Abstract
The strobilurin class of fungicides comprises a variety of synthetic plant-protecting compounds with broad-spectrum antifungal activity. In the present study, we demonstrate that a strobilurin fungicide, F 500 (Pyraclostrobin), enhances the resistance of tobacco (Nicotiana tabacum cv Xanthi nc) against infection by either tobacco mosaic virus (TMV) or the wildfire pathogen Pseudomonas syringae pv tabaci. F 500 was also active at enhancing TMV resistance in NahG transgenic tobacco plants unable to accumulate significant amounts of the endogenous inducer of enhanced disease resistance, salicylic acid (SA). This finding suggests that F 500 enhances TMV resistance in tobacco either by acting downstream of SA in the SA signaling mechanism or by functioning independently of SA. The latter assumption is the more likely because in infiltrated leaves, F 500 did not cause the accumulation of SA-inducible pathogenesis-related (PR)-1 proteins that often are used as conventional molecular markers for SA-induced disease resistance. However, accumulation of PR-1 proteins and the associated activation of the PR-1 genes were elicited upon TMV infection of tobacco leaves and both these responses were induced more rapidly in F 500-pretreated plants than in the water-pretreated controls. Taken together, our results suggest that F 500, in addition to exerting direct antifungal activity, may also protect plants by priming them for potentiated activation of subsequently pathogen-induced cellular defense responses.
When plants encounter pathogen attack, they activate diverse cellular defense responses that are aimed at resisting disease. In the case of gene-for-gene resistance, successful pathogen restriction is frequently accompanied by localized cell death of host tissue (hypersensitive response [HR]; Richberg et al., 1998). This localized cell death mostly results in the formation of visible necrotic lesions. After lesion formation, many plants develop an enhanced (acquired) resistance to a broad spectrum of pathogens, both in the tissue surrounding the primary infection site (local acquired resistance [LAR]) and often also in formerly uninoculated organs (systemic acquired resistance [SAR]; Hunt and Ryals, 1996; Ryals et al., 1996; Sticher et al., 1997; Dempsey et al., 1999). In NN genotype tobacco (Nicotiana tabacum), both forms of induced resistance become obvious, for example, by a significant reduction in the size of necrotic lesions produced upon secondary infection with tobacco mosaic virus (TMV) compared with lesions that develop after primary TMV attack (Ross, 1961). Alternatively, both LAR and SAR may be reflected by absence, reduced number, or delayed appearance of necrotic lesions that form either as a part of the HR or during disease development.
The identity of the long-distance signal that travels from the site of primary pathogen infection to the uninoculated parts of the plant to enhance disease resistance is still unknown (for review, see Ryals et al., 1996; Sticher et al., 1997). However, in the past decade, it became clear that in tobacco and some other plants, development of LAR and SAR requires the endogenous accumulation of salicylic acid (SA; Ryals et al., 1996; Dempsey et al., 1999). When SA accumulation is disrupted, tobacco and Arabidopsis plants display increased susceptibility to pathogen attack and fail to express both LAR and SAR (Delaney et al., 1994; Mauch-Mani and Slusarenko, 1996; Pallas et al., 1996).
In many plants, enhanced disease resistance is frequently accompanied by the activation of genes encoding pathogenesis-related (PR) proteins (van Loon and van Strien, 1999). Because some of these proteins display antimicrobial activity, their accumulation has often been assumed to contribute to acquired disease resistance. In tobacco, acidic PR-1 is the predominant PR protein and accumulates to 1% to 2% of the total leaf protein in infected leaf tissue (Alexander et al., 1993). The biological activity of the PR-1 protein is still unclear. However, overexpressing the PR-1 gene in transgenic tobacco plants enhanced their resistance against two oomycete pathogens (Alexander et al., 1993). This result suggested an important role for PR-1 in acquired disease resistance of tobacco.
In addition to the accumulation of PR proteins, some enhanced plant disease resistance is associated with a primed state causing potentiated activation of various cellular defense responses, but only once the protected tissue becomes attacked by a pathogen (for recent review, see Conrath et al., 2002). For example, in systemically resistant Arabidopsis plants, pathogen infection caused stronger activation of PR-1 genes when compared with plants without acquired disease resistance (Kohler et al., 2002). Similarly, in induced transgenic tobacco plants, the pathogen-elicited expression of an Asparagus officinalis PR-10::β-glucuronidase chimeric gene was stronger than in plants with no induced resistance (Mur et al., 1996).
The strobilurin class of fungicides comprises a variety of synthetic plant protecting compounds with broad-spectrum antifungal activity and structural similarity to basidiomycete antibiotics (Sauter et al., 1999). The mode of action of strobilurin fungicides is the inhibition of mitochondrial respiration by binding to the ubihydrochinone oxidation center of the mitochondrial bc1 complex (complex III), thereby blocking electron transfer (Sauter et al., 1999). Over the past years, there has also been increasing evidence for direct influences of strobilurins on plant physiology (Koehle et al., 2002). This physiological effect includes the so-called “greening,” that is, even in the absence of challenge by pathogen attack, plants treated with strobilurins are intense green and look healthier than plants that have not been treated with strobilurin fungicides (Koehle et al., 2002). This suggested to us that, in addition to their fungicidal activity, strobilurins might also enhance the capability of plants to ward off pathogens. In the present paper, we demonstrate that a novel synthetic strobilurin fungicide, F 500 (Pyraclostrobin), accelerates the TMV-induced activation of PR-1 genes in tobacco (Nicotiana tabacum cv Xanthi nc) plants, enhances their resistance against TMV and Pseudomonas syringae pv tabaci, and delays responses that are associated with resistance against P. syringae pv tomato DC3000.
