Abstract
This study examined the effect of neuropeptide Y Y1-receptor blockade both alone, and in interaction with α1-adrenoceptor antagonism, on basal hindlimb vascular conductance in male and female Sprague-Dawley rats. Hindlimb vascular conductance was measured during infusion of BIBP3226 (Y1-receptor antagonist; 100 μg kg−1), prazosin (α1-receptor antagonist; 20 μg kg−1), and combined blockade. In males, vascular conductance increased 1.1 ± 0.3 μl min−1 mmHg−1 above baseline with BIBP3226, and 2.4 ± 0.4 μl min−1 mmHg−1 above baseline with prazosin (both P < 0.05). The increase in vascular conductance during combined blockade (5.1 ± 0.7 μl min−1 mmHg−1) was greater than the sum of the independent BIBP3226 and prazosin responses (P < 0.05). In females, basal hindlimb vascular conductance was unaffected by Y1-receptor blockade. However, α1-receptor blockade resulted in a 3.5 ± 0.6 μl min−1 mmHg−1 increase in vascular conductance above baseline, which was not different than the combined blockade condition. Males had greater skeletal muscle neuropeptide Y concentration (P < 0.05; ELISA) than females. Furthermore, compared with females, male skeletal muscle contained greater Y1-receptor expression (P < 0.05; Western blot). It was concluded that, under baseline conditions, agonist and receptor-based mechanisms for Y1-receptor dependent control of vascular conductance in skeletal muscle was greater in male versus female rats.
Peripheral sympathetic neurones regulate microvessel tone through the release of noradrenaline (norepinephrine; NA), neuropeptide Y (NPY), and purines. Noradrenaline has been deemed the classical and primary transmitter substance involved in basal arteriolar tone (Zukowska-Grojec, 1995) through activation of α-receptors (αRs) on vascular smooth muscle cells. In turn, NPY exerts important vasomotor control in resistance vessels via activation of Y1 receptors (Y1Rs) which produces potent and prolonged vasoconstriction (Zukowska-Grojec & Wahlestedt, 1993; Ekelund & Erlinge, 1997; Malmstrom, 1997). Post-synaptically, the co-activation of Y1R and α1R by NPY and NA, respectively, leads to synergistic vasoconstrictive effects (Zukowska-Grojec & Wahlestedt, 1993). Nerve fibres storing NPY are abundant around resistance vessels and become more dense in distal (2nd and 3rd order) arterioles (Sundler et al. 1993). In addition to direct vasomotor control, NPY can presynaptically regulate the release of NA as well as autoregulate its own release via NPY Y2 receptors (Y2Rs) (Zukowska-Grojec & Wahlestedt, 1993). Despite evidence that NPY exerts important vasoactive effects there is debate regarding the conditions under which these effects are manifested. NPY is not generally associated with baseline vascular control or organ perfusion but its role during shock and/or chronic stress (e.g. sepsis, haemorrhage, cold stress) is well regarded (Zukowska-Grojec & Vaz, 1988; Qureshi et al. 1998). Thus, some have hypothesized that NPY release is dependent upon discharge properties of sympathetic nerve activity (Lundberg et al. 1990). In addition, there appears to be tissue specificity in NPY vascular control. For example, in early studies NPY was shown to cause a potent concentration-dependent constriction of cerebral blood vessels, skeletal muscle blood vessels, rat tail arteries, and guinea pig uterine arteries (Edvinsson et al. 1984b; Morris et al. 1985; Pernow et al. 1987; Neild, 1987). However, in other preparations it has been shown to have little or no isolated effect (Edvinsson et al. 1984a; Ekblad et al. 1984; Glover, 1985; Hanko et al. 1986).
Skeletal muscle is a tissue that has received minimal attention in terms of NPY's endogenous vascular effects, yet offers considerable sympathetically mediated control over a wide range of blood flow and levels of vascular conductance (VC), including baseline. Recently Buckwalter et al. observed that exogenous stimulation of Y1Rs in the hindlimb of mongrel dogs produced vasoconstriction at baseline and during exercise (Buckwalter et al. 2004). We have recently observed marked endogenous Y1R modulation of basal VC in the hindlimb of anaesthetized male Sprague-Dawley rats (Jackson et al. 2004); suggesting that Y1Rs in the male skeletal muscle vasculature are under chronic activation. Whether these findings are explained by the differential release hypothesis and discharge patterns of sympathetic neurones remains to be determined. However, based on this hypothesis there is reason to consider sex-dependent dimorphism of Y1R control of vascular tone. Namely, in humans, females have lower levels of baseline (Ng et al. 1993; Jones et al. 1996) and reflex-mediated changes in sympathetic nerve activity (Shoemaker et al. 2001) that might be expected to elicit less Y1R vascular control, compared with males. Nonetheless, the impact of gender on NPY-induced modulation of blood vessels remains uncertain. The greater increase in BP, heart rate and mesenteric vasoconstriction in males compared with females during cold stress (Zukowska-Grojec, 1995) has been directly associated with increases of plasma NPY immunoreactivity (Zukowska-Grojec, 1995). However, where some have observed greater increases in blood pressure during NPY infusion in male versus female rats (Zukowska-Grojec et al. 1991) others have not (Glenn et al. 1997; Bischoff et al. 2000).
Therefore, the aim of the present investigation was to determine the impact of gender on Y1R control of rat hindlimb skeletal muscle perfusion. Based on differences in baseline and reflexive sympathetic responses in males and females, noted above, we tested the hypothesis that compared with males, females would demonstrate lower Y1R vascular control. Furthermore, using a repeated measures model, this experiment addressed the interaction between Y1R and α1R control on baseline vascular conductance and how this might be different in male and female rats. The results indicated that endogenous Y1R activation has both independent and interactive effects on baseline vascular conductance in male, but not female, rats.
Methods
The Council on Animal Care at the University of Western Ontario approved the experimental protocol.
