Abstract
Septo-hippocampal cholinergic fibres ramify extensively throughout the hippocampal formation to release acetylcholine upon a diverse range of muscarinic and nicotinic acetylcholine receptors that are differentially expressed by distinct populations of neurones. The resultant modulation of cellular excitability and synaptic transmission within hippocampal circuits underlies the ability of acetylcholine to influence the dynamic properties of the hippocampal network and results in the emergence of a range of stable oscillatory network states. Recent findings suggest a multitude of actions contribute to the oscillogenic properties of acetylcholine which are principally induced by activation of muscarinic receptors but also regulated through activation of nicotinic receptor subtypes.
Physiology and structure of the cholinergic input
The medial septal nucleus provides the major source of cholinergic innervation to the hippocampus (reviewed by Dutar et al. 1995) and presents a direct synaptic input to both principal neurones and interneurones (Frotscher & Leranth, 1985; Leranth & Frotscher, 1987). In addition to this directed input, a significant proportion of cholinergic release sites do not associate with distinct postsynaptic specializations suggesting an additional bulk transmission role (Vizi & Kiss, 1998). Thus, the widespread nature of the cholinergic input contrasts with the parallel septo-hippocampal GABAergic projection which is more discerning by selectively targeting discrete populations of interneurones (Freund & Antal, 1988). Aside from this extrinsic cholinergic input, the hippocampus contains a numerically sparse population of cholinergic interneurones (Frotscher et al. 2000).
The activity of the septo-hippocampal projection has been the subject of much interest with regard to a possible pacemaker function in phasing hippocampal network activities; most notably the hippocampal theta rhythm (Stewart & Fox, 1990). Whilst there is evidence that a phasic GABAergic septo-hippocampal projection may entrain hippocampal principal cells (Toth et al. 1997) there is, as yet, no conclusive evidence that a rhythmic cholinergic input is necessary for hippocampal oscillatory activities in vivo. This aside, the precise discharge pattern of cholinergic septo-hippocampal cells has not been established unequivocally, although putative cholinergic ‘long-spike cells’ discharge in rhythmical bursts whilst even irregular firing cells discharge in phase relation to the theta cycle (Brazhnik & Fox, 1999).
To enable effective transmission of patterned cholinergic input, the hippocampus expresses a broad range of muscarinic acetylcholine receptors (mAChRs), with the m1 and m3 receptors being mainly expressed in principal neurones and m2 and m4 receptors on interneurones (Levey et al. 1995). Whilst the m2 receptor is highly localized at discrete interneurone subtypes (Hajos et al. 1998) it also exists on septo-hippocampal cholinergic terminals where it plays an auto-regulatory role (Rouse et al. 1999).
The septo-hippocampal pathway is also thought to activate nicotinic acetylcholine receptors (nAChR), although the precise expression pattern of nAChR subunits with respect to the afferent cholinergic input has not been fully established. Populations of interneurones receiving direct septo-hippocampal innervation bind the nAChR ligand α-bungarotoxin indicating the existence of α7 nAChRs (Freedman et al. 1993). In particular, this nAChR subtype is highly expressed at multiple loci including somata, dendrites, spines and axon fibres, as well as both glutamatergic and GABAergic axon terminals and postsynaptic sites (Fabian-Fine et al. 2001).
Cholinergic modulation of hippocampal neurones
Acetylcholine classically excites hippocampal pyramidal cells (Dodd et al. 1981; Cole & Nicoll, 1983) and the ionic basis of this excitation has now been elucidated in some detail. Specifically, mAChRs modulate a large number of ionic conductances in pyramidal neurones through both direct and indirect biochemical interactions. The conductances known to be modified include several K+ conductances (IM, the muscarine sensitive K+ current; IAHP, the Ca2+-activated K+ current responsible for slowing action potential discharges; Ileak, the background leak current) (Halliwell, 1990). In addition, mAChR activation also potentiates two mixed cation currents (Ih, the hyperpolarization-activated cation current; Icat, the Ca2+-dependent non-specific cation current) (Halliwell, 1990; Colino & Halliwell, 1993) and modulates the activity of both voltage-dependent Ca2+ currents (Toselli et al. 1989) and several ligand-gated receptors including the N-methyl-d-aspartate (NMDA) receptor (Markram & Segal, 1990).
