Abstract
In the superficial dorsal horn (SDH) processing of noxious and innocuous stimuli is critically dependent on the input–output relationship of its component neurones. Such relationships are routinely examined by assessing neuronal responses to somatic current injection or activation of synaptic inputs. A more complete understanding of input–output relationships would be achieved by comparing, in the same neurone, how the two forms of activation contribute to neuronal output. Therefore, we examined how SDH neurones transform depolarizing current injections and synaptic excitation via peripheral cutaneous stimuli (brush and pinch of the hindpaw) into trains of action potentials, in an in vivo preparation of the adult mouse spinal cord. Under whole-cell current clamp recording conditions four action potential discharge patterns were observed during depolarizing current injection: tonic firing neurones (21/93) discharged spikes throughout the step; initial bursting neurones (35/93) discharged several spikes at step onset; single spiking neurones (16/93) discharged one or two spikes at step onset; and delayed firing neurones (21/93) discharged spikes delayed from the step onset. Four characteristic profiles were observed in response to application of noxious (pinch) and innocuous (brush) cutaneous stimuli: nociceptive neurones (20/37) responded maximally to pinch stimulation; light touch neurones (9/37) responded maximally to brush stimulation; subthreshold neurones (4/37) exhibited depolarizing responses without firing action potentials; and hyperpolarizing neurones (4/37) exhibited a sustained pinch-induced hyperpolarization. Comparisons of current-evoked discharge patterns with peripherally evoked responses indicate SDH neurones expressing each of the four discharge patterns could receive, and therefore participate in the processing of information concerning, either noxious or innocuous stimuli. These data suggest that a neurone's response to current injection does not necessarily help identify or predict how the same neurone will respond to physiologically or functionally relevant stimuli.
The superficial dorsal horn (SDH: Rexed's laminae I and II) receives noxious information via fine myelinated and unmyelinated primary afferents (Light & Perl, 1979), and is the first site in the central nervous system where noxious signals undergo synaptic processing before transmission to higher brain centres (Willis & Coggeshall, 1991). Thus, understanding the input–output relationships in SDH neurones, specifically how these neurones respond to incoming signals and transform them into action potentials, is essential for understanding how the SDH processes noxious stimuli under normal and abnormal conditions.
In various studies of the SDH two commonly applied, but very distinct, experimental approaches have been employed to investigate input–output relationships in SDH neurones. The first approach has been to study the responses of neurones to depolarizing/hyperpolarizing current injection using in vitro spinal cord preparations. (Thomson et al. 1989; Yoshimura & Jessell, 1989a; Lopez-Garcia & King, 1994; Ruscheweyh & Sandkuhler, 2002; Lu & Perl, 2003). Using this approach SDH neurones are grouped into several categories based on their action potential discharge patterns and frequencies. Although a generally accepted terminology has not emerged, neurones have been identified that respond to depolarization with either sustained action potential discharge, marked spike frequency adaptation, or pronounced delays before spike onset. The functional significance of these different discharge patterns is unclear.
The second approach has been to study SDH neurone responses evoked during applications of peripheral cutaneous stimuli (both noxious and innocuous) using in vivo preparations such as monkey (Kumazawa & Perl, 1978), cat (Christensen & Perl, 1970; Steedman et al. 1985; Rethelyi et al. 1989; Steedman & Zachary, 1990) and rat (Woolf & Fitzgerald, 1983; Furue et al. 1999; Light & Willcockson, 1999). Although once again differences in terminology exist, these studies show that SDH neurones display a variety of responses to functionally relevant noxious (mechanical, chemical and thermal) and innocuous (mechanical and thermal) stimuli.
To date, no study has concurrently examined the response of SDH neurones to current injection and controlled peripherally applied stimuli in an intact animal preparation, despite several laboratory groups recently highlighting the need for such investigations (Grudt & Perl, 2002; Ruscheweyh & Sandkuhler, 2002). In this report we use an in vivo preparation of the mouse spinal cord to examine the relationship between responses evoked by current injection and peripherally applied cutaneous stimulation. We show that the in vivo responses of SDH neurones to current injection fall into four categories. In response to noxious and innocuous cutaneous stimuli four characteristic response profiles were also observed. We conclude, however, that the discharge pattern observed during depolarizing current injection does not predict an SDH neurones' discharge profile evoked by cutaneous stimulation. Rather, SDH neurones expressing each of the four discharge patterns can process information concerning noxious or innocuous modalities.
Methods
Animal preparation
All experimental procedures were approved by the University of Newcastle Animal Care and Ethics Committee. Experiments were carried out on C57/Bl6 mice aged 26–42 days (both sexes) with body weights ranging from 10.1 to 18.4 g. Details of the in vivo mouse spinal cord preparation have been previously described (Graham et al. 2004) and are shown schematically in Fig. 1. Similar preparations have also recently been described in rat (Furue et al. 1999; Light & Willcockson, 1999). Animals were anaesthetized with urethane (2.2 g kg−1 i.p.). The level of surgical anaesthesia was assessed 20 min after the initial dose by testing hindlimb withdrawal and corneal reflexes. If these reflexes were not abolished a supplemental dose (25% initial dose i.p.) of anaesthetic was administered. The level of anaesthesia was monitored throughout the experiment by testing the above reflexes. If reflexes recovered during the experiment further anaesthetic was administered (25% initial dose i.p.).
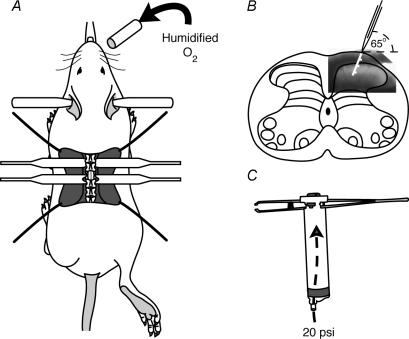
Figure 1. Patch-clamp recording from the mouse spinal cordin vivo.
A, schematic of the urethane-anaesthetized mouse, prepared for in vivo patch-clamp recording from the spinal cord. The animal's head is stabilized with ear and palate bars, while humidified 100% O2 is blown over the nostrils (curved arrow). Skin and musculature over the lumbosacral enlargement is retracted to expose the vertebral column. Vertebral clamps stabilize the T13−L1 and L2–3 vertebrae and a laminectomy exposes the L4–5 segment of the lumbosacral enlargement. The right hindlimb is positioned for subsequent cutaneous brush and pinch stimulation of the plantar surface of the hindpaw. B, schematic depicting electrode penetration of the spinal cord in the transverse plane. The electrode penetrates the dorsal surface of the spinal cord at an angle of 65 deg and is advanced into the SDH (approx. 100 µm). Subsequently, searching for neurones is carried out by advancing in 3 µm steps for a further 250 µm. The white line indicates electrode trajectory through an overlayed image of the dorsal horn (obtained from a fresh transverse slice). Tick marks indicate 100 µm intervals. C, a customised pneumatic pinching device was made from a pair of forceps attached to the plunger of a 10 ml syringe. The tips of the forceps closed during delivery of compressed air to the syringe barrel (dashed arrow). This device allowed delivery of reproducible pinching forces for controlled durations (see Graham et al. 2004).
