Abstract
We have examined the effect of regulated overexpression of AGL15, a member of the MADS domain family of regulatory factors, on reproductive tissues. Using molecular and physiological markers, we show that constitutive overexpression of AGL15 in Arabidopsis leads to delay and down-regulation of senescence programs in perianth organs and developing fruits and alters the process of seed desiccation. Through genetic crosses, we show that the rate of water loss in the maturing seeds is dictated by the genetic composition and physiological state of the maternal tissue, rather than the embryo. To define the developmental time and/or place when senescence programs are most affected by elevated AGL15 levels, we expressed AGL15 under the control of various promoters. Expression during senescence or in abscission zone cells did not produce delays in floral organ senescence or abscission. Using a glucocorticoid-inducible expression system, we show that an increase in AGL15 levels around the time of flower opening is necessary to delay senescence and increase floral organ longevity.
Members of the MADS domain family of transcriptional regulators participate in a variety of developmental processes in Arabidopsis, including regulation of floral organ identity (Yanofsky et al., 1990; Goto and Meyerowitz, 1994; Weigel and Meyerowitz, 1994; Krizek and Meyerowitz, 1996), inflorescence meristem identity (Irish and Sussex, 1990; Weigel and Meyerowitz, 1994; Kempin et al., 1995; Mandel and Yanofsky, 1995; Weigel and Nilsson, 1995), the transition to flowering (Michaels and Amasino, 1999, 2001; Borner et al., 2000; Lee et al., 2000; Sheldon et al., 2000; Smach et al., 2000), fruit wall differentiation (Gu et al., 1998), and lateral root differentiation (Zhang and Forde, 1998). All members of this family contain a highly conserved motif of 55 to 60 amino acids, known as the MADS domain, that is essential for the DNA-binding activity of these factors (Theissen et al., 2000). Many of the family members identified in plants also contain a less-conserved domain called the K domain, which may facilitate protein-protein interactions. The C domain, which constitutes approximately the last one-third of each protein, has a more variable sequence and is likely to contribute to differences in function.
Members of the MADS domain family have been shown recently to be important for developmental processes that involve cell separation. For example, SHP1 (SHATTERPROOF1) and SHP2 (SHATTERPROOF2) act redundantly to specify cell identity in the dehiscence zone in Arabidopsis fruits and to promote the lignification of cells adjacent to the dehiscence zone (Liljegren et al., 2000). The MADS domain protein JOINTLESS is necessary to specify pedicel abscission zones in tomato. Abscission zones are absent in the jointless mutant (Mao et al., 2000). The Arabidopsis seedstick (agl11) mutant shows defects in the formation of the funiculus, resulting in a persistent connection between the fruit wall and developing seeds (Pinyopich et al., 2001). The accumulated information on shp1/shp2, jointless, and stk mutants indicate that these genes are involved in regulating differentiation of abscission or dehiscence zone cells.
In a previous study, we showed that constitutive overexpression of the MADS domain protein AGL15 (AGAMOUS like 15) results in delays in abscission of sepals and petals in Arabidopsis (Fernandez et al., 2000). In wild type, AGL15 is expressed in developing embryos, in the vegetative shoot apex, at the base of leaf petioles, and in developing floral organs (Fernandez et al., 2000). Plants overexpressing AGL15 show no structural defects in abscission zones, unlike shp1/shp2, jointless, and stk mutants. If a strong stimulus in the form of exogenous ethylene is added, abscission can occur (Fernandez et al., 2000). In the absence of this stimulus, the plants show a significant delay in floral organ abscission, which is coupled to a delay in floral organ senescence. This combination of features indicates that AGL15's effect on cell separation processes is fundamentally different from that of other MADS domain proteins known to affect abscission or dehiscence.
AGL15 preferentially accumulates in young tissues, and our working hypothesis is that endogenous AGL15 operates to maintain or enhance an immature or juvenile or non-senescent state in those tissues. Constitutive overexpression of AGL15 might result in repression of some aspect of programmed senescence in aging floral tissues. Abscission of an organ is often coupled to its senescence, although petals appear to be shed in a turgid state in Arabidopsis (Bleecker and Patterson, 1997). The longevity of perianth organs is dramatically increased when AGL15 is overexpressed (Fernandez et al., 2000). Therefore, plants overexpressing AGL15 present a unique opportunity to explore the control of tissue longevity, the relationship between programmed senescence and abscission, and interactions between senescing organs and adjacent tissues.
In the first part of the work reported here, we examined the impact of AGL15 overexpression on senescence programs in floral organs and fruits and explored the relationship between fruit maturation and the process of seed desiccation. We then generated transgenic plants where AGL15 was expressed only in abscission zones or only during senescence to examine the contribution of cell- or program-specific expression to the effect on senescence. Finally, we set up a system where overexpression of AGL15 could be induced by the addition of glucocorticoid. Using this system, we show that AGL15 overexpression must be induced before the onset of senescence and abscission to produce subsequent delays in these processes.
RESULTS
The Effect of Overexpression of AGL15 on Perianth Senescence
Arabidopsis plants that constitutively express AGL15 exhibit increased perianth organ longevity (Fernandez et al., 2000), possibly due to some combination of effects on abscission and senescence. To examine the effect of AGL15 overexpression on senescence molecular programs, we used a β-glucuronidase (GUS) reporter construct to monitor the activity of the SAG12 (Senescence Associated Gene 12) promoter in the sepals and petals. Previous studies showed that expression of SAG12 is correlated with the onset of visible senescence symptoms in both vegetative (Lohman et al., 1994) and reproductive (Gan, 1995) tissues. Plants that were homozygous for an SAG12: GUS reporter construct were crossed to plants that were hemizygous for a 35S:gAGL15 transgene, which drives constitutive expression of AGL15 (Fernandez et al., 2000). Progeny that carried the AGL15 transgene, which represented 50% of the population, could be distinguished from their siblings due to differences in leaf shape and petiole length (Fernandez et al., 2000). In progeny with a wild-type phenotype, SAG12 promoter activity could be detected in stamens, petals, and sepals at 2 d after pollination (DAP) (not shown) and intense GUS staining was visible by 3 DAP (Fig. 1A). By 4 DAP, the floral organs had generally abscised, and no promoter activity was associated with the remaining tissues of the gynoecium (Fig. 1B). In progeny showing the AGL15 overexpression phenotype, activation of the SAG12 promoter was delayed in all floral organs (Fig. 1C). Promoter activity could be detected in the stamens around 4 DAP (not shown). In the sepals and petals however, no sign of strong promoter activity was visible at 4 DAP (Fig. 1D) or over a 4-week interval after pollination. Faint staining could sometimes be detected at the base of the still-attached perianth organs in older flowers (not shown). We conclude that overexpression of AGL15 affects senescence programs in sepals and petals, leading to down-regulation of the SAG12 gene.