RESULTS
F 500 Reduces TMV Lesion Size
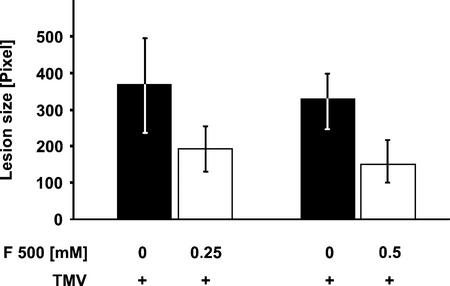
To investigate whether F 500 enhances the resistance of tobacco cv Xanthi nc (genotype NN) against TMV infection, on two tobacco leaves, one-half was infiltrated with aqueous suspensions of F 500. As a control, the remaining halves of the leaves were infiltrated with water. Twenty-four hours later, both halves of the two treated leaves were infected with TMV (1 μg mL−1) as described in “Materials and Methods.” The area of developing lesions was determined 7 d post TMV infection by fluorimaging.
As shown in Figure 1, infiltrating tobacco leaf tissue with F 500 caused a significant (Student's t test, P < 0.01, n = 26, F 500 = 0.5 mm) reduction in TMV lesion size. The reduction was about 50% when the leaf halves had been infiltrated with an aqueous suspension of F 500 at 0.25 mm and was only slightly more pronounced upon infiltrating F 500 at 0.5 mm. It should be noted that in all the experiments performed, we did not detect any effect of the pretreatment with F 500 on the number of developing TMV lesions. Furthermore, TMV lesion-reducing activity could also be observed when pure F 500 instead of the F 500 formulation was used for leaf pretreatment (data not shown). Thus, the TMV-protecting activity of the F 500 formulation is likely due to the F 500 fungicide.
Figure 1.
Reduction of TMV lesion size by pretreatment with F 500. On two tobacco plants, one-half of a leaf was infiltrated with an aqueous suspension of F 500 at 0.25 or 0.5 mm. The remaining halves of the leaves were infiltrated with water. Twenty-four hours later, both halves of the two leaves were infiltrated with a suspension of TMV (1 μg mL−1) by gently rubbing two layers of cheesecloth soaked with TMV suspension over the carborundum-covered leaf surfaces. The leaves then were washed under a stream of tap water. The size of developing lesions (±se) was examined with a fluorimager 7 d later as described in “Materials and Methods.”
F 500 Enhances the Resistance against P. syringae pv tabaci and Delays Resistance Responses to P. syringae pv tomato
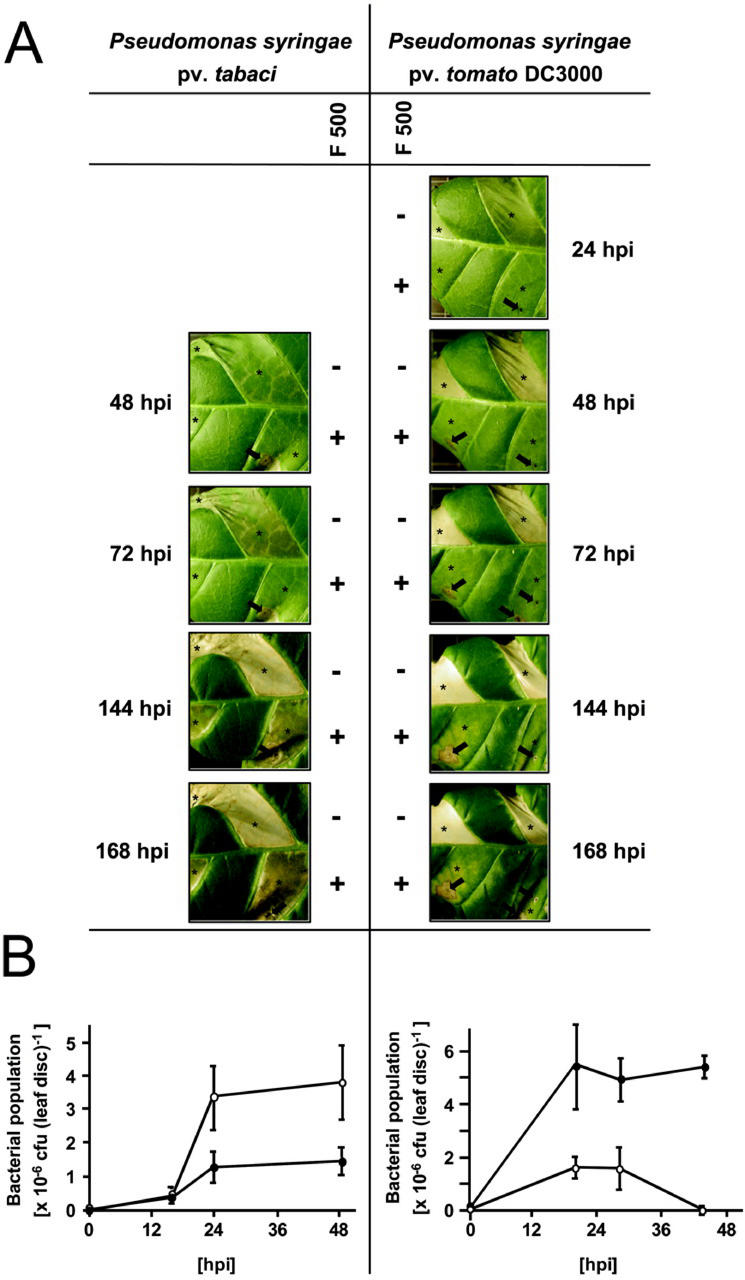
To assay the spectrum of pathogens against which F 500 can affect the resistance or diminish disease symptoms in tobacco, we next included two phytopathogenic bacteria in our experiments. By doing so, we found that pure F 500 delayed the appearance of disease symptoms induced by the virulent wildfire pathogen P. syringae pv tabaci (Fig. 2A). Bacteria-inoculated control tissue areas initially were chlorotic (within 72 h postinfection), then subsequently water soaked and necrotic (Fig. 2A), whereas the bacterial population in control leaf halves increased at least over the first 24 to 48 h postinfection (Fig. 2B). In the F 500-pretreated leaf panels, bacterial population size increased to a much lesser extent (Fig. 2B) and the appearance of disease symptoms induced by P. syringae pv tabaci was delayed (Fig. 2A).
