Abstract
Rhythmic electrical activity is ubiquitous in neuronal networks of the brain and is implicated in a multitude of different processes. A prominent example in the healthy brain is electrical oscillations in the gamma-frequency band (20–80 Hz) in hippocampal and neocortical networks, which play an important role in learning, memory and cognition. An example in the pathological brain is electrographic seizures observed in certain types of epilepsy. Interestingly the activation of kainate receptors (KARs) plays an important role in synaptic physiology and plasticity, and can generate both gamma oscillations and electrographic seizures. Electrophysiological recordings of extracellular gamma oscillations and intracellular currents in a hippocampal slice combined with computer modelling can shed light on the expression loci of KAR subunits on single neurones and the distinct roles subunits play in rhythmic activity in the healthy and the pathologicalal brain. Using this approach in wild-type (WT) and KAR knockout mice it has been shown that KAR subunits GluR5 and GluR6 have similar functions during gamma oscillations and epileptiform bursts and that small changes in the overall activity in the hippocampal area CA3 can tilt the balance between excitation and inhibition and cause the neuronal network to switch from gamma oscillations to epileptiform bursts.
Gamma oscillations in the in vitro hippocampus
Over the last decade or so the investigation of gamma oscillations in in vitro slice preparations of the hippocampus and neocortex has intensified. This has been largely due to the discovery of suitable induction protocols for this rhythmic activity. Generally two induction methods can be distinguished: (1) induction by electrical stimulation, which generates transient episodes of gamma oscillations (Traub et al. 1996; Whittington et al. 1997) and (2) induction by chemically activating muscarinic receptors (Fisahn et al. 1998), group I metabotropic glutamate receptors (Fisahn, 1999) or kainate receptors (KARs) (Buhl et al. 1998; Fisahn, 1999; Hormuzdi et al. 2001), which results in the generation of the sustained gamma oscillations reported on here. All of these induction protocols target receptor families whose activation leads to an increased excitation of pyramidal neurones and/or interneurones. Genetic deletion of a receptor subtype contributing to the excitation of pyramidal neurones and/or interneurones prevents induction of gamma oscillations by agonists of that receptor family (Fisahn et al. 2002, 2004). However, because of the redundancy of largely excitatory receptor families gamma oscillations in hippocampal slices of those knockout mice can still be induced by agonists of one of the other receptor families (Fisahn et al. 2002, 2004). In contrast, all induction protocols for gamma oscillations crucially depend on intact inhibitory neurotransmission. Hence altering the time course of inhibitory events leads to alterations in the oscillation frequency (Wilson & Bower, 1992; Whittington et al. 1995; Traub et al. 1996; Fisahn et al. 1998, 2004) and blocking GABAARs results in the loss of rhythmic activity (Buhl et al. 1998; Fisahn et al. 1998, 2002, 2004; Fisahn, 1999).
Kainate receptors
Amongst pharmacological induction protocols for gamma oscillations the activation of KARs is especially interesting. Firstly, activation of KARs induces not only gamma oscillations in hippocampal and neocortical slice preparations but also epileptogenic bursts, and kainate injection has long been used as an animal model for epileptogenesis (Nadler, 1981; Ben-Ari, 1985; Ben-Ari & Cossart, 2000). Since both disrupted or altered gamma oscillations (Ribary et al. 1991) as well as some types of epilepsy (Prince, 1978) are implicated in learning and memory deficits as well as cognitive decline (Teitelbaum et al. 1990; Viskontas et al. 2000), questions arise about possible common mechanisms underlying the oscillogenic and epileptogenic effects of KAR activation. Secondly, KARs have long been the little-thought-of small brother of AMPARs and NMDARs. Only in recent years has the investigation of their role in synaptic and network function intensified. But the roles of specific KAR subunits in generating rhythmic activity in neuronal networks are only beginning to emerge.
