Abstract
Although rhythmic behaviour of mammalian spinal ventral horn networks has been extensively studied little is known about oscillogenesis in the spinal dorsal horn. The aims of this in vitro study were to record and determine the underlying mechanisms of potassium-evoked network field oscillations in the substantia gelatinosa of the neonatal rat dorsal horn, a lamina involved in nociceptive processing. Transient pressure ejection of a potassium solution evoked reproducible rhythmic activity in discrete areas of the substantia gelatinosa which lasted for 5–15 s with a single prominent peak in the 4–12 Hz frequency band (7.7 ± 0.1 Hz, n = 60). Oscillations of similar frequency and amplitude were also observed in isolated dorsal horn quadrants. Application of CNQX (10 μm) reduced peak power amplitude and integrated power area (from 4 to 12 Hz) of the power spectrum, whereas d-AP5 (50 μm) had no effect on the potassium-evoked rhythm. Bicuculline (30 μm) or strychnine (10 μm) reduced the power amplitude and area. On combination of bicuculline (30 μm) and strychnine (10 μm) the reductions in power amplitude and area were not significantly different (P > 0.05) when compared with application of either drug alone. The gap junction blockers carbenoxolone (100 μm) or octanol (1 mm) significantly reduced power amplitude and area. Although TTX (1 μm) or a calcium-free perfusate both caused reductions in the power amplitude and area, potassium-evoked rhythmic activity persisted. However, this persistent rhythm was further reduced on combination of calcium-free perfusate with octanol (1 mm) and was abolished using a cocktail of drugs. Blockade of the potassium delayed rectifier current by tetraethylammonium (5 mm) or the hyperpolarization-activated current (Ih) by ZD7288 (10 μm) disrupted the synchronization of the potassium-induced oscillation. The frequency of potassium-induced rhythms was unaffected by any of the drugs tested. These novel findings demonstrate that transient pressure ejection of potassium evokes oscillatory activity in the substantia gelatinosa in vitro. This rhythm is partly dependent upon various receptors (AMPA/kainate, GABAA and glycine), ion channels (potassium delayed rectifier and Ih) and gap junctions. Oscillatory behaviour in the substantia gelatinosa could potentially play a role in the processing of nociceptive signals.
Rhythmic activity attributable to neuronal networks has been extensively documented in the ventral horn of the spinal cord of many mammalian species both in vivo and in vitro (Kudo & Yamada, 1987; Smith & Feldman, 1987; Dale & Kuenzi, 1997; Kiehn et al. 2000; Butt et al. 2002; Kiehn & Butt, 2003). Such ventral horn networks or central pattern generators typically drive slow co-ordinated activity within motoneurone pools (< 1 Hz) which elicit locomotor outputs (Dale & Kuenzi, 1997). Upon electrical stimulation of dorsal root fibres, much faster oscillations with a peak of ∼8 Hz have been described using intracellular recordings from neonatal rat motoneurones (Baranauskas & Nistri, 1995). In striking contrast, there have only been a few investigations which have sought to study rhythmic behaviour of neuronal networks in the spinal dorsal horn. Such studies are particularly warranted since the spinal dorsal horn plays a crucial role in the processing of somatosensory information including nociception.
In vivo studies in the dorsal horn using spectral analysis of background activity reveal rhythmic behaviour in populations of rat nociceptive and non-nociceptive neurones (Sandkühler & Eblen-Zajjur, 1994; Eblen-Zajjur & Sandkühler, 1997). The distribution of fundamental frequencies of these background neuronal oscillations was bimodal with peaks around 2 and 10 Hz (Sandkühler & Eblen-Zajjur, 1994). A functional role for rhythmic discharging in information transfer across these networks which subserve somatosensation was inferred from qualitative changes in cross-correlation patterns between neuronal pairs after sensory stimuli (Eblen-Zajjur & Sandkühler, 1997). Large voltage spontaneous oscillations of 10 Hz have also been recorded in vivo from rat dorsal roots upon section of the dorsalateral funiculus (Lidierth & Wall, 1996). In a recent in vitro study application of 4-aminopyridine induced rhythmic epileptiform activity at a frequency of 1.2 Hz in rat nociceptive spinal dorsal horn neurones (Ruscheweyh & Sandkuhler, 2003). Such rhythmic activity was thought to arise from the dorsal horn network where there was synchrony of many neurones rather than simply arising from the intrinsic membrane properties of neurones (Ruscheweyh & Sandkuhler, 2003). Taken together, the few electrophysiological studies of rhythmic behaviour in the spinal dorsal horn show that this region can produce oscillatory activity in the frequency range 1–10 Hz.
The mammalian neonatal rat spinal cord in vitro has proven utility as a model for elucidation of empirical features of motor networks (Nishimaru & Kudo, 2000). In this in vitro model, patterned activity is triggered by pharmacological strategies that enhance neuronal excitability (Cowley & Schmidt, 1994; Barthe & Clarac, 1997; Nishimaru & Kudo, 2000). An alternative means to activate the locomotor network in the mammalian ventral horn is elevation of the extracellular potassium concentration to induce a general depolarization of spinal neurones and a presumptive release of endogenous neurotransmitters such as glutamate that normally drive the network (Cazalets et al. 1992; Bracci et al. 1996, 1998; Beato et al. 1997). Studies of oscillatory network behaviours in the ventral horn of the spinal cord have demonstarted the importance of glutamatergic (Beato et al. 1997; Nishimaru et al. 2000; Whelan et al. 2000), GABAergic (Nishimaru & Kudo, 2000) and glycinergic (Kremer & Lev-Tov, 1997) mediated synaptic transmission in mammalian motor networks. In addition, the contribution of electrical coupling via gap junctions is established for motoneurones in the developing spinal cord and may play a role in facilitating co-ordination of motor output (Kiehn & Tresch, 2002). In spinal dorsal horn, the 1.2 Hz rhythm induced by 4-aminopyridine was inhibited by antagonism of AMPA/kainate or GABAA receptors and there was a reduction in the frequency on blockade of glycine receptors (Ruscheweyh & Sandkuhler, 2003), suggesting a role for glutamatergic, GABAergic and glycinergic transmission in dorsal horn rhythms.
In the present study, we have utilized an in vitro spinal cord transverse slice preparation from the neonatal rat and extracellular field analysis to characterize network activity within the substantia gelatinosa of the dorsal horn. To elicit rhythmic activity, brief pressure ejection of a high molarity potassium solution was applied to the substantia gelatinosa. Given their importance in spinal cord ventral horn networks, we determined the contributions of ionotropic glutamate receptors, GABA/glycine receptors and gap-junction electrical connectivity to potassium-induced oscillations within rat substantia gelatinosa in vitro. Since blockade of ionic currents such as the potassium delayed rectifier or the hyperpolarization-activated current, Ih, can modulate firing patterns of neurones in the substantia gelatinosa (Yoshimura & Jessell, 1989; Melnick et al. 2004a, b) we also determined the effect of blocking these currents on the potassium-evoked rhythm. Some of these data have been presented in abstract form (Asghar et al. 2002a, b, c; Cilia La Corte et al. 2003).
Methods
Spinal cord slices
All animal procedures were in accord with current UK legislation and covered by Home Office project and personal licences under the Animals (Scientific Procedures) Act 1986. Female Wistar rats aged 12–16 days were terminally anaesthetized with urethane (2 g kg−1, i.p.) and a dorsal laminectomy performed. The spinal cord was removed and submerged in ice-cold artificial cerebrospinal fluid (ACSF) containing sucrose (252 mm), substituted for NaCl, to improve cell viability during slicing. The standard normal ACSF contained (mm): 126 NaCl, 2.5 KCl, 1.4 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 25 NaHCO3, 11 glucose and was gassed with 95% O2–5% CO2 to maintain a pH of 7.4. The lumbar spinal cord was embedded in 3% agar and transverse slices were cut (300 μm) using a vibratome (Intracell, Royston, UK) and placed into normal ACSF at 35°C for 1 h. In some experiments, a dorsal horn quadrant was isolated from an intact slice by removal of the contralateral dorsal horn quadrant and both ventral horn segments by careful ablation using a scalpel blade. Complete spinal cord slices or dorsal horn quadrants were transferred to a Perspex recording chamber that maintained slices in an interface between warm humidified carbogen (95% O2–5% CO2) and ACSF that bathed the surface of the preparation at a flow rate of 1–1.5 ml min−1 (32°C). Spinal slices were equilibrated in ACSF for > 60 min before recording.
