Abstract
To investigate the effect of exercise on protein kinase C (PKC) activity and localization in human skeletal muscle, eight healthy men performed cycle ergometer exercise for 40 min at 76 ± 1% the peak pulmonary O2 uptake (V̇O2peak), with muscle samples obtained at rest and after 5 and 40 min of exercise. PKC expression, phosphorylation and activities were examined by immunoblotting and in vitro kinase assays of fractionated and whole tissue preparations. In response to exercise, total PKC activity was slightly higher at 40 min in an enriched membrane fraction, and using a pSer-PKC-substrate motif antibody it was revealed that exercise increased the serine phosphorylation of a ∼50 kDa protein. There were no changes in conventional PKC (cPKC) or PKCΘ activities; however, atypical PKC (aPKC) activity was ∼70% higher at 5 and 40 min, and aPKC expression and Thr410/403 phosphorylation were unaltered by exercise. There were no effects of exercise on the abundance of PKCα, PKCδ, PKCΘ and aPKC within cytosolic or enriched membrane fractions of skeletal muscle. These data indicate that aPKC, but not cPKC or PKCΘ, are activated by exercise in contracting muscle suggesting a potential role for aPKC in the regulation of skeletal muscle function and metabolism during exercise in humans.
The protein kinase C (PKC) enzymes are multifunctional Ser/Thr protein kinases involved in many cellular responses (Dempsey et al. 2000). There are at least 10 isoforms of PKC grouped into three classes based upon structural differences and cofactor requirements for activation (for review see Newton, 1995). In human skeletal muscle, nearly all of the PKC isoforms are expressed (Itani et al. 2000), and there is evidence that some PKC isoforms have a role in insulin resistance (Cortright et al. 2000; Itani et al. 2000, 2002; Beeson et al. 2003) as well as insulin- (Bandyopadhyay et al. 2000; Braiman et al. 2001; Beeson et al. 2003) and exercise- (Chen et al. 2002; Beeson et al. 2003) mediated glucose transport.
PKC activation occurs during contractions of rat skeletal muscle, as demonstrated by the translocation of PKC activity to a membrane-enriched fraction from a cytosolic fraction (Richter et al. 1987; Cleland et al. 1989). More recently, it has been demonstrated that there is higher activity of the atypical isoforms of PKC in skeletal muscle during exercise in mice (Chen et al. 2002) and in humans (Beeson et al. 2003; Nielsen et al. 2003). Given that PKC may be involved in the exercise-induced activation of glucose transport (Cleland et al. 1990; Wojtaszewski et al. 1998) and hormone-sensitive lipase (Donsmark et al. 2003) in skeletal muscle, the aim of the present study was to examine the effect of exercise on PKC activity in human skeletal muscle.
Methods
Experimental protocol
Healthy, active but untrained, men (n= 8; 24 ± 5 years; body mass index, 23 ± 2 kg m−2; mean ±s.d.) were recruited, and written and verbal information about the purpose, nature and potential risks relating to the experimental procedures was given to the subjects before they provided consent to participate. The protocol was reviewed and approved by the Deakin University Human Research Ethics Committee. One to two weeks prior to testing, subjects completed an incremental exercise test to volitional exhaustion on an electromagnetically braked cycle ergometer (Lode, Groningen, the Netherlands) to determine their peak pulmonary O2 uptake (V̇O2peak), which averaged 51 ± 2 ml kg−1 min−1 (mean ±s.e.m.). Expired air was analysed by O2 and CO2 analysers (AEI Technologies, Pittsburgh, PA, USA) and expired volume by a turbine ventilometer (Flow transformer K 520, KL Engineering, Australia). The gas analysers were calibrated against gases of known composition prior to each test.
Subjects were asked to refrain from exercise as well as caffeine, nicotine and alcohol ingestion for at least 24 h prior to the study. Subjects were provided with a standardized meal (∼80% carbohydrate) for the evening prior to testing and reported to the laboratory in the morning after an overnight fast (plasma glucose, 4.9 ± 0.1 mm). Subjects rested for at least 20 min in the supine position before a muscle sample was obtained from the vastus lateralis by percutaneous needle biopsy under local anaesthesia and immediately frozen in liquid N2. Subjects exercised for 40 min at 76 ± 1% V̇O2peak with biopsies taken at 5 and 40 min of exercise and frozen within 20 s after the last contraction. Muscle samples were stored in liquid N2 until analysis.
Analytical techniques
All chemicals were purchased from Sigma-Aldrich (St Louis, MI, USA) unless otherwise stated. Muscle samples were homogenised as outlined previously (Rose & Hargreaves, 2003) in a homogenization buffer containing (mm): Tris-HCl 50 (pH 7.5), sucrose 250, EDTA 1, EGTA 1, phenylmethylsulfonyl fluoride 1, dithiothreitol 1, sodium flouride 5, sodium pyrophosphate 5 and benzamidine 1, with 10% glycerol, 5 μl ml−1 protease inhibitor cocktail and Nonidet P-40 (NP-40; 1%), and mixed well at 4°C. To examine protein localization and PKC activity, muscle samples (40–50 mg) were homogenised in 1 : 8 volumes of homogenization buffer not containing NP-40 until no visible particles remained. These samples were spun at 350 000 g for 30 min and the resultant supernatant comprised the cytosolic fraction. The pellet was resuspended and mechanically disrupted in homogenization buffer containing NP-40 and after 30 min on ice, the pellet fraction was subjected to centrifugation at 100 000 g for 60 min, and the supernatant (particulate fraction) removed. For all samples, aliquots were taken for total protein assay (Pierce BCA, Rockford, IL, USA) and the remaining extract was stored at −80°C until required for analysis.