Stepping back from the complexities of how mAChRs modify specific ionic conductances the overriding effect of exogenously applied acetylcholine on hippocampal pyramidal cells is a pronounced membrane potential depolarization and increased membrane resistance (Cole & Nicoll, 1984). A comparable slow mAChR-dependent membrane potential depolarization, can be evoked in pyramidal neurones by direct electrical stimulation of cholinergic afferents in the hippocampus (Cole & Nicoll, 1983; Madison et al. 1987; Segal, 1988; Pitler & Alger, 1990; Morton & Davies, 1997) or medial septal nucleus in septo-hippocampal slices (Fig. 1). This response often results in a sustained action potential discharge, in part arising from a pronounced reduction in spike frequency adaptation (Cole & Nicoll, 1983; Morton & Davies, 1997). Whilst these effects represent the overt electrophysiological phenotype of cholinergic innervation, physiological activation of mAChRs also produce profound alterations in second messenger cascades and intracellular calcium mobilization (Power & Sah, 2002), suggesting longer term consequences for neuronal excitability and synaptic plasticity.
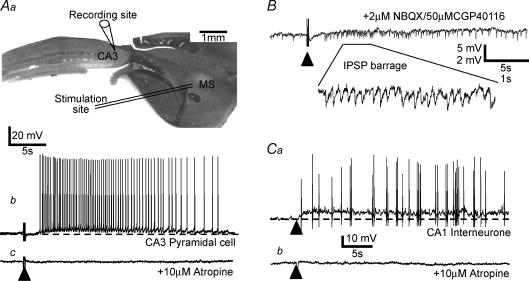
Figure 1. Activation of septo-hippocampal afferents excites hippocampal pyramidal cells and interneurones.
Aa, diagram of septo-hippocampal slice showing relative position of stimulating (within medial septum, MS) and recording electrode (CA3 pyramidal cell). Ab, intracellular recording from a CA3 pyramidal cell reveals an isolated slow depolarizing response to electrical stimulation (indicated by ▴) within the septal nucleus in the presence of a cocktail of AMPA/kainate, NMDA, GABAA and GABAB receptor antagonists (4 μm NBQX, 50 μm CGP40116, 50 μm picrotoxin and 1 μm CGP55845A, respectively). Ac, the action potential discharge and underlying slow depolarizing waveform were abolished upon application of the mAChR antagonist atropine (10 μm). B, example of a similar experiment in which only the AMPA/kainate and NMDA receptor antagonists were present to block glutamatergic EPSPs. The presence of a barrage of IPSPs (shown also in expanded inset) following afferent stimulation suggests a direct cholinergic excitation of presynaptic GABAergic interneurones. Subsequent application of 10 μm atropine abolished IPSP trains in this cell following afferent stimulation (data not shown). Ca, recording from a putative fast-spiking interneurone within area CA1 in which a similar slow depolarizing response is evoked following stimulation of cholinergic afferents. Cb, as with the pyramidal cell response, the slow depolarizing potential is completely abolished upon subsequent coapplication of the mAChR antagonist atropine (10 μm). Detail of evoked cholinergic EPSP methodology given in Morton & Davies (1997).
Moving to GABAergic interneurone populations, it is clear that synaptic or pharmacological activation of mAChRs produces more complex responses than are immediately obvious in pyramidal neurones. In this respect, pharmacological activation of mAChRs directly increases the frequency and amplitude of spontaneous IPSCs whilst at the same time depressing monosynaptically evoked IPSCs and the frequency of miniature IPSCs (Behrends & ten Bruggencate, 1993). Taken together these results suggest that, whilst activation of mAChRs directly excites GABAergic interneurones, it also has a depressant effect on the synaptic release of GABA. More recent studies have shown that in the majority of identified GABAergic interneurones, pharmacological activation of mAChRs results in a similar membrane depolarization to that seen in pyramidal cells but with a less prominent change in cell input resistance. However, there also appear to be several subpopulations of GABAergic interneurones in which mAChR activation produces (a) a pure hyperpolarizing response, (b) a biphasic response in which an initial hyperpolarization is followed by a secondary depolarizing phase, (c) a slow membrane potential oscillatory response, or (d) no response (in terms of membrane potential/conductance) (McQuiston & Madison, 1999a). Preliminary studies using physiological stimulation of septo-hippocampal afferents have identified a similar diversity of response in interneurones (Ferrigan et al. 2003). Given that GABAergic interneurones represent a highly heterogenous population with respect to their connectivity and neurochemistry, it is perhaps not surprising that they exhibit a more varied response to activation of mAChRs compared to that observed in the relatively homogeneous population of principal neurones. So far to date, however, there appears to be no strong correlation between the nature of the membrane potential response to mAChR activation and the morphological characteristics of cells in terms of soma/dendrite location and axonal arbour (Parra et al. 1998; McQuiston & Madison, 1999a).