Animals
Eight adult male (body weight, 284 ± 12 g) and seven adult female (body weight, 261 ± 14 g) (mean ±s.d.), age matched Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were used in this study. Rats were housed in a light (12 h cycle) and temperature (22°C) controlled room in Plexiglas cages. Rats were allowed to eat (Prolab Rat chow, Mouse and Hamster 3000 Diet) and drink water ad libitum. Prior to surgery animals were anaesthetized with an intraperitoneal injection of thiobutabarbital sodium (Inactin; 100 mg kg−1; Sigma-Aldrich). Internal body (rectal) temperature was monitored continuously and was maintained at 37 ± 0.5°C with a water-perfused heating pad (mean ±s.d.). Animals showed no signs of pain or distress throughout the experiment, as adequate depth of anaesthesia was assessed every half hour by the absence of flexor withdrawal reflex to a foot pinch.
Surgery
The trachea was intubated to facilitate spontaneous respiration, and end-tidal CO2 (ETCO2) measures were made from expired air at periods throughout the experiment. A polyethylene (PE50) cannula was inserted into the left common carotid artery to permit arterial blood pressure (ABP) recording from the amplified signal (ML118 Powerlab Quad Bridge Amplifier; ADInstruments, Colorado Springs, CO, USA) of a pressure transducer (MLT844; ADInstruments). Through a midline abdominal incision, the gut was carefully moved aside and covered with sterile gauze moistened with sterile saline (0.9% NaCl). Using cotton swabs a small portion (∼1 cm) of the right iliac artery was exposed and a cannula (PE50) was precisely advanced to the bifurcation of the aorta using microscopic guidance. This cannula was used for drug delivery to the left hindlimb, such that perfusion of drug was directed into the flow of blood travelling from the descending aorta to the left hindlimb. Immediately following these procedures, the gauze was removed and contents of the gut were repositioned, and incisions were closed (Becton-Dixon, 9 mm stainless steel wound clips).
Femoral artery blood flow (Qfem) was measured from the left hindlimb using a Transonic flowmeter (TS420 Perivascular Flowmeter Module; Transonic Systems Inc., Ithaca, NY, USA) and Transonic flowprobe (0.7 mm; 0.7PSB acute model) placed approximately 3 mm distal to the femoral triangle. Specifically, with the animal placed on its back, a small ∼1 cm medial incision was made through the shaved skin of the left thigh. Under microscopic guidance blunt dissection was used to clear the fascia and tissue overlying the femoral artery and vein, care was taken to ensure that nerves and vessels were not damaged during this procedure. With a small portion of the artery free from the nerve and vein the vessel was carefully placed into the flowprobe using blunt microforceps. Utilizing a custom-made manipulative device the probe and vessel were held in a natural anatomical position so as not to interfere with blood flow. Finally, innocuous water-soluble gel was spread over the opened area (∼1 cm2) of the hindlimb to maintain hydration of exposed tissue and quality of blood flow signal.
Experimental protocol
Animals recovered for 1 h following surgery; however, blood pressure (BP) and heart rate (HR) were stable and at normal levels within 30 min. After recovery, a 10 s vehicle (0.9% saline) infusion (160 μl) was carried out followed by 10 min recovery. In an effort to address the existence of endogenous synergy between α1- and Y1-receptor activation a repeated measures design was used (i.e. all animals received all three drug infusions). Baseline data were recorded for 5 min followed by the infusion of: (1) 100 μg kg−1N2-(diphenylacetyl)-N-[(4-hydroxyphenyl) methyl]-d-arginine amide (BIBP3226; specific non-peptide NPY Y1-receptor antagonist; Sigma-Aldrich); (2) 20 μg kg−1 prazosin (specific α1-adrenoceptor antagonist; Sigma-Aldrich); and (3) combined 100 μg kg−1 BIBP3226 + 20 μg kg−1 prazosin. The infusion speed and volume of each drug infusion was held constant at 10 s for the 160 μl infusion. Because prazosin elicits prolonged effects, BIBP3226 infusion was infused first in all animals. In order to control for possible effects caused by sequence of drug delivery, after a 5 min baseline collection, either prazosin (n = 4 males, 4 females) or BIBP3226 + prazosin (n = 4 males, 3 females) was delivered. Following a minimum of 2 h recovery and when all measured variables returned to baseline, the final drug infusion was carried out (either prazosin or BIBP3226 + prazosin, depending on the preceding infusion). The length of recovery period from prazosin infusion was based on observations in Sprague-Dawley rats indicating that the rate at which blood pressure returned to control levels was very similar to the elimination rate of prazosin from tissues and plasma (Dynon et al. 1983).
Our observations with BIBP3226 are assumed to be a direct result of Y1R antagonism on vascular smooth muscle and not an effect of locally altered metabolism. We have previously reported that 100 μg kg−1 BIBP3226 provided the maximal dilator response in male animals (Jackson et al. 2004). This dose was confirmed in preliminary experiments on a separate group of male (n = 3) and female (n = 3) animals (Fig. 1). These preliminary data also established that 20 μg kg−1 prazosin caused the least systemic effect (i.e. drop in BP) for the greatest increase in hindlimb blood flow in both male and female rats. Furthermore, it was confirmed that the drug doses utilized completely blocked the Y1- and α1-receptors in our model. Specifically, the agonist-induced decrease in vascular conductance (by either 100 μm NPY or 3 μm NA; Sigma-Aldrich) was completely abolished by a subsequent dose of the respective antagonist (100 μg kg−1 BIBP3226 or 20 μg kg−1 prazosin). Moreover after receptor blockade, an ensuing infusion of the corresponding agonist (either 100 μm NPY or 3 μm NA) had no effect on hindlimb vascular conductance. At the end of each experiment animals were killed with an overdose of thiobutabarbital sodium.
