Abstract
Transcranial magnetic stimulation (TMS) can produce effects not only at the site of stimulation but also at distant sites to which it projects. Here we examined the connection between supplementary motor area (SMA) and the hand area of the primary motor cortex (M1Hand) by testing whether prolonged repetitive TMS (rTMS) over the SMA can produce changes in excitability of the M1Hand after the end of the stimulus train. We evaluated motor-evoked potentials (MEPs) and the cortical silent period (CSP) evoked by a single-pulse TMS, short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) produced by a paired-pulse TMS, and forearm flexor H reflexes before and after 750 pulses of 5 Hz rTMS over SMA at an intensity of 110% active motor threshold (AMT) for the first dorsal interosseous (FDI) muscle. The amplitude of MEPs recorded from the right FDI muscle at rest as well as during voluntary contraction increased for at least 10 min after the end of rTMS, although the duration of the CSP, SICI and ICF did not change. There was no effect on H reflexes in the flexor carpi radialis muscle, even though the amplitude of the MEP obtained from the same muscle increased after rTMS. The effects on MEPs depended on the intensity of rTMS and were spatially specific to the SMA proper. We suggest that 5 Hz rTMS over SMA can induce a short-lasting facilitation in excitability of the M1Hand compatible with the anatomical connections between SMA and the M1Hand.
A series of studies from this laboratory and from other groups have shown that it is possible to distinguish the effects of transcranial magnetic stimulation (TMS) of primary motor cortex (M1) from those seen after stimulation over the presumed dorsal premotor cortex (PMd), some 1.5–2 cm anterior. Thus, single-pulse conditioning stimuli over PMd reduce the excitability of the primary motor cortex hand area (M1Hand) some 6 ms later (Civardi et al. 2001), whereas the same conditioning stimuli applied over M1Hand have a maximum effect at 1–2 ms (Kujirai et al. 1993). Repetitive TMS (rTMS) of PMd at an intensity of 90% active motor threshold (AMT) can either increase (5 Hz rTMS; Rizzo et al. 2003) or decrease (1 Hz rTMS; Gerschlager et al. 2001) the excitability of M1Hand for several minutes depending on the frequency of the conditioning rTMS, whilst the same stimulation applied directly over M1Hand has no effect. Behavioural studies have also revealed differences between stimulation of PMd and M1. For example, single-pulse TMS over M1 can delay reaction times if it is given late in the reaction period between stimulus and response, whereas the pulse has to be applied early in the reaction period for effects to be seen after stimulation of PMd (Schluter et al. 1998; Day et al. 1989). Finally, functional imaging studies, which show the effects of TMS both at the site of stimulation and at connected sites at a distance, reveal that rTMS over M1 produces quite a different pattern of after-effects on rCBF than stimulation over PMd (Siebner et al. 2003; Lee et al. 2003). Indeed, the most recent work with combined TMS and functional magnetic resonance imaging (fMRI) shows that it is possible to distinguish between both the cortical and subcortical structures activated by connections from motor and premotor cortex (Bestmann et al. 2004).
The question we ask here is whether it is possible to see a similar distinction between TMS over the supplementary motor area (SMA) and M1. If so, then it opens the possibility of studying the full pattern of electrophysiological interactions between the three main motor areas of the human cortex in awake, behaving subjects. The SMA, however, is likely to be a more difficult area to target than the PMd, since it is located in the interhemispheric fissure rather than being exposed on the lateral surface of the hemisphere. Nevertheless, effective stimulation should be possible given that TMS can target the adjacent leg area of the M1, albeit at a higher threshold than structures on the lateral surface of the cortex. In support of this, previous behavioural studies strongly suggest that TMS over the SMA produces effects that are different from those seen over M1. For example, short trains of TMS over SMA disrupt complex sequences of hand movements more readily than simple movements, whereas TMS of M1 affects both equally well (Gerloff et al. 1997). Similar distinctive effects have been seen on eye movement control and bimanual hand movements (Müri et al. 1995; Meyer-Lindenberg et al. 2002; Serrien et al. 2002; Steyvers et al. 2003). There are only two electrophysiological studies of SMA stimulation in healthy individuals. Civardi et al. (2001) found that single-pulse TMS over SMA could reduce the excitability of M1Hand some 6 ms later, indicating that SMA stimulation is likely to lead to changes in activity in anatomically connected regions in a way similar to that seen after stimulation of M1 and PMd. A similar effect was described by Oliveri et al. (2003) and found to be modulated by emotional stimulation.
The aim of the present paper was to extend these electrophysiological observations on remote effects of SMA stimulation using an rTMS approach. We applied 5 Hz rTMS to SMA and tested for after-effects on the excitability of M1Hand. The results suggest that it may be possible to target the SMA and its connections in a manner very similar to that seen after PMd or M1 stimulation.
Methods
Subjects
Twenty healthy volunteers participated in the experiments (9 women, 11 men; mean age 30.9 ± 7.5 years). Subjects were all right-handed and in good health at the time of study and were seated in a comfortable chair during the experiment. All subjects gave their written informed consent for this experiment, which was approved by the local ethical committee and conformed to the requirements of the Declaration of Helsinki.
Recording the EMG activity
In all experiments, the EMG activity was recorded from Ag–AgCl surface electrodes over the right or left first dorsal interosseous (FDI) or right flexor carpi radialis (FCR) muscles. The signal was amplified and band-pass filtered (10–1000 Hz) by a Digitimer D150 amplifier (Digitimer Ltd, Welwyn Garden City, Herts, UK) and acquired at a sampling rate of 5 kHz on a personal computer for off-line analysis (SigAvg Software, Cambridge Electronic Design, Cambridge, UK). During the experiments EMG activity was continuously monitored with visual feedback to ensure either complete relaxation at rest or a constant level of EMG activity during tonic contraction.
Assessment of cortical excitability of the M1Hand
Excitability of the left or right M1Hand was assessed with single- and paired-pulse TMS before and after rTMS. Measurements were performed with a High Power Magstim 200 machine and a figure-of-eight coil with mean loop diameters of 9 cm (Magstim Co., Whitland, Dyfed, UK). The magnetic stimulus had a nearly monophasic pulse configuration with a rise time of approximately 100 μs, decaying back to zero over approximately 0.8 ms. The coil current during the rising phase of the magnetic field flowed towards the handle. The coil was placed tangentially to the scalp with the junction region pointing backwards and laterally at a 45 deg angle away from the mid-line, approximately perpendicular to the line of the central sulcus, inducing a posterior–anterior current in the brain (Fig. 1). We chose this orientation because motor threshold is minimum when the induced electrical current in the brain flows approximately perpendicular to the line of the central sulcus (Brazil-Neto et al. 1992; Mills et al. 1992). We determined the optimum position for activation of the FDI or FCR muscle by moving the coil in 1 cm steps around the presumed M1. The site at which stimuli of slightly suprathreshold intensity consistently produced the largest MEPs in the target muscle was marked with a grease pencil as the ‘motor hot spot’. Baseline and post-rTMS measurements were performed over this marked area.