Figure 1.
GUS activity in flowers of transgenic plants carrying SAG12:GUS, a senescence reporter construct. A and B, GUS activity at 3 (A) and 4 (B) DAP in flowers of plants that do not overexpress AGL15. Perianth organs abscise around 4 DAP in these plants. C and D, Little or no GUS activity can be detected at 3 (C) or 4 (D) DAP in the flowers of plants that carry the 35S:gAGL15 transgene and constitutively overexpress AGL15. Perianth organs are retained for long periods in these plants.
The Effect of Overexpression of AGL15 on Fruit Wall Senescence
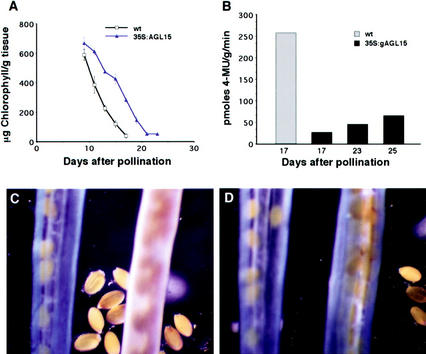
Overexpression of AGL15 also affects maturation processes in developing fruits. In Arabidopsis, the gynoecium, i.e. fourth whorl floral organs, develops into a silique that undergoes senescence when the seeds become mature. The siliques of plants constitutively overexpressing AGL15 remain green for an extended period of time. To compare the rates of silique maturation in these plants with wild type, we measured changes in chlorophyll content over time. Wild-type siliques contained approximately 1,200 μg chlorophyll g−1 of silique tissue at 9 DAP. The chlorophyll content declined rapidly over the next few days, and was reduced by one-half by 11 to 13 DAP, as shown in Figure 2A. By 17 DAP, there was approximately 80 μg chlorophyll g−1 of silique tissue. At this stage, the siliques were brownish yellow in color and started to dehisce.
Figure 2.
Analysis of fruit maturation in plants that constitutively overexpress AGL15. A, Chlorophyll content of maturing siliques of wild-type (wt) plants versus plants that constitutively overexpress AGL15 (35S:AGL15). Each data point represents the mean of three experimental replicas. Bars indicate the sd of the mean. B through D, GUS activity in the silique tissues of plants carrying the senescence reporter SAG12:GUS. B, Quantitative assays. Siliques were collected at 17 DAP from plants that do not overexpress AGL15 (gray bar) and at 17, 23, or 25 DAP from plants that overexpress AGL15 (black bars). C and D, Histochemical staining. The siliques on the left in each image were collected at 17 DAP from plants that do not overexpress AGL15. The siliques on the right in each image were collected at 17 (C) or 23 (D) DAP from plants that overexpress AGL15. SAG12 promoter activity was down-regulated and delayed in plants that overexpress AGL15.
Siliques from plants that constitutively overexpress AGL15 had a higher chlorophyll content at all stages. As Figure 2A shows, the maturation process had three distinct phases, marked by different rates of chlorophyll loss. From 9 to 13 DAP, chlorophyll content declined fairly rapidly. From 13 to 15 DAP, chlorophyll content declined very slowly, and then from 15 to 23 DAP, the chlorophyll content declined rapidly again. At 17 DAP, when the siliques of wild-type plants are fully mature, the siliques of plants carrying the AGL15 transgene still contain approximately one-half of the amount of chlorophyll present at 9 DAP. These siliques finally become yellow or brownish yellow around 21 to 23 DAP, when they contained approximately 100 μg chlorophyll g−1 of silique tissue. We conclude that the siliques remain green longer on plants constitutively overexpressing AGL15, partly because they have a higher chlorophyll content initially and partly because chlorophyll loss is slower, particularly during the middle phase of fruit maturation.
The effect of AGL15 overexpression on activation of the SAG12 promoter in maturing siliques was also examined. The same population of plants used for the floral organ analysis (35S:gAGL15 segregating in the presence of an SAG12: GUS reporter) was used in this study. In progeny that lacked the 35S:gAGL15 transgene, GUS activity could be detected in tissues of the fruit wall by 16 to 17 DAP (Fig. 2C). This is quite late in the senescence process and coincides with the final stages of chlorophyll loss in the siliques. In siblings that carried the AGL15 transgene, SAG12 promoter activity could not be detected in the fruits until 21 to 23 DAP (Fig. 2D), which corresponds to the final stages of chlorophyll loss in these siliques. Thus, based on the chlorophyll loss profiles and SAG12 promoter activity, senescence of fruit tissues appears to be delayed by almost 5 to 6 d when AGL15 is constitutively overexpressed.
To determine whether SAG12 promoter activity is down-regulated as well as delayed in the silique tissues, GUS activity was quantified by measuring emitted fluorescence after incubations with 4-methyl umbelliferyl glucuronide. At 17 DAP, the GUS activity in silique tissues of control plants was approximately 10-fold higher than the GUS activity in silique tissues of plants with the AGL15 transgene (Fig. 2B). The GUS activity increased with age in the silique tissues of the plants constitutively expressing AGL15, but never reached the levels observed in the control plants. The maximal activity, measured at 25 DAP, was approximately 25% of that in control plants at 17 DAP. After 25 DAP, the siliques tended to shatter during harvest, and no further measurements of GUS activity were made.
The Effect of Overexpression of AGL15 on Seed Desiccation
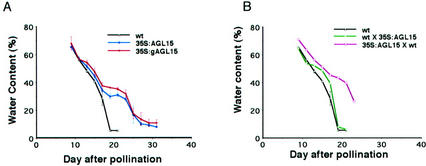
What are the consequences of increased silique tissue longevity? One possible effect, because the silique tissues have higher water potentials for a longer period, is an alteration in the decline in water content that marks the later stages of seed development. We compared the water content of seeds collected from self-pollinated wild-type plants and seeds from self-pollinated transgenic plants that contain a single copy of either 35S:AGL15 or 35S:gAGL15 in the hemizygous condition. As shown in Figure 3A, the initial water content and decline in water content from 9 to 15 DAP were very similar in seeds of wild-type plants (black line) and seeds of plants carrying AGL15 transgenes (blue and red lines). After 15 DAP, the water content of seeds of wild-type plants continued to drop rapidly and reached approximately 5% at 19 DAP. For seeds developing on plants constitutively overexpressing AGL15, the decline in seed water content slowed around 15 DAP. The seed water content was maintained at approximately 30% to 40% from 17 to 23 DAP and then declined rapidly after 23 DAP to reach 6% to 8% at 31 DAP. We conclude that constitutive overexpression of AGL15 leads to alterations in water relations in the developing fruit.