Field recordings
Extracellular field recordings were made using glass microelectrodes (2–4 MΩ) filled with normal ACSF and placed at a depth of approximately 5–10 μm into the substantia gelatinosa. To pressure eject the potassium solution, glass micropipettes (2–4 MΩ) were filled with 1.5 m potassium methylsulphate which was ejected using a picopump (World Precision Instruments) at pressures of 20–40 p.s.i. for 5–30 ms. The potassium solution was pressure ejected in close proximity to the extracellular recording electrode.
Waveforms were recorded and amplified (× 10) through an Axoclamp 2A (Axon Instruments, Union City, CA, USA) with further amplification (× 1000) provided by a Neurolog NL106 module (Digitimer, UK). Signals were initially filtered using a low pass band filter set at 120 Hz (Neurolog NL125). The signals in all the pharmacological experiments were filtered using the low pass band filter set at 40 Hz (Neurolog NL125).
Data capture and analysis
Waveforms were digitized at 5 kHz and captured for further analysis on Spike 2 software (Cambridge Electronic Design, Cambridge, UK). Power spectra were generated using a 2–3 s epoch and the amplitude of the peak frequency measured to give the power of the oscillation. In addition, the integrated power area in the 4–12 Hz frequency band was calculated from power spectra. As the resolution of the frequency domain of the power spectra was only 1.2 Hz, autocorrelation analysis was used as an alternative means to accurately calculate the frequency of the potassium-induced oscillation. All of the stated n values refer to the number of spinal slices used from different rats. Data values are expressed as mean ± s.e.m. Statistical analysis of power amplitude, power area and frequency were performed using either a paired or unpaired t test using the basal values which passed the normality test. Values of P < 0.05 were considered statistically significant.
Drugs
Drugs were dissolved in ACSF and bath applied via separate gravity-fed inlets. Bicuculline methiodide (a GABAA receptor antagonist), strychnine (a glycine receptor antagonist), d(–)-2-amino-5-phosphonopentanoic acid (d-AP5, an NMDA receptor antagonist), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, an AMPA/kainate receptor antagonist), tetrodotoxin (TTX, a sodium channel blocker) and 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288, a selective blocker for the Ih current) were all purchased from Tocris, UK. Octanol/carbenoxolone (gap junction uncouplers), tetraethylammonium (TEA, a blocker for delayed rectifier potassium channels) and ryanodine (an internal calcium store blocker) were purchased from Sigma, UK. Potassium methylsulphate was purchased from ICN (Aurora, OH, USA). Calcium-free perfusate was obtained by substituting CaCl2 (2.4 mm) in the ACSF for MgCl2 (increased from 1.2 to 3.6 mm).
Results
Pressure ejection of potassium elicits rhythmic activity in the substantia gelatinosa in vitro
Analysis of the extracellular recorded baseline in the substantia gelatinosa of spinal cord slices in vitro prior to pressure ejection of potassium did not reveal the presence of any spontaneous oscillations (n = 60). However, upon pressure ejection (20–40 p.s.i. for 5–30 ms) of potassium methylsulphate (1.5 m) onto the substantia gelatinosa an initial negative extracellular DC offset potential of 2–10 mV was elicited which quickly recovered to baseline within < 1.5 s (Fig. 1A). Subsequent to this potential, two profiles of activity could be recorded. In the first profile, which was the predominant type observed in ∼90% of slices and could be found with relative ease, rhythmic activity was observed with a single prominent peak in the 4–12 Hz frequency band (mean peak frequency of 7.7 ± 0.1 Hz, n = 60) with a duration of 5–15 s (see example shown in Fig. 1A). To ascertain the reproducibility of the potassium-evoked oscillation, in six slices pressure ejections of potassium were repeated every 10 min over a 90 min period. Oscillations were found to be reproducible as there were no significant differences (P > 0.05) in the peak power amplitude, integrated power area and frequency between the first potassium ejection and any of the subsequent ejections over the 90 min time period.
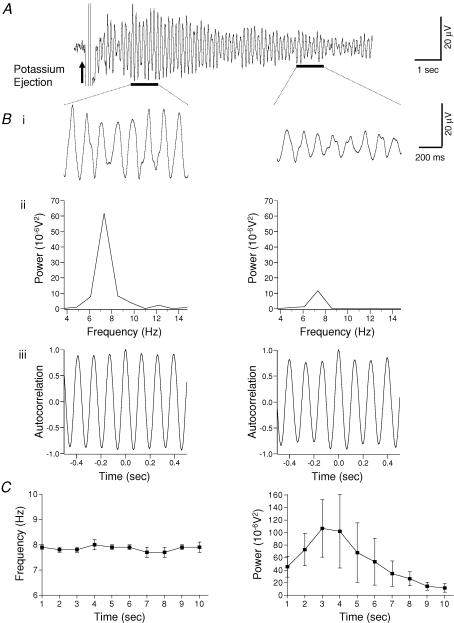
Figure 1. Potassium-induced rhythmic activity in the substantia gelatinosa of the rat spinal cord slice.
A, extracellular field recording of rhythmic activity after brief pressure ejection of 1.5 m KCH3SO4 (arrow). Evoked oscillatory activity was maintained for approximately 10 s. Bi, expanded records of records presented in A (black bars). Bii, power spectral analysis of records in Bi reveals a time-dependent decay of the amplitude of the power. Biii, autocorrelation analysis of 1 s epochs (black bars in A) was used to determine the frequency of the oscillation. Note that the frequency was unchanged despite a substantive decrease in power (Bi and Bii). C, pooled data (n = 6) showing that the peak frequency was unchanged across the duration of the evoked activity (left panel). In contrast, there was an initial increase in power that gradually decayed with time (right panel).
In the second profile of activity (seen in ∼10% of slices), application of potassium induced multiple (5–15) peaks of varying amplitude and frequency in the 13–100 Hz frequency band of the power spectrum as well as a prominent peak (in 3/6 experiments) in the 4–12 Hz band (mean peak frequency of 7.6 ± 0.1 Hz, n = 3). On repeat ejections of potassium the frequency and/or amplitude of the various peaks in the 13–100 Hz band of the power spectrum were variable although the peak in the 4–12 Hz band remained constant both in terms of frequency and power amplitude. When this mixed profile of activity was observed in slices, a preferential search was made to find locations in the substantia gelationsa which only displayed the first profile and this we managed to do in ∼80% of these slices. In this investigation we have only included results from the first profile of rhythmic activity as this was the dominant profile present in the majority of slices tested which could be found relatively easily and displayed good reproducibility.
In six preparations where the potassium-induced oscillations lasted for at least 10 s the profile of the peak power amplitude and the peak frequency was calculated in 1 s epochs from the start of the rhythm. The amplitude of the mean peak power increased gradually to reach a maximum 2–3 s after response onset and then steadily declined in the final 7 s (Fig. 1C). In contrast, the peak frequency showed no change within the 10 s examined (Fig. 1C). For example, the peak frequency between 1 and 2 s was 7.8 ± 0.1 Hz which was not significantly different from that of 7.9 ± 0.2 Hz at 9–10 s (P > 0.05, paired t test).