Kainate receptors are widely expressed in the hippocampal formation, with the five subunits (GluR5–7, KA1–2) being expressed in distinct patterns in different areas of the hippocampus (Wisden & Seeburg, 1993; Bureau et al. 1999). Functional KARs are expressed at both presynaptic and postsynaptic sites and their activation has a multitude of effects (Chittajallu et al. 1996; Castillo et al. 1997; Clarke et al. 1997; Rodriguez-Moreno et al. 1997, 2000; Vignes & Collingridge, 1997; Cossart et al. 1998, 2001; Frerking et al. 1998, 1999; Contractor et al. 2000, 2001; Schmitz et al. 2000, 2001; Semyanov & Kullmann, 2001; for review see Lerma et al. 2001; Lerma, 2003). KAR antagonists prevent induction of mossy fibre LTP (Bortolotto et al. 1999), which is considered important for learning and memory (Muller et al. 2002). The distinct distribution pattern of KAR subunits strongly suggests that KARs fulfil different roles in the neuronal network that depend on their localization. However knowledge about expression loci of KAR subunits within single neurones is sparse, owing to the lack of sufficiently specific antibodies. Equally limited is our understanding of the role of the different KAR subunits in synaptic and network function, both in the healthy brain (i.e. gamma oscillations) and the pathological brain (epileptiform bursts). In the absence of specific histological labelling tools electrophysiological recordings of kainate-induced gamma oscillations and synaptic currents in wild-type (WT) and KAR knockout mice (GluR5−/− (Mulle et al. 2000), GluR6−/− (Mulle et al. 1998), GluR7−/−, KA2−/− (Contractor et al. 2003)) can yield information about KAR subunit expression on single neurones.
KAR knockout mice: gamma oscillations and epilepsy
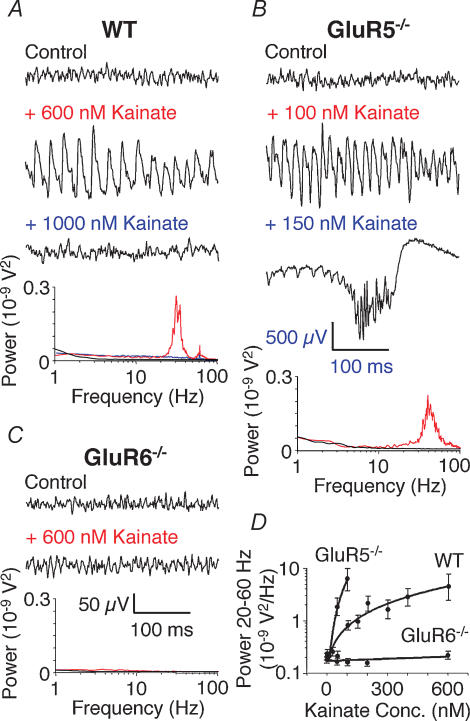
Activation of KARs induces gamma oscillations in the CA3 area of WT (Fig. 1A and D) as well as GluR7−/− and KA2−/− hippocampal slices. Increasing doses of KAR agonist eventually lead to a breakdown of the oscillation pressumably due to depolarization block of pyramidal neurones. In GluR5−/− KARs are activated and induce gamma oscillations of comparable power by much lower agonist concentrations and further increase of agonist concentration leads to epileptiform bursts (Fig. 1B and D). In contrast, in GluR6−/− KAR agonists fail to induce either gamma oscillations or epileptiform bursts (Fig. 1C and D). Gamma oscillations induced by KAR activation do not depend on NMDARs, mGluRs or AMPARs (Fig. 3B) but on KARs and GABAARs (Fisahn et al. 2004).
Figure 1. Kainate-induced gamma oscillations are disrupted in GluR6−/− but not GluR5−/− hippocampal slices.
A, example traces of extracellular field recordings in the CA3 area. In WT slices no rhythmic network activity is seen in control conditions (no drug; black line). Bath application of kainate induces gamma oscillations (red line). Increasing the kainate concentration leads to a breakdown of gamma oscillations (blue line). Power spectra of the recorded traces are shown below. B, in GluR5−/− slices maximal amplitude gamma oscillations are induced by much lower concentrations of kainate compared to WT (red line). Increasing the kainate concentration leads to the occurrence of epileptiform burst activity (500 μV scale bar applies only to 150 nm kainate trace). Power spectra of the recorded traces are shown below (the power spectrum of the trace showing an epileptiform burst is too large to be displayed). C, in GluR6−/− slices kainate fails to induce either gamma oscillations or epileptiform bursts (red line). Power spectra of the recorded traces are shown below. D, summary diagram showing the dependence of gamma oscillation power (integrated between 20 and 60 Hz) on kainate concentration. WT and GluR5−/− data points are shown only for kainate concentrations that resulted in gamma oscillations (n = 6 for WT, GluR5−/− and GluR6−/−). Figure redrawn from Fisahn et al. (2004); © Journal of Neuroscience 2004.