After reaching a deep level of anaesthesia, animals were transferred to a customised frame and stabilized with ear and palate bars. A thermal pad was placed under the animal to maintain body temperature between 34 and 37°C. Humidified 100% O2 was continuously blown over the animals' nostrils (Fig. 1A). Surgery was completed under a dissecting microscope (16 × and 40 ×). The vertebral column was exposed and stabilized with vertebral clamps on the arches of T13−L3 vertebrae. A laminectomy exposed the spinal cord at the L4–L5 level of the lumbosacral enlargement. The dura was reflected, and a small incision was made in the pia to allow penetration of the underlying dorsal horn with a recording pipette. Throughout the experiment the exposed surface of the spinal cord was irrigated with artificial cerebrospinal fluid containing (mM): 118 NaCl, 25 NaHCO3, 11 glucose, 2.5 KCl, 1 NaH2PO4, 1 MgCl2 and 2.5 CaCl2, maintained at 37°C and equilibrated with 95% O2–5% CO2 gas to achieve a final pH of 7.3. The mean duration of experiments (±s.d.) was 4.5 ± 1 h, and at the completion of experiments animals were overdosed with Nembutal (100 mg kg−1 i.p.).
Electrophysiology
Recording electrodes (8–12 MΩ) were fabricated from thick-wall (o.d. 1.5 mm, i.d. 0.86 mm) borosilicate glass capillaries using a horizontal pipette puller (P-97, Sutter Instrument Co., Novato, CA, USA). Electrodes were filled with a K+-based internal solution containing (mM): 135 KMeSO4, 6 NaCl, 2 MgCl2, 10 Hepes, 0.1 EGTA, 2 MgATP, 0.3 NaGTP, pH 7.3 (with KOH). The pipette tip was lowered to the surface of the spinal cord at an angle of 65 deg (to horizontal) and then advanced 100 µm into the spinal cord (Fig. 1B). This distance ensured the pipette tip had passed through the white matter overlying the SDH as determined by viewing transverse slices obtained from the same spinal region of age-matched animals. During penetration of the dorsal white matter continuous positive pressure (∼50 kpa; see Graham et al. 2004) was applied to the pipette to ensure its tip remained clear of debris. Following penetration of the white matter, the positive pressure on the pipette tip was reduced to approximately 10 kpa and the pipette was advanced (3 µm steps) for a further 250 µm (which corresponds to the border of lamina II and III: see above and Fig. 1B). This search strategy was carried out in voltage-clamp mode with an AxoClamp 2B amplifier (Axon Instruments, Forster City, CA, USA). While the pipette was advanced, the current response to a voltage step (+20 mV, 10 ms, delivered at 10 Hz) was used to monitor changes in pipette resistance. An increase in pipette resistance indicated contact with a neurone, glial cell, blood vessel or cell debris (Margrie et al. 2002). At this point the positive pressure was released and the holding potential was adjusted to −50 mV, and the voltage step polarity and magnitude changed from +20 mV to −50 mV. When a tight seal was obtained (range 2–7 GΩ) the membrane patch was ruptured using gentle suction establishing the whole-cell recording configuration (series resistance 10–84 MΩ). The amplifier was switched to bridge mode (current-clamp) and balanced for pipette resistance (bridge balanced). The membrane potential was noted at this point and designated as resting membrane potential (RMP). In some experiments, small hyperpolarizing bias currents (< 50 pA) were injected through the recording electrode to maintain RMP. All data were digitized online (sampled at 20 kHz, filtered at 10 kHz) via an ITC-16 computer interface (Instrutech, Long Island, NY, USA) and stored on a personal computer using Axograph v4.6 software (Axon Instruments, Foster City, CA, USA). All membrane potential values have been corrected for a 10 mV liquid junction potential (Barry & Lynch, 1991).
Stimulation protocols
Where possible neurones were activated in two ways: (1) via direct current injection through the recording electrode and (2) application of peripheral cutaneous stimuli to an appropriate receptive field on the hindlimb. Depolarizing and hyperpolarizing current steps (800 ms duration, 20 pA increments, delivered every 8 s) were applied to determine the neurone's response during depolarization and hyperpolarization. For cutaneous stimulation a neurone's receptive field was first determined by applying innocuous stimuli with a soft-bristle brush across the pedal surface of the hindpaw. The response to noxious stimulation was assessed by pinching the skin over the neurone's receptive field with a custom-built computer-controlled pincher (Graham et al. 2004). Briefly, the pincher consisted of a 10 ml syringe that was modified to accommodate a pair of forceps in the open position (Fig. 1C). Compressed air (20 p.s.i.) delivered to the syringe drove the plunger, which acted as a piston to close the forceps at a reproducible force for a prescribed duration of 1 s.
Data analysis
Data analysis was performed offline using semi-automated procedures within the Axograph v4.6 software package. Input resistance was estimated from the steady-state voltage response to a small hyperpolarizing current step (20–40 pA for 800 ms) from RMP. To capture individual APs elicited by depolarizing current injection or naturally applied stimuli we used a derivative threshold method (threshold set at dVm/dt = 25 V s−1 on traces digitized at 20 kHz). The membrane potential at derivative threshold was defined as firing threshold for captured APs. Rheobase current was determined as the smallest current step to elicit at least one AP. The height of each AP was measured as the difference between the firing threshold and its maximum positive peak. AP half-width was measured at the mid-point of AP height. AP afterhyperpolarization (AHP) amplitude was measured as the difference between firing threshold and the maximum negative peak following the AP.
Several parameters were measured to describe the preferred AP discharge pattern of each neurone. Firing latency was measured as the time interval from depolarizing step or pinch onset to the first evoked AP in a train. Firing duration was taken as the time interval between the onset of the first and last AP during a depolarizing step or pinch application. Instantaneous AP frequency was calculated, for all current steps or pinch stimuli evoking two or more APs, as the reciprocal of the time interval between successive APs. For multiple APs, mean frequency was calculated as the average of all instantaneous AP frequencies for single depolarizing steps or pinch stimulus applications.
We used SPSS v.10 software package for statistical analysis (SPSS Inc., Chicago, IL, USA). One-way analysis of variance was used to compare variables between/across SDH neurone groupings based on discharge pattern or peripheral stimulus-evoked responses. Student-Neuman-Keuls post hoc tests were used to determine which groups differed. Data that failed Levene's test of homogeneity of variance were compared using the non-parametric Kruskal Wallace test. Statistical significance was set at P < 0.05. All values are presented as means ±s.e.m.
Results
Stable whole-cell patch-clamp recordings were obtained from 93 neurones in 39 animals. Neuronal input resistance ranged from 89 to 1450 MΩ (370 ± 22 MΩ, n= 93), and resting membrane potentials were between −36 and −75 mV (− 59 ± 7 mV, n = 93). These values are comparable to those reported for rat SDH neurones using similar in vivo recording techniques (Furue et al. 1999; Light & Willcockson, 1999). The depth of recorded neurones, measured from the dorsal surface of the spinal cord, ranged from 100 to 350 µm (239 ± 52 µm). Thus, all recorded neurones were located within the SDH (see Methods and Fig. 1B).
All 93 neurones in the sample were classified according to their action potential (AP) discharge patterns during depolarizing current injection. In 40% (37/93) of neurones, stable recording conditions were maintained long enough to also classify SDH neurones according to their responses to peripheral innocuous and noxious mechanical stimuli.