When searching for potassium-evoked oscillations in the 4–12 Hz frequency range it was found that discrete locations within the substantia gelatinosa responded in a rhythmic manner. Moreover, there was no apparent clustering of these discrete locations in either the medial–lateral or dorsal–ventral axes of the substantia gelatinosa. Multiple extracellular field recordings using microelectrodes spanning approximately 100 μm were used to determine the spatial spread of the potassium-evoked oscillation within the substantia gelatinosa (n = 6). Rhythmic activity could only be recorded when the field electrode was located < 100 μm distant from the potassium ejection electrode.
In order to confirm that oscillatory activity was not a consequence of pressure artifacts, 1.5 m NaCl or ACSF was pressure ejected onto the substantia gelatinosa. Although short duration (< 200 ms) pressure artefacts were recorded, neither solution could induce any oscillatory activity (n = 3, 10–50 p.s.i., 5–30 ms, data not shown). However, oscillatory activity was induced in these slices upon pressure ejection of potassium.
Potassium elicits rhythmic activity in isolated dorsal horn quadrants
Isolated dorsal horn quadrants were utilized to determine whether they retained autonomous circuitry that was capable of producing oscillatory behaviour. On brief pressure ejection of potassium in the substantia gelatinosa of these dorsal horn quadrants, rhythmic activity lasting for 5–15 s was induced. There was no significant difference between dorsal horn quadrants slices and intact slices in the mean peak frequency of the induced oscillation. For example, at the 2–3 s time point following oscillation initiation the peak frequency in the intact slice (taken from the same spinal cord as the quadrants) was 7.5 ± 0.1 Hz (n = 6), and 7.4 ± 0.2 Hz (n = 6) in the quadrants (P > 0.05, unpaired t test). Similarly, there was no significant difference between the power amplitudes in both types of slice tested (intact slices: 16.2 ± 10.5 10−6V2, n = 6versus quadrant slices: 24.0 ± 9.3 10−6V2, n = 6, at 2–3 s following initiation of the rhythm; P > 0.05, unpaired t test).
Contribution of excitatory and inhibitory chemical synaptic transmission to the potassium-evoked rhythm
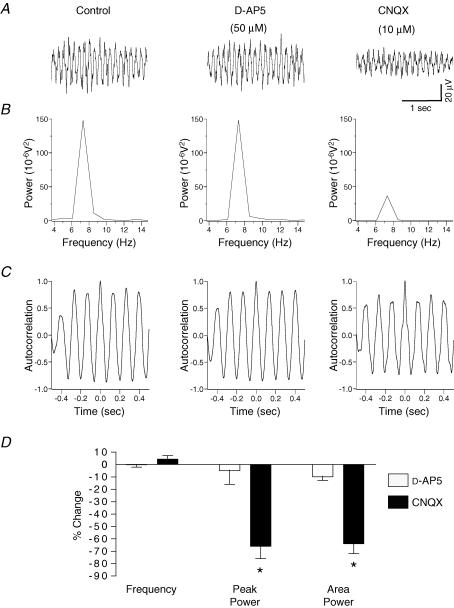
Studies were made using selective pharmacological receptor antagonists to determine the contribution of excitatory (fast glutamatergic) or inhibitory (GABAergic and glycinergic) neurotransmitter systems which may underlie the potassium-evoked oscillation in the substantia gelatinosa. In a spinal cord slice preparation, a protocol was used whereby sequential bath applications were made of d-AP5 (50 μm) alone followed by a combination of d-AP5 (50 μm) and CNQX (10 μm). Whereas application of d-AP5 alone had no effect on the potassium-induced rhythm in the substantia gelatinosa of this slice, subsequent addition of CNQX reduced the amplitude of the power by 75% and the power area by 77% (Fig. 2A–C). In other experiments, bath application of the selective AMPA/kainate receptor antagonist CNQX (10 μm, n = 6) alone caused a significant reduction (P < 0.05) of 66 ± 10% in the amplitude of the peak power of the oscillation and a 64 ± 8% reduction in the integrated power area (4–12 Hz) of the power spectrum (Fig. 2D). In contrast, in a separate series of experiments, bath application of the selective NMDA receptor antagonist d-AP5 (50 μm, n = 6) had no significant effect (P > 0.05) on either peak amplitude or area of the power spectrum (Fig. 2). There was no significant effect (P > 0.05) of either d-AP5 or CNQX upon the frequency of the evoked oscillation (control 7.4 ± 0.2 Hz versusd-AP5 7.5 ± 0.3 Hz; control 7.7 ± 0.1 Hz versus CNQX 8.1 ± 0.1 Hz, n = 6 both groups).
Figure 2. Potassium-evoked oscillations in the rat substantia gelatinosa in vitro are partly dependent upon AMPA/kainate receptors but not on NMDA receptors.
A, example of a single experiment where bath application of 50 μmd-AP5 for 30 min (middle panel) was followed by 10 μm CNQX for 30 min (right panel). B, CNQX reduced the peak amplitude and area of the power spectrum (B, right panel), without affecting frequency (C, right panel). In contrast, d-AP5 had no effect on either parameter (middle panels of B and C). D, quantified data (n = 6) reveal that CNQX but not d-AP5 significantly reduces the peak power and power area. Neither antagonist had an effect on the frequency of the oscillation. *P < 0.05 paired t test versus control.
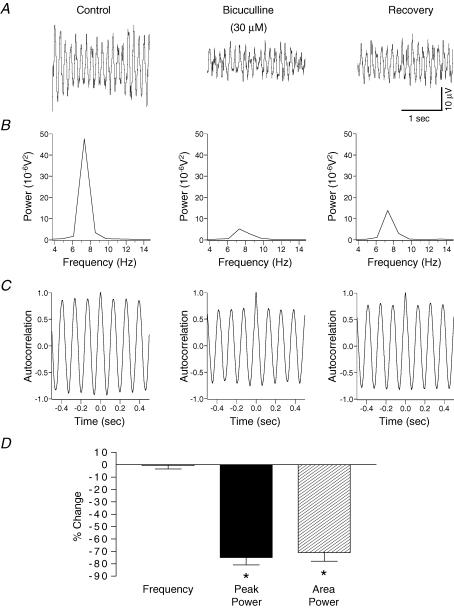
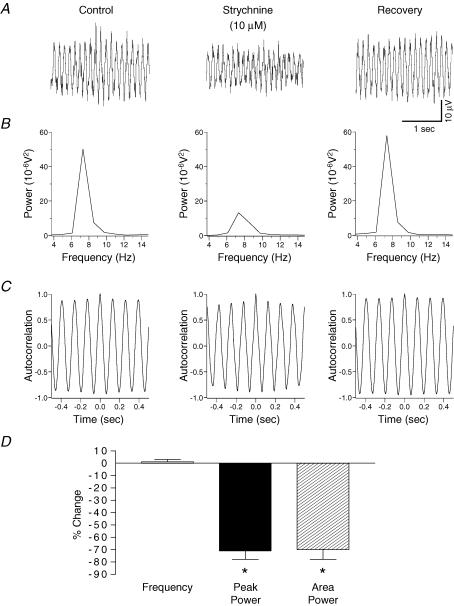
To assess the role of inhibitory neurotransmission in the generation of the potassium-evoked rhythm, the selective GABAA receptor antagonist bicuculline, and the glycine receptor antagonist strychnine were utilized. Bath application of bicuculline (30 μm, n = 6), or in a separate series of experiments strychnine (10 μm, n = 6), caused a significant reduction (P < 0.05) in the amplitude of the peak power by 75 ± 6% and 71 ± 7%, respectively (Figs 3 and 4). Both antagonists also caused a significant reduction (P < 0.05) in the area of the power spectrum (bicuculline: 71 ± 7%; strychnine: 70 ± 8%). Figures 3 and 4 show that neither bicuculline nor strychnine had any significant effect (P > 0.05) on the frequency of the potassium-evoked rhythm (control 7.9 ± 0.4 Hz versus bicuculline 7.9 ± 0.3 Hz; control 7.8 ± 0.5 Hz versus strychnine 7.9 ± 0.1 Hz, n = 6 both groups). Bath application (30 min) of a combination of bicuculline (30 μm) and strychnine (10 μm) caused a significant reduction (P < 0.05) in the amplitude of the peak power by 78 ± 7.4% and a 70 ± 7.8% reduction in the integrated power area (n = 6). These reductions were not significantly different (P > 0.05) when compared with application of either bicuculline (30 μm) or strychnine (10 μm) alone. The drug combination had no significant effect (P > 0.05) on the frequency of the potassium-evoked rhythm (control 7.6 ± 0.1 Hz versus bicuculline and strychnine 7.5 ± 0.1 Hz, n = 6).