In contrast to the slow sustained mAChR-mediated modulation of both pyramidal cells and interneurones, activation of nAChRs produces a fast and cell type-specific response. Thus, application of nAChR agonists generally produces either no or only a barely detectable response in pyramidal cells (Frazier et al. 1998b; McQuiston & Madison, 1999b), while both pharmacological (Jones & Yakel, 1997) and synaptic activation (Frazier et al. 1998a) of nAChRs in interneurones produce a brief depolarization or inward current. The kinetics and pharmacology of the response varies between cell types. The predominant response that is observed in interneurones whose axons ramify throughout dendritic layers is a fast depolarization mediated by α7 subunit-containing nAChRs. A second group of cells localized within stratum oriens and having axons which ramify within stratum lacunosum–moleculare display a dual component response with an initial fast phase followed by a slower non-α7 nAChR subunit-dependent depolarizing phase. A third category of interneurone with axons that provide perisomatic inhibition is insensitive to nAChR agonists (McQuiston & Madison, 1999b).
Clearly the pattern of activity of septo-hippocampal cholinergic afferents could have a potentially wide reaching impact on the excitability of the hippocampal network. By differentially gating inhibitory circuits through both nAChR- and mAChR-mediated mechanisms, cholinergic afferents may switch synaptic inhibition between perisomatic and pathway-specific dendritic domains. One important consideration is whether different patterns of cholinergic afferent input can differentially recruit separate receptor populations and cell types? In this respect, single action potentials in cholinergic fibres are effective at evoking nAChR-mediated postsynaptic potentials in interneurones but are relatively inefficient at evoking mAChR-mediated membrane potential depolarizations. In contrast, trains of stimuli delivered at 10–20 Hz, within the range at which putative septal cholinergic cells discharge during theta rhythm (Brazhnik & Fox, 1999), result in a robust recruitment of a mAChR-mediated synaptic response in interneurones and pyramidal neurones (Morton & Davies, 1997). A second, and less well answered consideration is whether particular cholinergic septo-hippocampal fibres or cholinergic interneurones preferentially target discrete cell types?
Cholinergic modulation of hippocampal synaptic transmission
Acetylcholine is a powerful presynaptic modulator of synaptic transmission at both glutamatergic and GABAergic synapses through both mAChRs and nAChRs. Such modulation is both cell type and pathway specific (Kahle & Cotman, 1989; Hasselmo & Schnell, 1994; Radcliffe et al. 1999). Furthermore, GABAergic interneurones can provide reciprocal presynaptic inhibition of cholinergic inputs through activation of GABAB receptors (Morton et al. 2001); an effect that is similar in magnitude to that produced by activation of adenosine A1 (Morton & Davies, 1997), μ-opioid (Kearns et al. 2001) and galanin receptors (Dutar et al. 1989). Clearly, this level of regulation of amino acid-mediated synaptic transmission adds further complexity to the control of network dynamics by cholinergic systems. Moreover, modulation of network activity by acetylcholine is additionally complicated by the ability of mAChR activation to promote longer term synaptic plasticity either directly (Auerbach & Segal, 1994) or through associative interaction with glutamatergic synaptic inputs (Huerta & Lisman, 1996). This aspect of modulation, like that of short-term modulation at the pre- and postsynaptic level, is also pathway specific in that mAChR activation can enhance long-term potentiation (LTP) within the dentate gyrus (Burgard & Sarvey, 1990) whilst enhancing or depressing LTP within CA3 pyramidal neurones (Maeda et al. 1993).