SDH neurone responses to current injection
Depolarizing current injections
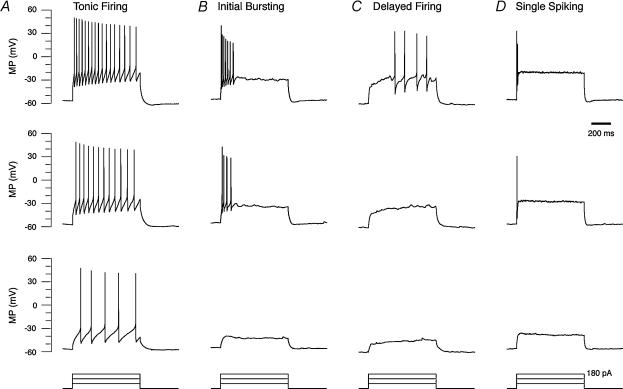
The present sample of SDH neurones was divided into four classes similar to those proposed by Ruscheweyh & Sandkuhler (2002) based on AP discharge pattern during depolarizing current injections. Tonic firing neurones constituted 22% (21/93) of the sample, and exhibited AP firing throughout the duration of current injection (Fig. 2A). Initial bursting neurones represented 37% (35/93) of the sample and responded with a burst of APs at the onset of depolarization followed by rapid adaptation (Fig. 2B). Delayed firing neurones made up 15% (14/93) of the sample and only fired APs after a considerable delay from the depolarization onset (Fig. 2C). This characteristic delay to AP firing decreased as injected current amplitude increased. Finally, single spiking neurones comprised 17% (16/93) of the sample, and fired one or occasionally two APs at depolarizing current onset and then remained silent. The single spiking pattern persisted despite increasing current injection amplitude.
Figure 2. Four discharge patterns are observed in SDH neurones in vivo.
A–D, examples of discharge patterns recorded in four SDH neurones during current injections (all recordings made from RMP). For each column the bottom trace indicates a series of current steps injected through the recording pipette. In each column, the upper three traces are voltage responses recorded during current injections of increasing magnitude (60, 120 and 180 pA). A, the tonic firing pattern was characterized by sustained spiking throughout the duration of the depolarization. Spike frequency increased as current step magnitude was increased. B, the initial bursting pattern was characterized by spiking restricted to the onset of depolarization. Under increasing levels of depolarization spiking remained confined to the onset of the stimulus. C, the delayed firing pattern was characterized by spiking onset in the latter stages of the depolarization. Increasing levels of depolarization reduced the delay to spike onset. D, the single spiking pattern was characterized by the discharge of one or two spikes at the onset of depolarization. Increasing levels of depolarization failed to change this discharge pattern.
Nine per cent of neurones (7/93) in the sample did not initially fire APs in response to depolarizing current injections (Fig. 3A, left). Such recordings may have been made from damaged neurones or non-excitable cells (glia); however, a number observations support another interpretation. First, all these cells appeared to be healthy when compared with the overall population, as evidenced by their RMPs (− 61.7 ± 1.3 versus −57.7 ± 0.8 mV) and input resistances (248 ± 52 versus 371 ± 22 MΩ). Second, despite failure of depolarizing current injection to elicit AP firing in these cells, spontaneous subthreshold excitatory postsynaptic potentials were observed (Fig. 3A, left panel) indicating that they received synaptic inputs. Further, in three cells a second sequence of depolarizing current steps elicited a delayed discharge pattern (Fig. 3A, right). Finally, pinch stimulation of the hindpaw evoked subthreshold excitatory responses in 4/7 cells (Fig. 3B) and action potential discharge in 2/7 cells (Fig. 3C). Taken together, we believe that these 7 cells were neurones and assigned them to the delayed firing class. Similar observations of ‘reluctant to fire’ SDH neurones have been recently reported in vitro (Prescott & De Koninck, 2002). In that study such neurones were subsequently placed into a single spiking category since they only managed to elicit a single AP when depolarizing steps were repeated from more depolarized membrane potentials. The significant difference in recording conditions, in vivo versus in vitro, may account for these findings (see Discussion).
Figure 3. Neurones reluctant to discharge during depolarizing current injection were classified asdelayed firing neurones.
A, voltage responses recorded in an SDH neurone during two identical depolarizing current injection protocols from the same membrane potential (separated by 5 min). In this neurone the first protocol failed to elicit AP discharge despite depolarization to −25 mV; however, in the second trial the neurone discharged APs in the delayed firing pattern. B, a subthreshold response, recorded from the same neurone as in A, during pinch stimulation of the hindpaw (horizontal bar indicates pinch duration). C, an example of a recording from another neurone, which also failed to discharge during depolarizing current injection (not shown). This neurone, however, discharged vigorously during pinch stimulation of the hindpaw. The data presented in A, B and C summarize the basis for assigning reluctant-to-fire neurones in the delayed firing class.
Comparisons between recording depth and membrane and action potential characteristics for neurones exhibiting the four discharge patterns (described above) are summarized in Table 1. Across the four groups presented in Table 1 and Fig. 2 no difference was observed in recording depth, input resistance, RMP, or AP threshold. In contrast, the rheobase current required to elicit AP firing in delayed firing and single spiking neurones was significantly greater than that of tonic firing and initial bursting neurones. AP height was significantly greater in tonic firing and initial bursting neurones, while AP half-width was shorter in tonic firing neurones compared with those in the remaining three groups. Finally, AHP amplitude for single spiking neurones was significantly smaller than that observed in the other groups.
Table 1.
Location, membrane and action potential properties of SDH neurones classified by action potential discharge pattern during depolarizing current injection
| Tonic firer (TF) | Initial burster (IB) | Delayed firer (DF) | Single spiker (SS) | Group | |
|---|---|---|---|---|---|
| n | 21 | 35 | 21 | 16 | 93 |
| Recording depth (µm) | 240 ± 10 | 240 ± 10 | 250 ± 10 | 230 ± 20 | 240 ± 10 |
| (range) ψ | (100–340) | (180–320) | (160–320) | (120–300) | (100–340) |
| Input resistance (MΩ) | 464 ± 57 | 328 ± 27 | 353 ± 42 | 325 ± 46 | 361 ± 21 |
| RMP (mV) | −58.1 ± 1.5 | −57.7 ± 1.5 | −59.3 ± 1.2 | −57.1 ± 1.8 | −58.1 ± 0.7 |
| Rheobase current (pA) | 55 ± 9 | 72 ± 9 | 98 ± 14† | 129 ± 15 | 84 ± 6 |
| * (D, SS) | * (D, SS) | * (TF, IB, SS) | * (TF, IB, D) | ||
| AP threshold (mV) | −34.7 ± 1.1 | −31.4 ± 1.2 | −29.6 ± 3.0† | −33.9 ± 1.4 | −32.3 ± 0.8 |
| AP height (mV) | 57.4 ± 3.3 | 57.0 ± 2.0 | 43.5 ± 2.8† | 47.6 ± 2.7 | 52.8 ± 1.4 |
| * (D, SS) | * (D, SS) | * (TF, IB) | * (TF, IB) | ||
| AP halfwidth (ms) | 0.62 ± 0.03 | 0.77 ± 0.03 | 0.83 ± 0.05† | 0.81 ± 0.06 | 0.75 ± 0.02 |
| * (IB, D, SS) | * (TF) | * (TF) | * (TF) | ||
| AHP amplitude (mV) | − 16.1 ± 1.4 | − 16.7 ± 0.9 | − 16.7 ± 1.3† | − 11.7 ± 1.6 | − 16.1 ± 0.1 |
| * (SS) | * (SS) | * (SS) | * (TF, IB, D) |
Values are means ±s.e.m. unless indicated. ψDepth represents actual electrode travel from cord surface (not corrected for 65 deg electrode angle).
