Abstract
The cerebral cortex encodes, stores and combines information about the internal and external environment in rhythmic activity of multiple frequency ranges. Neurones of the cortex can be defined, recognized and compared on the comprehensive application of the following measures: (i) brain area- and cell domain-specific distribution of input and output synapses, (ii) expression of molecules involved in cell signalling, (iii) membrane and synaptic properties reflecting the expression of membrane proteins, (iv) temporal structure of firing in vivo, resulting from (i)–(iii). Spatial and temporal measures of neurones in the network reflect an indivisible unity of evolutionary design, i.e. neurones do not have separate structure or function. The blueprint of this design is most easily accessible in the CA1 area of the hippocampus, where a relatively uniform population of pyramidal cells and their inputs follow an instantly recognizable laminated pattern and act within stereotyped network activity patterns. Reviewing the cell types and their spatio-temporal interactions, we suggest that CA1 pyramidal cells are supported by at least 16 distinct types of GABAergic neurone. During a given behaviour-contingent network oscillation, interneurones of a given type exhibit similar firing patterns. During different network oscillations representing two distinct brain states, interneurones of the same class show different firing patterns modulating their postsynaptic target-domain in a brain-state-dependent manner. These results suggest roles for specific interneurone types in structuring the activity of pyramidal cells via their respective target domains, and accurately timing and synchronizing pyramidal cell discharge, rather than providing generalized inhibition. Finally, interneurones belonging to different classes may fire preferentially at distinct time points during a given oscillation. As different interneurones innervate distinct domains of the pyramidal cells, the different compartments will receive GABAergic input differentiated in time. Such a dynamic, spatio-temporal, GABAergic control, which evolves distinct patterns during different brain states, is ideally suited to regulating the input integration of individual pyramidal cells contributing to the formation of cell assemblies and representations in the hippocampus and, probably, throughout the cerebral cortex.
Introduction
The cerebral cortex connects mammals with their environment by collecting data, storing information from previous experience and comparing the incoming information with stored patterns, which are continuously updated and recombined. Neuronal activity in the cerebral cortex, as in many other parts of the brain, shows rhythmic activity in behaviour-dependent frequency ranges (Singer, 1999; Steriade & Timofeev, 2003; Buzsaki & Draguhn, 2004). Rhythmic activity reflects both subthreshold events and the co-ordinated firing of neurones, which code information in the rate and, at least in some cases, in the phase of action potential discharge in relation to oscillations (O’Keefe & Recce, 1993). As a consequence of the behavioural context, the frequency of oscillations also depends on the role of the cortical area as exemplified by the occipital alpha rhythm (Berger, 1929), or the theta rhythm of the hippocampus (see below). Some brain states lead to coherent global oscillatory activity throughout the cortical mantle. For example, slow wave sleep is characterized by a 0.5–2 Hz coherent oscillation (Steriade, 2001). Notwithstanding some area-specific oscillations, the similarity of frequencies across species and areas implies that rhythmic activity is supported by an underlying similarity in biophysical parameters and synaptic and network organization both in terms of the spatial layout of the cortical circuits and in the temporal structure of their activation.
The cerebral cortex is able to generate rhythmic activity dependent on stimuli from the external and internal environment, and also when the animal is asleep and disconnected from the environment. Some of the interactions between cortex and the thalamus (Steriade, 1997), and the subcortical inputs modulating cortical activity in awake and sleep states are fairly well understood. Much less is known about the local interactions of the cortical cells, and the specific contribution of the different cell types to the intracortical rhythms is only beginning to emerge. In the present review we focus on the CA1 area of the rat hippocampus, as potentially the simplest area of the cerebral cortex with particularly well-characterized oscillatory activity patterns (Vanderwolf, 1969), in order to dissect the potential contribution of different cell types to distinct network states.
What constitutes a type of neurone?
There is no agreement on the number and identity of neuronal species in the cerebral cortex. This is partly due to the lack of agreement on criteria of what is necessary to define a cell type, and consequently different authors use partial criteria or sets of non-overlapping data for studying the same or a mixture of several neuronal populations. The lack of adequate definition of cell types mainly results from the small number of cells and cortical areas studied in a comprehensive manner, despite the availability of long established methods of proven value. In other words, relative to the challenge of defining the likely number of cell types that occupy a distinct position in the spatio-temporal structure of the cortex, little effort has been devoted to defining them in a rigorous manner. In addition, some statistical variability is expected within a single population of cells in all measures, which may have profound biological significance (Aradi et al. 2002; Foldy et al. 2003), but at the same time may make the recognition of individual cells more difficult.
The hypothesis that neurones with different shapes have distinct roles in the cortex was implicit in the earliest studies of Ramon y Cajal and was later elaborated by many, most elegantly by Janos Szentagothai (1975). Key evidence for neuronal shape being indicative of role came from neurotransmitter identification, e.g. GABA via the immunocytochemical demonstration of glutamate decarboxylase (GAD) in smooth dendritic neurones and its absence from pyramidal cells (Ribak, 1978). A further useful predictor for distinct roles was found in the differential and highly selective location of output synapses on target neurones (Ramon y Cajal, 1893; Szentagothai, 1975; Somogyi et al. 1998). The use of biophysical measures, expression patterns of proteins involved in cell signalling, and, more recently, in vivo firing patterns have all proved useful in defining cell types (Freund & Buzsaki, 1996; Kawaguchi & Kubota, 1997; Thomson et al. 2002; Markram et al. 2004). Probably all neurones of the cortex can be defined, recognized and compared on the comprehensive application of the following measures: (i) brain area- and cell domain-specific distribution of input and output synapses, (ii) expression of proteins involved in cell signalling, (iii) intrinsic membrane properties reflecting the expression of ion channel proteins, and (iv) temporal distribution of firing in vivo. Based on the above range of parameters, each cell will fall into a tight cluster, the cell type, in this multidimensional space. Of course, in most cases only some of the measures will be available in a given experiment, and one of the important current tasks is to establish which partial measures are sufficient for the correct recognition of a class of cell, in one cortical area, across areas and across different species. Initially, progress towards this goal is expected from simple cortical areas such as the CA1 region. Here we attempt to define cell types first on the distribution of input and output synapses coupled with protein expression, then show that at least some cell types defined this way exhibit distinct firing patterns in vivo, placing each interneurone type to a distinct position in a multidimensional space of spatial, molecular and temporal measures.
The hippocampal CA1 area
One of the basic obstacles to explaining why cortical neurones produce action potentials in a particular pattern is the lack of knowledge of the identity and number of input neurones in the required detail. In most cortical areas many populations of input axons and several distinct populations of recipient neurones are mixed in space, making synaptic connections difficult to predict. The cortical area with the least heterogeneous neuronal population and the smallest number of extrinsic inputs is probably the CA1 area, one reason for its popularity for studying the cortical network. The alignment of the somata and dendrites of pyramidal cells into defined layers and the laminar segregation of much of the extrinsic and intrinsic inputs provide the best chance for defining the synaptic relationships of distinct cell types and the basic cortical circuit.
The pyramidal cells are generally considered to form a single population, but there may be at least three distinct groups, which do not necessarily share the same inputs and response properties. Pyramidal cells in the compact layer of stratum pyramidale next to stratum radiatum are weakly immunopositive for calbindin and are smaller than pyramidal cells more loosely arranged towards stratum oriens, which are calbindin immunonegative (Baimbridge & Miller, 1982). Soma size usually correlates with the size of the axonal arborization, but, to our knowledge, the difference in axonal projections between calbindin negative and positive neurones has not been tested. A third population of pyramidal cells is located in stratum radiatum (Maccaferri & McBain, 1996; Gulyas et al. 1998), some of them being at the border with stratum lacunosum-moleculare. These cells are distinct from the other two populations as they project uniquely to the accessory olfactory bulb (Van Groen & Wyss, 1990). Unlike the other two populations they may have local axon collaterals also within stratum radiatum, in addition to str. oriens.
There are five known significant glutamatergic inputs to CA1 pyramidal cells (Fig. 1): from CA3 pyramidal cells, entorhinal cortical pyramidal cells, the thalamus, CA1 pyramidal cells and the amygdala. Their spatio-temporal interactions and modulation hold the key to explaining the role of this cortical area. Numerically the largest extrinsic glutamatergic input to the CA1 area is from the ipsi- and contralateral CA3 pyramidal cells terminating in stratum radiatum and oriens, with a sharp cut off at the radiatum–lacunosum-moleculare border. This input has a topographical organization with as yet unknown consequences, as the CA3 pyramids closest to the CA1 area innervate only stratum oriens, whereas those closest to the dentate hilus innervate only stratum radiatum (Ishizuka et al. 1990; Li et al. 1994). Therefore, the basal and apical dendrites of pyramidal cells and interneurones with dendrites restricted to str. oriens or radiatum receive input from different individual CA3 pyramidal cells.