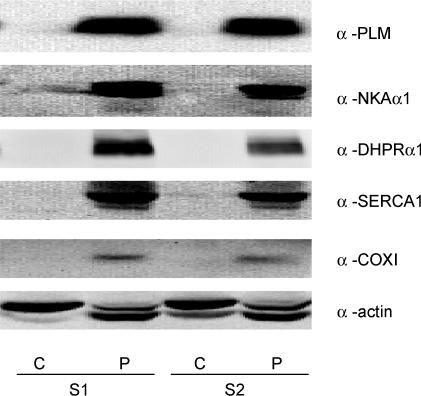
In preliminary experiments equal amounts of protein from both fractions were immunoprobed using antibodies for proteins specific to skeletal muscle subcellular organelles/structures. These included antibodies against sarcolemmal proteins: phospholemman (PLM; antibody provided by Professor Randall Moorman, University of Virginia, VA, USA) and Na+,K+-ATPase-α1 (NKAα1; B. Fambrough, Johns Hopkins University, MD, USA); t-tubule protein: dihydropyridine receptor-α1 (DHPRα1; Santa Cruz Biotechnology, CA, USA); sarcoplasmic reticulum protein: Ca2+-ATPase-1 (SERCA1; Santa Cruz Biotechnology); mitochondrial protein: cytochrome C-oxidase subunit I (COXI; Molecular Probes, OR, USA); and general marker: actin. The particulate fraction represents a preparation in which membranous structures are enriched, and the cytosolic fraction is devoid of membranes (Fig. 1).
Figure 1. Immunoblots of marker proteins for subcellular membrane structures in human skeletal muscle after fractionation.
Muscle samples (n = 2; S1, S2) were fractionated as described in the Methods, and equal amounts of protein (35 μg) were subjected to immunoblotting procedures and probed with antibodies specific to skeletal muscle subcellular/membrane structures. Blots were deliberately overexposed to detect the slightest immunoreactivity. Prefix α, anti; S, sample; P, particulate; C, cytosolic.
PKC activity was measured in skeletal muscle extracts (10–20 μg protein, 5 μl) in kinase assay buffer containing (mm): Hepes 10 (pH 7.2), MgCl2 5, EGTA 1, sodium pyrophosphate 0.1, ATP 0.1 (0.2 Ci mmol−1 5′[γ32P]ATP; Amersham Biosciences, Uppsala, Sweden); with 40 μm epidermal growth factor (EGF) receptor substrate peptide (H2N-KRTLRR-OH), 1.2 mm CaCl2, 10 μm phorbol-12-myristate-13-acetate (PMA), and 20 μg ml−1 phosphatidylserine in a final reaction volume of 25 μl. The EGF receptor peptide was chosen as it has the minimal requirements for conventional, novel and atypical PKC substrates including basic residues at −2 and +3, as well as a hydrophobic residue at the +1 position (Nishikawa et al. 1997). The reaction procedure was conducted as outlined previously (Rose & Hargreaves, 2003), except that the reaction time was 5 min.
To directly measure isoform-specific PKC activity, PKC isoforms and isotypes were immunoprecipiated (IP) from muscle extracts (500 μg) incubated with anti-PKCΘ or -PKCζ/ι antibodies (2 μg; Santa Cruz Biotechnology) in a final volume of 600 μl with gentle mixing for 2 h at 4°C. Following this 40 μl of 50% (v:v) of protein-A-sepharose (Amersham Biosciences) was added and further incubated with mixing for 2 h at 4°C. To immunopurify conventional PKC (cPKC), extracts (500 μg) were incubated with agarose-conjugated PKC-MC5 antibodies (20 μl; Santa Cruz Biotechnology) in a final volume of 600 μl with gentle mixing for 2 h at 4°C. The beads were washed and resuspended (40 μl) in buffer containing (mm): Tris 10 (pH 7.2), sodium pyrophosphate 1, EGTA 1. cPKC and PKCΘ activities were assayed for 10 min as described above. PKCζ/ι activity was assayed as described by Bandyopadhyay et al. (1997b) with minor modifications. Assays were performed in kinase assay buffer at 30°C with 40 μm[S25]-PKC(19–31) substrate peptide (H2N-RFARKGSLRQKNV-OH), and 40 μg ml−1 phosphatidylserine in a final reaction volume of 25 μl. Assays were conducted as previously described (Rose & Hargreaves, 2003). The assay time and PKC concentration were within a linear range for lysate and IP assays (data not shown). Preliminary experiments demonstrated that exercise did not alter the efficiency of immunoprecipitation of any of the PKC isoforms investigated (data not shown).