Calculation includes delayed firing neurones where current injections elicited APs (n = 14).
Significant difference (P < 0.05), abbreviation indicates groups comparisons.
Hyperpolarizing current injections
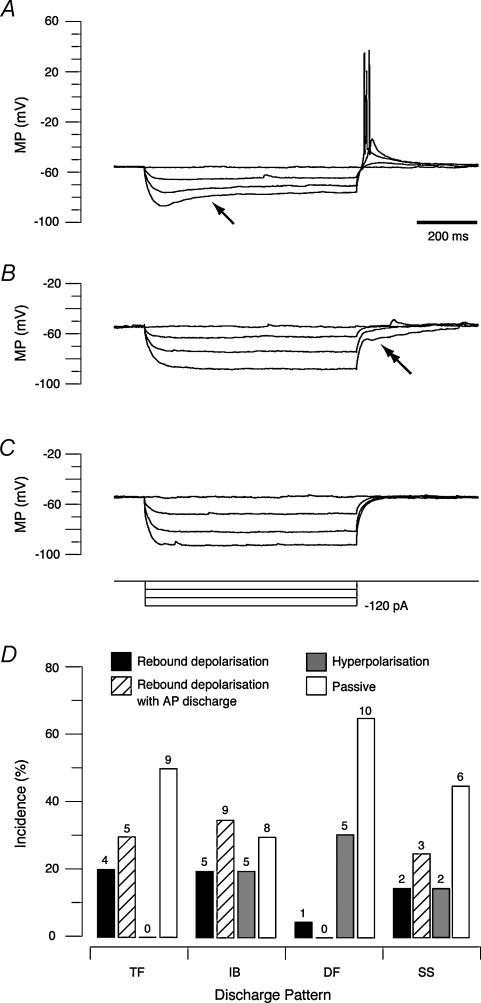
In addition to a diversity of discharge patterns elicited by depolarizing current injection, SDH neurones showed variable responses to hyperpolarizing current injection (Fig. 4). Three characteristic responses were observed upon release from hyperpolarization. In 39% of neurones (29/74) a rebound depolarization occurred, which elicited AP firing in 59% (17/29) of these neurones (Fig. 4A). In 41% (12/29) of cases these characteristics were accompanied by a sag in membrane potential during the hyperpolarizing current injection (arrow in Fig. 4A). Alternatively, in 16% of neurones (12/74) a long-lasting membrane hyperpolarization was observed following cessation of current injection (double arrow in Fig. 4B). In the remaining 45% (33/74) neither of the above responses were observed (Fig. 4C).
Figure 4. SDH neurones exhibit three response profiles to hyperpolarizing current injection in vivo.
A–C, representative recordings taken from three neurones exhibiting the different response profiles observed following release from hyperpolarization. In each panel, the overlayed traces are voltage responses recorded during current injections of increasing magnitude (−40, −80, −120 pA; square steps at bottom of C). A, after release from hyperpolarization 39% (29/74) of the neurones exhibited rebound depolarization with some (17/29) firing action potentials. In 41% (12/29) of these neurones a sag (arrow) in membrane potential occurred during the hyperpolarizing current injection. B, in 16% (12/74) of neurones a long-lasting hyperpolarizing response was observed following release from hyperpolarization (double arrow). C, in 45% (33/74) of neurones the voltage response returned passively towards RMP. D, proportions of neurones, grouped according to their current-evoked discharge pattern (shown in Fig. 2), that exhibit the various hyperpolarizing responses. Rebound depolarization was observed in approximately half the tonic firing (TF; 9/18) and initial bursting (IB; 14/27) and 38% (5/13) of single spiking (SS) neurones. Long-lasting hyperpolarization was most commonly observed in delayed firing (DF) neurones (5/16) and never in TF neurones. A passive return to RMP was observed in at least 30% of the neurones in each firing class (TF 9/18, IB 8/27, DF 10/16 and SS 6/13).
The relationship between hyperpolarization-activated responses and the depolarizing responses described above is shown in Fig. 4D. Approximately half the tonic firing (9/18), initial bursting (14/27) and single spiking (5/13) neurones expressed rebound depolarization, which could in nearly half these cases elicit APs (Fig. 4A). On the other hand few delayed firing neurones exhibited rebound depolarization and when present it did not cause AP firing. Delayed firing neurones were most likely to express long-lasting hyperpolarization at the cessation of current injection (30%, 5/16). This response was also observed in some initial bursting (20%, 5/27) and single spiking neurones (15%, 2/13) but not in tonic firing neurones (Fig. 4B). The remaining neurones in each class did not express either hyperpolarization-activated response (Fig. 4C).
SDH neurone responses to peripheral cutaneous stimulation
Classification using peripheral cutaneous stimuli proceeded for neurones that remained stable after assessment of their response during depolarizing and hyperpolarizing current injection. Both innocuous (light brush) and noxious (pinch) stimuli were applied to the receptive field of each neurone on the hindpaw. Only neurones where responses to both brush and pinch stimuli could be characterized (37/93) are included in this analysis.
In 29 of the 37 neurones APs were evoked in response to peripheral stimuli. These neurones were classified as nociceptive (Fig. 5A and 20/29) if they responded maximally to noxious ‘pinch’ stimulation. On seven occasions nociceptive neurones also exhibited depolarizations in response to ‘brush’, which evoked AP firing in four instances. These four neurones probably correspond to wide dynamic range or multi-receptive neurones that have been described in the SDH by other workers (Bennett et al. 1980; Woolf & Fitzgerald, 1983; Rethelyi et al. 1989; Light & Willcockson, 1999). Neurones were classed as light touch (Fig. 5B and 9/29) if they responded maximally to innocuous brush. Light touch neurones also fired APs in response to pinch on all but one occasion; however, these responses were largely restricted to the pinch onset and were qualitatively similar to the corresponding brush responses (Fig. 5B). Consequently, these have been interpreted as responses to the initial ‘touch’ contact of the forceps during pinching consistent with previous observations (Narikawa et al. 2000).
Figure 5. SDH neurones exhibit four response profiles during innocuous brush and noxious pinch.
A–D, voltage responses from four different neurones during application of brush (upper trace) and pinch (lower trace). A, nociceptive neurones responded maximally (fired APs) to pinch stimulation and in most recordings responded only with subthreshold depolarizations during brush. B, light touch neurones responded maximally to brush (upper trace). Typically, these neurones also responded to pinch stimulation, but APs were restricted to pinch onset and resembled brush-evoked responses (lower trace). C, subthreshold neurones responded to brush and pinch with small depolarizations that failed to reach AP threshold. D, hyperpolarizing neurones responded to both brush and pinch with membrane hyperpolarization, most evident during pinch stimulation. E, the proportion of neurones, grouped according to discharge patterns (as in Fig. 2), exhibiting each of the peripherally evoked response profiles. Each firing pattern group (TF, IB, DF and SS) included neurones with light touch and nociceptive profiles. Subthreshold responses were only observed in DF and SS neurones, whereas hyperpolarizing responses were restricted to IB and SS neurones. Together, these data suggest that SDH neurones expressing the four discharge patterns play a role in processing both noxious and innocuous peripheral stimuli.