Figure 2.
Enhanced resistance against P. syringae pv tabaci and impaired appearance of resistance responses to P. syringae pv tomato DC3000 upon pretreatment of tobacco leaf tissue with F 500. Tobacco leaf halves were infiltrated with 0.5 mm pure F 500 in 1% (v/v) dimethyl sulfoxide (DMSO; +) or with DMSO (1%, v/v) only (−). Twenty-four hours later, individual leaf panels (asterisks) were infiltrated with P. syringae pv tabaci or with P. syringae pv tomato DC3000 at 1 × 105 colony-forming units (cfu) mL−1 (corresponding to 20 × 103 cfu per leaf disc) through small holes punctured with a needle (some of which are marked with an arrow). A, Symptoms were recorded at the indicated time points with a digital camera. B, The increase in bacterial population size (±se) was determined in discs from infected leaf panels of F 500-pretreated (black symbols) and F 500-non-pretreated (white symbols) leaf halves at the indicated times. hpi, Hours postinfection.
When avirulent P. syringae pv tomato DC3000 was used for tobacco leaf infection, there was activation of an HR associated with the onset of necrosis and dehydration of the tissue (Fig. 2A), along with a slight and transient increase in bacterial growth that was followed by a dramatic reduction in bacterial population size (Fig. 2B) within 48 h after infection of control leaf areas. Interestingly, the F 500-pretreated and then P. syringae pv tomato DC3000-infected leaf panels, within 48 to 72 h postinfection, remained essentially free of detectable symptoms of necrosis (Fig. 2A), even upon microscopic examination, though there was multiplication and increased presence of bacteria within the first 20 to 44 h postinfection of this tissue (Fig. 2B). At later time points (144–168 h postinfection), tissue dehydration proceeded on control leaf halves, whereas the F 500-pretreated and subsequently P. syringae pv tomato DC3000-infected leaf tissue was subject to some localized chlorosis (Fig. 2A). Necrotic leaf areas, if present, were confined to the tissue surrounding the holes punctured with a needle to infiltrate bacteria (Fig. 2A).
Similar effects on bacterial population size and symptom development were seen when the F 500 formulation was used instead of pure F 500 for the pretreatment of tobacco leaf tissue (data not shown).
F 500 Does Not Affect the Potency of TMV to Infect Tobacco Leaves
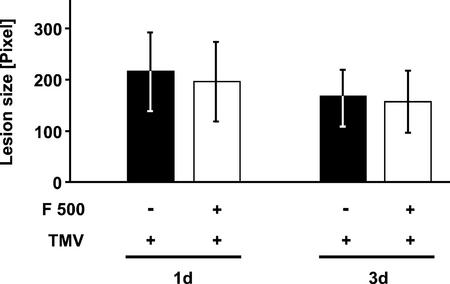
The F 500-caused reduction in TMV lesion size (Fig. 1) can either be due to an enhanced ability of the treated tobacco leaves to ward off TMV attack or may be due to a direct toxic effect of F 500 on TMV. To address the latter question, aliquots of the TMV suspension were incubated, for 1 and 3 d, in the absence or presence of 0.5 mm F 500 in the growth rooms of the plants. Then, tobacco leaf halves were infected with the respective F 500-pretreated or -non-pretreated TMV suspension as described above and the area of the developing lesions determined 5 d later.
