Abstract
Eccentric exercise is unique in that it can lead to muscle damage and soreness. Concentric exercise is not accompanied by evidence of damage. There are reports in the literature that muscle fatigue is a factor determining the amount of damage from eccentric exercise. Our theory for the damage process predicts that susceptibility for damage is independent of fatigue. Experiments were carried out to test this prediction as well as to seek other evidence in support of our theory. Comparisons were made between the effects of eccentric and concentric contractions. The nerve supply to the medial gastrocnemius muscle of the anaesthetized cat was divided into three equal portions in terms of the tension they generated. In the first experiment a muscle portion was fatigued by giving it 200 shortening contractions over 12 mm at a shortening speed of 50 mm s−1. This led to a mean fall in isometric tension (37 ± 4%) without a significant shift in the optimum length for peak active tension. Giving the fatigued muscle 10 eccentric contractions, active stretches over 6 mm at 50 mm s−1, beginning from the muscle's optimum length led to a further fall in tension (11%± 7%) and a significant shift in optimum length (3.7 mm ± 0.6 mm) in the direction of longer muscle lengths. The shift in optimum was taken as an indicator of muscle damage. This shift was not significantly different from that seen after eccentric contractions carried out on an unfatigued muscle. After a series of eccentric or concentric contractions, tension at the end of a ramp shortening of 6 mm at 10 mm s−1 fell more than isometric tension, and by near equal amounts for the two kinds of contractions. In an unfatigued muscle, if tension was altered by changing the rate of stimulation, the fall in shortening tension was greater than after either concentric or eccentric contractions. These observations were seen to be consistent with predictions of the proposed mechanism for the damage process.
Eccentric contractions, where active muscle lengthens, form part of activities such as running, kicking a ball and walking downhill. Eccentric exercise is distinct from other forms of exercise in that it routinely produces muscle damage in untrained individuals. The damage expresses itself by changes in the mechanical properties of the muscle as well as by delayed onset of muscle soreness (Proske & Morgan, 2001). One of us has put forward a theory for the mechanism for the initial event leading to the damage, the sarcomere disruption hypothesis, some aspects of which remain controversial (Morgan, 1990). For an alternate view, see Warren et al. (2001).
One important prediction of the theory is an increase in series compliance following the eccentric contractions. It is postulated that as a result of the nonuniform lengthening of sarcomeres during an active stretch on the descending limb of the muscle's length–tension relation, the region of sarcomere instability, some sarcomeres are stretched to beyond myofilament overlap. These sarcomeres are at risk of becoming disrupted during muscle relaxation. Following a series of eccentric contractions the presence of disrupted sarcomeres in series with still functioning sarcomeres increases the muscle's series compliance. This manifests itself as a shift in the direction of longer muscle lengths of the optimum length for peak active tension (Katz, 1939; Wood et al. 1993; Jones et al. 1997; Brockett et al. 2001). Here we have compared shifts in optimum length after a series of eccentric and concentric contractions of the muscle, so arranged to produce similar falls in active tension. The aim was to determine whether, as predicted by theory, a shift in optimum was a consequence exclusively of damage from eccentric contractions, whether there were any shifts in optimum after concentric contractions and whether fatigue from concentric contractions altered the susceptibility of the muscle for damage from eccentric contractions.
It has been reported that the damage from eccentric exercise is reduced following a series of fatiguing concentric contractions (Nosaka & Clarkson, 1997). This has led to the view that warm-up and fatigue produce some protection against the damage. Other reports emphasize the energy absorption of muscle undergoing active stretches and that in fatigued muscle, energy absorption is less, leading to an increase in vulnerability for eccentric damage (Mair et al. 1996). In addition, it has been reported that damage is proportional to force levels or the amount of work done, under certain conditions (McCully & Faulkner, 1986). Our theory for the mechanism of the damage process predicts that damage should be essentially independent of levels of force and fatigue, provided that activation is sufficient to produce a descending limb to the length–tension relationship. The experiments described here put that prediction to the test as well as seeking additional evidence in support of our theory.
If our interpretation is correct, that the shift in optimum length after a series of eccentric contractions is the result of overextended, disrupted sarcomeres lying in series with still functioning sarcomeres, to increase series compliance, it leads to two further specific predictions. First, the muscle should be less able to sustain tension during an active shortening. This is a direct consequence of having a smaller number of functioning force generators in series. That is, the remaining active sarcomeres would be shortened more rapidly and so would generate less force. Second, the rate of rise of tension during an isometric contraction should be slowed as an expression of both the increase in series compliance and the reduced number of active sarcomeres. In an alternative proposal (Warren et al. 2001), the initial event in the chain leading to damage and soreness from eccentric contractions is excitation–contraction (E–C) uncoupling. The subsequent shift in optimum length and reduced rate of rise are both interpreted in terms of changes in E–C coupling.
A further consideration is that there are well-known effects of fatigue on shortening velocity, independent of muscle damage (Edman & Mattiazzi, 1981; de Haan et al. 1989). We have therefore compared the muscle's ability to maintain tension during shortening following a series of eccentric contractions, and compared it with tension during shortening after a series of concentric contractions. To test the hypothesis that damage is predominantly the result of changes in E–C coupling, we have measured the tension during shortening and the rate of rise of tension using a range of stimulation rates. The data were found to support the nonuniform lengthening of sarcomeres hypothesis (Morgan, 1990) as an explanation for the initial event in the injury process triggered by eccentric exercise.