PKC abundance and phosphorylation were determined in equal amounts of protein in muscle extracts and IP samples using immunoblot analysis as previously described (Rose & Hargreaves, 2003) with anti-PKCα (H7), anti-PKCβII (C-18), anti-PKCδ (C-20), anti-PKCɛ (C-15), anti-PKCΘ (C-19), anti-PKCζ/ι (C-20) (Santa Cruz Biotechnology), anti-pT410/403-PKCζ/ι and anti-pS-PKC substrate motif (R/K-X-SPO4-Hyd-R/K; Cell Signalling Technology, Beverly, MA, USA) antibodies.
To provide a control sample for assays, L6 skeletal myotubes were treated with PMA (0.1 μm; PKC activity) or insulin (1 μm; PKCζ/ι activity). All media solutions were purchased from Invitrogen (Carlsbad, CA, USA). L6 myotubes were prepared as previously described (Rose & Hargreaves, 2003). For PMA treatment, cells (n= 3 dishes per treatment) were treated with PMA (0.1 μm) and control cells were treated with vehicle (0.1% ethanol) for 30 min. The cells were lysed in homogenization buffer without NP-40 and collected after 10 min on ice. PKC activity in the cytosolic and particulate fractions was determined as described above. The percentage of PKC activity in the particulate fraction relative to total PKC activity was 46 ± 5% in vehicle and 64 ± 3% in PMA-treated myotubes (n= 3, P < 0.05). Furthermore, a significant translocation of PKCα from the cytosolic fraction to the particulate fraction was shown by immunoblot analysis (P < 0.01; data not shown) as observed in other studies (Braiman et al. 1999). In addition, PMA induced increases (0.7- to 1.3-fold, P < 0.05) in the density of four out of five bands detected when particulate extracts were analysed by immunoblotting with a pSer-PKC substrate motif antibody (data not shown). These data indicate that the PKC assay and immunoblot analyses of fractionated cell lysates are sensitive measures of PKC activity and localization.
Myotubes (n= 3 dishes per treatment) were treated with or without insulin (1 μm) for 5 min and cells were lysed with homogenization buffer containing NP-40 and collected after 10 min on ice. IP PKCζ/ι activity was determined as described above. An increase in aPKC activity (control, 12.3 ± 1.3 pmol mg −1min−1; insulin, 28.3 ± 1.3 pmol mg−1min−1; P < 0.001) was measured with insulin treatment when compared with control cells, as observed in other studies (Bandyopadhyay et al. 1997a).
Calculations and statistics
Kinase activity was calculated as described by Goueli et al. (2001). The molecular weights of immunoreactive proteins were estimated by fitting their relative mobility in a curve generated by plotting the relative mobility against the log of the molecular weights of protein standards (Precision Plus Protein Standards, Bio-Rad, CA, USA). Arbitrary units for protein (total or phosphorylated) abundance were expressed as a ratio of sample band intensity relative to an internal control band intensity. The relative PKC activity and abundance between fractions are expressed as a ratio of particulate units and total (particulate + cytosolic) units. Statistical testing was done with unpaired t tests (MS Excel) or one-way ANOVA with repeated measures with post hoc (Student–Newman–Keuls) testing performed when differences were significant as appropriate (GraphPad Prism, v.2.01). Data are expressed as mean ±s.e.m. and differences were considered to be significant when P < 0.05.
Results
PKC isoform expression in human skeletal muscle
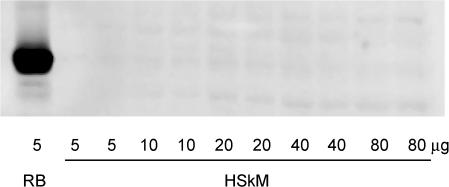
In the present study, PKCα, PKCδ, PKCɛ (data not shown), PKCΘ and PKCζ/ι were expressed in human skeletal muscle. These findings are similar to observations made by Itani et al. (2000). Despite being able to obtain a strong signal in rat brain, PKCβII was not detected in human skeletal muscle (Fig. 2), which is in contrast to the findings of others (Itani et al. 2002).
Figure 2. Protein kinase CβII is not expressed in human skeletal muscle.
Rat brain (RB) and human skeletal muscle (HSkM) proteins were subjected to immunoblotting procedures and probed using a polyclonal anti-PKCβII antibody.