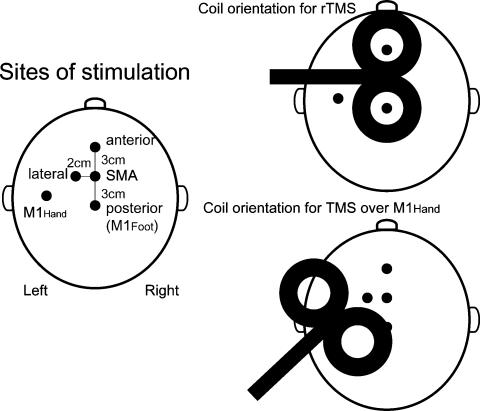
Figure 1. Sites of stimulation (left) and coil orientations for rTMS (top right) or for single-pulse TMS over the M1Hand (bottom right).
5Hz rTMS was given at four different scalp positions (SMA, 3 cm anterior, 3 cm posterior or 2 cm left from the SMA) all using the same coil orientation.
Stimulus intensities for TMS were determined at the beginning of each experiment. Resting motor threshold (RMT) was defined as the minimum output of the stimulator that induced a reliable MEP (about 50 μV in amplitude) in at least five of ten consecutive trials when the FDI muscle was completely relaxed. AMT was defined as the lowest stimulus intensity at which five of ten consecutive stimuli elicited reliable MEPs (about 200 μV in amplitude) during slight (10–15% maximum) tonic contraction of the target muscle.
Motor cortex excitability at rest (experiment 1)
Short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) were studied using the conditioning–test protocol introduced by Kujirai et al. (1993) with recordings from the right FDI muscle. Two monophasic magnetic stimuli were given through the same stimulating coil over the left M1Hand and the effect of the first (conditioning) stimulus on the second (test) stimulus was investigated. To avoid any floor or ceiling effect, we set the intensity of the conditioning stimulus to a relatively low value of 80% AMT. The test stimulus was adjusted to an intensity that, when given alone in control trials before rTMS, would evoke an EMG response of about 1 mV peak to peak. The following interstimulus intervals (ISIs) were tested: 2, 3, 10 and 15 ms. The five conditions (test pulse given alone and 4 conditioned pulses at different ISIs) were applied in a single block of 50 trials with an interval of 4 s between trials. In this block, which lasted approximately 3 min, the control condition (test pulse given alone) and each of the conditioning–test stimuli were tested ten times. The order of the conditions was randomised. Measurements were made on each individual trial. The mean peak-to-peak amplitude of the conditioned MEP at each ISI was expressed as a percentage of the mean peak-to-peak size of the unconditioned MEP in that block. The peak-to-peak amplitude of the unconditioned MEP in the relaxed right FDI muscle was used as a measure of corticospinal excitability. SICI was taken as the mean percentage inhibition of conditioned MEPs at ISIs of 2 and 3 ms, whilst ICF was taken as the mean facilitation at ISIs of 10 and 15 ms.
In experiment 2, we recorded the MEP from the right as well as the left FDI muscles after a single-pulse TMS of the contralateral M1Hand. The amplitude of the MEP was also set at about 1 mV peak to peak for the baseline before the rTMS.
Motor cortex excitability during contraction
In experiment 3, we measured the peak-to-peak amplitude of MEPs recorded during slight (10–15% maximum) tonic contraction of the right FDI muscle using an intensity of 120% RMT. In addition, we measured the duration of the cortical silent period (CSP), which is a marker for the excitability of long-lasting intracortical inhibition. For CSP measurements, EMG traces were rectified but not averaged. The mean length of the CSP was determined on the basis of measurements from each individual trial and defined as the interval between the onset of the MEP and the recovery of continuous EMG activity after the period of EMG suppression.
Assessment of spinal cord excitability
In experiment 4, we recorded the MEP and H reflex from the right FCR muscle at rest. MEP and H reflex stimuli were intermixed in random trials, with an interstimulus interval of 5 s. The H reflex was obtained by stimulating the median nerve at the elbow with a 500 μs electric pulse. For H reflex recording, the intensity of the stimulation was adjusted to produce half-maximal responses in each subject. For MEPs, the intensity of the stimulation was adjusted to produce responses of about 0.5 mV peak to peak. The peak-to-peak amplitude of the MEP and H reflex was measured for the analysis of rTMS parameters.
Five Hertz rTMS was performed over the hand area of the SMA. The site for SMA stimulation was determined in each subject, using a figure-of-eight coil with mean loop diameters of 9 cm, connected to a High Power Magstim 200 machine (monophasic pulse) as follows. First, the optimal position for activation of the right abductor hallucis (AH) muscle was determined by moving the coil in 1 cm steps along the sagittal mid-line around scalp vertex (Cz) with the handle pointing to the right. The AMT for this muscle was then determined. Next, stimuli at 120% AMT were given, moving the coil anteriorly along the sagittal mid-line in 1 cm steps. The SMA was defined as being 1 cm anterior to the last site from which MEPs could be evoked during contraction. Following these criteria, the site for the SMA stimulation was determined to be 3 cm anterior from the optimal position for activation of the AH muscle in most of the subjects (Fig. 1). This equated to a position of 1–4 cm (2–3 cm in most of the subjects) anterior to Cz.
Focal rTMS was performed using a figure-of-eight coil with mean loop diameters of 9 cm, connected to a Magstim Rapid stimulator (Magstim Co.). The magnetic stimulus had a biphasic waveform with a pulse width of approximately 300 μs. During the first phase of the stimulus, the current in the centre of the coil flowed towards the handle. Each individual's AMT over the M1Hand for the FDI muscle was determined prior to rTMS using the Magstim Rapid stimulator and the coil orientation with the handle pointing backwards and laterally at a 45 deg angle away from the mid-line. For the rTMS of the SMA, the coil was held tangentially to the skull with the handle pointing to the left to stimulate the left SMA predominantly (Fig. 1). A total of 750 single stimuli at 110% AMT for the FDI muscle were applied during a single rTMS session.
The 5 Hz rTMS session consisted of five trains of 150 stimuli separated by an intertrain interval of 30 s (5 min in total). The stimulation protocol was in accordance with published safety recommendations (Wassermann, 1998).
Experimental protocols
Seven different experiments were performed. Interstimulus intervals of 4 or 5 s were used in each experiment. In order to assess the time course of the effects of 5 Hz rTMS over the SMA, the following parameters were assessed before and immediately (0), 5, 10, 15 and 20 min after the end of the 5 Hz rTMS trains in experiments 1–6. In experiments 2–6, 20–40 responses before and 20 responses at each time point after the conditioning trains were recorded. The same subjects took part in several of the experiments, each on different days.