Figure 1. Innervation of pyramidal cells by 12 types of GABAergic interneuron and interneurons by 4 types of interneuron specific cell in the CA1 area of the hippocampus.
The main lamina specific glutamatergic inputs are indicated on the left. The somata and dendrites of interneurons innervating pyramidal cells are shown in orange, those innervating mainly or exclusively other interneurons are shown in pink. Axons are shown in light green and the main termination zone of GABAergic synapses are shown by yellow symbols. The proposed names of neurons, some of them abbreviated, are under each schematic cell and a minimal list of molecular cell markers is given, which in combination with the axonal patterns help the recognition and characterisation of each class. Note that one molecular cell marker may be expressed by several distinct cell types. Some cells are listed on the basis of limited data from one study and further data may lead to lumping of some classes (see text). Some additional cell types, which have not been reported in sufficient detail, are not indicated. Note the association of the output synapses of different sets of cell types with the perisomatic region, and either the Schaffer collateral, commissural or the entorhinal pathway termination zones, respectively. CB, calbindin; CR, calretinin; LM-PP, lacunosum-moleculare–perforant path; LM-R-PP; lacunosum-moleculare–radiatum–perforant path; m2, muscarinic receptor type 2; NPY, neuropeptide tyrosine; PV, parvalbumin; SM, somatostatin; VGLUT3, vesicular glutamate transporter 3.
In stratum lacunosum-moleculare the distal dendritic tufts of pyramidal cells are innervated by glutamatergic input from the entorhinal cortex (Amaral & Witter, 1989) and by thalamic input, mostly from the nucleus reuniens (Dolleman-van der Weel & Witter, 1996). Both of these pathways also innervate interneurones, but whether they have preference for certain types is unknown. There is a significant entorhinal input to stratum oriens, but it is not known if these axons originate from the same populations of entorhinal cells that innervate mostly str. lacunosum-moleculare. The synaptic targets of the boutons of entorhinal axons outside str. lacunosum-moleculare are also unknown. Some of these fibres turn radially and pass through the pyramidal layer, then join the more numerous axons in str. lacunosum-moleculare. On the way, they make synapses also in str. radiatum.
The fourth glutamatergic input comes from the local axon collaterals of CA1 pyramidal cells, mostly forming boutons in stratum oriens and innervating both pyramidal basal dendrites (Deuchars & Thomson, 1996) and interneurones (Buhl et al. 1994). The fifth input, which is likely to be glutamatergic and mostly terminates in stratum oriens, is from the amygdala with as yet unknown synaptic targets (Pikkarainen et al. 1999).
From the above it follows that each layer contains boutons from several distinct populations of glutamatergic presynaptic cell, even though one input may provide the majority of synapses. For example, stratum radiatum has glutamatergic boutons mostly form CA3 pyramidal cells, but local axon collateral of pyramidal cells located in this layer and entorhinal afferents also contribute. In stratum oriens, boutons of CA3 pyramids, CA1 pyramids, entorhinal and amygdala afferents are mixed, even though most glutamatergic synapses are probably formed by CA3 axons. The minor populations may not be statistically distributed amongst all potential glutamatergic targets, but they may innervate specifically only certain pyramidal cells or interneurones. Very little data exist on this issue, but one example shows that direct tests are necessary to explain the roles of particular connections. The so called O-LM GABAergic interneurone with dendrites restricted to stratum oriens (Fig. 1) receives at least 70% of its glutamatergic input from CA1 pyramidal cells (Blasco-Ibanez & Freund, 1995), although CA3 pyramidal boutons probably form a much larger fraction of synapses in this layer.
The GABAergic interneurones also show well recognizable laminar differences in both their dendritic and axonal arborizations. It is not accidental that Ramon y Cajal (1893) recognized specialized cells, later called basket cells, with short axons arborizing amongst the cell bodies of pyramidal cells in the hippocampus. The basket cell then was the first inhibitory neurone identified in the brain 70 years later. Andersen et al. (1963) exploited the precise laminar organization of the axons in ingenious in vivo field potential and intracellular measurements and, based on Ramon y Cajal's drawings, concluded that the effect of basket cells was hyperpolarizing. Although the identity of the basket cell has not been questioned, the many other cell types described by Ramon y Cajal (1893) and Lorente de No (1934) have either been ignored and all non-pyramidal cells called basket cells, or non-pyramidal cells were named by the laminar location of their soma, or some other simple feature, as if that would define a cell type as outlined above. A rigorous analysis of individual cells with several methods has shown that even basket cells, as defined by terminating on pyramidal somata, belong to several distinct classes (see below), and the laminar location of an interneurone soma does not define it. We survey below the interneurone types that have been reported in the CA1 area and most likely fulfil distinct roles in the CA1 hippocampal network. Homologous cells may have similar roles throughout the cortex.
Diversity of interneurones
We summarize our current view of interneurone classes in an order of termination sites from the axon initial segment to the most distal dendrites of pyramidal cells (Fig. 1). All non-pyramidal cells will be called interneurones for brevity, irrespective of whether their axon remains in or leaves the CA1 area. Due to space limitation we cannot deal critically with all the controversial data or divergent views (see Parra et al. 1998). We try to refer to what we consider the most salient and reproducible features, and emphasize some of the missing information needed for unequivocal identification. Additional molecular constituents, which completely overlap with another one that has already been in widespread use for delineating subpopulations of cell, will not be listed for lack of space. We indicate molecular constituents, which have tested negative in a cell type only if they have proved useful for differentiating between classes of cell (e.g. basket cells express either parvalbumin or CCK). With the cited evidence we hope to convince our readers that some frequently used criteria about the identity of interneurones are unsustainable. (i) Somatic location in a layer does not identify an interneurone class, as the same class may have somata in several layers and all layers contain several distinct classes. (ii) Dendritic shape does not necessarily identify an interneurone class, as indistinguishable axonal output can be made by cells having diverse dendritic fields. (iii) A single molecular cell marker, such as a calcium binding protein or a neuropeptide, does not identify an interneurone class on its own, as most molecules have been shown to be expressed in several different cell types with distinct inputs and spatio-temporal outputs. For example, parvalbumin is expressed by at least four distinct classes of interneurone (see below). Currently, we recognize 12 types of interneurone innervating mainly specific domains of pyramidal cells and four types of interneurone innervating mostly other interneurones (Fig. 1).
(1) Axo-axonic cell (parvalbumin (PV)+)
It provides GABAergic synapses exclusively to the axon initial segments of up to 1200 pyramidal cells (Somogyi et al. 1983; Li et al. 1992) acting on GABAA receptors (Buhl et al. 1994a) that include the α1, α2, β2/3 and γ2 subunits (Nusser et al. 1996; Somogyi et al. 1996). In most but not all cases, axo-axonic cells can be recognized from the shape of the axon terminal segments; therefore, without electron microscopic examination, in some cases, the cells cannot be distinguished from parvalbumin positive basket cells. The expression of parvalbumin in the soma, dendrites and terminals (Katsumaru et al. 1988) is common with some of the basket cells; no molecular marker has been reported to separate axo-axonic and PV+ basket cells. A small number of putative axo-axonic cells were found to innervate axon initial segments, somata and dendrites of pyramidal cells to different degrees (P. Somogyi, unpublished observation), but these unusual cells have only been seen in a few rats bred in laboratory conditions, which represent a deprived environment. The radial dendrites of axo-axonic cells form a more extensive tuft in str. lacunosum-moleculare than the dendrites of basket or bistratified cells (Buhl et al. 1994b). A few cells with exclusively horizontal dendrites in str. oriens have been reported (Ganter et al. 2004).
(2) Basket cell (PV+, CCK−)
It provides GABAergic synapses to the somata and proximal dendrites of pyramidal cells as well as to other PV+ basket cells and to as yet unidentified interneurones, and forms autapses (Cobb et al. 1997; Pawelzik et al. 2003). It acts on postsynaptic GABAA receptors (Buhl et al. 1994a; Buhl et al. 1995; Ali et al. 1999; Pawelzik et al. 2002) mainly of the α1 subunit containing type (Thomson et al. 2000; Nyiri et al. 2001; Klausberger et al. 2002). Fast postsynaptic response kinetics of interconnected PV+ basket cells ensure high frequency synchronization (Bartos et al. 2002) probably enhanced by gap junctional coupling (Fukuda & Kosaka, 2000). The axonal arbour may be restricted to the pyramidal layer or spread to varying degrees to str. oriens and radiatum with consequent shift in the proportion of output synapses on dendrites (Pawelzik et al. 2002). The consequences, if any, of such differences in the effect and role of the cells are unknown. Those cells terminating exclusively in the pyramidal layer may be misidentified as axo-axonic cells without EM data. The radial dendrites enter str. lacunosum-moleculare, but they rarely branch (Buhl et al. 1994a; Pawelzik et al. 2002).