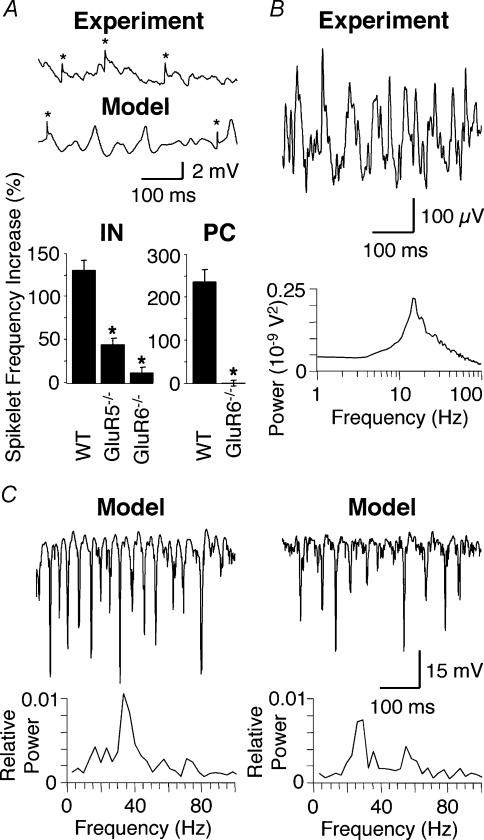
Figure 3. Ectopic action potentials and computer modelling of kainate-induced gamma oscillations.
A, the two example traces show ectopic action potentials recorded as spikelets (*) in pyramidal cells during an ongoing gamma oscillation. The upper trace is a physiological recording during kainate-induced gamma oscillations in WT; the lower trace is computer generated. The summary bar graphs show the kainate-induced increase of spikelet frequency recorded in interneurones (IN) and pyramidal cells (PC). The increase of spikelet frequency seen in WT IN is significantly reduced in GluR5−/− (*P < 0.0002) and GluR6−/− IN (P < 0.0001). It is notable that the increase of spikelet frequency in GluR5−/− is significantly larger than in GluR6−/− (P < 0.012). Likewise, the increase of spikelet frequency seen in WT PC is significantly reduced in GluR6−/− (*P < 0.0001) (IN: n = 5 for WT, n = 6 for GluR5−/−, n = 5 for GluR6−/−; PC: n = 6 for WT, n = 5 for GluR6−/−). B, physiological example trace of extracellular gamma oscillations induced by 100 nm kainate in the presence of 50 μm GYKI53655 in a WT hippocampal slice (n = 3). Compared to kainate-induced gamma oscillations without the AMPA receptor antagonist present (see Fig. 1A) the oscillation frequency has decreased to around 18 Hz. C, computer-generated example trace of extracellular gamma oscillations. The power spectrum of the example trace is shown below and exhibits a prominent peak around 40 Hz. For comparison with physiological recordings see Fig. 1A). D, computer-generated example trace of extracellular gamma oscillations in the absence of the ‘AMPA receptor’ component. The power spectrum of the example trace is shown below and exhibits a prominent peak around 25 Hz. Figure redrawn from Fisahn et al. (2004); © Journal of Neuroscience 2004.
Direct support for the involvement of KARs in status epilepticus comes from studies on KAR knockout mice and human studies. GluR6−/− mice are less susceptible to seizures following kainate injections than WT mice (Mulle et al. 1998). In addition GluR5 mRNA levels are decreased in patients suffering from temporal lobe epilepsy (Mathern et al. 1998) and activation of GluR5-containing receptors can reduce the propagation of seizures (Khalilov et al. 2002). The results by Mulle et al. (1998) and Khalilov et al. (2002) mesh with the gamma oscillation phenotype of the GluR6−/− and GluR5−/− hippocampal network. Taken together these in vitro gamma oscillation and in vivo status epilepticus argue for a common network mechanism underlying both rhythmic activities. Another common factor between gamma oscillations and status epilepticus is the importance of recurrent connectivity in hippocampal area CA3. Gamma oscillations are generated in area CA3 but not CA1, which lacks recurrent connectivity (Fisahn, 1999), and the high level of KAR expression in area CA3 (Wisden & Seeburg, 1993; Bureau et al. 1999) as well as its inherent recurrent connectivity render this region especially sensitive to the epileptogenic and neurotoxic effects of kainate (Westbrook & Lothman, 1983).