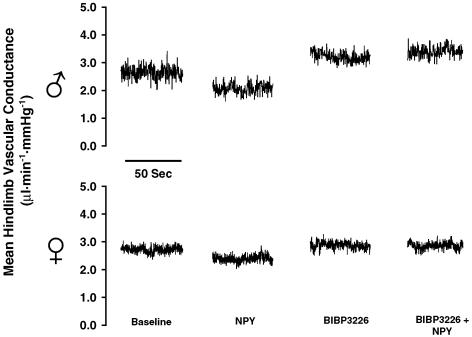
Figure 1.
Representative responses of mean hindlimb vascular conductance in males (top) and females (bottom) to illustrate complete Y1R blockade using BIBP3226 (100 μg kg−1). NPY (100 μm; 160 μl)-mediated vasoconstriction (Δ=−1.0 ± 0.03 μl min−1 mmHg−1 in males and −0.6 ± 0.03 μl min−1 mmHg−1 in females) was abolished by BIBP3226 (100 μg kg−1). This dose of BIBP3226 blocked any effect of a subsequent 100 μm infusion of NPY (n = 3 males, 3 females).
Data analysis
Functional consequences of Y1R and α1R blockade (n = 8 males, n = 7 females)
A Powerlab data acquisition system (ADInstruments) was used for real-time data collection. The pulsatile arterial blood pressure (ABP) signal was used to calculate heart rate (HR) and mean arterial pressure (MAP). Left hindlimb vascular conductance (VC) was calculated as the ratio of Qfem/MAP. For all conditions, Qfem, VC, MAP and HR were calculated as a 5 min stable average during the baseline period (Baseline) and as a 1 min average at the peak of the drug response (Drug).
The treatment effect was assessed using one-way analysis of variance (ANOVA) with repeated measures (Statistical Analysis System V.8.0.2, SAS Institute Inc., Cary, NC, USA). In the event of statistical significance (P < 0.05) Tukey's post hoc test was used to identify conditions that differed. The potential synergy between Y1R and α1R activation was assessed by comparing the sum of the drug responses from the BIBP3226 condition and prazosin condition against those of the BIBP3226 + prazosin condition using a paired t test. Data are presented as mean (± s.e.m.).
Cellular level analysis of NPY ligand and receptor (n = 6 males, n = 6 females)
Analyses were carried out on three different skeletal muscle groups known to contain differing expression of slow-twitch oxidative (SO), fast-twitch glycolytic (FG), and fast-twitch oxidative-glycolytic (FOG) fibre types. Skeletal muscle samples were taken from soleus (S; expressing SO > FOG fibres), white medial gastrocnemius (MG; expressing FG > FOG), and lateral gastrocnemius (LG; FOG > SO > FG) (Laughlin & Armstrong, 1983). The use of skeletal muscle groups expressing differing ratios of fibre types was based on early work by others illustrating that blood flow to such muscles is distributed differently at rest (Terjung & Engbretson, 1988) and during exercise (Armstrong & Laughlin, 1984; Terjung & Engbretson, 1988).
Y1R Western blotting
Approximately 70 mg of tissue was cut from the mid-belly of frozen muscle samples (from each of S, MG and LG), immediately homogenized in 15 volumes of extraction buffer (25% glycerol, 0.42 m NaCl, 1.5 mm MgCl2, 0.2 m EDTA, 20 mm Hepes, 10 μg ml−1 aprotinin; pH = 7.5), and centrifuged at 16000 g for 20 min in order to collect the supernatant as the tissue extract. Sample homogenates were then stored at −70°C until the time of total protein concentration determination and electrophoresis. Total protein concentration was accomplished using the Bradford protein assay (Bradford, 1976). Equal amounts of total protein (60 μg) were run on a 12% acrylamide mini gel (Bio-Rad, Hercules, CA, USA) overlaid with a 4% acrylamide stacking gel. After electrophoresis, the proteins were transferred at constant voltage in cold transfer buffer (10% running buffer, 20% methanol in ddH2O) to nitrocellulose membranes. The membranes were blocked in a 5% non-fat milk solution in Tris buffered saline + 0.05% Tween 20 (TTBS) (80 mm Tris Base, 0.5 m NaCl) overnight. Membranes were then incubated overnight in primary antibody specific to rat, human or mouse Y1-receptor (affinity purified rabbit antimouse Y1R IgG, Cat no. NPY1R11-A, Alpha Diagnostic International, San Antonio, TX, USA) at a dilution of 3 μg per ml in TTBS with 2% non-fat milk. Membranes were washed again in TTBS then incubated in secondary antibody (goat antirabbit antibody conjugated to horseradish peroxidase (HRP), BIO-RAD, Product no. 170-6518) in TTBS with 2% non-fat milk for 1 h. After washing, the blots were developed using enhanced chemiluminescent (ECL) Western blotting detection reagents (Amersham, Product no. RPN2106) and exposed to Kodak BioMax Light film. The films were scanned and subjected to densitometric quantification (Scion Image analysis software).
NPY immunoassay
NPY concentration was determined in whole muscle tissue homogenates (from each of S, MG and LG; see above for preparation of homogenate and total protein determination) and standards (50 μl duplicate samples) using a competitive immunoassay (Bachem Bioscience, King of Prussia, PA, USA). All samples were were incubated at room temperature for 2 h. The immunoplate was then washed 5 times with 300 μl per well of assay buffer. Wells were incubated at room temperature with 100 μl of streptavidin-HRP for 1 h. The immunoplate was washed again 5 times with 300 μl per well of assay buffer. Following washing, 100 μl of a tetramethylbenzidine (TMB) peroxidase substrate solution was added to all wells. After a 40 min incubation at room temperature the reaction was terminated by the addition of 100 μl 2 n HCl. Finally, the optical absorbance of each well was read at 450 nm (Bio-Rad Ultramark Microplate Imaging System, Bio-Rad, Hercules, CA, USA). Absorbance measures were converted to NPY concentration by comparison with the 10-point standard curve. Results are given as a ratio of pg NPY (μg tissue)−1, as computed from amount of total protein loaded per well. The assay has a minimum detectable concentration of 0.04–0.06 ng per ml or 2–3 pg per well (manufacturer's data).