(3) Basket cell (CCK+, VIP+, vesicular glutamate transporter 3 (VGLUT3)−, PV−)
It provides GABAergic synapses to the somata and proximal dendrites of pyramidal cells as well as to other CCK+ cells and to as yet unidentified interneurones (Harris et al. 1985; Nunzi et al. 1985). It acts on postsynaptic GABAA receptors (Buhl et al. 1996; Thomson et al. 2000; Pawelzik et al. 2002) mainly of the α2-subunit-containing type (Thomson et al. 2000; Nyiri et al. 2001). The axonal arbour may be restricted to the pyramidal layer or spread to varying degrees to str. oriens and radiatum. Synaptic targets have not been quantitatively reported. The combined expression of VIP and CCK is thought to define the class (Acsady et al. 1996a), and, because VIP and the VGLUT3 were found to be mutually exclusive in CCK+ interneurones (Somogyi et al. 2004), it is likely that two types of CCK+ basket cell exist. The radial dendrites enter str. lacunosum-moleculare, but rarely branch (Pawelzik et al. 2002).
(4) Basket cell (CCK+, VGLUT3+, VIP−, PV−)
Except for the difference in VGLUT3 and VIP expression, no other property has been differentiated between nos (3) and (4) (Somogyi et al. 2004). Because the presence of a vesicular transporter is likely to have significant consequences for the effect of the cell, we list them separately. As in further cases below, when so little information is available, it cannot be excluded that the expression of VIP and VGLUT3 is dynamically regulated in time and that types (3) and (4) are two states of one cell type. Further data on synaptic connectivity, additional molecular constituents known to occur in cortical CCK+ interneurones such as CRF, preprotachikinin B, 5-HT3 receptor, etc., intrinsic membrane properties and in vivo firing patterns are needed to test this possibility. However, VGLUT3 is a protein that seems to be expressed by a single class of GABAergic neurone.
(5) Bistratified cell (PV+, somatostatin+, NPY+, GABAA receptor α1 subunit+, CCK−)
The cell was named by Buhl et al. (1994a), because the axonal arbour matches the distribution in str. oriens and radiatum of the Schaffer collateral, commissural pathway. They act on dendritic GABAA receptors (Buhl et al. 1994a; Pawelzik et al. 1999, 2002; Maccaferri et al. 2000) likely to include the α5 and γ2 subunits (Pawelzik et al. 1999), and also innervate interneurones including basket cells (Halasy et al. 1996; Pawelzik et al. 2003). We emphasize the plasma membrane expression of the α1 subunit of the GABAA receptor as seen in immunocytochemical preparations outlining at high intensity the soma and dendrites of a subset of interneurones (Gao & Fritschy, 1994), which includes the bistratified cells (Klausberger et al. 2004), and the parvalbumin positive basket cells (A. Baude, P. Somogyi & T. Klausberger, unpublished observations). This does not mean that other interneurones do not express the α1 subunit, but only a subset expresses it at high level, in synapses and the extrasynaptic membrane (Nusser et al. 1995; Somogyi et al. 1996). The radial dendrites rarely enter str. lacunosum-moleculare (Buhl et al. 1994a). Three cells with horizontal dendrites and innervating pyramidal cells through GABAA receptor-mediated responses were reported as oriens-bistratified (O-Bi) cells (Maccaferri et al. 2000) from in vitro labelling. It remains to be seen if the in vivo firing pattern of such cells is similar to radial bistratified cells or they comprise a separate cell type.
(6) O-LM cell (PV+, somatostatin+, strongly mGluR1α+, presynaptic input mGluR7a decorated)
The horizontal dendrites in str. oriens and the axon mostly distributed in str. lacunosum-moleculare make them universally accepted and have given them their name (McBain et al. 1994), but they were already described as ‘celula de cilindro-eje ascendente’ by Ramon y Cajal (1893). They have been reported from other layers with identical axonal output under different name (Oliva et al. 2000), but whether they received different input from those in str. oriens was not tested. O-LM cells innervate the distal dendrites of pyramidal cells through GABAA receptors (Maccaferri et al. 2000) as well as other interneurones (Katona et al. 1999a). They express lower levels of PV (Klausberger et al. 2003; Ferraguti et al. 2004) than nos (1), (2) and (5), and a uniquely high level of mGluR1α in the extrasynaptic membrane (Baude et al. 1993) combined with a high level of presynaptic mGluR7 both in their glutamatergic (Shigemoto et al. 1996) and GABAergic (Somogyi et al. 2003) input terminals. Other interneurones also express mGluR1α at a distinctly lower level (Ferraguti et al. 2004) and mGluR7 is present at various density in terminals innervating other cell types, including pyramidal cells. Nevertheless, the high level of both mGluRs in combinations is unique to O-LM cells.
(7) Schaffer collateral associated cell (CCK+, calbindin+, somatostatin−, NPY−)
The name was given to cells with cell body in stratum radiatum and at the border with lacunosum-moleculare, innervating pyramidal cell apical and, to a lesser extent, basal dendrites in conjunction with the Schaffer collateral and commissural pathways (Vida et al. 1998). They also innervate interneurones (Vida et al. 1998), which include other Schaffer collateral associated cells (Pawelzik et al. 2002). The axonal arbour resembles those of bistratified and O-bistratified cells, but the latter cells are immunopositive for somatostatin and parvalmumin. The dendrites are mainly in str. radiatum but can enter all layers including the alveus (Cossart et al. 1998; Vida et al. 1998; Pawelzik et al. 2002). They activate postsynaptic GABAA receptors in pyramidal cells and interneurones (Vida et al. 1998; Pawelzik et al. 2002). In addition to CCK (Pawelzik et al. 2002) at least some of them express calbindin (Cope et al. 2002).
(8) Lacunosum-moleculare–radiatum perforant path-associated cell
Cell bodies are at the border of str. radiatum and lacunosum-moleculare or in str. radiatum and the cells have a wider dendritic field than no. (9), reaching the alveus and practically covering all layers (Hájos & Mody, 1997; Vida et al. 1998). The axon is centred on str. lacunosum-moleculare and may spread significantly into the dentate gyrus and str. radiatum (T. Klausberger, E. Papp, L. Marton & P. Somogyi unpublished observation). It is likely that they express CCK and calbindin, but this remains to be documented. More data are required on all aspects of neurones, particularly on the synaptic targets, to delineate distinct cell types in and close to the str. lacunosum-moleculare.
(9) Lacunosum-moleculare perforant path-associated cell
Cell bodies are in str. lacunosum-moleculare or at the border with str. radiatum and the axon associated with the entorhinal input in the CA1 area, sometimes also spreading to the subiculum, presubiculum and crossing the fissure into the dentate gyrus (Hajos & Mody, 1997; Cossart et al. 1998; Vida et al. 1998; Pawelzik et al. 2002). We list this cell separately based on the apparent difference in synaptic target domains from no. (8), although, due to the scarcity of data, it is not yet clear if they might form a continuum with no. (8). The postsynaptic elements were mainly dendritic shafts and to a lesser extent spines (Vida et al. 1998); the innervation of other interneurones has not been reported. Str. radiatum receives little, if any, innervation from this cell type in contrast to no. (8). The dendrites are biased to str. lacunosum-moleculare and enter radiatum only to a limited extent. Cholecystokinin immunoreactivity has been reported in one cell possibly of this type (Pawelzik et al. 2002).
(10) Neurogliaform cell
Originally described in the isocortex (for references see Tamás et al. 2003), identified on the basis of a small dense dendritic field and an extremely dense local axonal cloud. Three cells have been documented close to str. lacunosum-moleculare in the CA1 area from in vitro labelling (Khazipov et al. 1995; Vida et al. 1998), one under a different name (Khazipov et al. 1995). The axonal arbour, when sufficiently revealed, identifies the cell. The synaptic targets were pyramidal cell dendritic shafts and some spines, and the postsynaptic response in a pyramidal cell was suggested to be mediated by GABAA receptors (Vida et al. 1998). No molecular markers have been reported in the hippocampus.
(11) Trilaminar cell (m2 receptor+, presynaptic input mGluR8a decorated, calbindin−)
One cell has been reported from in vivo intracellular recording (Sik et al. 1995). Because of its properties, which appear to be distinct from all other reported cells that we are aware of, and our recent in vivo recording of an identical cell with additional striking properties (T. Klausberger, A. Baude, P. Szucs, L. Marton & P. Somogyi, unpublished observations), we designate it as a separate cell type. The soma and long horizontal dendrites were in str. oriens, and the axon densely innervated str. oriens, pyramidale and radiatum, hence the name (Sik et al. 1995). The same name has been used for various other cells with axon in at least three laminae (Hajos & Mody, 1997; Pawelzik et al. 2002), but we suggest retaining this name for this distinct type of cell. The cell projects to the subiculum and possibly to other brain areas as well. Our immunocytochemical study identified strong immunoreactivity for the m2 receptor in the somato-dendritic domain and intense presynaptic mGluR8a decoration on a trilaminar cell (T. Klausberger, A. Baude, Y. Dalezios, P. Szucs, L. Marton & P. Somogyi unpublished observations), molecules which reveal a distinct population of cell in str. oriens, as reported by Hájos et al. (1998) for m2 receptor positive cells. The cell reported by Sik et al. (1995) was calbindin immunonegative.