Effect of exercise on PKC activity
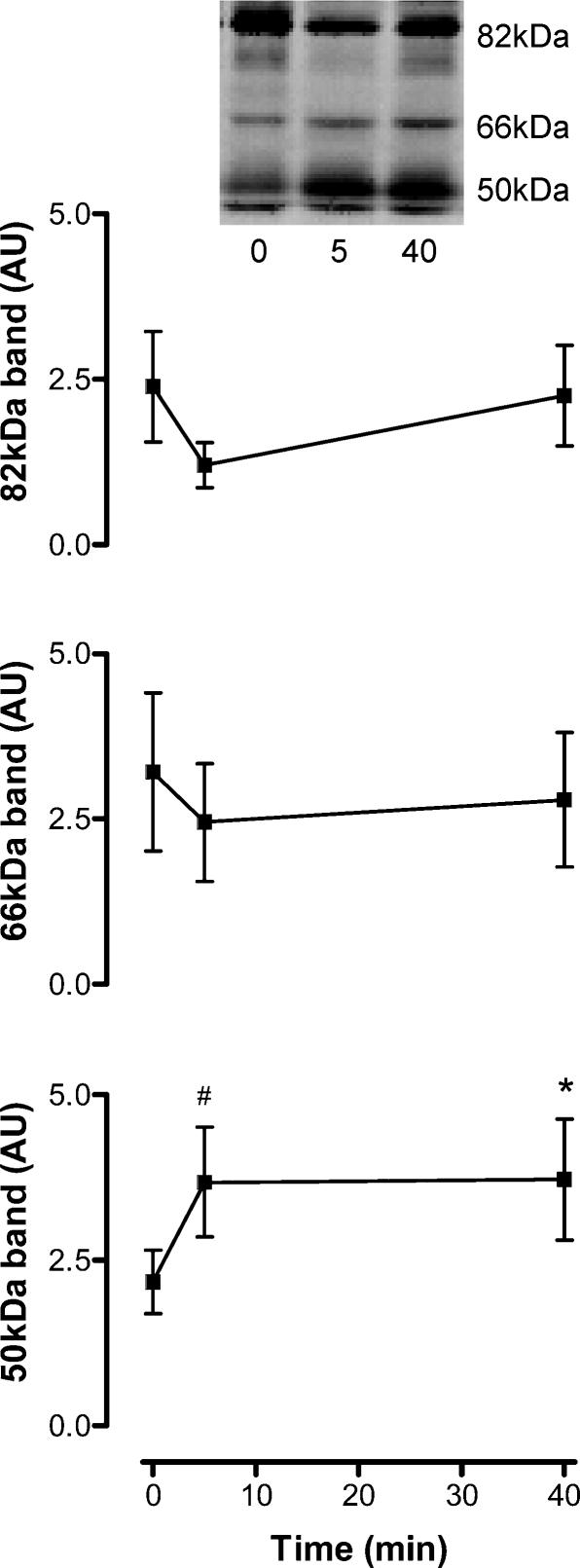
With 40 min ergometer exercise a slight increase in total PKC activity was observed in the particulate fraction only (P < 0.05;Fig. 3). No change was detected with 5 min of exercise in cytosolic or particulate PKC (P > 0.1; Fig. 3). As there were no changes in the ratio of PKC activity between fractions (Fig. 3), this suggests that there was no translocation of total PKC activity, and thus total PKC abundance, between the fractions. Rather, there may be an increase in the intrinsic activity of PKCs in membrane structures. To obtain an index of PKC activity, whole tissue extracts from basal and exercise samples were subjected to immunoblotting with a phospho-PKC substrates motif antibody. There were three consistently strongly immunoreactive bands detected with this antibody, when reacted with proteins from human skeletal muscle (Fig. 4). While there were no changes in the serine phosphorylation (pS) of the 82- and 66-kDa proteins, there was a 73 ± 18% and 75 ± 25% increase in pS of a 50-kDa protein at 5 and 40 min, respectively (P < 0.05; Fig. 4), indicating that there may be an increase in PKC activity towards this protein substrate/s.
Figure 3. Protein kinase C (PKC) activity of skeletal muscle extracts at rest and after exercise.
Cytosolic and particulate skeletal muscle extracts were assayed in vitro for PKC activity. Data are mean ±s.e.m., n = 8. Significantly different from 0: *P < 0.05.
Figure 4. Exercise increases the serine phosphorylation of a 50-kDa protein, but not 66- or 82-kDa proteins, in contracting human skeletal muscle.
Solubilised protein from crude skeletal muscle extracts was subjected to immunoblotting procedures and probed using a polyclonal phospho-serine PKC substrate motif antibody. Data are mean ±s.e.m., n = 8. Significantly different from 0: *P < 0.05, #P < 0.01. Insert shows a representative blot from one subject.