NPY concentration and Y1R expression data were analysed using two-way analysis of variance (ANOVA) with the two factors being sex and muscle group (Statistical Analysis System V.8.0.2, SAS Institute Inc.). In the event of statistical significance (P < 0.05) Tukey's post hoc test was used to identify conditions that differed.
Results
Baseline
Animals were normotensive (93–104 mmHg) and exhibited normal HR (360–374 beats min−1) during the baseline period (Table 1). Furthermore, end tidal CO2 in all conditions remained in the normal range throughout the experiment (38–42 mmHg). Within each group baseline vascular tone was not different prior to each of the three conditions (Table 2). Therefore, functional responses are represented as the change in vascular conductance (ΔVC) as needed. Furthermore, vehicle infusion did not affect MAP, HR or vascular conductance. Representative tracings of hindlimb vascular conductance to the pharmacologic interventions are shown for a single male and female animal in Fig. 2.
Table 1.
Heart rate and blood pressure responses associated with each condition
| BIBP3226 | Prazosin | BIBP3226 + prazosin | |||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | ||
| Heart rate | Baseline | 362 ± 21 | 372 ± 23 | 360 ± 14 | 374 ± 28 | 371 ± 17 | 374 ± 29 |
| (beats min−1) | Drug | 351 ± 25 | 371 ± 23 | 358 ± 16 | 369 ± 28 | 377 ± 19 | 369 ± 31 |
| Mean arterial pressure | Baseline | 98 ± 3 | 104 ± 2 | 93 ± 3 | 99 ± 4 | 97 ± 3 | 96 ± 4 |
| (mmHg) | Drug | 92 ± 4 | 105 ± 3 | 81 ± 2* | 78 ± 5* | 88 ± 4* | 78 ± 4* |
Values represent mean ± s.e.m.
Significant difference from baseline (P < 0.05).
Table 2.
Baseline values of hindlimb vascular conductance before three pharmacological treatments
| BIBP3226 | Prazosin | BIBP3226 + prazosin | |
|---|---|---|---|
| Male | 3.5 ± 0.5 | 3.6 ± 0.4 | 4.2 ± 0.5 |
| Female | 2.5 ± 0.6 | 2.1 ± 0.7 | 2.1 ± 0.6 |
Values are means ± s.e.m. and are measured in μl min−1 mmHg−1.
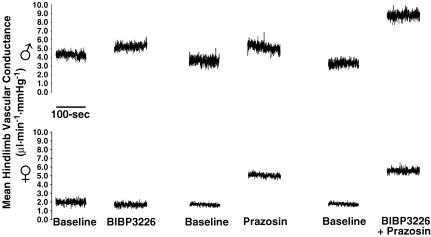
Figure 2.
Representative responses of mean hindlimb vascular conductance for each of the BIBP3226, prazosin, and BIBP3226 + prazosin treatments in males (top) and females (bottom).
Functional consequences of Y1R and α1R blockade
Effect of Y1R blockade (BIBP3226; Fig. 3)
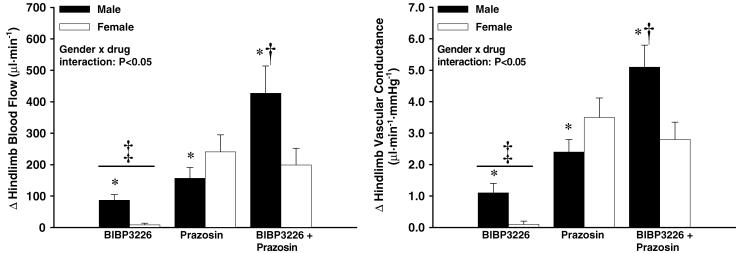
Figure 3.
Change in hindlimb blood flow (left; BF) and vascular conductance (right; VC) from baseline following each drug infusion. There was an increase in BF and VC from baseline in all conditions (P < 0.05) except in females with BIBP3226 infusion. * Indicates intracondition difference from female response (P < 0.05); † indicates different from all other conditions (male and female) (P < 0.05); ‡ indicates different from prazosin, and BIBP3226 + prazosin conditions (male and female) (n = 8 males, 7 females; P < 0.05).
MAP and HR were unchanged with Y1R antagonism (Table 1). Qfem and VC increased in males (87 ± 18 μl min−1 and 1.1 ± 0.3 μl min−1 mmHg−1, respectively; P < 0.05) but not in females.
Effect of α1R blockade (prazosin; Fig. 3)
Compared with baseline, infusion of prazosin caused a decrease in MAP for both males (from 93 ± 3 to 81 ± 2 mmHg; P < 0.05) and females (from 99 ± 4 to 78 ± 5 mmHg; P < 0.05). As expected (Hess, 1975; Massingham & Hayden, 1975; Oates et al. 1976; Mancia et al. 1980; Jackson et al. 2004) prazosin infusion did not affect HR in either group despite moderate hypotension that was greater in females than males (P < 0.05; see Table 1).
Compared with baseline, Qfem and VC increased in males (157 ± 34 μl min−1 and 2.4 ± 0.4 μl min−1 mmHg−1, respectively; P < 0.05) and also in females (241 ± 54 μl min−1 and 3.5 ± 0.6 μl min−1 mmHg−1, respectively; P < 0.05). The effect of prazosin on Qfem and VC was greater in females.
Effect of simultaneous Y1R and α1R blockade (BIBP3226 + prazosin; Fig. 3)
Compared with baseline, combined blockade caused a decrease in MAP for both males (from 97 ± 3 to 88 ± 4 mmHg; Table 1, P < 0.05) and females (from 96 ± 4 to 78 ± 4 mmHg; Table 1, P < 0.05) and no change in HR for either group. The drug-induced decrease in MAP was greater in females than males (Table 1, P < 0.05).
