66. doi: 10.1016/s0168-0102(99)00109-1. 10.1016/S0168-0102(99)00109-1. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Sakai K, Jouvet M. Specific neurons for wakefulness in the posterior hypothalamus in the cat. C R Acad Sci III. 1984;298:195–200. [PubMed] [Google Scholar]
- Vizuete ML, Traiffort E, Bouthenet ML, Ruat M, Souil E, Tardivel-Lacombe J, Schwartz JC. Detailed mapping of the histamine H2 receptor and its gene transcripts in guinea-pig brain. Neuroscience. 1997;80:321–343. doi: 10.1016/s0306-4522(97)00010-9. 10.1016/S0306-4522(97)00010-9. [DOI] [PubMed] [Google Scholar]
- Wada H, Inagaki N, Yamatodani A, Watanabe T. Is the histaminergic neuron system a regulatory center for whole-brain activity? Trends Neurosci. 1991;14:415–418. doi: 10.1016/0166-2236(91)90034-r. 10.1016/0166-2236(91)90034-R. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Taguchi Y, Hayashi H, Tanaka J, Shiosaka S, Tohyama M, Kubota H, Terano Y, Wada H. Evidence for the presence of a histaminergic neuron system in the rat brain: an immunohistochemical analysis. Neurosci Lett. 1983;39:249–254. doi: 10.1016/0304-3940(83)90308-7. 10.1016/0304-3940(83)90308-7. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Taguchi Y, Shiosaka S, Tanaka J, Kubota H, Terano Y, Tohyama M, Wada H. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984;295:13–25. doi: 10.1016/0006-8993(84)90811-4. 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Gaykema RP, Steinbusch HW, Watanabe T, Wada H. The connections between the septum-diagonal band complex and histaminergic neurons in the posterior hypothalamus of the rat. Anterograde tracing with Phaseolus vulgaris-leucoagglutinin combined with immunocytochemistry of histidine decarboxylase. Neuroscience. 1988;26:827–845. doi: 10.1016/0306-4522(88)90103-0. 10.1016/0306-4522(88)90103-0. [DOI] [PubMed] [Google Scholar]
- Wu M, Hajszan T, Leranth C, Alreja M. Nicotine recruits a local glutamatergic circuit to excite septohippocampal GABAergic neurons. Eur J Neurosci. 2003a;18:1155–1168. doi: 10.1046/j.1460-9568.2003.02847.x. 10.1046/j.1460-9568.2003.02847.x. [DOI] [PubMed] [Google Scholar]
- Wu M, Newton SS, Atkins JB, Xu C, Duman RS, Alreja M. Acetylcholinesterase inhibitors activate septohippocampal GABAergic neurons via muscarinic but not nicotinic receptors. J Pharmacol Exp Ther. 2003b;307:535–543. doi: 10.1124/jpet.103.052514. 10.1124/jpet.103.052514. [DOI] [PubMed] [Google Scholar]
- Wu M, Shanabrough M, Leranth C, Alreja M. Cholinergic excitation of septohippocampal GABA but not cholinergic neurons: implications for learning and memory. J Neurosci. 2000;20:3900–3908. doi: 10.1523/JNEUROSCI.20-10-03900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Zaborszky L, Hajszan T, van den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci. 2004;24:3527–3536. doi: 10.1523/JNEUROSCI.5364-03.2004. 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Zhang Z, Leranth C, Xu C, van den Pol AN, Alreja M. Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition. J Neurosci. 2002;22:7754–7765. doi: 10.1523/JNEUROSCI.22-17-07754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Datta S, Wu M, Alreja M. Hippocampal theta rhythm is reduced by suppression of the H-current in septohippocampal GABAergic neurons. Eur J Neurosci. 2004;19:2299–2309. doi: 10.1111/j.0953-816X.2004.03316.x. 10.1111/j.0953-816X.2004.03316.x. [DOI] [PubMed] [Google Scholar]