Figure 3 demonstrates that there was no significant (Student's t test, P = 0.74, n = 6, 1-d treatment of TMV with F 500) difference in the size of necrotic lesions induced by F 500-treated or -non-treated TMV. Therefore, direct inhibition by F 500 of the capacity of TMV to infect tobacco leaves is unlikely. As in the present experiment (Fig. 3), there was no prolonged pretreatment with F 500 of the leaf tissue before TMV infection and because F 500 was present only in the soaked cheesecloth used for leaf infection (white bars), lesion-reducing activity of F 500 was not significant in this experiment (Fig. 3).
Figure 3.
F 500 does not affect the infection potency of TMV. A TMV suspension (1 μg mL−1) was incubated, for 1 and 3 d, in the absence (−) or presence (+) of 0.5 mm F 500 under plant growth conditions. Tobacco leaf halves then were infected with the F 500-pretreated or -non-pretreated TMV suspension. TMV lesion size (±se) was determined 5 d postinfection.
F 500-Induced TMV Resistance Is Independent of SA Accumulation
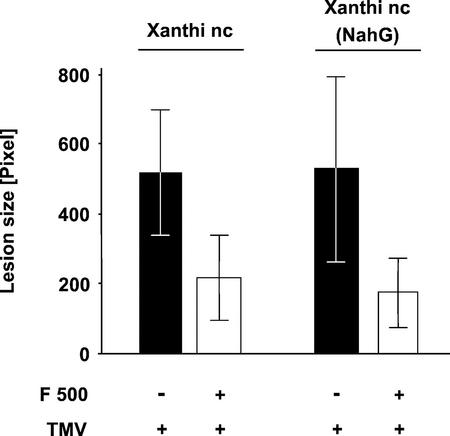
When enhancing the resistance of tobacco to TMV infection, F 500 could act independently of SA or affect steps upstream or downstream of SA accumulation. If F 500 acts before SA accumulation, it would not enhance TMV resistance in NahG transgenic tobacco plants. Due to the presence of the NahG gene from Pseudomonas putida, which encodes an SA-hydroxylase, these plants are unable to accumulate significant amounts of SA and do not express LAR or SAR in response to pathogen attack (Gaffney et al., 1993). If F 500 acts downstream or independently of the SA accumulation step, NahG plants should respond identically to nontransformed plants. To distinguish between these possibilities, halves of leaves of NahG plants were treated with 0.5 mm F 500 for 1 d before the resistance against TMV was evaluated.
As shown in Figure 4, F 500 enhanced the resistance against TMV infection in NahG transgenic tobacco to the same degree as in nontransformed tobacco cv Xanthi nc plants. Statistical analysis (Student's t test) revealed that the difference in the size of TMV lesions between F 500-pretreated and -non-pretreated NahG tobacco leaves was significant (P < 0.01, n = 16). Thus, the result from this experiment indicates that the F 500-induced TMV resistance of tobacco is independent of SA accumulation.
Figure 4.
F 500-induced TMV resistance does not depend on SA accumulation. Leaf halves of nontransformed (tobacco cv Xanthi nc) and transformed NahG (tobacco cv Xanthi nc [NahG]) plants were infiltrated with water (−; control halves) or with 0.5 mm F 500 (+). One day later, the entire leaves were infected with TMV. Lesion size (±se) was determined 3 d postinfection.
F 500 Does Not Directly Induce PR-1 Protein Accumulation
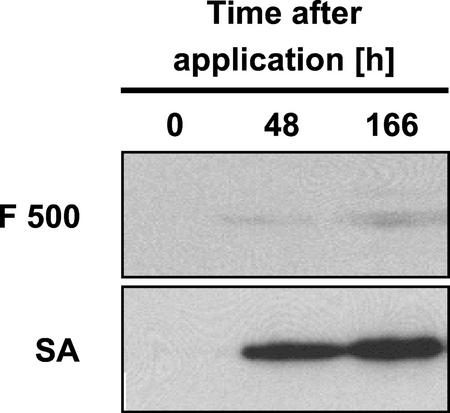
Next, we were interested in whether the F 500-induced resistance of tobacco against TMV is associated with the accumulation of known molecular markers of acquired disease resistance. For this purpose, F 500-treated tobacco leaf tissue was assayed for the accumulation of PR-1 proteins by western-blotting analysis at various time points after treatment. By doing so, we found that, although there was a prominent and time-dependent accumulation of PR-1 proteins in tobacco leaf halves that had been infiltrated with 0.5 mm SA, induction of these proteins was only very low when 0.5 mm F 500 was injected into tobacco leaf halves (Fig. 5). Thus, the F 500-induced TMV resistance of tobacco does not depend on a significant, pre-infectional accumulation of the prominent disease resistance marker protein PR-1.
Figure 5.
F 500 does not significantly induce the accumulation of PR-1 proteins in tobacco leaves. One-half of a leaf of a tobacco plant was infiltrated with 0.5 mm F 500, whereas the other one-half was infiltrated with 0.5 mm SA (positive control). At the indicated time points post treatment, leaf tissue was assayed for the accumulation of PR-1 proteins by western-blotting analysis.