Figure 3. Involvement of GABAA receptor-mediated inhibition in rhythmic activity within rat substantia gelatinosa in vitro.
A, potassium-induced rhythmic activity before (left panel) and after 30 min 30 μm bicuculline (middle panel). B, bicuculline reduced the power amplitude and area of the spectrum (middle panel) and on drug washout there was a partial reversal of this effect (right panel). C, bicuculline had no effect on the frequency of the oscillation. D, quantified data (n = 6) reveal that bicuculline reduces the peak power amplitude and the power area with no significant effect on the frequency of activity. *P < 0.05 paired t test versus control.
Figure 4. Contribution of glycine receptor-mediated inhibition to potassium-evoked rhythmic activity in rat substantia gelatinosa in vitro.
A, potassium-induced rhythm before (left panel) and after 30 min 10 μm strychnine (middle panel). B, strychnine reversibly reduced the power of the spectrum. C, there was no effect of strychnine on the frequency of the oscillation. D, quantified data (n = 6) reveal that strychnine significantly reduces the parameters of peak power amplitude and power area with no significant effect on the frequency of activity. *P < 0.05 paired t test versus control.
Effects of blocking chemical synaptic transmission and the role of gap junctions in potassium-evoked rhythms
To test whether or not potassium-evoked rhythmic activity could persist after blocking action potential conduction or by inhibiting action potential-dependent neurotransmitter release the effects of TTX (1 μm) and a calcium-free extracellular ACSF perfusate were determined, respectively. Although there was a significant reduction (P < 0.05) in the amplitude and area of the power spectrum with TTX (amplitude: 59 ± 12%; area: 53 ± 11%, n = 6) or calcium-free perfusate (amplitude: 56 ± 5%; area: 54 ± 7%, n = 6), potassium-induced rhythmic activity was found to persist with either treatment. Neither TTX nor the calcium-free perfusate had any significant effect (P > 0.05) on the peak frequency of the potassium-evoked rhythm (control 7.6 ± 0.2 Hz compared with TTX 7.9 ± 0.4 Hz; control 7.9 ± 0.2 Hz compared with calcium-free perfusate 8.1 ± 0.1 Hz, n = 6 both groups).
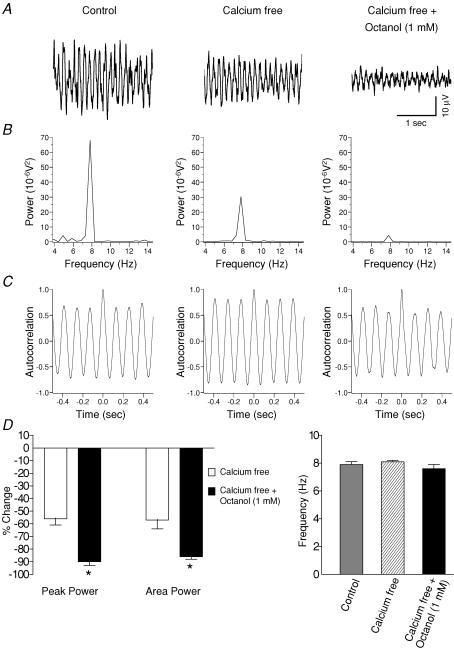
To ascertain whether gap junctions could underlie the persistent potassium-induced rhythmic activity following calcium-free perfusate the putative gap junction blocker octanol (1 mm) was subsequently perfused in combination with calcium-free perfusate. With this combination, there was a further significant reduction (P < 0.05) in the amplitude of the power spectrum; a reduction of 56 ± 5% (n = 6) with calcium-free perfusate was further taken to 90 ± 3% (n = 6) when combined with octanol (Fig. 5). Similarly, there was a significantly greater reduction (P < 0.05) in the power area from 54 ± 7% in calcium-free perfusate (n = 6) to 86 ± 2% (n = 6) with the octanol. Figure 5D shows that there was no significant change in the peak frequency between calcium perfusate and when combined with octanol (P > 0.05, n = 6).
Figure 5. Potassium-evoked oscillations without action potential dependent chemical neurotransmission in the rat substantia gelatinosa in vitro and the involvement of gap junction coupling.
A, example traces from the same experiment showing potassium-induced oscillations before (left panel) and after bath application of calcium-free perfusate either alone (middle panel) or in subsequent combination for 45 min with the gap junction blocker 1 mm octanol (right panel). B, calcium-free perfusate caused a reduction in the peak amplitude of the power spectrum (middle panel). There was a further reduction in peak amplitude on subsequent addition of octanol to the calcium-free perfusate (right panel). C, autocorrelograms showing that frequency of the oscillation was unchanged. D, quantified data (n = 6) showing that the peak power and area power are significantly reduced (P < 0.05) by the calcium-free perfusate (left panel). A combination of calcium-free perfusate and octanol caused a further significant reduction in the peak power and area (left panel). Neither the calcium-free perfusate alone or in combination with octanol had any significant effect (P > 0.05) on the frequency of the oscillation (right panel). *P < 0.05 paired t test versus calcium-free perfusate.
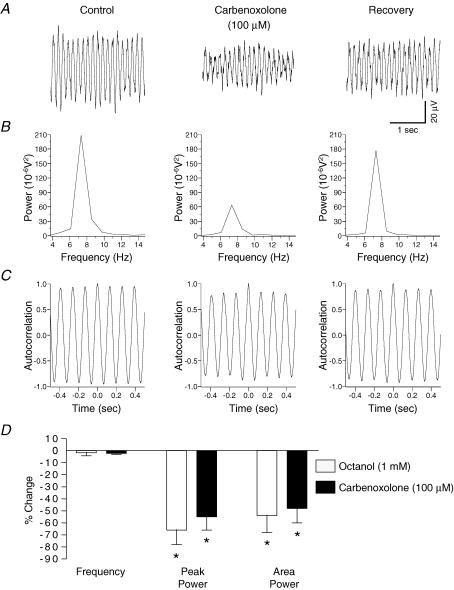
In a separate series of experiments the effects of the putative gap junction blockers carbenoxolone and octanol on potassium-evoked oscillations were tested in spinal slices perfused with normal ASCF. Bath application of carbenoxolone (100 μm, n = 6) caused significant reductions (P < 0.05) in amplitude of the peak power by 55 ± 11% and in the power area by 48 ± 12% of the potassium-evoked oscillation (Fig. 6). Octanol (1 mm, n = 6) caused similar significant reductions (P < 0.05) in the peak power amplitude (66 ± 12%) and in the area (54 ± 14%) of the power spectrum (Fig. 6). There was no significant effect (P > 0.05) on the frequency of the oscillation upon application of either carbenoxolone or octanol (Fig. 6D).
Figure 6. Partial attenuation of rhythmic activity in the rat substantia gelatinosa in vitro by the gap junction uncoupling agents carbenoxolone and octanol.