Experiment 1: assessment of the MEP, SICI and ICF recorded in the right relaxed FDI muscle
Eleven subjects (5 women, 6 men; mean age 31.7 ± 8.6 years) were studied in this experiment.
In four of them, we also examined SICI and ICF with three different intensities of test pulse in order to evoke test MEP amplitudes that covered the same range as those seen before and after rTMS to SMA. No rTMS was applied in this set of control experiments.
Experiment 2: assessment of the MEP in the right and left relaxed FDI muscles
Eleven subjects (5 women, 6 men; mean age 31.5 ± 8.6 years) were studied in this experiment.
Twenty responses were collected on each side at each time point.
Experiment 3: assessment of the MEP and CSP recorded in the right active FDI muscle
Eight subjects (4 women, 4 men; mean age 30.5 ± 4.2 years) were studied in this experiment.
Experiment 4: assessment of the MEP and H reflex recorded in the right relaxed FCR muscle
Nine subjects (4 women, 5 men; mean age 30.4 ± 8.8 years) were studied in this experiment. The intensity of the rTMS was set at 110% AMT for the FCR muscle.
Twenty responses were collected for each response type at each time point.
Experiment 5: effect of the position of the rTMS coil
Seven subjects (3 women, 4 men; mean age 33.7 ± 8.9 years) were studied in this experiment. The effect of 5 Hz rTMS over three different scalp positions (SMA, 3 cm anterior or 3 cm posterior to the SMA; see Fig. 1) on the amplitude of the MEP in the right relaxed FDI muscle was assessed on separate days in all seven subjects. The effect of 5 Hz rTMS over another scalp position (2 cm left of the SMA) on the amplitude of the MEP was also assessed on the separate days and compared to the effect of the rTMS over the SMA in four of them. We used the same coil orientation for rTMS at all of these positions.
Experiment 6: effect of the intensity of the rTMS
Eight subjects (3 women, 5 men; mean age 33.0 ± 8.5 years) were studied in this experiment.
The effect of 5 Hz rTMS over the SMA at two different intensities of 100 and 110% of AMT for the FDI muscle on the amplitude of the MEP in the right relaxed FDI muscle was assessed on separate days.
Experiment 7: effect of a conditioning stimulus over the foot motor area on the MEP in the right relaxed FDI muscle
Six subjects (1 women, 5 men; mean age 34.3 ± 8.2 years) were studied in this experiment using a paired-pulse technique. The test response in the right relaxed FDI muscle evoked by stimulation of the left M1Hand was conditioned by the stimulation of the left foot motor area (M1Foot) at ISIs of 2 and 3 ms. This experiment was designed to investigate the intensity needed for the conditioning stimulus over the left M1Foot to spread to M1Hand. We argued that if SICI could be produced in the FDI by a conditioning stimulus over the leg area, then the stimulus may have been sufficient to spread to the hand area and activate circuits involved in SICI. The equivalent intensity at the hand area of the conditioning stimulus would then be at least 70% AMT (the usual threshold for SICI). We then argued that if the current was insufficient to spread from the leg to hand area of primary motor cortex, then it was also unlikely that rTMS over SMA could spread to PMd, since the mediolateral distance is very similar. The experimental design relies on the fact that SICI is thought to be relatively focal within motor cortex (see intracortical stimulation experiments of Ashby et al. 1999).
The left M1Foot was defined as the optimum position for activation of the right abductor hallucis muscle as described in the rTMS parameters section. For the conditioning stimulation, the same coil connected to a Magstim Rapid stimulator and the same coil orientation over the M1Foot as those used for the rTMS were used and the intensity was set at 110, 130 and 150% of the AMT for the FDI muscle. For the test stimulus, a small (internal loop diameters of 3.5 cm) figure-of-eight coil connected to a High Power Magstim 200 machine (monophasic pulse) was used and the intensity was adjusted to evoke an MEP of about 1 mV peak to peak. The three conditions (test pulse given alone and two conditioned pulses) were tested ten times each in a single block. The order of the conditions was randomized. The mean peak-to-peak amplitude of the all conditioned MEPs at ISIs of 2 and 3 ms was expressed as a percentage of the mean peak-to-peak size of the unconditioned MEPs in each block performed using each conditioning stimulus intensity.
Data analysis
Mean values of each measure of the MEP and CSP before and at a given time point after the rTMS were measured in each subject. The mean MEP or H reflex amplitude at each time point after rTMS was expressed as a percentage of the mean amplitude before rTMS in experiments 2, 4, 5 and 6. The amplitudes of the MEP at rest and during contraction, and the duration of the CSP in experiments 1 and 3 were entered in separate one-way repeated measures ANOVA (analysis of variance) with ‘time’ as the within-subject factor. Percentage changes of the conditioned MEP relative to the unconditioned MEP were also entered in one-way repeated measures ANOVA with ‘intensity of conditioning stimulation’ (110 versus 130 versus 150 of AMT) in experiment 7 as the within-subject factor. Percentage changes of the conditioned MEP relative to the unconditioned MEP were entered in two-way repeated measures ANOVA with ‘time’ and ‘stimulation condition’ (SICI versus ICF) in experiment 1 as within-subject factors. SICI or ICF at baseline were entered in separate one-way repeated measures ANOVA with ‘test MEP amplitude’ in experiment 1. In addition, the amplitudes of the MEP or H reflex were entered in separate two-way repeated measures ANOVA with ‘time’ and ‘side of recording’ (right versus left FDI muscles) in experiment 2; ‘response type’ (MEP versus H reflex) in experiment 4; ‘position for rTMS’ (SMA versus sites 3 cm anterior or posterior to the SMA, SMA versus site 2 cm lateral to SMA) in experiment 5; and ‘intensity of rTMS’ (100 versus 110% of AMT) in experiment 6 as the within-subject factors. Post hoc analyses were performed with Student's paired t test. These t tests were not corrected for multiple comparisons because the main conclusions of our experiments are based solely on the ANOVAs. The Greenhouse-Geisser correction was used when necessary to correct for non-sphericity in the ANOVAs. A P value of < 0.05 was considered significant for all statistical analysis. Data are expressed as means ± s.e.m.
Results
None of the subjects reported adverse effects during and after rTMS.
Experiment 1: assessment of the MEP, SICI and ICF recorded in the right relaxed FDI muscle
Mean AMT was 32.6 ± 1.1% of maximum stimulator output. Mean intensities used in the paired-pulse TMS protocol were 57.5 ± 2.3% for the test pulse and 26.1 ± 0.8% for the conditioning stimulus. Mean AMT as determined with the rapid magnetic stimulator was 47.6 ± 1.4%. Mean rTMS intensity was 52.5 ± 1.5%.