(12) Back-projection cell
The cell was named for its widespread axon innervating the CA1 and CA3 areas and the dentate hilus (Sik et al. 1994, 1995). Only two cells have been reported, but the extensive axon is so striking that this cell type probably has little overlap with other interneurones, except possibly with the hippocampo-septal projection cells (see no. (13) below). No molecular cell marker has been directly identified in back-projection cells, but because of the similarity of their axon to intensely NADPH diaphorase positive cells, which probably express high level of neuronal nitric oxide synthase, it was suggested that they are the same cell type (Sik et al. 1994). The cell bodies of the two in vivo recorded cells were in stratum oriens with horizontal dendrites and an axon running through stratum radiatum and lacunosum-moleculare and freely crossing the hippocampal fissure. The synaptic connections of these cells have not been studied in detail, but they form type 2 synapses on dendrites and somata possibly of pyramidal cells (Sik et al. 1994).
(13) Hippocampo-septal cell (calbindin+, somatostatin+)
This and the following three interneurone classes have been reported to innervate mainly or exclusively other interneurones (Freund & Buzsaki, 1996; Gulyas et al. 2003). In the CA1 area these cells are located in str. oriens (Zappone & Sloviter, 2001; Jinno & Kosaka, 2002) having horizontal dendrites and a local axon in str. oriens, pyramidale and radiatum (Gulyas et al. 2003). Retrograde labelling shows that they project to the septum, and to other areas of the hippocampal formation, and the axon of some of them was traced into the CA3 area. The latter projection may indicate overlap or identity with back-projection cells (see no. (12)). Electron microscopic examination showed that the postsynaptic targets were other interneurones (Gulyas et al. 2003). Hippocampo-septal cells innervate mainly GABAergic neurones also in the medial septum (Toth et al. 1993). At least some of them express mGluR1α at a lower level than O-LM cells (see above) and may be decorated by mGluR7a enriched input.
(14) Interneurone specific cell I (IS-I). (calretinin+)
The soma may be in str. oriens pyramidale or radiatum with dendrites spanning most layers and the axon innervating mainly calbindin positive and other calretinin positive cells (Acsady et al. 1996b; Gulyas et al. 1996). The possible expression of VIP by these cells is not known.
(15) Interneurone specific cell II (IS-II), (VIP+)
The soma was reported mainly in str. radiatum and the border with lacunosum-moleculare and the dendrites biased to the latter layer (Acsady et al. 1996a, b). The axon innervates mainly CCK/VIP positive basket cells (Acsady et al. 1996b; Gulyas et al. 1996). The possible expression of calretinin by these cells is not known.
(16) Interneurone specific cell III (IS-III), (VIP+, calretinin+, terminals mGluR7a+)
The soma was located mainly in str. pyramidale and radiatum with radial dendrites crossing most layers (Acsady et al. 1996a, b). The axon innervates mainly O-LM cells (Acsady et al. 1996a; Ferraguti et al. 2004) and the terminals express high level of mGluR7a in the presynaptic active zone (Somogyi et al. 2003).
We would like to emphasize that on some cells very little information exists and sometimes even that only from one laboratory; but revealing how little we know may facilitate more detailed tests. Undoubtedly, ongoing studies will modify this list, but the number of distinct sources of GABA is unlikely to become smaller. Some of the above listed cell classes may be lumped or split further when sufficient information becomes available on all aspects that define a cell class with our criteria. In particular, the backprojection and septo-hippocampal cells (nos (12) and (13)), the two classes of perforant path-associated cells (nos (8) and (9)) and some of the three classes of interneurone specific cells (nos (14)–(16)) may prove to be members of a single population. We are aware of additional findings of axonal and dendritic patterns which we have not been able to assess in sufficient detail. The coexistence patterns of molecular cell markers predict some clear separations, but also a large as yet unexplained heterogeneity, indicating possible further distinct cell classes. For example, 5-HT3 receptor, CCK and CB1 receptor expression strongly overlaps (Morales & Bloom, 1997, Morales et al. 2004; Katona et al. 1999b; Tsou et al. 1999), but it is not yet clear whether all CCK expressing cells express the receptors or only some of the cell types (nos (3), (4) and (7)–(9)) defined above. Only mapping these molecules to cells with full axonal and dendritic arborizations and revealing their firing patterns can lead to clear cell identification.
Even a minimalist view leads to at least 10 types of distinct interneurone innervating a single pyramidal cell, although whether all pyramidal cells are uniformly innervated remains to be tested. Why are so many types of cell needed in the cortex, presumably releasing GABA independently, when in other regions of the nervous system one or two types innervating all parts of the postsynaptic cell suffice? One can argue that the different domains of the pyramidal cell have distinct roles and need independent modulation. For example, as the axon initial segment is thought to generate the action potential, its GABAergic regulation by the axo-axonic cell must be independent of the distal dendrite innervated by the O-LM cells, due to the different times that GABA is needed there. But even the same domain may receive input from several distinct cell types with similar specialization, as the bistratified and Schaffer collateral-associated cells converging on pyramidal dendrites in stratum radiatum and oriens, or CCK or parvalbumin expressing basket cells converging on pyramidal somata, the domain of integration. Why these inputs to the same postsynaptic domain need to be independent is not understood, but a temporal division of labour is a possibility.
Pyramidal cell activity is synchronized on different time scales (see below). Because the perisomatic region integrates inputs that lead to action potential generation presumably in the axon initial segment, the GABAergic inputs to the soma/proximal dendrites and axon initial segment are well placed to synchronize pyramidal cells. Tests conducted in vitro (Cobb et al. 1995) demonstrate that single basket and axo-axonic cells are able to entrain and synchronize tonically discharging hippocampal pyramidal cells by resetting the phase of intrinsic membrane potential oscillations at the physiologically relevant theta (4–7 Hz) frequency (Fig. 2). If a single basket cell innervating up to 2000 pyramidal cells (Sik et al. 1995; Halasy et al. 1996) can reset the rhythmic firing of the cells and synchronize them in interaction with intrinsic membrane oscillations, how would the approximately 25 basket cells converging on a single pyramidal cell (Buhl et al. 1994a) affect firing in different brain states? In attempting to answer this question first we briefly summarize the oscillatory activities of pyramidal cells and then examine how the firing of some of the much less understood interneurones is related to them.
Figure 2. Basket cells phase and synchronize pyramidal cell firing in the CA1 areain vitro.
A, paired intracellular recording of an electron microscopically defined basket cell (orange traces) and a pyramidal cell (blue traces). When the pyramidal cell was depolarized to fire at low rate, the action potentials following the basket cell-evoked IPSP (onset, orange triangle) fell in a narrow window (top trace), due to the resetting of an intrinsic membrane potential oscillation (superimposition of 12 sweeps). This is apparent in the average of those sweeps in which the pyramidal cell did not fire (middle trace), showing a depolarizing overshoot at the time interval when the pyramidal cell produces rebound spikes. Hyperpolarizing the pyramidal cell by constant current injection eliminates the oscillation (bottom trace). B, a single IPSP of unitary amplitude, evoked by extracellular stimulation close to the pyramidal cell layer (onset, orange triangle), synchronized the firing of two tonically discharging intracellularly recorded pyramidal cells (PC1, PC2) at theta frequency for several cycles due to the resetting of intrinsic membrane oscillations (traces superimposed on the onset of the IPSP). C, when the IPSPs were evoked at theta frequency (triangles), the synchrony became much tighter due to the sequential inhibition and facilitation of firing probability in narrow time windows. Scales in A: A, B refers to two top panels; C, D refers to bottom two panels. Data from Cobb et al. (1995) with permission (http://www.nature.com); panel A was prepared by the late Eberhard Buhl.
Brain state dependent oscillations and temporal patterns of pyramidal cell discharge
Given the enormous number of neurones in the cerebral cortex a temporally structured synchronous activity might be an organizational necessity for neuronal integration, coincidence detection, discrimination of unlinked information and binding the different aspects of discrete objects or events (Singer, 1999). Hippocampal network oscillations at various frequencies are reliably correlated with certain behaviours.