The remaining neurones (8/37) did not fire action potentials during application of test stimuli, i.e. they were ‘functionally silent’. It is possible that: (1) recordings were made from damaged neurones; (2) the neurone's receptive field could not be located; or (3) the receptive field was not sensitive to the two stimulus modalities used in this study. These ‘functionally silent’ neurones, however, had input resistances and RMPs similar to the overall population (Table 2). In addition, these neurones fired action potentials when depolarized by current injection. Finally, subthreshold receptive fields were located for these neurones, making it unlikely that a suprathreshold receptive field was overlooked. Therefore these functionally silent neurones were further analysed and separated into two groups based on their response characteristics. Subthreshold neurones (Fig. 5C and 4/8) responded to brush and pinch with depolarizations but failed to fire APs. Hyperpolarizing neurones (Fig. 5D and 4/8) responded to application of the test stimuli with a membrane hyperpolarization, which was most apparent during ‘pinch’.
Table 2.
Location and membrane properties of SDH neurones classified by peripherally evoked responses
| Functionally silent | |||||
|---|---|---|---|---|---|
| Nociceptive | Light touch | Subthreshold | Hyperpolarizing | Group | |
| n | 20 | 9 | 4 | 4 | 37 |
| Recording depth (µm) | 230 ± 10 | 250 ± 20 | 240 ± 20 | 240 ± 30 | 240 ± 10 |
| (range) ψ | (100–300) | (160–320) | (200–280) | (180–300) | (100–320) |
| Input resistance (MΩ) | 313 ± 46 | 434 ± 64 | 481 ± 73 | 399 ± 119 | 404 ± 38 |
| RMP (mV) | − 58.8 ± 1.3 | − 56.7 ± 1.7 | − 55.6 ± 3.0 | − 54.4 ± 1.5 | − 57.5 ± 0.9 |
Values are means ±s.e.m. unless indicated. ψDepth represents actual electrode travel from cord surface (not corrected for 65 deg electrode angle).
The recording depth, RMP and input resistance of the classified neurones were similar for all groups and are summarized in Table 2. This sample also had a similar distribution to the overall population of tonic firing (25 versus 22%), initial bursting (35 versus 37%), delayed firing (25 versus 24%) and single spiking (16 versus 17%) neurones. Figure 5E compares the current-evoked and peripheral stimulation-evoked classifications of SDH neurones. Neurones exhibiting all discharge patterns (tonic firing, initial bursting, delayed firing and single spiking) exhibited both light touch and nociceptive response profiles. In contrast, only delayed firing and single spiking neurones exhibited subthreshold response profiles and only initial bursting and single spiking neurones exhibited the hyperpolarizing responses.
Pinch-evoked versus current-evoked responses
An important feature of the pinch stimulus employed in the present study is its reproducible pinching force and duration (Graham et al. 2004). Together these features permit comparison of pinch-evoked responses across recordings. Here, we examined pinch-evoked discharge in 11 nociceptive neurones recorded during three consecutive pinch trials. Data from these neurones were subsequently divided into two groups, based on the current-evoked discharge pattern of each neurone, and compared (tonic firing, n= 5; initial bursting, n= 6; Fig. 6). Peri-stimulus histograms of pinch-evoked discharge (1 s pinch) indicate that both tonic firing and initial bursting neurones respond similarly to the pinch stimulus (Fig. 6A).
Figure 6. Comparison of current- and pinch-evoked responses in tonic firing and initial bursting SDH neurones.
A, overlaid peri-stimulus histograms of pinch-evoked AP discharge (1 s pinch) in neurones classified according to their current-evoked AP discharge as tonic firing (black) or initial bursting (grey). Inset (upper right) shows representative voltage responses for a tonic firing and initial bursting neurone. Plots in B, C and D compare attributes of pinch-evoked AP discharge and current-evoked AP discharge (elicited by current steps of increasing magnitude; from rheobase to 80 pA above rheobase). B, the duration of pinch-evoked AP discharge was similar in tonic firing and initial bursting neurones. In contrast, current-evoked AP discharge duration during all depolarizing steps above rheobase was significantly greater in tonic firing versus initial bursting neurones (*P < 0.05). C, the number of pinch-evoked APs elicited in tonic firing neurones and initial bursting neurones was similar, despite the number of APs discharged during all depolarizing current steps above rheobase being significantly greater in tonic firing versus initial bursting neurones (*P < 0.05). D, the mean pinch-evoked AP firing frequency elicited at all current steps was similar in tonic firing and initial bursting neurones. Thus, neurones with vastly different current-evoked discharge characteristics (tonic firing versus initial bursting) can exhibit similar pinch-evoked discharge profiles.
Further comparison of pinch-evoked AP discharge in tonic firing and initial bursting neurones revealed that the duration (Fig. 6B), number of APs (Fig. 6C), and mean AP firing frequency (Fig. 6D) were all similar. In contrast several quantitative measures of current-evoked discharge differed between tonic firing and initial bursting neurones. For example, AP firing duration and number of APs generated during current-evoked discharge were significantly greater in tonic firing versus initial bursting neurones at all current injections above rheobase (Fig. 6B and C). Only mean AP firing frequency was similar in tonic firing and initial bursting neurones for each depolarizing current amplitude (Fig. 6D).
Discussion
Two distinct approaches have been used to examine signal processing in SDH neurones. The first has been to assign neurones into classes, based on their discharge patterns during depolarizing current injection using in vitro spinal cord slices, and then make inferences about how they might respond to activation of peripheral inputs (Prescott & De Koninck, 2002; Ruscheweyh & Sandkuhler, 2002). The second has used in vivo preparations and examined responses during the application of peripheral cutaneous stimuli (Woolf & Fitzgerald, 1983; Furue et al. 1999; Light & Willcockson, 1999). In this study we examined the in vivo responses of SDH neurones, in an intact preparation of the adult mouse spinal cord, to both depolarizing current injection and functionally relevant peripheral cutaneous stimuli. The major findings are: (1) the discharge patterns displayed by neurones in the in vivo mouse SDH are similar to those reported in previous in vitro rodent studies (Thomson et al. 1989; Lopez-Garcia & King, 1994; Grudt & Perl, 2002; Prescott & De Koninck, 2002; Ruscheweyh & Sandkuhler, 2002; Ruscheweyh et al. 2004); (2) the responses of SDH neurones, to noxious and innocuous stimuli, are qualitatively similar to those obtained in other in vivo rodent preparations (Woolf & Fitzgerald, 1983; Furue et al. 1999; Light & Willcockson, 1999); and (3) no predictive relationship exists between SDH neuronal responses during current injection and functionally relevant stimulation.
Responses of SDH neurones to current injection
Injection of current steps via a microelectrode has been employed widely throughout the CNS to study the membrane properties of neurones (Larkman & Mason, 1990; Faber et al. 2001; Powers & Binder, 2001). This approach, although artificial when compared with synaptically driven excitation, has provided an effective means of separating SDH neurones into various classes in vitro (Thomson et al. 1989; Yoshimura & Jessell, 1989a; Lopez-Garcia & King, 1994; Grudt & Perl, 2002; Prescott & De Koninck, 2002; Ruscheweyh & Sandkuhler, 2002; Ruscheweyh et al. 2004). For example, SDH neurones are commonly classified by the discharge pattern evoked during depolarization or alternatively their response during and following release from hyperpolarization (Yoshimura & Jessell, 1989a; Lopez-Garcia & King, 1994). It is well established that discharge pattern heterogeneity is underpinned by a differential expression of various voltage-activated ionic conductances (Yoshimura & Jessell, 1989a; Ruscheweyh & Sandkuhler, 2002; Hu & Gereau, 2003; Melnick et al. 2004b; Ruscheweyh et al. 2004). How these in vitro findings relate to SDH neurones in living animals has not been examined.