Compared with baseline, Qfem and VC increased in males (427 ± 87 μl min−1 and 5.1 ± 0.7 μl min−1 mmHg−1, respectively; P < 0.05) and also in females (199 ± 53 μl min−1 and 2.8 ± 0.6 μl min−1 mmHg−1, respectively; P < 0.05). The effect of combined blockade on Qfem and VC was greater in males (P < 0.05; Fig. 3).
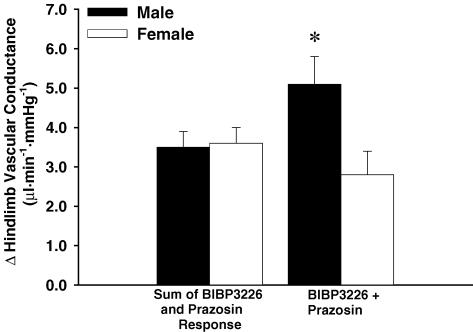
In males, combined blockade resulted in a greater increase in VC than the sum of the effects from the BIBP3226 and prazosin conditions (5.1 ± 0.7 versus 3.5 ± 0.6 μl min−1 mmHg−1; P < 0.05; Fig. 4).This effect was not observed in females.
Figure 4.
Comparison of the change in hindlimb vascular conductance between the sum of responses from BIBP3226 and prazosin conditions and the BIBP3226 + prazosin condition. * Indicates different from all other responses (male and female) (n = 8 males, 7 females; P < 0.05).
Tissue NPY and Y1 receptors
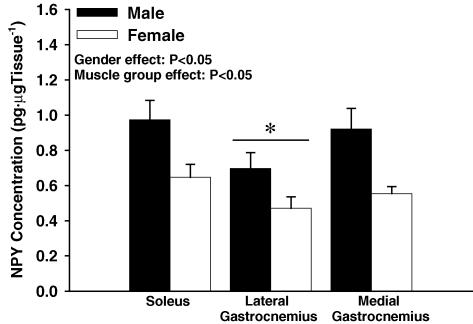
NPY concentration (Fig. 5)
Figure 5.
NPY concentration from whole muscle tissue homogenate in male (n = 6) and female (n = 6) rats measured using ELISA. Male muscle tissue had 35% greater NPY concentration than females (P < 0.05). * Indicates that the lateral gastrocnemius contained lower NPY concentration than the medial gastrocnemius and soleus (P < 0.05).
Males exhibited greater NPY concentration than females in whole muscle tissue homogenate (P < 0.05). Specifically, male versus female NPY concentration for each muscle group tested was: 0.97 ± 0.11 versus 0.65 ± 0.07 pg NPY (μg tissue)−1 for soleus; 0.70 ± 0.09 versus 0.47 ± 0.06 pg NPY (μg tissue)−1 for lateral gastrocnemius; 0.92 ± 0.12 versus 0.55 ± 0.04 pg NPY (μg tissue)−1 for medial gastrocnemius.
Y1R expression (Fig. 6)
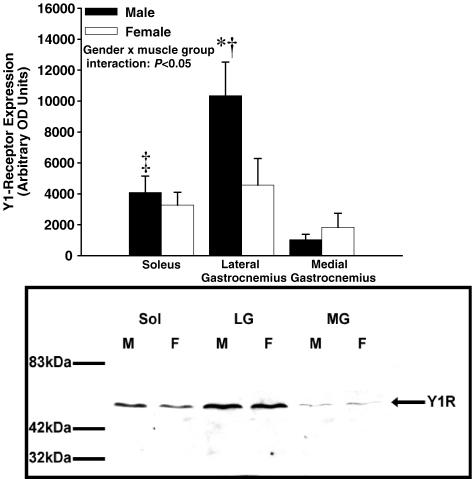
Figure 6.
Western blot analysis of Y1R from whole muscle tissue homogenate in male (n = 6) and female (n = 6) rats. Males tended to have greater overall Y1R expression; this can be attributed to the difference observed in the lateral gastrocnemius. * Indicates different from female (P < 0.05); † indicates different from all other muscle groups (male and female) (P < 0.05); ‡ indicates different from medial gastrocnemius for (P < 0.05; male and female).
A gender–muscle group interaction was observed for Y1R expression (P < 0.05). Post hoc analysis indicated that, compared with females, males exhibited greater overall Y1R expression in whole muscle tissue homogenate. This greater overall Y1R expression in males was due mainly to the high Y1R concentration in male lateral gastrocnemius (P < 0.05). Furthermore, male soleus muscle had greater Y1R expression than medial gastrocnemius (P < 0.05).
Discussion
Previous attempts to assess the role of Y1 receptors in vascular control have primarily relied on the functional response to the infusion of NPY, the receptor agonist. Although essential for determining the existence of receptors and interactions within physiological systems, the infusion of agonists does not address autogenous ligand–receptor interactions. In the current investigation highly selective receptor antagonists BIBP3226 and prazosin were used to unmask the Y1R and α1R synergism that may exist endogenously under baseline conditions. Therefore, the key novel finding in the present investigation was that, in contrast to males, female rats did not exhibit basal endogenous Y1R control of hindlimb vascular conductance (VC). In addition, males exhibited endogenous synergistic control of basal hindlimb VC via Y1R and α1R interactions where females did not. Compensatory elevations in α1R vascular regulation appeared to occur in the female animals so that baseline hindlimb haemodynamics were not dramatically different in the two groups. To the best of our knowledge, this is the first investigation to address the existence of gender-dependent differences in basal vascular control in skeletal muscle.