F 500 Primes Tobacco Leaves for Accelerated PR-1 Induction after TMV Attack
As a next step toward elucidating the mode of action of F 500 in the enhancement of disease resistance in tobacco, we examined whether the pretreatment with F 500 may prime the leaves to better activate cellular defense responses once attacked by TMV. To this end, halves of tobacco leaves were infiltrated with 0.5 mm F 500, whereas control halves were infiltrated with water. After 1 d, both halves of the leaves were infected with TMV. At various time points post-TMV application, leaf tissue was harvested and analyzed for the accumulation of both PR-1 mRNA and protein by RNA gel blot and western-blotting analysis, respectively.
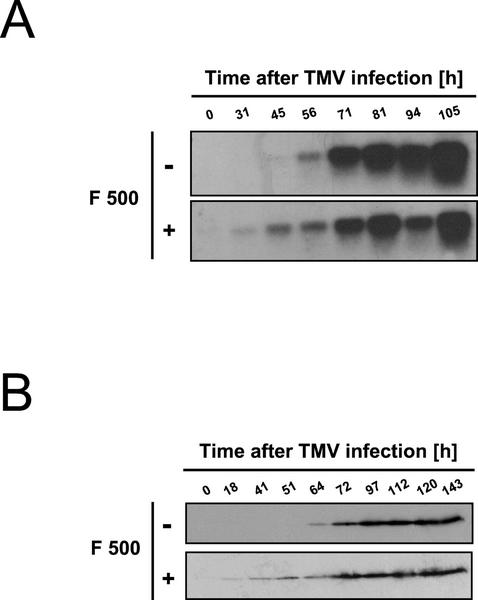
Consistent with the results in Figure 5, there was only faint, if any, accumulation of PR-1 mRNA and protein in leaf halves treated with F 500 only. Interestingly, however, in the F 500-pretreated and then TMV-infected leaf halves, the virus-induced accumulation of both PR-1 mRNA and protein was detectable at least 12 h earlier than in the water-pretreated and then TMV-infected control halves (Fig. 6). Thus, the pretreatment with F 500 enables the tobacco leaves to react faster with PR-1 gene expression and PR-1 protein accumulation but only after the protected tissue becomes attacked by TMV (Fig. 6).
Figure 6.
Pretreatment with F 500 primes tobacco leaf tissue for accelerated accumulation of both PR-1 mRNA (A) and PR-1 protein (B). One-half of a tobacco leaf was infiltrated with water (−), whereas the other one-half was infiltrated with F 500 (0.5 mm; +). After 24 h, the entire leaf was infected with TMV. At the indicated time points, leaf tissue was analyzed for the accumulation of PR-1 transcripts (A) and PR-1 protein (B) by RNA gel-blot and western-blotting analysis, respectively.
DISCUSSION
In this study, we demonstrated that tobacco cv Xanthi nc plants treated with the synthetic strobilurin derivative F 500 showed enhanced resistance against infection by either TMV (Fig. 1) or virulent P. syringae pv tabaci (Fig. 2). In contrast, the pretreatment with F 500 delayed induction of the HR and necrosis by P. syringae pv tomato DC3000 (Fig. 2A) and also led to an increase in bacterial population size (Fig. 2B), indicating that F 500 interferes with resistance responses of tobacco cv Xanthi nc to avirulent P. syringae pv tomato DC3000. The reason for the paradoxical result with the two bacterial strains remains unclear. One possible explanation for the observed increase in P. syringae pv tomato DC3000 growth in F 500-pretreated leaf panels is the fact that this tissue, in contrast to the untreated control, does not collapse but remains viable, possibly due to F 500 affecting the perception of the avirulent pathogen by the host. This assumption is supported by the highly delayed appearance of visible symptoms on F 500-pretreated and then P. syringae pv tomato DC3000-infected tobacco leaf tissue. Because P. syringae pv tomato DC3000 that has been re-isolated from F 500-pretreated and then infected tobacco leaves was fully avirulent when infiltrated into tobacco leaf tissue (data not shown), we can exclude the possibility that F 500 causes genetic changes in the bacteria that affect avirulence.
The lack of correlation between the dose of F 500 applied and the observed reduction in TMV lesion size (Fig. 1) might be due to the poor solubility of F 500 in water (0.19 mg 100 mL−1 at 20°C). Because of this poor solubility, increasing the content of formulated F 500 in the aqueous suspension from a certain amount does not further enhance the concentration of dissolved F 500 and, therefore, may not further reduce the size of TMV lesions (Fig. 1). Alternatively, the induced TMV resistance might already be fully triggered at the lower F 500 doses.
F 500 had no direct inhibitory effect on TMV' s infection potency (Fig. 3) and per se did not affect in vitro the multiplication of P. syringae pv tabaci (data not shown). Thus, the enhanced protection against these two pathogens (Figs. 1, 2, and 4) very likely results from the activation by the F 500 fungicide of an induced disease resistance mechanism in the plant. This conclusion is consistent with an earlier report demonstrating impaired activity of the fungicides Metalaxyl, Cu(OH)2, and Fosethyl in Arabidopsis plants blocked in their disease resistance signal transduction mechanism (Molina et al., 1998). Furthermore, induction of SAR in Arabidopsis was reported recently as a mode of action of the plant protecting fungicide Probenazole (Yoshioka et al., 2001).