Theta oscillations (4–10 Hz) occur during whole body movement, memory tasks and rapid-eye-movement sleep (Grastyan et al. 1959; Vanderwolf, 1969) and a form can also be produced in vitro (Konopacki et al. 1987; Gillies et al. 2002). During theta oscillations the majority of the pyramidal cells in the CA1 area exhibit a very low firing rate with the highest firing probability during or shortly after the trough of the theta cycles recorded extracellularly in the pyramidal cell layer. This average firing pattern is common in theta oscillations during rapid eye movement sleep (Csicsvari et al. 1999), walking (Harris et al. 2002) and also during theta oscillations recorded in anaes-thetized animals (Buzsaki et al. 1983; Soltesz & Deschenes, 1993), as shown in Fig. 5. However, the mean theta phase distribution is largely the sum of the discharge of those CA1 pyramidal cells, which are so-called place cells during navigation. When the rat enters the place field of a given cell, the cell will start to fire before the positive peak of the theta oscillations recorded extracellularly in the pyramidal cell layer (O'Keefe & Recce, 1993). As the animal advances through the place field, the place cell fires at earlier phases during consecutive theta cycles resulting in a phase precession of the firing of 180 degrees and more. The theta phase of the action potentials predicts the position of the animal within the place field (O'Keefe & Recce, 1993; Skaggs et al. 1996). Thus the specific phase of firing of pyramidal cells not only might serve the organization of the network but could additionally encode parts of the information content in itself (O'Keefe & Recce, 1993).
Figure 5. Synaptic connectivity and distinct in vivo firing patterns of pyramidal cells and four types of interneurone embedded in the hippocampal network.
The schematic drawing summarizes the main synaptic connections in the CA1 area of pyramidal cells (blue), parvalbumin expressing basket, axo-axonic, bistratified and O-LM cells. The cells have differential temporal firing patterns during theta and ripple oscillations (mean of several cells). For clarity two theta cycles are shown in the firing probability histograms. The Y axis of the spike probability plots was constructed by including all events and cycles in the analysed period irrespective of whether the individual recorded cell fired or not. The phase relationship of the extracellularly recorded field potential (schematic white wave) used in the spike alignments and the phase shifted oscillation in the membrane potential oscillation of pyramidal cells reported from intracellular studies (blue waves) is shown schematically. For the ripples, time was normalized to the beginning, highest amplitude and end of ripple episode. The spike probability plots show that during different network oscillations representing two distinct brain states, interneurones of the same connectivity class show different firing activities and therefore modulate their specific postsynaptic target-domain in a brain-state-dependent manner. Interneurones belonging to different connectivity classes fire preferentially at distinct time points during a given oscillation. Because the different interneurones innervate distinct domains of the pyramidal cells, the respective compartments will receive GABAergic input at different time points. This suggests a role for interneurones in the temporal structuring of the activity of pyramidal cells and their inputs via their respective target domain in a co-operative manner, rather than simply providing generalized inhibition. (Firing probability histograms modified from Klausberger et al. 2003, 2004.)
Sharp wave-associated high frequency ripples (120–200 Hz) of around 100 ms duration occur in the CA1 area of the hippocampus during slow-wave sleep and consummatory behaviours (Buzsaki et al. 1983). During ripples, initiated by the co-ordinated discharge of CA3 pyramidal cells, a CA1 pyramidal cell fires one or a few action potentials phased locked to the trough of the cycles, but not every pyramidal cell will be active during a given sharp wave event. This is consistent with the hypothesis that pyramidal cells forming cell assemblies fire together reinforcing their connections in the network by the high synchrony of discharge within a few milliseconds in each ripple cycle (Buzsaki, 1989).
Gamma oscillations (30–80 Hz) are not restricted to a single brain state. They occur during network activity at lower frequencies (Csicsvari et al. 2003) and also simultaneously with theta oscillations, the power of the gamma oscillations being modulated by the phase of the theta cycles. Such a coexistence of two or more oscillations at different frequencies, the power of the faster-frequency oscillation being modulated by the phase of the slower-frequency oscillation might be a general principal of the architecture of the cerebral cortex.
Small irregular activity occurs when an animal is startled out of sleep without moving and during active waking when it abruptly freezes (see Jarosiewicz et al. 2002). In this network state only a small subset of CA1 pyramidal cells having place fields in the rat's location are active (Jarosiewicz et al. 2002).
Slow oscillations at 1 Hz or less have not been investigated in detail in the hippocampus and little is known about any regularity in the firing of pyramidal cells or interneurones outside the ripple oscillations during cortical slow oscillations (Penttonen et al. 1999).
In interaction with the glutamatergic inputs, local GABAergic interneurones sculpt the firing pattern of active pyramidal cells and orchestrate the subthreshold events reflected in the extracellular field potential oscillations. But, considering the multitude of interneurones, can we predict which type(s) are responsible for contributing to the stereotyped pyramidal firing patterns, and do all interneurones participate in all network states?
Network and oscillation phase related discharge of interneurones in vivo
In vivo recordings of putative interneurones indicated that their firing is strongly correlated with various network oscillations (Ranck, 1973; O'Keefe, 1976). Many, but not all, interneurones receive GABAergic theta modulated input from the medial septum (Toth et al. 1997) and discharge phase locked to theta activity; hence they were named theta cells in single cell recordings from behaving animals. Interestingly, even during one network state several different firing patterns of putative interneurones have been reported. For example, individual unidentified interneurones were found to fire at distinct phases of the theta oscillations (Skaggs et al. 1996) and in different patterns during sharp wave-associated ripple events (Csicsvari et al. 1999). These observations raised the possibility that the different firing patterns of interneurones might be correlated with their spatial specificity in innervating distinct domains of pyramidal cells and other interneurones. We have tested this hypothesis by recording the firing patterns of anatomically identified interneurones during network oscillations in anaesthetized rats (Klausberger et al. 2003, 2004).
Theta activity
During theta oscillations axo-axonic cells (see no. (1)) exhibited the highest firing probability around the peak of the pyramidal layer extracellular theta, i.e. when the average pyramidal cell is most hyperpolarized (Fig. 4). Parvalbumin immunopositive basket cells (see no. (2)) fired at the descending phase of the oscillations recorded extracellularly in the pyramidal cell layer. There is considerable variability in the mean theta phase of individual cells. The bistratified cells (see no. (5)) innervating str. radiatum and oriens fired at the trough of the extracellular theta in the pyramidal cell layer (Figs 3, 4, 5). The O-LM cells terminating on the most distal dendrites in conjunction with the entorhinal input also fired at the trough of the field potential (Fig. 5).
Figure 4. In vivo firing patterns of four types of interneurone during two oscillatory network states.
Action potentials (filtered 0.8–5 kHz) of a parvalbumin immunopositive basket, an axo-axonic, a bistratified and an O-LM cell are shown during theta oscillations (3–6 Hz) and ripple episodes (filtered 90–140 Hz) recorded extracellularly in the pyramidal cell layer. During theta oscillations the PV basket cell fired at the descending phase, the axo-axonic cell around the peak and the bistratified and the O-LM cell discharged mainly at the trough of theta wave. Note the different inter-spike intervals during single theta cycles between bistratified and O-LM cells. During ripple episodes the PV basket cell fired preferentially at the highest amplitude of the ripple, the axo-axomic cell sometimes fired before the ripple but was depressed afterwards, the bistratified cell fired throughout, before and after the ripple episode and the O-LM cell was silent. Scales: ripples, 0.1 mV, 50 ms; spikes, 0.5 mV; theta, 0.2 mV, 0.3 s. (Data modified from Klausberger et al. 2003, 2004.)
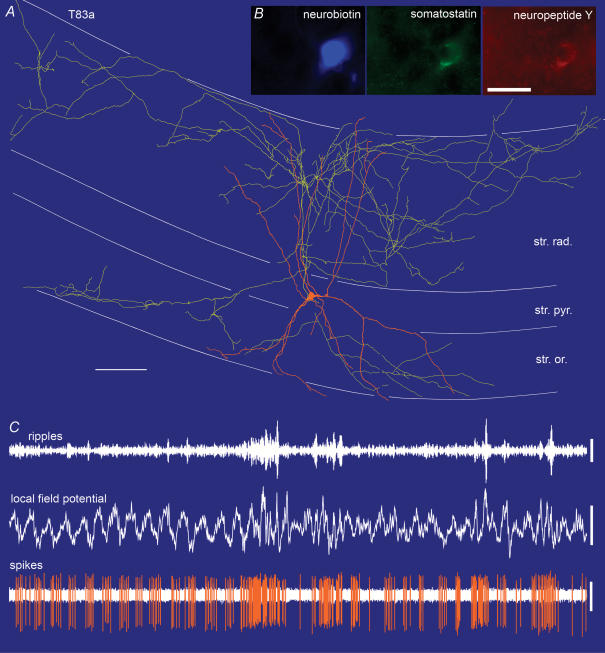
Figure 3. In vivo firing patterns and visualization of a bistratified cell (T83a).