Figure 3.
Analysis of seed desiccation in plants that overexpress AGL15. Seeds were excised from maturing siliques at various times (DAP) and seed water content was calculated from measurements of the fresh and dry weight of each sample. A, The water content of seeds of self-pollinated wild-type plants (black line) declines more rapidly than the water content of seeds of self-pollinated transgenic plants that overexpress AGL15. The transgenic plants carried one copy of either of two transgenes (35S:AGL15, blue line; or 35S:gAGL15, red line) in the hemizygous condition. B, The water content of seeds of self-pollinated wild-type plants (black line) was compared with the water content of seeds derived from reciprocal crosses between wild-type plants and plants that overexpress AGL15. The green line shows the water content of seeds derived from crosses where wild-type plants were pollinated with transgenic pollen. The pink line shows the water content of seeds derived from crosses where plants that overexpress AGL15 were pollinated with wild-type pollen.
Does expression of the AGL15 transgene in the embryo contribute to this effect? We addressed this question genetically in two ways. First, we pollinated wild-type plants with pollen from transgenic plants carrying a single copy of the 35S:AGL15 transgene in the homozygous condition. The resulting seeds contained embryos with one copy of the AGL15 transgene and wild-type seed coats, and were surrounded by wild-type silique tissues. As shown in Figure 3B, the decline in water content in the seeds resulting from such crosses (green line) was similar to that in the seeds from self-pollinated wild-type plants (black line). Thus, presence of the AGL15 transgene in embryos is not sufficient to cause a delay in seed desiccation.
Second, we pollinated transgenic plants that were hemizygous for the AGL15 transgene with wild-type pollen. This increased the frequency of wild-type embryos in developing fruits from 25% (selfed 35S:AGL15 plant, blue line, Fig. 3A) to 50% (pink line, Fig. 3B). However, the rate and pattern of water content decline of the seeds, shown by the pink line in Figure 3B, did not change. On the basis of these results, we conclude that the genotype of the embryo contributes little to the effect of AGL15 overexpression on seed desiccation.
The Effect of Abscission Zone-Specific Expression of AGL15
In our previous study, we showed that constitutive overexpression of AGL15 leads to delays in perianth organ abscission (Fernandez et al., 2000). Does overexpression of AGL15 in cells outside of the abscission zone contribute to this effect? To address this question, we generated transgenic plants in which overexpression of AGL15 was restricted to the abscission zone cells.
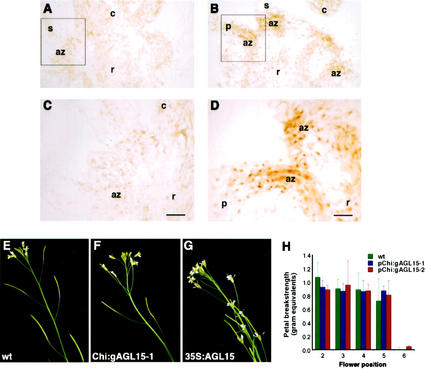
To overexpress AGL15 in the abscission zone, we transformed plants with a construct designated Chi: gAGL15, which consisted of the promoter of a bean (Phaseolus vulgaris) chitinase gene (Broglie et al., 1989) fused to sequence encoding AGL15 with 14 additional amino acids at the N terminus. Plants that constitutively overexpress this form of AGL15 show the same phenotype (not shown) as plants that constitutively overexpress unmodified AGL15. The bean chitinase gene promoter shows strong activity in the floral organ abscission zones of Arabidopsis floral organs immediately after bud anthesis (Bleecker and Patterson, 1997). In plants carrying the Chi:gAGL15 construct, AGL15 is overexpressed in the abscission zone cells and targeted to the nuclei, as confirmed with AGL15-specific antibodies (Fig. 4, A–D). A total of 28 independent transgenic lines were isolated, and a subset of these was followed for two generations. In all cases, floral organs abscised at the same time as in wild type (Fig. 4, E and F) and earlier than in plants where AGL15 was constitutively overexpressed (Fig. 4G). To determine whether more subtle changes in tissue integrity had occurred, the petal break strength was measured for successively older flowers along the length of the inflorescence. The petal break strength profiles for individuals from two independent lines, Chi:gAGL15-1 and Chi:gAGL15-2 (each line contains a single Chi:gAGL15 locus in the homozygous condition), were indistinguishable from wild type (Fig. 4H). We conclude that overexpression of AGL15 in the abscission zone is not sufficient to delay abscission of perianth organs.
Figure 4.
Analysis of perianth organ senescence and abscission in transgenic plants that overexpress AGL15 in abscission zones. A through D, Immunolocalization of AGL15 in perianth organ abscission zones. No AGL15 accumulation can be detected in cells in the abscission zones in wild-type plants (A and C). AGL15 accumulates at elevated levels in the nuclei of cells in the abscission zones in transgenic plants carrying the Chi:gAGL15 construct (B and D). The location of the abscission zones shown at higher magnification in C and D are indicated by the boxes in A and B. az, Abscission zone; c, carpel; p, petal; r, receptacle; s, sepal. Bars = 20 μm. E through G, Inflorescences of wild-type (E) and transgenic plants carrying either the Chi:gAGL15 transgene (F) or 35S:AGL15 transgene (G). Overexpression of AGL15 in the abscission zones does not produce the visible delay in perianth organ abscission and senescence associated with constitutive overexpression. H, Analysis of the force needed to remove petals in progressively older flowers (in different positions relative to the youngest open flower) in wild-type plants and two lines of transgenic plants carrying the Chi:gAGL15 transgene. Each data point represents the mean of eight to 12 measurements. Bars indicate the sd.