Cholinergic modulation of network properties
Given the complexities by which cholinergic innervation can influence different neuronal components that integrate with one another to generate and sustain network oscillatory states it is not surprising that few studies have been able to pinpoint the role that each aspect of modulation plays in shaping neuronal oscillations. That activation of cholinergic systems is capable of doing this, however, is particularly important since coherent network oscillations in vivo are believed to provide a temporal context against which the precise firing of cells may encode information. In this regard, hippocampal oscillations may be of importance in associative learning (Buzsaki, 2002) and as a reference for coding by place cells (O'Keefe & Recce, 1993). To date, investigation of the mechanistic aspects of oscillatory network behaviour has been most widely studied using an assortment of hippocampal oscillations created in vitro by a variety of induction paradigms (Traub et al. 2004). Activation of ACh receptors, like other pharmacological manipulations (e.g. activation of metabotropic glutamate receptors (mGluRs)) induces a range of synchronized oscillatory responses in hippocampal slices (Fig. 2) including low frequency bursting, intermittent theta frequency oscillations (MacVicar & Tse, 1989), beta frequency oscillations (Shimono et al. 2000) and gamma frequency oscillations (Fisahn et al. 1998). Most of these oscillatory states require intact excitatory and inhibitory circuits, being disrupted by blockade of fast glutamatergic and GABAergic neurotransmission. In addition to network-driven responses, intrinsic membrane potential oscillations (Leung & Yim, 1991; Strata, 1998), resonance (Pike et al. 2000) and low frequency oscillatory plateau potential-like responses (Williams & Kauer, 1997; Cobb et al. 1999) have been reported in pyramidal cells. Furthermore, mAChR-driven intrinsic theta frequency oscillations have been reported in specific interneurones (Chapman & Lacaille, 1999) which, in turn, synchronize pyramidal cell activity through phasic inhibition (Cobb et al. 1995). The ionic basis for these intrinsic oscillatory activities probably involves activation of the hyperpolarization-activated inward ‘pacemaker’ current (Ih) such that mAChRs excite pyramidal neurones through increasing Ih (Fisahn et al. 2002) and conversely, selective blockers of Ih suppress mAChR-induced theta frequency oscillations (Cobb et al. 2003).
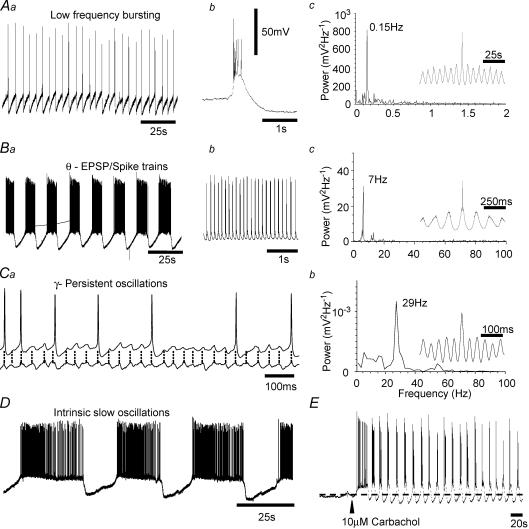
Figure 2. Pharmacological activation of acetylcholine receptors induces a variety of stable cellular and network oscillatory state.
Aa, intracellular recording from a CA3 pyramidal cell reveals a low frequency synchronous burst discharge in response to 1 μm carbachol application. Individual bursts (b) occur within a dominant frequency of 0.15 Hz, as shown in the power spectrum (c). Ba, higher concentration of carbachol (10 μm) results in the appearance of periodic episodes of rhythmic oscillatory depolarization. During oscillatory episodes, rhythmic depolarization was commonly suprathreshold resulting in a phasic dischage of action potentials (b) around the theta frequency range (c). Ca, in some slices, the predominant response to carbachol application is a persistent membrane potential oscillation within the high beta low–gamma frequency range, with the dominant frequency in this example (b) being 29 Hz. D, pharmacological uncoupling of fast AMPA receptor-mediated synaptic transmission (4 μm NBQX) reveals a very slow, presumably intrinsic oscillatory state in a subpopulation of pyramidal neurones, often resembling repeated plateau potentials. Oscillatory states described in A–C developed gradually as carbachol washed into the recording chamber. Each represents a sustained coherent activity within the hippocampal CA3 network that could be readily detected by extracellular field recordings. Oscillatory activity could also be induced rapidly as shown in E where arrowhead indicates fast application of 10 μm carbachol. Methodological details are given in Cobb et al. (1999) and Cobb et al. (2000).