A, reconstruction of the neurobiotin-labelled bistratified cell. The soma and dendrites (orange) are shown complete; the axon (yellow) is shown only from 3 sections of 65 µm thickness for clarity. Note that the axon branches preferentially in stratum radiatum (str. rad.) and stratum oriens (str. or.), but avoids stratum pyramidale (str. pyr.) and stratum lacunosum-moleculare. Reconstruction made by and presented courtesy of Peter Szucs. Scale bar, 100 µm. B, immunofluorescence micrographs showing that the bistratified cell expressed the neuropeptides somatostatin and neuropeptide Y. Scale bar 20 µm. C, in vivo firing patterns of the cell showing the ripples (local field potential filtered 90–140 Hz), local field potential in the pyramidal cell layer (filtered 0.3–200 Hz) and spikes of the labelled cell (filtered 0.8–5 kHz). Note that during the initial theta oscillations (4 Hz) the cell fired rhythmically on the trough of the theta cycles. Subsequently, the local field potential became more irregular and sharp wave-associated ripples appeared, around which the cell strongly increased firing. Scales: 0.5 s; ripples 0.1 mV; local field potential 1 mV; spikes 0.2 mV.
Putting pyramidal cell firing into interneuronal context, it is necessary to consider the complex theta-phase relationship between intracellular compartments, the extracellular field potential and the changes of theta phase as a function of depth between the CA1 pyramidal cell layer and the hippocampal fissure (Buzsaki, 2002). Briefly (Fig. 5), the peak of the theta recorded intracellularly in the soma of pyramidal cells lags the trough of the extracellular theta recorded in the pyramidal layer by 0–60 degrees (Soltesz & Deschenes, 1993). The peak of the intracellular theta recorded in the dendrites of pyramidal cells slightly lags the peak of the extracellular theta in the pyramidal cell layer (Kamondi et al. 1998). Pyramidal cells fire, on average, at a time when the soma is most depolarized and after the highest firing probability of PV+ basket and axo-axonic cells. Therefore, these interneurones might time and synchronize the subthreshold theta oscillations in pyramidal cells similar to in vitro experiments cited above (Cobb et al. 1995). Because the time course of the activation of pyramidal cells due to the rebound effect may be faster in vivo than in vitro, it is possible that IPSPs evoked by basket cells in pyramidal cells can contribute to phase resetting on intrinsic oscillations and pyramidal cell firing 90 degrees later in vivo. However, pyramidal cells also fire at lower probability at all phases of the oscillation as seen in the shallow theta modulation of their activity. Furthermore, as mentioned above, the theta modulation of phase precessing cells is faster than the frequency of the average theta field potential. Curiously, the highest probability of pyramidal cell firing coincides with maximal O-LM and bistratified cell firing (Klausberger et al. 2003, 2004) and relative dendritic hyperpolarization (Kamondi et al. 1998) (Fig. 5). Thus, although GABA release from O-LM and bistratified cells may contribute to the phase shift of the oscillation from the pyramidal layer to str. lacunosum-moleculare, it may make the dendrites paradoxically least excitable at the pyramidal cell highest firing probability, assuming that GABA is inhibitory. Such dendritic inhibition may provide a local threshold effect on inputs with only the strongest inputs leading to pyramidal cell firing. In parallel, GABAergic hyperpolarization of dendrites may contribute to deinactivation of voltage sensitive sodium and low threshold calcium channels and rebound dendritic spikes in those cells receiving the strongest excitatory inputs.
Ripple oscillations
During sharp wave-associated ripple episodes (Fig. 5) parvalbumin-expressing basket cells and bistratified cells increase their discharge frequency, but there are subtle yet significant differences between them (Klausberger et al. 2003, 2004). On average, basket cells fired preferentially at the highest amplitude of the ripple and bistratified cells fired with high frequency throughout the whole ripple episodes. Furthermore, unlike basket cells, bistratified cells increased their firing rate even before ripples can be detected in the extracellular field potential. Axo-axonic cells slightly increased their firing activity at the beginning of the ripples, but became silent after the highest amplitude of the ripple episode, and appeared suppressed even after the ripple episodes. In contrast, O-LM cell firing was specifically suppressed for the duration of ripple episodes. The silence of O-LM cells during ripple episodes is surprising because they receive their major glutamatergic input from local CA1 pyramidal cells, which are most active as a population during ripples. The pyramidal input to O-LM cells has a very low resting release probability, which is increased only by repetitive firing (Ali et al. 1999). Because pyramidal cells rarely fire more than one or two action potentials, this network state is not optimal for O-LM cell activation. However, the specific depression of firing of O-LM cells during ripple episodes cannot be explained by weak excitation alone. Additional mechanisms for silencing must be present in the network. From the major, and apparently independent, GABAergic input of O-LM cells from IS-III cells (see no. (16)), we predict that IS-III cells fire strongly during ripples.
The consequences of the depressed firing of O-LM cells during ripple episodes remain unknown. It can be speculated that the absence of hyperpolarization and shunting could promote action potentials to back-propagate into the most distal apical dendrites (Magee & Johnston, 1997), leading to potentiation of the few synapses from the entorhinal cortex that are active during ripple episodes. The biphasic firing of axo-axonic cells might inhibit pyramidal cells from firing at the beginning of the ripple episode, but allow them to discharge at the highest amplitude of the oscillation, focusing highly synchronous firing to a short period.
The high firing frequency of bistratified cells during the ripple episodes seemed rather surprising (Klausberger et al. 2004), given that they innervate the dendrites of pyramidal cells with GABAergic synapses at the same site, where the strongly activated glutamatergic input from the Schaffer collateral commissural input terminates and initiates the ripples. The analysis of the exact spike timing during single ripple cycles indicated that both bistratified and also parvalbumin expressing basket cells fire at the ascending phase of the ripple cycle (Csicsvari et al. 1999; Klausberger et al. 2004), on average 1–2 ms after the firing of pyramidal cells. This specific spike timing indicates that the role for the interneurones during ripple episodes lies not in the shunting of action potentials, but, more likely, in providing hyperpolarization well-timed to de-inactivate voltage-dependent ion channels and therefore promote subsequent firing of pyramidal cells. In addition, the high firing frequency and specific spike timing of bistratified and parvalbumin positive basket cells indicate their prominent role in generating ripple oscillations in the CA1 area during sharp waves. The synchronized firing of networks of basket and bistratified cells at the ascending phase of the ripple cycles will lead to population IPSCs in pyramidal cells most likely reflected by a subsequent intracellular hyperpolarization and the extracellular positive deflection in the ripple cycle. In particular, the early ripple frequency firing of bistratified cells, before the pyramidal cells, could structure dendritic oscillations in the pyramidal cell dendrites and with the build-up of excitation lead to fast dendritic spikes actively propagating to the soma (Kamondi et al. 1998; Golding & Spruston, 1998) and evoking the phase locked pyramidal cell discharge. Thus, a strong excitation from the CA3 area and rhythmic hyperpolarization (Csicsvari et al. 2000) originating from parvalbumin positive basket and bistratified cells (Klausberger et al. 2004), together with a possible contribution of axo-axonal gap junctions between pyramidal cells (Traub et al. 2002), might produce the CA1-specific phenomenon of sharp wave-associated ripples.
These results indicate that interneurones within a connectivity class (nos (1), (2), (5) and (6) reported so far) exhibit similar firing patterns during a given network oscillation. In addition, during different network oscillations representing two distinct brain states, interneurones of the same connectivity class show different firing activities and therefore modulate their specific postsynaptic target-domain in a brain-state-dependent manner. Interneurone type-specific firing patterns point to a role in structuring the activity of pyramidal cells via their respective target domain and accurately timing and synchronizing pyramidal cell discharge rather than simply providing generalized inhibition. And finally, interneurones belonging to different connectivity classes may fire preferentially at distinct time points during a given oscillation. Because the different interneurones innervate distinct domains of the pyramidal cells, the different compartments will receive differential GABAergic input in time. Such a dynamic spatio-temporal GABAergic control, which evolves distinct patterns during different brain states, is ideally suited to regulating the input integration of individual pyramidal cells and contribute to the formation of cell assemblies and representations in the hippocampus.
Future directions
So far, the in vivo firing patterns of only four distinct classes of interneurone have been reported, although many more types of interneurone exist. In particular, it is apparent that dendrite-targeting O-LM and bistratified cells discharge with highest probability at the trough of the pyramidal layer theta, whereas the basket and axo-axonic cells innervating the perisomatic region of pyramidal cells discharge, on average, at earlier phases of the theta cycle. Does this mean that the postsynaptic target of an interneurone (e.g. the specific subcellular domain of a pyramidal cell) determines the firing patterns of this interneurone? This can be tested by recording additional interneurone classes with target preference similar to those already presented, such as basket cells expressing CCK and innervating the soma similar to PV expressing basket cells, or Schaffer collateral-associated cells innervating the same dendritic domain as the reported bistratified cells.