The current observation of endogenous Y1R control in the hindlimb of basal male Sprague-Dawley rats supports our earlier report (Jackson et al. 2004). The fact that only males exhibited Y1R modulation of VC illustrates sexual dimorphism in the control and maintenance of vascular tone in skeletal muscle. Although there was no observable difference in baseline flow in males and females, baseline vascular tone in males appears to be established by a balance and/or synergy between Y1R and α1R. This interaction was unmasked with simultaneous antagonism of Y1R and α1R, an effect that has been established, using exogenous agonists, in studies of blood pressure (Itoi et al. 1986) and vessel tone in human omental arteries, guinea pig uterine arteries and rabbit gastroepiploic, pulmonary and femoral arteries (Edvinsson et al. 1984a; Wahlestedt et al. 1985; Fallgren et al. 1993; Bergdahl et al. 1996). Thus, this Y1–α1 adrenoceptor ‘cross-talk’ mechanism appears to exist in several tissue types, at least in males. In contrast, females appear to compensate for the lower NPY control through the up-regulation of α1R modulation on hindlimb VC.
The functional data indicating reduced Y1R activation in females versus males are supported by the additional findings that males exhibited greater Y1R expression and NPY concentration in whole muscle tissue homogenate compared with females. These findings are consistent with observations of overall lower sympathetic nerve traffic in female versus male humans (Shoemaker et al. 2001). These data are also congruent with, and provide the mechanistic basis for, earlier findings of (a) greater and sustained pressor responses and NPY increases during stress in male versus female rats, and (b) greater pressor responses to exogenous NPY in areflexive pithed male versus female rats (Zukowska-Grojec et al. 1991). To our knowledge this is the first study to quantitatively measure Y1R expression in skeletal muscle. Available evidence indicates that peripheral Y1Rs exist solely as vascular receptors and are coupled to cAMP/phosolipase C, with increases in intracellular calcium upon activation causing constriction (Franco-Cereceda & Liska, 1998). Therefore, although the current observations were made in whole muscle homogenate, they should represent the actual potential for Y1R-induced smooth muscle contraction in the rat hindlimb. The low concentration of NPY in the interstitial space is difficult to assess with current technology. Therefore, in this study, the assessment of NPY was made from whole tissue homogenates, an approach that is not sensitive to contributions from neural, endothelial or interstitial sources of the neuropeptide. Although these details could not be examined the findings are consistent with the overall reduced Y1R activation in the female animals.
Nonetheless, Y1R expression was apparent in the females. Also, NPY concentration in the females was 65% of the level observed in the males in each muscle group. Thus, it was surprising that despite the presence of mechanisms for endogenous Y1R control of baseline vascular tone in both groups, there was no effect of Y1R blockade in the females. Based on pilot data and earlier work, it is believed that the dose of BIBP3226 was sufficient (see Fig. 1) (Jackson et al. 2004).
A possible explanation for the lack of Y1R control in the females, despite the presence of NPY and Y1 receptors, is that the bioavailability of NPY was limited in females. Specifically, sex differences in the modulation of prejunctional control of NPY release and/or its metabolism may exist. The complete NPY1 − 36 molecule binds and activates Y1R; however, the conversion of vasoconstrictive NPY1 − 36 to non-vasoconstrictive NPY3 − 36 or NPY2 − 36 occurs endogenously in the presence of endothelial NPY-converting enzyme dipeptidyl peptidase IV (DPPIV) (Lee et al. 2003) or aminopeptidase P (Mentlein & Roos, 1996), respectively. Each of NPY1 − 36 and its metabolites will activate prejunctional Y2 receptor-mediated inhibition of NPY release (Mentlein & Roos, 1996). Using rat tail arterial ring segments, Glenn et al. (1997) concluded that NPY-converting enzymes (peptidases) may be more active in females. This effect may reduce NPY availability for Y1R binding and enhance Y2 activation. Such differences in NPY metabolism may also explain some of the confusion regarding sex differences in sensitivity to exogenous NPY (Zukowska-Grojec et al. 1991; Glenn et al. 1997; Bischoff et al. 2000). The details of how gender modulates the impact of these peptidases on neurogenic vasomotor control, NPY metabolism and receptor binding affinities of NPY and its fragments will require additional and detailed experimentation. In a related manner, differences in autoreceptor expression must also be considered. Such data are not available.
Limitations
Blood vessels of skeletal muscle respond to changes in distending pressure that may interact with neurogenic constrictor control (Johnson, 1989). This myogenic effect may have contributed to the current results. For example, there was a greater drop in BP with prazosin infusion in females compared with males. However, it is unlikely that this was a major factor in defining the differences in male and female responses. Certainly, it cannot explain the absence of an effect of Y1R blockade or Y1R–α1R synergism in females because combined blockade (BIBP3226 + prazosin) still produced a greater increase in VC in males versus females despite a greater decrease in BP in females.
Examinations of NPY effects on vasopressin release suggest that menstrual phase may affect physiological responses to this neuropeptide (Sato et al. 1995). Whether or not this effect extends to endogenous Y1R activation has not been addressed and was not examined in the current study. However, we have observed previously that the generalized effect of gender on sympathetic nerve activity overwhelms any subtle effects of menstrual phase, should they occur (Shoemaker et al. 2001). Therefore, we expect that the current observations represent a robust gender effect that may only be slightly modified by menstrual phase.
We do not believe that the observed gender difference in the current study was dependent on the type of anaesthesia used (i.e. barbiturate versus non-barbiturate). In a previous study, as a control, the effect of barbiturate (Inactin) versus non-barbiturate (urethane and α-chloralose) anaesthetic on BIBP3226-induced vasodilation in male rats was addressed. The type of anaesthetic had no effect on the response (Jackson et al. 2004). In the current study, a control experiment was conducted on a different group of female rats anaesthetized with α-chloralose (80 mg kg−1) and urethan (500 mg kg−1). Again, the type of anaesthetic did not change the absence of a BIBP3226 effect in these females (i.e. the change in hindlimb vascular conductance from baseline with BIBP3226 infusion: α-chloralose and urethan (n = 4 females), 0.1 ± 0.06 μl min−1 mmHg−1; Inactin (n = 7 females), 0.1 ± 0.05 μl min−1 mmHg−1). These data cannot confirm whether the anaesthetic was impacting basal sympathetic nerve activity; however, they add confidence that the gender difference observed with Y1R blockade was not dependent on the type of anaesthesia.