Methods
The experiments were carried out on four cats of both sexes with a weight range of 3.5–4.2 kg. The experiments were undertaken with approval from the Monash University Committee for Ethics in Animal Experimentation.
General anaesthesia was induced by an intraperitoneal injection of sodium pentobarbitone (40 mg kg−1), and was maintained throughout the experiment with additional doses of 12 mg ml−1 given into the cephalic vein when necessary. The total supplementary dose given varied between animals and was approximately 80 mg over the 12 h period of the experiment. Depth of anaesthesia was assessed at half-hourly intervals by testing reflex responses including the eye-blink, flexor withdrawal to a toe pinch and ear-flick. In addition the level of muscular tone was noted, as were the rate of respiration and end-tidal CO2 concentration. At the end of the experiments, animals were killed with an overdose of anaesthetic. The trachea was cannulated and end-tidal CO2 concentration was monitored, to indicate the adequacy of ventilation. Rectal temperature was measured and core body temperature maintained at ∼38°C by means of a feedback-regulated heating blanket.
The animal was secured to a metal frame, with steel pins in the pelvis and tibia. A laminectomy was performed to expose ventral roots L7−S1, which were cut at their point of entry into the spinal cord. The left lower limb was dissected and the medial gastrocnemius muscle (MG) was separated from the lateral gastrocnemius and soleus. The exposed muscle and spinal cord were covered by pools of paraffin oil in baths fashioned from skin flaps, the temperature of which was maintained between 35 and 38°C. The nerve to the MG was located and carefully dissected free from the posterior tibial nerve. All hind limb nerves, including nerves to hip muscles, were then cut, except for the nerve to the MG.
Markers were placed on the Achilles tendon and the distal end of the tibia, and the distance between them measured when the ankle was maximally flexed and the leg was in the position it would occupy later in the experiment. This defined the maximal physiological length of the muscle (Lm) and allowed muscle length to be expressed subsequently in relation to Lm. The distal tendon of the MG was identified and separated from the rest of the Achilles tendon, which was severed, leaving only the MG tendon attached to the calcaneum. The calcaneum was cut and a 2 mm diameter hole drilled through it. A threaded rod was passed through the hole and secured to the bone with a pair of nuts and washers. At the other end, the rod was attached to the shaft of an electromagnetic servomotor, which regulated muscle length changes. Muscle tension was measured with an Entran strain gauge attached to the metal rod. The system had a compliance of 5 μm N−1.
Experimental procedure
The tensions produced in the MG by single-shock stimulation of the muscle nerve and of the collected ventral roots were measured at the start of each experiment, and the ventral roots were then divided into three parts, approximately equal in terms of the tension produced when stimulated separately. In any particular experiment, the tetanic tension at optimal length (Lopt) differed by no more than 13% between the piece generating the highest tension and the piece generating the smallest tension. Values of Lopt of each portion differed by no more than 0.6 mm. For all 12 muscle parts, Lopt was between Lm− 11.1 mm and Lm− 12.8.
For each part of the muscle, the length–tension relation and the tension during a shortening contraction were measured before and after a series of contractions. These were 10 eccentric contractions alone, 200 concentric contractions alone or 10 eccentric contractions preceded by 200 concentric contractions.
The length–tension relation was measured over a length range between Lm and Lm− 20 mm. Muscle length was increased in computer-controlled steps of 2 mm amplitude and 30 s duration. At the end of each step, the muscle was stimulated via its ventral root supply for 0.25 s at 140, 80 or 50 pulses per second (pps). Tension was measured as the difference between the peak tension during stimulation and the preceding passive tension at the same length.
The tension remaining at the end of a slow shortening was taken as a measure of any change in shortening velocity. The procedure was to stimulate the muscle for 0.3 s at 50, 80 or 140 pps at an initial length of Lopt+ 3 mm, and after 0.25 s to shorten it by 6 mm at 10 mm s−1. The shortening rate was kept low to avoid tension from falling to zero at any time during the shortening. The isometric tension at the start of the shortening ramp and the concentric tension at the end of the ramp were measured, after subtraction of the passive tension measured during shortening without stimulation.
The eccentric protocol comprised a series of 10 contractions at an interval of 30 s. Each contraction consisted of a 0.4 s period of stimulation at 80 pps, during which the muscle was stretched at 50 mm s−1 from an initial length of Lopt to Lopt+ 6 mm, the stretch starting 0.15 s after the start of the stimulation.
The fatiguing concentric contractions were a series of 200 separate contractions at an interval of 15 s. Each contraction consisted of stimulating the muscle for 0.4 s at 80 pps while shortening it from Lopt+ 6 mm to Lopt− 6 mm at a velocity of 60 mm s−1.