The Effect of Senescence-Induced Expression of AGL15
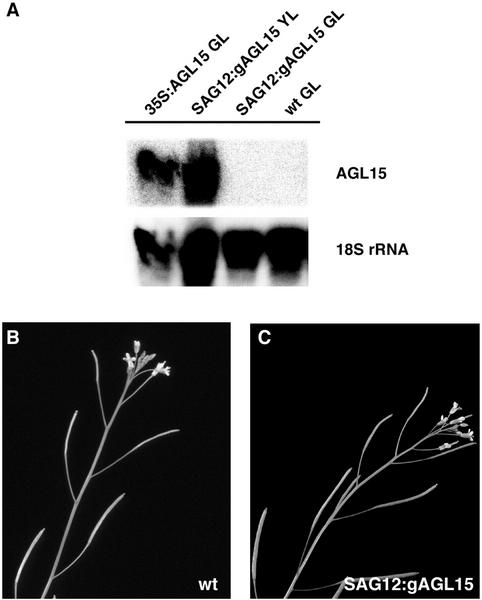
We have shown that overexpression of AGL15 leads to delays in floral organ senescence and fruit maturation. Does overexpression of AGL15 have the same effect if it occurs after the start of tissue senescence? To address this question, transgenic plants in which AGL15 is expressed under the control of the SAG12 promoter were generated. Previous studies showed that placing expression of bacterial cytokinin synthesis genes under the control of this promoter leads to delayed leaf senescence (Gan and Amasino, 1995). We generated a total of 84 independent lines carrying the SAG12:gAGL15 construct, which consists of a fusion between the SAG12 promoter and AGL15 coding sequence. A subset of these lines was followed through two generations. AGL15 mRNA accumulated in senescing tissues in these plants, as confirmed by RNA gel-blot analysis of leaf samples (Fig. 5A). As Figure 5A shows, the relative level of mRNA accumulation was comparable with that achieved in younger tissues using the 35S cauliflower mosaic virus (CaMV) promoter. AGL15 mRNA cannot be detected in the younger green leaves of the SAG12:gAGL15 transgenic plants (Fig. 5A), indicating that accumulation occurs in an age-dependent manner.
Figure 5.
Analysis of perianth organ senescence and abscission in wild-type plants and transgenic plants that overexpress AGL15 during senescence. A, Gel-blot analysis of total RNA samples (5 μg lane−1) isolated from leaves of wild-type or transgenic plants and hybridized with probes specific for AGL15 or 18S rRNA sequences. AGL15 transcripts accumulate in a senescence-dependent manner in transgenic plants carrying SAG12:gAGL15 constructs. AGL15 transcripts can be detected in non-senescing, green leaves (GL) of plants that constitutively overexpress AGL15 (35S:AGL15), but cannot be detected in green leaves of either wild-type plants (wt) or transgenic plants that carry SAG12:AGL15 constructs (SAG12:AGL15). AGL15 transcripts accumulate at higher levels in the yellow, senescing leaves (YL) of plants carrying SAG12:gAGL15 constructs. Hybridization with 18S rRNA probes indicates equal loading of samples in each lane. B and C. The inflorescences of wild-type plants (B) and plants that carry SAG12:gAGL15 transgenes (C) are indistinguishable in terms of floral organ senescence and abscission.
Plants that overexpress AGL15 in senescing tissues resembled wild-type plants in most regards. Leaf senescence was not affected and floral organ senescence and abscission were not visibly altered (Fig. 5, B and C). Fruit maturation appeared to be slightly delayed, however. The siliques turned yellow 2 to 4 d later (relative to the time of pollination) in the transgenic plants than in wild-type plants (not shown). Based on these results, we conclude that, although it is possible to see some effects in fruits, senescence-induced overexpression of AGL15 is not equivalent to constitutive overexpression in terms of enhancing organ longevity.
The Effect of Elevation of AGL15 at Different Stages
We used a glucocorticoid-inducible expression system to more clearly define when overexpression of AGL15 exerts its effects. A line carrying a transcriptional regulator activated by the application of the glucocorticoid dexamethasone (35S:GVG), obtained from Dr. Nam-Hai Chua (Rockefeller University, New York), was crossed to a line carrying the regulatory “target” (UAS:AGL15). Plants carrying both constructs were selected in the F3 generation. Overexpression of AGL15 was induced at various times by applying dexamethasone, either by bottom watering (for systemic induction) or by spraying (for local induction).
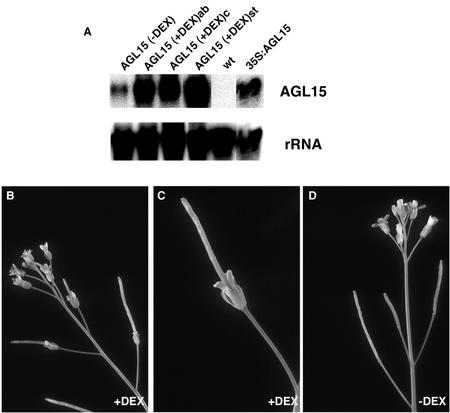
The effectiveness of the induction system was established through gel-blot analysis (Fig. 6A) of total RNA samples isolated from induced and uninduced plants. AGL15 transcripts accumulated at higher levels in plants carrying the induction system than in wild-type plants, even in the absence of dexamethasone induction. However, the perianth organs of these plants senesced and abscised normally (not shown), indicating that the constitutive expression due to the leakiness of the system is below the threshold needed for overexpression effects. In plants treated with dexamethasone, AGL15 transcripts accumulate at higher levels than in plants carrying 35S:AGL15 constructs (Fig. 6A).
Figure 6.
Effects of glucocorticoid-induced overexpression of AGL15. A, Gel-blot analysis of total RNA samples (5 μg lane−1) isolated from plants in which AGL15 overexpression can be induced by treatment with dexamethasone (+DEX) or controls, hybridized with probes specific for AGL15 or 18S rRNA sequences. Plants carrying the induction system were either not treated (AGL15 [−DEX]) or were treated after bolting (AGL15 [+DEX] ab) or continuously (AGL15 [+DEX] c) with dexamethasone. AGL15 mRNAs were particularly abundant in lines that showed strong phenotypic changes after treatment ([+DEX] st). AGL15 transcripts were less abundant in plants that constitutively overexpress AGL15 (35S:AGL15) and were undetectable in wild-type plants (wt). Hybridization with 18S rRNA probes indicates equal loading of samples in each lane. B and C, Inflorescence (B) and individual flower (C) after induction of AGL15 overexpression by spraying with dexamethasone. D, Inflorescence of a similar plant sprayed with control solution lacking dexamethasone.
To determine when AGL15 overexpression is most critical for extended organ longevity, AGL15 was induced systemically by introducing dexamethasone into the solution used for watering at various times after the start of germination. We found that systemic induction during the reproductive phase was sufficient to produce the overexpression effects in floral organs. The plants did not need to be exposed to dexamethasone during the vegetative phase or during the transition to flowering. Spraying the inflorescence with dexamethasone was just as effective as systemic induction. All of the unopened floral buds sprayed with dexamethasone gave rise to flowers showing delayed abscission and senescence of perianth organs (Fig. 6, B and C). Buds sprayed with solutions lacking dexamethasone showed no effects on either senescence or abscission (Fig. 6D). Open flowers sprayed with dexamethasone also showed no effects, even though AGL15 transcripts accumulated in these flowers (not shown). We conclude that stage 13 (flower opening) marks a period of transition with regard to the sensitivity of floral tissues and senescence programs to increased AGL15 levels. Induction of AGL15 overexpression can occur as late as stage 11 or 12 but must occur before stage 13 (bud anthesis) to increase floral organ longevity.