From the above results alone we can already conclude that the KAR subunit GluR6 is involved in mediating kainate-induced excitation in the hippocampal network. If this subunit is lacking, KAR activation fails to generate gamma oscillations in the slice preparation and kainate-injected mice are less likely to develop electrographic seizures. In contrast the GluR5 subunit may be involved in setting the level of inhibition within the network. If it is missing, kainate-induced excitation mediated by other KARs results in unchecked depolarization of pyramidal neurones. This activity could overpower its lessened inhibitory restraints and lead to electrographic seizures.
KAR knockout mice: cellular and synaptic currents
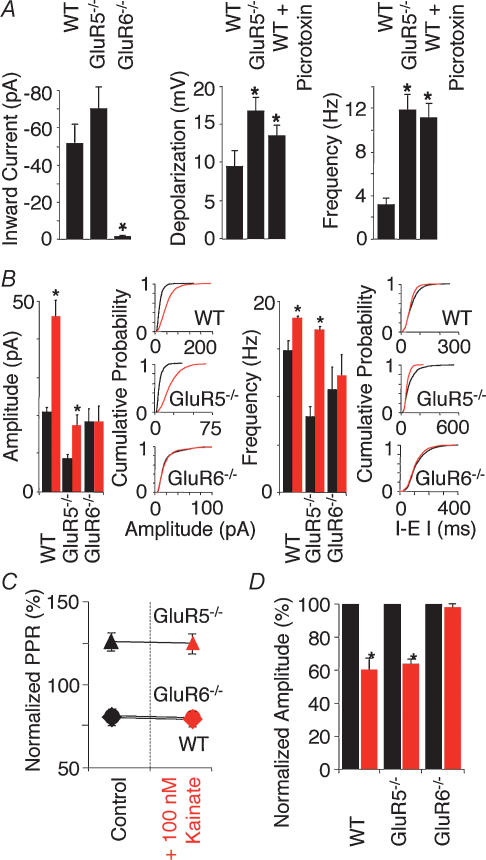
Activation of KARs causes inward currents and hence depolarization and increase in action potential firing in pyramidal neurones and interneurones in WT and GluR5−/− but not GluR6−/− (Fisahn et al. 2004) (Fig. 2A). Interestingly the kainate-induced depolarization and concomitant increase in action potential firing in GluR5−/− interneurones is significantly larger than observed in WT (Fisahn et al. 2004) (Fig. 2A). It is possible that GluR5-containing KARs play a role in promoting spontaneous GABA release from interneurones as previously described (Semyanov & Kullmann, 2001). A lack of GluR5 would result in reduced GABA release and a decrease in hippocampal inhibitory tone permitting a greater depolarization by excitatory afferents. This appears to be the case since partial block of inhibitory neurotransmission in WT results in increased depolarization and action potential firing in WT interneurones (Fisahn et al. 2004) (Fig. 2A). Taken together, these data suggest that KARs containing the GluR6 but not the GluR5 subunit, are essential for the depolarization and increased excitability of pyramidal cells and interneurones, and highlight a role for GluR5 receptors in regulating the inhibitory tone of the local circuit as mediated by interneurones.
Figure 2. Kainate-induced changes of inward current, sIPSC and eIPSC are absent in GluR6−/− but not WT and GluR5−/− interneurones and pyramidal neurones.