Data collection and statistics
A PowerMac G3 computer with a NIDAQ card (National Instrument Corp., Austin TX, USA) running IgorPro (WaveMetrics, Lake Oswego, OR, USA) and custom-written software were used to acquire, process and analyse all data, as well as being used to control stimulus timing and generate the waveforms controlling the muscle stretcher.
For all parameters measured, the mean and standard error of the mean (s.e.m.) were calculated. Statistical significance was set at P < 0.05. A two- or three-factor analysis of variance (ANOVA) with interactions was used to determine the significant factors determining the effects of the various procedures.
The statistical program used was Data Desk (Ithaca, NY, USA).
Results
A total of four animals were used in this study. Each muscle was subdivided into three portions, making a total of 12 preparations for study. In the first animal a variety of stimulus conditions and experimental sequences were tried and, since therefore not all the data were directly comparable to those acquired in subsequent experiments, they have not all been included in pooled values. The observations were, however, consistent with the subsequent conclusions. Each third of the muscle had near-identical mechanical properties, at least in terms of the measurements made here (see Methods). This was achieved by subdividing the ventral root rather than the motor nerve, and thereby ensuring a free mixing of motor axons (see also Whitehead et al. 2001, 2003).
Shift in optimum length and fall in force
The first questions posed were whether a shift in optimum was unique to eccentric exercise, and whether the damaging effects of eccentric contractions were altered in any way by muscle fatigue. We chose as a damage indicator the shift in optimum length for peak active tension in the direction of longer muscle lengths (Wood et al. 1993; Jones et al. 1997; Whitehead et al. 2003). This has the advantage of being present immediately after the eccentric contractions, while other reported indicators, the swelling, stiffness and soreness appear later. The force drop from the damage is also immediate but is likely to include fatigue effects. The specific hypotheses tested here were that only eccentric exercise shifted the optimum length, and that the force drop from fatigue did not alter the muscle's susceptibility to damage from the eccentric contractions, as measured by the shift in optimum length.
To fatigue the muscle without producing any damage, it was subjected to a series of concentric contractions (Newham et al. 1983a,b). The muscle was stimulated at 80 pps during shortening at 60 mm s−1 over 12 mm, the length change being symmetrical about the optimum length for that muscle portion. It took 200 shortening contractions, each lasting 0.4 s and separated from the next by 15 s before muscle tension had fallen to levels comparable to those seen after 10 eccentric contractions.
Fall in force
In the experiment illustrated in Fig. 1, one muscle portion was subjected to 10 eccentric contractions while a second portion received 200 concentric contractions followed by 10 eccentric contractions. The eccentric contractions carried out on the unfatigued muscle led to a drop in isometric force to 70% of its original value, as measured at the new optimum length. For four preparations, the eccentric contractions led to a mean fall in force to 68% (±2%) of its initial value (compare □ and ▪, Fig. 2).
Figure 1. Length–tension curves before and after eccentric or concentric contractions.
Upper panel, length–tension relation for one-third of the MG muscle, before (□) and after (▪) 10 eccentric contractions. The optimum length for peak active tension for each curve is shown by an arrow. The stretches for the eccentric contractions (6 mm at 50 mms−1) were arranged to lie on the descending limb of the control length–tension curve, starting at the optimum length. Muscle length has been expressed relative to the maximum physiological length (Lm). Lower panel, length–tension curves before (□), after 200 concentric contractions (○) and after a further 10 eccentric contractions of the fatigued muscle (•). The concentric contractions were active shortenings of 12 mm at 60 mms−1 lying symmetrically across the muscle optimum length.
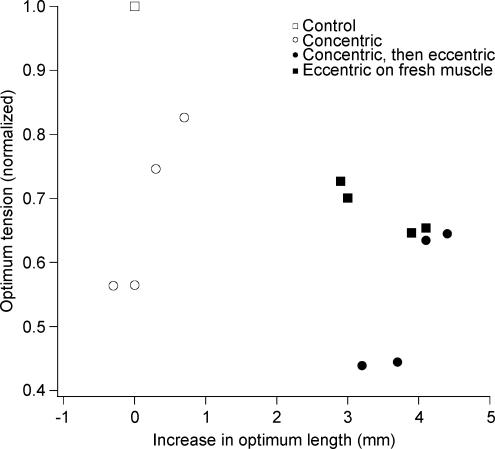
Figure 2. Changes in optimum length after eccentric and concentric contractions.
Data for eight muscle portions from three experiments. The change in optimum length is expressed relative to the optimum for the control length–tension curve (□). The isometric tension at the new optimum length is given as a fraction of the control optimum tension, assigned a value of 1.0 (□). ○, values after 200 concentric contractions, ▪, values after 10 eccentric contractions on unfatigued muscle, •, values for the muscles fatigued by 200 concentric contractions, then given an additional 10 eccentric contractions.
The 200 concentric contractions carried out on the second muscle portion led to a fall in force to 58% of its original value. For four muscles the mean value was 68% (± 7%). See Fig. 2 (open circles). When the fatigued muscle was subjected to 10 eccentric contractions, force fell further to a value of 46% of control tension which was 76% of its pre-eccentric value. For the four preparations the mean remaining force was 54% (± 6%). See Fig. 2 (filled circles). This represented a fall to 89% (± 7%) of the pre-eccentric value.