F 500 protected tobacco cv Xanthi nc leaf tissue from TMV (Figs. 1 and 4) and P. syringae pv tabaci attack (Fig. 2), but did not induce TMV resistance in tobacco cv Xanthi and also failed to induce resistance against tobacco etch virus and potato virus Y in tobacco cv Xanthi nc plants (data not shown). Furthermore, F 500 obviously interferes with the resistance of tobacco cv Xanthi nc to P. syringae pv tomato DC3000. Thus, the F 500-induced resistance acts against various, yet not all, pathogens. Similar findings have been made before with induced resistance phenomena, including SAR (Ryals et al., 1996) and rhizobacteria-induced systemic resistance (van Loon et al., 1998).
F 500 was active at the enhancement of TMV resistance in NahG transgenic tobacco plants (Fig. 4). In addition, F 500 did not induce the accumulation of SA in wild-type tobacco (data not shown). Therefore, F 500 enhances the TMV resistance of tobacco either by acting downstream of SA in the SA signal transduction network or by functioning independently of SA. The latter possibility is more likely because F 500 did not directly cause significant accumulation of the SA-responsive PR-1 proteins in infiltrated tobacco leaves (Fig. 5). The mechanism by which F 500 accelerates the activation of PR-1 genes and enhances the pathogen resistance of tobacco remains unclear, however.
In various fungi, strobilurins were reported to stimulate alternative respiration (Affourtit et al., 2000). Interestingly, Carr and coworkers (Chivasa et al., 1997) found that SA-mediated enhancement of TMV resistance in tobacco is sensitive to salicylhydroxamic acid, an inhibitor of the alternative oxidase pathway. Thus, it is possible that the F 500-induced increase in TMV resistance and the associated acceleration of PR-1 gene expression in tobacco cv Xanthi nc is mediated, at least in part, by activation of the alternative respiratory pathway as a response to F 500-induced inhibition of mitochondrial respiration. However, alternative oxidation does not confer resistance against bacterial pathogens in tobacco cv Xanthi nc (Chivasa et al., 1997) and, thus, the F 500-induced resistance against P. syringae pv tabaci (Fig. 2) requires another explanation.
The TMV-induced activation of PR-1 defense genes and the associated accumulation of the PR-1 proteins occurred much earlier in F 500-pretreated plants than in the water-pretreated controls (Fig. 6). Thus, F 500 may enhance disease resistance in tobacco by accelerating the plant's ability for the induction of normal defense responses that occur once the pathogen is sensed by the plant. A similar conclusion has been drawn from earlier experiments demonstrating potentiated activation of various elicitor-induced defense responses in parsley (Petroselinum crispum) culture cells primed with SA or synthetic SA analogs (Kauss et al., 1992; Katz et al., 1998; Thulke and Conrath, 1998). Reports on stronger induction of pathogen-activated defense responses in intact tobacco (Mur et al., 1996) and Arabidopsis (Kohler et al., 2002) plants pre-incubated with inducers of enhanced plant disease resistance also supported the conclusion that a primed state for potentiated activation of cellular defense responses might play a crucial role in acquired plant disease resistance. In this context, it is interesting that Zimmerli et al. (2000) demonstrated accelerated accumulation of PR-1 transcripts in P. syringae pv tomato DC3000-infected Arabidopsis plants made resistant by pretreatment with the nonprotein amino acid β-aminobutyric acid. Furthermore, pretreatment of cultured tomato (Lycopersicon esculentum) cells with the wound-generated systemic peptide messenger systemin was reported to enhance the hydrogen peroxide burst subsequently induced by oligogalacturonides or by osmotic stress (Stennis et al., 1998).
MATERIALS AND METHODS
Materials
Pure F 500, as well as a formulation of F 500 (BAS 500 F DI) containing 20% (w/w) active ingredient, were provided by BASF Inc. (Limburgerhof, Germany). The formulation was suspended in water as a stock suspension containing 1 mm F 500. The suspension was left on the bench for exactly 15 min to sediment insoluble materials. The F 500 content in the resulting supernatant was determined by HPLC analysis and found to be 0.5 mm, indicating saturation of the F 500 solution. The supernatant was used, partly after further dilution with water, for infiltration of the tobacco leaf apoplast as described below. Pure F 500 was suspended in 1% (v/v) DMSO at 0.5 mm (final concentration) and infiltrated into leaf tissue also as described below. SA was purchased from Sigma (St. Louis), dissolved in water as a 10 mm stock solution, and adjusted to pH 5.8 with KOH.
Pseudomonas syringae pv tomato (strain DC3000) and P. syringae pv tabaci were provided by Brian Staskawicz (University of California, Berkeley) or bought at the German Collection of Microorganisms and Cell Cultures, Inc. (Braunschweig, Germany), respectively.