Whilst the principal mechanism(s) by which mAChRs induce oscillatory states is not fully understood, it is unlikely that the effect is simply one of exciting (depolarizing) the neuronal population since direct interventions such as disinhibition or elevation of extracellular potassium concentration do not produce similar patterns of activity. That said, activation of other excitatory neurotransmitter receptors including kainate receptors and mGluRs, can induce similar patterns of activity, although each pharmacological manipulation may generate oscillatory activity through distinct cellular pathways. Thus, mAChR- and mGluR-induced gamma oscillations in vitro arise from different underlying mechanisms and circuitries (Palhalmi et al. 2004) whereas mAChR- and mGluR-induced intermittent theta frequency oscillations exhibit near-identical charateristics (Cobb et al. 2000) and, indeed, demonstrate cooperativity between neurotransmitter systems. Whether this can be replicated through physiological activation of receptor systems and whether it occurs in vivo has yet to be determined. In this respect, it is notable that direct application of mAChR or mGluR agonists in vivo produces predominant theta or gamma frequency oscillations, respectively (Martin, 2001). It should also be noted that multiple coincident oscillatory patterns of activity can be induced by mAChR activation within a given hippocampal region (Fellous & Sejnowski, 2000), with modelling studies predicting further combinations of oscillatory patterns that have yet to be observed in vitro (Tiesinga et al. 2001).
Whilst the oscillogenic action of ACh appears to be primarily mediated via mAChRs, the lack of highly selective mAChR subtype receptor ligands has hampered progress in identifying the role of individual mAChRs as well as interactions between mAChR subtypes. The involvement of the nicotinic class of AChR in oscillatory events contrasts with that of mAChRs, as this receptor population appears not to participate in the genesis of coherent oscillatory network states per se but instead modulates pre-existing oscillatory states (Williams & Kauer, 1997; Cobb et al. 1999). Whilst further investigation is required to assess the full extent to which nAChRs are involved in modifying physiologically relevant network oscillations, progress has been made from a pathological standpoint in that nAChRs have been shown to potentiate oscillatory bursting activity within area CA3 in vitro (Fig. 3; Roshan-Milani et al. 2003), an effect consistent with the observation that excessive activation of nAChRs in vivo induces seizure activity (Damaj et al. 1999).
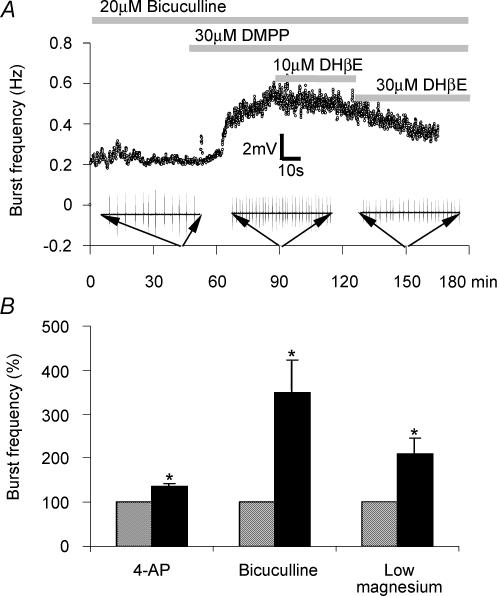
Figure 3. Pharmacological activation of nicotinic acetylcholine receptors modulates synchronized bursting activity in area CA3.
A, scatter plot showing instantaneous burst frequency in response to continuous 20 μm bicuculline-induced disinhibition. Following a period of stable burst frequency, application of the selective nAChR agonist DMPP (10 μm) as indicated by the horizontal bar results in a pronounced burst frequency potentiation which is reversed upon subsequent coapplication of the selective nAChR antagonist dihydro-β-erythroidine (30 μm). B, bar chart showing that pharmacological activation of nAChRs produces a significant enhancement of CA3 pyramidal bursting brought about by a range of pharmacological regimes including direct excitation of CA3 neurones through potassium channel blockade-induced depolarization (4-aminopyridine, 10–30 μm 4-AP), reduction of fast GABAergic inhibition (20 μm bicuculline) and potentiation of NMDA receptor-mediated excitation (0 mm Mg2+ perfusion medium). Methodological details given in Roshan-Milani et al. (2003) from which B is reproduced from Epilepsy Research, 56, Roshan-Milani et al., Regulation of epileptiform activity in hippocampus by micotinic acetylcholine receptor activation. 51–65 © 2003 with permission from Elsevier.