In response to depolarizing current, SDH neurones in vivo expressed one of four discharge patterns; tonic firing, initial bursting, delayed firing or single spiking. All firing patterns have been previously described in vitro (at room temperature ∼23°C), using rodent preparations (Thomson et al. 1989; Yoshimura & Jessell, 1989a; Lopez-Garcia & King, 1994; Grudt & Perl, 2002; Prescott & De Koninck, 2002; Ruscheweyh & Sandkuhler, 2002; Hantman et al. 2004). In these studies, however, one or two discharge patterns tend to predominate and all four patterns are not always described. The incidence of each discharge pattern in our study differs from these previous in vitro reports, specifically all discharge patterns have been identified and are well represented.
In response to hyperpolarizing current injection, SDH neurones in vivo expressed one of three responses in vivo: post-inhibition depolarization, post-inhibition hyperpolarization, or no post-inhibition response. As with the discharge patterns, described above during depolarization, similar observations of hyperpolarization-activated responses have been made in in vitro spinal cord slices (Thomson et al. 1989; Yoshimura & Jessell, 1989a; Lopez-Garcia & King, 1994; Prescott & De Koninck, 2002). Our study differed from those above in that the proportion of neurones exhibiting hyperpolarization-activated responses (45%) was substantially lower than reported in vitro (76%; Yoshimura & Jessell, 1989). Generally, SDH neurones that are more excitable during depolarization (tonic firing and initial bursting) are also the most likely to show enhanced excitability following release from hyperpolarization (Fig. 4D). Conversely, neurones that are less excitable during depolarization (delayed firing) display reduced excitability after release from hyperpolarization (Fig. 4D).
Details of the voltage-dependent currents underlying depolarization- and hyperpolarization-activated responses in SDH neurones have been studied extensively in vitro. The expression of a TTX-sensitive Na+ current and a TEA-sensitive delayed rectifying K+ current is sufficient to generate tonic firing in SDH neurones (Melnick et al. 2004b). Other voltage-dependent conductances (e.g. Ca2+-dependent K+ current) are, however, important in modulating the features of tonic firing (e.g. interspike interval). The spike frequency adaptation observed in initial bursting and single spiking has been suggested to be due largely to a reduction in the TTX-sensitive Na+ current in some SDH neurones (Melnick et al. 2004a), although a role for a K+ current with D-current like characteristics has also been suggested to contribute to single spiking (Ruscheweyh & Sandkuhler, 2002). Two of IA-like K+ currents have been identified in SDH neurones and have been shown to underlie various forms of delayed firing (Yoshimura & Jessell, 1989a; Ruscheweyh & Sandkuhler, 2002; Ruscheweyh et al. 2004).
During hyperpolarization, a time-dependent Ih-like current contributes to membrane sag, rebound depolarization and rebound AP firing. In addition, a second fast activating/inactivating hyperpolarization-activated current has been identified that does not contribute to membrane sag or rebound depolarization. In 61% of substantia gelatinosa (lamina II) neurones this current has been shown to co-exist with the Ih-like current (Yoshimura & Jessell, 1989a). Finally the IA-like current, which is capable of delaying action potential firing (see above), can, when activated following release from hyperpolarization, manifest as an extended hyperpolarization (Yoshimura & Jessell, 1989a; Grudt & Perl, 2002; Ruscheweyh & Sandkuhler, 2002; Ruscheweyh et al. 2004).
Taken together, our data on SDH neurone responses during depolarizing and hyperpolarizing current steps provide evidence for the presence and subsequent influence of currents described in vitro on action potential spike generation in vivo. Detailed analysis and separation of these currents in vivo would require voltage-clamp and pharmacological analysis. Performing both these manipulations in vivo is difficult. However, such studies can now be performed in vitro with the knowledge that the currents described above are relevant for spike generating mechanisms in vivo.
There are several possible reasons for the observed differences in the proportion of SDH neurones expressing these responses to depolarizing and hyperpolarizing current injection in vivo versus in vitro. In a number of systems (e.g. vertebrate locomotor pattern generators; reviewed in Heckman et al. (2003))) it has been shown that the electrophysiological response of neurones depends critically on recording conditions. In this study mice were anaesthetized with urethane, a non-specific drug that affects the action of the three major fast neurotransmitter systems in the spinal cord (Hara & Harris, 2002). Furthermore, the effects of urethane on slow neurotransmitter systems and voltage-dependent conductances is unknown. Another major difference between in vitro and in vivo recording conditions is the presence of descending monoaminergic (e.g. serotonin and noradrenaline) input in the in vivo preparation. These descending pathways play important roles in modulating SDH neurone responses (Fields & Basbaum, 1978; Millan, 2002). Hence in the in vivo preparation both anaesthetic action and descending monoaminergic drive may significantly influence the excitability ‘set-point’ of SDH neurones.
Some in vitro studies have shown that altering the membrane potential of a neurone can change its discharge pattern (Yoshimura & Jessell, 1989a; Prescott & De Koninck, 2002; Ruscheweyh & Sandkuhler, 2002; Ruscheweyh et al. 2004). SDH neurones in this study had slightly more depolarized membrane potentials than those reported in vitro and this might have contributed to the different representation of various discharge patterns. For example, the voltage sensitivity of the A-current underlying the delayed discharge pattern means these neurones discharge tonically at more depolarized potentials (Ruscheweyh & Sandkuhler, 2002).
The phosphorylation state of important intracellular signalling pathways can also change the discharge properties of SDH neurones. For example, activation of various kinases (e.g. protein kinase A, protein kinase C and extracellular signal-related kinase) can modulate the functioning of certain potassium channels in SDH neurones (Hu & Gereau, 2003). It is unknown whether differences in the activation state of these pathways exist between in vivo and in vitro spinal cord preparations. In addition, the activity of intracellular pathways and protein–protein interactions will differ at in vivo temperatures (37°C) versusin vitro slice work at room temperature. This temperature difference will also influence the kinetics of voltage-gated ion channels whose activation combines to determine the discharge pattern of a neurone (Hille, 1992). With these issues in mind it will be important to assess the influence of elevated temperature on action potential discharge in SDH neurones using in vitro preparations.
Responses of SDH neurones to peripheral cutaneous stimulation
It is well established that neurones in the SDH respond to noxious cutaneous stimuli of mechanical, chemical and thermal origins (Willis & Coggeshall, 1991). SDH neurones also respond to innocuous stimuli such as light touch, warming, cooling and itch (Sugiura et al. 1986; Carstens, 1997). We chose brush and pinch stimuli for classification of SDH neurones, as a recent in vivo study in rat showed that most light touch neurones responded to brush and all nociceptive neurones responded to pinch (Light & Willcockson, 1999). Hence, these select stimuli allowed a rapid assay of the responsiveness of individual SDH neurones. This was an important consideration as one goal of this study was to obtain both the current-evoked discharge pattern and response to peripheral stimulation data in the same neurone. Due to technical issues our average recording duration in the mouse was shorter (Graham et al. 2004) than those recently made in rat (Furue et al. 1999; Light & Willcockson, 1999) (approximately 10 versus 30 min). Therefore, we could only obtain both type types of recordings for approximately 40% of the recorded neurones.