A, in voltage clamp (Vh = −60 mV) bath application of 100 nm kainate induces an inward current in WT (−50.8 ± 9.5 pA; n = 8) and GluR5−/− (−71.4 ± 11.6 pA; n = 5), but not GluR6−/− interneurones (−1.9 ± 1.3 pA; n = 5; P = 0.002). In current clamp (interneurones held below firing threshold) bath application of 100 nm kainate leads to a depolarization and increase in firing frequency in WT interneurones (9.5 ± 2.0 mV; 3.2 ± 0.6 Hz; n = 11). Kainate-induced depolarization and increase in firing frequency are significantly bigger in GluR5−/− interneurones (16.8 ± 1.7 mV; 11.9 ± 1.4 Hz; n = 9; P < 0.02 depolarization, P < 0.0001 frequency) than in WT interneurones. Partly compromising inhibitory neurotransmission by application of 10 μm picrotoxin results in increased depolarization and firing frequency in WT interneurones (13.6 ± 1.4 mV; 11.2 ± 2.1 Hz; n = 4; P < 0.02 depolarization, P < 0.0001 frequency). B, in voltage clamp (Vh = 0 mV) bath application of 100 nm kainate (red) induces an increase in sIPSC amplitude and frequency in both WT and GluR5−/− but not GluR6−/− pyramidal neurones. Summary histogram and representative cumulative probability plots are shown. Kainate increases sIPSC amplitude and frequency in WT. In GluR5−/− sIPSC control amplitude and frequency is approximately half of WT control values. In GluR6−/− sIPSC amplitude and frequency remains unchanged by kainate at WT control levels. (n = 6 for WT and GluR5−/−, n = 5 for GluR6−/−; amplitude: P = 0.02 for WT and P < 0.09 for GluR5−/−; frequency: P < 0.1 for WT and P < 0.02 for GluR5−/−). C, paired eIPSCs show paired-pulse depression in WT and GluR6−/− but facilitation in GluR5−/− pyramidal cells in control conditions (black) as well as after application of 100 nm kainate (red). Paired pulse ratios (PPR) remain unchanged after the application of kainate. D, concomitantly, kainate (red) depresses eIPSC amplitude in WT and GluR5−/− but has no effect in GluR6−/− (n = 5 for WT and GluR6−/−, n = 4 for GluR5−/−; P < 0.0001). Figure redrawn from Fisahn et al. (2004); © Journal of Neuroscience 2004.
Activation of KARs leads to a significant increase of sIPSC amplitude and frequency in WT and GluR5−/− but not GluR6−/− pyramidal neurones (Fisahn et al. 2004) (Fig. 2B). The absence of sIPSC changes in GluR6−/− again points towards a location of the GluR6 subunit with maximum influence on action potential generation, i.e. the cell soma. It is interesting to note that the reported basal level of IPSC amplitude and frequency is significantly lower in GluR5−/− compared to WT (Fisahn et al. 2004) (Fig. 2B). This could indicate a lowered excitability in the inhibitory axonal network and a resulting lowered efficacy of GABAergic synapses as recently described by Jiang et al. (2001). Unlike sIPSC amplitude and frequency, action potential-independent transmitter release (mIPSC) is not affected by KAR activation (Cossart et al. 1998; Frerking et al. 1998, 1999; Semyanov & Kullmann, 2001; Fisahn et al. 2004; but see Rodriguez-Moreno et al. 1997). This argues against an expression of KARs, possibly containing the GluR5 subunit, directly on the terminal of interneurone-to-pyramidal neurone synapses.
Several studies of KAR physiology have indicated that their activation increases sIPSC frequency while decreasing eIPSC amplitude (Rodriguez-Moreno et al. 1997; Cossart et al. 1998; Frerking et al. 1998, 1999). Explanations to resolve this apparent contradiction include direct actions of KAR agonists on GABA release via presynaptic KARs (Rodriguez-Moreno et al. 1997; Rodriguez-Moreno & Lerma, 1998), or a kainate-induced increase in GABA release, which acts on both pre- and postsynaptic GABARs (Frerking et al. 1998, 1999; Fisahn et al. 2004). In the latter scenario both presynaptic GABABRs and postsynaptic GABAARs are activated, which subsequently depress further GABA release and decrease the postsynaptic input resistance (increase postsynaptic shunting), respectively (Frerking et al. 1999; Fisahn et al. 2004). Evoked IPSCs show paired-pulse depression in WT and GluR6−/− but paired-pulse facilitation in GluR5−/− pyramidal neurones (Fisahn et al. 2004) (Fig. 2C). This points to a lower initial release probability from interneurone-to-pyramidal neurone synapses compared to WT and GluR6−/− and a role for the GluR5 subunit in setting the depolarizational level of the axonal network. Furthermore, activation of KARs depresses eIPSC amplitude in WT and GluR5−/− but not GluR6−/− pyramidal neurones (Fig. 2D) while leaving the paired-pulse ratio unaffected in all three mouse strains (Fisahn et al. 2004) (Fig. 2C).