DISCUSSION
Overexpression of AGL15 Affects Multiple Developmental Processes
Overexpression of AGL15 results in an overall delay in abscission and senescence in reproductive tissues. In addition to the previously reported effects on perianth organ longevity and abscission (Fernandez et al., 2000), we have shown that processes such as fruit maturation, silique dehiscence, and seed desiccation are also affected. Each of these processes involves tissues that undergo senescence or maturation (perianth organs, fruit walls, and seed coats) and cell layers that express programs leading to cell separation (floral organ abscission zones, silique dehiscence zones, and funiculi). In this work, we have used plants overexpressing AGL15 to explore issues related to the regulation of tissue longevity, interactions between organs and separation zones, and the relationship between programmed senescence and abscission.
Overexpression of AGL15 leads to both down-regulation and delays in the molecular programs associated with senescence in floral organs and maturing siliques. In wild-type plants, the senescence reporter SAG12:GUS is activated as early as 3 DAP in the floral organs and during the final stages of chlorophyll loss in silique tissues. When AGL15 is overexpressed, SAG12 promoter activation is delayed by up to 3 d in floral organs. SAG12 promoter activation and chlorophyll loss are both delayed in the fruits of plants overexpressing AGL15, and GUS activity is significantly lower at the final stages of fruit maturation than in wild-type plants. We conclude that AGL15 serves as a repressor of senescence when it is constitutively overexpressed, and is likely to act at a point upstream of both the final stages of chlorophyll loss and SAG12 promoter activation.
Overexpression of AGL15 results in an extended desiccation phase during seed development. During the maturation phase, seed fresh and dry weight increase rapidly due to synthesis and deposition of storage reserves. During the desiccation phase, both the fresh weight and water content drop as the seeds lose water. The desiccation phase is longer in the seeds produced on plants overexpressing AGL15 largely because of changes in the profile of water loss. Although seed water content usually declines at a steady rate throughout the desiccation phase in wild type, seed water content is maintained around the 30% to 40% level for several days (from 17–23 DAP) in plants overexpressing AGL15. Although the decline in water content later resumes, seed maturation is delayed. The seeds remain green several days longer and will not germinate readily if they are excised during this period (not shown), indicating that they are not yet fully mature. Although an extension of the maturation period might be expected to enhance reserve accumulation, this does not appear to be the case here. The levels of storage proteins in mature seeds obtained from plants that overexpress AGL15 are not elevated relative to the levels in mature wild-type seeds (K.W. Nichols and D.E. Fernandez, unpublished data).
Effects on Fruit Maturation and Seed Desiccation Can Be Attributed to AGL15 Overexpression in Maternal Tissues
Can any part of the effects on fruit maturation and seed desiccation be attributed to signals coming from embryos that carry the AGL15 transgene? We found that introducing the AGL15 transgene into embryos is not sufficient to produce the overexpression effects in wild-type females. In addition, when crosses are performed with transgenic females such that the dosage and/or the frequency of embryos carrying the AGL15 transgene is reduced, no alteration is seen in the pattern of water content decline or rate of fruit senescence relative to self-pollinated transgenic plants. Hence, we conclude that transgene expression in the maternal tissues is necessary, and may be sufficient, to direct the changes seen with AGL15 overexpression.
Overexpression of AGL15 affects several features that may contribute to changes in water balance and the delay in seed desiccation. First, delays in fruit wall senescence may result in the maintenance of higher water potentials inside the silique. Seed desiccation is associated with senescence of the pod or capsule in legumes (Greenwood and Bewley, 1982; Murray and Noodén, 1986), and it has been suggested that water loss in seeds is primarily due to transpiration of water from the surfaces of surrounding tissues (Kermode, 1990). Both chlorophyll loss and the decline in seed water content transiently slow for a period of several days in plants overexpressing AGL15. When chlorophyll loss resumes, the decline in seed water content does as well. This correlation suggests the two parameters are linked and would be consistent with a cause-and-effect relationship. Alternatively, fruit wall senescence and seed desiccation may be controlled by regulatory elements that are similarly impacted by AGL15 overexpression.
Another possible contributing factor in this developmental context would be an effect on seed detachment. The final stages of seed desiccation occur after the vascular supply to the seed is severed through funiculus “abscission” (Kermode, 1990). In plants overexpressing AGL15, the seeds remain green and are connected to the fruit tissues for a longer period. This suggests that the onset of processes that lead to cell separation are delayed in the funiculi, although we have not determined, by direct measurements, when flow through the vascular system ceases. Just as in floral organ abscission zones, AGL15 protein can be immunolocalized at early stages of development in the nuclei of the small, densely cytoplasmic cells that form the detachment zone at the distal end of the funiculus (S. Perry and D. Fernandez, unpublished data). Whether AGL15 plays any direct role in maintenance or specification of these cells has not yet been determined.
The Effects of Abscission- and Senescence-Induced Expression of AGL15
Abscission is frequently associated with senescence of the organ distal to the point of cell separation, and both processes can be initiated in response to the same developmental and environmental factors (Taylor and Whitelaw, 2001). In Arabidopsis, the floral organs of Arabidopsis are shed in a turgid state (Bleecker and Patterson, 1997); however, based on our senescence marker analysis, the senescence process has already initiated in these organs when abscission occurs. Overexpression of AGL15 affects both abscission and senescence. Because extensive cross talk within and between cells is likely in this situation, secondary effects are difficult to distinguish from primary effects. By supplying “extra” AGL15 in particular cells or at specific times, we sought to determine the relationship between effects on abscission, effects on senescence, and other possible primary effects.
If AGL15's primary effect is on genes controlling abscission, we might expect that overexpression of AGL15 in abscission zone cells would be sufficient to achieve the floral overexpression phenotype. We found, however, that when AGL15 is supplied ectopically only in the abscission zone, the perianth organs on such plants both senesce and abscise as in wild type. We cannot rule out the possibility that abscission zone-specific expression at an earlier stage, before the chitinase promoter is active, would have the desired effect; however, our results suggest that the effect on abscission is a secondary effect.
If AGL15 regulates senescence genes directly, we might expect to see aspects of the overexpression phenotype if AGL15 is supplied at elevated levels during the course of senescence. However, plants that express AGL15 in a senescence-activated manner show no change in the timing of perianth organ senescence and abscission relative to wild type. In fact, in subsequent experiments with glucocorticoid-inducible expression, we found that AGL15 overexpression that occurs anytime after the flowers open is ineffective. Fruit senescence, on the other hand, is somewhat delayed relative to wild type when AGL15 is expressed in a senescence-activated manner, although not to the extent it is in plants that constitutively overexpress AGL15. Thus, although AGL15 may affect some aspects of late senescence programs, it cannot act to halt or reverse the senescence process in either flowers or fruits if it is expressed after senescence is initiated.