The size of the fall in force from eccentric contractions carried out on fresh muscle was significantly larger than from eccentric contractions given after the concentric contractions, presumably because in the fresh unstimulated muscle there was more opportunity for fatigue. The fall in force, for the eccentric-only protocol was less than after combined concentric and eccentric contractions (P < 0.02). The tension remaining after eccentric-only contractions was not significantly different from the tension remaining after concentric-only contractions (P = 0.3; post hoc test after one-way ANOVA).
Shift in optimum length
The muscles were subjected to active stretches that were arranged to lie entirely on the descending limb of the length–tension relation. The stretch began at the optimum length and extended to Lopt+ 6 mm. For the unfatigued muscle in Fig. 1 this led to a shift in optimum length, in the direction of longer muscle lengths, of 3.0 mm. For the four preparations, the average value was 3.5 ± 0.3 mm, which was significant (P < 0.001) See Fig. 2 (▪).
The 200 concentric contractions given to the preparation in Fig. 1 led to only a slight shift in optimum length from 12.0 to 12.3 mm below Lm. The average shift for the four muscles (Fig. 2, ○) was 0.18 ± 0.21 mm longer than at the start of the experiment. This was not significantly different from zero (P= 0.98, post hoc test after one-way ANOVA). The muscle shown in Fig. 1 was then subjected to 10 eccentric contractions. This was accompanied by a shift in optimum length of 3.2 mm. For the four preparations the average shift was 3.7 ± 0.6 mm. This was significant (P < 0.001). Values for each preparation are shown in Fig. 2 (•). The shift in optimum, when measured for eccentric contractions given to fatigued muscles, was found to be not significantly different from that measured on fresh muscle, regardless of whether the shift was adjusted back to the muscle's original optimum (P = 0.73) or the value immediately before the eccentric contractions (P = 0.54).
So the conclusion from this series of experiments was that susceptibility of the muscle to damage from eccentric exercise, with damage measured by the shift in optimum length, was no different if the muscle had been fatigued beforehand. If susceptibility was measured from the size of the force drop, this was complicated by some force recovery from the previous concentric contractions. The additional important conclusion was that damage did not depend on force levels under these conditions. It was just as extensive after force in the muscle had been dropped by almost a half.
Tension during shortening
The hypothesis to be tested in this experiment was that in a damaged muscle, with fewer force-generating sarcomeres in series in myofibrils, for a given level of isometric tension, the muscle would be able to sustain less tension during shortening than an undamaged, fatigued muscle.
Examples of shortening contractions are shown in Fig. 3. For some preparations we have included the additional variable of altering the rate of stimulation of the muscle, as a means of testing the possibility that any changes were the result of E–C coupling dysfunction rather than sarcomere disruption. In the example of Fig. 3, active tension at the end of shortening of the unfatigued muscle, for 140 pps, was 29% of the preshortening, isometric tension. After the concentric contractions it was 20% of the prefatigue value; after the eccentric contractions it was 16%. So the trend of the data was in the anticipated direction, in the sense that shortening tension was lowered by fatigue, but not as much as by a small number of eccentric contractions. Notice too, the much slower rate of rise for the isometric contraction after the eccentric contractions (Fig. 3). These trends were analysed across eight preparations.
Figure 3. Shortenings after a series of concentric or eccentric contractions.
Tension changes during a 6 mm shortening of the muscle at 10 mm s−1. The shortening step began after 0.25 s of isometric contraction, using stimulation at 140 pps (continuous trace) or 80 pps (dotted trace) or 50 pps (dashed trace). Measurements were made at the start of the experiment (Pre), after a series of 200 concentric contractions (Conc) or after a series of 10 eccentric contractions (Ecc). The continuous trace at the bottom indicates muscle length, the shaded bar, the period of stimulation.
Values for tension at the end of shortening were normalized relative to the peak isometric tension measured at the start of the experiment. These have been plotted against optimum isometric tension measured after the concentric or eccentric contractions, also normalized relative to tension at the start of the experiment (Fig. 4). Shortening tension values for the unexercised muscle have been shown for three rates of stimulation, 50 pps, 80 pps and 140 pps while for the fatigued and damaged muscles, only the 140 pps values have been shown.
Figure 4. Force during a shortening after concentric and eccentric contractions.
Shortening force and isometric force before the shortening are given as fractions of the isometric force measured at optimum length at the start of the experiment using a rate of 140 pps stimulation. □, unexercised muscle, ○, muscles fatigued using concentric contractions, •, eccentric contractions carried out on fatigued muscles, ▪, eccentric contractions carried out on unfatigued muscles. The dotted line indicates the relation if the fall in shortening force was proportional to the fall in isometric force. The coarse dashed line is drawn through points for 140 pps values for the control muscles and after eccentric, concentric or both kinds of contraction. The slope of the line was 0.44 ± 0.02. For the unfatigued muscle values are also shown for 50 pps and 80 pps simulation rates. Here the smaller symbols are for the lower stimulation rates. These values have the fine dashed line drawn through them (slope 1.30 ± 0.13).