The proportion of nociceptive and light touch responding SDH neurones described here is similar to that reported in rats, cats and monkeys (Kumazawa & Perl, 1978; Light et al. 1979; Woolf & Fitzgerald, 1983; Rethelyi et al. 1989; Furue et al. 1999; Light & Willcockson, 1999). Furthermore, their location in the SDH appears to be similar across these species, with light touch neurones located deeper within the SDH and nociceptive neurones tending to be located more superficially (Table 2). Together, these observations suggest that the organization of inputs to the SDH may be similar among species investigated so far. This study also identified a group of SDH neurones that only exhibit subthreshold responses during cutaneous stimulation in vivo (Fig. 5C and D). Subthreshold responses have been observed previously in the SDH (Light & Willcockson, 1999); however, their physiological role remains unclear. These functionally ‘silent’ neurones responded during pinch stimulation and thus could be considered nociceptive despite their lack of AP discharge.
Noxious evoked hyperpolarizing responses have also been described in SDH neurones (Lopez-Garcia & King, 1994); however, like subthreshold depolarizing responses it is difficult to speculate on the role played by these neurones. A recent study described an inhibitory connection between two classes of SDH neurones with distinct discharge properties (Lu & Perl, 2003). Specifically, the recipient neurones of this inhibitory connection all expressed an initial bursting response (termed ‘phasic’ by these investigators) during depolarizing steps. Given neurones exhibiting hyperpolarizing responses in the present study were predominantly initial bursting, it is possible that these responses represent activation of a similar inhibitory connection.
Physiological significance
Neurones in the SDH have been classified into various categories using a wide variety of characteristics including discharge properties, intrinsic membrane properties, primary afferent input, neuronal morphology, transmitter content (Todd & Spike, 1993) and presence of calcium binding proteins (Ren & Ruda, 1994). Assimilating these multiple classification schemes is now essential for a better understanding of neuronal function in this region. A number of studies have begun to address this issue; for example a correlation is known to exist between neurotransmitter content and calcium binding protein content of SDH neurones (Laing et al. 1994; Albuquerque et al. 1999). More recently, a number of studies have described correlations between morphological and electrophysiological features in SDH neurones (Han et al. 1998; Grudt & Perl, 2002; Prescott & De Koninck, 2002).
Taken together, our study failed to find any obvious correlations between the current-evoked discharge patterns and peripherally evoked responses in SDH neurones. Rather, among neurones displaying each current-evoked discharge pattern (i.e. tonic firing, initial bursting, delayed firing and single spiking) both nociceptive and light touch response profiles were observed. This suggests neurones expressing each discharge pattern play a role in the processing of information concerning noxious as well as innocuous modalities. Of course, these findings must be considered in light of the well-established neuronal diversity in the SDH. Our sample population would have included local interneurones (both excitatory and inhibitory), short and long propriospinal neurones and projection neurones (Willis & Coggeshall, 1991). Future studies combining in vivo physiological recording with subsequent examination of neuronal morphology and neurotransmitter phenotype may in fact uncover a relationship between these parameters and an SDH neurone's discharge pattern and/or its response to peripheral cutaneous stimuli.
Our finding is in contrast to a study using an ex vivo hemisected spinal cord–hindlimb preparation of the neonatal rat (P10–14). This investigation reported associations between discharge patterns and peripherally evoked responses (Lopez-Garcia & King, 1994). Specifically, this study identified a strong association between neurones that exhibited rebound firing at the end of a hyperpolarizing step (also observed in our data, see Fig. 4A) and pinch-induced hyperpolarization. A weak association was also reported between initial bursting and nociceptive response profiles. Nevertheless, the associations reported were not definitive and their findings may have been influenced by species, methodological (ex vivo versus in vivo; dorsal horn versus SDH), and developmental (P10–14 versus P26–42) differences.
The question, however, remains to what extent different intrinsic membrane properties contribute to a SDH neurones' discharge profile during more physiologically relevant stimuli? Our data suggest that in vivo, the mechanisms underlying tonic firing and initial bursting in SDH neurones during current injection have little impact on their peripherally evoked responses (Fig. 6). This is perhaps not surprising as the current injected into the somata of a neurone will have greater access to somatic versus dendritic conductances (Rall, 1977; Powers & Binder, 2001). This point is important in the context of this study as rodent SDH neurones have extensive dendritic trees (Grudt & Perl, 2002), and thus would receive most of their synaptic inputs at locations electrotonically distant from their somata. In spinal motoneurones it has been shown that dendritic conductances generated by the activation of dendritic synapses can be a major source of depolarizing drive. It is not known if voltage-gated channels or persistent inward currents (Schwindt & Crill, 1977) are present on the dendrites of SDH neurones. These factors may explain why we failed to observe any equivalence between responses to a simple square step depolarization versus the complex mixture of transient depolarizations and hyperpolarizations evoked by a volley of primary afferent activation during pinch.
The complexity of in vivo activation of peripheral receptive fields means synaptic inputs arrive at a recorded SDH neurone via monosynaptic and polysynaptic pathways with differing latencies (Yoshimura & Jessell, 1989b). Thus, the variation of the incoming signals generated by our pinch stimuli, which presumably activates individual peripheral receptors at different times during the stimuli, may explain why there appears to be no strong association between current-evoked discharge patterns and peripherally evoked responses.
In summary, this study confirms that the current-evoked discharge patterns described in rat spinal cord in vitro also exist in mouse SDH neurones in vivo. Furthermore, while neurones expressing each discharge pattern participate in the processing of innocuous and noxious information in the SDH, their firing patterns do not necessarily predict a similar response during noxious stimulation. These findings support the notion that heterogeneity of many cellular properties in the SDH endows it with the capacity to carry out multiple functions (Light et al. 1979; Grudt & Perl, 2002). Studies are now required to analyse the factors that underlie neuronal heterogeneity in the SDH. In this regard, the laboratory mouse provides significant advantages for such experiments because the availability of transgenic animals permits the targeted study of neurones of known phenotype (Hantman et al. 2004) and altered synaptic connectivity (Graham et al. 2003).
Acknowledgments
The authors would like to thank Dr T. W. Margrie for assistance in establishing the in vivo patch-clamp recording techniques used in this study. Professor M. B. Calford is thanked for providing critical comments on the manuscript. We thank the National Health and Medical Research Council of Australia, The Hunter Medical Research Institute and the University of Newcastle Small Grants Scheme for support.