Growth and Treatment of Plants, Determination of TMV Lesion Size, and Evaluation of Symptoms
Nontransgenic and NahG transgenic tobacco plants were grown at 23°C in a 16-h-light cycle and used for experimentation at 6 to 8 weeks. One-half of a well-developed leaf of a tobacco plant was infiltrated, through small holes punctured with a needle, by a syringe with either pure F 500 in 1% (v/v) DMSO, an aqueous suspension of F 500 formulation, or with an aqueous solution of SA. The second one-half of the leaf was infiltrated with the respective control solution; that is, either 1% (v/v) DMSO or water.
For TMV infection, the entire leaf was inoculated, 24 h later, with a suspension of the U1 strain of TMV (1 μg mL−1) in 50 mm sodium phosphate (pH 7.0) by gently rubbing two layers of cheesecloth soaked with TMV suspension over the carborundum-covered leaf surface. The leaf was then washed under a stream of tap water. After an additional 3 to 7 d, the area (average ± se) of lesions on the leaf halves was determined based on the fluorescence of phenolic compounds in the lesions with a FluorImager 595 (Molecular Dynamics, Inc., Sunnyvale, CA) and ImageQuant software (Molecular Dynamics, Inc.). This computer-based method for determining TMV lesion size was found to be highly reliable and less prone to subjective evaluation errors than the classical determination of lesion size with a ruler.
P. syringae pv tomato (strain DC3000) and P. syringae pv tabaci were grown at 30°C in King's B media for 1 d. After centrifugation, bacterial cells were washed and resuspended to 1 × 105 cfu mL−1 in 10 mm MgCl2. Bacterial suspensions were infiltrated into the apoplast of tobacco leaf tissue, through small holes punched with a needle, using a plastic syringe. Appearing symptoms were recorded with a digital camera (model C-3030, Olympus, Tokyo) for 1 week.
Estimation of Bacterial Populations
Increases in bacterial populations were estimated in two leaf discs (1 cm in diameter) taken from infected leaf areas of two different plants at the indicated time points postinfection (n = 4). The discs were homogenized in 500 μL of sterile water, thoroughly mixed, and serial dilutions of the slurry were plated out on King's B agar. After incubation at 30°C for 2 d, colonies were counted and the original population size deducted. Population sizes are given as cfu per leaf disc.
Analysis of PR-1 Induction
To determine the accumulation of PR-1 proteins by western-blotting analysis, two leaf discs (1 cm in diameter) of respectively treated tobacco leaf halves were homogenized and fractionated by SDS-PAGE as described (Conrath et al., 1995). The separated proteins were electrophoretically transferred to a polyvinylidene difluoride membrane. Immunoblot analysis was performed as described with mouse monoclonal antibody 33G1, which specifically recognizes PR-1 proteins (Conrath et al., 1995).
To evaluate the accumulation of PR-1 mRNA by RNA gel-blot analysis, total RNA was isolated from frozen leaf discs using TRI-Reagent (Molecular Research Center, Cincinnati) according to the manufacturer's instructions. Ten micrograms of total RNA was denatured and separated on a 1.2% (w/v) agarose-2.5% (v/v) formaldehyde gel essentially as described (Thulke and Conrath, 1998). After blotting to a positively charged nylon membrane (Nytran-Plus, Schleicher & Schull, Dassel, Germany) by downstream capillary transfer using 10× SSC (1.5 m sodium chloride and 0.15 m sodium citrate, pH 7.0), RNA was cross-linked to the membrane by UV irradiation. Prehybridization and hybridization were performed at 65°C in 0.25 m Na2HPO4, pH 7.2; 1 mm EDTA; 7% (w/v) SDS; and 1% (w/v) bovine serum albumin. Hybridization with a 32P-labeled tobacco PR-1 cDNA probe was for 16 h. After hybridization, the membrane was washed at 65°C for 1 h with two changes of the washing solution (40 mm Na2HPO4, pH 7.2; 1 mm EDTA; 5% [w/v] SDS; and 0.5% [w/v] bovine serum albumin). Finally, the blot was exposed to maximum sensitivity x-ray film (Eastman-Kodak, Rochester, NY) at −70°C.
All experiments shown in this study were performed at least three times with similar results.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
ACKNOWLEDGMENTS
We thank Regine Hakenbeck for allowing us to use the fluorimager. We are also grateful to Dan Klessig for providing the tobacco PR-1 cDNA clone and the PR-1 antibody. We greatly appreciate the provision of NahG plants by John Ryals and of P. syringae pv tomato DC3000 by Brian Staskawicz. We thank Heinrich Kauss, Jean Greenberg, and the two unknown reviewers for valuable suggestions and comments on the manuscript.
Footnotes
This work was supported by BASF Inc. (grant to U.C.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.004432.