Overexpression of AGL15 before the Onset of Senescence and Abscission Produces Pronounced Effects
To investigate the contribution the timing of AGL15 overexpression makes to the total picture of overexpression effects, we set up a system that allowed us to inducibly express AGL15 at high levels. Although the system showed some “leakiness” (i.e. AGL15 is expressed at low levels even in the absence of the glucocorticoid inducer), phenotypic changes were associated exclusively with induction and high-level expression. Our studies showed that induction before the transition to flowering or in very young floral buds is not necessary. Induction immediately (1 d) before the flower opens is sufficient to achieve the full overexpression effect on perianth longevity and retention. This corresponds to the period when AGL15 promoter activity normally ceases in wild-type flowers (Fernandez et al., 2000). We suggest that the effects may be due to ectopic expression that adds “extra” AGL15 to a shrinking pool of functional molecules in normal target cells. Induction after the flowers open is ineffective, even though AGL15 transcripts still accumulate in these older flowers after induction (not shown). We conclude that the direct targets of AGL15 action are likely to be in tissues that have largely completed the process of differentiation but are in a presenescent state. Further molecular studies to determine these targets and the primary effects of AGL15 overexpression should focus on this stage and tissues.
In summary, overexpression of AGL15 affects the progression of developmental programs, particularly those that involve both senescence and abscission/cell separation, in flowers, fruits, and seeds. Transgenic plants can often provide new insights into important physiological relationships. In this case, we see a particularly striking demonstration of the strong connection between senescence/maturation of the maternal tissues and the process of seed desiccation. Based on our analysis of the effects of regulated AGL15 overexpression, we conclude that programs operating in cells in the presenescent state or just initiating senescence are most sensitive to changes in AGL15 levels. The phenotypic changes likely reflect changes in gene regulation that occur at a stage before obvious signs of abscission or senescence appear.
MATERIALS AND METHODS
Plant Material
Arabidopsis ecotype Wassilewskija (Ws) was used in all experiments unless otherwise specified. Plants were grown to maturity in a growth chamber (Enconair Ecological Chamber, Winnipeg, Canada) with a 16-h-light (approximately 125 μEm−2 s−1) and 8-h-dark regime, 22°C, and 70% to 80% relative humidity, as described previously (Fernandez et al., 2000).
Chlorophyll Content Measurement
Newly opened flowers were hand pollinated and siliques were collected at various DAP. Two siliques from each of two individual plants were collected, weighed, quickly frozen with liquid nitrogen, and stored at −80°C until analysis. Each sample was homogenized in 1.2 mL of 80% (v/v) acetone using a pestle in an Eppendorf tube (Eppendorf Scientific, Westbury, NY). After removing cellular debris by centrifugation, the supernatant containing chlorophyll was removed and diluted 1:1 [v/v] with 100% (v/v) acetone. The absorption at 663 and 646 nm was measured for each sample using a spectrophotometer. The chlorophyll content was calculated according to Hanfrey et al. (1996), as follows:
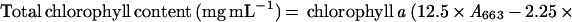 |
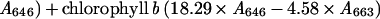 |
Senescence Reporter Gene Analysis
Seeds of a line (Landsberg erecta ecotype) carrying a single insertion of the SAG12:GUS reporter gene construct (Gan and Amasino, 1995), which consists of a fusion between the SAG12 promoter and the coding sequence of GUS, were obtained from Susheng Gan (University of Kentucky, Lexington). Plants carrying SAG12:GUS in the homozygous condition were crossed with plants carrying a single insertion of 35S:gAGL15 (CaMV 35S promoter fused to the coding sequence of AGL15 and the first three introns; Fernandez et al., 2000) in the hemizygous condition. GUS activities were assayed in the progeny. All of the plants carried the SAG12:GUS construct and one-half of the population showed the phenotypic changes associated with AGL15 overexpression (Fernandez et al., 2000).
GUS Activity Assays
For histochemical assays, tissues were incubated with 0.5 mg mL−1 5-bromo-4-chloro-3-indolyl glucuronide as previously described (Fernandez et al., 2000). The tissues were then incubated in 70% (v/v) ethanol until the chlorophyll was completely extracted.
For fluorometric assays, siliques at different developmental stages were harvested, weighed, frozen in liquid nitrogen, and stored at −80°C. For each data point, two to three siliques were homogenized with 200 μL of extraction buffer (50 mm NaH2PO4, [pH 7.0], 10 mm EDTA, 0.1% [v/v] Triton X-100, 0.1% [w/v] sodium lauryl sarcosine, and 10 mm β-mercaptoethanol) in an Eppendorf tube using a pestle. Cellular debris was removed by centrifugation. Five microliters of plant extract was added to 1 mL of extraction buffer containing 1 mm 4-methyl umbelliferyl glucuronide, and the reaction was incubated at 37°C. At 0, 5, 15, 30, and 60 min, 200 μL was removed, and the fluorometric reaction was carried out as previously described (Jefferson et al., 1987). The emission at 455 nm was measured after excitation at 365 nm in a fluorometer (Hoefer TKO100, San Francisco).
Water Content Analysis
For each data point, a total of eight to 12 staged siliques were collected from eight to 12 individual plants. To determine fresh weight, the seeds were removed from the siliques using forceps, pooled, and weighed. To determine dry weight, each seed sample was then baked at 80°C for 5 h and weighed again. The water content was calculated as follows:
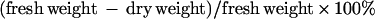 |
Generation of Transgenic Arabidopsis
To express AGL15 in the floral organ abscission zones, AGL15 was placed under the control of regulatory sequences from a bean (Phaseolus vulgaris) abscission chitinase gene (Chi:gAGL15). A 1.4-kb fragment, representing the chitinase upstream regulatory region plus 24 bp of the coding sequence, was excised from pBD3226 (Broglie et al., 1989) using HindIII and BamHI restriction enzymes and cloned into a pPZP221 transformation vector (Hajdukiewicz et al., 1994), which includes the nopaline synthase terminator (NOS). The AGL15 DNA sequence was amplified from 35S:gAGL15 (Fernandez et al., 2000) using PCR and was inserted downstream of the chitinase sequence. This created an in-frame fusion that added 14 amino acids to the N terminus of the AGL15 gene product. Plants that constitutively express this version of AGL15 show the AGL15 overexpression phenotype (S.-C. Fang, unpublished data), indicating that the extra amino acids do not interfere with AGL15 function. The AGL15 coding region was also sequenced to confirm that no mutations had been introduced during PCR amplification. The Chi:gAGL15 construct was introduced into Arabidopsis (Ws ecotype) by vacuum infiltration with Agrobacterium tumefaciens strain GV3101, as described by Bechtold et al. (1993). T1 plants were selected on germination medium (GM) medium supplemented with 100 μg L−1 gentamycin. Plants carrying Chi:gAGL15 insertions in the homozygous condition were identified in the T2 generation and used for immunohistochemistry studies.