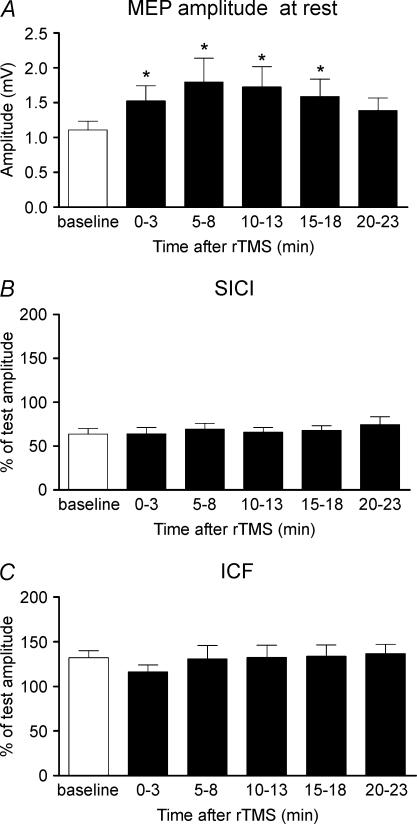
Figure 2 summarizes the effects of 5 Hz rTMS over the SMA on the unconditioned MEP, SICI and ICF as evaluated at rest during the paired-pulse protocol. Five hertz rTMS increased the amplitude of MEPs evoked by a single suprathreshold TMS stimulus in the right relaxed FDI muscle. A repeated measures ANOVA demonstrated a significant effect of ‘time’ on the mean MEP amplitude (F2,20= 4.4, P= 0.025). Post hoct tests revealed that the increase in MEP amplitude lasted at least for 15 min after the end of stimulation (0 min t=−3.1, P= 0011; 5 min t=−2.7, P= 0.022; 10 min t=−3.3, P= 0.008; and 15 min t=−3.75, P= 0.004; Fig. 2A). There was no lasting effect on the relative strength of SICI or ICF expressed as a percentage of unconditioned values (Fig. 2B and C).
Figure 2. Conditioning effects of 5 Hz rTMS over SMA at 110% AMT on MEP amplitude (A), SICI (B) and ICF (C) recorded from right relaxed first dorsal interosseous (FDI) muscle.
SICI was assessed using interstimulus-intervals (ISIs) of 2 and 3 ms. ICF was estimated using ISIs of 10 and 15 ms. Error bars are s.e.m. Asterisks denote a significant change relative to baseline.
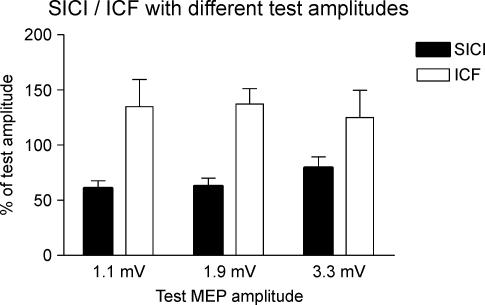
The lack of change in percentage SICI/ICF in Fig. 2 is difficult to interpret in view of the fact that the amplitude of the test MEP also changes after rTMS. In order to quantify how this might affect measures of SICI/ICF we conducted a separate control experiment in four of the same subjects who participated in experiment 1. In separate blocks of trials we adjusted the test intensity to evoke three different amplitudes of test MEP and then measured how much SICI/ICF was evoked by a constant conditioning pulse (Fig. 3). The first two amplitudes of the test MEP (1.1 and 1.9 mV) were the same as the pre- and post-rTMS test MEPs in these four subjects; the largest test MEP (3.3 mV) was larger than in any of the trials of experiment 1. A one-way repeated measures ANOVA with a factor of ‘test MEP amplitude’ revealed a significant main effect on SICI (F2,6= 5.2, P= 0.049), but not on ICF (F2,6= 0.56, P= 0.6). This was due to the fact that SICI with a test MEP amplitude of 1.9 mV was the same as SICI with a test MEP of 1.1 mV, whereas SICI with a test MEP of 3.3 mV was reduced. We conclude that the lack of change in SICI/ICF seen in Fig. 2 was unlikely to have been caused by the difference in test MEP sizes.
Figure 3. Control experiment evaluating the percentage SICI/ICF with three different amplitudes of test MEP.
Test MEPs were 1.1, 1.9 or 3.3 mV.
Experiment 2: assessment of the MEP in the right and left relaxed FDI muscles
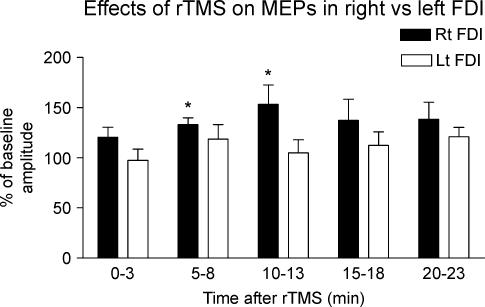
Figure 4 compares the effect of the 5 Hz rTMS over the SMA on the amplitude of MEPs recorded from right versus left relaxed FDI muscle. Five Hertz rTMS increased the amplitude of MEPs in the right FDI muscle, but had little effect on the left FDI muscle. Two-way repeated measures ANOVA revealed a significant main effect of ‘side of recording’ (F1,10= 7.49, P= 0.021) on the percentage changes of the post-rTMS amplitudes relative to the baseline values. Post hoct tests revealed that the increase in MEP amplitude in the right FDI muscle was present at 5 min (t=−5.3, P < 0.001) and 10 min (t=−2.7, P= 0.024) after rTMS, but that there was no lasting effect on MEP amplitude in the left muscle.
Figure 4. Conditioning effects of 5 Hz rTMS over SMA at 110% AMT on the amplitude of MEPs recorded from the rightvs. left relaxed FDI muscles.
MEP amplitude was expressed as a percentage of the baseline value (right FDI, 1.10 ± 0.13 mV; left FDI, 1.11 ± 0.13 mV). Error bars are s.e.m. Asterisks denote a significant change relative to baseline.
Experiment 3: assessment of the MEP and CSP recorded in the right active FDI muscle
The mean RMT in this experiment (without the bistim connector inserted) was 37.1 ± 3.2% of maximum stimulator output in eight subjects. Mean intensity for test MEPs was 44.5 ± 3.9%.