LITERATURE CITED
- Affourtit C, Heaney SP, Moore AL. Mitochondrial electron transfer in the wheat pathogenic fungus Septoria tritici: on the role of alternative respiratory enzymes in fungicide resistance. Biochim Biophys Acta. 2000;1459:291–298. doi: 10.1016/s0005-2728(00)00157-2. [DOI] [PubMed] [Google Scholar]
- Alexander D, Goodmann RM, Gut-Rella M, Glascock C, Weymann K, Friedrich L, Maddox D, Ahl-Goy P, Luntz T, Ward E et al. Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proc Natl Acad Sci USA. 1993;90:7327–7331. doi: 10.1073/pnas.90.15.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivasa S, Murphy AM, Naylor M, Carr JP. Salicylic acid interferes with tobacco mosaic virus replication via a novel salicylhydroxamic acid-sensitive mechanism. Plant Cell. 1997;9:547–557. doi: 10.1105/tpc.9.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U, Chen Z, Ricigliano JR, Klessig DF. Two inducers of plant defense responses, 2,6-dichloroisonicotinic acid and salicylic acid, inhibit catalase activity in tobacco. Proc Natl Acad Sci USA. 1995;92:7143–7147. doi: 10.1073/pnas.92.16.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U, Pieterse CMJ, Mauch-Mani B. Priming in plant-pathogen interactions. Trends Plant Sci. 2002;7:210–216. doi: 10.1016/s1360-1385(02)02244-6. [DOI] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E et al. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Crit Rev Plant Sci. 1999;18:547–575. [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Hunt MD, Ryals JA. Systemic acquired resistance signal transduction. Crit Rev Plant Sci. 1996;15:583–606. [Google Scholar]
- Katz VA, Thulke OU, Conrath U. A benzothiadiazole primes parsley cells for augmented elicitation of defense responses. Plant Physiol. 1998;117:1333–1339. doi: 10.1104/pp.117.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Theisinger-Hinkel E, Mindermann R, Conrath U. Dichloroisonicotinic and salicylic acid, inducers of systemic acquired resistance, enhance fungal elicitor responses in parsley cells. Plant J. 1992;2:655–660. [Google Scholar]
- Koehle H, Grossmann K, Jabs T, Stierl R, Gerhard M, Kaiser W, Glaab J, Conrath U, Seehaus K, Herms S. Physiological effects of the strobilurin fungicide F 500 on plants. In: Lyr H, Russell PE, Dehne H-W, Sisler HD, editors. Modern Fungicides and Antifungal Compounds III. Andover, UK: Intercept; 2002. (in press) [Google Scholar]
- Kohler A, Schwindling S, Conrath U. Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis. Plant Physiol. 2002;128:1046–1056. doi: 10.1104/pp.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch-Mani B, Slusarenko A. Production of salicylic acid precursors is a major function of phenylalanine ammonia lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell. 1996;8:203–212. doi: 10.1105/tpc.8.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina A, Hunt MD, Ryals JA. Impaired fungicide activity in plants blocked in disease resistance signal transduction. Plant Cell. 1998;10:1903–1914. doi: 10.1105/tpc.10.11.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LA, Naylor G, Warner SAJ, Sugars JM, White RF, Draper J. Salicylic acid potentiates defense gene expression in tissue exhibiting acquired resistance to pathogen attack. Plant J. 1996;9:559–571. [Google Scholar]
- Pallas JA, Paiva NL, Lamb C, Dixon RA. Tobacco plants epigenetically suppressed in phenylalanine ammonia lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J. 1996;10:281–293. [Google Scholar]
- Richberg MH, Aviv DH, Dangl JL. Dead cells do tell tales. Curr Opin Plant Biol. 1998;1:480–485. doi: 10.1016/s1369-5266(98)80039-3. [DOI] [PubMed] [Google Scholar]
- Ross AF. Systemic acquired resistance induced by localized virus infection in plants. Virology. 1961;13:340–358. doi: 10.1016/0042-6822(61)90319-1. [DOI] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner H-Y, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter H, Steglich W, Anke T. Strobilurins: evolution of a new class of active substances. Angew Chem Int Ed. 1999;38:1328–1349. doi: 10.1002/(SICI)1521-3773(19990517)38:10<1328::AID-ANIE1328>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Stennis MJ, Chandra S, Ryan CA, Low P. Systemin potentiates the oxidative burst in cultured tomato cells. Plant Physiol. 1998;117:1031–1036. doi: 10.1104/pp.117.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L, Mauch-Mani B, Métraux J-P. Systemic acquired resistance. Annu Rev Phytopathol. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- Thulke OU, Conrath U. Salicylic acid has a dual role in the activation of defense-related genes in parsley. Plant J. 1998;14:35–42. doi: 10.1046/j.1365-313X.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- van Loon LC, van Strien EA. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol. 1999;55:85–97. [Google Scholar]
- Yoshioka K, Nakashita H, Klessig DF, Yamaguchi I. Probenazole induces systemic acquired resistance in Arabidopsis with a novel type of action. Plant J. 2001;25:149–157. doi: 10.1046/j.1365-313x.2001.00952.x. [DOI] [PubMed] [Google Scholar]
- Zimmerli L, Jakab G, Métraux J-P, Mauch-Mani B. Potentiation of pathogen-specific defense mechanisms in Arabidopsis by β-aminobutyric acid. Proc Natl Acad Sci USA. 2000;97:12920–12925. doi: 10.1073/pnas.230416897. [DOI] [PMC free article] [PubMed] [Google Scholar]