After the eccentric or concentric contractions, if there had been no change in the muscle's ability to sustain tension during shortening, any fall in shortening tension would have been expected to be proportional to the accompanying fall in isometric tension (dotted line, Fig. 4). It turned out that after both concentric and eccentric contractions, values lay below the line of proportionality, indicating that shortening tension had fallen more than expected. Here it should be remembered that there were 200 concentric contractions and only 10 eccentric contractions.
Statistical analysis used an ANOVA with tension during shortening as the dependent variable, and type of contraction and preshortening isometric tension as independent variables. Eccentric and concentric exercise, adjusted in intensity to produce similar falls in tension could not be distinguished on the basis of changes in tension during shortening.
The relationship between shortening tension and isometric tension for the unexercised muscle, the concentrically exercised muscle, and the eccentrically exercised muscle, all using a stimulation rate of 140 pps is shown by the coarsely dashed line in Fig. 4. With 140 pps the muscle had reached its maximum tension. The slope of the line was 0.44 ± 0.02. This compared with a slope of 0.32 for the proportionality line between isometric tension and shortening tension (dotted line, Fig. 4).
By comparison, when isometric tension was altered in the unexercised muscle, by changing stimulation rate, shortening tension measured for the three rates of stimulation had a much steeper dependence on isometric tension (Fig. 4, fine dashed line). The line drawn through the values had a slope of 1.3 ± 0.13. That is, for a given fall in isometric tension, shortening tension fell almost four times more steeply than predicted from the proportionality line and three times more steeply than for the relation between unfatigued and fatigued muscle using the higher rate of stimulation.
Rate of rise of tension
The final measurements made compared the rate of rise of isometric tension after a series of eccentric or concentric contractions, using different rates of stimulation (Figs 3 and 5). The specific hypothesis tested was that after eccentric contractions, rates of rise would fall much more than after concentric contractions, as a result of the increased series compliance from the presence of disrupted sarcomeres in damaged muscle fibres. The hypothesis was supported by the observations.
Figure 5. Maximum rates of rise of tension after concentric and eccentric contractions.
The maximum rate of rise was measured for a particular contraction at the muscle optimum length. It is given in optimum force (Piso) per second (s). Rate of rise has been plotted against the tension at the end of a shortening, expressed as a fraction of the isometric tension measured at the start of the experiment. Symbols as in previous figures. The smaller open squares are for lower stimulation rates.
Fatigue from concentric contractions, although reducing tension during shortening, had no effect on the rate of rise of isometric tension (Fig. 5). Lowering the rate of stimulation also produced little change (Figs 3 and 5). Eccentric contractions, on the other hand, produced both a fall in shortening tension and a reduced rate of rise. The size of the effect on rate of rise was the same, regardless of whether or not the eccentric contractions had been preceded by a series of concentric contractions (Figs 3 and 5).
Measurements of maximum rate of rise for a stimulation rate of 140 pps, gave a mean of 22.0 ± 3.0 s−1 for control muscles, 21.0 ± 3.5 s−1 after concentric contractions, 12.2 ± 1.1 s−1 after eccentric contractions on unfatigued muscle, and 12.8 ± 1.2 s−1 after eccentric exercise on fatigued muscle, all normalized to the final tension in that contraction.
The above trends were all confirmed by an ANOVA with rate of rise of tension as the dependent variable, and type of exercise and stimulation rate as independent variables. Both were significant with P < 0.001, showing that rate of rise depended both on the type of contraction and on stimulation rate. The post hoc test on experimental condition showed that control and concentric contractions were indistinguishable (P = 0.69), as were eccentric contractions on unfatigued muscle and eccentric contractions after concentric contractions (P = 0.75), while all other pairs were clearly different with P < 0.001.
To summarize, these experiments have shown that muscle damage from a series of eccentric contractions, as indicated by a shift in optimum length, is unaffected by muscle fatigue. Secondly concentrically and eccentrically exercised muscles show similar reductions in the force they can generate during slow shortening, for similar reductions in isometric force, even though the drop in force was achieved with very different numbers of contractions. Finally, rate of force development is slowed to a much greater extent in the eccentrically exercised muscle than in the concentrically exercised muscle, or in the unexercised muscle when stimulation rate is reduced.
Discussion
The aim of these experiments was to test the predictions of theories for the mechanism of muscle damage from eccentric contractions. Our theory predicts that susceptibility for damage is independent of the level of muscle fatigue. This prediction was put to the test. In addition, any damage would be expected to significantly reduce the muscle's capacity to bear loads during shortening, and to slow the rate of rise of isometric tension. These ideas were tested by comparing the effects of eccentric contractions or concentric contractions, arranged to produce similar falls in force. It is known that concentric contractions produce muscle fatigue without any accompanying damage (Newham et al. 1983a,b). In addition, in the unexercised muscle, the effect of falls in force from lowering stimulation rate was tested. In the event, our predictions were, in large part, confirmed by the observations.