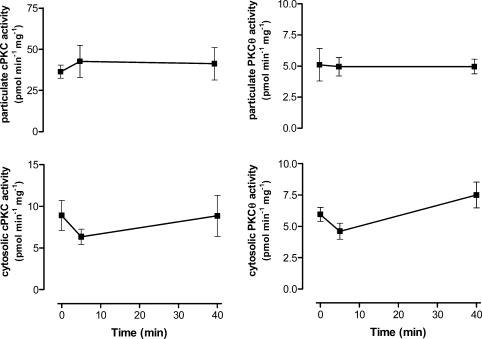
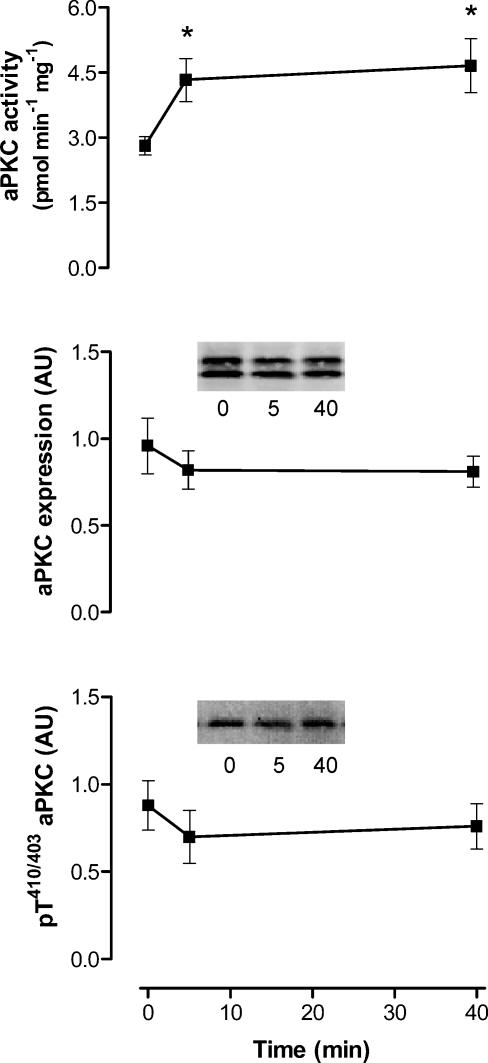
To examine whether select PKC isoforms were activated by exercise in contracting skeletal muscle, PKCs were immunopurified from tissue lysates and assayed for activity. There were no differences in cPKC or PKCΘ activity in particulate or cytosolic fractions when basal and exercise samples were compared at both time points (Fig. 5). In contrast, there were 65 ± 26% and 80 ± 38% increases in aPKC activity immunopurified from whole tissue lysates at 5 and 40 min, respectively (Fig. 6). These increases in aPKC activity occurred despite no changes in aPKC protein expression or phosphorylation at threonine residue 410/403 (Fig. 6) measured from the same tissue extracts.
Figure 5. Conventional protein kinase C (cPKC) and PKCΘ activity of skeletal muscle fractions at rest and after exercise.
Cytosolic and particulate extracts were prepared and cPKC (left panel) and PKCΘ (right) were immunoprecipitated from skeletal muscle extracts (500 μg) and assayed for PKC activity in vitro. Data are mean ±s.e.m., n = 6.
Figure 6. Atypical protein kinase C (aPKC or PKCζ/ι) activity, expression and Thr410/403 phosphorylation of contracting skeletal muscle before and during exercise in humans.
aPKC was immunoprecipitated from skeletal muscle tissue extracts (500 μg) and assayed in vitro (top panel). Equal amounts of solubilised protein from crude skeletal muscle tissue extracts were also subjected to immunoblotting procedures and probed using a polyclonal PKCζ/ι (middle panel) and phospho-Thr410/403-PKCζ/ι (bottom panel) antibodies. Data are mean ±s.e.m., n = 8. Significantly different from 0: *P < 0.05. Inserts show representative blots from one subject.
Effect of exercise on PKC isoform localization
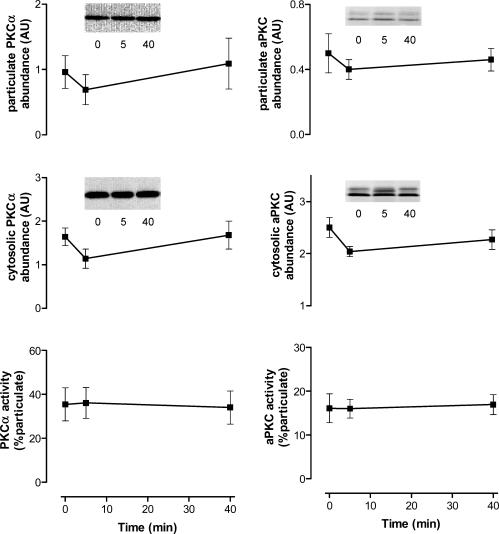
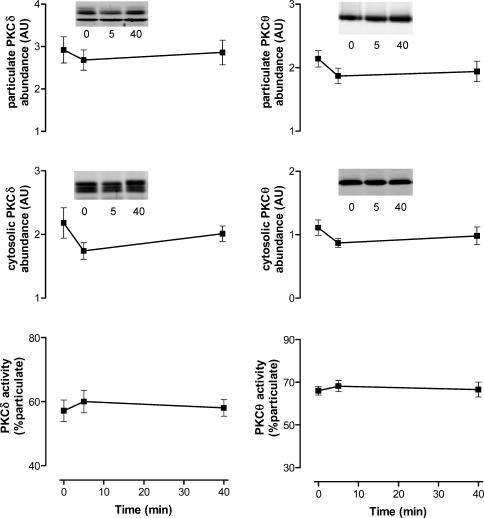
The relative PKC isoform abundance between skeletal muscle membrane and cytosol fractions from basal and exercise samples were determined by immunoblotting using isoform-specific antibodies. There were no differences in the abundance of PKCα, aPKC, PKCδ and PKCΘ, within cytosolic or particulate fractions when comparing basal and exercise samples (Figs 7 and 8). Nor were there any differences in the fraction ratio (% in the particulate extract) induced by exercise. These data suggest that exercise does not induce translocation from the cytosol to membranes of these PKC isoforms in human skeletal muscle.