Concluding remarks
Through its complex innervation and signalling pathways, ACh is ideally placed to orchestrate oscillatory network activity. Key determinants of its influence on this activity will include the pattern of afferent activity, the target map of afferent innervation, subtypes of ACh receptors recruited and the ongoing activity in non-cholinergic aspects of the network. As these and other parameters change through time with alterations in behaviour, the network may dynamically switch oscillogenic and plastic properties. Considerable effort will be required to understand fully the intricacies by which cholinergic systems operate within this context. However, current development of ACh-based pharmacological strategies to rectify abnormalities in oscillatory activity associated with CNS disease, e.g. gamma oscillations in schizophrenia, continue to move forward at pace. In this respect, research into so called ‘oscillopathies’, a field pioneered by the late Eberhard Buhl, is likely to make a significant impact in the development of future therapeutic strategies to treat cortical dysfunction.
Acknowledgments
We are grateful to the Wellcome Trust for support.
References
- Auerbach JM, Segal M. A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol. 1994;72:2034–2040. doi: 10.1152/jn.1994.72.4.2034. [DOI] [PubMed] [Google Scholar]
- Behrends JC, ten Bruggencate G. Cholinergic modulation of synaptic inhibition in the guinea pig hippocampus in vitro: excitation of GABAergic interneurons and inhibition of GABA-release. J Neurophysiol. 1993;69:626–629. doi: 10.1152/jn.1993.69.2.626. [DOI] [PubMed] [Google Scholar]
- Brazhnik ES, Fox SE. Action potentials and relations to the theta rhythm of medial septal neurons in vivo. Exp Brain Res. 1999;127:244–258. doi: 10.1007/s002210050794. [DOI] [PubMed] [Google Scholar]
- Burgard EC, Sarvey JM. Muscarinic receptor activation facilitates the induction of long-term potentiation (LTP) in the rat dentate gyrus. Neurosci Lett. 1990;116:34–39. doi: 10.1016/0304-3940(90)90382-j. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Lacaille JC. Cholinergic induction of theta-frequency oscillations in hippocampal inhibitory interneurons and pacing of pyramidal cell firing. J Neurosci. 1999;19:8637–8645. doi: 10.1523/JNEUROSCI.19-19-08637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Bulters DO, Davies CH. Coincident activation of mGluRs and mAChRs imposes theta frequency patterning on synchronised network activity in the hippocampal CA3 region. Neuropharmacology. 2000;39:1933–1942. doi: 10.1016/s0028-3908(00)00036-8. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Bulters DO, Suchak S, Riedel G, Morris RG, Davies CH. Activation of nicotinic acetylcholine receptors patterns network activity in the rodent hippocampus. J Physiol. 1999;518:131–140. doi: 10.1111/j.1469-7793.1999.0131r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Larkman PM, Bulters DO, Oliver L, Gill CH, Davies CH. Activation of Ih is necessary for patterning of mGluR and mAChR induced network activity in the hippocampal CA3 region. Neuropharmacology. 2003;44:293–303. doi: 10.1016/s0028-3908(02)00405-7. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983;221:1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. The pharmacology of cholinergic excitatory responses in hippocampal pyramidal cells. Brain Res. 1984;305:283–290. doi: 10.1016/0006-8993(84)90434-7. [DOI] [PubMed] [Google Scholar]
- Colino A, Halliwell JV. Carbachol potentiates Q current and activates a calcium-dependent non-specific conductance in rat hippocampus in vitro. Eur J Neurosci. 1993;5:1198–1209. doi: 10.1111/j.1460-9568.1993.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glassco W, Dukat M, Martin BR. Pharmacological characterization of nicotine-induced seizures in mice. J Pharmacol Exp Ther. 1999;291:1284–1291. [PubMed] [Google Scholar]
- Dodd J, Dingledine R, Kelly JS. The excitatory action of acetylcholine on hippocampal neurones of the guinea pig and rat maintained in vitro. Brain Res. 1981;207:109–127. doi: 10.1016/0006-8993(81)90682-x. [DOI] [PubMed] [Google Scholar]
- Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev. 1995;75:393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- Dutar P, Lamour Y, Nicoll RA. Galanin blocks the slow cholinergic EPSP in CA1 pyramidal neurones from ventral hippocampus. Eur J Pharmacol. 1989;164:355–360. doi: 10.1016/0014-2999(89)90477-9. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous JM, Sejnowski TJ. Cholinergic induction of oscillations in the hippocampal slice in the slow (0.5–2 Hz), theta (5–12 Hz), and gamma (35–70 Hz) bands. Hippocampus. 2000;10:187–197. doi: 10.1002/(SICI)1098-1063(2000)10:2<187::AID-HIPO8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ferrigan L, Khoshnood J, Cobb SR. Isolation of a slow cholinergic synaptic response in CA1 hippocampal interneurons. Soc Neurosci Abstract. 2003;247:2. [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, McBain CJ, Wess J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron. 2002;33:615–624. doi: 10.1016/s0896-6273(02)00587-1. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci. 1998a;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998b;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Wetmore C, Stromberg I, Leonard S, Olson L. Alpha-bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J Neurosci. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Vida I, Bender R. Evidence for the existence of non-GABAergic, cholinergic interneurons in the rodent hippocampus. Neuroscience. 2000;96:27–31. doi: 10.1016/s0306-4522(99)00525-4. [DOI] [PubMed] [Google Scholar]
- Hajos N, Papp EC, Acsady L, Levey AI, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1998;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- Halliwell JV. Physiological mechanisms of cholinergic action in the hippocampus. Prog Brain Res. 1990;84:255–272. doi: 10.1016/s0079-6123(08)60910-3. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: computational modeling and brain slice physiology. J Neurosci. 1994;14:3898–3914. doi: 10.1523/JNEUROSCI.14-06-03898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Low-frequency stimulation at the troughs of theta-oscillation induces long-term depression of previously potentiated CA1 synapses. J Neurophysiol. 1996;75:877–884. doi: 10.1152/jn.1996.75.2.877. [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol. 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle JS, Cotman CW. Carbachol depresses synaptic responses in the medial but not the lateral perforant path. Brain Res. 1989;482:159–163. doi: 10.1016/0006-8993(89)90554-4. [DOI] [PubMed] [Google Scholar]
- Kearns IR, Morton RA, Bulters DO, Davies CH. Opioid receptor regulation of muscarinic acetylcholine receptor-mediated synaptic responses in the rat hippocampus. Neuropharmacology. 2001;41:565–573. doi: 10.1016/s0028-3908(01)00108-3. [DOI] [PubMed] [Google Scholar]
- Leranth C, Frotscher M. Cholinergic innervation of hippocampal GAD- and somatostatin-immunoreactive commissural neurons. J Comp Neurol. 1987;261:33–47. doi: 10.1002/cne.902610104. [DOI] [PubMed] [Google Scholar]
- Leung LW, Yim CY. Intrinsic membrane potential oscillations in hippocampal neurons in vitro. Brain Res. 1991;553:261–274. doi: 10.1016/0006-8993(91)90834-i. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci. 1995;15:4077–4092. doi: 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Muscarinic receptor activity has multiple effects on the resting membrane potentials of CA1 hippocampal interneurons. J Neurosci. 1999a;19:5693–5702. doi: 10.1523/JNEUROSCI.19-14-05693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999b;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Tse FW. Local neuronal circuitry underlying cholinergic rhythmical slow activity in CA3 area of rat hippocampal slices. J Physiol. 1989;417:197–212. doi: 10.1113/jphysiol.1989.sp017797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Lancaster B, Nicoll RA. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987;7:733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Kaneko S, Satoh M. Bidirectional modulation of long-term potentiation by carbachol via M1 and M2 muscarinic receptors in guinea pig hippocampal mossy fibre-CA3 synapses. Brain Res. 1993;691:324–330. doi: 10.1016/0006-8993(93)91628-6. [DOI] [PubMed] [Google Scholar]
- Markram H, Segal M. Acetylcholine potentiates responses to N-methyl-D-aspartate in the rat hippocampus. Neurosci Lett. 1990;113:62–65. doi: 10.1016/0304-3940(90)90495-u. [DOI] [PubMed] [Google Scholar]