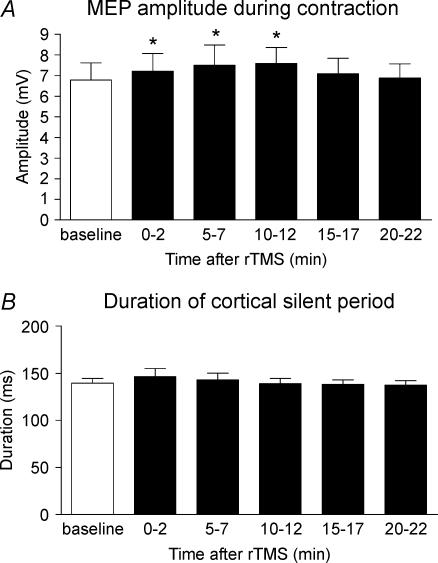
Figure 5 shows the effects of 5 Hz rTMS over the SMA on the MEP and CSP in the active right FDI. Five Hertz rTMS increased the amplitude of the MEP slightly, although baseline MEP amplitudes in the active FDI muscle were considerably larger than those evoked in the relaxed muscle. A repeated measures ANOVA demonstrated a significant effect of ‘time’ on the amplitude of the MEP (F5,35= 2.6, P= 0.04). Post hoct tests revealed a significant increase in MEP amplitude for at least 10 min after rTMS (0 min t=−3.1, P= 0.017; 5 min t=−2.4, P= 0.048; 10 min t=−3.3, P= 0.012; Fig. 5A). In contrast with the effect on the MEP, there was no significant effect on the duration of the CSP (Fig. 5B).
Figure 5. Conditioning effects of 5 Hz rTMS over SMA at 110% AMT on the amplitude of the MEP during contraction (A) and the duration of cortical silent period (B) recorded from right FDI muscle.
Error bars are s.e.m. Asterisks denote a significant change relative to baseline.
Experiment 4: assessment of the MEP and H reflex recorded in the right relaxed FCR muscle
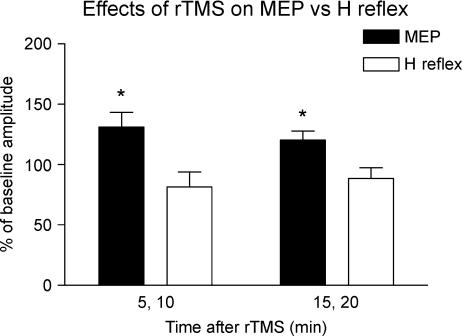
Figure 6 compares the effects of 5 Hz rTMS over the SMA on the amplitudes of the MEP and H reflex in the right FCR muscle. Five Hertz rTMS increased the amplitude of the MEP, but not that of the H reflex. A two-way repeated measures ANOVA demonstrated a significant main effect of ‘response type’ on the amplitudes (F1,8= 12.8, P= 0.007). Post hoct tests revealed a significant increase of MEP amplitude at 5–10 min (t=−2.6, P= 0.033) and at 15–20 min (t=−2.8, P= 0.024) after rTMS. There was no significant change in H reflex amplitude after rTMS.
Figure 6. Conditioning effects of 5 Hz rTMS over SMA at 110% AMT on the amplitude of the MEPs or H reflexes recorded from right relaxed flexor carpi radialis (FCR) muscle.
Error bars are s.e.m. Asterisks denote a significant change relative to baseline.
Experiment 5: effect of the position of the rTMS coil
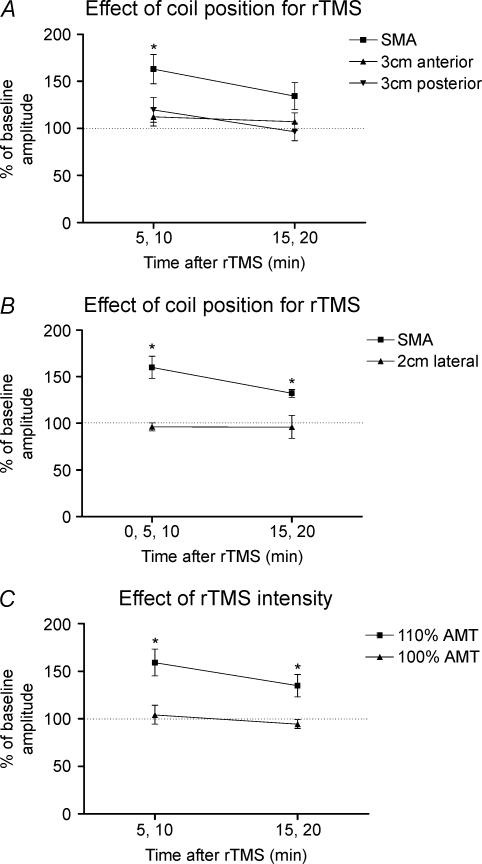
Figure 7A shows the effects of 5 Hz rTMS over three different anteroposterior scalp positions on percentage changes in post-rTMS MEP amplitudes relative to baseline values (see Fig. 7 legend for mean amplitudes of baseline MEPs) in the right relaxed FDI muscle. A two-way repeated measures ANOVA revealed a significant main effect of ‘position’ (F2,12= 11.9, P= 0.001). This was due to the fact that 5 Hz rTMS over SMA but not over the anterior or posterior scalp positions increased MEP amplitude. Post hoct tests revealed a significant increase in MEP amplitude at 5–10 min (t=−4.1, P= 0.007) after rTMS over SMA.
Figure 7. Effect of coil position and stimulus intensity on responses to rTMS.
A, conditioning effects of 5 Hz rTMS over SMA at 110% AMT vs. sites 3 cm anterior or posterior to SMA on MEP amplitudes 5–10 and 15–20 min after the end of rTMS. Data are given as percentages of pre-rTMS baseline values in the right relaxed FDI muscle of 7 subjects. Note that because the experiments examining differences in the effects from the three stimulations were performed on different days, the amplitudes of the baseline MEPs varied slightly (baseline MEPs before rTMS over SMA, 1.19 ± 0.14 mV; 3 cm anterior, 1.14 ± 0.11 mV; 3 cm posterior, 1.02 ± 0.13 mV). B, conditioning effects of 5 Hz rTMS over SMA at 110% AMT vs. a site 2 cm lateral to SMA on MEP amplitudes relative to the baseline values (baseline MEPs before rTMS over SMA, 1.16 ± 0.19 mV; 2 cm lateral, 1.22 ± 0.16 mV) in the right relaxed FDI muscle of 4 subjects. C, conditioning effects of 5 Hz rTMS over SMA at 110% AMT vs. 100% AMT on the percentage changes of the MEP amplitude relative to the baseline values (110% AMT, 1.18 ± 0.12 mV; 100% AMT, 1.09 ± 0.1 mV) in the right relaxed FDI muscle of 8 subjects. Error bars are s.e.m. Asterisks denote a significant change relative to baseline.
Figure 7B compares the effect of 5 Hz rTMS over the SMA with 5 Hz rTMS over a position 2 cm lateral to SMA. Only rTMS over SMA resulted in a change in size of MEP compared with baseline values. This was confirmed by a two-way repeated measures ANOVA that revealed a significant main effect of ‘position’ (F1,3= 25.9, P= 0.015). Post hoct tests revealed a significant increase in MEP amplitude at 0–10 min (t=−5.0, P= 0.015) and at 15–20 min (t=−7.6, P= 0.005) after rTMS over SMA. There was no significant change in MEP amplitude after rTMS over the lateral scalp position.