Figure 7. Effect of exercise on isoform-specific protein kinase C (PKC) localization in contracting skeletal muscle during exercise in humans.
Equal amounts of protein from skeletal muscle extracts were subjected to immunoblotting procedures and probed using PKCα (left panel) and PKCζ/ι (right panel) antibodies. Data are mean ±s.e.m., n = 7. Inserts show representative blots from one subject.
Figure 8. Effect of exercise on isoform-specific protein kinase C (PKC) localization in contracting skeletal muscle during exercise in humans.
Equal amounts of protein from skeletal muscle extracts were subjected to immunoblotting procedures and probed using PKCδ (left panel) and PKCΘ (right panel) antibodies. Data are mean ±s.e.m., n = 7. Inserts show representative blots from one subject.
Discussion
The major finding of this study was that atypical PKC (aPKC) activity increased early (5 min) after the commencement of exercise in contracting skeletal muscle, and remained elevated after 40 min of exercise. This result is similar to findings of others that show higher activity of aPKC in contracting skeletal muscle during exercise in humans (Beeson et al. 2003; Nielsen et al. 2003). Furthermore, studies in rodents have also demonstrated a similar time-course for increased aPKC activity in skeletal muscle of exercised animals (Chen et al. 2002).
The mechanism for increased aPKC in contracting skeletal muscle is not clear. It has been demonstrated that phosphatidic acid (PA; 5–50 μm) increases aPKC (immunopurified from rodent skeletal muscle) activity in vitro (Chen et al. 2002). It is important to note that when L6 myotubes were incubated with 2,4-dinitrophenol (DNP), which activated aPKC in these cells via phospholipase D production of PA, incubation with a phospholipase D inhibitor reduced immunopurified aPKC activity (Chen et al. 2002), suggesting that the effects of PA are conserved even after the process of immunopurification. Given that PA concentrations are higher in hindlimb muscles of rodents after nerve electrical stimulation in situ (Cleland et al. 1989), this is a likely mechanism for higher aPKC activity in contracting muscle. The mechanism by which insulin activates aPKC is dependent, at least in part, on phosphorylation of a threonine residue within the activation loop by 3-phosphoinositide-dependent protein kinase-1 (PDK1; Standaert et al. 2001). However, there is evidence that activation of aPKC via PLD does not require PDK1-mediated phosphorylation of aPKC (Sajan et al. 2002). Furthermore, while the effect of exercise on muscle PDK1 activity has not been investigated, exercise does not activate insulin receptor substrate 1 (IRC1)-associated PI3-kinase (Wojtaszewski et al. 1997), and therefore presumably PDK1, in contracting muscle. In the present study, no change in phosphorylation of these threonine residues of aPKC was observed when comparing exercise and basal samples. Furthermore, there was no translocation of aPKC from the cytosol to membranes with exercise. These findings are consistent with those reported by others (Richter et al. 2004). However, the present findings on aPKC phosphorylation and localization are in contrast to a study by Perrini et al. (2004), where skeletal muscle aPKC phosphorylation and abundance in membranes were higher after 30 and 60 min of exercise, respectively. While the reason for the discrepancies between the present findings and those by Perrini et al. (2004) are not readily apparent, they may be related to sample preparation (phosphorylation) and time-dependent effects (localization).
It has been suggested that aPKC may be involved in the exercise-induced increase in glucose transport (Chen et al. 2002). However, there is little evidence linking aPKC and glucose transport in muscle during exercise. Studies using the microbial agent calphostin-C as an inhibitor of PKC (Wojtaszewski et al. 1998; Ihlemann et al. 1999) or down-regulation of 1,2-diacylglycerol (DAG)-sensitive PKC enzymes by chronic phorbol ester treatment (Cleland et al. 1990) have demonstrated a role for PKC in glucose transport during exercise. However, neither of these treatments is likely to affect aPKC (Kobayashi et al. 1989; Bandyopadhyay et al. 1997a), and therefore results of these studies should not be extended to suggest a role for aPKC in contraction-mediated glucose transport. Clearly, further studies are warranted to investigate the potential role of aPKC in exercise-mediated glucose transport in skeletal muscle given that there is strong evidence linking aPKC to insulin-mediated glucose transport and glucose transporter isoform 4 (GLUT4) translocation (Farese, 2002).
In the present study there was a slight increase in general PKC activity within membranes and increased serine phosphorylation of a 50 kDa protein with exercise. While not specific, these data indicate an increase in PKC activity with exercise. Given that the majority of PKC within skeletal muscle consists of conventional and novel isoforms (Itani et al. 2000), their activity/localization was investigated. Unlike in previous studies in rodents (Richter et al. 1987; Cleland et al. 1989), there was no evidence of a translocation of PKC activity or protein from a cytosolic fraction to a particulate/membrane fraction. In particular, there were no alterations in cPKC or PKCΘ activities within or between these fractions with exercise. Neither were there any changes in the abundance of PKC isoforms within or between fractions with exercise. The lack of effect of exercise on cPKC activity and PKCα between cytosolic and membrane fractions was unexpected as increases in Ca2+ concentration by DNP in L6 myotubes induced increases in cPKC activity and higher cPKC protein abundance in membranes (Khayat et al. 1998). Furthermore, exposure of amphibian skeletal muscle to agents that raise intracellular free Ca2+ resulted in translocation of cPKC activity towards membranes (Sun & Zhu, 1998). It is unlikely that the different mode of Ca2+ stimulation (oscillatory vs. constant) is an explanation as Ca2+ oscillations are also known to stimulate PKCα translocation (Oancea & Meyer, 1998).