Consistent with the hypothesis that the kainate-induced increase in spontaneous GABA release increases postsynaptic shunting, a concomitant reduction in input resistance in both WT and GluR5−/− pyramidal neurones and interneurones was observed (Fisahn et al. 2004). In GluR6−/− the input resistance change is significantly smaller in both pyramidal neurones and interneurones. Taken together these data argue against a direct action of kainate at the presynaptic terminal in modulating GABA release and suggest a GluR6-dependent mechanism, involving postsynaptic shunting via GABAARs (Frerking et al. 1999; Fisahn et al. 2004).
KAR knockout mice: modulation of neuronal firing properties
Increasing tonic excitation of pyramidal neurones and interneurones is not everything that is needed to induce gamma oscillations, however. Changes to neuronal firing characteristics such as switching from burst- to single-spike mode in pyramidal neurones may be another contributing factor (Fisahn, 1999). The length and frequency of bursts of action potentials is governed by a number of conductances including a calcium-activated potassium current with slow decay time, which hyperpolarizes the cell membrane (IsAHP; Madison & Nicoll, 1984; Lancaster & Adams, 1986; Traub et al. 1993). Likewise the length and frequency of single action potentials is influenced by calcium-activated hyperpolarizing potassium currents, which in pyramidal neurones have been designated as having medium and fast decay times (ImAHP, IfAHP; Brown & Griffith, 1983; Storm, 1987). A recent study showed that IsAHP is decreased by direct activation of KARs on CA1 pyramidal cells (Melyan et al. 2002, 2004). A related study published in this issue shows that the medium and slow AHP currents are modulated by GluR6-containing KARs but not GluR5-containing KARs. Therefore GluR6-containing KARs appear to have an important influence on the firing frequency of pyramidal neurones.
Electrophysiology and computer modelling
One tool that has been very beneficial to the investigation of gamma oscillations and their underlying mechanisms is the computer modelling of large neuronal networks (Traub et al. 1996, 2000, 2001, 2003; Wang & Buzsáki, 1996). In the simulation of gamma oscillations ectopic spikes in the axonal network are an essential component (Traub et al. 2000, 2003) (Fig. 3A). By laterally communicating through gap-junction-connected axonal networks, ectopic action potentials are thought to facilitate synchronization in the neuronal network. Testing this in experiments shows that KAR activation increases the frequency of ectopic action potentials in both WT interneurones and pyramidal neurones. The increase in spikelet frequency seen in WT is significantly reduced in GluR5−/− and GluR6−/− interneurones and pyramidal neurones (Fisahn et al. 2004) (Fig. 3A). This suggests the existence of two populations of spikelets: one GluR5 dependent and presumably originating from axon-generated action potentials (Semyanov & Kullmann, 2001); the other GluR6 dependent and presumably originating from soma-generated action potentials in adjacent cells. Both axon- and soma-generated action potentials could cross via gap junctions into axons of neighbouring cells where they travel antidromically to the soma and are recorded as spikelets, or orthodromically to synapses to initiate transmitter release.
Just as computer simulations can suggest directions for experimental research so can experimental data in turn provide directions for refinement of the network computer models. In previous models (Traub et al. 2000) the excitatory component was assumed to be carried by AMPAR-like receptors and modelled accordingly. However, gamma oscillations induced by the activation of KARs are independent of AMPARs (Fisahn et al. 2004) (Fig. 3B). This has resulted in an alteration of the computer model to allow a two component synaptic excitation of interneurones: a fast ‘AMPA’ component (τ = 1 ms) and a slower and smaller ‘kainate’ component (τ = 5 ms) (Fisahn et al. 2004). The adapted model correctly simulates the moderate decrease in oscillation frequency when AMPARs are selectively blocked in the hippocampal slice (Fig. 3C and D).