A, field recording of potassium-induced rhythmic activity before (left panel) and after 45 min 100 μm carbenoxolone (middle panel). B, carbenoxolone reversibly reduced the power of the spectrum (middle panel), without affecting frequency (C). D, quantified data (n = 6) reveal that carbenoxolone reduces the peak power and area with no significant effect on the frequency of activity. A similar profile was obtained with octanol (45 min, 1 mm), another gap junction uncoupler (n = 6). *P < 0.05 paired t test versus control.
Since a combination of a calcium-free ASCF perfusate and octanol did not cause an abolition of the potassium-induced rhythm, a calcium-free ASCF perfusate containing a cocktail of drugs was bath applied (30 min, n = 6): TTX (1 μm), carbenoxolone (100 μm), bicuculline (30 μm), strychnine (10 μm) and CNQX (10 μm). Despite using a calcium-free perfusate in this drug cocktail, internal calcium stores could potentially be a source of calcium. To counter this, the internal calcium store blocker ryanodine (20 μm) was also added to the cocktail. This cocktail caused a virtual abolition of the potassium-induced rhythm (reduction in the power amplitude by 96 ± 2% and in the power area by 95 ± 2%).
Ion channel blockers and potassium-evoked rhythms
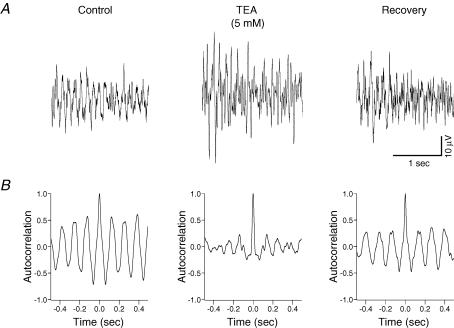
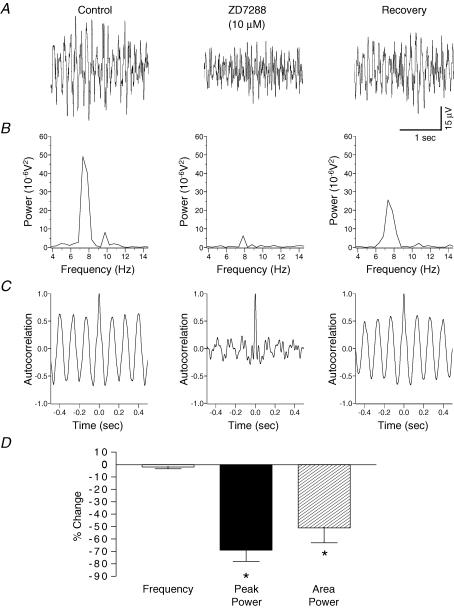
To determine the role of the delayed-rectifier potassium current on potassium-evoked oscillations, the delayed-rectifier potassium ion channel blocker, TEA, was bath applied. TEA (5 mm, 30–45 min, n = 6) induced random spiking activity of the baseline and during the response to pressure ejection of potassium (Fig. 7). Autocorrelation analysis of the potassium-evoked activity revealed a disruption of synchronization with TEA (Fig. 7). To ascertain whether the hyperpolarization-activated current (Ih) could underlie potassium-induced oscillations in the substantia gelatinosa the effects of the selective Ih blocker ZD7288 was tested. Bath application of ZD7288 (10 μm, 30–45 min, n = 6) caused a significant reduction (P < 0.05) in the amplitude of the peak power by 63 ± 9% and in the power area by 51 ± 12% (Fig. 8). Autocorrelation analysis of the potassium-evoked activity showed a disruption in synchronization with ZD7288 (Fig. 8). There was no significant effect (P > 0.05) of ZD7288 on the frequency of the potassium-evoked rhythm (control 7.6 ± 0.1 Hz versus ZD7288 7.4 ± 0.1 Hz; n = 6).
Figure 7. Block of the delayed rectifier potassium current by TEA disrupts synchronization of the potassium-evoked rhythmic activity in rat substantia gelatinosa in vitro.
A, potassium-induced rhythmicity before (left panel) and after 45 min 5 mm TEA (middle panel). TEA also caused spontaneous activity in the baseline prior to potassium ejection (not shown). Such spontaneous activity was also observed during the potassium-evoked rhythm (middle panel) and consequently the power amplitude for the potassium-induced rhythm could not be determined from the power spectrum. B, TEA causes a disruption in the synchronization of the rhythm.
Figure 8. Block of the Ih current by ZD7288 reduces potassium-evoked rhythmic activity and synchronization in rat substantia gelatinosa in vitro.
A, potassium-induced rhythmicity before (left panel) and after 45 min 10 μm ZD7288 (middle panel). B, ZD7288 reversibly reduced the power of the spectrum. C, there was no effect of ZD7288 on the frequency of the oscillation although there was a disruption in the synchrony of the rhythm. D, quantified data (n = 6) reveal that ZD7288 significantly reduces the parameters of peak power amplitude and power area with no significant effect on the frequency of activity. *P < 0.05 paired t test versus control.
Discussion
Characteristics of potassium-evoked rhythmic activity in the substantia gelatinosa
This study is the first to demonstrate potassium-induced extracellular field oscillations constrained to a narrow frequency band peaking at approximately 8 Hz in neonatal rat substantia gelatinosa neurones of the lumbar spinal cord in vitro. This peak frequency is similar to the frequency band of rhythms described in dorsal horn in vivo. For example, spinalization of rat spinal cord unmasks large amplitude spontaneous dorsal root potentials that oscillate at around 10 Hz (Lidierth & Wall, 1996). In decerebrate spinalized rats, dorsal horn neurones display spontaneous activity with a fundamental frequency of 6–13 Hz (Sandkühler & Eblen-Zajjur, 1994). These studies speculatively proposed that the basis of such rhythmic activity was the existence of intrinsic local neuronal networks subject to extrinsic modulation by sensory afferent input, propriospinal or descending systems. The concept of rhythmic activity as an emergent property of interconnected dorsal horn neurones is supported by the present in vitro investigation in the substantia gelatinosa which was isolated from such extrinsic influences. Since in the current study there was preservation of potassium-evoked ∼8 Hz rhythmic activity in dorsal horn quadrants, this mitigates against ventral horn networks as the primary source of dorsal activity and suggests that sufficient circuitry exists within the unilateral dorsal horn for rhythmogenesis. In line with this observation are the in vitro studies showing persistence of rhythmic activity in dorsal horn quadrants of spinal cord slices (Demir et al. 2002) or in dorsal horn segments after ablation of the ventral horn (Kremer & Lev-Tov, 1998).
Despite the spatial distribution of the potassium-induced oscillation being restricted to a region < 100 μm, such activity is likely to reflect synchronous activity of a network of dorsal horn neurones rather than oscillatory activity from a single neurone since (1) rhythmic activity was recorded as a field potential which only reflects the added activity of many neurones, and (2) blocking synaptic transmission by TTX or a calcium-free ACSF perfusate reduced rhythmic activity indicating, at least, a partial dependence on a neuronal network. In order to evaluate whether potassium-induced rhythmic activity is dependent on intrinsic membrane properties or is a unique emergent property of the neuronal network it will be necessary to perform electrophysiological studies such as intracellular recordings in individual substantia gelatinosa neurones. In spinal cord, the existence of putative conditional oscillator neurones (Aiken et al. 2003) or neurones with bistable membrane properties (Schmidt et al. 1998) that may contribute to rhythmic motor output implies a role for intrinsic voltage-gated conductances. Such intrinsic oscillatory mechanisms may act to reinforce or modify activity within a neuronal network and their involvement is not incompatible with the operation of other central pattern generator mechanisms.