No change was detected in the activity and localization of the other DAG-sensitive PKCs (PKCΘ and PKCδ) in skeletal muscle with exercise. Similarly, acute exercise does not affect skeletal muscle PKCδ activity of the Israeli sand rat (Heled et al. 2003) or humans (AJ Rose & M Hargreaves, unpublished observations); nor translocation of PKCδ and PKCɛ in human skeletal muscle (Perrini et al. 2004). While not all of the PKC isoforms have been examined, the present results suggest that exercise does not alter the activity of conventional or novel PKCs in contracting human skeletal muscle.
DAG is an important cofactor required for complete activation and translocation of DAG-sensitive PKCs (Newton, 1995). While it has been demonstrated that DAG concentration increases in electrically stimulated rodent hindlimb muscle (Cleland et al. 1989) other studies have shown no effect of contraction on skeletal muscle DAG concentration (Turinsky et al. 1990). Whether a lack of DAG accumulation accounts for the unresponsiveness of skeletal muscle cPKC and nPKC to exercise in the present study requires further investigation.
In summary, aPKC, but not cPKC or PKCΘ, is activated by exercise in contracting human skeletal muscle and thus may play a role in regulating skeletal muscle function and metabolism during exercise in humans.
Acknowledgments
The medical and technical assistance of Dr Andrew Garnham, Ms Janelle Mollica and Mr Sean McGee is greatly appreciated. The authors are grateful to Professor Randall Moorman for the provision of the PLM antibodies. Prof. Bruce Kemp is an Australian Research Council Federation Fellow supported by grants from the Australian Research Council, National Health and Medical Research Council and National Heart Foundation.
References
- Bandyopadhyay G, Kanoh Y, Sajan MP, Standaert ML, Farese RV. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-λ on insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 2000;141:4120–4127. doi: 10.1210/endo.141.11.7766. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Standaert ML, Galloway L, Moscat J, Farese RV. Evidence for involvement of protein kinase C (PKC)-ζ and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997a;138:4721–4731. doi: 10.1210/endo.138.11.5473. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Standaert ML, Zhao LYuB, Avignon A, Galloway L, Karnam P, Moscat J, Farese RV. Activation of protein kinase C (α, β, ζ) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-ζ in glucose transport. J Biol Chem. 1997b;272:2551–2558. doi: 10.1074/jbc.272.4.2551. 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson SR. Activation of protein kinase C ζ induces serine phosphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle. Mol Cell Biol. 2001;21:7852–7861. doi: 10.1128/MCB.21.22.7852-7861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman L, Sheffi-Friedman L, Bak A, Tennenbaum T, Sampson SR. Tyrosine phosphorylation of specific protein kinase C isoenzymes participates in insulin stimulation of glucose transport in primary cultures of rat skeletal muscle. Diabetes. 1999;48:1922–1929. doi: 10.2337/diabetes.48.10.1922. [DOI] [PubMed] [Google Scholar]
- Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV, Jr, Farese RV. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and aminoimidazole-4-carboxamide-1-beta-D-riboside (AICAR)-stimulated glucose transport. J Biol Chem. 2002;277:23554–23562. doi: 10.1074/jbc.M201152200. [DOI] [PubMed] [Google Scholar]
- Cleland PJ, Abel KC, Rattigan S, Clark MG. Long-term treatment of isolated rat soleus muscle with phorbol ester leads to loss of contraction-induced glucose transport. Biochem J. 1990;267:659–663. doi: 10.1042/bj2670659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland PJ, Appleby GJ, Rattigan S, Clark MG. Exercise-induced translocation of protein kinase C and production of diacylglycerol and phosphatidic acid in rat skeletal muscle in vivo. Relationship to changes in glucose transport. J Biol Chem. 1989;264:17704–17711. [PubMed] [Google Scholar]
- Cortright RN, Azevedo JL, Jr, Zhou Q, Sinha M, Pories WJ, Itani SI, Dohm GL. Protein kinase C modulates insulin action in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E553–E562. doi: 10.1152/ajpendo.2000.278.3.E553. [DOI] [PubMed] [Google Scholar]
- Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–L438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- Donsmark M, Langfort J, Holm C, Ploug T, Galbo H. Contractions activate hormone-sensitive lipase in rat muscle by protein kinase C and mitogen-activated protein kinase. J Physiol. 2003;550:845–854. doi: 10.1113/jphysiol.2003.042333. 10.1113/jphysiol.2003.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV. Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. Am J Physiol Endocrinol Metab. 2002;283:E1–E11. doi: 10.1152/ajpendo.00045.2002. [DOI] [PubMed] [Google Scholar]