Conclusions
These data provide evidence that Y1R activation contributes to the maintenance of basal hindlimb vascular tone in intact male but not in female rats. In addition, the control of basal male hindlimb vasculature is subject to the synergistic effects of Y1R and α1R co-activation. The observed gender differences can be partially explained by lower overall NPY concentration and lower Y1R expression. The chronic nature of Y1R activation in the male animals, and the differential distribution of Y1R in the various muscle types, suggests that this mechanism has a role in the distribution of microvascular perfusion and resistance, at least in males. The role of sex-specific dimorphism on NPY bioavailability due to differences in the release and/or metabolism of this neuropeptide remains to be addressed.
Acknowledgments
This research was supported by The Natural Science and Engineering Research Council of Canada (NSERC) and The Academic Development Fund from the University of Western Ontario (J. Kevin Shoemaker); The Heart and Stroke Foundation of Ontario (HSFO grant T5036, Earl G. Noble); D.N.J. was the recipient of a Heart and Stroke Foundation of Canada (HSFC)/Canadian Institutes of Health Research (CIHR) Institute of Gender and Health (IGH) Doctoral Research Award.
References
- Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. Am J Physiol. 1984;246:H59–68. doi: 10.1152/ajpheart.1984.246.1.H59. [DOI] [PubMed] [Google Scholar]
- Bergdahl A, Nilsson T, Cantera L, Nilsson L, Sun XY, Hedner T, Erlinge D, Valdemarson S, Edvinsson L. Neuropeptide Y potentiates noradrenaline-induced contraction through the neuropeptide Y Y1 receptor. Eur J Pharmacol. 1996;316:59–64. doi: 10.1016/s0014-2999(96)00636-x. [DOI] [PubMed] [Google Scholar]
- Bischoff A, Gerbracht A, Michel MC. Gender and hypertension interact to regulate neuropeptide Y receptor responsiveness. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:173–180. doi: 10.1007/s002109900175. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Hamann JJ, Kluess HA, Clifford PS. Vasoconstriction in exercising skeletal muscles: a potential role for neuropeptide Y? Am J Physiol Heart Circ Physiol. 2004;287:H144–149. doi: 10.1152/ajpheart.00071.2004. [DOI] [PubMed] [Google Scholar]
- Dynon MK, Jarrott B, Louis WJ. Tissue distribution and hypotensive effect of prazosin in the conscious rat. J Cardiovasc Pharmacol. 1983;5:235–239. doi: 10.1097/00005344-198303000-00012. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Ekblad E, Hakanson R, Wahlestedt C. Neuropeptide Y potentiates the effect of various vasoconstrictor agents on rabbit blood vessels. Br J Pharmacol. 1984a;83:519–525. doi: 10.1111/j.1476-5381.1984.tb16516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Emson P, McCulloch J, Tatemoto K, Uddman R. Neuropeptide Y: immunocytochemical localization to and effect upon feline pial arteries and veins in vitro and in situ. Acta Physiol Scand. 1984b;122:155–163. doi: 10.1111/j.1748-1716.1984.tb07493.x. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Hakanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984;8:225–235. doi: 10.1016/0167-0115(84)90064-8. 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- Ekelund U, Erlinge D. In vivo receptor characterization of neuropeptide Y-induced effects in consecutive vascular sections of cat skeletal muscle. Br J Pharmacol. 1997;120:387–392. doi: 10.1038/sj.bjp.0700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallgren B, Arlock P, Edvinsson L. Neuropeptide Y potentiates noradrenaline-evoked vasoconstriction by an intracellular calcium-dependent mechanism. J Auton Nerv Syst. 1993;44:151–159. doi: 10.1016/0165-1838(93)90027-r. 10.1016/0165-1838(93)90027-R. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A, Liska J. Neuropeptide Y Y1 receptors in vascular pharmacology. Eur J Pharmacol. 1998;349:1–14. doi: 10.1016/s0014-2999(98)00242-8. 10.1016/S0014-2999(98)00242-8. [DOI] [PubMed] [Google Scholar]
- Glenn TC, Krause DN, Duckles SP. Vascular responses to neuropeptide Y are greater in female than male rats. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:111–118. doi: 10.1007/pl00004908. [DOI] [PubMed] [Google Scholar]
- Glover WE. Increased sensitivity of rabbit ear artery to noradrenaline following perivascular nerve stimulation may be a response to neuropeptide Y released as cotransmitter. Clin Exp Pharmacol Physiol. 1985;12:227–230. doi: 10.1111/j.1440-1681.1985.tb02636.x. [DOI] [PubMed] [Google Scholar]
- Hanko JH, Tornebrandt K, Hardebo JE, Kahrstrom J, Nobin A, Owman C. Neuropeptide Y induces and modulates vasoconstriction in intracranial and peripheral vessels of animals and man. J Auton Pharmacol. 1986;6:117–124. doi: 10.1111/j.1474-8673.1986.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Hess HJ. Prazosin: biochemistry and structure-activity studies. Postgrad. Medical. 1975 Spec No 9–17. [PubMed] [Google Scholar]
- Itoi K, Mouri T, Takahashi K, Sasaki S, Imai Y, Yoshinaga K. Synergistic pressor action of neuropeptide Y and norepinephrine in conscious rats. J Hypertens Suppl. 1986;4:S247–250. [PubMed] [Google Scholar]
- Jackson DN, Noble EG, Shoemaker JK. Y1- and α1-receptor control of basal hindlimb vascular tone. Am. J. Physiol Regul. Integr. Comp Physiol. 2004;287:R228–R223. doi: 10.1152/ajpregu.00723.2003. [DOI] [PubMed] [Google Scholar]