In this study, brief transient elevations in extracellular potassium elicited reproducible oscillatory activity in substantia gelatinosa neurones in vitro. The external concentration of potassium that results from transient pressure ejection of the potassium methysulphate solution was not determined in this investigation but previous studies in hippocampal slices using an identical protocol and ion-sensitive electrodes detected an elevation in potassium of 0.5–2.0 mm (LeBeau et al. 2002; Towers et al. 2002). This level of extracellular potassium is similar to the 4–5 mm range reported for potassium transients elicited in the neonate dorsal horn in vitro after single dorsal root stimuli and considerably lower than the 10–11 mm levels associated with repetitive dorsal root stimuli (Walton & Chesler, 1988). Elevations in potassium concentration are used as an oscillogenic strategy in spinal ventral horn studies (Cazalets et al. 1992; Beato et al. 1997; Bracci et al. 1998; Kremer & Lev-Tov, 1998) although the mechanism whereby elevated extracellular potassium elicits rhythmic activity is unknown. One possibility is that the elevated levels of potassium mimic neuronal depolarization normally induced by localized neurotransmitter release and this may have occurred in the present experiments.
Contribution of glutamatergic neurotransmission to network activity
With regard to central pattern generators in the ventral horn, glutamatergic neurotransmission participates in rhythmogenesis (Cazalets et al. 1992; Cowley & Schmidt, 1994) and attenuation of rhythmicity by selective non-NMDA and NMDA receptor antagonists reinforces the importance of glutamate receptor excitatory drive to network behaviour (Beato et al. 1997; Bracci et al. 1998). In our model of potassium-evoked rhythmic activity within substantia gelatinosa in vitro, the AMPA/kainate antagonist CNQX significantly attenuated, but did not abolish, the amplitude of the peak power of the oscillation and the area of the power spectrum without affecting the peak frequency. However, the selective NMDA receptor antagonist d-AP5 had no influence on either parameter of the power spectra. Taken together, these data imply a role for non-NMDA excitatory amino acid receptors in potassium-evoked oscillatory activity within substantia gelatinosa in vitro. Interestingly, afferent-induced synaptic transmission in the substantia gelatinosa in vitro is strongly reliant on non-NMDA receptors (Yoshimura & Jessell, 1990) although NMDA receptors may participate under certain conditions (Bardoni et al. 2000). An absolute requirement for functional AMPA/kainate but not NMDA receptors to generate rhythmic activity was demonstrated in mouse spinal cord in vitro (Whelan et al. 2000). The finding that combined application of d-AP5 and CNQX did not abolish potassium-evoked activity within substantia gelatinosa infers that fast glutamate-mediated excitation cannot fully account for the oscillatory response and that other excitatory or inhibitory mechanisms must contribute.
Contribution of inhibitory interneurones to network activity
In the current investigation, the attenuation of oscillatory power by either the GABAA receptor antagonist, bicuculline, or the glycine receptor antagonist, strychnine, implies a role for inhibitory interneurones in the manifestation of potassium-evoked substantia gelatinosa oscillatory activity. This finding is contrary to what would be expected on the basis of data for ventral horn that describes the emergence of distinctive slow synchronized bursting after disinhibition with GABA or glycine antagonists (Bracci et al. 1996; Tscherter et al. 2001) and degradation of synchronous activity by GABA agonists (Cazalets et al. 1998). Our data therefore suggest crucial differences in the cellular basis of rhythmicity and the contribution of inhibition of ∼8 Hz rhythms in substantia gelatinosa compared with the slower oscillations of central pattern generators in the ventral horn. Interestingly, bicuculline sensitivity is a feature of gamma frequency rhythms in cortical networks that rely on mutually coupled inhibitory interneurones for synchrony (Whittington et al. 2000) and such a mechanism may also be relevant in substantia gelatinosa rhythms.
Antagonism of both GABAA and glycine receptors using a combination of bicuculline and strychnine caused a similar inhibition of the potassium-induced oscillation as compared with each individual antagonist. It is possible that such similar levels of inhibition are a reflection of the co-release and transmission of GABA and glycine from inhibitory interneurones. Immunoreactive investigations have shown co-localization of GABA and glycine in the spinal dorsal horn (Todd et al. 1996) and in an electrophysiological study by Jonas et al. (1998) it was established that there was co-release of GABA and glycine from spinal interneurones in the mammalian spinal dorsal horn.
Contribution of electrical signalling to network activity
Potassium-induced oscillations in the substantia gelatinosa were decreased (reduction in power amplitude and area) but not abolished when action potential conduction was blocked by TTX or when action potential-dependent neurotransmitter release was inhibited by calcium-free ACSF perfusate. A further significant reduction (total reduction ∼90%) in the power amplitude and power area of the potassium oscillation was evident when the putative gap junction blocker octanol was subsequently added in combination with the calcium-free perfusate. Furthermore, in preparations perfused with normal ACSF, application of the gap junction blockers octanol or carbenoxolone caused a reduction in the power amplitude and area of the potassium-induced oscillation. Together these results demonstrate that the potassium-induced rhythm in substantia gelatinosa persists despite blocking chemical synaptic transmission and that gap junction-mediated electrical coupling partly underlies the rhythm. An abolition of the potassium-induced rhythm could be achieved, however, following perfusion with calcium free ACSF in conjunction with gap junction blockers and a cocktail of receptor antagonists.
Notwithstanding the issue of pharmacological specificity, at the concentrations used both octanol (1 mm) and carbenoxolone (100 μm) are recognized as gap junction uncouplers (Rozental et al. 2001). They have been used extensively to reveal the contribution of gap junctions and electrical synapses to synchronicity in many CNS areas including spinal cord (Dermietzel, 1998; Rozental et al. 2000; Tresch & Kiehn, 2000; LeBeau et al. 2002). Another potential problem with the use of octanol and carbenoxolone in spinal cord tissue is that comparatively long time periods (> 2 h) are required to obtain a maximal degree of gap junction uncoupling (Tresch & Kiehn, 2000; Kiehn & Tresch, 2002). Thus, the reductions in the potassium-evoked oscillation with octanol or carbenoxolone in the present investigation using a 45 min perfusion time may have been greater following a longer incubation period with these uncouplers.
Although the present findings showing that potassium-induced oscillations persist in the absence of action potentials (blocked with TTX) or in the absence of chemical synaptic transmission (blocked using a calcium-free perfusate) is counterintuitive, such a phenomenon has also been reported in the slower motor rhythms of the neonatal rat spinal ventral horn. For example, robust motor rhythms induced by NMDA and 5-HT (∼1.5 Hz) could still be recorded after elimination of all chemical action potential-dependent synapses by TTX in the neonatal rat spinal cord (Tresch & Kiehn, 2000). Such action potential-independent rhythms were proposed to be attributable to the intrinsic oscillatory capability of motoneurones (pacemaker properties) and neural co-ordination across gap junctions (Tresch & Kiehn, 2000; Kiehn & Tresch, 2002). The suppression of NMDA or 5-HT-induced ventral root oscillations by carbenoxolone further supports motoneuronal synchronization via gap junctions (Tresch & Kiehn, 2000). Moreover, such a phenomenon is not restricted to the neonate as a role for electrical connectivity has been proposed for adult mammalian ventral horn networks (Kiehn & Tresch, 2002). There is currently scant data available for the function of gap junctions within the dorsal horn. To our knowledge, the data of our study are the first to imply a role for gap junctions within substantia gelatinosa network behaviour. In dorsal and ventral grey matter, there is evidence for mixed electrical/chemical synapses and expression of connexin proteins, the molecular constituents of gap junctions (Rash et al. 1996, 1998). Moreover, connexin 36 a neuronal protein implicated in electrical coupling has been localized to the spinal dorsal horn (Rash et al. 2000).