The method
We have chosen the medial gastrocnemius muscle of the anaesthetized cat to make the comparisons between the effects of concentric and eccentric contractions. It allowed us to place the findings within the context of other observations using this preparation (Whitehead et al. 2001, 2003). To maximize the amount of data acquired from each preparation and to keep peak force levels within a manageable range, the muscle's nerve supply was subdivided into three near-equal portions, in terms of the tension they generated. Given that MG contains motor units with different susceptibilities to fatigue (Burke et al. 1971), it was important to also achieve near-equal distributions of motor units. This was done by subdividing the nerve at the level of the ventral root, where experience has shown, axons of different motor units are freely intermingled (Wise et al. 2001). Direct measurements confirmed that the three portions were similar in their mechanical properties.
A second consequence of making observations on only one-third of the muscle was that the optimum length for peak active tension of each piece was a little shorter than for the whole muscle (0.6 mm, see Methods). This is due to the fact that contraction of a part of the muscle will not stretch the series elastic component as much as activation of the whole muscle (Proske & Morgan, 1984; Sandercock, 2000). However, within the limitations imposed by these considerations it was possible to make meaningful and useful observations on each part of the muscle.
The effect of fatigue
Against a background of suggestions that fatigue either increased or decreased the susceptibility of muscle for the damage from eccentric exercise (see Introduction), we considered the following hypothesis: A central tenet of the (Morgan, 1990) hypothesis was that overstretch and disruption of sarcomeres from an active lengthening took place only on the descending limb of the sarcomere length–tension relation because this was known to be a region of sarcomere instability (Gordon et al. 1966). We considered that the lower tension in fatigued muscle could be compared to stimulating an unfatigued muscle at a less than optimal rate. It is known that submaximal activation of a muscle leads to a shift in optimum length for active tension in the direction of longer lengths (Rack & Westbury, 1969; Morgan et al. 2000). At lower stimulation rates, less Ca2+ would be released into the sarcoplasm and the shift in optimum would therefore be an expression of the length dependence of myofilament sensitivity to Ca2+ (Endo, 1973). According to these ideas we predicted that muscle fatigue could reduce the amount of damage from eccentric contractions because of a fatigue-related shift in optimum in the direction of longer lengths. It would mean that less of the active stretch would extend onto the descending limb of the muscle's length–tension relation and so less damage would result. So a result where there was some evidence of protection from fatigue would have been compatible with our hypothesis provided the fatigue was accompanied by a significant shift in optimum length.
A 35% drop in isometric tension was observed after 200 concentric contractions and this was accompanied by a nonsignificant shift of the optimum length. It therefore makes the above mechanism for a protective effect, unlikely. Furthermore, when eccentric contractions were given to already fatigued muscles, such muscles showed just as much evidence of damage as did fresh, unstimulated muscles subjected to the same number of contractions. Two important points arise from this conclusion. One, it is confirmed that concentric contractions are not accompanied by a shift in optimum length. It means that the muscle remains vulnerable to damage from any subsequent eccentric contractions. Secondly, the result emphasizes that damage from eccentric contractions, under the conditions of our experiments, is not dependent on the levels of force reached during the contractions (McCully & Faulkner, 1986). It is the length range covered by the eccentric contractions that is the critical factor, not the tension (Talbot & Morgan, 1998).
Given that in this study only 10 eccentric contractions were used, the amount of fatigue produced by these contractions, independent of damage, would have been rather small. Yet there were still significant shifts in optimum length accompanying similar falls in tension to those seen after 200 concentric contractions. The result emphasizes the point that the shift in optimum is associated exclusively with the damage from eccentric exercise and that only a small number of eccentric contractions is required to effect it (Gregory et al. 2003).
The conclusion is that fatigue from concentric exercise does not alter the muscle's susceptibility to damage from eccentric exercise. It has been proposed in the past that warm-up may be one strategy for minimizing damage from eccentric exercise (Nosaka & Clarkson, 1997). The importance of such a proposal has recently been brought into sharp focus by evidence in support of the view that eccentric damage can, at times, lead to a muscle strain injury (Brockett et al. 2004). Since the evidence presented here suggests that fatigue does not alter the susceptibility for eccentric damage, we would predict that muscle warm-up and fatigue are not effective strategies for preventing strain injuries. It may be, of course, that there are changes in motor control in a fatigued athlete that reduce their risk of damage and injury. But such changes would be independent of any mechanism concerned with eccentric damage.
Tension during shortening
Changes in mechanical properties of muscle following a series of fatiguing contractions include a decrease in peak isometric tension, a slowing of shortening velocity and a slower rate of relaxation. For a review see Fitts (1996). Here we chose to determine the effects of a series of eccentric contractions on levels of tension maintained by the muscle at the end of a constant-velocity shortening and to compare these with effects of a large enough number of concentric contractions to effect a similar fall in isometric tension. It is currently believed that the reduced shortening velocity during fatigue is the result of an accumulation of ADP, which competes with ATP-binding sites on myosin and thereby slows down cross-bridge detachment (Siemankowski et al. 1985; Pate & Cooke, 1989).
We considered an additional mechanism for the eccentric contractions. We have postulated that, as a result of muscle damage, there are some disrupted sarcomeres in myofibrils in series with still functioning ones. It means that the number of force generators in series is effectively reduced, leading to a reduced ability to maintain tension during shortening. Notice in Fig. 4 that all values of shortening tension after fatigue or damage lie below the line of proportionality between shortening tension and the control isometric tension. That is, shortening tension after both eccentric and concentric contractions fell more than predicted from the fall in isometric tension. Furthermore the two kinds of contractions, arranged to produce similar falls in isometric tension, showed similar falls in shortening tension, in spite of the large difference in the number of contractions involved in achieving the falls.