Experiment 6: effect of rTMS intensity
Figure 7C shows the effects of different intensities of 5 Hz rTMS over the SMA on MEPs in the right relaxed FDI muscle. A two-way repeated measures ANOVA revealed a significant main effect of ‘intensity’ (F1,7= 31.9, P= 0.001). This was due to the fact that 5 Hz rTMS over the SMA at 110% AMT but not at 100% AMT increased the MEP amplitude. Post hoct tests revealed a significant increase in MEP amplitude at 5–10 min (t=−4.2, P= 0.004) and at 15–20 min (t=−2.7, P= 0.03) after rTMS over SMA at 110% AMT. There was no significant change in MEP amplitude after rTMS at 100% AMT.
Experiment 7: effect of a conditioning stimulus over the foot motor area on the MEP in the right relaxed FDI muscle
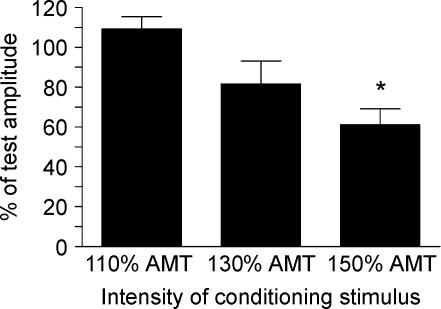
Figure 8 shows the mean percentage changes of the conditioned MEP relative to the unconditioned MEP in each block using three different conditioning intensities. Conditioning stimuli of the left M1Foot at stronger intensities tended to inhibit the MEP evoked by test stimuli of the left M1Hand. A repeated measures ANOVA revealed a significant main effect of ‘intensity’ on the percentage changes of the conditioned MEP relative to the unconditioned MEP (F2,10= 10.1, P= 0.004). A post hoct test revealed that the conditioning stimuli at 150% AMT (t= 4.84, P= 0.005) but not at 110 or 130% AMT significantly inhibited the MEP evoked in the right FDI muscle.
Figure 8. Effect of a conditioning stimulus over the left foot motor area on MEPs in the right relaxed FDI muscle using a paired-pulse TMS technique.
The test response in the right relaxed FDI muscle evoked by the stimulation of the left M1Hand was conditioned by stimulation of the left foot motor area (M1Foot) at ISIs of 2 and 3 ms. Error bars are s.e.m. Asterisks denote a significant change relative to unconditioned control values.
Discussion
The present results show that rTMS over a spatially distinct region of the SMA can lead to lasting changes in the excitability of the M1Hand. The particular rTMS parameters that we used (750 pulses of 5 Hz rTMS at 110% AMT for the FDI muscle) led to an increase in the amplitude of MEPs evoked in the right FDI in both the relaxed and active state for a period of at least 10 min after the end of rTMS. Since the H reflex in the right forearm was unchanged by rTMS, we suggest that the effects occurred because of changes in the excitability of the M1 rather than the cervical spinal cord.
Cortical area targeted by rTMS
It is difficult to be completely certain about the precise site of our SMA stimulus. Müri et al. (1995) centred the coil on the midsagittal line 5 cm anterior to the optimal position for leg muscle stimulation, whereas other studies found that the optimal positions for SMA stimulation were between 2 and 4 cm anterior to the Cz (Cunnington et al. 1996; Terao et al. 2001; Verwey et al. 2002; Serrien et al. 2002; Oliveri et al. 2003). Neuroimaging studies also have identified the hand area of the SMA proper, 2–3 cm anterior to Cz (Hikosaka et al. 1996; Lee et al. 1999). Thus the positions that we used for SMA stimulation, which were between 1 and 4 cm (2 and 3 cm in most subjects) anterior to Cz, correspond well with previous estimates. We shall refer to this site as the SMA proper, to distinguish it from the more anterior pre-SMA.
Are the after-effects on MEPs due to stimulation of SMA or to spread of the stimulus to other sites?
We used a relatively low intensity of rTMS (110% AMT of the FDI muscle) to try to avoid spread of the stimulus to other structures. In particular, given the pattern of the effects, we were concerned that the rTMS stimulus current might have spread to the premotor area or M1Hand. Because of this we performed a control experiment to try to estimate how much physical spread of the stimulus could occur from the SMA. The experiment was a version of the short-interval paired-pulse conditioning protocol of Kujirai et al. (1993). In that protocol a single conditioning stimulus over the M1Hand suppresses MEPs in the hand if the ISI is around 1–5 ms. The minimum intensity of conditioning stimulus required to produce this effect is about 70% AMT (Orth et al. 2003). We repeated the experiment with a conditioning coil over the M1Foot, and a test coil over the M1Hand, and found that conditioning stimuli of 110% AMT had no effect on MEPs from the M1Hand at ISIs of 2 and 3 ms. However, if we increased the intensity to 150% AMT, then inhibition occurred.
We argue that at 110% AMT, there could have been no physical spread of the stimulus from the M1Foot to the M1Hand since we saw no MEP suppression. Thus the effective intensity of any stimulus spread to the M1Hand must have been less than 70% AMT (the threshold for paired-pulse inhibition; Orth et al. 2003). Since the distance from M1Foot to M1Hand is similar to the distance between the point of SMA stimulation and the point that previous workers have used to activate PMd (e.g. Rizzo et al. 2003), we can apply similar reasoning and suggest that if the rTMS in the present experiments spread physically to PMd, then its effective intensity would be less than 70% AMT. Indeed, the coil orientation we used (lateromedial) is not as effective in eliciting after-effects from PMd on M1Hand as the usual anterior–posterior orientation (Gerschlager et al. 2001), and this would reduce the effectiveness of any stimulus spread even further. No previous studies have shown that there are any effects on the excitability of MEPs from M1Hand at such low intensities (Gerschlager et al. 2001; Rizzo et al. 2003), so we conclude that the effects we observed in the present experiments were due to rTMS around SMA and not to spread of the stimulus to PMd.
There were no after-effects on motor cortex excitability if the rTMS coil was moved 3 cm anterior or posterior or 2 cm lateral to the optimal point for SMA stimulation. This is consistent with the idea that the effects were induced by activation of the SMA proper rather than the pre-SMA or M1Foot, which are about 3 cm anterior or posterior to that location (Ikeda et al. 1999; Terao et al. 2001), or due to stimulation of the most medial part of the dorsal premotor cortex, which is immediately lateral to the SMA.
The intensity of rTMS that produced effects on the MEP was around 110% AMT (in the motor cortex hand area). At first sight this seems rather low, given that the threshold for evoking MEPs from the adjacent leg area of motor cortex is usually quite high and often requires a larger stimulating coil. However, paired-pulse experiments have shown that a conditioning stimulus of similar low intensity can lead to short-interval intracortical inhibition of leg muscle MEPs (Chen et al. 1998). Presumably, the threshold for activating a sufficient number of corticospinal output neurones to produce an MEP in the leg is higher than the threshold for activating other intracortical circuits. If the same is true for the SMA then this may explain the apparently low intensity needed in the present study. It should also be noted that similar low intensities of SMA stimulation have been successfully used by others to induce not only changes in corticospinal excitability (Civardi et al. 2001, 90% AMT; Oliveri et al. 2003, 70–90% RMT) but also to interrupt behavioural tasks (Terao et al. 2001, 110% AMT; Serrien et al. 2002, 90% AMT; Verwey et al. 2002, 90% RMT).