Contribution of ion channels to network activity
In this investigation, block of the delayed rectifier potassium current by TEA or blocking the hyperpolarization-activated Ih current by ZD7288, unlike any of the other pharmacological agents tested in this study, caused strong disruptions in the synchronization of the potassium induced oscillation as revealed by the autocorrelograms (Figs 7 and 8). Thus, both the delayed-rectifier potassium and Ih currents could represent intrinsic components which could regulate the timing of potassium-induced oscillations in substantia gelatinosa neurones. It is possible that TEA could have affected ion channels other than the delayed-rectifier potassium channels. However, the concentration of 5 mm TEA used in the current investigation was within the range of 1–10 mm used in voltage-clamp studies to selectively study delayed-rectifier potassium currents in the rat subtantia gelationosa in vitro (Olschewski et al. 2001; Melnick et al. 2004b).
Further electrophysiological investigations in single substantia gelatinosa neurones are needed to understand further the role of delayed-rectifier potassium currents and Ih in potassium-evoked oscillogenesis. Moreover, our present results cannot exclude the possibility that other types of potassium ion channel currents or the various types of sodium or calcium conductances can also affect or modulate the potassium-evoked rhythm.
Functional significance of network synchrony within substantia gelatinosa
The spinal dorsal horn plays an important part in the processing of somatosensory and sensorimotor afferent inputs. It is strategically placed to modulate information transfer to functionally relevant target areas, e.g. thalamo-cortical or ventral motor networks. The substantia gelatinosa is conceptualized as a system comprised of heterogeneous populations of interneurones that integrate and process sensory afferent inputs arising principally, but not exclusively, from peripheral nociceptors. Neurones of the substantia gelatinosa contribute either directly or indirectly to ascending somatosensory, propriospinal or motor reflex pathways. Substantia gelatinosa neurones also provide feed-forward transmission to deep dorsal horn laminae that receive and process both nociceptive and non-nociceptive afferent inputs. In considering the role of rhythmicity within this region, the spatio-temporal correlations of co-activated neurones could encode for distinct sensory modalities, e.g. nociceptive versus low threshold inputs from muscle versus skin (Coghill et al. 1993). A role for rhythmically discharging nociceptive neurones in information transfer within the dorsal horn has been proposed previously (Sandkühler & Eblen-Zajjur, 1994). In this scenario, modulation of synchronous firing by intrinsic or extrinsic factors could strongly influence information transfer across dorsal horn laminae. The finding that skin inflammation induced qualitative and quantitative alterations in dorsal horn synchronicity prompted the suggestion of a role for dynamic network synchronicity in the central pathophysiology of hyperalgesia and allodynia (Eblen-Zajjur & Sandkuhler, 1997). Moreover, cross-correlograms of simultaneous recordings in superficial and deep dorsal horn neurones have revealed a loss of functional coherence in rats with peripheral neuropathic injury indicating a modulation of nociceptive spinal inputs in neuropathic pain (Biella et al. 1997).
In conclusion, the in vitro data presented in this study reveal that the substantia gelatinosa displays a property common to many areas of the CNS, namely a rhythmic capability derived from synaptic and non-synaptic interactions within a network. Specifically, the data demonstrates a part dependence of the potassium-evoked oscillation upon AMPA/kainate, GABAA and glycine receptors but not NMDA receptors. A partial role for gap junctions is also indicated in the oscillation induced by potassium. In addition, blockade of the potassium delayed rectifier and Ih currents can disrupt the synchronization of the potassium-evoked rhythm.
Acknowledgments
This work was supported in part by the University of Leeds and The Wellcome Trust. SCR was funded by a summer research bursary from The Nuffield Foundation. PFCLC is in receipt of a BBSRC Committee Studentship. Technical support was provided by Mrs J. Daniel.
References
- Aiken SP, Kuenzi FM, Dale N. Xenopus embryonic spinal neurons recorded in situ with patch clamp electrodes – conditional oscillators after all? Eur J Neurosci. 2003;18:333–343. doi: 10.1046/j.1460-9568.2003.02755.x. 10.1046/j.1460-9568.2003.02755.x. [DOI] [PubMed] [Google Scholar]
- Asghar AUR, Al Dawoud M, LeBeau FEN, Buhl EH, King AE. The role of GABAA and glycine receptor-mediated inhibition of theta frequency oscillations in substantia gelatinosa neurones of the rat spinal cord in vitro. J Physiol. 2002a;544.P:78P. [Google Scholar]
- Asghar AUR, LeBeau FE, Buhl EH, King AE. A role for gap junctions in theta oscillations in substantia gelatinosa neurones of the young rat spinal cord in vitro. FENS Abstracts. 2002b;1:273. [Google Scholar]
- Asghar AUR, LeBeau FE, Buhl EH, King AE. Potassium-induced theta oscillations in rat substantia gelatinosa neurones of the spinal cord in vitro. J Physiol. 2002c;539.P:152P. [Google Scholar]
- Baranauskas G, Nistri A. Membrane potential oscillations of neonatal rat spinal motoneurons evoked by electrical stimulation of dorsal root fibres. Eur J Neurosci. 1995;7:2403–2408. doi: 10.1111/j.1460-9568.1995.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Magherini PC, MacDermott AB. Activation of NMDA receptors drives action potentials in superficial dorsal horn from neonatal rats. NeuroReport. 2000;11:1721–1727. doi: 10.1097/00001756-200006050-00025. [DOI] [PubMed] [Google Scholar]
- Barthe JY, Clarac F. Modulation of the spinal network for locomotion by substance P in the neonatal rat. Exp Brain Res. 1997;115:485–492. doi: 10.1007/pl00005718. [DOI] [PubMed] [Google Scholar]
- Beato M, Bracci E, Nistri A. Contribution of NMDA and non-NMDA glutamate receptors to locomotor pattern generation in the neonatal rat spinal cord. Proc R Soc Lond B Biol Sci. 1997;264:877–884. doi: 10.1098/rspb.1997.0122. 10.1098/rspb.1997.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biella G, Riva L, Sotgiu ML. Interaction between neurons in different laminae of the dorsal horn of the spinal cord. A correlation study in normal and neuropathic rats. Eur J Neurosci. 1997;9:1017–1025. doi: 10.1111/j.1460-9568.1997.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Bracci E, Ballerini L, Nistri A. Localization of rhythmogenic networks responsible for spontaneous bursts induced by strychnine and bicuculline in the rat isolated spinal cord. J Neurosci. 1996;16:7063–7076. doi: 10.1523/JNEUROSCI.16-21-07063.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci E, Beato M, Nistri A. Extracellular K+ induces locomotor-like patterns in the rat spinal cord in vitro: comparison with NMDA or 5-HT induced activity. J Neurophysiol. 1998;79:2643–2652. doi: 10.1152/jn.1998.79.5.2643. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Lebret JM, Kiehn O. Organization of left-right coordination in the mammalian locomotor network. Brain Res Brain Res Rev. 2002;40:107–117. doi: 10.1016/s0165-0173(02)00194-7. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Bertrand S, Sqalli-Houssaini Y, Clarac F. GABAergic control of spinal locomotor networks in the neonatal rat. Ann N Y Acad Sci. 1998;860:168–180. doi: 10.1111/j.1749-6632.1998.tb09047.x. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia La Corte PF, LeBeau FE, Buhl EH, King AE, Asghar AUR. Oscillatory activity in the substantia gelatinosa of the rat spinal cord in vitro is dependent on both chemical and electrical neurotransmission. BNA Abstracts. 2003;68.04:153. [Google Scholar]