References
- Albuquerque C, Lee CJ, Jackson AC, MacDermott AB. Subpopulations of GABAergic and non-GABAergic rat dorsal horn neurons express Ca2+-permeable AMPA receptors. Eur J Neurosci. 1999;11:2758–2766. doi: 10.1046/j.1460-9568.1999.00691.x. 10.1046/j.1460-9568.1999.00691.x. [DOI] [PubMed] [Google Scholar]
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membrane Biol. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Abdelmoumene M, Hayashi H, Dubner R. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. J Comp Neurol. 1980;194:809–827. doi: 10.1002/cne.901940407. [DOI] [PubMed] [Google Scholar]
- Carstens E. Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. J Neurophysiol. 1997;77:2499–2514. doi: 10.1152/jn.1997.77.5.2499. [DOI] [PubMed] [Google Scholar]
- Christensen BN, Perl ER. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970;33:293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Faber ESL, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. J Neurophysiol. 2001;85:714–723. doi: 10.1152/jn.2001.85.2.714. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI. Brainstem control of spinal pain-transmission neurons. Annu Rev Physiol. 1978;40:217–248. doi: 10.1146/annurev.ph.40.030178.001245. 10.1146/annurev.ph.40.030178.001245. [DOI] [PubMed] [Google Scholar]
- Furue H, Narikawa K, Kumamoto E, Yoshimura M. Responsiveness of rat substantia gelatinosa neurones to mechanical but not thermal stimuli revealed by in vivo patch-clamp recording. J Physiol. 1999;521:529–535. doi: 10.1111/j.1469-7793.1999.00529.x. 10.1111/j.1469-7793.1999.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BA, Brichta AM, Callister RJ. An in vivo mouse spinal cord preparation for patch-clamp analysis of nociceptive processing. J Neurosci Meth. 2004;136:221–228. doi: 10.1016/j.jneumeth.2004.01.014. 10.1016/j.jneumeth.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Graham BA, Schofield PR, Sah P, Callister RJ. Altered inhibitory synaptic transmission in superficial dorsal horn neurones in spastic and oscillator mice. J Physiol. 2003;551:905–916. doi: 10.1113/jphysiol.2003.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZS, Zhang ET, Craig AD. Nociceptive and thermoreceptive lamina I neurons are anatomically distinct. Nat Neurosci. 1998;1:218–225. doi: 10.1038/665. 10.1038/665. [DOI] [PubMed] [Google Scholar]
- Hantman AW, van den Pol AN, Perl ER. Morphological and physiological features of a set of spinal substantia gelatinosa neurons defined by green fluorescent protein expression. J Neurosci. 2004;24:836–842. doi: 10.1523/JNEUROSCI.4221-03.2004. 10.1523/JNEUROSCI.4221-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analgesia. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland MA USA: Sinauer Associates; 1992. [Google Scholar]
- Hu H-J, Gereau RW, Gereau IV. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J Neurophysiol. 2003;90:1680–1688. doi: 10.1152/jn.00341.2003. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978;177:417–434. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- Laing I, Todd AJ, Heizmann CW, Schmidt HH. Subpopulations of GABAergic neurons in laminae I–III of rat spinal dorsal horn defined by coexistence with classical transmitters, peptides, nitric oxide synthase or parvalbumin. Neuroscience. 1994;61:123–132. doi: 10.1016/0306-4522(94)90065-5. 10.1016/0306-4522(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Larkman A, Mason A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. J Neurosci. 1990;10:1407–1414. doi: 10.1523/JNEUROSCI.10-05-01407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Perl ER. Re-examination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979;186:117–131. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- Light AR, Trevino DL, Perl ER. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979;186:151–171. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Light AR, Willcockson HH. Spinal laminae I–II neurons in rat recorded in vivo in whole cell, tight seal configuration: properties and opioid responses. J Neurophysiol. 1999;82:3316–3326. doi: 10.1152/jn.1999.82.6.3316. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia JA, King AE. Membrane properties of physiologically classified rat dorsal horn neurons in vitro: correlation with cutaneous sensory afferent input. Eur J Neurosci. 1994;6:998–1007. doi: 10.1111/j.1460-9568.1994.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margrie TW, Brecht M, Sakmann B. vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 2002;444:491–498. doi: 10.1007/s00424-002-0831-z. 10.1007/s00424-002-0831-z. [DOI] [PubMed] [Google Scholar]
- Melnick IV, Santos SFA, Safronov BV. Mechanism of spike frequency adaptation in substantia gelatinosa neurones of rat. J Physiol. 2004a;559:383–395. doi: 10.1113/jphysiol.2004.066415. 10.1113/jphysiol.2004.066415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick IV, Santos SFA, Szokol K, Szucs P, Safronov BV. Ionic basis of tonic firing in spinal substantia gelatinosa neurons of rat. J Neurophysiol. 2004b;91:646–655. doi: 10.1152/jn.00883.2003. 10.1152/jn.00883.2003. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. 10.1016/S0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Narikawa K, Furue H, Kumamoto E, Yoshimura M. vivo patch-clamp analysis of IPSCs evoked in rat substantia gelatinosa neurons by cutaneous mechanical stimulation. J Neurophysiol. 2000;84:2171–2174. doi: 10.1152/jn.2000.84.4.2171. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol. 2002;539:817–836. doi: 10.1113/jphysiol.2001.013437. 10.1113/jphysiol.2001.013437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Core conductor theory and cable properties of neurons. In: Kandel ER, editor. Handbook of Physiology, Cellular Biology of Neurons. Bethesda: American Physiological Society; 1977. pp. 39–77. [Google Scholar]
- Ren K, Ruda MA. A comparative study of the calcium-binding proteins calbindin-D28K, calretinin, calmodulin and parvalbumin in the rat spinal cord. Brain Res Rev. 1994;19:163–179. doi: 10.1016/0165-0173(94)90010-8. 10.1016/0165-0173(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Rethelyi M, Light AR, Perl ER. Synaptic ultrastructure of functionally and morphologically characterized neurons of the superficial spinal dorsal horn of cat. J Neurosci. 1989;9:1846–1863. doi: 10.1523/JNEUROSCI.09-06-01846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R, Ikeda H, Heinke B, Sandkuhler J. Distinctive membrane and discharge properties of rat spinal lamina I projection neurones in vitro. J Physiol. 2004;555:527–543. doi: 10.1113/jphysiol.2003.054049. 10.1113/jphysiol.2003.054049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R, Sandkuhler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol. 2002;541:231–244. doi: 10.1113/jphysiol.2002.017756. 10.1113/jphysiol.2002.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt P, Crill WE. A persistent negative resistance in cat lumbar motoneurons. Brain Res. 1977;120:173–178. doi: 10.1016/0006-8993(77)90510-8. 10.1016/0006-8993(77)90510-8. [DOI] [PubMed] [Google Scholar]
- Steedman WM, Molony V, Iggo A. Nociceptive neurones in the superficial dorsal horn of cat lumbar spinal cord and their primary afferent inputs. Exp Brain Res. 1985;58:171–182. doi: 10.1007/BF00238965. [DOI] [PubMed] [Google Scholar]
- Steedman WM, Zachary S. Characteristics of background and evoked discharges of multireceptive neurons in lumbar spinal cord of cat. J Neurophysiol. 1990;63:1–15. doi: 10.1152/jn.1990.63.1.1. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Headley MP. Membrane characteristics and synaptic responsiveness of superficial dorsal horn neurons in a slice preparation of adult rat spinal cord. Eur J Neurosci. 1989;1:479–488. doi: 10.1111/j.1460-9568.1989.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I–III of the mammalian spinal dorsal horn. Prog Neurobiol. 1993;41:609–645. doi: 10.1016/0301-0082(93)90045-t. 10.1016/0301-0082(93)90045-T. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. New York: Plenum Press; 1991. [Google Scholar]
- Woolf CJ, Fitzgerald M. The properties of neurones recorded in the superficial dorsal horn of the rat spinal cord. J Comp Neurol. 1983;221:313–328. doi: 10.1002/cne.902210307. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Membrane properties of rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989a;62:109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989b;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]