Summary
The lack of the KAR subunits GluR5 or GluR6 produces distinct phenotypes of gamma oscillations: heightened susceptibility to epileptiform bursts in response to kainate in the absence of GluR5, and complete failure of kainate to induce gamma oscillations or epileptiform bursts in the absence of GluR6. These distinct phenotypes suggest differential roles played by the GluR5 and GluR6 subunits in kainate-induced gamma oscillations and epileptiform bursts and, together with intracellular recordings, point towards likely loci of expression on single neurones: KARs containing GluR5 expressed on axons of interneurones and for GluR6-containing KARs expressed in the somato-dendritic region of both interneurones and pyramidal cells. The distinct roles the KAR subunits GluR5 and GluR6 play in rhythmic activity in the healthy brain (i.e. gamma oscillations) also extend to rhythmic activity in the pathological brain (epileptiform bursts) and it appears that small changes in the overall activity of the area CA3 network can tilt the balance between excitation and inhibition and cause the neuronal network to switch from gamma oscillations to epileptiform bursts.
The in vitro hippocampal network when challenged with suitable concentrations of kainate receptor agonists such as domoate or kainate generates gamma oscillations. When the make-up of the network is altered by genetic engineering (e.g. knocking out a KAR subunit (Fisahn et al. 2004) or a gap junction subtype (Hormuzdi et al. 2001)) the response of the network to KAR activation, its ‘phenotype’, can be altered. From these changes in electrophysiologically recorded extracellular field activity insights can be obtained about the role the deleted structure plays in the wild-type network. Gamma oscillations in in vitro slice preparations can therefore serve as a tool to help investigate the role of receptor subunits (Fisahn et al. 2002, 2004), gap junctions (Hormuzdi et al. 2001), etc. in the neuronal network using electrophysiological means. Coupled with intracellular patch clamp recordings this approach can yield clues as to the locus of expression of these structures on single neurones.
References
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neurosci. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Brown DA, Griffith WH. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Tamás G, Fisahn A. Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J Physiol. 1998;513:117–126. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Bischoff S, Heinemann SF, Mulle C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J Neurosci. 1999;19:653–663. doi: 10.1523/JNEUROSCI.19-02-00653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GM, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- Clarke V, Ballyk B, Hoo K, Mandelzys A, Pellizzari A, Bath C, Thomas J, Sharpe E, Davies C, Ornstein P, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2-/- mice. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Sailer A, O'Gorman S, Heinemann SF. Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J Neurosci. 2000;20:8269–8278. doi: 10.1523/JNEUROSCI.20-22-08269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Cossart R, Tyzio R, Dinocourt C, Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron. 2001;29:497–508. doi: 10.1016/s0896-6273(01)00221-5. [DOI] [PubMed] [Google Scholar]
- Fisahn A. Oxford University: 1999. An investigation into cortical gamma frequency oscillations in vitro. DPhil Thesis. [Google Scholar]
- Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann S, McBain CJ. Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci. 2004;24:9658–9668. doi: 10.1523/JNEUROSCI.2973-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Pike F, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, McBain CJ, Wess J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron. 2002;33:615–624. doi: 10.1016/s0896-6273(02)00587-1. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- Frerking M, Petersen CCH, Nicoll RA. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus. Proc Natl Acad Sci U S A. 1999;96:12917–12922. doi: 10.1073/pnas.96.22.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormuzdi S, Pais I, LeBeau FEN, Towers SK, Rozov A, Buhl EH, Whittington M, Monyer H. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- Jiang L, Xu J, Nedergaard M, Kang J. A kainate receptor increases the efficacy of GABAergic synapses. Neuron. 2001;30:503–513. doi: 10.1016/s0896-6273(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Hirsch J, Cossart R, Ben-Ari Y. Paradoxical anti-epileptic effects of a GluR5 agonist of kainate receptors. J Neurophysiol. 2002;88:523–527. doi: 10.1152/jn.2002.88.1.523. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Rodriguez-Moreno A, Lopez-Garcia JC. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA1 pyramidal neurones in vitro. J Physiol. 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Kornblum HI, Mendoza D, Lozada A, Leite JP, Chimelli L, Born DE, Fried I, Sakamoto AC, Assirati JA, Peacock WJ, Ojemann GA, Adelson PD. Altered hippocampal kainate-receptor mRNA levels in temporal lobe epilepsy patients. Neurobiol Dis. 1998;5:151–176. doi: 10.1006/nbdi.1998.0200. [DOI] [PubMed] [Google Scholar]
- Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron. 2002;34:107–114. doi: 10.1016/s0896-6273(02)00624-4. [DOI] [PubMed] [Google Scholar]
- Melyan Z, Wheal HV, Lancaster B. Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors. J Neurosci. 2004;24:4530–4534. doi: 10.1523/JNEUROSCI.5356-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Swanson GT, Brana C, O'Gorman S, Bettler B, Heinemann SF. Subunit composition of kainate receptors in hippocampal interneurons. Neuron. 2000;28:475–484. doi: 10.1016/s0896-6273(00)00126-4. [DOI] [PubMed] [Google Scholar]
- Muller D, Nikonenko I, Jourdain P, Alberi S. LTPmemory and structural plasticity. Curr Mol Med. 2002;2:605–611. doi: 10.2174/1566524023362041. [DOI] [PubMed] [Google Scholar]
- Nadler JV. Minireview. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- Prince DA. Neurophysiology of epilepsy. Annu Rev Neurosci. 1978;1:395–415. doi: 10.1146/annurev.ne.01.030178.002143. [DOI] [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, Mogilner A, Llinas R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci U S A. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron. 1998;20:1211–1218. doi: 10.1016/s0896-6273(00)80501-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, López-Garcia JC, Lerma J. Two populations of kainate receptors with separate signaling mechanisms in hippocampal interneurons. Proc Natl Acad Sci U S A. 2000;97:1293–1298. doi: 10.1073/pnas.97.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Frerking M, Nicoll RA. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Kullmann DM. Kainate receptor-dependent axonal depolarization and action potential initiation in interneurons. Nat Neurosci. 2001;4:718–723. doi: 10.1038/89506. [DOI] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, Cashman NR. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N Engl J Med. 1990;322:1781–1787. doi: 10.1056/NEJM199006213222505. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, Fisahn A, LeBeau FEN, Whittington MA, Buhl EH. A model of gamma-frequency network oscillations induced in the rat CA3 region by charbachol in vitro. Eur J Neurosci. 2000;12:4093–4106. doi: 10.1046/j.1460-9568.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Traub RD, Kopell N, Bibbig A, Buhl EH, LeBeau FEN, Whittington MA. Gap junctions between interneuron dendrites can enhance long-range synchrony of gamma oscillations. J Neurosci. 2001;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Miles R, Jefferys JGR. Synaptic and intrinsic conductances shape picrotoxin-induced synchronized after-discharges in the guinea-pig hippocampal slice. J Physiol. 1993;461:525–547. doi: 10.1113/jphysiol.1993.sp019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Pais I, Bibbig A, LeBeau FEN, Buhl EH, Hormuzdi SG, Monyer H, Whittington MA. Contrasting roles of axonal (pyramidal cell) and dendritic (interneuron) electrical coupling in the generation of gamma oscillations in the hippocampus in vitro. Proc Natl Acad Sci U S A. 2003;100:1370–1374. doi: 10.1073/pnas.0337529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsáki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996;493:471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, McAndrews MP, Moscovitch M. Remote episodic memory deficits in patients with unilateral temporal lobe epilepsy and excisions. J Neurosci. 2000;20:5853–5857. doi: 10.1523/JNEUROSCI.20-15-05853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Buzsáki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook GL, Lothman EW. Cellular and synaptic basis of kainic acid-induced hippocampal epileptiform activity. Brain Res. 1983;273:97–109. doi: 10.1016/0006-8993(83)91098-3. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Stanford IM, Jefferys JG, Traub RD. Spatiotemporal patterns of gamma frequency oscillations tetanically induced in the rat hippocampal slice. J Physiol. 1997;502:591–602. doi: 10.1111/j.1469-7793.1997.591bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Wilson M, Bower JM. Cortical oscillations and temporal interactions in a computer simulation of piriform cortex. J Neurophysiol. 1992;67:981–995. doi: 10.1152/jn.1992.67.4.981. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]