To express AGL15 during senescence, AGL15 was placed under the control of regulatory sequences from the SAG12 gene (Gan and Amasino, 1995). A 2.2-kb fragment of the SAG12 upstream regulatory sequence was excised from pSG506 (Gan and Amasino, 1995) using EcoRV and NcoI restriction enzymes. After treatment with S1 nuclease, the SAG12 sequence was inserted immediately upstream of the AGL15 coding sequence in a PZP212 transformation vector (Hajdukiewicz et al., 1994) to create SAG12:gAGL15, which includes a NOS terminator. The SAG12:gAGL15 construct was introduced into Arabidopsis (Ws ecotype) using A. tumefaciens strain GV3101 and the floral dip method, as described by Clough and Bent (1998). T1 plants were selected on GM medium supplemented with 50 μg L−1 kanamycin.
Immunohistochemistry
Arabidopsis tissues were fixed with 4% (w/v) freshly prepared paraformaldehyde and 0.02% (v/v) Triton X-100 in 50 mm potassium phosphate buffer, pH 7.0, at 4°C overnight. Fixed tissues were embedded in paraffin medium (Paraplast Plus, Sigma, St. Louis) and sectioned (7 μm thick) with a steel knife. Immunolocalization was performed using AGL15-specific antiserum, as described previously (Perry et al., 1996).
Analysis of Petal Break Strength
Petal break strength was measured in flowers of the primary inflorescence at the stage when at least 10 flowers had opened but before global proliferative arrest (Hensel et al., 1994) of the inflorescence meristems. Petal break strength was quantified using a stress transducer as described previously (Patterson, 1998; Fernandez et al., 2000).
System for Glucocorticoid-Inducible Expression of AGL15
The AGL15 coding sequence amplified from 35S:gAGL15 (Fernandez et al., 2000) using PCR was cloned into a pPZP212 transformation vector (Hajdukiewicz et al., 1994), which includes the NOS terminator. A DNA fragment, UAS-35S, containing six copies of the GAL4 upstream activation site (UAS) and −46 to +9 region of the CaMV 35S promoter, was excised from pTA7001 (Aoyama and Chua, 1997) using HindIII and XhoI restriction enzymes and inserted upstream of the AGL15 sequence. The resulting construct was designated UAS-35S:gAGL15. The UAS-35S:gAGL15 construct was introduced into Arabidopsis (Ws ecotype) by vacuum infiltration with A. tumefaciens strain GV3101, as described by Bechtold et al. (1993). T1 plants were selected on GM medium supplemented with 50 μg L−1 kanamycin (Sigma).
To introduce the glucocorticoid-regulated transcriptional activator GVG, transgenic plants carrying the UAS-35S:gAGL15 construct were crossed to transgenic plants carrying the TA7002:LUC construct (seed obtained from Dr. Nam-Hai Chua, Rockefeller University). TA7002:LUC contains the complete two-component glucocorticoid-inducible system (Aoyama and Chua, 1997) and, therefore, provides the GVG transcriptional factor for induction of AGL15. F1 plants containing both UAS-35S:gAGL15 and TA7002:LUC were selected on GM medium supplemented with 50 μg L−1 kanamycin (Sigma) and 50 μg L−1 hygromycin (Sigma). The induction of AGL15 mRNA in F1 seedlings by glucocorticoid was confirmed by RNA gel-blot analysis (not shown). Plants in the F3 generation were used for further analyses.
Glucocorticoid Treatments
Dexamethasone (Sigma), an analog of glucocorticoid, was dissolved in 100% (v/v) ethanol to make a 30 mm stock solution and stored at −20°C. For systemic induction, the plants were bottom watered every other day with 10% (v/v) Hoagland solution containing 30 μm dexamethasone and 0.01% (v/v) Silwet L-77 (OSI Specialties, Inc., Sistersville, WV). To treat inflorescence tissues, the pots were covered by plastic wrap and the inflorescences were allowed to grow through holes in the plastic. The exposed inflorescences were sprayed with a solution containing 30 μm dexamethasone and 0.01% (v/v) Silwet L-77 every other day. Control plants were sprayed with a solution containing 0.1% (v/v) ethanol and 0.01% (v/v) Silwet L-77.
RNA Gel-Blot Analysis
Total RNA was isolated according to the method described by Verwoerd et al. (1989). Five micrograms of total RNA was denatured and sized-fractionated in a 1% (v/v) formaldehyde-agarose gel following the protocol described in Ausubel et al. (1988). RNA was then transferred to a nylon membrane (Nytran Supercharge, Schleicher & Schuell, Keene, NH). The DNA template used to make AGL15 probe was generated by PCR amplification from an Arabidopsis AGL15 cDNA clone using primers 5′-CTTGAAGAATCACGCCTC-3′ and 5′-TTAGCGGCCGCAGAGAACCTTTGTCTTTTGGCTTC-3′. The amplified DNA product contained 24 bp of the K box region and the full C-terminal region of AGL15. The DNA template used to make the 18S rRNA probe was excised from pRE12 (Delseny et al., 1983) using the EcoRI restriction enzyme. The template DNAs were gel purified and labeled with 32P-labeled dCTP by random priming as described by Ausubel et al. (1988). Prehybridization, hybridization, and wash conditions were as described previously (Heck et al., 1995). The membrane was exposed to a phosphoscreen and the hybridization pattern was visualized by using a Cyclone Storage Phosphor Imaging System (Hewlett-Packard, Palo Alto, CA). The membrane was stripped before the second hybridization by washing twice with boiling 0.1% (w/v) SDS and 0.1× SSC.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
ACKNOWLEDGMENTS
We thank Melissa Lehti-Shiu for helpful comments on the manuscript, Dr. Susheng Gan for the SAG12 reporter construct, Drs. Anthony Bleecker and Sara Patterson for the Chitinase construct, Dr. Nam-Hai Chua for the GVG constructs, and Claudia Lipke for assistance with plant photography.
Footnotes
This work was supported by the University of Wisconsin (Madison) Graduate School, by the U.S. Department of Agriculture-National Research Initiative Competitive Grants Program (grant no. 96–35304–3699), and by the Department of Energy/National Science Foundation/U.S. Department of Agriculture Collaborative Program on Research in Plant Biology (grant no. DBI 96–02222).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.004721.