Effects on MEP versus H reflexes
Changes in the MEP can occur because of changes in cortical as well as spinal excitability. The SMA proper sends direct projections to the spinal cord (Dum & Strick, 1996) and so rTMS could potentially have activated this projection and changed the excitability of spinal circuits that participate in generating the MEP. However, direct activation of corticospinal neurones from the SMA seems unlikely given the low intensity of stimulation, especially in comparison with the intensity needed to activate corticospinal output from the leg area of motor cortex. Nevertheless, rTMS could have resulted in a change in the tonic level of corticospinal output from SMA, which could have had a similar lasting effect on the spinal cord. To clarify this, we tested whether the conditioning train affected spinal H reflexes recorded from the FCR muscle. It did not, even though the MEP was increased in the same muscle. The simplest explanation of this result is that changes in the MEP amplitude are not due to direct effects of the conditioning stimulus on excitability of spinal motoneurones. However, since MEPs and H reflexes can recruit different fractions of the spinal motoneurone pool in some muscle groups (Morita et al. 1999), and since presynaptic effects on the terminals of Ia afferents involved in the H reflex could have compensated for changes in excitability of spinal motoneurones, we cannot rule out entirely the possibility that rTMS over SMA produced some changes in spinal excitability.
No after-effects on paired-pulse excitability
We evaluated the excitability of circuits in the left M1Hand involved in producing the MEP, SICI/ICF and CSP. The only effect was on the MEP amplitude. Although such selectivity is unusual, it is not incompatible with the known differences in mechanism of these various effects, all of which have separate neural circuits within the cortex. There are other examples of rTMS being given over a distant site that projects to M1Hand in which changes in MEP amplitude also occur without any changes in SICI/ICF (Gilio et al. 2003; Rizzo et al. 2003). So the present results are not unique. Rizzo et al. (2003) reported that the effects on MEP or paired-pulse excitability of 5 Hz premotor rTMS depend on the intensity of rTMS: at 90% AMT, MEP amplitude increased without any changes in paired-pulse excitability; at 80% AMT, paired-pulse excitability at an ISI of 7 ms decreased without any changes in MEP amplitude. We used only one intensity for rTMS over SMA and evaluated only four ISIs of 2, 3, 10 and 15 ms for paired-pulse excitability. Thus, we do not feel able to draw any definitive conclusion about the effects of intensity on changes in paired-pulse excitability.
How does rTMS over SMA result in changes in corticospinal excitability in the hand area of the primary motor cortex?
Anatomical and physiological studies in animals (Dum & Strick, 1991; Luppino et al. 1993) have shown that there are large bilateral connections between M1 and SMA. The paired-pulse study of Civardi et al. (2001) suggested that these can be accessed by TMS of SMA, whilst recent imaging studies show they may also be activated by TMS over M1 (Siebner et al. 2000; Bestmann et al. 2003). So, were the present results due to the fact that rTMS directly activates cortico-cortical projections from SMA to M1 thus leading to secondary changes in circuits of M1? Conversely, did rTMS of SMA change the local balance of activity within SMA itself and secondarily lead to a change in the amount of tonic activity in connections between SMA and M1? The present experiments were not designed to distinguish between these possibilities. However, given the low intensity of rTMS, we suspect that stimulation did not activate any direct outputs from SMA, and that it is more likely to have changed the level of on-going activity in any connections that are tonically active. If so, the after-effects on M1 excitability are due to after-effects on the level of on-going activity in this connection. Interestingly, the data showed that the effects on MEP amplitude took several minutes to develop, implying that rTMS over SMA sets up a rather slow reaction in the motor cortical circuits responsible for the MEP. The fact that effects on the MEP were present whether the muscles were relaxed or actively contracting indicates that facilitation at rest was not simply due to a raised level of resting excitability, as could be produced, for example, by subthreshold depolarization of cortical motorneurones. The effects seem more likely to be due to a change in effectiveness of synaptic connections between neurones activated by a TMS pulse and the corticospinal output responsible for the MEP.
As noted at the start of this section, the double-pulse TMS experiments of Civardi et al. (2001) showed a predominant inhibitory effect of single-pulse stimulation of SMA on M1. Why did we observe a net excitatory effect with rTMS? The most likely answer is that the final outcome depends on the frequency and the intensity of the rTMS. In the experiments of Civardi, increasing the intensity of the conditioning pulse reversed the effect from inhibition (at 90% AMT) to facilitation (at 120% AMT). Similarly, the effects of rTMS itself depend on the frequency of stimulation, as illustrated by results from PMd. Repetitive TMS of the PMd in healthy subjects, at 1 Hz (90% AMT for the M1Hand) reduces MEP amplitude (Gerschlager et al. 2001), whereas MEPs are enhanced after 5 Hz rTMS (Rizzo et al. 2003). We conclude that the direction of the after-effect is not easily predictable and depends on the precise combination of neural elements activated by rTMS.
Another question is to what extent medial premotor cortex contributed to the effects we obtained. When we moved the rTMS coil 2 cm lateral we saw no lasting effects on MEPs, suggesting that premotor effects are small. In fact, previous studies in monkeys show that the somatotopy of premotor cortex lies roughly parallel to that of the M1, so the medial premotor area would contain mainly hindlimb or trunk representation areas rather than hand (Godschalk et al. 1995; Tokuno & Nambu, 2000). In addition, recent functional imaging studies have also shown a strong link between the M1Hand and lateral premotor cortex, but not between the M1Hand and medial premotor cortex in humans (Bestmann et al. 2003). Thus we speculate that the effects were produced mainly via functional interaction between SMA and M1Hand, although we cannot rule out the possibility that some of the effects had some contribution from the medial premotor cortex.
Finally it should be noted that we orientated the rTMS so that the most effective phase of the induced stimulus current flowed into the left SMA. This led to the largest effects on MEPs evoked in the right hand, and would be consistent with activation of connections between left SMA and left M1Hand.