The simplest explanation for these findings is that both kinds of contractions lead to a reduction in unloaded shortening velocity, but the mechanisms involved are likely to be different. We propose that in concentrically fatigued muscles there was no change in the number of active sarcomeres compared with the unexercised muscle, but unloaded shortening speed has slowed for each sarcomere. By contrast, after the eccentric contractions there was little change in unloaded shortening speed of sarcomeres but the number of active sarcomeres in series had become fewer.
The other result to emerge from this experiment was that for an unfatigued muscle, reducing isometric tension by reducing the stimulation rate led shortening tension to fall much more steeply than after a similar fall in tension from fatigue or eccentric damage (Fig. 4). Reducing the stimulation rate is likely to lower tension in unexercised muscle by reducing Ca2+ release. Since our data show a proportionately smaller fall in tension after eccentric or concentric contractions it suggests that a fall in Ca2+ release is not primarily responsible. It has been reported that there is a stimulation rate-dependent reduction in Ca2+ release in single fibres of mouse muscle after a series of eccentric contractions (Balnave & Allen, 1995). However, in other studies on single frog fibres, after a series of eccentric contractions there was no reduction in Ca2+ transients during stimulation, and tension at longer lengths was higher, suggesting that the observed shift in optimum was not due to submaximal activation (Morgan et al. 1996). The different findings may, of course, represent a species difference.
We conclude that the reduction in shortening velocity from concentric and eccentric contractions is similar when these are arranged to produce similar falls in active tension. However, neither is due primarily to reduced Ca2+ release since otherwise the falls in shortening velocity would have been greater, as judged from the effect of stimulus rate on unexercised muscle. Since only 10 eccentric contractions were given, it implies that the effect on shortening velocity of such exercise is much more severe that for concentric exercise. It means that athletes exercising eccentrically, if not protected by training, risk losing control over their movements as forces drop and shortening speed slows.
Rate of rise of tension
In his account of the effect of active lengthenings applied to the frog sartorius muscle, Katz (1939) noted that ‘the isometric tetanus tension developed 2–3 times more slowly …’ Here we have confirmed this observation (Figs 3 and 5). In addition we have shown that while fatigue from concentric contractions reduces force output, the maximum rate of rise of tension (normalized to the final tension reached) is only slightly reduced from that seen in unfatigued muscle (Fig. 5). By contrast, after the eccentric contractions, the rate of rise was significantly reduced. Reducing tension in the unexercised muscle by lowering stimulation rate had only a small effect on the maximum rate of rise (Fig. 5).
Katz's (1939) original interpretation of his findings was that there had been a partial transformation of active contractile into passive elastic tissue. That explanation still holds today. As a result of the eccentric contractions, some disrupted and nonfunctioning sarcomeres lie scattered amongst the still-functioning sarcomeres. This would slow the speed of shortening of a myofibril for a given load and therefore slow the rate of rise of tension in the tendon. In addition, the total series compliance of the muscle fibre would be expected to be increased by the presence of the disrupted sarcomeres and there would also be some slowing of cross-bridge turnover rate associated with fatigue, although this latter effect would be relatively small since only 10 eccentric contractions were used.
To conclude, we have compared changes in mechanical properties of mammalian muscle after a series of eccentric or concentric contractions and from changing stimulation rate. It has emerged that a shift in optimum length is unique to eccentric contractions while slowing of unloaded shortening for a similar fall in isometric tension is common to both eccentric and concentric contractions. In an unfatigued muscle, changing stimulation rate reduces tension during shortening to a much greater extent, relative to isometric tension, than after a series of concentric or eccentric contractions. Finally the rate of rise of isometric tension is greatly reduced after eccentric contractions. Concentric contractions and lower stimulation rates had much less effect. All of this emphasizes the major differences in the mechanisms underlying the response of the muscle to the two kinds of contractions. Since most sports involve components of both concentric and eccentric exercise, the kinds of changes described here are likely to occur routinely. What also emerges from this work is the severity of the effects on muscle properties produced by eccentric exercise. Just a few contractions, if carried out at long enough lengths, lead to large falls in active tension, slowing of muscle shortening speed and slowing of the rate of rise of tension. Such changes will reduce control of movements and so interfere with athletic performance. The data emphasize the importance for athletes of adequate training programmes that provide protection against the damage from eccentric exercise and so allow achievement of peak performance.
Acknowledgments
The work was carried out with support from the National Health and Medical Research Council of Australia.