Another fascinating question is how the different firing patterns of distinct interneurones are generated. The results so far indicate that the in vivo firing of a cell is not simply determined by its main excitatory drive as predicted from the laminar location of the dendrites, but is governed by complex network phenomena (see Freund & Buzsaki, 1996). Therefore, we need a much better knowledge of which types of cells are connected with GABAergic synapses and gap junctions, what the different sources of excitation and modulation are and how accurately timed input from different sources is integrated into a specific firing output. In other words, a much better spatio-temporal definition of the network is needed to understand the processing of information in the cerebral cortex.
Most of the GABAergic cell types defined in the CA1 area have homologues in the isocortex, which is particularly apparent in the supragranular layers. For example, the somata of supragranular pyramidal cells are innervated by basket cells immunopositive for PV, or CCK, or CCK and VGLUT3 (Kawaguchi & Kubota, 1997; Somogyi et al. 2004) as in the hippocampus, and the axo-axonic cell was discovered in the rat visual cortex. Somatostatin positive bitufted cells with molecular expression profile (Dalezios et al. 2002) and response properties similar to hippocampal O-LM cells innervate the distal dendrites of isocortical pyramidal cells. A full comparison is beyond the scope of this review. The spatial relationships of pre- and postsynaptic cells are more difficult to decipher because several populations of pyramidal cells are present in the same space and they may or may not share a given interneurone (Rockland & Ichinohe, 2004). A great deal of information is available on interneurones of the isocortex, but a comprehensive picture is only slowly emerging (Kawaguchi & Kubota, 1997; Somogyi et al. 1998; Thomson et al. 2002; Markram et al. 2004)) due to partial data sets, the lack of directly tested synaptic connections, the inconsistent delineation of cell identity and the limited information on in vivo firing patterns of defined cell types. It will be interesting to see the contribution of the same well-defined interneurone classes to distinct network oscillations and brain states across different cortical areas.
Acknowledgments
The authors thank Drs Agnes Baude, Yannis Dalezios, Francesco Ferraguti, Laszlo Marton and Peter Szucs for discussions that contributed to this review and for allowing us to refer to some of their unpublished results. Peter Szucs provided the unpublished bistratified cell reconstruction for Fig. 2. We are grateful for the constructive critical comments of Drs Laszlo Acsady, Gyorgy Buzsaki, Shozo Jinno, Istvan Katona and Imre Vida on an earlier version of the manusript. T.K. is a Junior Research Fellow at St John's College, Oxford University and is supported by grant P16637 of the Austrian Science Fund.
References
- Acsady L, Arabadzisz D, Freund TF. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience. 1996a;73:299–315. doi: 10.1016/0306-4522(95)00610-9. [DOI] [PubMed] [Google Scholar]
- Acsady L, Gorcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996b;73:317–334. doi: 10.1016/0306-4522(95)00609-5. [DOI] [PubMed] [Google Scholar]
- Ali AB, Bannister AP, Thomson AM. IPSPs elicited in CA1 pyramidal cells by putative basket cells in slices of adult rat hippocampus. Eur J Neurosci. 1999;11:1741–1753. doi: 10.1046/j.1460-9568.1999.00592.x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Loyning Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963;198:540–542. doi: 10.1038/198540a0. [DOI] [PubMed] [Google Scholar]
- Aradi I, Santhakumar V, Chen K, Soltesz I. Postsynaptic effects of GABAergic synaptic diversity: regulation of neuronal excitability by changes in IPSC variance. Neuropharm. 2002;43:511–522. doi: 10.1016/s0028-3908(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–229. doi: 10.1016/0006-8993(82)90804-6. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger H, et al. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Nat Acad Sci U S A. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Berger H. Ueber das Elektroenkephalogram des Menschen. Erste Mitteilung Arch Psychiat Nervenkr. 1929;87:529–570. [Google Scholar]
- Blasco-Ibanez JM, Freund TF. Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feed-back activation. Eur J Neurosci. 1995;7:2170–2180. doi: 10.1111/j.1460-9568.1995.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Cobb SR, Halasy K, Somogyi P. Properties of unitary IPSPs evoked by anatomically identified basket cells in the rat hippocampus. Eur J Neurosci. 1995;7:1989–2004. doi: 10.1111/j.1460-9568.1995.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994a;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Han Z-S, Lorinczi Z, Stezhka VV, Karnup SV, Somogyi P. Physiological properties of anatomically identified axo-axonic cells in the rat hippocampus. J Neurophysiol. 1994b;71:1289–1307. doi: 10.1152/jn.1994.71.4.1289. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Szilagyi T, Halasy K, Somogyi P. Physiological properties of anatomically identified basket and bistratified cells in the CA1 area of the rat hippocampus in vitro. Hippocampus. 1996;6:294–305. doi: 10.1002/(SICI)1098-1063(1996)6:3<294::AID-HIPO7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Two-stage model of memory trace formation: a role of ‘noisy’ brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Leung L-W, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res Rev. 1983;6:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Halasy K, Vida I, Nyiri G, Tamas G, Buhl EH, et al. Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience. 1997;79:629–648. doi: 10.1016/s0306-4522(97)00055-9. [DOI] [PubMed] [Google Scholar]
- Cope DW, Maccaferri G, Márton LF, Roberts JDB, Cobden PM, Somogyi P. Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurones target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience. 2002;109:63–80. doi: 10.1016/s0306-4522(01)00440-7. [DOI] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A, Buzsaki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron. 2000;28:585–594. doi: 10.1016/s0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Dalezios Y, Lujan R, Shigemoto R, Roberts JDB, Somogyi P. Enrichment of mGluR7a in the presynaptic active zones of GABAergic and non-GABAergic terminals on interneurons in the rat somatosensory cortex. Cereb Cortex. 2002;12:961–974. doi: 10.1093/cercor/12.9.961. [DOI] [PubMed] [Google Scholar]
- Deuchars J, Thomson AM. CA1 pyramid-pyramid connections in rat hippocampus in vitro: dual intracellular recordings with biocytin filling. Neuroscience. 1996;74:1009–1018. doi: 10.1016/0306-4522(96)00251-5. [DOI] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Witter MP. Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. J Comp Neurol. 1996;364:637–650. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Cobden P, Pollard M, Cope D, Shigemoto R, Watanabe M, et al. Immunolocalization of metabotropic glutamate receptor 1a (mGluR1a) in distinct classes of interneuron in the CA1 region of the rat hippocampus. Hippocampus. 2004;14:193–215. doi: 10.1002/hipo.10163. [DOI] [PubMed] [Google Scholar]
- Földy C, Aradi I, Howard A, Soltesz I. Diversity beyond variance: modulation of firing rates and network coherence by GABAergic subpopulations. Eur J Neurosci. 2003;19:119–130. doi: 10.1046/j.1460-9568.2003.03096.x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kosaka T. Gap junctions linking dendritic network gabaergic interneurons hippocampus. J Neurosci. 2000;20:1519–1528. doi: 10.1523/JNEUROSCI.20-04-01519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter P, Szücs P, Paulsen O, Somogyi P. Properties of horizontal axo-axonic cells in stratum oriens of the hippocampal CA1 area of rats in vitro. Hippocampus. 2004;14:232–243. doi: 10.1002/hipo.10170. [DOI] [PubMed] [Google Scholar]
- Gao B, Fritschy JM. Selective allocation of GABAA receptors containing the α1 subunit to neurochemically distinct subpopulations of rat hippocampal interneurons. Eur J Neurosci. 1994;6:837–853. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Gillies MJ, Traub RD, LeBeau FE, Davies CH, Gloveli T, Buhl EH, et al. A model of atropine-resistant theta oscillations in rat hippocampal area CA1. J Physiol. 2002;543:779–793. doi: 10.1113/jphysiol.2002.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Spruston N. Dendritic sodium spikes are variable triggers of axonal action potentials in hippocampal CA1 pyramidal neurons. Neuron. 1998;21:1189–1200. doi: 10.1016/s0896-6273(00)80635-2. [DOI] [PubMed] [Google Scholar]
- Grastyan E, Lissak K, Madarasz I, Donhoffer H. Hippocampal electrical activity during the development of conditioned reflexes. Electroencephalogr Clin Neurophysiolsupplement. 1959;11:409–430. doi: 10.1016/0013-4694(59)90040-9. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AL, Hajos N, Katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17:1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Toth K, McBain CJ, Freund TF. Stratum radiatum giant cells: a type of principal cell in the rat hippocampus. Eur J Neurosci. 1998;10:3813–3822. doi: 10.1046/j.1460-9568.1998.00402.x. [DOI] [PubMed] [Google Scholar]
- Hájos N, Mody I. Synaptic communication among hippocampal interneurons: Properties of spontaneous IPSCs in morphologically identified cells. J Neurosci. 1997;17:8427–8442. doi: 10.1523/JNEUROSCI.17-21-08427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Papp EC, Acsady L, Levey AL, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1998;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- Halasy K, Buhl EH, Lorinczi Z, Tamas G, Somogyi P. Synaptic target selectivity and input of GABAergic basket and bistratified interneurons in the CA1 area of the rat hippocampus. Hippocampus. 1996;6:306–329. doi: 10.1002/(SICI)1098-1063(1996)6:3<306::AID-HIPO8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Hirase H, Leinekugel X, Dragol G, Czurkó A, et al. Spike train dynamics predicts theta-related phase precession in hippocampal pyramidal cells. Nature. 2002;417:738–741. doi: 10.1038/nature00808. [DOI] [PubMed] [Google Scholar]