- Martin SJ. Activation of metabotropic glutamate receptors induces gamma frequency oscillations in the rat dentate gyrus in vivo. Neuropharmacology. 2001;40:634–637. doi: 10.1016/s0028-3908(00)00190-8. [DOI] [PubMed] [Google Scholar]
- Morton RA, Davies CH. Regulation of muscarinic acetylcholine receptor-mediated synaptic responses by adenosine receptors in the rat hippocampus. J Physiol. 1997;502:75–90. doi: 10.1111/j.1469-7793.1997.075bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton RA, Manuel NA, Cobb SR, Davies CH. Regulation of muscarinic acetylcholine receptor-mediated EPSPs by GABAB receptors in the rat hippocampus. J Physiol. 2001;535:757–766. doi: 10.1111/j.1469-7793.2001.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Palhalmi J, Paulsen O, Freund TF, Hajos N. Distinct properties of carbachol- and DHPG-induced network oscillations in hippocampal slices. Neuropharmacology. 2004;47:381–389. doi: 10.1016/j.neuropharm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Parra P, Gulyas AI, Miles R. How many subtypes of inhibitory cells in the hippocampus? Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- Pike FG, Goddard RS, Suckling JM, Ganter P, Kasthuri N, Paulsen O. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J Physiol. 2000;529:205–213. doi: 10.1111/j.1469-7793.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Activation of the pharmacologically defined M3 muscarinic receptor depolarizes hippocampal pyramidal cells. Brain Res. 1990;534:257–262. doi: 10.1016/0006-8993(90)90137-z. [DOI] [PubMed] [Google Scholar]
- Power JM, Sah P. Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. J Neurosci. 2002;22:3454–3462. doi: 10.1523/JNEUROSCI.22-09-03454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe KA, Fisher JL, Gray R, Dani JA. Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Ann N Y Acad Sci. 1999;868:591–610. doi: 10.1111/j.1749-6632.1999.tb11332.x. [DOI] [PubMed] [Google Scholar]
- Roshan-Milani S, Ferrigan L, Khoshnood MJ, Davies CH, Cobb SR. Regulation of epileptiform activity in hippocampus by nicotinic acetylcholine receptor activation. Epilepsy Res. 2003;56:51–65. doi: 10.1016/j.eplepsyres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Rouse ST, Marino MJ, Potter LT, Conn PJ, Levey AI. Muscarinic receptor subtypes involved in hippocampal circuits. Life Sci. 1999;64:501–509. doi: 10.1016/s0024-3205(98)00594-3. [DOI] [PubMed] [Google Scholar]
- Segal M. Synaptic activation of a cholinergic receptor in rat hippocampus. Brain Res. 1988;452:79–86. doi: 10.1016/0006-8993(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Shimono K, Brucher F, Granger R, Lynch G, Taketani M. Origins and distribution of cholinergically induced beta rhythms in hippocampal slices. J Neurosci. 2000;20:8462–8473. doi: 10.1523/JNEUROSCI.20-22-08462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M, Fox SE. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990;13:163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- Strata F. Intrinsic oscillations in CA3 hippocampal pyramids: physiological relevance to theta rhythm generation. Hippocampus. 1998;8:666–679. doi: 10.1002/(SICI)1098-1063(1998)8:6<666::AID-HIPO9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tiesinga PH, Fellous JM, Jose JV, Sejnowski TJ. Computational model of carbachol-induced delta, theta, and gamma oscillations in the hippocampus. Hippocampus. 2001;11:251–274. doi: 10.1002/hipo.1041. [DOI] [PubMed] [Google Scholar]
- Toselli M, Lang J, Costa T, Lux HD. Direct modulation of voltage-dependent calcium channels by muscarinic activation of a pertussis toxin-sensitive G-protein in hippocampal neurons. Pflugers Arch. 1989;415:255–261. doi: 10.1007/BF00370874. [DOI] [PubMed] [Google Scholar]
- Toth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol. 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, LeBeau FE, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Kiss JP. Neurochemistry and pharmacology of the major hippocampal transmitter systems: synaptic and nonsynaptic interactions. Hippocampus. 1998;8:566–607. doi: 10.1002/(SICI)1098-1063(1998)8:6<566::AID-HIPO2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Williams JH, Kauer JA. Properties of carbachol-induced oscillatory activity in rat hippocampus. J Neurophysiol. 1997;78:2631–2640. doi: 10.1152/jn.1997.78.5.2631. [DOI] [PubMed] [Google Scholar]