- Goueli SA, Hsiao K, Goueli BS. Assaying activity of individual protein kinases in crude tissue or cellular extracts. Methods Enzymol. 2001;333:16–27. doi: 10.1016/s0076-6879(01)33040-9. [DOI] [PubMed] [Google Scholar]
- Heled Y, Shapiro Y, Shani Y, Moran DS, Langzam L, Braiman L, Sampson SR, Meyerovitch J. Physical exercise enhances protein kinase C δ activity and insulin receptor tyrosine phosphorylation in diabetes-prone psammomys obesus. Metabolism. 2003;52:1028–1033. doi: 10.1016/s0026-0495(03)00154-9. 10.1016/S0026-0495(03)00154-9. [DOI] [PubMed] [Google Scholar]
- Ihlemann J, Galbo H, Ploug T. Calphostin C is an inhibitor of contraction, but not insulin-stimulated glucose transport, in skeletal muscle. Acta Physiol Scand. 1999;167:69–75. doi: 10.1046/j.1365-201x.1999.00591.x. 10.1046/j.1365-201x.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- Itani SI, Zhou Q, Pories WJ, MacDonald KG, Dohm GL. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes. 2000;49:1353–1358. doi: 10.2337/diabetes.49.8.1353. [DOI] [PubMed] [Google Scholar]
- Khayat ZA, Tsakiridis T, Ueyama A, Somwar R, Ebina Y, Klip A. Rapid stimulation of glucose transport by mitochondrial uncoupling depends in part on cytosolic Ca2+ and cPKC. Am J Physiol. 1998;275:C1487–C1497. doi: 10.1152/ajpcell.1998.275.6.C1487. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. 10.1016/0006-291X(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Frosig C, Sajan MP, Miura A, Standaert ML, Graham DA, Wojtaszewski JF, Farese RV, Richter EA. Increased atypical PKC activity in endurance-trained human skeletal muscle. Biochem Biophys Res Commun. 2003;312:1147–1153. doi: 10.1016/j.bbrc.2003.11.041. 10.1016/j.bbrc.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95:307–318. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- Perrini S, Henriksson J, Zierath JR, Widegren U. Exercise-induced protein kinase C isoform-specific activation in human skeletal muscle. Diabetes. 2004;53:21–24. doi: 10.2337/diabetes.53.1.21. [DOI] [PubMed] [Google Scholar]
- Richter EA, Cleland PJ, Rattigan S, Clark MG. Contraction-associated translocation of protein kinase C in rat skeletal muscle. FEBS Lett. 1987;217:232–236. doi: 10.1016/0014-5793(87)80669-5. 10.1016/0014-5793(87)80669-5. [DOI] [PubMed] [Google Scholar]
- Richter EA, Vistisen B, Maarbjerg SJ, Sajan M, Farese RV, Kiens B. Differential effect of bicycling exercise intensity on activity and phosphorylation of atypical protein kinase C and extracellular signal-regulated protein kinase in skeletal muscle. J Physiol. 2004;560:911–920. doi: 10.1113/jphysiol.2004.071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Hargreaves M. Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol. 2003;553:303–309. doi: 10.1113/jphysiol.2003.054171. 10.1113/jphysiol.2003.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajan MP, Bandyopadhyay G, Kanoh Y, Standaert ML, Quon MJ, Reed BC, Dikic I, Farese RV. Sorbitol activates atypical protein kinase C and GLUT4 glucose transporter translocation/glucose transport through proline-rich tyrosine kinase-2, the extracellular signal-regulated kinase pathway and phospholipase D. Biochem J. 2002;362:665–674. doi: 10.1042/0264-6021:3620665. 10.1042/0264-6021:3620665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert ML, Bandyopadhyay G, Kanoh Y, Sajan MP, Farese RV. Insulin and PIP3 activate PKC-ζ by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and autophosphorylation (T560) sites. Biochemistry. 2001;40:249–255. doi: 10.1021/bi0018234. [DOI] [PubMed] [Google Scholar]
- Sun JH, Zhu PH. Effects of high potassium and caffeine exposure on activities of Ca2+-dependent and Ca2+-independent protein kinase C in frog skeletal muscle. Cell Signal. 1998;10:569–574. doi: 10.1016/s0898-6568(97)00193-9. 10.1016/S0898-6568(97)00193-9. [DOI] [PubMed] [Google Scholar]
- Turinsky J, Bayly BP, O'Sullivan DM. 1,2-Diacylglycerol and ceramide levels in rat skeletal muscle and liver in vivo. Studies with insulin, exercise, muscle denervation, and vasopressin. J Biol Chem. 1990;265:7933–7938. [PubMed] [Google Scholar]
- Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes. 1997;46:1775–1781. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Laustsen JL, Derave W, Richter EA. Hypoxia and contractions do not utilize the same signaling mechanism in stimulating skeletal muscle glucose transport. Biochim Biophys Acta. 1998;1380:396–404. doi: 10.1016/s0304-4165(98)00011-7. [DOI] [PubMed] [Google Scholar]