- Coghill RC, Mayer DJ, Price DD. The roles of spatial recruitment and discharge frequency in spinal cord coding of pain: a combined electrophysiological and imaging investigation. Pain. 1993;53:295–309. doi: 10.1016/0304-3959(93)90226-F. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett. 1994;171:147–150. doi: 10.1016/0304-3940(94)90626-2. [DOI] [PubMed] [Google Scholar]
- Dale N, Kuenzi F. Ionic currents, transmitters and models of motor pattern generators. Curr Opin Neurobiol. 1997;7:790–796. doi: 10.1016/s0959-4388(97)80137-7. 10.1016/S0959-4388(97)80137-7. [DOI] [PubMed] [Google Scholar]
- Demir R, Gao BX, Jackson MB, Ziskind-Conhaim L. Interactions between multiple rhythm generators produce complex patterns of oscillation in the developing rat spinal cord. J Neurophysiol. 2002;87:1094–1105. doi: 10.1152/jn.00276.2001. [DOI] [PubMed] [Google Scholar]
- Dermietzel R. Gap junction wiring: a ‘new’ principle in cell-to-cell communication in the nervous system? Brain Res Rev. 1998;26:176–183. doi: 10.1016/s0165-0173(97)00031-3. 10.1016/S0165-0173(97)00031-3. [DOI] [PubMed] [Google Scholar]
- Eblen-Zajjur AA, Sandkühler J. Synchronicity of nociceptive and non-nociceptive adjacent neurons in the spinal dorsal horn of the rat: stimulus-induced plasticity. Neuroscience. 1997;76:39–54. doi: 10.1016/s0306-4522(96)00286-2. 10.1016/S0306-4522(96)00286-2. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol. 2003;70:347–361. doi: 10.1016/s0301-0082(03)00091-1. 10.1016/S0301-0082(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O, Tresch MC, Harris-Warrick RM. Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord. Brain Res Bull. 2000;53:649–659. doi: 10.1016/s0361-9230(00)00398-1. 10.1016/S0361-9230(00)00398-1. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Tresch MC. Gap junctions and motor behavior. Trends Neurosci. 2002;25:108–115. doi: 10.1016/s0166-2236(02)02038-6. 10.1016/S0166-2236(02)02038-6. [DOI] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol. 1997;77:1155–1170. doi: 10.1152/jn.1997.77.3.1155. [DOI] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. GABA-receptor-independent dorsal root afferents depolarization in the neonatal rat spinal cord. J Neurophysiol. 1998;79:2581–2592. doi: 10.1152/jn.1998.79.5.2581. [DOI] [PubMed] [Google Scholar]
- Kudo N, Yamada T. N-methyl-D,1-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett. 1987;75:43–48. doi: 10.1016/0304-3940(87)90072-3. 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- LeBeau FE, Towers SK, Traub RD, Whittington MA, Buhl EH. Fast network oscillations induced by potassium transients in the rat hippocampus in vitro. J Physiol. 2002;542:167–179. doi: 10.1113/jphysiol.2002.015933. 10.1113/jphysiol.2002.015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidierth M, Wall PD. Synchronous inherent oscillations of potentials within the rat lumbar spinal cord. Neurosci Lett. 1996;220:25–28. doi: 10.1016/s0304-3940(96)13231-6. 10.1016/S0304-3940(96)13231-6. [DOI] [PubMed] [Google Scholar]
- Melnick IV, Santos SF, Safronov BV. Mechanism of spike frequency adaptation in substantia gelatinosa neurones of rat. J Physiol. 2004a;559:383–395. doi: 10.1113/jphysiol.2004.066415. 10.1113/jphysiol.2004.066415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick IV, Santos SF, Szokol K, Szucs P, Safronov BV. Ionic basis of tonic firing in spinal substantia gelatinosa neurons of rat. J Neurophysiol. 2004b;91:646–655. doi: 10.1152/jn.00883.2003. 10.1152/jn.00883.2003. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Kudo N. Formation of the central pattern generator for locomotion in the rat and mouse. Brain Res Bull. 2000;53:661–669. doi: 10.1016/s0361-9230(00)00399-3. 10.1016/S0361-9230(00)00399-3. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Takizawa H, Kudo N. 5-Hydroxytryptamine-induced locomotor rhythm in the neonatal mouse spinal cord in vitro. Neurosci Lett. 2000;280:187–190. doi: 10.1016/s0304-3940(00)00805-3. 10.1016/S0304-3940(00)00805-3. [DOI] [PubMed] [Google Scholar]
- Olschewski A, Hempelmann G, Vogel W, Safronov BV. Suppression of potassium conductance by droperidol has influence on excitability of spinal sensory neurons. Anesthesiology. 2001;94:280–289. doi: 10.1097/00000542-200102000-00018. 10.1097/00000542-200102000-00018. [DOI] [PubMed] [Google Scholar]
- Rash JE, Dillman RK, Bilhartz BL, Duffy HS, Whalen LR, Yasumura T. Mixed synapses discovered and mapped throughout mammalian spinal cord. Proc Natl Acad Sci U S A. 1996;93:4235–4239. doi: 10.1073/pnas.93.9.4235. 10.1073/pnas.93.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Nagy JI, Yasumura T. Freeze-fracture immunogold labeling and histological mapping of connexins Cx43, Cx30, and Cx32 in gap junctions of ependymocytes, astrocytes, and oligodendrocytes in rat brain and spinal cord. Molec Biol Cell. 1998;9:550. [Google Scholar]
- Rash JE, Staines WA, Yasumura T, Patel D, Furman C, Stelmack GL, Nagy JI. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin-36 but not connexin-32 or connexin-43. Proc Natl Acad Sci U S A. 2000;97:7573–7578. doi: 10.1073/pnas.97.13.7573. 10.1073/pnas.97.13.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozental R, Giaume C, Spray DC. Gap junctions in the nervous system. Brain Res Rev. 2000;32:11–15. doi: 10.1016/s0165-0173(99)00095-8. 10.1016/S0165-0173(99)00095-8. [DOI] [PubMed] [Google Scholar]
- Rozental R, Srinivas M, Spray DC. How to close a gap junction channel. Efficacies and potencies of uncoupling agents. Meth Mol Biol. 2001;154:447–476. doi: 10.1385/1-59259-043-8:447. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Sandkuhler J. Epileptiform activity in rat spinal dorsal horn in vitro has common features with neuropathic pain. Pain. 2003;105:327–338. doi: 10.1016/s0304-3959(03)00248-3. 10.1016/S0304-3959(03)00248-3. [DOI] [PubMed] [Google Scholar]
- Sandkühler J, Eblen-Zajjur AA. Identification and characterization of rhythmic nociceptive and non-nociceptive spinal dorsal horn neurons in the rat. Neuroscience. 1994;61:991–1006. doi: 10.1016/0306-4522(94)90419-7. 10.1016/0306-4522(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Hochman S, MacLean JN. NMDA receptor-mediated oscillatory properties: potential role in rhythm generation in the mammalian spinal cord. Ann NY Acad Sci. 1998;860:189–202. doi: 10.1111/j.1749-6632.1998.tb09049.x. [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Meth. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Watt C, Spike RC, Sieghart W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J Neurosci. 1996;16:974–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers SK, LeBeau FE, Gloveli T, Traub RD, Whittington MA, Buhl EH. Fast network oscillations in the rat dentate gyrus in vitro. J Neurophysiol. 2002;87:1165–1168. doi: 10.1152/jn.00495.2001. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Motor coordination without action potentials in the mammalian spinal cord. Nat Neurosci. 2000;3:593–599. doi: 10.1038/75768. 10.1038/75768. [DOI] [PubMed] [Google Scholar]
- Tscherter A, Heuschkel MO, Renaud P, Streit J. Spatiotemporal characterization of rhythmic activity in rat spinal cord slice cultures. Eur J Neurosci. 2001;14:179–190. doi: 10.1046/j.0953-816x.2001.01635.x. 10.1046/j.0953-816x.2001.01635.x. [DOI] [PubMed] [Google Scholar]
- Walton K, Chesler M. Activity-related extracellular potassium transients in the neonatal rat spinal cord: an in vitro study. Neuroscience. 1988;25:983–995. doi: 10.1016/0306-4522(88)90051-6. 10.1016/0306-4522(88)90051-6. [DOI] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O'Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol. 2000;84:2821–2833. doi: 10.1152/jn.2000.84.6.2821. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. 10.1016/S0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell T. Membrane properties of rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]