References
- Balnave CD, Allen DG. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol. 1995;488:25–36. doi: 10.1113/jphysiol.1995.sp020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockett CL, Morgan DL, Proske U. Human hamstring muscles adapt to eccentric exercise by changing optimum length. Med Sci Sports Exercise. 2001;33:783–790. doi: 10.1097/00005768-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Brockett CL, Morgan DL, Proske U. Predicting hamstring strain injury in elite athletes. Med Sci Sports Exercise. 2004;36:379–387. doi: 10.1249/01.mss.0000117165.75832.05. [DOI] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Zajac FE., III Mammalian motor units: physiological–histochemical correlation in three types in cat gastrocnemius. Science. 1971;174:709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Edman KA, Mattiazzi AR. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motility. 1981;2:321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Endo M. Length dependence of activation of skinned muscle fibres by calcium. Cold Spring Harb Symp Quant Biol. 1973;37:505–510. [Google Scholar]
- Fitts RH. Muscle fatigue: the cellular aspects. Am J Sports Med. 1996;24:S9–S13. [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Tendon organs as monitors of muscle damage from eccentric contractions. Exp Brain Res. 2003;151:346–355. doi: 10.1007/s00221-003-1508-3. [DOI] [PubMed] [Google Scholar]
- de Haan A, Jones DA, Sargeant AJ. changes in velocity of shortening, power output and relaxation rate during fatigue of rat medial gastrocnemius muscle. Pflugers Arch. 1989;413:422–428. doi: 10.1007/BF00584493. [DOI] [PubMed] [Google Scholar]
- Jones C, Allen T, Talbot J, Morgan DL, Proske U. Changes in the mechanical properties of human and amphibian muscle after eccentric exercise. Eur J Appl Physiol Occup Physiol. 1997;76:21–31. doi: 10.1007/s004210050208. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully KK, Faulkner JA. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol. 1986;61:293–299. doi: 10.1152/jappl.1986.61.1.293. [DOI] [PubMed] [Google Scholar]
- Mair SD, Seaber AV, Glisson RR, Garrett WE., Jr The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24:137–143. doi: 10.1177/036354659602400203. [DOI] [PubMed] [Google Scholar]
- Morgan DL. New insights into the behavior of muscle during active lengthening. Biophys J. 1990;57:209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Claflin DR, Julian FJ. The effects of repeated active stretches on tension generation and myoplasmic calcium in frog single muscle fibres. J Physiol. 1996;497:665–674. doi: 10.1113/jphysiol.1996.sp021798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U. Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. J Physiol. 2000;522:503–513. doi: 10.1111/j.1469-7793.2000.t01-2-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newham DJ, McPhail G, Mills KR, Edwards RH. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983a;61:109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Newham DJ, Mills KR, Quigley BM, Edwards RH. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond) 1983b;64:55–62. doi: 10.1042/cs0640055. [DOI] [PubMed] [Google Scholar]
- Nosaka K, Clarkson PM. Influence of previous concentric exercise on eccentric exercise-induced muscle damage. J Sports Sci. 1997;15:477–483. doi: 10.1080/026404197367119. [DOI] [PubMed] [Google Scholar]
- Pate E, Cooke R. A model of crossbridge action: The effects of ATP, ADP and Pi. J Muscle Res Cell Motility. 1989;10:181–196. doi: 10.1007/BF01739809. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Stiffness of cat soleus muscle and tendon during activation of part of muscle. J Neurophysiol. 1984;52:459–468. doi: 10.1152/jn.1984.52.3.459. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537:333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack PMH, Westbury DR. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol. 1969;204:443–460. doi: 10.1113/jphysiol.1969.sp008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandercock TG. Nonlinear summation of force in cat soleus muscle results primarily from stretch of the common-elastic elements. J Appl Physiol. 2000;89:2206–2214. doi: 10.1152/jappl.2000.89.6.2206. [DOI] [PubMed] [Google Scholar]
- Siemankowski RF, Wiseman MO, White HD. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci U S A. 1985;82:658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JA, Morgan DL. The effects of stretch parameters on eccentric exercise – induced damage to toad skeletal muscle. J Muscle Res Cell Motility. 1998;19:237–245. doi: 10.1023/a:1005325032106. [DOI] [PubMed] [Google Scholar]
- Warren GL, Ingalls CP, Lowe DA, Armstrong RB. Excitation–contraction uncoupling: Major role in contraction-induced muscle injury. Exercise Sports Sci Rev. 2001;29:82–87. doi: 10.1097/00003677-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Morgan DL, Gregory JE, Proske U. Rises in whole muscle passive tension of mammalian muscle after eccentric contractions at different muscle lengths. J Appl Physiol. 2003;95:1224–1234. doi: 10.1152/japplphysiol.00163.2003. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Weerakkody NS, Gregory JE, Morgan DL, Proske U. Changes in passive tension of muscle in humans and animals after eccentric exercise. J Physiol. 2001;533:593–604. doi: 10.1111/j.1469-7793.2001.0593a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Morgan DL, Gregory JE, Proske U. Fatigue in mammalian skeletal muscle stimulated under computer control. J Appl Physiol. 2001;90:189–197. doi: 10.1152/jappl.2001.90.1.189. [DOI] [PubMed] [Google Scholar]
- Wood SA, Morgan DL, Proske U. Effects of repeated eccentric contractions on structure and mechanical properties of toad sartorius muscle. Am J Physiol Cell Physiol. 1993;265:C792–C800. doi: 10.1152/ajpcell.1993.265.3.C792. [DOI] [PubMed] [Google Scholar]