Differences between the after-effects of rTMS to SMA and PMd
Rizzo et al. (2003) reported that 5 Hz rTMS over lateral premotor area at 90% AMT not only increased MEP amplitude but also decreased the duration of the CSP. However, 5 Hz rTMS over SMA increased MEP amplitude but did not change the duration of the CSP in the present study, even though we used a similar intensity of TMS (120% RMT) to evoke the CSP to Rizzo et al. (2003; about 115–125%). In order to confirm the difference between the effects of SMA and PMd on the CSP we made a direct comparison of the present data with the original data from Rizzo et al. (2003) and found them to be significantly different (premotor rTMS percentage change in CSP duration 89.4 ± 3.3% (n = 10), SMA rTMS 104.8 ± 5.1% (n = 8), Student's unpaired t test, t= 1.7, P= 0.016). This is therefore further evidence that the effects we observed from rTMS over SMA are mediated by different mechanisms to those from rTMS over PMd.
A final question is why rTMS over the premotor area can modulate CSP whereas rTMS over SMA has no effect? One possibility is that the high intensity of stimuli that are conventionally used to produce the CSP (120–150% RMT) spread from M1 and activate neurones outside M1, perhaps in the PMd. If this additional activation contributes to the CSP, then changes in the excitability of these neurones may be the substrate for the changes in CSP that occur after rTMS over PMd.
Conclusion
Five hertz rTMS over SMA at 110% AMT for the hand muscle can increase the amplitude of MEPs evoked from the left M1Hand at rest as well as during tonic voluntary contraction for at least 10 min. Although there may have been some contribution from the medial premotor cortex, we suggest that the majority of these after-effects are produced by modulating activity in the connections between M1Hand and SMA.
Acknowledgments
K.M. was supported by the Magnetic Health Science Foundation. The work was funded by the Medical Research Council.
References
- Ashby P, Reynolds C, Wennberg R, Lozano AM, Rothwell J. On the focal nature of inhibition and facilitation in the human motor cortex. Clin Neurophysiol. 1999;110:550–555. doi: 10.1016/s1388-2457(98)00082-0. 10.1016/S1388-2457(98)00082-0. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage. 2003;20:1685–1696. doi: 10.1016/j.neuroimage.2003.07.028. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9:832–842. [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Thickbroom GW, Laing BA, Mastaglia FL, et al. Effects of magnetic stimulation over supplementary motor area on movement in Parkinson's disease. Brain. 1996;119:815–822. doi: 10.1093/brain/119.3.815. [DOI] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Maertens de Noordhout A, Nakashima K, et al. Delay in the excution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain. Brain. 1989;112:649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallet M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120:1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Gilio F, Rizzo V, Siebner HR, Rothwell JC. Effects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J Physiol. 2003;551:563–573. doi: 10.1113/jphysiol.2003.044313. 10.1113/jphysiol.2003.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godschalk M, Mitz AR, Van Duin B, Van der Burg H. Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci Res. 1995;23:269–279. doi: 10.1016/0168-0102(95)00950-7. 10.1016/0168-0102(95)00950-7. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Putz B. Activation of human presupplementary motor area in learning of sequential procedures: a functional MRI study. J Neurophysiol. 1996;76:617–621. doi: 10.1152/jn.1996.76.1.617. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Yazawa S, Kunieda T, Ohara S, Terada K, Mikuni N, et al. Cognitive motor control in human pre-supplementary motor area studied by subdural recording of discrimination/selection-related potentials. Brain. 1999;122:915–931. doi: 10.1093/brain/122.5.915. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Chang KH, Roh JK. Subregions within the supplementary motor area activated at different stages of movement preparation and excution. Neuroimage. 1999;9:117–123. doi: 10.1006/nimg.1998.0393. 10.1006/nimg.1998.0393. [DOI] [PubMed] [Google Scholar]
- Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, et al. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:5308–5318. doi: 10.1523/JNEUROSCI.23-12-05308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Ziemann U, Hajak G, Cohen L, Berman KF. Transitions between dynamical states of differing stability in the human brain. Proc Natl Acad Sci U S A. 2002;99:10948–10953. doi: 10.1073/pnas.162114799. 10.1073/pnas.162114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-t. 10.1016/0168-5597(92)90096-T. [DOI] [PubMed] [Google Scholar]
- Morita H, Baumgarten J, Petersen N, Christensen LOD, Nielsen JB. Recruitment of extensor-carpi-radialis motor unit by transcranial magnetic stimulation and radial-nerve stimulation in human subjects. Exp Brain Res. 1999;128:557–562. doi: 10.1007/s002210050881. 10.1007/s002210050881. [DOI] [PubMed] [Google Scholar]
- Müri RM, Rivaud S, Vermersch AI, Léger JM, Pierrot-Deseilligny C. Effects of transcranial magnetic stimulation over the region of the supplementary motor area during sequences of memory-guided saccades. Exp Brain Res. 1995;104:163–166. doi: 10.1007/BF00229866. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Babiloni C, Filippi MM, Caltagirone C, Babiloni F, Cicinelli P, et al. Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Exp Brain Res. 2003;149:214–221. doi: 10.1007/s00221-002-1346-8. [DOI] [PubMed] [Google Scholar]
- Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114:2362–2369. doi: 10.1016/s1388-2457(03)00243-8. 10.1016/S1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Siebner HR, Modugno N, Pesenti A, Münchau A, Gerschlager W, et al. Shaping the excitability of human motor cortex with premotor rTMS. J Physiol. 2003;554:483–495. doi: 10.1113/jphysiol.2003.048777. 10.1113/jphysiol.2003.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Strens LH, Oliviero A, Brown P. Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neurosci Lett. 2002;328:89–92. doi: 10.1016/s0304-3940(02)00499-8. 10.1016/S0304-3940(02)00499-8. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Filipovic SR, Rowe JB, Cordivari C, Gerschlager W, Rothwell JC, et al. Patients with focal arm dystonia have increased sensitivity to slow-frequency repetitive TMS of the dorsal premotor cortex. Brain. 2003;126:2710–2725. doi: 10.1093/brain/awg282. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, et al. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54:956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- Steyvers M, Etoh S, Sauner D, Levin O, Siebner HR, et al. High-frequency transcranial magnetic stimulation of the supplementary motor area reduces bimanual coupling during anti-phase but not in-phase movements. Exp Brain Res. 2003;15:309–317. doi: 10.1007/s00221-003-1490-9. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Enomoto H, Furubayashi T, Shiio Y, Machii K, et al. Hemispheric lateralization in the cortical motor preparation for human vocalization. J Neurosci. 2001;21:1600–1609. doi: 10.1523/JNEUROSCI.21-05-01600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuno H, Nambu A. Organization of nonprimary motor cortical inputs on pyramidal and nonpyramidal tract neurons of primary motor cortex: an electrophysiological study in the macaque monkey. Cerebral Cortex. 2000;10:58–68. doi: 10.1093/cercor/10.1.58. 10.1093/cercor/10.1.58. [DOI] [PubMed] [Google Scholar]
- Verwey W, Lammens R, van Honk J. On the role of the SMA in the discrete sequence production task: a TMS study. Neuropsychologia. 2002;40:1268–1276. doi: 10.1016/s0028-3932(01)00221-4. 10.1016/S0028-3932(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. 10.1016/S0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]