- Johnson PC. The myogenic response in the microcirculation and its interaction with other control systems. J Hypertens Supplement. 1989;7:S33–S39. [PubMed] [Google Scholar]
- Jones PP, Snitker S, Skinner JS, Ravussin E. Gender differences in muscle sympathetic nerve activity: effect of body fat distribution. Am J Physiol. 1996;270:E363–366. doi: 10.1152/ajpendo.1996.270.2.E363. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Rat muscle blood flows as a function of time during prolonged slow treadmill exercise. Am J Physiol. 1983;244:H814–824. doi: 10.1152/ajpheart.1983.244.6.H814. [DOI] [PubMed] [Google Scholar]
- Lee EW, Michalkiewicz M, Kitlinska J, Kalezic I, Switalska H, Yoo P, Sangkharat A, Ji H, Li L, Michalkiewicz T, Ljubisavljevic M, Johansson H, Grant DS, Zukowska Z. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J Clin Invest. 2003;111:1853–1862. doi: 10.1172/JCI16929. 10.1172/JCI200316929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JM, Franco-Cereceda A, Hemsen A, Lacroix JS, Pernow J. Pharmacology of noradrenaline and neuropeptide tyrosine (NPY)-mediated sympathetic cotransmission. Fundam Clin Pharmacol. 1990;4:373–391. doi: 10.1111/j.1472-8206.1990.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Malmstrom RE. Neuropeptide Y Y1 receptor mechanisms in sympathetic vascular control. Acta Physiol Scand Supplement. 1997;636:1–55. [PubMed] [Google Scholar]
- Mancia G, Ferrari A, Gregorini L, Ferrari MC, Bianchini C, Terzoli L, Leonetti G, Zanchettie A. Effects of prazosin on autonomic control of circulation in essential hypertension. Hypertension. 1980;2:700–707. doi: 10.1161/01.hyp.2.5.700. [DOI] [PubMed] [Google Scholar]
- Massingham R, Hayden ML. A comparsion of the effects of prazosin and hydrallazine on blood pressure, heart rate and plasma renin activity in conscious renal hypertensive dogs. Eur J Pharmacol. 1975;30:121–124. doi: 10.1016/0014-2999(75)90213-7. 10.1016/0014-2999(75)90213-7. [DOI] [PubMed] [Google Scholar]
- Mentlein R, Roos T. Proteases involved in the metabolism of angiotensin II, bradykinin, calcitonin gene-related peptide (CGRP), and neuropeptide Y by vascular smooth muscle cells. Peptides. 1996;17:709–720. doi: 10.1016/0196-9781(96)00066-6. 10.1016/0196-9781(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Morris JL, Gibbins IL, Furness JB, Costa M, Murphy R. Co-localization of neuropeptide Y, vasoactive intestinal polypeptide and dynorphin in non-noradrenergic axons of the guinea pig uterine artery. Neurosci Lett. 1985;62:31–37. doi: 10.1016/0304-3940(85)90280-0. 10.1016/0304-3940(85)90280-0. [DOI] [PubMed] [Google Scholar]
- Neild TO. Actions of neuropeptide Y on innervated and denervated rat tail arteries. J Physiol. 1987;386:19–30. doi: 10.1113/jphysiol.1987.sp016519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- Oates HF, Graham RM, Stoker LM, Stokes GS. Haemodynamic effects of prazosin. Arch Int Pharmacodyn Ther. 1976;224:239–247. [PubMed] [Google Scholar]
- Pernow J, Ohlen A, Hokfelt T, Nilsson O, Lundberg JM. Neuropeptide Y: presence in perivascular noradrenergic neurons and vasoconstrictor effects on skeletal muscle blood vessels in experimental animals and man. Regul Pept. 1987;19:313–324. doi: 10.1016/0167-0115(87)90173-x. 10.1016/0167-0115(87)90173-X. [DOI] [PubMed] [Google Scholar]
- Qureshi NU, Dayao EK, Shirali S, Zukowska-Grojec Z, Hauser GJ. Endogenous neuropeptide Y mediates vasoconstriction during endotoxic and hemorrhagic shock. Regul Pept. 1998;75–76:215–220. doi: 10.1016/s0167-0115(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Sato K, Crofton JT, Wang YX, Share L. Effects of gender on the central actions of neuropeptide Y and norepinephrine on vasopressin and blood pressure in the rat. Brain Res. 1995;689:71–78. doi: 10.1016/0006-8993(95)00454-x. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol. 2001;281:H2028–2035. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- Sundler F, Böttcher G, Ekblad E, Håkanson R. PP, PYY, and NPY: Occurance and distribution in the periphery. In: Colmers WF, Wahlestedt C, editors. The Biology of Neuropeptide Y and Related Peptides. Tolowa: Humana Press Inc; 1993. [Google Scholar]
- Terjung RL, Engbretson BM. Blood flow to different rat skeletal muscle fiber type sections during isometric contractions in situ. Med Sports Exerc. 1988;20:S124–130. doi: 10.1249/00005768-198810001-00006. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Edvinsson L, Ekblad E, Hakanson R. Neuropeptide Y potentiates noradrenaline-evoked vasoconstriction: mode of action. J Pharmacol Exp Ther. 1985;234:735–741. [PubMed] [Google Scholar]
- Zukowska-Grojec Z. Neuropeptide Y. A novel sympathetic stress hormone and more. Ann N Y Acad Sci. 1995;771:219–233. doi: 10.1111/j.1749-6632.1995.tb44683.x. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Shen GH, Capraro PA, Vaz CA. Cardiovascular, neuropeptide Y, and adrenergic responses in stress are sexually differentiated. Physiol Behav. 1991;49:771–777. doi: 10.1016/0031-9384(91)90317-h. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Vaz AC. Role of neuropeptide Y (NPY) in cardiovascular responses to stress. Synapse. 1988;2:293–298. doi: 10.1002/syn.890020319. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Wahlestedt C. Origin and actions of neuropeptide Y in the cardiovascular system. In: Colmers WF, Wahlestedt C, editors. The Biology of Neuropeptide Y and Related Peptide. Totowa: Humana Press; 1993. pp. 315–388. [Google Scholar]