- Harris KM, Marshall PE, Landis DMD. Ultrastructural study of cholecystokinin-immunoreactive cells and processes in area CA1 of the rat hippocampus. J Comp Neurol. 1985;233:147–158. doi: 10.1002/cne.902330202. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jarosiewicz B, McNaughton BL, Skaggs WE. Hippocampal population activity during the small-amplitude irregular activity state in the rat. J Neurosci. 2002;22:1373–1384. doi: 10.1523/JNEUROSCI.22-04-01373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Immunocytochemical characterization of hippocamposeptal projecting GABAergic nonprincipal neurons in the mouse brain: a retrograde labeling study. Brain Res. 2002;945:219–231. doi: 10.1016/s0006-8993(02)02804-4. [DOI] [PubMed] [Google Scholar]
- Kamondi A, Acsady L, Wang X-J, Buzsaki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: Activity-dependent phase-precession of action potentials. Hippocampus. 1998;8:244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Katona I, Acsady L, Freund TF. Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience. 1999a;88:37–55. doi: 10.1016/s0306-4522(98)00302-9. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999b;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumaru H, Kosaka T, Heizmann CW, Hama K. Gap junctions on GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus (CA1 region) Exp Brain Res. 1988;72:363–370. doi: 10.1007/BF00250257. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cerebral Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Congar P, Ben-Ari Y. Hippocampal CA1 lacunosum-moleculare interneurons: modulation of monosynaptic GABAergic IPSCs by presynaptic GABAB receptors. J Neurophysiol. 1995;74:2126–2137. doi: 10.1152/jn.1995.74.5.2126. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton L, Roberts JDB, Cobden PM, Buzsáki G, et al. Brain state- and cell type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, Baude A, Roberts JDB, Magill P, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopacki J, Bland BH, Maclver MB, Roth SH. Cholinergic theta rhythm in transected hippocampal slices: independent CA1 and dentate generators. Brain Res. 1987;436:217–222. doi: 10.1016/0006-8993(87)91664-7. [DOI] [PubMed] [Google Scholar]
- Li X-G, Somogyi P, Tepper JM, Buzsaki G. Axonal and dendritic arborization of an intracellularly labeled chandelier cell in the CA1 region of rat hippocampus. Exp Brain Res. 1992;90:519–525. doi: 10.1007/BF00230934. [DOI] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- Maccaferri G, McBain CJ. Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci. 1996;16:5334–5343. doi: 10.1523/JNEUROSCI.16-17-05334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JDB, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol. 2000;524:91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McBain CJ, DiChiara TJ, Kauer JA. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J Neurosci. 1994;14:4433–4445. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Wang SD, DiazRuiz O, Jho DHJ. Cannabinoid CB1 receptor and serotonin 3 receptor subunit A (5-HT3A) are co-expressed in GABA neurons in the rat telencephalon. J Comp Neurol. 2004;468:205–216. doi: 10.1002/cne.10968. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Gorio A, Milan F, Freund TF, Somogyi P, Smith AD. Cholecystokinin-immunoreactive cells form symmetrical synaptic contacts with pyramidal and non-pyramidal neurons in the hippocampus. J Comp Neurol. 1985;237:485–505. doi: 10.1002/cne.902370406. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Roberts JDB, Baude A, Richards JG, Sieghart W, Somogyi P. Immunocytochemical localization of the α1 and β2/3 subunits of the GABAA receptor in relation to specific GABAergic synapses in the dentate gyrus. Eur J Neurosci. 1995;7:630–646. doi: 10.1111/j.1460-9568.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Stephenson FA, Somogyi P. The α6 subunit of the GABAA receptor is concentrated in both inhibitory and excitatory synapses on cerebellar granule cells. J Neurosci. 1996;16:103–114. doi: 10.1523/JNEUROSCI.16-01-00103.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of α2-subunit-containing GABAA receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra P, Gulyas AI, Miles R. How many subtypes of inhibitory cells in the hippocampus. Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Bannister AP, Deuchars J, Ilia M, Thomson AM. Modulation of bistratified cell IPSPs and basket cell IPSPs by pentobarbitone sodium, diazepam and Zn2+: dual recordings in slices of adult rat hippocampus. Eur J Neurosci. 1999;11:3552–3564. doi: 10.1046/j.1460-9568.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Hughes DI, Thomson AM. Physiological and morphological diversity of immunocytochemically defined parvalbumin- and cholecystokinin-positive interneurones in CA1 of the adult rat hippocampus. J Comp Neurol. 2002;443:346–367. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Hughes DI, Thomson AM. Modulation of inhibitory autapses and synapses on rat CA1 interneurones by GABAA receptor ligands. J Physiol. 2003;546:701–716. doi: 10.1113/jphysiol.2002.035121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Nurminen N, Miettinen R, Sirvio J, Henze DA, Csicsvari J, et al. Ultra-slow oscillation (0.025 Hz) triggers hippocampal afterdischarges in Wistar rats. Neuroscience. 1999;94:735–743. doi: 10.1016/s0306-4522(99)00367-x. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Ramon y Cajal S. Estructura del asta de ammon y fascia dentata. Anal Soc Espan Historia Natural. 1893;22:53–114. [Google Scholar]
- Ranck JB. Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. Part 1. Behavioral correlates and firing repertoires. Exp Neurol. 1973;42:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Ribak CE. Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase. J Neurocytol. 1978;7:461–478. doi: 10.1007/BF01173991. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Ichinohe N. Some thoughts on cortical minicolumns. Exp Brain Res. 2004;158:265–277. doi: 10.1007/s00221-004-2024-9. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JDB, Ohishi H, Nusser Z, Kaneko T, et al. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996a;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsaki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsaki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265:1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Deschenes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, et al. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19:552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Dalezios Y, Luján R, Roberts JDB, Watanabe M, Shigemoto R. High level of mGluR7 in the presynaptic active zones of select populations of GABAergic terminals innervating interneurones in the rat hippocampus. Eur J Neurosci. 2003;17:2503–2520. doi: 10.1046/j.1460-9568.2003.02697.x. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Fritschy J-M, Benke D, Roberts JDB, Sieghart W. The γ2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the α1 and β2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Nunzi MG, Gorio A, Smith AD. A new type of specific interneuron in the monkey hippocampus forming synapses exclusively with the axon initial segments of pyramidal cells. Brain Res. 1983;259:137–142. doi: 10.1016/0006-8993(83)91076-4. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cerebral Cortex. 1997;7:583–604. doi: 10.1093/cercor/7.6.583. [DOI] [PubMed] [Google Scholar]
- Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. 2001;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–576. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Szentagothai J. The ‘module-concept’ in cerebral cortex architecture. Brain Res. 1975;95:475–496. doi: 10.1016/0006-8993(75)90122-5. [DOI] [PubMed] [Google Scholar]
- Tamás G, Lörincz A, Simon A, Szabadics S. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP, Hughes DI, Pawelzik H. Differential sensitivity to Zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur J Neurosci. 2000;12:425–436. doi: 10.1046/j.1460-9568.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layer 2–5 of adult rat and cat neocortex: Triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- Toth K, Borhegyi Z, Freund TF. Postsynaptic targets of GABAergic hippocampal neurons in the medial septum diagonal band of broca complex. J Neurosci. 1993;13:3712–3724. doi: 10.1523/JNEUROSCI.13-09-03712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol. 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Draguhn A, Whittington MA, Baldeweg T, Bibbig A, Buhl EH, et al. Axonal gap junctions between principal neurons: a novel source of network oscillations, and perhaps epileptogenesis. Rev Neurosci. 2002;13:1–30. doi: 10.1515/revneuro.2002.13.1.1. [DOI] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;9:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol. 1990;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol. 1998;506:755–773. doi: 10.1111/j.1469-7793.1998.755bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappone CA, Sloviter RS. Commissurally projecting inhibitory interneurons of the rat hippocampal dentate gyrus: a colocalization study of neuronal markers and the retrograde tracer Fluoro-Gold. J Comp Neurol. 2001;441:324–344. doi: 10.1002/cne.1415. [DOI] [PubMed] [Google Scholar]