LITERATURE CITED
- Aoyama T, Chua N-H. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997;11:605–612. doi: 10.1046/j.1365-313x.1997.11030605.x. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Struhl K. Current Protocols in Molecular Biology. New York: Greene Publishing Associates; 1988. [Google Scholar]
- Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Ser III Sci Vie. 1993;360:1194–1199. [Google Scholar]
- Bleecker AB, Patterson SE. Last exit: senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell. 1997;9:1169–1179. doi: 10.1105/tpc.9.7.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner R, Kampmann G, Chandler J, Gleibner R, Wisman E, Apel K, Melzer S. A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J. 2000;24:591–599. doi: 10.1046/j.1365-313x.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- Broglie KE, Biddle P, Cressman R, Broglie R. Functional analysis of DNA sequences responsible for ethylene regulation of a bean chitinase gene in transgenic tobacco. Plant Cell. 1989;1:599–607. doi: 10.1105/tpc.1.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Delseny M, Cooke R, Penon P. Sequence heterogeneity in radish nuclear ribosomal-RNA genes. Plant Sci Lett. 1983;30:107–119. [Google Scholar]
- Fernandez DE, Heck GR, Perry SE, Patterson SE, Bleecker AB, Fang S-C. The embryo MADS domain factor AGL15 acts postembryonically: inhibition of perianth senescence and abscission via constitutive expression. Plant Cell. 2000;12:183–197. doi: 10.1105/tpc.12.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S. Molecular characterization and genetic manipulation of plant senescence. PhD thesis. Madison: University of Wisconsin; 1995. [Google Scholar]
- Gan S, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- Goto K, Meyerowitz EM. Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev. 1994;8:1548–1560. doi: 10.1101/gad.8.13.1548. [DOI] [PubMed] [Google Scholar]
- Greenwood JS, Bewley JD. Seed development in Ricinus communis (castor bean): I. Descriptive morphology. Can J Bot. 1982;60:1751–1760. [Google Scholar]
- Gu Q, Ferrándiz C, Yanofsky MF, Martienssen R. The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development. 1998;125:1509–1517. doi: 10.1242/dev.125.8.1509. [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol. 1994;25:989–994. doi: 10.1007/BF00014672. [DOI] [PubMed] [Google Scholar]
- Hanfrey C, Fife M, Buchanan-Wollaston V. Leaf senescence in Brassica napus: expression of genes encoding pathogenesis-related proteins. Plant Mol Biol. 1996;30:597–609. doi: 10.1007/BF00049334. [DOI] [PubMed] [Google Scholar]
- Heck GR, Perry SE, Nichols KW, Fernandez DE. AGL15, a MADS domain protein expressed in developing embryos. Plant Cell. 1995;7:1271–1282. doi: 10.1105/tpc.7.8.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel LL, Nelson MA, Richmond TA, Bleecker AB. The fate of inflorescence meristems is controlled by developing fruits in Arabidopsis. Plant Physiol. 1994;106:863–876. doi: 10.1104/pp.106.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish VF, Sussex IM. Function of the apetala-1 gene during Arabidopsis floral development. Plant Cell. 1990;2:741–753. doi: 10.1105/tpc.2.8.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempin SA, Savidge B, Yanofsky MF. Molecular basis of the cauliflower phenotype in Arabidopsis. Science. 1995;267:522–525. doi: 10.1126/science.7824951. [DOI] [PubMed] [Google Scholar]
- Kermode AR. Regulatory mechanisms involved in the transition from seed development to germination. Crit Rev Plant Sci. 1990;9:155–496. [Google Scholar]
- Krizek BA, Meyerowitz EM. The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development. 1996;122:11–22. doi: 10.1242/dev.122.1.11. [DOI] [PubMed] [Google Scholar]
- Lee H, Suh SS, Park E, Cho D, Ahn JH, Kim S-G, Lee HS, Kwon M, Lee I. The AGAMOUS-like 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 2000;14:2366–2376. doi: 10.1101/gad.813600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren SJ, Ditta GS, Eshed Y, Savidge B, Bowman JL, Yanofsky MF. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature. 2000;404:766–770. doi: 10.1038/35008089. [DOI] [PubMed] [Google Scholar]
- Lohman KN, Gan S, John MC, Amasino RM. Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiol Plant. 1994;92:322–328. [Google Scholar]
- Mandel MA, Yanofsky MF. A gene triggering flower formation in Arabidopsis. Nature. 1995;377:522–524. doi: 10.1038/377522a0. [DOI] [PubMed] [Google Scholar]
- Mao L, Begum D, Chuang H, Budiman MA, Szymkowiak EJ, Irish EE, Wing RA. JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature. 2000;406:910–913. doi: 10.1038/35022611. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell. 2001;13:935–941. doi: 10.1105/tpc.13.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BJ, Noodén LD. Xylem dye movement in relation to desiccation in soybean pods (abstract no. 104) Plant Physiol. 1986;80:S-20. [Google Scholar]
- Patterson SE. Characterization of delayed floral organ abscission and cell separation in Arabidopsis thaliana L. Heynh. PhD thesis. Madison: University of Wisconsin; 1998. [Google Scholar]
- Perry SE, Nichols KW, Fernandez DE. The MADS domain protein AGL15 localizes to the nucleus during early stages of seed development. Plant Cell. 1996;8:1977–1989. doi: 10.1105/tpc.8.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinyopich A, Ditta GS, Yanofsky MF. 12th International Conference on Arabidopsis Research, WI: Madison; 2001. Roles of SEEDSTICK MADS-box gene during ovule and seed development (abstract no. 54) [Google Scholar]
- Sheldon CC, Rouse DT, Finnegan EJ, Peacock WJ, Dennis ES. The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC) Proc Natl Acad Sci USA. 2000;97:3753–3758. doi: 10.1073/pnas.060023597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- Taylor JD, Whitelaw CA. Signals in abscission. New Phytol. 2001;151:323–339. [Google Scholar]
- Theissen G, Becker A, Di Rosa A, Kanno A, Kim JT, Münster T, Winter K, Sadler H. A short history of MADS-box genes in plants. Plant Mol Biol. 2000;42:115–149. [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BMM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Meyerowitz EM. The ABCs of floral homeotic genes. Cell. 1994;78:203–209. doi: 10.1016/0092-8674(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Weigel D, Nilsson O. A developmental switch sufficient for flower initiation in diverse plants. Nature. 1995;377:495–500. doi: 10.1038/377495a0. [DOI] [PubMed] [Google Scholar]
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 1990;346:35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]